Abstract
In an earlier published telephone interview study (n > 1,000) we have shown that retired shift workers subjectively report worse sleep than retired day workers. This laboratory study sought to determine whether these findings held up when objective polysomnograhic (PSG) measures of sleep were taken and whether retirees' circadian temperature rhythms differed as a function of shift work exposure. All completers of the telephone interview were invited to attend a 36-hour laboratory study for which participants were paid. This involved continuous core body temperature measurement (using an ingestible pill-based system) and 2 nights of PSG. Shift work exposure (plus other measures) was collected by taking a detailed work history. The second laboratory night was scored into sleep stages. Post hoc, we divided participants into 4 shift work exposure groups: 0 years (ie, no exposure to shift work), 1 to 7 years, 7 to 20 years, and >20 years. Sample sizes were 11, 16, 15, and 15, respectively, with approximate equality in mean age (71.7 years of age, 69.1 years of age, 70.0 years of age, and 70.4 years of age, respectively) and percent male (63%, 50%, 67%, and 73%, respectively). Shift work exposure was associated with worse PSG sleep in a dose-related fashion. The percentages of participants with sleep efficiency, 80% for the 0 years, 1 to 7 years, 7 to 20 years, and >20 years groups were 36%, 63%, 67%, and 73%, respectively (P < 0.01), and the percentages with total sleep time (TST), 6 hours were 36%, 56%, 53%, and 73%, respectively (P < 0.01). From the circadian rhythm record, shift work exposure appeared to result (P = 0.06) in an increased spread of phase angles (difference between habitual bedtime and time of temperature trough). In conclusion, it appears likely that shift work may be related to a scarring of sleep and circadian rhythms. This may be associated with a change in the relationship between habitual sleep timing and the phase of the circadian pacemaker.
Keywords: sleep, circadian, aging, shiftwork, night work
Introduction
For the night shift worker, having to work at night and sleep during the day is an unnatural act, often requiring up to a 10-hour delay in circadian phase.1 Moreover, even permanent night workers invariably revert to a diurnal orientation during their days off and are thus, in practice, experiencing repeated circadian phase shifts on a weekly basis, much as is the case for rotating shift workers.2 For this and other reasons related to a day oriented society, the sleep of working shift workers is usually impaired.3,4
Sleep also changes as a function of age. In older adults, the deeper levels of sleep are less evident, there are more bouts of unwanted wakefulness during the night, and there is a tendency to wake earlier than desired.5,6 Thus, the retired shift worker over age 65 represents an individual whose general age-related sleep fragility might allow any residual sleep impairment from shift work exposure to be revealed when compared with that of an equivalently aged retired dayworker.
In studying retired shift workers, possible “survivor effects” need to be considered. Shift work intolerant individuals can often switch to a day job. Thus, any study of retirees that has shift work exposure duration as an independent variable must take into account the likelihood that individuals finding shift work particularly difficult might switch to daywork early on in their careers. They would then have less experience with shift work by the time they reach retirement. People who are able to do shift work for >20 years are the survivors who are still “left standing,” as it were. Thus, these survivors might comprise a particularly resilient group relative to those who quit shift work early. This effect has been observed in the cardiovascular findings of Knutsson and colleagues7 and in our own study of the effects of shift work exposure on clinical depression,8 both of which showed lessened risk in those with >20 years of shift work exposure. Thus, rather than use solely a simple dichotomy between retired shift worker and retired dayworker, in the present analysis, the shift worker group was further divided into 3 exposure bins (1 to 7 years, 7 to 20 years, and >20 years). Each bin was then compared with a retired dayworker (0 year) group, that is, a zero exposure group (see below).
In a recent telephone survey study,9 we have shown that even in retirement, shift workers report subjectively poorer sleep than dayworkers (with a survivor effect leading to a slightly reduced difference in the higher exposure group). These findings were in agreement with an earlier survey from Sweden of retired monozygotic twins with and without shift work exposure.10 This study showed poorer self-rated sleep in the twins with shift work exposure relative to their corresponding nonexposed twin. This twins study can be cited as evidence that there may indeed be some scarring effects in sleep from a working life involving shift work.
With regard to circadian rhythms, there is less evidence of scarring in the literature. However, sleep and circadian rhythms are inexorably linked in the human, and it would not be surprising were some circadian dysfunction to be apparent in retired shift workers. Like the sleep findings, these effects may be amplified by the age of the participants, since age has been shown to affect circadian rhythms.11,12 Melatonin is an integral part of the circadian process, and melatonin pills are often used by shift workers as a countermeasure.1
The present laboratory study sought to confirm our earlier telephone survey finding regarding subjective sleep and also to test two hypotheses: the first hypothesis was that the polysomnographically recorded sleep of retired shift workers is worse than that of retired day workers; and the second hypothesis that this difference in sleep is associated with differences in the circadian temperature rhythm (as measured while on a normal sleep/wake routine).
