Abstract
Purpose
The purpose of this study was to evaluate whether the bone morphology of the hip affects the range of motion (ROM) in total hip arthroplasty (THA).
Methods
Using the CT data of 63 patients who underwent THA, we calculated the ROM of flexion (Flex), internal rotation (Int-R) and external rotation (Ext-R) using 3D dynamic analysis software. We measured the distance between the anterior surface of the stem and anterior aspect of the greater trochanter (GTa length) at the cutting point and between the tip of the antero-inferior iliac spine (AIIS) and coronal plane of both femoral heads (AIIS length), as a parameter of the femur and pelvis, respectively. The relationship between the ROM, bone anatomy and impingement site was evaluated.
Results
We found a significant decrease in the ROM of Flex and the Int-R to be inversely proportional to the GTa and AIIS length. In Flex and Int-R, the anterior intertrochanteric region often impinges on the AIIS in patients with larger bone anatomy.
Conclusions
We demonstrated that the bone morphology of the hip substantially affects the ROM of Flex and Int-R, especially in patients with large bone anatomy. For these patients we should consider bony impingement in THA.
Introduction
Total hip arthroplasty (THA) has been not only the most popular procedure for patients with severe degenerative osteoarthritis of the hip joints but has also been one of the most successful operative interventions for improving patient quality of life. However, there are several complications in THA such as loosening, dislocation, infection, thromboembolic disease, fracture and so on. Dislocation is one of the most serious complications and also has continued to be a frequent complication over the past several years [1–4]. The factors that influence dislocation include implant design, implant orientation and alignment, surgical approach, and status of the soft tissues [2, 3]. In addition, patient factors that are associated with increased risk of dislocation include female gender, advanced age, and history of previous hip surgery [5, 6]. Bartz et al. [7] noted three different mechanisms of dislocation: (1) prosthetic impingement, (2) osseous impingement and (3) spontaneous dislocation. The cause of spontaneous dislocation is not fully known. It might be due to soft tissue imbalance, muscle weakness or contracture of the joint. With respect to the prosthetic impingement, this problem depends on the position of the implanted total hip prosthesis, and we can overcome this problem by using the optimal position of the acetabular and femoral implant or a larger size of femoral head [8–10]. As for bony impingement, it has been recommended that surgeons should resect the osteophytes and bony prominence completely or increase the stem offset [9]. However, despite using these recommended implant positions and the osteophytes and bony prominence, we sometimes experience cases that are easily dislocated in surgery, especially in patients with larger morphology of the bone. Furthermore, the extent of the morphological contribution of the bone anatomy around the hip to bony impingement is not fully known.
Nowadays, preoperative planning is commonly used before THA and computed simulation analysis is often used by many investigators to predict the optimal implant orientations and to analyse the ROM after THA [11–14]. In our study, we evaluated the influence of the bone morphology of the pelvis and femur on ROM after THA using CT-based 3D dynamic motion analysis.
Patients and methods
In this study, we retrospectively reviewed a total of 63 patients (63 hips) who underwent THA, including 17 men and 46 women with a mean age of 64.5 years (range, 54–84 years). The hip diagnoses were osteoarthritis in 63 joints. We excluded patients who had had a previous operation, severely dislocated hip, or post-osteotomy from our study. The implant used was Accolade TMZF stem and the acetabular side had Trident acetabular PSL and the hemispherical cup systems (Stryker Orthopedics/Howmedica, Mahwah, NJ). All patients had a preoperative computed tomographic (CT) scan from the anterior superior iliac spine (ASIS) to the knee joint through the distal femoral condyles using a 320-row multi-detector helical CT scanner (Aquilion ONE, Toshiba Medical Healthcare, Tochigi, Japan), with a reconstructed slice width of one millimetre and a slice interval of one millimetre. Ethics approval was granted by the Institutional Review Board.
Three-dimensional motion analysis
CT-based simulation software (ZedHip Lexi Co., Ltd., Tokyo, Japan) [15] was used to create virtual 3D bone models and perform virtual simulations using the preoperative THA planning mode. This software allows for the generation and separation of independent femoral and acetabular 3D models.
