Abstract
While habituation develops to a repeated psychological stressor, manipulating certain parameters of the stress challenge experience may lead to dishabituation of the stress response. In this experiment, we investigated whether the behavioral, endocrine, and neural responses (c-fos mRNA immediate early gene expression) to a psychological stressor (restraint) differ when the duration of the stressor given on the test day violates expectations based on prior stress experience. Rats experienced 10 min of daily restraint on Days 1-4 followed by challenge with either the same duration (10 min) or a longer duration (30 min) of restraint on Day 5. Rats’ behavior was video recorded during the Day 5 restraint episode, and trunk blood and brain tissue were collected 30 min following restraint onset. Struggling behavior was manually scored as active attempts to escape the restraint device. Rats who experienced the same duration of repeated restraint showed a significant decrease of plasma corticosterone (CORT) compared to the 10 min acute restraint group (habituation). In addition, these rats showed decreased active struggling over repeated restraint trials. Conversely, the rats showed an increased CORT response (dishabituation) when they experienced a longer duration of restraint on Day 5 than they had previously. These rats showed a habituated behavioral response during the first 10 min of restraint, however struggling behavior increased once the duration of restraint exceeded the expected duration (with a peak at 12 min). This peak in struggling behavior did not occur during 30 min acute restraint, indicating that the effect was related to memory of previous restraint experience and not due to a longer duration of restraint. In contrast, these animals showed habituated c-fos mRNA expression in the paraventricular nucleus (PVN), lateral septum (LS), and medial prefrontal cortex (mPFC) in response to the increased stressor duration. Thus, there was dissociation between c-fos mRNA expression in key stress responsive brain regions and the behavioral and endocrine response to increased stressor duration. This dissociation may have been due to a greater lag time for c-fos mRNA responses to reflect the impact of a dishabituation response. In conclusion, habituation of the endocrine and behavioral stress response occurred when the duration of the stressor matches previous experience, while dishabituation of the stress response was triggered (with remarkable temporal precision) by an unexpected increase in stress duration.
Keywords: Stress, restraint, habituation, struggling behavior, CORT, ACTH, immediate early genes, c-fos
Introduction
Stress plays a predisposing and exacerbating role in a number of pathological physiological and psychological conditions, such as impaired immunity, cardiovascular disorders, major depressive illness, and chronic anxiety [1-3]. However, the influence of stress on physiological and psychological disorders is often difficult to analyze because an individual’s perception of a stressor and subsequent responses differ based on prior experience [4-6]. For example, habituation of a variety of stress-related measures (struggling behavior [7], HPA-axis activity [8], and sympathetic adrenomedullary activity [9]) occurs after repeated exposure to the same, or homotypic, stressor. Dysregulation of the neural circuitry that supports stress-response habituation may be involved in the etiology of some of these physiological and psychological disorders.
Restraint is widely used as a rodent model of psychological stress [10]. Manipulation of certain stimulus parameters associated with a stressor challenge condition may disrupt the expression of stress response habituation if a mismatch is detected between the current stressor situation and expectations surrounding that situation due to prior experience of that stressor. For example, Grissom et al. [11] document the importance of novel contextual cues in disrupting habituation to repeated restraint. Changing multiple sensory cues between restraint experiences, however, complicates the interpretation of observed associated changes in neural activity, since it is unclear whether the changes reflect a violation of expectations versus simply a response to a novel sensory stimulus. Although many experiments have used cues to address the predictability of both the onset and termination of a physical stressor [12-15], these experiments do not address the extent to which rats generate expectations of a stressor outcome itself, without developing associations with external cues. This is a psychological dimension of stress that is largely untested, and one that may play an important role in the development and expression of habituation to repeated psychological stress.
One parameter of restraint experience that can be easily manipulated without changing the sensory experience of restraint and the surrounding context is the restraint duration. Therefore, to test whether rats generate expectations of a stressor’s outcome based on prior experience, we gave rats a consistent duration of restraint (10 min) for the first four days of repeated restraint experience, and then increased the duration to 30 min on the last day of restraint experience. Through behavioral, neuroendocrine, and immediate early gene analyses (used as an indicator of relative activity of the limbic-hypothalamic-pituitary-adrenal axis), we investigated the hypothesis that habituated responses to repeated restraint are disrupted when the duration of restraint on the test day violates expectations based on prior stress experience. We expected that rats would show increased struggling behavior in response to an unexpected increase in restraint duration, and that this increase in behavior would be paralleled by increased secretion of corticosterone, as well as increased immediate early gene expression in stress responsive brain regions.
A number of immediate early genes are rapidly induced in select brain regions by stress experience and show significant habituation to repeated stress [16, 8], however c-fos mRNA is the best characterized. The c-fos gene encodes a transcription factor protein that regulates the expression of other genes that may be involved in neural adaptation to a stressful stimulus [17]. We chose three key stress-responsive brain regions to measure changes in c-fos mRNA expression: the paraventricular nucleus of the hypothalamus (PVN), the lateral septum (LS), and the medial prefrontal cortex (mPFC, both prelimbic and infralimbic subregions) to determine which of these regions might be involved in dishabituation of the stress response.
Activation of the PVN represents the first step in the hypothalamic-pituitary-adrenal (HPA) axis neuroendocrine response to stress. Neural activation of the PVN represents the convergence of signals from a number of limbic brain regions projecting both directly and indirectly to the PVN [18-20], which are ultimately responsible for the perception of the stressfulness of an experience. Therefore, if an unexpected increase in restraint duration results in increased HPA axis activity, this increase should also be reflected by an increase in c-fos mRNA in the PVN [21].
A considerable amount of research has focused on which brain regions may be involved in perception of stress and dysregulation of the stress response [18-20]. We have chosen to focus on the mPFC and LS based on our recent study in which we found that transient inactivation of the mPFC during initial exposure to restraint can interfere with the subsequent expression of HPA axis stress response habituation [22]. Moreover, in our recent study we found that the subsequent impaired expression of stress response habituation was selectively associated with relative c-fos mRNA levels in the mPFC and LS. These findings are consistent with other studies that observe altered PFC neural activity in stress-related disorders [23-25]. The prelimbic and infralimbic subregions of the rat mPFC have also been shown to provide regulatory control over stress-induced HPA axis activity [26-30]. Less is known about the role of the LS in stress response adaptation, but there is some evidence that the lateral septum (LS) is an important mediator of stress-related behaviors [31,32].