Methodology
Telephone interview study
All participants for the present study were recruited from a large-scale telephone interview survey. Full details of that study are given in recent publications.9,13,14 To summarize, over a 5-year period, a total of 1,166 community resident retired seniors 65 to 97 years of age, with a mean age of 74.8 years participated. Of these, 658 were male, and 508 were female. The respondents completed a 20 to 30 minute telephone interview that included the taking of a detailed work history as well as a series of questionnaires about sleep, sleepiness, mood, and health. At the end of each completed telephone interview, every respondent was invited to take part in a 36-hour laboratory study for which an honorarium was to be given. Those expressing an interest were sent information about the laboratory study, contacted by the laboratory supervisor (BDB) regarding an in-person tour of the laboratory and given further explanation of the study and answers to any questions they had regarding the study. Those still interested in participating were asked by the laboratory supervisor to provide signed informed consent. Prior to any data collection, the study was approved by the University of Pittsburgh Internal Review Board (IRB) after the protocol and consent form had been submitted to the IRB for approval. The IRB approval was renewed annually.
Participants
All eligible participants volunteering from the telephone survey were admitted to the study without regard to shift work exposure (see below). Because the study involved living away from home in a sleep laboratory for 36 hours with detailed sleep and circadian rhythm measures being taken, it was expected that only a small fraction of the >1,000 participants agreeing to complete a simple 20 to 30 minute telephone interview would be willing or able to participate in the 36-hour laboratory study. The positive response rate was about 5%, which was close to the 6.25% rate expected in the original design. As such, it would be more accurate to view the present participants as a “convenience sample” recruited via the telephone study. The retired shift workers of the laboratory study had an identical mean Pittsburgh Sleep Quality Index (PSQI) score (5.9) to those in the telephone study. Participants for the telephone study were required to be 65 years of age or older, to have not done any shift work in the past 12 months, and to be now retired (ie, not working for pay for more than 10 hours per week outside the home). Both men and women were eligible, and race was not a factor in participant recruitment. Post hoc, we divided participants into 4 shift work exposure groups: 0 years (no exposure to shift work), 1 to 7 years, 7 to 20 years, and .20 years; those with some exposure to shift work, but <1 year were excluded. These groups were of roughly equal size, basing the bin intervals on those used while conducting the interviews. Sample sizes were 11, 16, 15, and 15, respectively, with approximate equality in mean age (71.7 years, 69.1 years, 70.0 years, and 70.4 years) and percent male (63%, 50%, 67%, and 73%). These figures were similar to those from the telephone survey study where the mean age was 74.7 years and percent male was 57%. The percentage of retired dayworkers was, however, lower in this laboratory study than in the telephone survey study (19% versus 35%).9
Screening
Medical screening on all participants took place 2 to 4 weeks prior to the laboratory evaluation and included a complete medical history and physical examination. This was conducted by a qualified nurse practitioner who examined the participant, took a detailed health history, and obtained a list of current medications. The nurse practitioner also obtained (with the participant's permission) current medical records from participant's personal physician, which were available to her prior to approving the participant for the study. Unless a medication indicated an unstable medical or psychiatric condition or represented a safety concern, particular medications were not used as exclusionary criteria. Participants with medical problems were included only if the problems were deemed to be stable, nonacute chronic medical problems, such as well-controlled hypertension or hypothyroidism. Potential participants who exhibited acute symptoms warranting treatment were not included until a stable treatment regimen had been implemented. Medical screening also included a routine laboratory panel (complete blood count, electrolytes, blood glucose, blood urea nitrogen, creatinine, liver function tests, thyroid function tests, urinalysis, and electrocardiogram).
Psychiatric screening was conducted to exclude potential participants who were suffering from psychotic disorder or substance abuse disorder as determined by administration of the Structured Clinical Interview for DSM-IV. Participants were also required to score 24 or greater on the Folstein Mini-mental State Examination to exclude patients with dementia. Participants were not screened for sleep apnea or periodic limb movements. Some limited polysomnographic oximetry on night 1 of the laboratory study was available, but the core body temperature (CBT) measurement equipment precluded any measure being taken from the participant's trunk. The inexact Apnea Hypopnea Index (AHI) scores we did obtain were insufficiently precise to be used in the findings, but there appeared to be no systematic worsening of this AHI measure with shift work exposure (mean values: 0 year, 21.8; 1–7 years, 17.3; 7–20 years, 13.8; and >20 years, 20.3). Unfortunately, habitual daytime napping was not assessed in either the telephone or laboratory studies. However, napping was forbidden during the 36-hour protocol (see below).
There were only 12 potential participants who dropped out or were excluded after signing consent. Their mean age was 70.3 years, 2 were retired day workers, 10 were retired shift workers, 6 were male, and 6 were female. Of these 12, 4 participants were no longer able or interested, and 8 had unstable medical conditions. Once having started the laboratory study, no participant quit before the end.
Repeat of telephone interview
Because of anonymity issues, the telephone interview had to be repeated for participants in the present study. The interview was given in person by a member of the team that had conducted the original telephone interviews. The interview started by collecting data from which an accurate estimate of the duration of shift work exposure could be calculated. This involved the taking of a detailed work history, progressing from position to position back through the participant's entire working life. Questions were asked as to what the work schedule for that job was (eg, permanent, weekly, or rotating), what the work involved as well as starting and ending dates (at least to the nearest year). The interviewers were skilled at helping participants give this information by suggesting time anchors such as the birth of a child to help them remember. Shift work and daywork were both defined very specifically with regard to working times and hours worked per month. Only jobs requiring at least 35 hours per week of work outside the home were included in the definitions of shift work and daywork. This reduced the heterogeneity that might have arisen from including part-time jobs. The question arose regarding how military service should be included. Essentially, military personnel are potentially on duty around the clock rather than being on a fixed (or even rotating) shift schedule. It would thus be almost impossible to accurately characterize such work schedules. Prior to the telephone study,9 the decision was made not to include military service in the calculation of shift work exposure, and this decision carried over to the present study. Shift work was defined as nonovertime scheduled work outside the home overlapping the midnight to 6 am window (ie, night work) on either a fixed or rotating basis (details of which were taken). From these data, a shift work exposure figure was calculated for each participant. In order to be included in the 0 year exposure group (former dayworker), the participant was required to answer “no” to the question, “Have you ever worked a job that required regular (nonovertime) work after 9 pm?”