Based on a CT scan date of pelvis and femur, we first digitised the reference points, then 3D reconstruction of the bone model was made semi-automatically. If there was noise, they were revised manually. Next, the sizes of the implants and their 3D positions and orientation relative to the host bones were planned, and implantation was performed in multi planner reconstructed (MPR) view. This software was capable of simulating and detecting both bone to bone, bone to implant and implant impingement, which allowed the maximum ROM to be defined as the number of degrees of movement before impingement of either bone or implant occurred. The location of this impingement on both the femoral and acetabular side can also be defined in the model.
The pelvic coordinate system was the anterior pelvic plane, which was defined by the ASIS and the pubic tubercle. The femoral coordinate system was defined by the centre of the femoral head, the knee centre, and both femoral condyles.
Implant positioning and setting
The simulated implant was the Accolade TMZF stem with a 36-mm-diameter alumina head and a neck of standard length in all cases. The implant size was chosen to maximise both fit and fill in the femoral metaphysis, taking into consideration the implant size used in the operation. The shaft axis of the femoral implant was placed in the centre of the original femoral dyaphysis. The acetabular side had a Trident acetabular PSL cup with a highly cross-linked polyethylene (HXLPE) insert without marginal lips in all cases. The acetabular component size was also chosen to maximise both fit and fill in the acetabulum. The acetabular component position was determined by placing the implant at the site of the original acetabulum. The anteversion of the femoral implant was set at 20°, cup anteversion at 20° (total anteversion was fixed as 40°) and cup abduction at 45° in a radiographic manner, and in all cases. Any acetabular osteophytes that were attached to the acetabular bone rim were removed.
Calculation of the ROM and impingement site
The centre of the femoral head was located by fitting a sphere to the articular surface of the femoral head. The pelvis was fixed in space, while the femur was free to translate in all directions but constrained to rotate around the centre of rotation of the hip. Based on this computerised analysis, the ROM was measured in those directions that are important for dislocation and ADL such as walking, running, squatting, climbing stairs and so on: flexion (Flex) with 0° of adduction and internal rotation, internal rotation (Int-R) in 90° of flexion with 0° of adduction and external rotation (Ext-R) in 0° of flexion with 0° of adduction.
Measurements of bony size of pelvis and femur
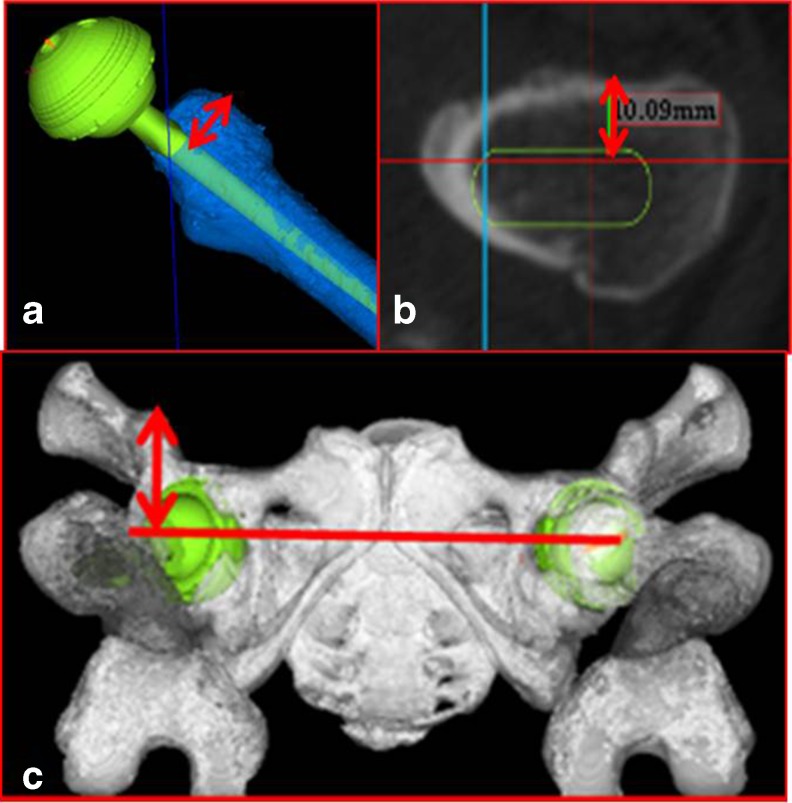
The horizontal distance between the anterior edge of the femoral implant and the most anterior part of the greater trochanter at the cutting point of the femur was measured on the axial plane of the simulation as a parameter of morphology of femoral bone anatomy, defined as the anterior aspect of the greater trochanter (GTa) length (Fig. 1a–b). The horizontal maximum distance between the anteroinferior iliac spine (AIIS) and coronal plane of the centre of both femoral heads on the frontal plane in the simulation was also measured as a parameter of morphology of pelvic bone anatomy, defined as the AIIS length (Fig. 1c). The total length that added GTa and AIIS length was also calculated as a parameter of morphology of total hip anatomy.
