Abstract
Purpose
We will test the hypothesis that ultrasound supported by polymerase chain reaction (PCR) could improve bacterial identification in non-infected prosthetic joint loosening. The aim was to detect bacterial species in non-infected prosthetic joint loosening using ultrasound and 16S rRNA gene sequencing.
Methods
A total of 16 patients (11 women and five men) aged 46–80 years (mean age 65.7) with diagnosed knee or hip implant loosening (mean implant survival of 102.1 months) were investigated. Bacterial culture and DNA sequencing were used to detect bacteria on the surface of failed implants removed during revision arthroplasty. The results of pre- and intraoperative culture and DNA sequencing were compared. Histopathological analysis was also performed.
Results
The number of positive cultures rises with a higher level of C-reactive protein (CRP). The results of the cultures from synovial fluid obtained through joint aspiration were consistent with sonicates from components of prostheses in 12 cases (75 %). Bacterial DNA was found in 90 % of patients with negative synovial fluid culture. PCR revealed two or more bacterial species, often of the same genus: Ralstonia pickettii, Pseudomonas spp., Brevibacterium spp., Lactobacillus spp., Propionibacterium spp. and Staphylococcus spp.These are micro-organisms present in the environment or on the human body and often associated with compromised immunity.
Conclusions
The ultrasound procedure followed by PCR and sequencing improve bacterial identification in silent prosthetic joint infection. The lack of clinical signs of infection and negative preoperative and intraoperative cultures do not exclude the presence of micro-organisms on the implants.
Keywords: Prosthesis, Infection, Ultrasound, Sonication, Polymerase chain reaction, Silent infection, Aseptic loosening
Introduction
The rise in the number of joint replacement procedures performed results in an increasing number of complications. The most severe complications include prosthetic joint infection (PJI). PJIs occur in 1–4 % of patients after primary total hip or knee replacements [1, 2] and 3.2–7 % of patients after revision arthroplasties [1]. The increasing number of primary arthroplasties is accompanied by a higher total number of revision operations (which include PJI). However, because of the demands for high microbiological purity in the operating room environment, we note a decreasing rate of PJI in relation to the total number of orthopaedic procedures.
The diagnosis of periprosthetic joint infection is based on history (fever, operations in the past, pain), clinical assessment (infiltration, sinus tract) and laboratory tests [elevated WBC, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP)]. Radiological signs of implant loosening such as scalloping, osteolysis, periostitis or collections of fluid detected on ultrasonography around the joint may facilitate the diagnosis of PJI. The laboratory criteria of PJI diagnosis have not yet been established. At present, the diagnosis is based on the presence of a sinus tract communicating with the prosthesis or a pathogen isolated by culture from at least two separate samples or when the results of laboratory studies meet at least four of six diagnostic criteria: elevated serum ESR and serum CRP, elevated serum leukocyte count, elevated synovial neutrophil percentage (PMN%), purulent fluid in the affected joint, isolation of a micro-organism in one culture (periprosthetic tissue or fluid) or greater than five neutrophils per high-power field observed in periprosthetic tissue [3].
Previous studies have demonstrated the presence of bacteria under a protective layer of biofilm that does not induce a systemic response with production of acute-phase proteins and an increased leukocyte count. Due to the location of bacteria, cultures obtained from the tissues around the joint are often negative. The tool which improves the sensitivity of cultures is ultrasound or sonication of the infected implants removed during revision surgery. The ultrasound destroys the protective biofilm covering a sessile group of bacteria and releases active cells, which promotes culture growth considerably and facilitates identification of pathogens [4]. It seems that ultrasound supported by polymerase chain reaction (PCR) tests could play a leading role in bacterial identification [5].
The aim of this study was to detect bacterial species in supposedly non-infected prosthetic joint loosening using sonication and 16S rRNA gene sequencing. This prospective study was constructed to detect and/or isolate bacteria present on the surface of the implant in cases of silent infection. We postulate that a sonication procedure followed by PCR will improve bacterial identification in non-infected prosthetic joint loosening. Diagnosis of aseptic loosening of the prosthesis does not exclude the presence of bacteria on the surface of the prosthesis.
