Abstract
Purpose
Kashin-Beck disease (KBD) is an endemic degenerative osteoarthritis associated with extracellular matrix degradation. The aim of this investigation was to evaluate the role of targeting genes in the pathogenesis of KBD and primary osteoarthritis (OA) involved in extracellular matrix degradation.
Methods
Agilent 44 K human whole-genome oligonucleotide microarrays were used to detect the gene expression in KBD and OA cartilage. The mRNA and protein expressions of CSGalNAcT-1 and Hapln-1 in chondrocytes were verified by reverse transcription polymerase chain reaction (RT-PCR) and western blot, and their expression in cartilage were verified with immunocytochemical analysis. Meanwhile, CSGalNAcT-1 and Hapln-1 protein levels in the selenium intervention group of KBD with different concentrations (0.25, 0.1and 0.05 μg/ml) were detected by western blot.
Results
CSGalNAcT-1 and Hapln-1 were down-regulated in KBD and OA at both mRNA and protein levels, and were increased in Se(Selenium) groups compared to KBD free-Se group. However, Wnt 3a, β-catenin and Runx-2 were up-regulated in OA and KBD at protein levels. Additionally, immunohistochemical staining showed that CSGalNAcT-1 and Hapln-1 were reduced in all zones of KBD and OA articular cartilage, but not significantly reduced in the up zone of OA articular cartilage.
Conclusions
The CSGalNAcT-1 and Hapln-1 were down-regulated in both KBD and OA cartilage. CSGalNAcT-1 may be involved in the damage of articular cartilage of KBD and OA by regulating Hapln-1 in the Wnt/β-catenin signalling pathway. It was indicated that CSGalNAcT-1 and Hapln-1 may play important roles in the pathogenesis of KBD and OA.
Introduction
Kashine Beck disease (KBD) is a chronic, endemic osteochondropathy, which is mainly distributed in Asia, including China, South Korea, Russia, etc. Osteoarthritis (OA) is a chronic degenerative joint disease with increasing prevalence with age. Compared to OA, KBD displays classic enlarged interphalangeal joints but not joint pain, stiffness, limitation of motion in clinical manifestations, and characteristic pathology changes with chondrocyte apoptosis and necrosis in deep zones of cartilage. Focal chondronecrosis and the impaired endochondral ossification in the deep zone of cartilage of KBD results in further chronic deforming osteoarthrosis and impaired skeletal development [1]. At present, there are three major environmental hypotheses of KBD [2–4]: selenium deficiency, serious cereal contamination by mycotoxin-producing fungi and high humic acid levels in drinking water. OA, as the most prevalent degenerative joint disease, causes chronic joint pain and disability by destruction and loss of articular cartilage. As the degenerated disease, necrosis and apoptosis of chondrocytes and degradation of extracellular matrix (ECM) in damaged articular cartilage have been observed in both KBD and OA [5], although referred to different pathogenesis.
Chondroitin sulfate N-acetylgalactosaminyl-transferase-1 (CSGalNAcT-1), an essential enzyme in chondroitin sulphate metabolism, participates in chondroitin sulphate (CS) chain formation of aggrecan, and plays an important role in degenerative extracellular matrix of cartilage. Recent studies have also highlighted the key role of CSGalNAcT-1 in chondrogenesis at early developmental stages [6]. Additionally, in experimental animal models, the cartilage of CSGalNAcT-1-null mice was significantly smaller than that of wild-type mice, suggesting the importance of CSGalNAcT-1 in endochondral ossification [7]. Although there are several studies about the biological function of CSGalNAcT-1 in vivo and in vitro, it has not been linked to human cartilage-like disease, such as KBD and OA. Hyaluronan and proteoglycan link protein 1 (Hapln-1), also named cartilage link protein 1 (Crtl-1) or link protein, is an important component of the cartilage matrix [8–10]. It stabilises aggregation of aggrecan and hyaluronic acid, two extracellular matrix components essential for cartilage development [9]. The disruption of Crtl-1 resulted in a severe chondrodysplasia [11]. However, the correlation between Hapln-1 with OA has been rarely performed, and there are even no reports on Hapln-1 in KBD. Therefore we investigated the differential expression of CSGalNAcT-1 and Hapln-1 among KBD, OA, and normal cartilage.
In this study, we screened and identified the differentially expressed genes (CSGalNAcT-1, Hapln-1, Wnt 3a, β-catenin and Sox-9) in KBD and OA cartilage, to understand their potential role in mechanism of disease.
Materials and methods
Target gene screening and CSGalNAcT gene network
Human glycosyltransferase related genes were screened from CFG (Consortium for Functional Glycomics) and CAZY database [12]. Differentially expressed glycosyltransferase related genes between KBD and OA chondrocytes were screened using Agilent 44 K human whole-genome oligonucleotide microarrays of KBD and OA cartilage [13], the selection criteria were defined as a more than two-fold difference in the level of expression (i.e., difference in up-regulated expression more than two-fold, and difference in down-regulated expression less than 0.5-fold) and further targeted genic screening had been investigated through their biological functions. The CSGalNAcT gene network was graphically displayed which described the relationships between a subset of genes and their neighbouring genes using IPA.