Materials and methods
2.1 Animal Procedures
Male Sprague-Dawley rats (285-320g at time of experimentation) were obtained from Harlan Sprague Dawley Inc. (Indianapolis, IN, USA) and were housed 2 per cage in polycarbonate tubs. All animals were given ad lib water and rodent chow and were given at least one week of acclimation after arrival to the animal facilities at the University of Colorado at Boulder. The colony room lights were maintained on a 12-h light/dark cycle, with lights on at 0700 h. Procedures for ethical treatment of animals conformed to the guidelines found in the “Guide for the Care and Use of Laboratory Animals,” DHHS Publication No. (NIH) 80-23, revised 2010 8th ed. and were approved by the University of Colorado Institutional Animal Care and Use Committee.
2.2 Experimental Design
Rats were divided into four treatment groups (n=12, N=48) according to restraint experience on Days 1-4 (repeated 10 min restraint vs. home cage) and duration of restraint on Day 5 (test day; 10 min vs. 30 min; see Table 1). Rats who experienced 10 min restraint on Days 1-4 and Day 5 were compared to rats that experienced 10 min acute restraint challenge for the first time to test for habituation. Conversely, rats that experienced 10 min restraint on Days 1-4 but experienced a longer duration (30 min) on Day 5, were compared to rats that experienced 30 min acute restraint challenge for the first time to test for dishabituation.
Table 1. Experimental design.
2×2 between groups factorial design: restraint experience on Days 1-4 (home cage vs. repeated restraint) by test day (Day 5) restraint challenge duration (10 min vs. 30 min) resulting in a total of 4 treatment groups (n = 12, N=48).
| n = 12 Treatment Group |
Repeated Restraint Experience (Days 1-4) | Restraint Challenge (Day 5) |
|---|---|---|
|
| ||
| Acute Stress 10 min challenge | — | 10 min |
| Repeated Restraint / 10 min Challenge | 10 min | 10 min |
| Acute Stress 30 min challenge | — | 30 min |
| Repeated Restraint / 30 min Challenge | 10 min | 30 min |
2.3 Restraint Procedures and Behavioral Recording
Rats were removed from their home cage and placed into a restrainer on a black tabletop in a room adjacent to their home cage room. Restrainers were cylindrical, adjustable length plexiglass tubes (15.5 ± 2.5 cm long and 6.3 cm diameter with air holes in the front, top and back). This version of restraint is considered to be primarily psychological in nature because it does not produce pain or direct physical insult [10]. Struggling behavior during restraint was recorded via a ceiling-mounted video camera. Light and heavy mobility were blindly scored in seconds and divided into 1 min bins using manual event recording software (courtesy of J. Christianson) according to criteria described by Grissom, Kerr, and Bhatnagar [7]. Since there were no treatment group differences in light mobility scores, only heavy mobility scores are reported as “active struggling.” All behavioral manipulations were performed between 0800 and 1400 with time of day counterbalanced between treatment conditions.
2.4 Tissue Preparation and Processing
Rats were sacrificed 30 min after restraint onset on Day 5 (10 min restraint groups were placed back in their home cages for 20 min before sacrifice, see Figure 1). Brains were flash frozen (isopentane bath maintained between -30 °C and -20 °C) and stored at -80 °C. Trunk blood was collected in ethylenediaminetetraacetic acid (EDTA)-coated tubes, placed on wet ice, and centrifuged for 15 minutes at 4 °C to collect plasma for hormone assays. Plasma aliquots were then snap frozen on dry ice and stored within 45 minutes of sacrifice.
Figure 1. Timeline of test day stress challenge.
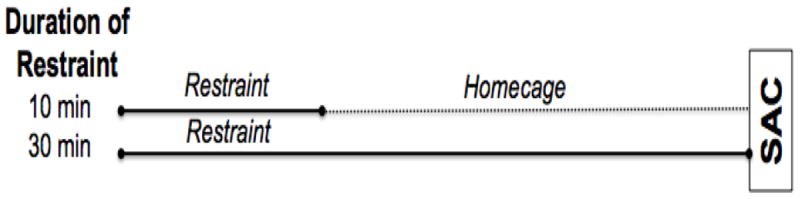
Rats challenged with 10 min of restraint on Day 5 were returned to their homecage for 20 min prior to sacrifice so that all treatment groups were killed 30 min after restraint onset. SAC=time of sacrifice.
2.5 Plasma Hormone Assays
Measurement of plasma corticosterone (CORT) was conducted in duplicate on 20 μl of heat-inactivated plasma with an enzyme immunoassay kit (Assay Designs, Ann Arbor, MI, USA) according to manufacturer’s instructions. Sensitivity for the corticosterone assay was 130 ng/100 ml and all samples were run in a single assay with a coefficient of variability of 7%. Plasma concentrations of adrenocorticotropic hormone (ACTH) were determined in duplicate (125 μl plasma) by competitive radioimmunoassay procedures previously described [33]. Radiolabeled 125I ACTH tracer was obtained from DiaSorin (Cat # 20515, Stillwater MN) and primary ACTH antiserum (rabbit antibody Rb7) was provided courtesy of Dr. Bill Engeland, University of Minnesota. The detection limit for this assay was 15 pg/ml and all samples were run in the same assay with an intra-assay coefficient of 10%.
2.6 In situ hybridization
We used in situ hybridization to examine c-fos mRNA expression in the brain. Coronal brain sections (12 μm) were cut on a cryostat (Leica Microsystems model 1850), thaw-mounted onto Colorfrost® plus microscope slides and stored at -80 °C. Series of sections were collected at the approximate rostral-caudal levels that contain the following brain regions as indicated in Paxinos and Watson [34]: (1) prefrontal cortex (3.2 mm anterior to bregma), (2) lateral septum (0.7 mm anterior to bregma), and (3) paraventricular nucleus of the hypothalamus (PVN; -1.8 mm posterior to bregma). In situ hybridization for c-fos mRNA was performed as described previously and utilized 35-S labeled riboprobes [8].
2.7 Autoradiographic image analysis
Semi-quantitative analyses of autoradiographs were performed on digitized images from X-ray films as described previously [35]. All analyses were performed with the aid of a rat brain atlas (Paxinos and Watson [34]) for guidance in determining proper anatomical placement of regions of interest (ROI) on digitized images with the following specifications. For prefrontal cortex, a square was centered within the dorsal medial PFC (dmPFC; approximate location of prelimbic cortex) or ventral medial PFC (vmPFC; approximate location of infralimbic cortex). For the lateral septum and PVN, the ROI was drawn around the perimeter of the visibly discriminable brain structure.
