Abstract
Objectives
This study aimed to examine expression profile of MUC4 in intraductal papillary mucinous neoplasm of the pancreas (IPMN).
Methods
We performed immonohistochemistry (IHC) of MUC4 in 142 IPMNs, with evaluation of the specificity of two anti-MUC4 monoclonal antibodies (MAbs), 8G7 and 1G8, in cancer cell lines.
Results
MAb 8G7 showed a clear immunoreactivity, whereas MAb 1G8 did not show any immunoreactivity, in the Western blotting and IHC for human pancreatic carcinoma cell lines expressing MUC4 mRNA. However, IHC signals detected by both MAbs were observed in the tissue specimens. The expression rates of MUC4/8G7 detected by MAb 8G7 and MUC4/1G8 detected by MAb 1G8 in the intestinal-type IPMNs were significantly higher than those in the gastric-type IPMNs. In the intestinal-type IPMNs, MUC4/8G7 was expressed mainly in the cytoplasm of the neoplastic cells, whereas MUC4/1G8 was expressed mainly at the cell apexes. Even in the gastric-type IPMNs with rare MUC4 expression in the low-grade dysplasia, both MUC4 expression rates increased when dysplasia advanced.
Conclusions
A significantly higher expression of MUC4 in intestinal-type IPMNs than in gastric-type IPMNs will be one of the biomarkers to discriminate between the intestinal-type IPMNs with high malignancy potential from gastric-type IPMNs with low malignancy potential.
Keywords: Intraductal papillary mucinous neoplasm, intestinal-type, gastric-type, MUC4/8G7, MUC4/1G8, immunohistochemistry
INTRODUCTION
Intraductal papillary mucinous neoplasm of the pancreas (IPMN) is a mucin producing cystic neoplasm. IPMN was recognized by the World Health Organization (WHO) in 1996, and the WHO renamed as IPMN in 2000. 1 Currently, IPMN is the most common of the cystic neoplasms of the pancreas. IPMNs are classified into the following four subtypes: gastric-type, intestinal-type, pancreatobiliary-type, and oncocytic-type.2, 3 In 1993, we had first reported that PDAC showed MUC1 (pan-epithelial membrane-associated mucin)-positive but MUC2 (intestinal-type secretory mucin)-negative expression, whereas IPMN (named as “intraductal papillary tumor of the pancreas” in our article, since the term of IPMN was not reported yet) showed MUC1-negative but MUC2-positive expression, in the immunohistochemistry (IHC) study.4 In 1999, we also demonstrated that intestinal-type IPMNs (named “IPMT-villous dark cell type” in our article) showed MUC1-negative but MUC2-positive expression, whereas gastric-type IPMNs (named “IPMT-papillary clear cell type”) showed MUC1-negative and MUC2-negative expression.5 We also reported that invasive carcinoma derived from the intestinal-type IPMN shows de novo MUC1 expression, despite the absence of MUC1 expression in non-invasive lesions.6 In contrast, gastric-type IPMN rarely develops into carcinoma, and the survival of the patients with intestinal-type IPMN is significantly worse than those with gastric-type IPMN.6-8 Consequently, our series of IHC studies for mucin expression showed that MUC1 expression is related to invasive proliferation of the neoplasms and a poor outcome for the patients, whereas MUC2 expression is related to non-invasive proliferation of neoplasms and a favorable outcome for the patients, not only in neoplasms of the pancreatobiliary system but also in neoplasms of the other organs.8
MUC4 was first reported as a tracheobronchial mucin and is one of the membrane-associated mcuins.9 Recently, we found that a high expression of MUC4 in PDAC,10 intrahepatic cholangiocarcinoma mass-forming type11 and extrahepatic bile duct carcinoma12 is a new independent poor prognosis factor. To date, however, there has been no extensive study of MUC4 expression in IPMNs. We examined the expression profile of MUC4 in 142 IPMNs and found that MUC4 expression is mainly observed in intestinal-type IPMNs.
MATERIALS and METHODS
Patients and Tissue Samples
Between 1985 and 2011, surgical specimens of 142 IPMNs were obtained from the files of the Department of Pathology, Kagoshima University Hospital, and Department of Pathology, Kagoshima-shi Medical Association Hospital. The samples were classified on their hematoxylin-eosin (HE) staining findings, with IHC analysis of the mucin expression. The mean age of the patients was 66.7 years (range 42-91 years). The present study was approved by the ethical committee of both hospitals. All specimens were fixed in formalin, embedded in paraffin and cut into 4μm thick sections for IHC, in addition to the HE staining.
Evaluation of Monoclonal Antibodies for MUC4
IHC for MUC4 was performed using two mouse monoclonal antibodies (MAbs), 8G7 and 1G8. The MAb 8G7 was generated by Dr. Batra’ group at the University of Nebraska Medical Center, Omaha, USA.13 It has been confirmed that this monoclonal antibody was strongly reactive against the MUC4 peptide and with native MUC4 from human tissues or pancreatic cancer cells in Western blotting, IHC and confocal analysis.13 The MAb 1G8 (purchased from Invitrogen, Camarillo, CA, USA) is raised against rat sequence (rat ASGP-2). The antibody recognizes an epitope on the rat ASGP-2 subunit, which is corresponds to the human MUC4β subunit, and shows a cross reactivity with human samples.14 We evaluated the specificity of the MAb 8G7 and MAb 1G8 by Western blotting and IHC of six pancreatic cancer cell lines.
Cells and Culture Conditions
Human pancreatic carcinoma cell lines MiaPaca2, Panc1, AsPC1, BxPC3, HPAF2 and Capan1 were purchased from the American Type Culture Collection (Manassas, VA, USA). MiaPaca2 and Panc1 cells were maintained in DMEM (Sigma-Aldrich, St. Louis, MO, USA); AsPC-1 and BxPC3 cells were maintained in RPMI-1640 medium (Sigma-Aldrich); HPAF2 cells were maintained in Eagle's minimum essential medium (Sigma-Aldrich) and Capan1 cells were maintained in DMEM/F-12 (Sigma-Aldrich). All media were supplemented with 10% fetal bovine serum (GIBCO, Breda, the Netherlands) and 100 U/mL penicillin/100 μg/mL streptomycin (Sigma-Aldrich). All cells were incubated in 5% CO2 at 37°C and maintained at sub-confluent levels.