Tissue preparation and groups
Cartilage samples collected were divided into three groups: (1) Normal control group: eight samples of articular cartilage were collected from individuals unaffected with KBD from non-KBD areas; five males and three females between 55 and 64 years old who had failed surgery; (2) OA group: six samples of articular cartilage were collected from hospitals of non-KBD areas; four males and two females between 56 and 65 years old who had accepted joint replacement surgery without KBD; and (3) KBD group: six samples of articular cartilage were collected from KBD diseased areas including four males and two females aged from 49 to 63 years old who had accepted joint replacement surgery. The patients with OA were diagnosed as having OA according to the Western Ontario and McMaster Universities OA Index of pain, stiffness, function [14], and some clinical examination, which allowed for exclusion of KBD and rheumatoid arthritis (RA). Patients with KBD were diagnosed as having grade II or III disease on the basis of the clinical criteria for the diagnosis of KBD in China.
Cell culture
Articular cartilage was obtained from KBD, OA patients and femoral head replacement for trauma as control samples. Samples were sequentially digested by trypsin (0.5%), and type II collagenase (0.2%). Cells were seeded at the density of 1.5 × 104cells per cm2 in DMEM/F12 with 10% FBS in 25 cm2 culture flasks and cultured until they reached confluence. Primary cells of OA and control samples were passaged in culture with 10% FBS. Primary cells of KBD were passaged in culture using trypsinisation (0.02% trypsin) and cultured for another 48 hours. Then the different concentrations of selenium (0.25, 0.1 and 0.05 μg/ml) were added and cultured for three weeks with every three days a change of the medium.
Quantitative analysis of CSGalNAcT-1 transcripts in OA, KBD and normal chondrocytes by real-time PCR
Total RNA was isolated from chondrocytes using Trizol protocol. cDNA was generated using RevertAid™ First Strand cDNA Synthesis Kit (Thermo Scientific Molecular Biology, Canada) according to the manufacturer’s instructions. The forward primer for CSGalNAcT-1 was 5′-GACTTCATCAATATAGGTGGGTTTGAT-3′, the reverse primer was 5′-GTCCGTACCACTATGAGGTTGCT-3′ [15]. The forward primer for Hapln-1 was 5′-AATGATGGTGCTCAGATTGCAA-3′, the reverse primer, 5′-ACAGCGGTCATATCCGAGAATTT-3′ [16]. Quantitative RT-PCR was performed using the FTC 3000 real-time PCR System (Funglyn Biotech Inc., Canada). Relative quantification of mRNA expression was calculated using the 2-ΔΔCt method, in which ΔΔCt = (Ctgene−Ctβ)KBD/OA−(Ctgene−Ctβ)control. The relative amount of each CSGalNAcT-1 and Hapln-1 were normalised to the amount of β-actin transcript in the same cDNA. All assays were performed in triplicate.
Immunohistochemistry
Cartilage slices (0.5–1 cm) were fixed with 4% paraformaldehyde for 24 hours after removal of the tissue and decalcified in 3% ethylenediaminetetraocetic acid (EDTA). The samples were dehydrated in a series of alcohol, cleared in xylene, and embedded in paraffin wax. Paraffin sections (4–5 μm) were cut, mounted on slides, pretreated with 2% with 10% poly-l-lysine, and stored at room temperature until used. Paraffin-embedded sections were deparaffinised with xylene and then rehydrated in graded ethanol. Endogenous peroxidase activity was blocked by 3% H2O2 for 15 minutes at room temperature. The method of 10 mol/L urea for 20 minutes at 37 ° C and complex enzyme digesting for 20 minutes was used for antigen retrieval. Then nonspecific binding was blocked by normal goat serum. For the primary antibody, polyclonal rabbit anti-human primary antibodies against CSGalNAcT-1 (1:50; ABGENT, San Diego, CA 92121, USA) and Hapln-1 (1:50; Abcam, UK) were used at 4 ° C overnight. After incubation for 20 min at room temperature with goat anti-rabbit IgG (Zhongshan Goldenbridge Biotechnology Co., Ltd., Beijing, China), the sections were reacted with 3, 3′-diaminobenzidine (DAB) solution for three minutes and then were counter-stained with hematoxylin for ten seconds.
Western blot analysis
Protein was extracted from chondrocytes using tissue protein extraction kit according to the manufacturer’s instructions. The concentration of total protein was quantified using the NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE, USA). Twelve uL of total protein was loaded per lane and electrophoresed in 12% SDS-PAGE using a constant voltage of 80 V for two hours. Then the proteins were transferred onto 0.45-nitrocellulose PVDF membranes (Hybond-ECL, Amersham) at 350 mA for 90 minutes and blocked with 5% nonfat milk at room temperature for two hours. The membranes were incubated overnight at 4 °C with 1:500-diluted primary antibodies to CSGalNAcT-1 (1:50; ABGENT, San Diego, CA 92121, USA) and Hapln-1 (1:50; Abcam(HK)Ltd, UK), Wnt 3a (rabbit polyclonal antibody, Cell Signalling), β-catenin (mouse monoclonal antibody, Santa), Runx-2 (rabbit polyclonal antibody, Bioworld) and Sox-9 (rabbit polyclonal anti-Hapln-1 antibody, Cell Signalling) in TBST containing 5% nonfat milk and β-actin (Mouse monoclonal anti-β-actin antibody, ABGENT, USA). After being incubated with anti-rabbit or anti-mouse IgG (1:4,000) and immunoreactive proteins were visualised on a film with an enhanced chemiluminescence kit (NEN Life Science Products, Boston, MA, USA). Quantification of band intensity was determined using image software.
Statistical analysis
Statistical differences in expressions of protein between OA and KBD were analysed statistically with SPSS 13.0 software. Differences in mRNA levels for OA and KBD were tested using the Student’s t test. Statistical significance was accepted at P<0.05. The correlation of CSGalNAcT-1 and Hapln-1 were tested using Spearman’s rank correlation.