For all ROI analyses, an average gray level was determined for each rat by subtracting the ROI gray level from a background control reading from white matter in the same hemisphere. For each cortical ROI, at least six independent measurements across separate tissue sections from each brain were averaged. For each subcortical ROI, an average of at least three independent measurements were averaged per brain. Average integrated density was expressed as an average percent difference from acute 30 min restrained rats in order to allow for direct comparison of relative c-fos mRNA expression levels across brain regions.
2.8 Statistical Analyses
Data from two cohorts (n=6 for each cohort, n=12 total) were pooled and separate two-way ANOVAs were performed (SPSS 15) for each dependent measure (CORT, ACTH, c-fos mRNA). Three-way ANOVAs including cohort as a cofactor were also performed, however, c-fos in the IL mPFC was the only measure to show significant cohort interaction with the other factors (restraint duration on test day and restraint experience; see Results). For behavioral results, separate repeated measures ANOVAs were performed on the whole duration of restraint, plus the duration excluding the first minute, for both the 10 min and 30 min restraint groups. Post hoc analyses using one-tailed independent samples t-tests on each individual minute of restraint duration were performed to test for differences between groups based on restraint experience. We predicted a priori that rats with prior restraint experience would struggle less than restraint naïve rats during the first 10 min of restraint on day 5, but they would struggle more than restraint naïve rats during the remainder of the 30 min restraint challenge.
In cases where there were overall significant F-test results, post hoc pairwise comparisons of group differences of interest using Fishers Least Significant Difference Test (FLSD) are indicated on the data figures (alpha level, P≤0.05). Small differences in within-group degrees of freedom within a given experiment are due to the loss of a few plasma and histological samples due to sample preparation or assay-related problems. Data presented represent group averages ±SEM.
Results
3.1 Behavioral Results
Significant habituation of struggling behavior occurred both between trials due to restraint experience, as well as within the first 2 min of a 10 min trial of acute restraint. Comparisons of struggling behavior of both groups of rats who experienced 10 min restraint on Day 5 revealed that struggling behavior in the first two minutes of restraint habituated due to prior restraint experience (Figure 2). Repeated measures ANOVA of the entire restraint duration (minutes 1-10) revealed a significant main effect for time in restraint [F(9,198) = 7.06, p<0.001], as well as a significant interaction between time in restraint and restraint experience [F(9,198) = 5.071, p<0.001] due to the difference in behavior during the first minute between restraint naïve and experienced rats. One-tailed independent samples t-test revealed a significantly higher response in the first minute [acute vs. repeated restraint, t = 6.33, p<0.001] and second minute [t = 2.39, p<0.05], but not the third minute [t = -0.53, p=0.302], indicating that struggling behavior of restraint naïve rats had largely dissipated by the third minute of restraint.
Figure 2. Struggling behavior of rats challenged with 10 min of restraint: within and between session habituation within the first 2 min of restraint onset.
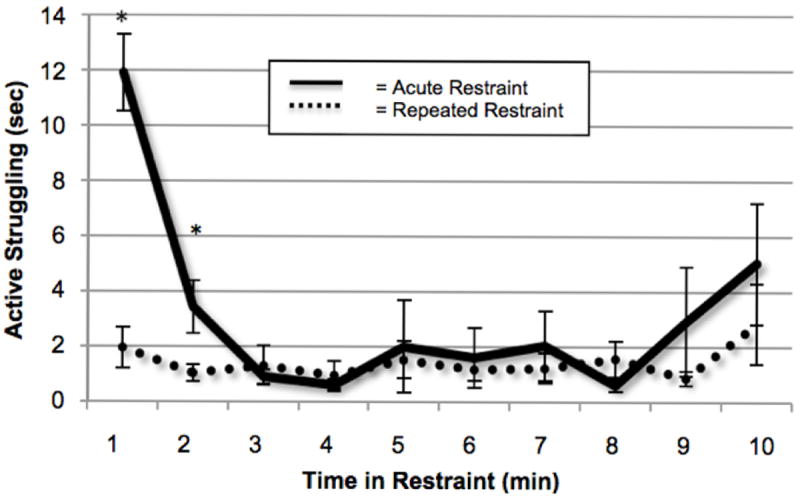
Graph shows struggling behavior on Day 5 of rats experiencing 10 min restraint for the first time (acute restraint, solid line) versus 10 min of restraint for the 5th time (repeated restraint, dashed line). * indicates significant one-tailed independent samples t-test between groups at that time interval, p<0.05, n=12.
For rats challenged with 30 min of restraint on the test day, we saw a similar habituation effect in struggling behavior in the first two minutes of restraint, both between trials due to restraint experience, as well as within the first 2 min of a 30 min trial of acute restraint (Figure 3). Repeated measures ANOVA for the whole duration of restraint (minutes 1-30) revealed a main effect for time in restraint [minutes 1 through 30, F(29,638) = 3.36, p<0.001] as well as an interaction between time in restraint and restraint experience [F(29,638) = 4.03, p<0.001] due primarily to the large difference in behavior during the first minute between naïve and experienced rats. One-tailed independent samples t-test revealed a significantly higher response in the first minute [acute vs. repeated restraint, t = 5.50, p<0.001] and second minute [t = 1.78, p<0.05], but not the third minute [t = 0.842, p=0.205], indicating that struggling behavior had habituated by the third minute of restraint.
Figure 3. Struggling behavior of rats challenged with 30 min of restraint: increased struggling when duration of restraint does not match previous experience (minutes 10 through 13).