RNA extraction and RT-PCR
Total RNA was extracted from the cells using the RNeasy mini kit (Qiagen, Hilden, Germany) and quantified by NanoDrop ND-1000 spectrophotometer. The obtained mRNA was reverse transcribed to cDNA with the High Capacity RNA to cDNA kit (Applied Biosystems, Foster City, CA, USA). The following primers were designed for the subsequent PCR: MUC4, 5’-TGGGACGATGCTGACTTCTC-3’, 5’-CCCCGTTGTTTGTCATCTTTC-3’; ACTB, 5’-CTCTTCCAGCCTTCCTTCCTG-3’, 5’-GAAGCATTTGCGGTGGACGAT-3’. PCR was performed with the AmpliTaq Gold Fast PCR Master Mix (Applied Biosystems) following the manufacturer's protocol. Gene expression was normalized to the β-actin mRNA level in each sample.
Protein Extraction and Western Blotting
Total cell lysates were prepared using RIPA buffer containing protease inhibitor cocktail (Nacalai Tesque, Tokyo, Japan). The protein concentration was measured by the BCA assay (Thermo Scientific, Rockford, IL, USA). An equal amount of protein lysate was resolved on 2 % agarose gel containing SDS and passively transferred onto PVDF membrane for overnight at room temperature. Membranes were blocked with 1% skim milk / PBST over 2 hours and subjected to the standard immunodetection procedure using specific primary antibodies. The primary antibodies are as follows: MAb 8G7 (1:1000) and MAb 1G8 (1:1000), and anti-human α-tubulin MAb DM1A (1:2,000, Sigma-Aldrich, St. Louis, MO, USA).
Immunocytochemistry for Cultured Cells
For MUC4 staining in cultured cells, cells were seeded in 8-chamber slides (Becton Dickinson and Company, Franklin Lakes, NJ, USA) and incubated for overnight. Cells were fixed with 3.7 % formaldehyde for 10min at room temperature and stained with MAb 8G7 (1:24,000) and MAb 1G8 (1:4,000) for overnight at 4°C, respectively. Signal detection was performed by an immunoperoxidase method using a Vectastain Elite ABC kit (Vector Laboratories, Inc., Burlingame, CA, USA) according to the manufacturer's instructions.
Immunohistochemistry for Human Tissues
In addition to MUC4 expression in IPMNs to confirm the histologic subtypes by IHC finding as well as HE staining.6, 8 IHC was performed by the immunoperoxidase method as follows. Antigen retrieval was performed using CC1 antigen retrieval buffer (Ventana Medical Systems, Tucson, AZ, USA) for all sections. Following incubation with the primary antibodies (MAb 8G7 diluted 1:3000 and MAb 1G8 diluted 1:500), sections were stained on a Benchmark XT automated slide stainer using a diaminobenzidine detection kit (Ventana Medical Systems). Reaction products were not present when non-immune serum or PBS was used instead of the primary antibodies. Positive controls for antibody staining were run using tissues of pulmonary bronchus for MUC4. For simplicity, MUC4/8G7 and MUC4/1G8 are used to indicate the MUC4 antigens detected by each MAbs 8G7 and 1G7.
Classification of Types of IPMNs
The 142 IPMNs were classified into the following four subtypes: gastric-type, intestinal-type, pancreatobiliary-type, and oncocytic-type; and also into the following dysplastic grades: low-grade dysplasia (L), intermediate-grade dysplasia (I), high-grade dysplasia including carcinoma in situ (H), and invasive carcinoma (IC) according to the WHO Classification of Tumours of the Digestive Syetem2 on the basis of their morphological features and their IHC reactivity.
In IPMNs, when there were overlapping of more than two in the four subtypes and different dysplastic grades in one IPMN, we evaluated each histological subtype and dysplasia independently. Therefore, in the 142 IPMN specimens, we could evaluate 236 foci of various histological subtypes and dysplastic grades in total.
Evaluation of the Results by Scoring of immunohistochemistry of IPMNs
Four blinded investigators (I.K., M.H., M.H., and S.Y.) evaluated the IHC staining data independently. When the evaluation differed among the four, a final decision was made by consensus. Since immunohistochemical reaction for MUC4 in IPMNs usually is an all-or-none phenomenon with anti-MUC4 MAbs, 8G7 and 1G8, we did not incorporate the intensity in the grading of the staining. In addition, MUC4/8G7 was expressed diffusely in the cytoplasm, whereas MUC4/1G8 was expressed linearly along the cell apexes. Thus, the intracytoplasmic MUC4/8G7 expression looks like to have a high intensity than the cell apical MUC4/1G8 expression. Therefore, the results were evaluated based on the percentage of positively stained neoplastic cells, irrespective of the cellular localization of the immunostaining, using the following scoring system: 0, <5% of neoplastic cells stained; 1+, ≥5% to <25%; 2+, ≥25% to <50%; 3+, ≥50% to <75%; and 4+: ≥75% stained. Cases with ≥5% of neoplastic cells stained were considered to be positive. Statistical analysis was performed using SPSS (version 12.8).