Fig. 1.
a Anterior aspect of the greater trochanter (GTa) length. The horizontal distance between the anterior surface of the femoral stem and the anterior aspect of the great trochanter at the cutting point. b In high power view. c AIIS length. The horizontal maximum distance between AIIS and coronal plane of the bilateral femoral head on the frontal plane
Evaluation design
To evaluate the relationship between the bone anatomy morphology around the hip, ROM after THA, and impingement site, three evaluations were performed in this study:
Analysis of the relationship between GTa length and AIIS length
Analysis of the relationship between ROM and the bone anatomy morphology of the pelvis and femur
Analysis of the relationship between impingement site and the bone anatomy morphology of the pelvis and femur
Statistical analysis
All data were expressed as mean ± standard deviation (SD) and statistical analysis was performed using Stat-View-J version 5.0 software (Hulinks, Tokyo, Japan). The correlations were evaluated using Pearson’s chi-squared test. A P value of less than 0.01 was considered statistically significant.
Results
Analysis of the relationship between GTa length and AIIS length
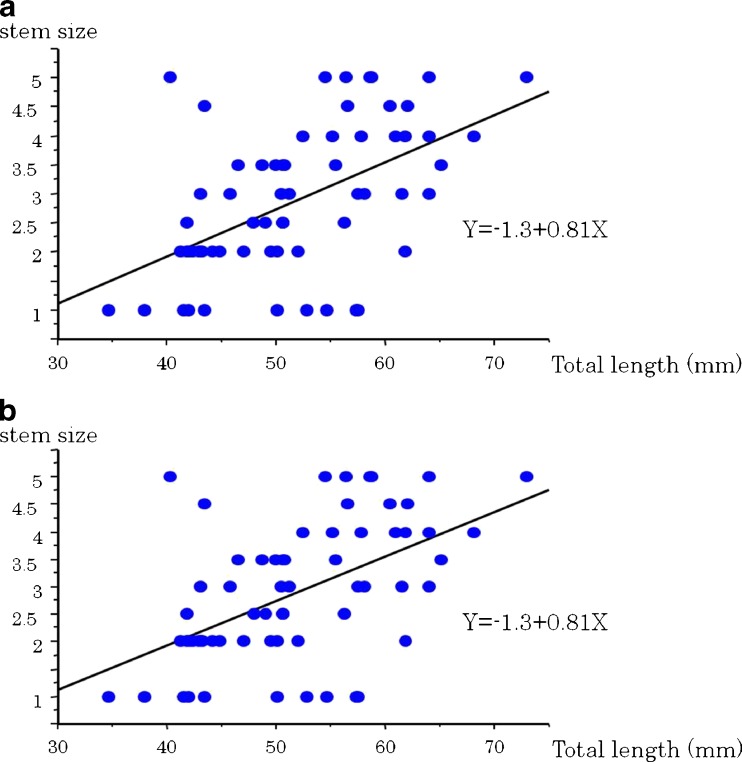
The mean GTa length is 13.7 ± 3.7 mm, the AIIS length is 38.1 ± 5.5 mm and the total length is 51.8 ± 8.3 mm. There is a positive correlation between the GTa length and AIIS length (R2= 0.38, P < 0.001) with statistical difference (Fig. 2a). Although the strength of correlations was not sufficient, there are positive correlations between the stem size and GTa length (R2 = 0.23, P < 0.001), AIIS length (R2= 0.22, P < 0.001), and total length (R2= 0.38, P < 0.0001) with statistical difference, respectively (Table 1, Fig. 2b).