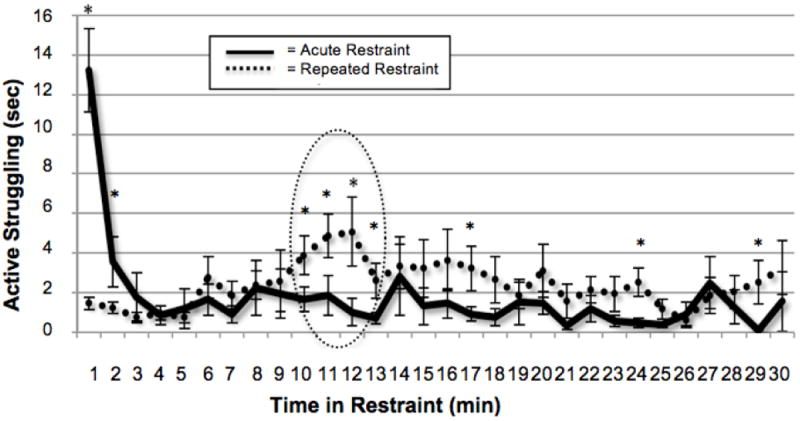
Graph shows struggling behavior of rats experiencing 30 min acute restraint (solid line) versus 10 min repeated restraint on Days 1-4, with 30 min restraint challenge on Day 5 (dashed line). Rats who experienced 10 min of repeated restraint on Days 1-4 show habituated struggling behavior during the first 10 min of restraint challenge on Day 5. However, between 10-13 min, when the duration exceeded what was previously experienced (dashed circle), the rats who were habituated to 10 min repeated restraint reinstate their struggling behavior, with a peak at 12 min. There is also a trend for increased struggling for the remainder of the duration of restraint, with significantly higher struggling at minutes 17, 24, and 29. * Indicates significant one-tailed independent samples t-test by restraint experience, p<0.05, n=12.
Importantly, rats challenged with an increase in restraint duration on Day 5 displayed increased struggling behavior that peaked at 12 min. Excluding the first minute of restraint from the repeated measures ANOVA revealed a significant main effect for restraint experience over the duration of restraint [minutes 2 through 30, F(1,22) = 6.10, p<0.05]. One-tailed independent samples t-test for minutes 2 through 30 revealed a significantly higher response at minutes 10, 11, 12, and 13, but not minute 14. There was a trend for increased struggling for the rest of the duration of restraint, with significantly higher struggling at minutes 17, 24, and 29.
3.2 Endocrine Results
The plasma CORT response habituated to repeated 10 min restraint, and this habituation was disrupted following increased stressor duration (Figure 4a). Two-way ANOVA of plasma CORT revealed significant main effects of both restraint experience [F(1,44) = 13.08, p<0.001] and duration of restraint on test day [10 min versus 30 min, F(1,44) = 42.60, p<0.001], with no significant interaction [F(1,44) = 1.70, p=0.199]. However, post hoc pairwise comparisons revealed no significant difference between groups experiencing 30 min restraint on test day (LSD p=0.109), supporting the presence of at least a partial dishabituation of the response of rats that had previously experienced 10 min of restraint each day. Plasma ACTH did not show habituation to repeated 10 min restraint (likely due to the timepoint of sacrifice; see discussion), but prior restraint experience did decrease response to 30 min restraint on the test day (LSD p<0.01) (Figure 4b). Two-way ANOVA of plasma ACTH revealed significant main effects of both restraint experience [F(1,44) = 6.20, p<0.05] and duration of restraint on test day [F(1,44) = 42.52, p<0.001], with no significant interaction [F(1,44) = 2.97, p=0.092].
Figures 4. CORT and ACTH responses to restraint challenge.
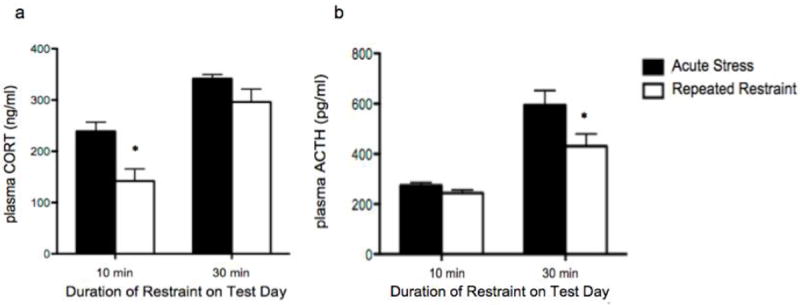
a and b Solid bars = acute stress, white bars = repeated restraint experience. a) Rats that received 10 min of repeated restraint show significant habituation of plasma CORT compared to rats challenged for the first time with 10 min restraint, but show no significant habituation when challenged with 30 min of restraint. b) No significant difference exists between plasma ACTH after 10 min acute or repeated restraint, however previous experience of 10 min restraint on Days 1-4 significantly decreases plasma ACTH response to 30 min restraint duration on test day. (* indicates significant LSD pair-wise comparison between differing restraint experience with same test day duration, p<0.05, n=12).
3.3 c-fos mRNA Expression
Each brain region included in the analysis showed a significant decrease in c-fos mRNA expression due to prior restraint experience, but each had a unique pattern of expression according to duration of restraint on test day (Figure 5). Most brain regions did not show a significant cohort interaction (with the exception of the infralimbic subregion of the medial prefrontal cortex; see below) therefore the F values reported are from a two-way ANOVA collapsed across cohorts.
Figure 5. All brain regions show significant habituation to repeated restraint experience (white bars), but show differing responses to acute restraint of varying duration (10 min vs. 30 min, solid bars).
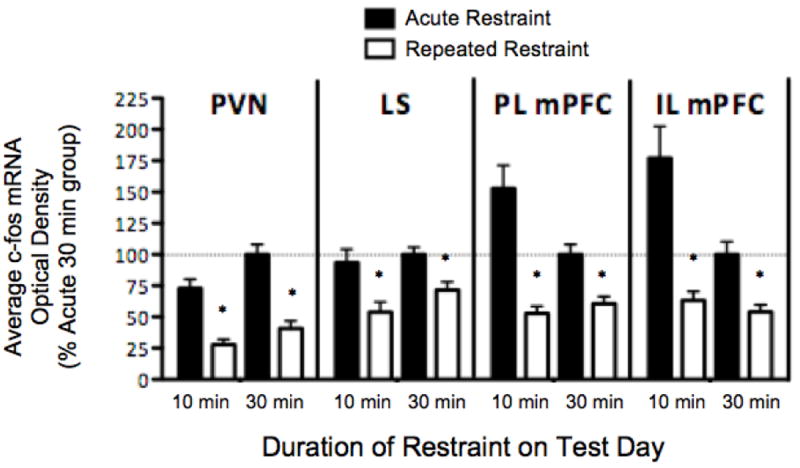
Data represent average optical density, reported as a % of the acute 30 min restraint group (100% set as the horizontal dashed line). * Indicates significant LSD pair-wise comparison between differing restraint experience with same test day duration, p<0.05, n=12.
Habituation of c-fos mRNA expression occurred in the paraventricular nucleus (PVN) regardless of increased restraint duration on test day. There was a significant main effect for restraint experience [F(1,44) = 62.96, p<0.001] and duration of restraint on test day [F(1,44) = 9.379, p<0.01], with no significant interaction [F(1,44) = 1.163, p=0.287].