RESULTS
Evaluation of Two Monoclonal Antibodies for MUC4
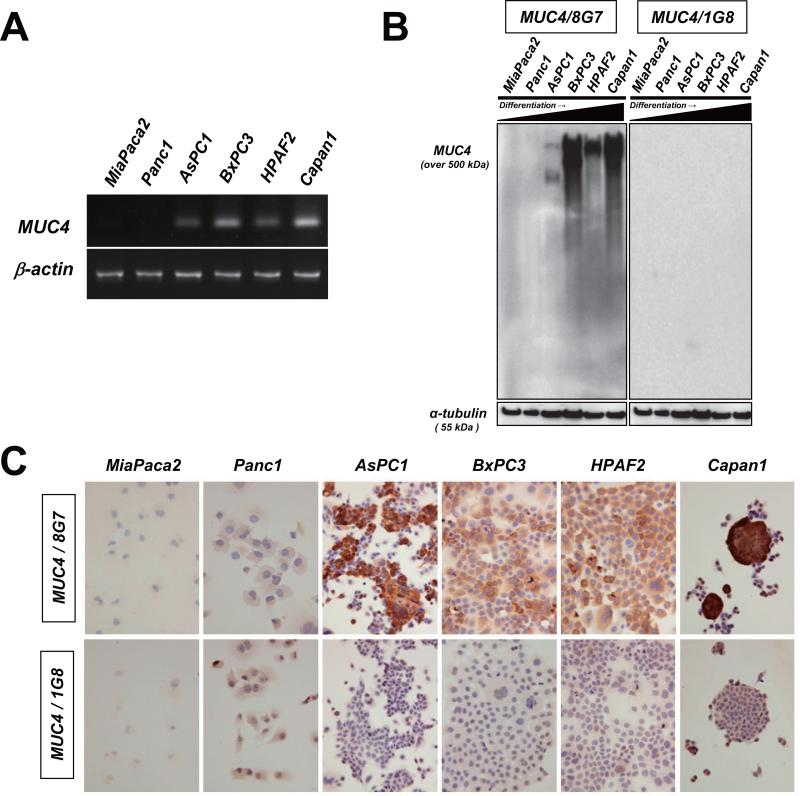
To examine the correlation between MUC4 expression and antibody specificities of two anti-MUC4 MAbs, 8G7 and 1G8, we carried out RT-PCR, Western blotting and IHC analysis using 6 pancreatic cancer cell lines. Consistent with the previous report,15 MUC4 mRNA was preferentially detected in cancer cells derived from well to moderately differentiated pancreatic cancer cell lines. (Fig. 1A). Our data also showed that MAb 8G7 recognized a very high molecular weight protein (over 500 kD, which was the expected size for native MUC4). Particularly, MUC4/8G7 expression was observed in cancer cells derived from well to moderately differentiated pancreatic cancers (Fig. 1B). In contrast, MAb 1G8 did not show any immunoreactive bands (Fig. 1B).
Figure 1.
The difference in antibody specificity between anti-human MUC4 monoclonal antibodie, 8G7 and 1G8. A: MUC4 mRNA expression was examined by RT-PCR. B: Cell lysates were immunoblotted and detected by the indicated antibodies, respectively. The α-tubulin served as a loading control. C: Formalin-fixed pancreatic cancer cells were processed for immunocytochemistry using the indicated antibodies, respectively. Original magnification x400.
The IHC analysis showed a similar pattern of MUC4 expression in 6 pancreatic cancer cell lines revealed by the Western blotting. MUC4/8G7 expression was observed in cancer cells derived from well to moderately differentiated pancreatic cancers (Fig. 1C). In contrast, MUC4/1G8 expression was not shown in any cell lines (Fig. 1C).
Expression Profiles of MUC4/8G7 and MUC4/1G8 in IPMNs according to the Classification by Subtype and Dysplasia
In the normal pancreatic tissue, MUC4/8G7 and MUC4/1G8 were not expressed, except for the constant expression of MUC4/1G8 at the vascular endothelium.
In 142 IPMNs, there were 90 pure gastric-type IPMNs, 39 pure intestinal-type IPMNs, 1 pure pancreatobiliary-type IPMN, 6 combine gastric and intestinal-type IPMNs, 6 combined gastric and pancreatobiliary-type IPMNs.
When we evaluated each histological subtype and dysplasia independently, there were 221 lesions of gastric-type IPMN [gastric-type IPMN-L (n=102), gastric-type IPMN-I (n=72), gastric-type IPMN-H (n=29) and gastric-type IPMN-IC (n=18)], 118 lesions of intestinal-type IPMN [intestinal-type IPMN-L (n=37), intestinal-type IPMN-I (n=44), intestinal-type IPMN-H (n=26) and intestinal-type IPMN-IC (n=11)], and 7 lesions of pancreatobiliary-type IPMN [pancreatobiliary-type IPMN-H (n=7)]. There was no oncocytic-type IPMN in the present study.
General difference in the expression rates of MUC4/8G7 and MUC4/1G8 between gastric-type IPMNs and intestinal-type IPMNs
The 7 lesions of the pancreatobiliary-type IPMN-H showed neither MUC4/8G7 nor MUC4/1G8 expression. Thus, we performed the statistical analyses for the MUC4/8G7 and MUC4/1G8 expression between intestinal-type IPMNs and gastric-type IPMNs.