Fig. 2.
a Relationship between the anterior aspect of the greater trochanter (GTa) length and anteroinferior iliac spine (AIIS) length. b Relationship between stem size and total length
Table 1.
Relationship between stem size and each parameter
| Parameter | R | P value |
|---|---|---|
| GTa length | −0.47 | <0.001 |
| AIIS length | −0.48 | <0.001 |
| Total length | −0.53 | <0.0001 |
GTa anterior aspect of the greater trochanter, AIIS anteroinferior iliac spine
Analysis of the relationship between ROM and the bone anatomy morphology of the pelvis and femur
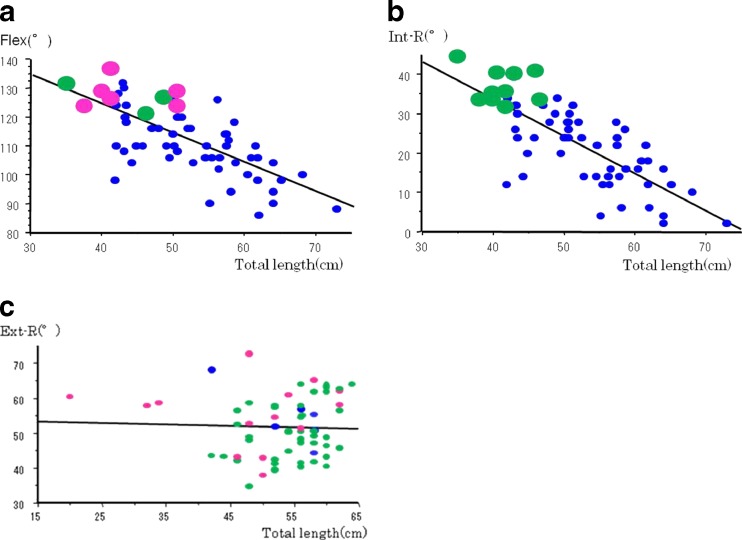
The mean ROM is 112° in Flex, 22.7° in Int-R and 53.2° in Ext-R (Table 2). With respect to Flex and Int-R, we found an obvious decrease in hip ROM, inversely proportional to the length of GTa, AIIS and total length, respectively. There are negative correlations between the ROM and GTa length, AIIS length with statistical difference, and especially between the ROM and total length (Tables 3 and 4, Fig. 3a–f). However there was no significant correlation between the ext-R and each of the three parameters (Table 5).
Table 2.
Average angle of each parameter (°)
| Flex | Int-R | Ext-R |
|---|---|---|
| 112 ± 12.1 | 22.7 ± 10.2 | 53.2 ± 7.8 |
Table 3.
Relationship between Flex and each parameter
| Parameter | R | P value |
|---|---|---|
| GTa length | −0.64 | <0.001 |
| AIIS length | −0.64 | <0.001 |
| Total length | −0.7 | <0.00001 |
GTa anterior aspect of the greater trochanter, AIIS anteroinferior iliac spine
Table 4.
Relationship between Int-R and each parameter
| Parameter | R | P value |
|---|---|---|
| GTa length | −0.69 | <0.001 |
| AIIS length | −0.7 | <0.001 |
| Total length | −0.77 | <0.00001 |
GTa anterior aspect of the greater trochanter, AIIS anteroinferior iliac spine
Fig. 3.