Habituation of c-fos mRNA expression also occurred in the lateral septum (LS) regardless of increased restraint duration on test day. There was a significant main effect for restraint experience [F(1,44) = 17.91, p<0.001], but not for duration of restraint on test day [F(1,44) = 2.283, p=0.138], nor an interaction [F(1,44) = 0.490, p=0.487]. Therefore, LS did not show an increased c-fos mRNA response to a longer duration of acute restraint, nor did it show an increased response to a restraint duration that was longer than previously experienced.
Habituation of c-fos mRNA expression occurred in the prelimbic subregion of the medial prefrontal cortex (PL mPFC) regardless of increased stressor duration on test day. There was a significant main effect for restraint experience (F(1,44) = 27.796, p<0.001], but not for duration of restraint on test day [F(1,44) = 0.397, p=0.532] or an interaction [F(1,44) = 3.846, p=0.056]. Although there was a trend for an increased response to 10 min vs. 30 min acute restraint (LSD p=0.074), this difference was not significant. Therefore, PL mPFC did not show an increased c-fos mRNA response to a longer duration of acute restraint, nor did it show an increased response to a restraint duration that was longer than previously experienced.
The infralimbic subregion of the medial prefrontal cortex (IL mPFC) showed a significant cohort interaction with duration of restraint on test day [three-way ANOVA, F(1,40) = 4.976, p<0.05]. However there was a significant habituation of c-fos mRNA in repeated restraint rats for both cohorts and consequently this cohort interaction did not influence our ultimate interpretation. Habituation of c-fos mRNA expression occurred in the IL mPFC regardless of increased restraint duration on test day. There was a main effect for restraint experience [F(1,44) = 20.829, p<0.001], but not for duration of restraint on test day [F(1,44) = 2.517, p=0.120] or an interaction [F(1,44) = 1.716, p=0.197]. Therefore, IL mPFC did not show an increased c-fos mRNA response to a longer duration of acute restraint, nor did it show an increased response to a restraint duration that was longer than previously experienced.
Discussion
4.1 An unexpected increase in restraint duration caused a negative behavioral response to restraint challenge
The most striking result of this study is that rats exposed to repeated restraint remember the duration of restraint experience, and manipulation of restraint duration can result in dishabituation of behavioral and neuroendocrine responses to restraint challenge. This suggests that rats develop an expectation of stressor duration based on prior stressor experience. We found that prior restraint experience decreased the struggling response to repeated restraint, as reported before [7], and an unexpected increase in restraint duration was associated with an increase of struggling behavior that may indicate a negative emotional state. This increased struggling behavior began to emerge around the 10th min of restraint, and peaked at the 12th min after restraint onset. The peak in struggling behavior beginning prior to the anticipated end of restraint (during the 10th min) possibly reflected a learned anticipatory response to the expected release from restraint that then intensified upon detection of the extended restraint duration. This could be a sign of behavioral shaping, meaning that occasionally the rats struggled just before the end of the 10min restraint duration, and struggling at this particular moment led to negative reinforcement (removing the rat from restraint). Even though there was no cue indicating the end of the first 10min interval of the extended 30min restraint period, the rats may have learned that if they struggle once they have been in restraint for 10 minutes (but not during any other time) they will be removed from restraint. Since they were not removed from the restraint during the extended restraint period, this may have increased their struggling behavior for the remaining duration of restraint.
These results illustrate that rats show remarkable temporal precision when perceiving an increase in restraint duration, and previous research shows that rats are comparable to humans in their perception of time [36]. Time perception is supported by a cortical-striatal-thalamic-cortical loop and involves a three-step information processing model: 1) the clock stage, in which physical time is transformed into psychological time, 2) the memory stage, in which attentional processes guide whether information is temporally significant enough to be stored as memory, and 3) the decision stage, in which the stored time memory is compared to a sample value of the expected time of the event (stored in reference memory) [36, 37]. This timing ability, termed interval timing, possesses great flexibility, but is lacking in precision [38]; however, stress may play an important role in enhancing the attentional processes necessary for accurate memories of restraint duration. Therefore, the duration of restraint appears to be an important perceptual parameter that is a component of expectations based on previous experiences of psychological stress.
4.2 Neuroendocrine response to restraint increased following an unexpected increase in restraint duration
In support of the behavioral data, CORT levels showed some dishabituation to an unpredicted increase in restraint duration. However, changes in ACTH response to repeated restraint showed no solid evidence supporting either the habituation or dishabituation effects seen in both the behavior and CORT data. The ACTH response is expected to closely follow the CORT response as part of a tightly coupled cascade of HPA-axis responses. The disparity in the CORT and ACTH profile is likely due to aspects of the experimental design that were not optimal for observing changes in ACTH secretion. For instance, rats that were challenged with 10 min restraint on test day (both acutely- and repeatedly-restrained groups) were returned to their home cage for 20 min before sacrifice in order to hold constant across treatment groups the interval of time between restraint onset and sacrifice. During this 20 min period, much of the ACTH response that was initiated by the restraint experience had likely decayed, due to the short half-life of ACTH in the blood of adult rats (~ 4.5 min) [39, 40]. The ACTH response profile to 10 min acute restraint is less informative than expected due to this time consideration. It should also be noted that there is a time lag in plasma CORT elevation after HPA axis activation due to the obligatory adrenal cortical de novo synthesis of this steroid hormone. Thus, it is possible that increased HPA axis activity that may have been triggered by a dishabituation response around 12 min after stress onset would not be fully manifest at the CORT secretion level until more than 20 min later. The fact that rats with previous restraint experience had less CORT after 10 min of restraint challenge and the equivalent amount of CORT after 30 min of restraint challenge compared to restraint naïve rats on Day 5 suggests that they had a greater amount of HPA axis activation during the last 20 min of restraint challenge. Further experiments that employ repeated blood sampling using indwelling jugular catheters are needed to tease apart the timing of the ACTH and CORT responses relative to an unanticipated increase in stress duration. In addition, measuring the response of the sympathetic nervous system (SNS), as another stress response indicator, could provide greater temporal resolution than plasma HPA axis hormone measures, and the SNS measures may therefore better reflect the physiological element of the emotional response surrounding an unexpected increase in stress duration.