Results
General findings screened by glycosyltransferase gene and CSGalNAcT gene network
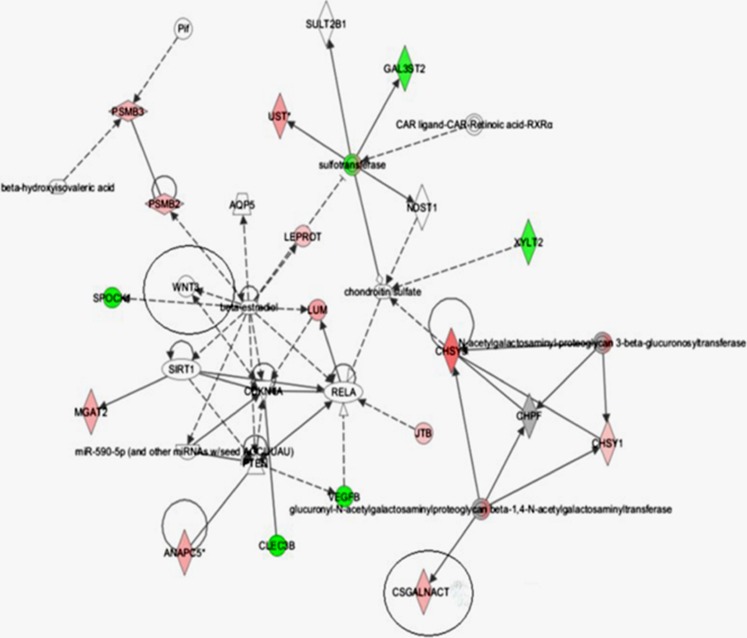
Fifteen up-regulated genes and ten down-regulated genes were screened from 416 genes related to glycosyltransferase using CFG (Consortium for Functional Glycomics) [12, 17], CAZY and Agilent 44 K human whole-genome oligonucleotide microarrays for KBD and OA (Tables 1 and 2). The CSGalNAcT gene network was graphically displayed which described potential relationships between a subset of genes and their neighboring genes (Fig. 1).
Table 1.
Selected list of genes with higher expression in Kashin-Beck disease (KBD) than osteoarthritis (OA)
| Function | Public ID | Gene symbol | Differential fold change, mean ± SEM |
|---|---|---|---|
| Cytokine/growth factor | NM_000597 | IGFBP2 | 3.51 ± 2.52 |
| Lipid metabolism | NM_004315 | ASAH1 | 17.35 ± 15.03 |
| Glycan biosynthesis and metabolism | |||
| Metabolism | NM_003779 | B4GALT3 | 2.86 ± 1.08 |
| Metabolism | NM_015170 | SULF1 | 14.28 ± 13.25 |
| Metabolism | NM_020692 | GALNTL1 | 25.53 ± 16.87 |
| Metabolism | NM_020474 | GALNT1 | 11.58 ± 8.93 |
| Metabolism | NM_022087 | GALNT11 | 25.43 ± 14.17 |
| Metabolism | NM_022167 | XYLT2 | 5.27 ± 2.05 |
| Metabolism | NM_175856 | CSS3 | 7.83 ± 3.97 |
| Cytoskeleton and cell movement | NM_001614 | ACTG1 | 29.09 ± 13.13 |
| Development process | NM_004450 | ERH | 5.52 ± 4.66 |
| Activin and inhibin | NM_002193 | INHBB | 5.65 ± 4.08 |
| Cytokine | NM_002727 | PRG1 | 33.04 ± 18.24 |
| Protein synthesis and modification | NM_000993 | RPL31 | 38.01 ± 11.65 |
| Oncogene related | NM_002966 | S100A10 | 4.84 ± 2.36 |
Table 2.
Selected list of genes with lower expression in Kashin-Beck disease (KBD) than osteoarthritis (OA)
| Function | Public ID | Gene symbol | Differential fold change, mean ± SEM |
|---|---|---|---|
| Macrophage related | NM_004864 | GDF15 | 0.23 ± 0.12 |
| Aggrecan Metabolism | |||
| Miscellaneous | AK098514 | CSGALNACT | 0.04 ± 0.007 |
| Metabolism | NM_003033 | ST3GAL1 | 0.04 ± 0.008 |
| Glucose metabolism | NM_005566 | LDHA | 0.32 ± 0.23 |
| Oncogene related | NM_001015049 | BAG5 | 0.26 ± 0.2 |
| DNA modification | NM_018371 | ChGn | 0.4 ± 0.22 |
| Cytokine | NM_003278 | CLEC3B | 0.33 ± 0.12 |
| Cytokine/growth factor | NM_000201 | ICAM1 | 0.34 ± 0.29 |
| Lysosome related | NM_005561 | LAMP1 | 0.015 ± 0.001 |
| Extracellular matrix relate | NM_001884 | HAPLN1 | 0.44 ± 0.13 |
Fig. 1.