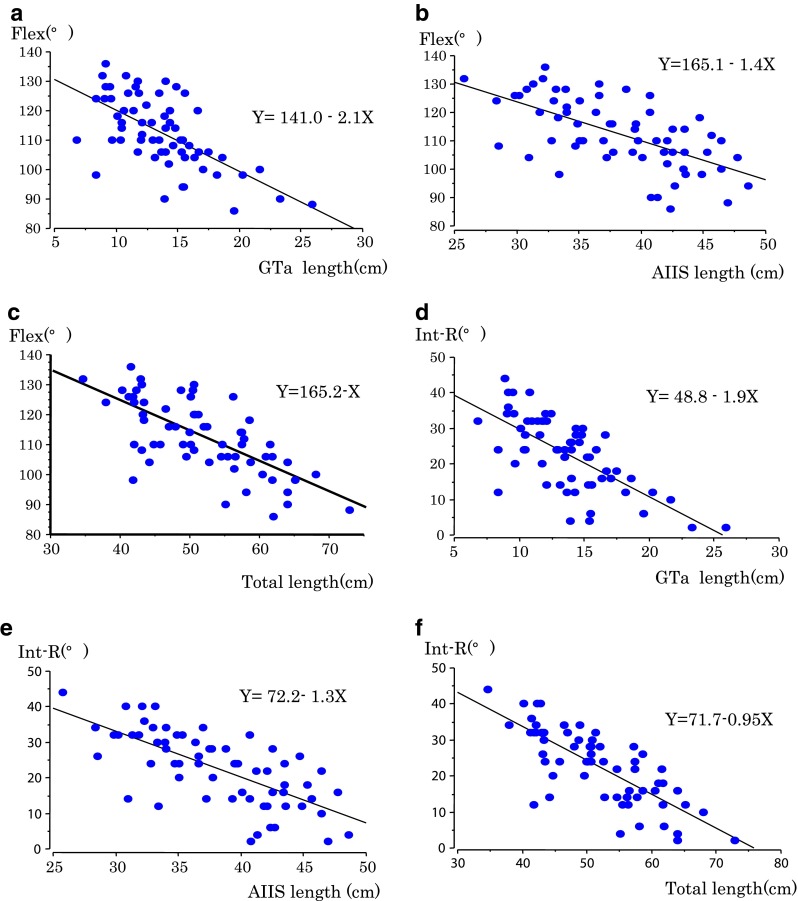
Relationship between ROM of Flex and Int-R and each of the three parameters. a Flex–anterior aspect of the greater trochanter (GTa) length. b Flex–anteroinferior iliac spine (AIIS) length. c Flex–total length. d Flex–GTa length. e Flex–AIIS length. f Flex–total length
Table 5.
The relationship between Ext-R and each parameter
| Parameter | R | P value |
|---|---|---|
| GTa length | 0.009 | 0.9 |
| AIIS length | −0.05 | 0.7 |
| Total length | −0.03 | 0.8 |
GTa anterior aspect of the greater trochanter, AIIS anteroinferior iliac spine
Analysis of the relationship between impingement site and the bone anatomy morphology of the pelvis and femur
In our study, impingement occurs in three ways: bone to bone impingement, cup–neck impingement and implant–bone impingement. Bony impingement preceded cup–neck impingement in many cases, especially in Flex and Int-R. In Flex, bony impingement was observed in 54 hips; the anterior greater trochanteric region of the femur impinges on AIIS (Fig. 4a). Bony impingement was also observed in six hips; however, the impingement site was different—the femoral shaft impinged on ASIS (Fig. 4b), and cup–neck impingement was observed in three hips (Fig. 4c). In Int-R, bony impingement was observed in 53 hips, where the anterior greater trochanteric region of the femur or the femoral neck at the cutting point impinged on AIIS and cup–neck impingement was observed in ten hips. In Ext-R, cup-neck impingement was observed in 44 hips, implant–bone impingement in 14 hips (Fig. 4d) and bony impingement–lesser trochanter on ischial bone in five hips.
Fig. 4.