The sleep and circadian questionnaires followed after the work history. We developed verbally presented versions of the Pittsburgh Sleep Quality Index (PSQI)15 and the Sleep Timing Questionnaire (STQ),16 as well as other instruments. The PSQI was used as a measure of sleep quality, the STQ as a means of determining habitual bedtime and rising time for each participant. Also included were questions to glean information regarding morningness, physical health, depressive symptoms, daytime sleepiness, and demographics. These procedures were completed after the consent form was signed (typically 2 to 4 weeks before the laboratory study).
Laboratory study
The laboratory study lasted about 36 hours, encompassing 2 nights and an intervening day. Participants were studied individually. Each session started at about 7 pm. After unpacking and settling in, core body temperature (CBT) monitoring commenced and continued for the entire 36 hours. Studies took place in the University of Pittsburgh Neuroscience Clinical and Translational Research Center (N-CTRC) time isolation laboratories. These laboratories comprise two 1-bedroom apartments with living and eating areas that are isolated from daylight, protected from noise, and that allow the precise monitoring of participants. Although the participant lived in a windowless apartment, temporal isolation was not enforced, and time pieces were allowed. Participants were not socially isolated; technicians and project staff regularly entered the apartment for monitoring and social interaction. Napping was forbidden, and participants were monitored to ensure this. The apartment had fluorescent mock windows and skylights which were on between rising time and dinner, yielding about 500 to 600 lux; between dinner and bedtime only incandescent lamps were used, yielding up to 100 lux. Between bedtime and rising time, the bedroom was dark.
Prior to the study, participants' food preferences were determined and breakfast (30–60 minutes after rising time), lunch (noon), and dinner (6 pm) were provided. Snacks were also continually available. If the participant habitually drank caffeinated beverages, then up to 2 cups were allowed at breakfast, otherwise caffeine was forbidden. If the participant requested exercise and/or a shower, it was taken at 10:30 am. The daily procedure allowed participants to pursue activities such as hobbies, reading, watching videotapes, and so on during the day as protocol allowed. The study ended following breakfast and/or a shower after the second polysomnography (PSG) (see below) night. Taxi transportation home was offered. Participants were recompensed $250 for the completed study.
Circadian measures
Core body temperature (CBT) values were measured every minute. An ingestible pill-based system (VitalSense®, Minimitter Corp., Bend, Oregon, USA) was used. The system is based on a radio pill that is swallowed and broadcasts the internal temperature to a receiver in a belt-pack device worn by the participant. The pill passes through the participant undigested and is then discarded with a bowel movement. Every hour during wakefulness (and immediately after each bowel movement), technicians entered the apartment and viewed the belt-pack to ensure that good data were still being collected. If the first pill was passed during the study, a second pill was initialized and swallowed. At the completion of the 36-hour study, data from the belt-pack were downloaded onto a computer for subsequent analysis. The initial (nonbiological and unreliable) values obtained during the first 40 to 100 minutes after swallowing the pill were discarded. For analysis, 30-minute bins were constructed starting at 19:45 (ie, centered on the top and bottom of each hour). Mean temperature curves for the 4 shift work exposure groups were plotted. For analysis, 24-hour and 12-hour sinusoids were fitted by least-squares.11,12 Using the fitted composite curve, circadian phase was estimated by the clock time of nocturnal fitted minimum (Tmin) and circadian amplitude by RANGE (fmaximum temperature value of the fitted curve minus minimum temperature value of the fitted curve). For one subject in the 7-to 20-year group there was no discernible nocturnal trough in the fitted curve, and the sample size for the circadian measures was thus reduced from 15 to 14 for this group. Also calculated for each participant was the phase angle (PA) between bedtime and Tmin. This was simply the number of minutes elapsing between bedtime (which was at habitual bedtime, see below) and the Tmin for that participant. The value of PA has been shown to be strongly related to sleep quality and sleep duration in seniors.17
Sleep measures
For each participant, bedtimes and rising times were enforced based upon the habitual times as determined by the Sleep Timing Questionnaire (STQ).16 Each of the 2 nights was recorded using polysomnography (PSG). PSG recordings included one channel of EEG (C3 or C4 referenced to [A1–A2]), two referential electrooculograms (EOG), and submental electromyogram (EMG). All electrode impedances were <5000 ohms, and the EEG band pass was 0.3 to 30 Hz. PSG recordings were directed onto a computer with sampling at a rate of 256 Hz. Following standard conventions of the University of Pittsburgh N-CTRC, all nocturnal polygraph records (stored on the computer) were scored visually by PSG technicians into 20-second epochs according to modifed Rechtschaffen and Kales criteria.18 For the present analysis, total sleep time (TST), percent sleep efficiency (SE), and percent slow wave sleep (%SWS) from night 2 were the three PSG variables considered. The PSQI score15 was used as a measure of subjective sleep quality.