Chondrocyte gene network of CSGalNAcT-1 was classified as glycan biosynthesis and metabolism and functional pathway
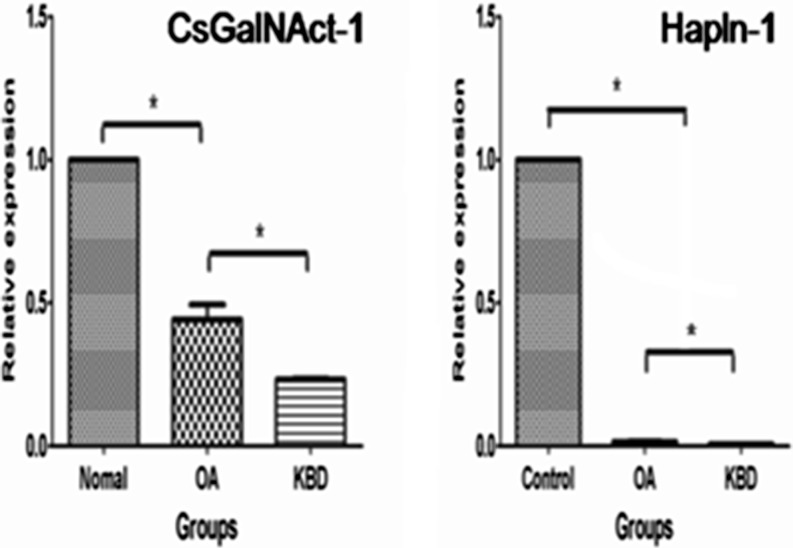
Lower expression of CSGalNAcT-1 and Hapln-1 mRNA in KBD and OA cartilages
CSGalNAcT-1 mRNA level was significantly lower in OA cartilage than normal cartilage (P = 0.037) using quantitative real-time PCR; meanwhile, the expression of CSGalNAcT-1 was less in KBD than OA cartilage (P = 0.014). Similarly, Hapln-1 mRNA expression level was also regularly decreased in OA and KBD compared with normal cartilage (P < 0.0001, P = 0.029, respectively) (Fig. 2).
Fig. 2.
CSGalNAcT-1 and Hapln-1 mRNA expression in osteoarthritis (OA) cartilage, Kashin-Beck disease (KBD) cartilage and normal cartilage. CSGalNAcT-1 and Hapln-1 mRNA levels reach statistical significance. * Denotes significant difference at P < 0.05
The expression of CSGalNAcT-1 and Hapln-1 in all morphological zones of articular cartilage from KBD and OA patients
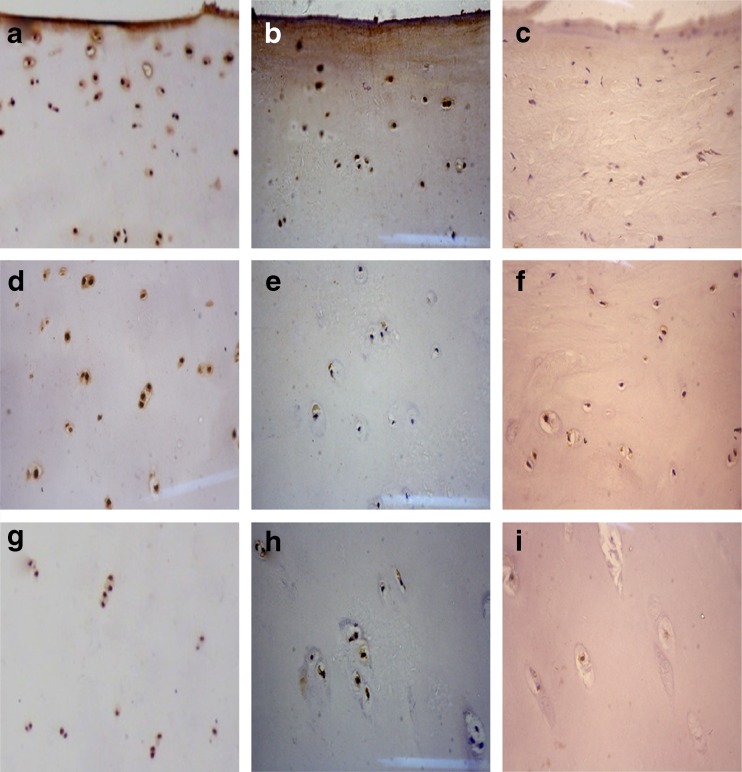
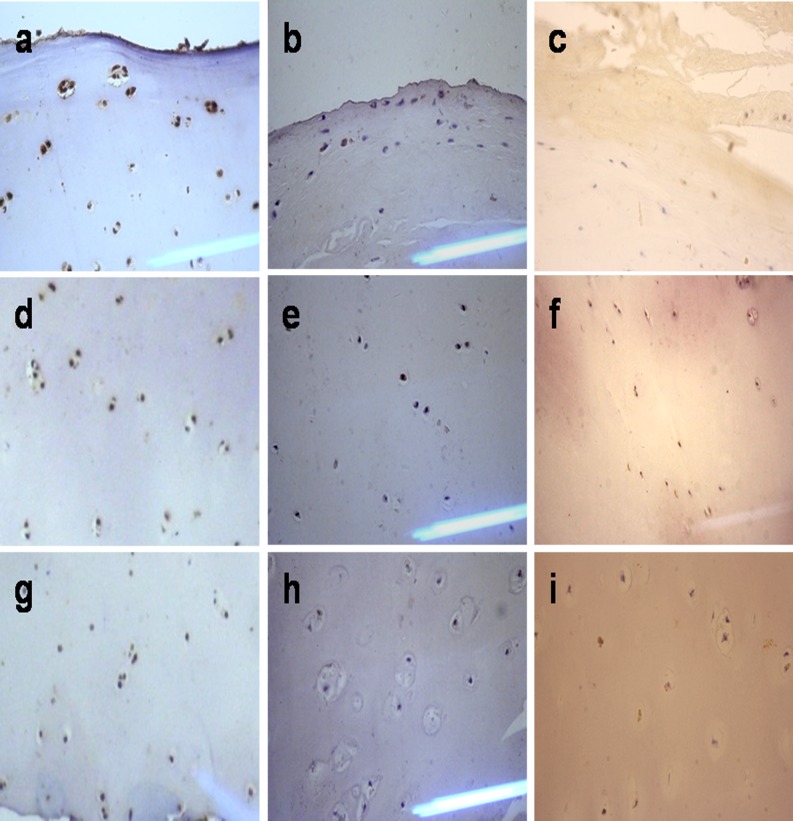
In contrast to the control group (Fig. 3a,d,g; Fig. 4a,d,g), CSGalNAcT-1 and Hapln-1 staining was remarkably reduced in the middle and deep zones of the OA group articular cartilage (Fig. 3b,e,h; Fig. 4b,e,h), but was hardly expressed in all morphological zones of KBD group articular cartilage (Fig. 3c,f,i; Fig. 4c,f,i).