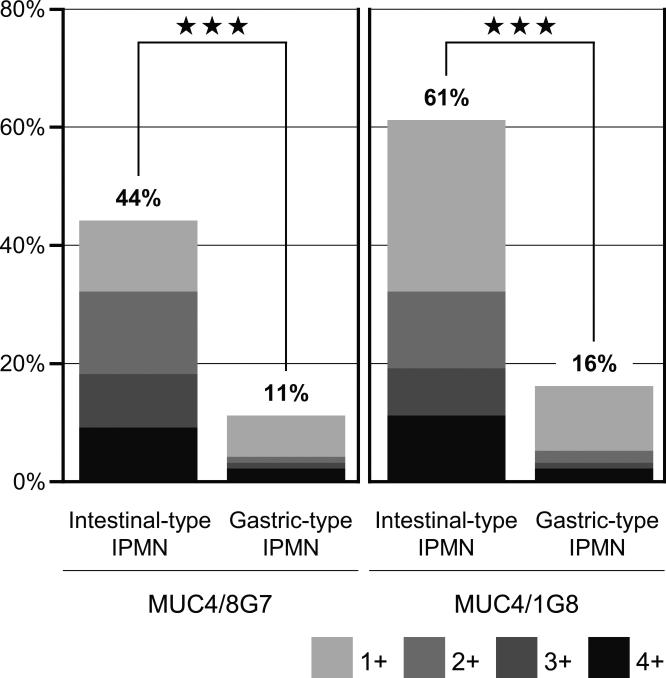
In total, the rates of positive expression (≥5% of the neoplastic cells stained) of MUC4/8G7 (44%, 52/118) and MUC4/1G8 (61%, 72/118) in intestinal-type IPMNs were significantly higher than those of MUC4/8G7 (11%, 24/221) and MUC4/1G8 (16%, 36/221) in the gastric-type IPMNs (p<0.0001, respectively) (Fig. 2). The detailed number and percentage of positively stained neoplastic cells using the scoring system were summarized in Table 1.
Figure 2.
The general difference in the expression rates of MUC4/8G7 (A) and MUC4/1G8 (B) between gastric-type IPMNs and intestinal-type IPMNs. In total, the rates of positive expression of MUC4/8G7 (44%, 52/118) and MUC4/1G8 (61%, 72/118) in intestinal-type IPMNs were significantly higher than those of MUC4/8G7 (11%, 24/221) and MUC4/1G8 (16%, 36/221) in the gastric-type IPMNs (A and B) . The detailed number and percentage of positively stained neoplastic cells using the grading system were summarized in Table 1. ★★★ indicate p<0.0001
Table 1.
Detailed number and percentage of positively stained neoplastic cells using the scoring system in IPMNs
| MUC4/8G7 | 0 | 1+ | 2+ | 3+ | 4+ | Total |
| Intestinal-type IPMN | 66 (56%) | 14 (12%) | 16 (14%) | 11 (9%) | 11 (9%) | 118 |
| Gastric-type IPMN | 197 (89%) | 16 (7%) | 2 (1%) | 2 (1%) | 4 (2%) | 221 |
| MUC4/1G8 | 0 | 1+ | 2+ | 3+ | 4+ | Total |
| Intestinal-type IPMN | 46 (39%) | 34 (29%) | 15 (13%) | 10 (8%) | 13 (11%) | 118 |
| Gastric-type IPMN | 185 (84%) | 24 (11%) | 5 (2%) | 3 (1%) | 4 (2%) | 221 |
*0 indicates <5% of neoplastic cells stained; 1+, ≥5% to <25%; 2+, ≥25% to <50%; 3+, ≥50% to <75%; 4+, ≥75%.
Detailed difference in the expression rates of MUC4/8G7 and MUC4/1G8 in each dysplastic lesions between gastric-type IPMNs and intestinal-type IPMNs
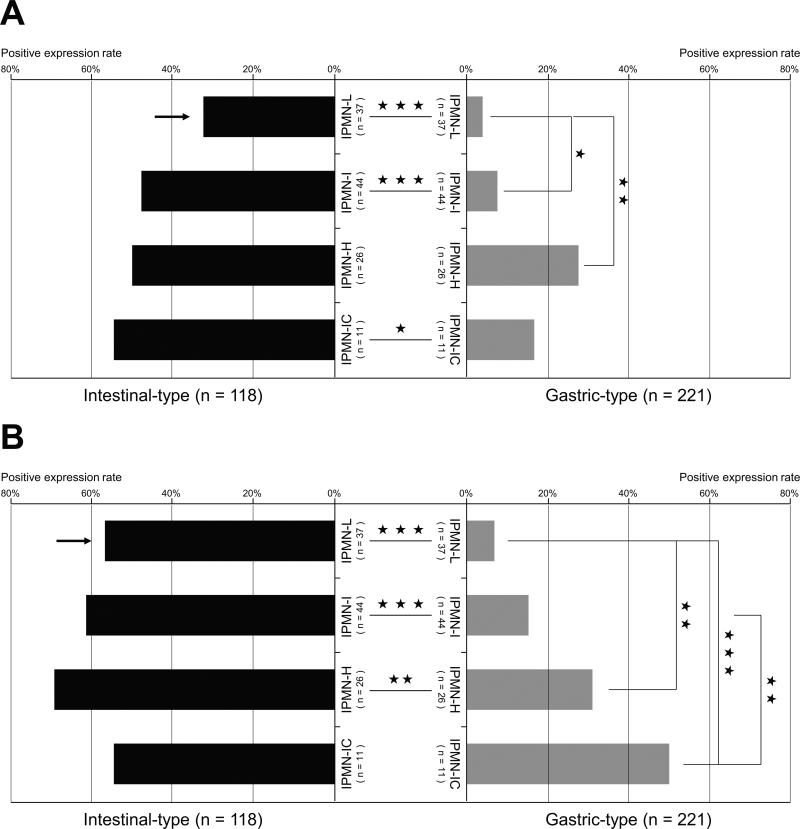
The independent evaluation of the 118 lesions of intestinal-type IPMN and the 221 lesions of gastric-type IPMN showed the following results, in the analysis of the rates of positive expression (≥5% of the neoplastic cells stained) (Fig. 3).
Figure 3.
A detailed difference of the expression rates of MUC4/8G7 (A) and MUC4/1G8 (B) in each dysplastic lesions between the 221 lesions of gastric-type IPMN and the 118 lesions of intestinal-type IPMN. The positive expression rates of MUC4/8G7 in the intestinal-type IPMNs were significantly higher than those in the gastric-type IPMNs (A). The positive expression rates of MUC4/1G8 in the intestinal-type IPMNs were significantly higher than those in the gastric-type IPMNs (B). In the gastric-type IPMNs, both MUC4/8G7 and MUC4/1G8 expression rates increased significantly with increasing the dysplastic grades (A and B). In each histological dysplastic grade, the positive expression rate of MUC4/8G7 in the intestinal-type IPMN-L was significantly lower than that of MUC4/1G8 in the intestinal-type IPMN-L (P<0.05) (A and B, arrows). L indicates low-grade dysplasia; I, intermediate-grade dysplasia; H, high-grade dysplasia including carcinoma in situ; IC, invasive carcinoma; ★★★, P<0.001; ★★, P<0.01; ★, P<0.05.