Data analysis
There were 4 sleep variables (PSQI, TST, SE, %SWS) and 3 CBT variables (Tmin, RANGE, PA). Means and standard deviations (SDs) of these 7 variables were calculated for each of the 4 exposure groups. The mean CBT was also graphed for the 4 exposure groups (0 years, 1 to 7 years, 7 to 20 years, and >20 years).
The following procedure was used to test statistical significance. This procedure used standard chi-square formulae as described in nonparametric statistical text books.19 A 2-tailed P value of 0.05 was taken as criterion. For each variable, the 0 year (former dayworker) group values was considered to be the “expected” pattern. The null hypothesis was that prior shift work exposure had no effect and that the 3 former shift worker groups (1 to 7 years, 7 to 20 years, and >20 years) did not differ from the 0 year group in the proportion of participants outside the criterion for that variable. Criteria were based on known normal values.12,17,20–22 The criterion for each variable is given in the second column of Table 2. Thus, for each variable, a count was made regarding how many participants in each of the 4 groups were outside that criterion. For each of the 3 former shift worker groups, there was then an observed frequency of participants outside the criterion, which was then compared using chi-squared statistics to the corresponding expected frequency based on the 0 year group figures. A chi-square value (with 2 df) was computed for each variable and the significance determined from chi-square tables. A secondary check was made of the study conclusions using continuous rather than categorical variables for each result deemed significant by the primary analysis. First, a comparison was made in mean values between the former dayworker (FDW) group, that is, the 0 year group (n = 11), and the former shift worker (FSW) group, that is, the group formed by pooling the 1 to 7 years, 7 to 20 years, and >20 years groups (n = 46). Two 1-tailed t tests were performed, one using the unbalanced comparison (n = 11 + 46) and the other after balancing the group sizes by repeating the FDW data 3 times (n = 44 + 46). The statistical software used for this analysis comprised part of the Sigmaplot Version 12® software.
Table 2.
summary of the statistical analysis procedure and results (see text).
| Variable | To be outside criterion is to be | % in 0y (n = 11) | % in 1y–7y (n = 16) | % in 7y–20y (n = 15) | % in >20y (n = 15) | Chi2 (P) |
|---|---|---|---|---|---|---|
| PSQI | >5 | 9.1 | 68.8 | 66.7 | 25.0 | 121.79 (<0.001) |
| TST | <360 min. | 36.4 | 56.3 | 53.3 | 75.0 | 9.50 (<0.01) |
| SE | <80% | 36.4 | 62.5 | 66.7 | 75.0 | 13.36 (<0.01) |
| %SWS | <1% | 45.5 | 50.0 | 46.7 | 43.8 | <1 (n.s.) |
| Tmin | Not 2 am–4 am | 36.4 | 43.8 | 42.9 | 43.8 | <1 (n.s.) |
| PA | Not 120–300 min. | 18.2 | 31.3 | 35.7 | 37.5 | 5.60 (0.06) |
| RANGE | <0.5 deg. | 18.2 | 25.5 | 28.6 | 12.5 | 1.52 (n.s.) |
Notes: For each of the 7 variables, the proportion gleaned from the 0 year group was used to calculate the expected number of participants meeting criterion for that variable within each of the other 3 groups (1 to 7 years, 7 to 20 years, and >20 years). This was compared with the observed number within the 3 shift work exposure groups using the chi-square test. The sample size was reduced from 15 to 14 for the circadian variables in the 7 to 20 year group (see text).
Abbreviations: PSQI, Pittsburgh Sleep Quality Index; TST, Total Sleep Time; SE, Sleep Efficiency (% night actually asleep); %SWS, percentage of the night spent in slow Wave sleep (ie, deep sleep); Tmin, clock time of the fitted minimum of the temperature rhythm; PA, Phase Angle between habitual bedtime and Tmin; RANGE, circadian amplitude as estimated by fitted maximum minus fitted minimum; n.s., not significant (P > 0.10).
Results
The summary statistics are tabulated in Table 1, and the CBT rhythms plotted in Figure 1. Both subjective and objective measures of sleep showed a detrimental effect of shift work exposure. PSQI values were higher (denoting worse sleep), and TST and SE were both reduced, as compared to the 0 year group values. As indicated in Table 2, the statistical analysis of the sleep data indicated that the 3 former shift worker groups were significantly worse than the former day-worker group in PSQI, TST, and SE, but not in %SWS. When a t test compared FDW and FSW groups in an unbalanced design (n = 11 + 46), all 3 measures showed trends toward a shift work exposure effect (PSQI: t = 1.65, P = 0.05; TST: t = 1.74, P = 0.07; SE: t = 1.75, P = 0.04). When the FDW group was packed with repetitions to balance the sample sizes (n = 44 + 46), highly significant differences emerged (PSQI: t = 2.60, P = 0.0005; TST: t = 2.44, P = 0.008; SE: t = 2.86, P = 0.003).
Table 1.