Fig 3.
Comparison of CSGalNAcT-1 expression in the cartilage from osteoarthritis (OA), Kashin-Beck disease (KBD) and normal groups. a, b, c Denote the upper of normal, OA and KBD cartilage, respectively. d, e, f Denote the middle of normal, OA and KBD cartilage, respectively. g, h, i Denote the deep of normal, OA and KBD cartilage, respectively
Fig. 4.
Comparison of Hapln-1 expression in the cartilage from osteoarthritis (OA), Kashin-Beck disease (KBD) and normal groups. a, b, c Denote the upper of normal, OA and KBD cartilage, respectively. d, e, f Denote the middle of normal, OA and KBD cartilage, respectively. g, h, i Denote the deep of normal, OA and KBD cartilage, respectively
The protein expressions of CSGalNAcT-1 and Hapln-1 were significantly reduced in all morphological zones of articular cartilage from KBD and OA patients compared with the controls (t = 3.556, 36.824, df = 16, P = 0.000, 0.016, respectively). The CSGalNAcT-1 and Hapln-1 expressions in articular cartilage from patients with KBD were weaker than that of OA in the upper zone (t = 13.895, 19.825, df = 14, P < 0.0001), but showed no significance in the middle and deep zone by t test (Table 3). Additionally, there were correlations between CSGalNAcT-1 and Hapln-1 by analysis using Spearman’s (r = 0.937, P < 0.0001).
Table 3.
Percentage of chondrocyte staining for CSGalNAcT-1 and Hapln-1 in articular cartilage and growth plate cartilage of Kashin-Beck disease (KBD), osteoarthritis (OA) patients and normal cartilage
| Groups | n | Upper zone | Middle zone | Deep zone |
|---|---|---|---|---|
| Positive (%) | Positive (%) | Positive (%) | ||
| CSGalNAcT-1 | ||||
| Normal group | 8 | 100.0 ± 0.0ab | 100.0 ± 0.0ab | 100.0 ± 0.0ab |
| OA group | 8 | 93.7 ± 4.3ac | 42.8 ± 8.2a | 20.4 ± 8.6a |
| KBD group | 8 | 46.0 ± 9.5bc | 40.9 ± 6.0b | 17.1 ± 5.5b |
| Hapln-1 | ||||
| Normal group | 8 | 100.0 ± 0.0ab | 100.0 ± 0.0ab | 100.0 ± 0.0ab |
| OA group | 8 | 90.9 ± 2.9ac | 45.8 ± 12.5a | 23.3 ± 5.4a |
| KBD group | 8 | 44.6 ± 9.2bc | 33.3 ± 8.2b | 18.6 ± 7.6b |
aNormal groups vs OA group, P < 0.01
bNormal groups vs KBD group, P < 0.01
cOA group vs KBD group, P < 0.01
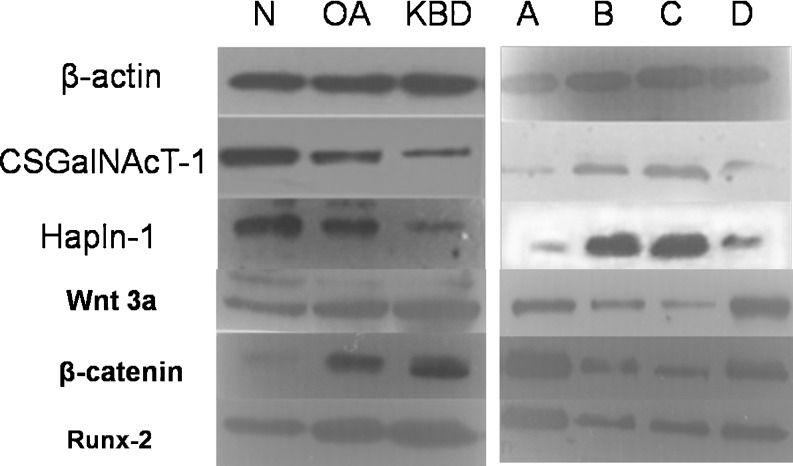
Expression of CSGalNAcT-1, Hapln-1, Wnt 3a, β-catenin and Runx-2 at protein levels in KBD and OA cartilage and Se KBD group.