The expression rates of MUC4/8G7 (Fig. 3A) and MUC4/1G8 (Fig. 3B) in the intestinal-type IPMN-L (32%, 12/37; 57%, 21/37) were significantly higher than those in the gastric-type IPMN-L (4%, 4/102; 7%, 7/102) (P<0.001, respectively).
The expression rates of MUC4/8G7 (Fig. 3A) and MUC4/1G8 (Fig. 3B) in the intestinal-type IPMN-I (48%, 21/44; 61%, 27/44) were significantly higher than those in the gastric-type IPMN-I (13%, 9/72; 15%, 11/72) (P<0.001, respectively).
The expression rate of MUC4/8G7 in the intestinal-type IPMN-H (50%, 13/26) was higher than that in the gastric-type IPMN-H (23%, 8/29) but not significant (Fig. 3A), whereas the expression rate of MUC4/1G8 in the intestinal-type IPMN-H (69%, 18/26) was significantly higher than that in the gastric-type IPMN-H (31%, 9/29) (P<0.01) (Fig. 3B).
The expression rate of MUC4/8G7 in the intestinal-type IPMN-IC (55%, 6/11) was significantly higher than that in the gastric-type IPMN-IC (17%, 3/18) (P<0.05) (Fig. 3A), whereas the expression rate MUC4/1G8 in the intestinal-type IPMN-IC (55%, 6/11) was similar to that in the gastric-type IPMN-IC (50%, 9/18) (Fig. 3B).
In each histological dysplastic grade, the positive expression rate of MUC4/8G7 in the intestinal-type IPMN-L (32%, 12/37) was significantly lower than that of MUC4/1G8 in the intestinal-type IPMN-L (57%, 21/37) (P<0.05) (Fig. 3A and 3B, arrows). There is no significant difference in the positive expression rates between MUC4/8G7 and MUC4/1G8 in the other histological dysplastic grades (Fig. 3).
Comparison of MUC4/8G7 and MUC4/1G8 expression between intestinal-type IPMNs and gastric-type IPMNs
In the intestinal-type IPMNs, both MUC4/8G7 and MUC4/1G8 expression rates show tendencies to increase, but not significant (Fig. 3). In contrast, in the gastric-type IPMNs, both MUC4/8G7 and MUC4/1G8 expression rates increased significantly with increasing dysplastic grades (Fig. 3), although the MUC4/8G7 expression rate in the invasive carcinoma lesions of the gastric-type IPMNs is not so high (Fig. 3A).
Expression patterns of MUC4/8G7 and MUC4/1G8 in intestinal-type IPMNs
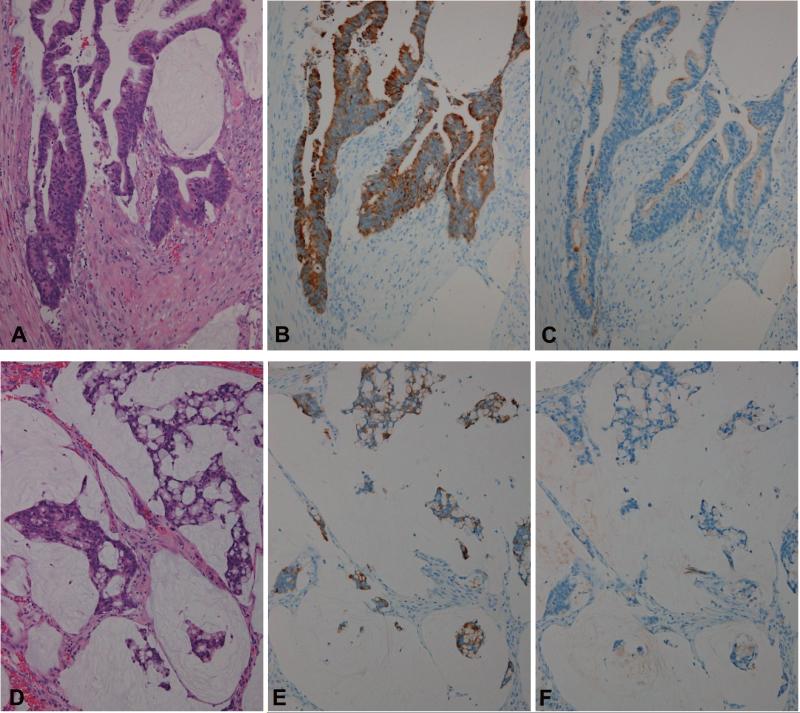
In the intestinal-type IPMNs (Fig. 4A), the early invasive carcinoma lesion (macroscopically noninvasive but microscopically showing focal stromal invasion) with mucin production derived from intestinal-type IPMN (Fig. 5A) and the mucinous/colloid carcinomas derived from intestinal-type IPMNs (Fig. 5D), MUC4/8G7 was expressed mainly in the cytoplasm of the neoplastic cells (Figs. 4B, 4C, 5B and 5E), whereas MUC4/1G8 was expressed mainly at the cell apexes (Figs. 4D, 4E, 5C and 5F).
Figure 4.
The expression pattern of MUC4/8G7 and MUC4/1G8 in intestinal-type IPMN-I (A-E) and gastric-type IPMN-L (F-K). Intestinal-type IPMN-I (A) showed MUC4/8G7 expression mainly in the cytoplasm of the neoplastic cells (B and C), whereas MUC4/1G8 expression was localized mainly at the cell apexes (D and E). Most of gastric-type IPMN-L (F) showed neither MUC4/8G7 expression (G and I) nor MUC4/1G8 expression (J and K). Note the expression of MUC4/1G8 at the vascular endothelium in the interstitial tissue (lower area of J). HE (A and F, Original magnification x100), MUC4/8G7 (B x100, C x400, G x100, I x400) and MUC4/1G8 (D x100, E x400, J x100, K x400).