Mean (SD) for each variable in the 3 shift work exposure groups.
| Variable | Units | 0y group | 1y–7y group | 7y–20y group | >20y group |
|---|---|---|---|---|---|
| PSQI | PSQI units | 4.0 (3.6) | 6.5 (2.3) | 7.5 (3.6) | 3.7 (2.8) |
| TST | Minutes | 368 (57) | 323 (79) | 348 (60) | 345 (35) |
| SE | Percent | 81.2 (9.7) | 74.2 (13.9) | 75.2 (9.7) | 74.3 (10.3) |
| %SWS | Percent | 4.7 (6.0) | 3.8 (5.6) | 4.2 (5.7) | 1.9 (2.3) |
| Tmin | hh:mm (mm) | 03:05 (68) | 04:12 (103) | 03:14 (128) | 03:36 (92) |
| PA | Minutes | 203 (82) | 225 (89) | 201 (102) | 227 (88) |
| RANGE | deg. C | 0.74 (0.33) | 0.70 (0.27) | 0.65 (0.16) | 0.75 (0.17) |
| N | 11 | 16 | 15 | 16 | |
| Mean age | 71.7y | 69.1y | 70.0y | 70.4y | |
| % male | 63% | 50% | 67% | 73% |
Note: The sample size was reduced from 15 to 14 for the circadian variables in the 7 to 20 year group (see text).
Abbreviations: PSQI, Pittsburgh Sleep Quality Index; TST, Total Sleep Time; SE, Sleep Efficiency (% night actually asleep); %SWS, percentage of the night spent in Slow Wave Sleep (ie, deep sleep); Tmin, clock time of the fitted minimum of the temperature rhythm; PA, Phase Angle between habitual bedtime and Tmin; RANGE, circadian amplitude as estimated by fitted maximum minus fitted minimum.
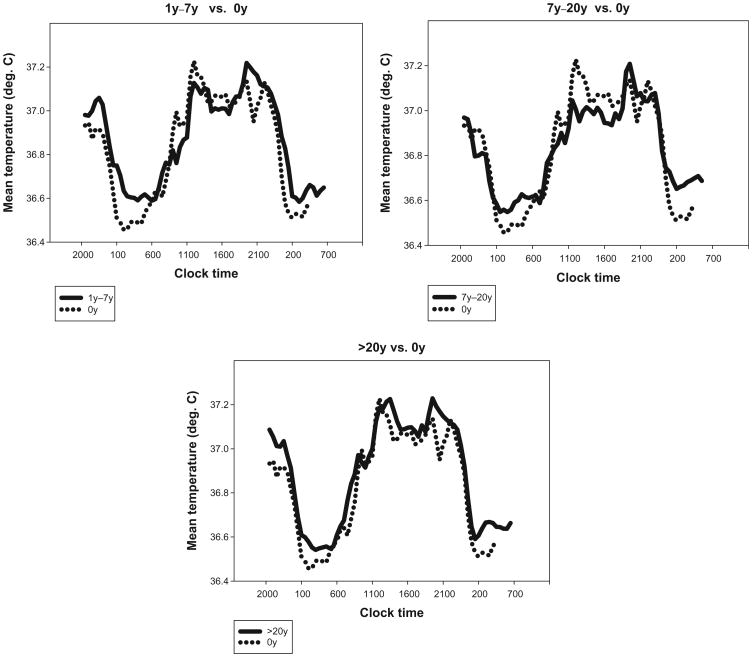
Figure 1.
Mean core body temperature (CBT) curves for each of three shift work exposure groups (1y–7y, 7y–20y, >20y) [solid lines], each plotted with the mean CBT for former day workers (0y exposure) [dotted lines].
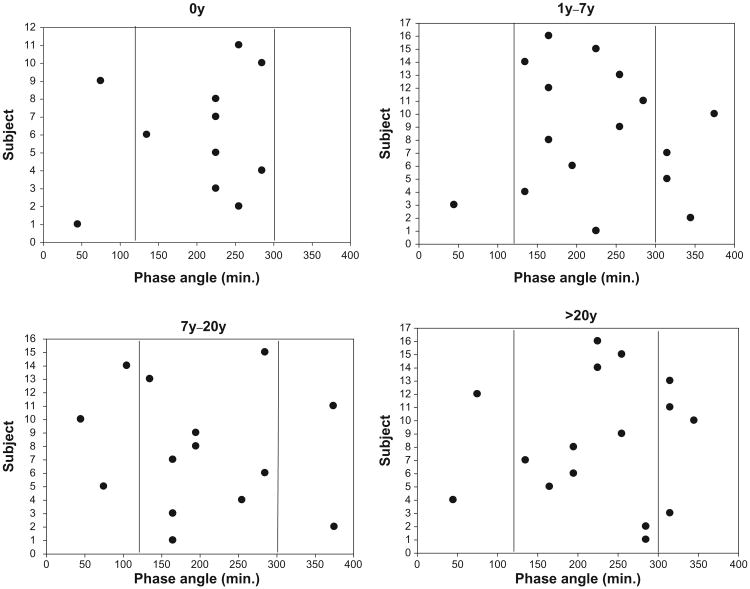
With regard to circadian rhythms, Figure 1 suggests that the 0 year group may have had a lower nocturnal trough than each of the former shift worker groups. However, the statistical analysis of the CBT data (Table 2) indicated that there was no significant shift work exposure effect in RANGE (or, indeed, in Tmin). However, when phase angles (PA) were considered, the shift work exposed groups differed (P = 0.06) from the 0 year group in the proportion of the sample outside of the normal 2 to 5 hour range (in either direction). This can be seen in the scatter plots of Figure 2. Thus, the second hypothesis (regarding the presence of shift work exposure effects in circadian rhythms) was weakly confirmed but only in respect to phase angles rather than as a simple difference in phase or amplitude.