4.3 Lack of changes in immediate early gene expression as a result of an unexpected increase in restraint duration
We investigated mRNA expression changes in the immediate early gene, c-fos as an indicator of the neural response profile underlying the negative behavioral and endocrine response to an unexpected increase in restraint duration. In general, the patterns of c-fos mRNA expression in our chosen regions of interest do not support the dishabituation effect seen in the behavior and CORT data. Induction of the c-fos gene in neural tissue is associated with increased excitation of the neuron above basal levels of activity, especially by excitation patterns that are associated with neuroplasticity [17, 41, 42]. The amount of c-fos induction within a number of stress-reactive brain regions has been shown to vary with acute stressor intensity [21, 43]. Repeated exposure to the same stressor (homotypic stressor) typically results in habituation of the c-fos mRNA response that is often associated with behavioral habituation [8, 16, 44]. There is some evidence for intrinsic neuronal adaptation of immediate early gene induction to repeated drug and stress exposure, which may result in a shift from a primary c-fos gene induction response to a progressive increased expression of other immediate early genes, most notably delta Fos B [45-47]. However, a number of studies observe a robust c-fos gene induction in the brain of rodents when they are challenged with a novel stressor (heterotypic stressor) after habituation to a homotypic stressor [48-50].
Although the transient increase in struggling behavior observed in this study during restraint seems to be consistent with increased CORT secretion, that response may not have been persistent enough to trigger changes in expression of the immediate early gene, c-fos, in our regions of interest. Alternatively, there may not have been enough time after the onset of a dishabituation response to be reflected in elevated c-fos mRNA levels measured 30 min after restraint onset. Our results likely illustrate the temporal limitations of immediate early gene brain mapping, especially when examining a single time-point.
Most brain regions in this study showed habituation of the c-fos mRNA response to repeated restraint, although the degree of this habituation varied between brain regions. Figure 6 portrays the percent habituation expressed for each dependent measure when the rat was challenged with 10 or 30 min of restraint (depicted as % habituation between acute and repeated restraint groups). Plasma CORT showed dishabituation following increased duration of restraint on the test day, however, plasma ACTH showed more relative habituation to the increased restraint duration (30 min) than to the predicted stressor duration (10 min). There was similar habituation of c-fos mRNA in the PVN in response to increased stressor duration, however, in LS and PL/IL mPFC, there was less habituation in response to an increase in stressor duration that may be indicative of a supportive trend toward dishabituation. Further experiments implementing intracranial cannula manipulations to transiently inactivate the mPFC during repeated restraint experience will help determine whether the mPFC is necessary for dishabituation of the behavioral and endocrine stress response observed with an unexpected increase in restraint duration.
Figure 6. Varying degrees of habituation, expressed as the % difference in mean response between groups experiencing the same duration of restraint on test day (acute restraint group divided by repeated restraint group).
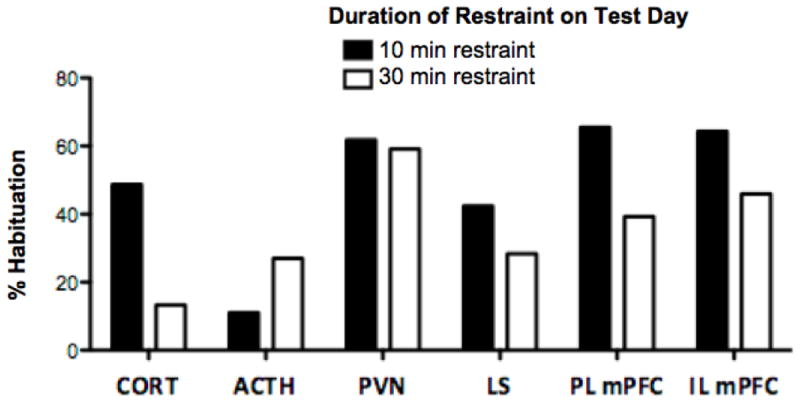
Solid bars = habituation between groups experiencing 10 min restraint on test day; White bars = habituation between groups experiencing 30 min restraint on test day. Plasma CORT shows much less habituation (dishabituation) following increased duration of restraint on test day, however, plasma ACTH shows more habituation to the increased restraint duration (30 min) than to the predicted stressor duration (10 min). C-fos mRNA expression profiles in all brain regions show habituation to repeated restraint, but to varying degrees. There is similar habituation in the PVN in response to increased stressor duration, however, in LS and PL/IL mPFC, there is less habituation in response to an increase in stressor duration.
In addition to a habituation effect, in some brain regions there was a significant effect of duration of acute restraint experience that occurred in the opposite direction as expected [51]. In PVN, 30 min acute restraint showed higher c-fos mRNA expression than 10 min acute restraint, as expected, however it did not show the dishabituation effect of CORT, as would be expected. This may be due to the difference in time course of induction between CORT and c-fos, where the c-fos mRNA induced around minute 12 did not have enough time to reach peak levels before the time of sacrifice (30 min after the beginning of restraint). An additional consideration is that c-fos mRNA is expressed in a number of other cells in the PVN that are not related to the release of CRH peptide (and thus, do not play a role in the initiation of the HPA-axis cascade of responses).
We did not see a difference in c-fos mRNA expression in the LS between 10 min and 30 min acute restraint, which may indicate a ceiling effect of expression. However, we expected the c-fos expression profile to follow the behavioral dishabituation response, since the LS plays an important role in regulating anxiety behavior [31, 32]. Changes in immediate early gene expression in LS may be dependent on the type of behavior observed, and may correlate more closely with other stress-related behaviors, such as stereotyped grooming [31], instead of with active attempts to escape the restraint tube.
There was an interesting effect of duration of acute restraint in the mPFC that did not follow our expectations. In both PL and IL subregions, 10 min acute restraint showed higher c-fos induction than 30 min acute stress. This may be due to the fact that the rats that were given only 10 min of restraint were put back in their home cage for 20 min before sacrifice. The mPFC has wide-reaching projections that include subcortical motor regions [52], therefore it may play a role in the increased motor activity seen in rats who have been returned to their home cage immediately following restraint experience (unpublished observation). Future experiments sampling multiple post restraint time-points or alternate immediate early genes, such as arc and deltaFosB [45,46, 53], in the analysis are needed to tease apart the relationship between neural activity and struggling behavior during restraint. It may also be fruitful to examine the potential role of the midbrain dopamine system in error detection and rule learning within this paradigm, particularly within the amygdala, nucleus accumbens and dorsal striatum [54]. This experiment presents a unique form of error detection in which an emotional response is triggered without an explicit environmental event. This differs from traditional associational learning in which a cue becomes associated with the termination of restraint. However, it is possible that the error detection processes that rats use to compare memories of previous restraint experience utilize very similar neural processes to traditional associational learning.