Figure 5.
The expression pattern of MUC4/8G7 and MUC4/1G8 in the early invasive carcinoma lesion with mucin production derived from intestinal-type IPMN-H (A-C) and mucinous/colloid carcinoma derived from intestinal-type IPMN (D-F). In the early invasive carcinoma lesion with mucin production derived from intestinal-type IPMN-H (A), MUC4/8G7 was expressed mainly in the cytoplasm of the neoplastic cells (B), whereas MUC4/1G8 was expressed mainly at the cell apexes (C). In the mucinous/colloid carcinoma derived from intestinal-type IPMN (D), MUC4/8G7 was also expressed mainly in the cytoplasm of the neoplastic cells (E), whereas MUC4/1G8 was expressed sparsely in a small number of the neoplastic cells in the secreted mucin in this case (F). HE (A and D, Original magnification x200; C, x100), MUC4/8G7 (B and E x200) and MUC4/1G8 (C and F x200).
Expression patterns of MUC4/8G7 and MUC4/1G8 in gastric-type IPMNs
In most of the gastric-type IPMNs-L (Figs. 4F and 6A-right side) to gastric-type IPMNs-I, neither MUC4/8G7 (Figs. 4G, 4I, and 6B-right side) nor MUC4/1G8 (Figs. 4J, 4K, and 6C-right side) was expressed.
Figure 6.
Expression pattern of MUC4/8G7 and MUC4/1G8 in gastric-type IPMN-H (A-C) and invasive tubular adenocarcinoma derived from gastric-type IPMN (D-F). In the gastric-type IPMN, MUC4/8G7 was expressed mainly in the cytoplasm of the neoplastic cells showing high-grade dysplasia (B, left side), whereas it was not expressed in the neoplastic cells showing low-grade dysplasia (B, right side). In contrast, MUC4/1G8 was expressed mainly at the cell apexes of the neoplastic cells showing high-grade dysplasia (C, left side), whereas it was not expressed in the neoplastic cells showing low-grade dysplasia (C, right side). In the invasive tubular adenocarcinoma derived from gastric-type IPMN (D), MUC4/8G7 was expressed mainly in the cytoplasm of the neoplastic cells (E), whereas MUC4/1G8 was expressed mainly at the cell apexes (F). HE (A and D, Original magnification x200), MUC4/8G7 (B and E x200) and MUC4/1G8 (C and F x200).
In the gastric-type IPMNs-H (Fig. 6A-left side) and the invasive tubular adenocarcinoma derived from gastric-type IPMN (Fig. 6D), MUC4/8G7 was expressed mainly in the cytoplasm of the neoplastic cells (Figs. 6B-left side and 6E), whereas MUC4/1G8 was expressed mainly at the cell apexes of the neoplastic cells (Figs. 6C-left side and 6F).
DISCUSSION
The expression of MUC4 has been studied in PDAC tissue samples and cell lines, and its relation to malignant progression has been elucidated.16 We have reported that MUC4 expression is a very useful predictor of a poor prognosis in patients with pancreatobiliary carcinomas.10-12 The expression of MUC4 is a useful indicator of malignancy potential in PDACs,10, 17 but there have been no studies of MUC4 expression in IPMNs. In the present study, our results indicate that the expression rates of MUC4/8G7 and MUC4/1G8 in intestinal-type IPMN were significantly higher than those in gastric-type IPMNs not only in total but also in most of the histological grades.
There are controversial results from clinicopathological studies of MUC4 expression in human carcinomas, which were determined by two MAbs, 8G7 or 1G8. In most studies using 8G7, MUC4/8G7 expression is related to aggressive tumor behavior or a poor outcome in human carcinomas.10-12, 18-20 In contrast, most of the studies using 1G8, described that loss of MUC4/1G8 expression is related to poor survival,21-23 expect for one study of PDAC.24 The major problem is due to the undefined differences of specificities between MAb 8G7 and MAb 1G8. Our evaluation of the MAb 8G7 and MAb 1G8 by Western blotting and IHC of pancreatic cancer cell lines revealed that MAb 8G7 showed specific immunoreactivity, whereas MAb 1G8 does not show any immunoreactivity. Expression of MUC4 mRNA was detected in the pancreatic cancer cell lines in the present study and also has previously been shown in pancreatic cancer cell lines by RT-PCR and northern blot analyses.15 These difference may be related to the low homology (about 60%) between human MUC4 detected by MAb 8G7 and rat Muc4 detected by MAb 1G8.13, 25
However, not only MAb 8G7 but also MAb 1G8 reacts with human IPMN tissues, although the locations of MUC4/8G7 and MUC4/1G8 expression showed marked difference. The cytoplasmic expression pattern of MUC4/8G7 observed in IPMNs is seen also in PDAC, the intrahepatic cholangiocarcinoma-mass forming type and extrahepatic bile duct carcinoma.10-12 The intracellular localization of MUC4/8G7 may be explained by the reason that MAb 8G7 is made against an unglycosylated form of MUC4. In contrast, the linear expression pattern of MUC4/1G8 along with the cell apexes of IPMNs may be a cross reaction with human tissues, since the expression pattern at the cell apexes is not consistent with the fact that MAb 1G8 recognizes an epitope corresponding to the human MUC4β subunit which is located in the intracytoplasmic C-terminal position of the MUC4 molecule.14
The distinction of IPMN subtypes is very important from the clinical perspective, in terms of the imaging diagnosis using US, CT and MRI, and also in the evaluation of the malignancy potential. Intestinal-type IPMNs with MUC2-positive expression are located mainly in the main pancreatic duct and show high frequencies of malignant transformation and invasive carcinoma (usually mucinous/colloid carcinoma) with de novo MUC1 expression in the invasive carcinoma lesions.3, 6, 8 In contrast, gastric-type IPMNs with MUC2-negative expression are usually located in the pancreatic branch duct and rarely show malignant transformation.3, 6, 7 The MUC4 expression mainly in the intestinal-type IPMNs may be a helpful marker to distinguish the dangerous intestinal-type IPMNs from the safe gastric-type IPMNs.