Figure 2.
Individual phase angles for each subject within the four shift work exposure groups (0y, 1y–7y, 7y–20y, >20y).
Notes: Plotted is the number of minutes elapsing between bedtime and the circadian temperature rhythm trough (see text). The two vertical lines represent the outer bounds of “usual” phase angles (120 mins [2h] and 300 mins [5h]).
Discussion
In our earlier paper reporting the telephone interview survey,9 we noted that shift work exposure appeared to be associated with worse subjective sleep quality (ie, higher PSQI scores). Moreover, secondary analyses confirmed that the pathway for this appeared not to be through differences in chronotype or subjective health. In that paper, we thus hypothesized that the shift work exposure effect may have been through changes in the characteristics of the retirees' sleep and circadian system. In the present study, we have replicated the PSQI result regarding subjective sleep. As mentioned in the introduction, these findings concerning subjective sleep are directly in line with a Swedish monozygotic twins study10 showing shift work exposure effects on sleep in elderly retired shift workers. In addition to this, however, there are two studies of young and middle-aged adults that directly pertain to the present subjective sleep results in that they concern subjective sleep data from workers not currently doing shift work. In 1997, Brugere et al23 reported data from a large scale French epidemiological study with 21,378 subjects, 9,584 of whom were shift workers. Sleep symptoms were extracted from a self-report questionnaire of health symptoms. Subjects were separated into those currently working shifts (“present”), those who previously worked shifts but were not current shift workers (“past”), and those who had never been shift workers (“never”), plotting the number of subjects with insomnia symptoms by different age groups. The observed frequency in sleep disorders revealed the expected worsening with age. Interestingly, though, the “past” line was intermediate between the “never” and “present” lines approaching the “present” line at the highest age (52 years of age). Thus, former shift workers in their fifties reported almost as much sleep disruption as current shiftworkers in that age group and substantially more than those who had never worked shifts. There is also the work of Dumont and colleagues24 who studied insomnia symptoms in currently dayworking nurses who had previously experienced shift work for several years. The greatest effect of shift work exposure on sleep was observed in the 57 nurses who had between 4 and 10 years of exposure. For these nurses, there was more evidence of insomnia than in the low (<4 years) exposure group. For nurses with more than 10 years of exposure, there appeared to be a survivor effect lessening the risk (see introduction). Thus, it would appear that the present subjective sleep findings are broadly in agreement with conclusions made by earlier authors.
We have also confirmed that a shift work exposure effect exists in objective sleep as measured by the gold standard polysomnography. Thus, the first hypothesis was confirmed, with total sleep time (TST) and sleep efficiency (SE) worse in the retired shift worker groups compared with the retired dayworkers. However, the percentage of slow wave sleep (%SWS) measure did not show any significant shift work exposure effect. When FDW and FSW participants were compared in terms of time in bed (TIB), the 2 groups were almost identical in mean values, at 454 minutes and 452 minutes, respectively. Although there are several previous studies showing shift work effects in the objective sleep of current shift workers,3,4,25 there appear to be none concerning the objective sleep of retired shift workers.
As noted in the introduction, survivor effects may lead to apparently less shift work exposure effect being evidenced in the highest shift work exposure group (>20 years). The present analysis confirmed such an effect in PSQI. Table 2 reveals that while two-thirds of the 1 to 7 year and 7 to 20 year groups had PSQI scores > 5 (indicating sleep problems), only a quarter of the >20 year group did (as compared with about 9% for the 0 year group). It is noteworthy that a survivor effect was not observed in the objective PSG-based sleep variables, where three-quarters of the >20 year group had TST values less than 6 hours and SE values less than 80%, which was a higher proportion than that seen in any of the other 3 exposure groups (for the 0 year group the figure was about one-third). One might speculate that for the individual concerned, the subjective, rather than the objective aspects of sleep are more salient and thus likely to lead to a switch to daywork. This is in line with current thinking about the general definition of insomnia, which has tended to focus particularly on subjective distress in sleep and daytime symptoms.26
Although it is possible that habitually poor sleepers chose more shift work, it seems more likely that shift work leads to scarring of sleep and circadian rhythms, thus confirming with objective gold standard polysomnographic measures, our telephone survey results regarding subjective sleep measures.
While not quite achieving statistical significance (P = 0.06), the circadian rhythm findings regarding a greater spread of phase angles in the shift work exposed groups (Fig. 2) do suggest that the etiology of this scarring may at least partially run through changes in the relation between the circadian pacemaker and the habitual timing of sleep. As noted in many different studies over the past few decades, the phase of the circadian cycle at which sleep is attempted is a powerful determinant of the quantity and quality of sleep obtained.17,27–30 Thus, a greater spread in phase angles between bedtime and Tmin may itself be regarded as a potential disruptor of sleep. Indeed, in current shift workers, there is a shift work disorder, defined in several different nosologies, which is always placed within the circadian rhythm sleep disorders (CRSD) group,4,31 It would not be unreasonable to hypothesize that the sleep disruption of retired shift workers could also (at least in part) be described as a CRSD. Were that to be the case, then behavioral circadian interventions such as the use of bright lights and/or melatonin pills as agents of circadian rhythm change might be as beneficial to retired shift workers as they have been shown to be for current shift workers.1,32,33
One must recognize, however, that the present results in the circadian domain are very preliminary and that much more data are needed before those conclusions can be regarded as anything but very tentative. In particular, what is needed is a study in which a constant wakeful bedrest (with no meals or knowledge of clock time) is enforced for 24 hours (known as unmasking)34,35 so that truly endogenous circadian measures can be obtained, uncontaminated by evoked effects due to meals, changes in posture, and so on. Such a routine would also allow the addition of salivary melatonin as a circadian measure, thus significantly strengthening the circadian part of the study.