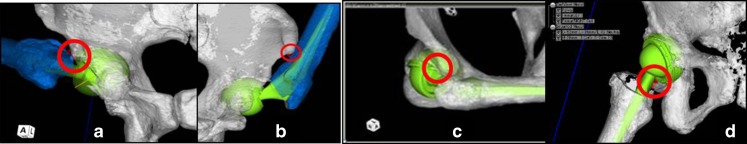
a Bony impingement. b Bony impingement (ASIS-femur). c. Cup–neck impingement. d Implant–bone impingement
When impingement site was plotted on the graph (Fig. 3c–f, the cup–neck impingement and bony impingement), the femoral shaft was seen to impinge on ASIS, hence showing a decrease in the morphology of bone size of the pelvis and femur. In other words, the ROM of Flex and Int-R decreased due to bony impingement (the anterior great trochanteric region of the femur impinges on AIIS) as the morphology of bone size of pelvis and femur increases (Fig. 5a–b). However, there was no significant correlation between the ROM of Ext-R and the impingement site (Fig. 5c).
Fig. 5.
Impingement site in relation to bony anatomy around hip. a Flex–total length graph. Blue point indicates bony impingement (AIIS on femoral neck or greater trochanter), green color cup–neck impingement and pink color bony impingement (ASIS on femoral shaft) Int-R–total length graph. Blue point indicates bony impingement (AIIS on femoral neck or great trochanter) and green color indicates cup–neck impingement
Discussion
Impingement is often the main aetiology for post-THA instability. Dislocation can occur subsequent to impingement between the two components or between the acetabulum and proximal femur. Even without dislocation, the patient may complain of clicking in various activities such as walking, climbing the stairs, squatting and so on, leading to anxiety and dissatisfaction. Many studies have analysed the variables that affect the ROM after THA. Recent studies have shown the optimal implant positions to acquire a satisfactory ROM. Widmer et al. [11] showed that a cup inclination between 40° and 42° combined with a cup anteversion between 23° and 28° and the stem antetorsion determined according to the formula “cup anteversion + 0.7 × stem antetorsion = 37°” fulfilled the severe ROM. Furthermore, in recent studies, alternative bearings with larger diameter of femoral heads have been reported to reduce the dislocation rates in primary or revision THA [16, 17], although the advantages of diameters beyond 38 mm have not been demonstrated clinically [18].
These appropriate orientations of the implant and size of the femoral head are important factors for the prevention of implant impingement, and bony impingement is also an important factor for post-THA stability and for prevention of dislocation. Suzuki et al. [19] mentioned the importance of bony impingement using still CT frames and reported that bony impingement frequently limits hip motion after THA, independently of the ROM of the prosthetic components. Kessler et al. [12] evaluated the ROM after THA in various orientations of cup abduction, anteversion and femoral anteversion using computer models. They reported that bony impingement became much more common than implant impingement when implants were positioned to minimise impingement. Although there are several reports about bony impingement, less has been written about the importance of variable bone anatomy affecting bony impingement. In this study, we took a subject-specific approach to analyse the effect of the bone anatomy around the hip on restricting hip ROM after THA.
In our study, we measured GTa and AIIS length as an indicator of individual bone anatomy of the femur and pelvis, based on our experiences and previous reports. Bartz et al. [7] found that osseous impingement was likely to occur between the greater trochanter and the iliac bone before implant impingement in a cadaver study, and several authors have previously mentioned a similar phenomenon regarding bony impingement [20, 21]. As for implant positioning in our study, all of the cases were inside the “safe zone” that Lewinnek proposed [13, 22]. Our results showed that the more the size of pelvis and femur increases, the more the ROM of Flex and Int-R decreases. Moreover, the location of the initial contact was not consistent at the region of cup–neck, but was more commonly located at the greater trochanteric region or femoral neck on AIIS in patients with a larger morphology of the pelvis and femur. These results indicate that hip ROM after THA reduces because of bony impingement in patients with larger skeletal morphology of the hip.