Our study series showed that the methylation status, mRNA expression, and mucin core protein expression were well correlated with each other for MUC1, MUC2, MUC4 and MUC5AC,8, 26, 27 which were identified as useful tumor markers in combination in our IHC studies of pancreatobiliary neoplasms.8 Recently, we developed a novel highly sensitive and specific Methylation Specific Electrophoresis (MSE) method to analyze the DNA methylation status of MUC1, MUC2, MUC4 and MUC5AC in not only cancer cell lines but also in human tissues and pancreatic juices.28 We are trying to apply the MSE method to the combined analyses of the epigenetic status of MUC1, MUC2, MUC4 and MUC5AC in pancreatic juice, for the early detection of the pancreatic lesions.8, 28
In conclusion, the significantly high expression of MUC4 in intestinal-type IPMNs than in gastric-type IPMNs will be one of the biomarkers to discriminate intestinal-type IPMNs with high malignancy potential from gastric-type IPMNs with low malignancy potential. These findings will be important information on the application of the MSE method for the analysis of pancreatic juice from patients with pancreatic cystic lesion.
ACKNOWLEDGEMENTS
We thank Mr. Y. Atsuchi, Mr. K. Matsuo, Ms. C. Baba, Ms. Y. Nishimura, Ms. S. Yoshimura and Ms. I. Houjou for their technical assistance. This study was supported in part by Princes Takamatsu Cancer Research Fund (11-24319) to S. Yonezawa; Grants-in-Aid for Scientific Research on Scientific Research (B) 23390085 to S. Yonezawa; Scientific Research (C) 21590399 to M. Higashi; Young Scientists (B) 24701008 to S. Yokoyama; Scientific Research on Priority Areas 239349 to S. Kitamoto (JSPS Fellowship) from the Ministry of Education, Science, Sports, Culture and Technology, Japan; Kagoshima Medical Association to I. Kitazono, Japan; a Pancreas Reserch Foundation of Japan to S. Yokoyama; and the Kodama Memorial Foundation, Japan to S. Yokoyama and M. Higashi. S.Batra is supported in part by the NIH grants (CA78590, CA163120, CA133944 and CA111294).
Footnotes
I.K. and M.H. contributed equally to this work.
Conflict of Interest Disclosures: The authors made no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Iwao Kitazono, Department of Human Pathology, Field of Oncology, Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan; Cardiovascular and Gastroenterological Surgery, Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan.
Michiyo Higashi, Department of Human Pathology, Field of Oncology, Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan.
Sho Kitamoto, Department of Human Pathology, Field of Oncology, Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan.
Seiya Yokoyama, Department of Human Pathology, Field of Oncology, Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan.
Michiko Horinouchi, Department of Human Pathology, Field of Oncology, Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan; Department of Pathology, Kagoshima-shi Medical Association Hospital, Kagoshima, Japan.
Masahiko Osako, Department of Surgery, Kagoshima-shi Medical Association Hospital, Kagoshima, Japan.
Takeshi Shimizu, Department of Pathology, Kagoshima-shi Medical Association Hospital, Kagoshima, Japan.
Mineo Tabata, Department of Surgery, Kagoshima-shi Medical Association Hospital, Kagoshima, Japan.
Surinder K. Batra, Departments of Biochemistry and Molecular Biology, Eppley Institute for Research in Cancer and Allied Diseases, University of Nebraska Medical Center, Omaha, USA.
Masamichi Goto, Department of Human Pathology, Field of Oncology, Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan; National Sanatorium Hoshizuka-Keiaien, Kanoya, Kagoshima, Japan.
Suguru Yonezawa, Department of Human Pathology, Field of Oncology, Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan.