This experiment addresses an important psychological parameter of repeated restraint in which rats appear to develop expectations based on memories of the duration of previous restraint experience. This experimental design may be useful for future studies investigating which aspects of stressor experience memories support the expression of habituation to repeated psychological stress.
Conclusions
In summary, rats can encode the duration of repeated homotypic stressor experiences and behave as if they have formed an expectation of the duration of that particular stressor upon subsequent exposure. In addition, they are able to perceive an extended duration of stressor exposure independent from external cues, and that perception leads to a behavioral response that may be consistent with a negative emotional response. While struggling behavior and plasma CORT show dishabituation in response to an unexpected increase in restraint duration, plasma ACTH concentrations and c-fos mRNA expression patterns show dissociated responses that do not directly support dishabituation. Further experiments, including repeated blood sampling, additional timepoints for sacrifice, and additional immediate early gene analyses with different profiles of induction, are needed to determine which brain regions show responses that parallel an unpredicted increase in restraint duration. Once these regions of interest are identified, further experiments can manipulate the function of those brain regions to determine which regions are necessary for detection of mismatch between past and present restraint experience, and which brain regions contribute to the emotional, physiological and behavioral responses triggered by the detection of this mismatch.
Highlights.
CORT and struggling behavior increase in response to unexpected restraint duration.
Timing of this increase suggests restraint duration is a salient stressor memory.
C-fos mRNA shows habituation in PVN, LS, and mPFC regardless of restraint duration.
Dissociation highlights varying dynamics of c-fos mRNA, CORT/ACTH, and behavior.
Expectations of duration are an important parameter of psychological stress.
Acknowledgments
We would like to thank John P. Christianson for the use of his event-recording software for manually scoring struggling behavior. This work was supported by National Institute of Mental Health grant MH75968.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McEwen B. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- 2.Esch T, Stefano G, Fricchione G, Benson H. The role of stress in neurodegenerative diseases and mental disorders. Neuroendocrinol Lett. 2002;23:199–208. [PubMed] [Google Scholar]
- 3.Pittenger C, Duman R. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacol. 2008;33(1):88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 4.McEwen B, Wingfield J. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43(1):2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 5.Grissom N, Bhatnagar S. Habituation to repeated stress: get used to it. Neurobiol Learn Mem. 2009;92(2):215–24. doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bale T. Stress sensitivity and the development of affective disorders. Horm Behav. 2006;50(4):529–33. doi: 10.1016/j.yhbeh.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Grissom N, Kerr W, Bhatnagar S. Struggling behavior during restraint is regulated by stress experience. Behav Brain Res. 2008;191(2):219–26. doi: 10.1016/j.bbr.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girotti M, Pace T, Gaylord R, Rubin B, Herman J, Spencer R. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138(4):1067–81. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Masini C, Nyhuis T, Sasse S, Day H, Campeau S. Effects of voluntary wheel running on heart rate, body temperature, and locomotor activity in response to acute and repeated stressor exposures in rats. Stress. 2011;14(3):324–34. doi: 10.3109/10253890.2010.548013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buynitsky T, Mostofsky D. Restraint stress in biobehavioral research: Recent developments. Neurosci Biobehav Rev. 2009;33(7):1089–98. doi: 10.1016/j.neubiorev.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Grissom N, Iyer V, Vining C, Bhatnagar S. The physical context of previous stress exposure modifies hypothalamic-pituitary-adrenal responses to a subsequent homotypic stress. Horm Behav. 2007;51(1):95–103. doi: 10.1016/j.yhbeh.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Lemoine A, Armando I, Brun J, Barontini M, Segura E. Stressor predictability influences open field behavior, pain sensitivity and brain MAO inhibitory activity (tribulin) in the rat. Behav Brain Res. 1994;61:91–5. doi: 10.1016/0166-4328(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 13.Mormede P, Dantzer R, Michaud B, Kelley K, Le Moal M. Influence of stressor predictability and behavioral control on lymphocyte reactivity, antibody responses, and neuroendocrine activation in rats. Physiol Behav. 1988;43:577–83. doi: 10.1016/0031-9384(88)90211-9. [DOI] [PubMed] [Google Scholar]
- 14.Tsuda A, Ida Y, Satoh H, Tsujimaru S, Tanaka M. Stressor predictability and rat brain noradrenaline metabolism. Pharmacol Biochem Behav. 1989;32:569–72. doi: 10.1016/0091-3057(89)90198-6. [DOI] [PubMed] [Google Scholar]
- 15.Christianson J, Jennings J, Ragole T, Flyer J, Benison A, Barth D, et al. Safety signals mitigate the consequences of uncontrollable stress via a circuit involving the sensory insular cortex and bed nucleus of the stria terminalis. Biol Psychiatry. 2011;70(5):458–64. doi: 10.1016/j.biopsych.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stamp J, Herbert J. Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience. 1999;94(4):1313–22. doi: 10.1016/s0306-4522(99)00368-1. [DOI] [PubMed] [Google Scholar]
- 17.Herdegen T, Leah J. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- 18.Herman J, Cullinan W. Neurocircuitry of stress: central control of the hypothalamopituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 19.Herman J, Figueiredo H, Mueller N, Ulrich-Lai Y, Ostrander M, Choi D, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo pituitary adrenocortical responsiveness. Front Neuroendocrin. 2003;24(3):151–80. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Herman J, Ostrander M, Mueller N, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1201–13. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Pace T, Gaylord R, Topczewski F, Girotti M, Rubin B, Spencer R. Immediate-early gene induction in hippocampus and cortex as a result of novel experience is not directly related to the stressfulness of that experience. Eur J Neurosci. 2005;22:1679–90. doi: 10.1111/j.1460-9568.2005.04354.x. [DOI] [PubMed] [Google Scholar]