We performed Western blot analysis to determine the protein levels of CSGalNAcT-1, Hapln-1, Wnt 3a, β-catenin and Runx-2. As shown in Fig. 5, CSGalNAcT-1 and Hapln-1 were weakly expressed in OA compared with normal cartilage, but more frequently overexpressed compared with KBD cartilage. Wnt 3a, β-catenin and Runx-2 were overexpressed in OA compared with normal cartilage, but more weakly expressed compared with KBD cartilage. Figure 5 also showed that CSGalNAcT-1 and Hapln-1 protein expressions were the lowest in the KBD free-Se group. There was no significant difference between the 0.25 μg/ml Se groups and 0.10 μg/ml Se group, but they were higher than the 0.05 μg/ml Se group, suggesting that Se increased CSGalNAcT-1, Hapln-1, Wnt 3a, β-catenin and Runx-2 expression in human chondrocyte. Quantification of band intensity was determined using image software (SensiCapture), and showed a decrease in CSGalNAcT-1 and Hapln-1 expressions compared with normal cartilage, and Wnt 3a, β-catenin and Runx-2 expressions were increased in OA and KBD compared with normal cartilage; however, CSGalNAcT-1 and Hapln-1 expressions were increased in the selenium KBD group compared with the KBD group; Wnt 3a, β-catenin and Runx-2 expression were decreased in the selenium KBD group compared with the KBD group.
Fig. 5.
CSGalNAcT-1 and Hapln-1 protein level expression reduced in osteoarthritis (OA) and Kashin-Beck disease (KBD) cartilage compared with normal controls. Wnt 3a, β-catenin and Runx-2 level expression increased in OA and KBD cartilage compared with normal controls. A KBD free-Se group. B 0.25 μg/ml Se + KBD group. C 0.10 μg/ml Se + KBD group. D 0.05 μg/ml Se + KBD group
Discussion
CSGalNAcT and Hapln-1 were selected as candidate genes from 25 glycosyltransferases related genes. CSGalNAcT-1 is a member of the glycosyltransferases family, and other genes of this family include, CSS [12], CSGlcAT [6], and CSGalNAcT-2 [13]. All of these enzymes participate in the CS chain elongation, whereas CS chain initiation probably involves only CSGalNAcT-1 and 2. Hapln-1, as a key link protein of cartilage matrix, is essential for cartilage proteoglycan aggregate formation. The network of CSGalNAcT graphically described the relationships between a subset of genes and their neighbouring genes using IPA to analyse the regulatory mechanism of CSGalNAcT. Recent studies showed that the Wnt/β-catenin signalling pathway could elicit matrix catabolism and occur in the hypertrophic zone of the growth plate in developing and growing long bones; activation or increased activity of the Wnt/β-catenin signalling pathway could play a causative role in joint diseases [14, 15, 17–19]. The changes of Wnt signalling may affect the balance of matrix GAG and CS in content [16], and CSGalNAcT-1 plays an important role in CS metabolism. Therefore, we speculated CSGalNAcT-1 may participate in the pathogenesis of KBD and OA by influencing the Wnt/β-catenin signalling pathway.
The mRNA expressions of CSGalNAcT-1 and Hapln-1 were significantly less in KBD and OA compared with normal cartilage tissue by real-time PCR. Additionally, we found that CSGalNAcT-1 and Hapln-1 protein levels in KBD and OA cartilage and chondrocytic were also obviously reduced compared with control groups by immunohistochemical staining and western blot. It was consistent with previous studies that Hapln-1 mRNA expression was downregulated in osteoarthritic cartilage [20], and CSGalNAcT-1 was highly expressed in the developing cartilage. Moreover, Yumi et al. reported that CSGalNAcT-1 was required for at least 50% of the total CS synthesis to preserve normal cartilage development based on histology of the epiphyses of E18.5 wild-type and CSGalNAcT-1-null (−/−) mice [21]. Taken together, CSGalNAcT-1 and Hapln-1 expression decreased at transcriptional and translational levels in the evolution process of cartilage degenerative disorders. We supposed that if CSGalNAcT-1 and Hapln-1 changed in amount, cartilage tissue may acquire pathological changes.
Although KBD and OA are chronic joint diseases with similar clinical manifestations and cartilage lesions, including cartilage and matrix degeneration, chondrocyte necrosis, and apoptosis, they have different pathogenesis [22]; therefore, there is an urgent need to better understand its pathogenesis to enable the development of new management, treatment strategies and new pathological diagnostic standards to distinguish between KBD and OA. In our study, CSGalNAcT-1 and Hapln-1 mRNA and protein levels in chondrocytes from patients with KBD were weaker than that of OA. Besides changes in the quantitative amounts and components in the degradation of cartilage, the increased proliferative activity of chondrocytes is primarily featured in the upper cartilage zones, because of proliferative factors from the synovial fluid [23]. This is consistent with our immunohistochemical staining (Figs. 3 and 4; Table 3). In KBD patients, CSGalNAcT-1 and Hapln-1 expression were lower in the upper zone of cartilage than in OA patients; however, there were no significant differences in the middle and deep zones. In OA pathological changes, besides the histological and morphological changes seen in the articular cartilage, the increased proliferative activity of chondrocytes is primarily featured in the upper cartilage zones, because of proliferative factors from the synovial fluid. In the KBD patients, Xiong [1] found that the deep zone of focal chondronecrosis and the impaired endochondral ossification results in further chronic deforming osteoarthrosis and impaired skeletal development. Taken together, we believe that it is better to understand the pathogenesis of KBD and OA by determining the location of protein expression of CSGalNAcT-1 and Hapln-1 in morphological zones.