REFERENCES
- 1.Longnecker DS, Albert G, Hruban RH. In: Intraductal papillary-mucinous neoplasms of the pancreas. Hamilton SA, Aaltonen LA, editors. World Health Organization Classification of Tumours, Pathology and Genetics of Tumours of the Digestive System; 2000. pp. 237–241. [Google Scholar]
- 2.Adsay NV, Fukushima N, Furukawa T, et al. In: Intraductal neoplasms of the pancreas. Hamilton SA, Aaltonen LA, editors. World Health Organization Classification of Tumours, Pathology and Genetics of Tumours of the Digestive System; 2010. pp. 304–313. [Google Scholar]
- 3.Furukawa T, Hatori T, Fujita I, et al. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut. 2011;60:509–516. doi: 10.1136/gut.2010.210567. [DOI] [PubMed] [Google Scholar]
- 4.Osako M, Yonezawa S, Siddiki B, et al. Immunohistochemical study of mucin carbohydrates and core proteins in human pancreatic tumors. Cancer. 1993;71:2191–2199. doi: 10.1002/1097-0142(19930401)71:7<2191::aid-cncr2820710705>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.Yonezawa S, Horinouchi M, Osako M, et al. Gene expression of gastric type mucin (MUC5AC) in pancreatic tumors: its relationship with the biological behavior of the tumor. Pathol Int. 1999;49:45–54. doi: 10.1046/j.1440-1827.1999.00823.x. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura A, Horinouchi M, Goto M, et al. New classification of pancreatic intraductal papillary-mucinous tumour by mucin expression: its relationship with potential for malignancy. J Pathol. 2002;197:201–210. doi: 10.1002/path.1109. [DOI] [PubMed] [Google Scholar]
- 7.Horinouchi M, Nagata K, Nakamura A, et al. Expression of Different Glycoforms of Membrane Mucin(MUC1) and Secretory Mucin (MUC2, MUC5AC and MUC6) in Pancreatic Neoplasms. Acta Histochem Cytochem. 2003;36:443–453. [Google Scholar]
- 8.Yonezawa S, Higashi M, Yamada N, et al. Mucins in human neoplasms: clinical pathology, gene expression and diagnostic application. Pathol Int. 2011;61:697–716. doi: 10.1111/j.1440-1827.2011.02734.x. [DOI] [PubMed] [Google Scholar]
- 9.Carraway KL, Ramsauer VP, Haq B, Carothers Carraway CA. Cell signaling through membrane mucins. Bioessays. 2003;25:66–71. doi: 10.1002/bies.10201. [DOI] [PubMed] [Google Scholar]
- 10.Saitou M, Goto M, Horinouchi M, et al. MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas. J Clin Pathol. 2005;58:845–852. doi: 10.1136/jcp.2004.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shibahara H, Tamada S, Higashi M, et al. MUC4 is a novel prognostic factor of intrahepatic cholangiocarcinoma-mass forming type. Hepatology. 2004;39:220–229. doi: 10.1002/hep.20031. [DOI] [PubMed] [Google Scholar]
- 12.Tamada S, Shibahara H, Higashi M, et al. MUC4 is a novel prognostic factor of extrahepatic bile duct carcinoma. Clinical Cancer Research. 2006;12:4257–4264. doi: 10.1158/1078-0432.CCR-05-2814. [DOI] [PubMed] [Google Scholar]
- 13.Moniaux N, Varshney GC, Chauhan SC, et al. Generation and characterization of anti-MUC4 monoclonal antibodies reactive with normal and cancer cells in humans. J Histochem Cytochem. 2004;52:253–261. doi: 10.1177/002215540405200213. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Perez A, Yasin M, et al. Presence of MUC4 in human milk and at the luminal surfaces of blood vessels. J Cell Physiol. 2005;204:166–177. doi: 10.1002/jcp.20277. [DOI] [PubMed] [Google Scholar]
- 15.Andrianifahanana M, Moniaux N, Schmied BM, et al. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res. 2001;7:4033–4040. [PubMed] [Google Scholar]
- 16.Balague C, Gambus G, Carrato C, et al. Altered expression of MUC2, MUC4, and MUC5 mucin genes in pancreas tissues and cancer cell lines. Gastroenterology. 1994;106:1054–1061. doi: 10.1016/0016-5085(94)90767-6. [DOI] [PubMed] [Google Scholar]
- 17.Swartz MJ, Batra SK, Varshney GC, et al. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am J Clin Pathol. 2002;117:791–796. doi: 10.1309/7Y7N-M1WM-R0YK-M2VA. [DOI] [PubMed] [Google Scholar]
- 18.Hamada T, Wakamatsu T, Miyahara M, et al. MUC4: a novel prognostic factor of oral squamous cell carcinoma. International journal of cancer. Journal international du cancer. 2012;130:1768–1776. doi: 10.1002/ijc.26187. [DOI] [PubMed] [Google Scholar]
- 19.Tsutsumida H, Goto M, Kitajima S, et al. MUC4 expression correlates with poor prognosis in small-sized lung adenocarcinoma. Lung Cancer. 2007;55:195–203. doi: 10.1016/j.lungcan.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Shanmugam C, Jhala NC, Katkoori VR, et al. Prognostic value of mucin 4 expression in colorectal adenocarcinomas. Cancer. 2010;116:3577–3586. doi: 10.1002/cncr.25095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weed DT, Gomez-Fernandez C, Pacheco J, et al. MUC4 and ERBB2 expression in major and minor salivary gland mucoepidermoid carcinoma. Head & neck. 2004;26:353–364. doi: 10.1002/hed.10387. [DOI] [PubMed] [Google Scholar]
- 22.Kwon KY, Ro JY, Singhal N, et al. MUC4 expression in non-small cell lung carcinomas: relationship to tumor histology and patient survival. Archives of Pathology & Laboratory Medicine. 2007;131:593–598. doi: 10.5858/2007-131-593-MEINCL. [DOI] [PubMed] [Google Scholar]
- 23.Weed DT, Gomez-Fernandez C, Yasin M, et al. MUC4 and ErbB2 expression in squamous cell carcinoma of the upper aerodigestive tract: correlation with clinical outcomes. The Laryngoscope. 2004;114:1–32. doi: 10.1097/00005537-200408001-00001. [DOI] [PubMed] [Google Scholar]
- 24.Westgaard A, Schjolberg AR, Cvancarova M, et al. Differentiation markers in pancreatic head adenocarcinomas: MUC1 and MUC4 expression indicates poor prognosis in pancreatobiliary differentiated tumours. Histopathology. 2009;54:337–347. doi: 10.1111/j.1365-2559.2009.03227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaturvedi P, Singh AP, Batra SK. Structure, evolution, and biology of the MUC4 mucin. FASEB J. 2008;22:966–981. doi: 10.1096/fj.07-9673rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada N, Nishida Y, Tsutsumida H, et al. MUC1 expression is regulated by DNA methylation and histone H3 lysine 9 modification in cancer cells. Cancer Res. 2008;68:2708–2716. doi: 10.1158/0008-5472.CAN-07-6844. [DOI] [PubMed] [Google Scholar]
- 27.Yamada N, Nishida Y, Tsutsumida H, et al. Promoter CpG methylation in cancer cells contributes to the regulation of MUC4. Br J Cancer. 2009;100:344–351. doi: 10.1038/sj.bjc.6604845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yokoyama S, Kitamoto S, Yamada N, et al. The application of methylation specific electrophoresis (MSE) to DNA methylation analysis of the 5' CpG island of mucin in cancer cells. BMC Cancer. 2012;12:67. doi: 10.1186/1471-2407-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]