- 22.Weinberg M, Johnson D, Bhatt A, Spencer R. Medial prefrontal cortex activity can disrupt the expression of stress response habituation. Neuroscience. 2010;168(3):744–56. doi: 10.1016/j.neuroscience.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanco E, Castilla-Ortega E, Miranda R, Begega A, Aguirre J, Arias J, et al. Effects of medial prefrontal cortex lesions on anxiety-like behaviour in restrained and non-restrained rats. Behav Brain Res. 2009;201(2):338–42. doi: 10.1016/j.bbr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 24.McEwen B. Mood disorders and allostatic load. Biol Psychiatry. 2003;54(3):200–7. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 25.Hains A, Arnsten A. Molecular mechanisms of stress-induced prefrontal cortical impairment: implications for mental illness. Learn Mem. 2008;15(8):551–64. doi: 10.1101/lm.921708. [DOI] [PubMed] [Google Scholar]
- 26.Jones K, Myers B, Herman J. Stimulation of the prelimbic cortex differentially modulates neuroendocrine responses to psychogenic and systemic stressors. Physiol Behav. 2011;104(2):266–71. doi: 10.1016/j.physbeh.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radley J, Arias C, Sawchenko P. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26(50):12967–76. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vertes R. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 29.Radley J, Gosselink K, Sawchenko P. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29(22):7330–40. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heidbreder C, Groenewegen H. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27(6):555–79. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Bakshi V, Newman S, Smith-Roe S, Jochman K, Kalin N. Stimulation of lateral septum CRF2 receptors promotes anorexia and stress-like behaviors: functional homology to CRF1 receptors in basolateral amygdala. J Neurosci. 2007;27(39):10568–77. doi: 10.1523/JNEUROSCI.3044-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zarrindast M, Valizadegan F, Rostami P, Rezayof A. Histaminergic system of the lateral septum in the modulation of anxiety-like behaviour in rats. Eur J Pharmacol. 2008;583(1):108–14. doi: 10.1016/j.ejphar.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Nicholson W, Davis D, Sherrell B, Orth D. Rapid radioimmunoassay for corticotropin in unextracted human plasma. Clin Chem. 1984;30(2):259–65. [PubMed] [Google Scholar]
- 34.Paxinos G, Watson C. The rat brain in stereotaxis coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- 35.Campeau S, Akil H, Watson S. Lesions of the medial geniculate nuclei specifically block corticosterone release and induction of c-fos mRNA in the forebrain associated with audiogenic stress in rats. J Neurosci. 1997;17(15):5979–92. doi: 10.1523/JNEUROSCI.17-15-05979.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matell M, Meck W. Neuropsychological mechanisms of interval timing behavior. BioEssays. 2000;22:94–103. doi: 10.1002/(SICI)1521-1878(200001)22:1<94::AID-BIES14>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 37.Meck W, Benson A. Dissecting the brain’s internal clock: how frontal-striatal circuitry keeps time and shifts attention. Brain Cogn. 2002;48(1):195–211. doi: 10.1006/brcg.2001.1313. [DOI] [PubMed] [Google Scholar]
- 38.Paule M, Meck W, McMillan D, McClure G, Bateson M, Popke E, et al. The use of timing behaviors in animals and humans to detect drug and/or toxicant effects. Neurotox Teratol. 1999;21(5):491–502. doi: 10.1016/s0892-0362(99)00015-x. [DOI] [PubMed] [Google Scholar]
- 39.Garcia A, Marti O, Valles A, Dal-Zotto S, Armario A. Recovery of the hypothalamic-pituitary-adrenal response to stress: effect of stress intensity, stress duration, and previous stress exposure. Neuroendocrinol. 2000;72:114–25. doi: 10.1159/000054578. [DOI] [PubMed] [Google Scholar]
- 40.Vázquez D, Morano M, Taylor L, Akil H. Kinetics of radiolabeled adrenocorticotropin hormone in infant and weanling rats. J Neuroendocrinol. 1997;9(7):529–36. doi: 10.1046/j.1365-2826.1997.00608.x. [DOI] [PubMed] [Google Scholar]
- 41.Kubik S, Miyashita T, Guzowski J. Using immediate-early genes to map hippocampal subregional functions. Learn Mem. 2007;14(11):758–70. doi: 10.1101/lm.698107. [DOI] [PubMed] [Google Scholar]
- 42.Kovács K. Measurement of immediate-early gene activation: c-fos and beyond. J Neuroendocrinol. 2008;20(6):665–72. doi: 10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 43.Campeau S, Watson S. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J Neuroendocrinol. 1997;9(8):577–88. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- 44.Campeau S, Dolan D, Akil H, Watson S. C-fos mRNA induction in acute and chronic audiogenic stress: possible role of the orbitofrontal cortex in habituation. Stress. 2002;5(2):121–30. doi: 10.1080/10253890290027895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tekumalla P, Calon F, Rahman Z, Birdi S, Raiput A, Hornykiewicz O, et al. Elevated levels of deltaFosB and RGS9 in striatum in Parkinson’s disease. Biol Psychiatry. 2001;50:813–16. doi: 10.1016/s0006-3223(01)01234-3. [DOI] [PubMed] [Google Scholar]
- 46.Nestler E, Barrot M, Self D. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci USA. 2001;98(20):11042–46. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McClung C, Ulery P, Perrotti L, Zachariou V, Berton O, Nestler E. DeltaFosB: a molecular switch for long-term adaptation in the brain. Mol Brain Res. 2004;132(2):146–54. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 48.Weinberg M, Bhatt A, Girotti M, Masini C, Day H, Campeau S, Spencer R. Repeated ferret odor exposure induces different temporal patterns of same-stressor habituation and novel-stressor sensitization in both hypothalamic-pituitary-adrenal axis activity and forebrain c-fos expression in the rat. Endocrinol. 2009;150(2):749–61. doi: 10.1210/en.2008-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84(4):1025–39. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 50.Melia K, Ryabinin A, Schroeder R, Bloom F, Wilson M. Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. J Neurosci. 1994;14(10):5929–38. doi: 10.1523/JNEUROSCI.14-10-05929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weinberg M, Girotti M, Spencer R. Restraint-induced fra-2 and c-fos expression in the rat forebrain: relationship to stress duration. Neuroscience. 2007;150(2):478–86. doi: 10.1016/j.neuroscience.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gabbott P, Warner T, Jays P, Salway P, Busby S. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492(2):145–77. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 53.Trneckova L, Rotllant D, Klenerova V, Hynie S, Armario A. Dynamics of immediate early gene and neuropeptide gene response to prolonged immobilization stress: evidence against a critical role of the termination of exposure to the stressor. J Neurochem. 2007;100(4):905–14. doi: 10.1111/j.1471-4159.2006.04278.x. [DOI] [PubMed] [Google Scholar]
- 54.Stefani M, Moghaddam B. Rule learning and reward contingency are associated with dissociable patterns of dopamine activation in the rat prefrontal cortex, nucleus accumbens, and dorsal striatum. J Neurosci. 2006;26(34):8810–18. doi: 10.1523/JNEUROSCI.1656-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