Conclusions
When sleep is measured polysomnographically, there appears to be a sleep scarring associated with a working life involving shift work. As compared with retired dayworkers, retired shift workers are more likely to achieve less sleep and spend a greater percentage of the night awake. This effect may possibly be associated with a change in the relationship between habitual sleep timing and the phase of the circadian pacemaker.
Recommendations
When treating older adults in the sleep clinic, it should be recognized that retired shift workers may experience increased sleep problems resulting from their shift work exposure. It may be important to question patients about their preretirement shift work exposure because retired shift workers might represent a more at risk group. Also, in view of the present results concerning circadian phase angles, such patients may particularly benefit from the adoption of a strictly regular sleep-wake routine, and/or the application of circadian therapies such as bright lights and melatonin pills.
Limitations of the Study
Of necessity, intensive laboratory studies cannot recruit hundreds of participants. Being such a small fraction of the larger telephone study, the present sample of 57 is better regarded as a convenience sample than a truly representative sampling of retirees. However, this convenience sample of 46 retired shift workers did have an identical mean PSQI score (5.9) to that of the telephone study sample of 726 retired shift workers. Clearly, the study would have benefitted from a larger sample size and, in particular, a larger 0 year group comparison sample. There were limitations concerning the definitions of work and shift work, and no account was made of habitual daytime napping. An unmasking protocol and the addition of other circadian markers such as salivary melatonin may have given a more accurate estimate of the circadian pacemaker than was provided by core body temperature measurement on a normal sleep-wake routine.
Acknowledgments
Special thanks are owed to Jean Miewald for help with data analysis, to our dedicated laboratory technicians, to Melissa Clark for pilot work, to the UCSUR team for their hard work, and to our participants for their time.
Funding: Support for this work was provided by U.S. National Institute on Aging Grants AG-13396 and AG-20677, and by RR-024153. Neither the University of Pittsburgh, nor the funding agencies, necessarily holds the views reported in this paper.
Footnotes
This is an open access article published under the Creative Commons CC-BY-NC 3.0 license.
Author Contributions: Originally conceived, designed and supervised the execution of the study and the analysis of the results: THM; collaborated in the design and execution of the study: DJB, KSK, BDB; provided medical supervision: DJB; supervised data collection BDB; supervised administration and subject recruitment KSK; supervised data processing and analysis: MEF; wrote the first draft of the manuscript: THM; read, edited and contributed text to the manuscript: DJB, BDB, KSK, MEF.
Competing Interests: Authors should state competing interests here in compliance with ICMJE conflict of interest rules (http://www.icmje.org/ethical_4conflicts.html) and must complete and return the disclosure form (http://www.icmje.org/coi_disclosure.pdf). If none exist this text will be shown: “Author(s) disclose no potential conflicts of interest.”
Disclosures and Ethics: As a requirement of publication authors have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
This article is available from http://www.la-press.com.
References
- 1.Monk TH. What can the chronobiologist do to help the shift worker? J Biol Rhythms. 2000;15(2):86–94. doi: 10.1177/074873040001500202. [DOI] [PubMed] [Google Scholar]
- 2.Knauth P, Hornberger S. Preventive and compensatory measures for shift workers. Occup Med (Lond) 2003;53(2):109–16. doi: 10.1093/occmed/kqg049. [DOI] [PubMed] [Google Scholar]
- 3.Akerstedt T. Shift work and sleep disorders. Sleep. 2005;28(1):9–11. [PubMed] [Google Scholar]
- 4.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27(8):1453–62. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 5.Ancoli-Israel S. Sleep and aging: prevalence of disturbed sleep and treatment considerations in older adults. J Clin Psychiatry. 2005;66(Suppl 9):24–30. [PubMed] [Google Scholar]
- 6.Bliwise DL. Normal Aging. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th. Philadelphia, PA: Elsevier Saunders; 2011. pp. 24–38. [Google Scholar]
- 7.Knutsson A. Health disorders of shift workers. Occup Med (Lond) 2003;53(2):103–8. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- 8.Scott AJ, Monk TH, Brink L. Shiftwork as a risk factor for depression: A Pilot Study. Int J Occup Environ Hlth. 1997;3(3):S2–9. [PubMed] [Google Scholar]
- 9.Monk TH, Buysse DJ, Billy BD, et al. Shiftworkers report worse sleep than day workers, even in retirement. J Sleep Res. 2013;22:201–8. doi: 10.1111/jsr.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ingre M, Akerstedt T. Effect of accumulated night work during the working lifetime, on subjective health and sleep in monozygotic twins. J Sleep Res. 2004;13(1):45–8. doi: 10.1111/j.1365-2869.2004.00390.x. [DOI] [PubMed] [Google Scholar]