We define ‘anterior offset’ as the distance between the line on the anterior aspect of the proximal femur and the centre of the stem head. In accordance with the prediction of Kessler et al. [12] that the head–neck ratio of the native femur would correlate with the overall hip ROM before bony impingement in THA, it is important to retain ‘anterior offset’ in order to avoid bony impingement and improve the ROM of Flex and Int-R, especially in patients with large skeletal morphology. With respect to the size of the femoral head, several studies have already shown that the ROM after THA is not limited by implant impingement with a larger diameter of femoral head but is limited by bony impingement [7, 12, 23]. From the viewpoint of ‘anterior offset’, once bony impingement is observed as a restricting factor, increasing head diameter has no further effect on improving ROM, because the centre of the femoral head does not change and anterior offset is not improved. In order to retain ‘anterior offset’, our results suggest that elongation of the stem offset or the use of a femoral implant with increased anteversion may increase the hip ROM after THA. Furthermore, the finding that bony impingement often occurs at AIIS also offer caution against excessive medialisation or heightening of the hip centre in cup positioning. If bony impingement is observed as a restricting factor in these conditions, the resection of the bony impingement site–anterior aspect of femoral neck and greater trochanter or AIIS may decrease the incidence of posterior dislocation by allowing an increase of ROM in Flex and Int-R until bone impingement, especially in patients with large bony anatomy. It can be assumed that bony impingement restricts ROM either after THA by CT data or by implant size, because of a positive correlation between the skeletal size and stem size, and therefore we have to take this phenomenon into consideration at the planning of THA.
There were several limitations in our study. First, in our study the influence of the surrounding soft tissue was not taken into account, which may have affected the actual hip ROM. Second, we only analysed the ROM until impingement. Hip dislocation involves levering of the head out of the cup after impingement, and a larger head size may have the advantage of reducing dislocation by way of acquiring jumping distance, even if the pre-impingement ROM remains the same.
In summary, our computed model allows for a clinically relevant assessment of the ROM after THA and provides supplements to clinical studies. We demonstrated that skeletal morphology of the hip substantially affects the ROM of Flex and Int-R especially in patients with large bone anatomy. For patients with large bone anatomy, bony impingement of the proximal femur on AIIS may become a significant risk factor for dislocation. Therefore, we have to take ‘anterior offset’ into consideration in THA for these patients.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Courpied JP, Caton JH. Total hip arthroplasty, state of the art for the 21st century. Int Orthop. 2011;35:149–150. doi: 10.1007/s00264-011-1207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Lima DD, Urquhart AG, Buehler KO, Walker RH, Colwell CW., Jr The effect of the orientation of the acetabular and femoral components on the range of motion of the hip at different head–neck ratios. J Bone Joint Surg Am. 2000;82:315–321. doi: 10.2106/00004623-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Nadzadi ME, Pedersen DR, Yack HJ, Callaghan JJ, Brown TD. Kinematics, kinetics, and finite element analysis of commonplace maneuvers at risk for total hip dislocation. J Biomech. 2003;36(5):77–91. doi: 10.1016/s0021-9290(02)00232-4. [DOI] [PubMed] [Google Scholar]
- 4.Caton J, Prudhon JL. Over 25 years survival after Charnley’s total hip arthroplasty. Int Orthop. 2011;35:185–188. doi: 10.1007/s00264-010-1197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrey BF. Difficult complications after hip joint replacement. Dislocation. Clin Orthop Relat Res. 1997;344:179–187. doi: 10.1097/00003086-199711000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Jolles BM, Zangger P, Leyvraz PF. Factors predisposing to dislocation after primary total hip arthroplasty. A multivariate analysis. J Arthroplasty. 2002;17:282–288. doi: 10.1054/arth.2002.30286. [DOI] [PubMed] [Google Scholar]