KBD, an endemic osteoarthropathy, principally occurs in childhood [24]. The many severe KBD patients also exhibited decreased limb length and short stature due to the focal and irregular closure of the growth plates [25]. CSGalNAcT-1 is necessary glycosyltransferase for normal levels of endochondral ossification. The decrease in the mRNA and protein levels of aggrecan and Hapln-1 in the ECM of Csgalnact1−/− cartilage, and Csgalnact1−/−mice exhibited slight dwarfism [7]. Dongxu et al. confirmed that Se deficiency does not cause KBD, but is closely related [26]. In our study, we investigated the effect of Se on the CSGalNAcT-1, Hapln-1, Wnt 3a, β-catenin and Runx-2 expressions and confirmed the protective effects of Se on the cartilage development through increasing the expressions of CSGalNAcT-1 and Hapln-1. Our findings suggested that the environmental factor of KBD areas may affect the Wnt/β-catenin signalling pathway and further decrease the level of CSGalNAcT-1 expression and formation of aggrecan and Hapln-1 of the ECM in the cartilage development stage, to become one of the important factors of KBD development.
There was positive correlation between CSGalNAcT-1 and Hapln-1 expressions by Spearman’s rank correlation in our study. This is consistent with Takashi Sato’s conclusions that aggrecan and Hapln-1 of the ECM are significantly reduced in Csgalnact-1−/− cartilage in an experimental animal model [7]. Hapln-1 is a stable macromolecular structure that induces compression resistance and shock absorption in the joints. Cartilage-specific expression of the Hapln-1 transgene can completely avert perinatal mortality in Hapln-1-null mice and can correct skeletal abnormalities depending on dose [11]. Therefore, we supposed that CSGalNAcT-1 could contribute to cartilage degenerative disorders by targeting the Wnt/β-catenin signalling pathway from environmental factors of KBD areas (Se deficiency).
Conclusions
The down-regulation of CSGalNAcT-1 and Hapln-1 was identified in both KBD and OA cartilage. CSGalNAcT-1 may participate in the pathogenesis of KBD and OA by targeting the Wnt/β-catenin signalling pathway from environmental factors of KBD areas. It may provide a better understanding of the molecular details in KBD pathogenesis for future research in osteochondrosis.
Acknowledgments
This study was supported by the National Natural Scientific Foundation of China (30972556, 81202157), the Specialised Research Fund for the Doctoral Program of Higher Education of China (20090201110049) and “13115” Major Program on Technology Science Innovation Project of Shaanxi Province (2009ZDKG-79).
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Jingjing Zheng, Xiong Guo and Tiezhou Hou designed the study and analyzed the data. Cuiyan Wu, Shixun Wu, Honghai Xu, Yongtao Zhang provided surgical specimens and clinical information. All authors have read and approved the manuscript.
Abbreviations
- KBD
Kashin-Beck disease
- OA
Osteoarthrosis
- CSGalNAcT-1
Chondroitin Sulphate N-acetylgalactosaminyl-transferase-1
- Hapln-1
Hyaluronan and proteoglycan link protein 1
- Se
selenium
References
- 1.Xiong G. Diagnostic, clinical and radiological characteristics of Kashin-Beck disease in Shaanxi Province, PR China. Int Orthop. 2001;25(3):147–150. doi: 10.1007/s002640100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan J, Zhu W, Wang W, Li R, Hou S, Wang D, Yang L. Selenium in soil and endemic diseases in China. Sci Total Environ. 2002;284(1):227–235. doi: 10.1016/S0048-9697(01)00889-0. [DOI] [PubMed] [Google Scholar]
- 3.Chasseur C, Suetens C, Nolard N, Begaux F, Haubruge E. Fungal contamination in barley and Kashin-Beck disease in Tibet. Lancet. 1997;350(9084):1074. doi: 10.1016/S0140-6736(05)70453-0. [DOI] [PubMed] [Google Scholar]
- 4.Peng A, Wang WH, Wang CX, Wang ZJ, Rui HF, Wang WZ, Yang ZW. The role of humic substances in drinking water in Kashin-Beck disease in China. Environ Health Perspect. 1999;107(4):293. doi: 10.1289/ehp.99107293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiong G, Hong Z, Chun-Xia C, Yan Z, Dong G, Zengtie Z, Yingang Z, von der Klaus M, von der Helga M. Abnormal expression of Col x, PTHrP, TGF-beta, bFGF, and VEGF in cartilage with Kashin-Beck disease. J Bone Miner Metab. 2006;24:319–328. doi: 10.1007/s00774-006-0690-3. [DOI] [PubMed] [Google Scholar]
- 6.Sakai K, Kimata K, Sato T, Gotoh M, Narimatsu H, Shinomiya K, Watanabe H. Chondroitin sulfate N-acetylgalactosaminyltransferase-1 plays a critical role in chondroitin sulfate synthesis in cartilage. J Biol Chem. 2007;282(6):4152–4161. doi: 10.1074/jbc.M606870200. [DOI] [PubMed] [Google Scholar]