- 11.Brown EN, Czeisler CA. The statistical analysis of circadian phase and amplitude in constant-routine core-temperature data. J Biol Rhythms. 1991;7(3):177–202. doi: 10.1177/074873049200700301. [DOI] [PubMed] [Google Scholar]
- 12.Monk TH, Buysse DJ, Reynolds CF, Kupfer DJ, Houck PR. Circadian temperature rhythms of older people. Exp Gerontol. 1995;30(5):455–74. doi: 10.1016/0531-5565(95)00007-4. [DOI] [PubMed] [Google Scholar]
- 13.Monk TH, Buysse DJ, Billy BD, et al. Circadian type and bed-timing regularity in 654 retired seniors: correlations with subjective sleep measures. Sleep. 2011;34(2):235–9. doi: 10.1093/sleep/34.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monk TH, Buysse DJ, Schlarb JE, Beach SR. Timing, duration and quality of sleep, and level of daytime sleepiness in 1166 retired seniors. Healthy Aging and Clinical Care in the Elderly. 2012;4:33–40. doi: 10.4137/HACCE.S10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 16.Monk TH, Buysse DJ, Kennedy KS, Potts JM, DeGrazia JM, Miewald JM. Measuring sleep habits without using a diary: The sleep timing questionnaire (STQ) Sleep. 2003;26(2):208–12. doi: 10.1093/sleep/26.2.208. [DOI] [PubMed] [Google Scholar]
- 17.Monk TH, Buysse DJ, Begley AE, Billy BD, Fletcher ME. Effects of a 2h change in bedtime on the sleep of healthy seniors. Chronobiol Int. 2009;26(3):526–43. doi: 10.1080/07420520902821119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rechtschaffen A, Kales AA. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Bethesda, MD: National Institute of Neurological Diseases and Blindness; 1968. [Google Scholar]
- 19.Siegel S. Nonparametric Statistics for the Behavioral Sciences. New York: McGraw-Hill; 1956. [Google Scholar]
- 20.Reynolds CF, Monk TH, Hoch CC, et al. Electroencephalographic sleep in the healthy “old old”: A comparison with the “young old” in visually scored and automated (period) measures. J Gerontol. 1991;46:M39–46. doi: 10.1093/geronj/46.2.m39. [DOI] [PubMed] [Google Scholar]
- 21.Ancoli-Israel S. Sleep and its disorders in aging populations. Sleep Med. 2009;10(Suppl 1):S7–11. doi: 10.1016/j.sleep.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Edinger JD, Fins AI, Sullivan RJ, Jr, et al. Sleep in the laboratory and sleep at home: comparisons of older insomniacs and normal sleepers. Sleep. 1997;20(12):1119–26. doi: 10.1093/sleep/20.12.1119. [DOI] [PubMed] [Google Scholar]
- 23.Brugere D, Barrit J, Butat C, Cosset M, Volkoff S. Shiftwork, age, and health: an epidemiologic investigation. Int J Occup Environ Health. 1997;3(Suppl 2):S15–9. [PubMed] [Google Scholar]
- 24.Dumont M, Montplaisir J, Infant-Rivard C. Sleep quality of former night-shift workers. Int J Occup Environ Hlth. 1997;3(3):S10–4. [PubMed] [Google Scholar]
- 25.Tepas DI, Walsh JK, Moss PD, Armstrong DR. Polysomnographic correlates of shiftworker performance in the laboratory. In: Reinberg A, Vieux N, Andlauer P, editors. Night and Shift Work: Biological and Social Aspects. Oxford, UK: Pergamon Press; 1981. pp. 179–86. [Google Scholar]
- 26.Buysse DJ. Chronic insomnia. Am J Psychiatry. 2008;165(6):678–86. doi: 10.1176/appi.ajp.2008.08010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weitzman ED, Kripke DF, Goldmacher D, McGregor P, Nogeire C. Acute reversal of the sleep-waking cycle in man. Effect on sleep stage patterns. Arch Neurol. 1970;22(6):483–9. doi: 10.1001/archneur.1970.00480240003001. [DOI] [PubMed] [Google Scholar]
- 28.Czeisler CA, Weitzman ED, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: Its duration and organization depend on its circadian phase. Science. 1980;210:1264–7. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- 29.Lavie P. The 24-hour Sleep propensity function (SPF): Practical and theoretical implications. In: Monk TH, editor. Sleep, Sleepiness and Performance. Chichester, UK: John Wiley & Sons Ltd; 1991. pp. 65–93. [Google Scholar]
- 30.Monk TH, Moline ML. The timing of bedtime and waketime decisions in free-running subjects. Psychophysiology. 1989;26(3):304–10. doi: 10.1111/j.1469-8986.1989.tb01922.x. [DOI] [PubMed] [Google Scholar]
- 31.Drake CL. The characterization and pathology of circadian rhythm sleep disorders. J Fam Pract. 2010;59(Suppl 1):S12–7. [PubMed] [Google Scholar]
- 32.Burgess HJ, Sharkey KM, Eastman CI. Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Med Rev. 2002;6(5):407–20. [PubMed] [Google Scholar]
- 33.Arendt J. Jet-lag and shift work: (2). Therapeutic use of melatonin. J R Soc Med. 1999;92(8):402–5. doi: 10.1177/014107689909200805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minors DS, Waterhouse JM. The use of constant routines in unmasking the endogenous component of human circadian rhythms. Chronobiol Int. 1984;1(3):205–16. doi: 10.3109/07420528409063897. [DOI] [PubMed] [Google Scholar]
- 35.Duffy JF, Dijk DJ. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms. 2002;17(1):4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]