- 7.Bartz RL, Nobel PC, Kadakia NR, Tullos HS. The effect of femoral component head size on posterior dislocation of the artificial hip joint. J Bone Joint Surg Am. 2000;82:1300–1307. doi: 10.2106/00004623-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Heithoff BE. Dislocation after total hip arthroplasty: a single surgeon’s experience. Orthop Clin North Am. 2001;32:587–591. doi: 10.1016/S0030-5898(05)70229-7. [DOI] [PubMed] [Google Scholar]
- 9.Malik A, Maheshwari A, Dorr LD. Impingement with total hip replacement. J Bone Joint Surg Am. 2007;89:1832–1842. doi: 10.2106/JBJS.F.01313. [DOI] [PubMed] [Google Scholar]
- 10.Crowninshield RD, Maloney WJ, Wentz DH, Humphrey SM, Blanchard CR. Biomechanics of large femoral heads: what they do and don’t do. Clin Orthop. 2004;429:102–107. doi: 10.1097/01.blo.0000150117.42360.f9. [DOI] [PubMed] [Google Scholar]
- 11.Widmer KH, Zurfluh B. Compliant positioning of total hip components for optimal range of motion. J Orthop Res. 2004;22:815–821. doi: 10.1016/j.orthres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Kessler O, Patil S, Stefan W, Mayr E, Colwell CW, D’Lima DD. Bony impingement affects range of motion after total hip arthroplasty: a subject-specific approach. J Orthop Res. 2008;26:443–452. doi: 10.1002/jor.20541. [DOI] [PubMed] [Google Scholar]
- 13.Rousseau MA, Lazennec JY, Boyer P, Mora N, Gorin M, Catonné Y. Optimization of total hip arthroplasty implantation: is the anterior pelvic plane concept valid? J Arthroplasty. 2009;24:22–26. doi: 10.1016/j.arth.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Incavo SJ, Thompson MT, Gold JE, Patel RV, Icenogle KD, Noble PC. Which procedure better restores intact hip range of motion: total hip arthroplasty or resurfacing? A combined cadaveric and computer simulation study. J Arthroplasty. 2011;26:391–397. doi: 10.1016/j.arth.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Tsukeoka T, Hyun Lee T. Sagittal flexion of the femoral component affects flexion gap and sizing in total knee arthroplasty. J Arthroplasty. 2012;27:1094–1099. doi: 10.1016/j.arth.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Lachiewicz PF, Soileau ES. Low early and late dislocation rates with 36- and 40-mm heads in patients at high risk for dislocation. Clin Orthop Relat Res. 2013;471:439–443. doi: 10.1007/s11999-012-2379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garbuz DS, Masri BA, Duncan CP, Greidanus NV, Bohm ER, Petrak MJ, Della Valle CJ, Gross AE. Dislocation in revision THA: do large heads (36 and 40 mm) result in reduced dislocation rates in a randomized clinical trial? Clin Orthop Relat Res. 2012;470:351–356. doi: 10.1007/s11999-011-2146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez JA, Rathod PA. Large diameter heads: is bigger always better? J Bone Joint Surg Br. 2012;94(11 Suppl A):52–54. doi: 10.1302/0301-620X.94B11.30508. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki K, Matsubara M, Morita S, Muneta T, Shinomiya K. CT image evaluation of the internal rotation limit prior to bony impingement after total hip arthroplasty. J Orthop Sci. 2002;7:433–438. doi: 10.1007/s007760200075. [DOI] [PubMed] [Google Scholar]
- 20.Dorr LD, Wan Z. Causes of and treatment protocol for instability of total hip replacement. Clin Orthop Relat Res. 1998;335:144–151. doi: 10.1097/00003086-199810000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Dorr LD, Wolf AW, Chandler R, Conaty JP. Classification and treatment of dislocations of total hip arthroplasty. Clin Orthop Relat Res. 1983;173:151–158. [PubMed] [Google Scholar]
- 22.Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217–220. [PubMed] [Google Scholar]
- 23.Burroughs BR, Hallstrom B, Golladay GJ, Hoeffel D, Harris WH. Range of motion and stability in total hip arthroplasty with 28-, 32-, 38-, and 44-mm femoral head sizes. J Arthroplasty. 2005;20:11–19. doi: 10.1016/j.arth.2004.07.008. [DOI] [PubMed] [Google Scholar]