- 7.Sato T, Kudo T, Ikehara Y, Ogawa H, Hirano T, Kiyohara K, Hagiwara K, Togayachi A, Ema M, Takahashi S. Chondroitin sulfate N-acetylgalactosaminyltransferase 1 is necessary for normal endochondral ossification and aggrecan metabolism. J Biol Chem. 2011;286(7):5803–5812. doi: 10.1074/jbc.M110.159244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudhia J, Bayliss MT, Hardingham TE. Human link protein gene: structure and transcription pattern in chondrocytes. Biochem J. 1994;303(Pt 1):329. doi: 10.1042/bj3030329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mörgelin M, Paulsson M, Hardingham T, Heinegård D, Engel J. Cartilage proteoglycans. Assembly with hyaluronate and link protein as studied by electron microscopy. Biochem J. 1988;253(1):175. doi: 10.1042/bj2530175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neame PJ, Christner J, Baker J. The primary structure of link protein from rat chondrosarcoma proteoglycan aggregate. J Biol Chem. 1986;261(8):3519–3535. [PubMed] [Google Scholar]
- 11.Czipri M, Otto JM, Cs-Szabó G, Kamath RV, Vermes C, Firneisz G, Kolman KJ, Watanabe H, Li Y, Roughley PJ. Genetic rescue of chondrodysplasia and the perinatal lethal effect of cartilage link protein deficiency. J Biol Chem. 2003;278(40):39214–39223. doi: 10.1074/jbc.M303329200. [DOI] [PubMed] [Google Scholar]
- 12.Kitagawa H, Uyama T, Sugahara K. Molecular cloning and expression of a human chondroitin synthase. J Biol Chem. 2001;276(42):38721–38726. doi: 10.1074/jbc.M106871200. [DOI] [PubMed] [Google Scholar]
- 13.Sato T, Gotoh M, Kiyohara K, Akashima T, Iwasaki H, Kameyama A, Mochizuki H, Yada T. Differential roles of two N-acetylgalactosaminyl transferases, CSGalNAcT-1, and a novel enzyme, CSGalNAcT-2. Initiation and elongation in synthesis of chondroitin sulfate. J Biol Chem. 2003;278(5):3063–3071. doi: 10.1074/jbc.M208886200. [DOI] [PubMed] [Google Scholar]
- 14.Enomoto-Iwamoto M, Kitagaki J, Koyama E, Tamamura Y, Wu C, Kanatani N, Koike T, Okada H, Komori T, Yoneda T. The Wnt antagonist Frzb-1 regulates chondrocyte maturation and long bone development during limb skeletogenesis. Dev Biol. 2002;251(1):142–156. doi: 10.1006/dbio.2002.0802. [DOI] [PubMed] [Google Scholar]
- 15.Tamamura Y, Otani T, Kanatani N, Koyama E, Kitagaki J, Komori T, Yamada Y, Costantini F, Wakisaka S, Pacifici M, Iwamoto M, Enomoto-Iwamoto M. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005;280(19):19185–19195. doi: 10.1074/jbc.M414275200. [DOI] [PubMed] [Google Scholar]
- 16.Jinping X, Wei W, Matt L, Kevin C, Takayuki H, Jeffrey CL, Sunil K. Chondrogenic differentiation of human mesenchymal stem cells in three-dimensional alginate gels. Tissue Eng. 2008;14(5):667–680. doi: 10.1089/tea.2007.0272. [DOI] [PubMed] [Google Scholar]
- 17.Shortkroff S, Yates KE. Alteration of matrix glycosaminoglycans diminishes articular chondrocytes’ response to a canonical Wnt signal. Osteoarthr Cartilage. 2007;15(2):147–154. doi: 10.1016/j.joca.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Yuasa T, Otani T, Koike T, Iwamoto M, Enomoto-Iwamoto M. Wnt/b-catenin signaling stimulates matrix catabolic genes and activity in articular chondrocytes: its possible role in joint degeneration. Lab Invest. 2008;88(3):264–274. doi: 10.1038/labinvest.3700747. [DOI] [PubMed] [Google Scholar]
- 19.Borovecki F, Pecina-Slaus N, Vukicevic S. Biological mechanisms of bone and cartilage remodelling-genomic perspective. Int Orthop. 2007;31(6):799–805. doi: 10.1007/s00264-007-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tew SR, Clegg PD, Brew CJ, Redmond CM, Hardingham TE. SOX9 transduction of a human chondrocytic cell line identifies novel genes regulated in primary human chondrocytes and in osteoarthritis. Arthritis Res Ther. 2007;9(5):R107. doi: 10.1186/ar2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe Y, Takeuchi K, Onaga SH, Sato M, Tsujita M, Abe M, Natsume R, Li M, Furuichi T, Saeki M. Chondroitin sulfate N-acetylgalactosaminyltransferase-1 is required for normal cartilage development. Biochem J. 2010;432(Pt 1):47. doi: 10.1042/BJ20100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schepman K, Engelbert RHH, Visser MM, Yu CL, de Vos R. Kashin Beck disease: more than just osteoarthrosis. Int Orthop. 2011;35(5):767–776. doi: 10.1007/s00264-010-1043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meachim G, Collins D. Cell counts of normal and osteo-arthritic articular cartilage in relation to the uptake of sulphate (35SO4) in vitro. Ann Rheum Dis. 1962;21(1):45. doi: 10.1136/ard.21.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nesterov A. The clinical course of Kashin-Beck disease. Arthritis Rheum. 1964;7(1):29–40. doi: 10.1002/art.1780070105. [DOI] [PubMed] [Google Scholar]
- 25.Cao J, Li S, Shi Z, Yue Y, Sun J, Chen J, Fu Q, Hughes C, Caterson B. Articular cartilage metabolism in patients with Kashin-Beck disease: an endemic osteoarthropathy in China. Osteoarthr Cartilage. 2008;16(6):680–688. doi: 10.1016/j.joca.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Dongxu M, Dexiu D, Zhilun W, Jianjun Z, Cai B. Twenty-year research on selenium related to Kashin-Beck disease. J Xi’an Med Univ. 1997;9(1):79–89. [Google Scholar]