Materials and methods
Patients and samples
A total of 16 patients (11 women and five men) aged 46–80 years (mean age 65.7) with a diagnosis of prosthetic knee or hip loosening qualified for this study. We recruited ten patients with hip and six patients with knee joint loosening attending the Department of Orthopaedics and Traumatology, Medical University of Silesia in Katowice, Poland. The average period between the primary joint replacement and the revision arthroplasty was calculated at 102.1 months (approx. 8.5 years). In three cases this period was shorter than six months and in one case it was 17 months. In the other 12 cases it was a late loosening that occurred ≥24 months after the primary procedure (Table 1).
Table 1.
Clinical details of patients
| Patient | Age/sex | Affected joint | CRP | Histopathology resultsa | Age of the implant, in months | Culture results | Molecular identification | |
|---|---|---|---|---|---|---|---|---|
| Bacteria identified by 16S rRNA gene sequencing | ||||||||
| Preoperative samples (joint fluid) | Sonicate | |||||||
| 1 | 72/M | Knee | < 5 | II | 17 | Negative | Negative | Brevibacterium frigoritolerans |
| 2 | 63/M | Knee | < 5 | II | 84 | Negative | Negative | Negative |
| 3 | 72/F | Knee | <5 | II | 50 | Negative | Negative | Ralstonia pickettii, Ralstonia mannitolilytica |
| 4 | 62/F | Hip | <5 | II | 168 | Negative | Negative | Enterobacter cancerogenus, Escherichia vulneris |
| 5 | 78/F | Hip | < 5 | II | 6 | Negative | Negative | Pseudomonas otitidis, Pseudomonas aeruginosa |
| 6 | 76/F | Hip | <5 | II | 168 | Negative | Negative | Pelomonas aquatica, Pelomonas saccharophila |
| Brachybacterium muris, Brachybacterium rhamnosum | ||||||||
| 7 | 80/F | Hip | <5 | II | 108 | Negative | Negative | Staphylococcus capitis, Staphylococcus caprae |
| 8 | 48/F | Hip | <5 | II | 96 | Negative | Negative | Lactococcus lactis |
| 9 | 46/M | Hip | < 5 | II | 120 | Negative | Ralstonia pickettii | Staphylococcus hominis |
| 10 | 64/F | Hip | 6 | III | 240 | Negative | Negative | Pseudomonas otitidis, Pseudomonas aeruginosa, Staphylococcus hominis, Propionibacterium acnes, Propionibacterium propionicum, Lactobacillus frumenti, Lactobacillus vaginalis |
| 11 | 54/F | Knee | 93.4 | II | 3 | S. aureus | S. aureus | |
| 12 | 50/M | Knee | 32.5 | II | 6 | S. aureus | S. aureus | |
| 13 | 73/F | Hip | 6.2 | II | 336 | S. warneri | Negative | |
| 14 | 80/F | Hip | 7 | II | 156 | S. epidermidis | Negative | |
| 15 | 77/F | Knee | 18.6 | III | 36 | S. hominis | S. hominis Ralstonia pickettii | |
| 16 | 56/M | Hip | 39 | II | 40 | Enterobacter cloacae | Enterobacter cloacae | |
Ethical approval was obtained from the Ethics Committee of the Medical University of Silesia in Katowice, Poland. The patients were informed about the aim and methods of the study and each of them gave written, informed consent to participate in this study.
Inclusion criteria were: presence of symptoms suggesting loosening of any of the components of the prosthetic hip or knee joint such as: pain in the hip or thigh region, knee pain, radiological symptoms of loosening (disintegration of prosthesis components with the bone, displaced components of the prosthesis) and elevated or normal level of the markers of infection (WBC, ESR, CRP).
Exclusion criteria were: administration of antibiotics less than two weeks before revision arthroplasty, periprosthetic fractures, other established infection sites in the organism, rheumatoid arthritis and lack of patient's consent for participation in the study.
Microbiological methods
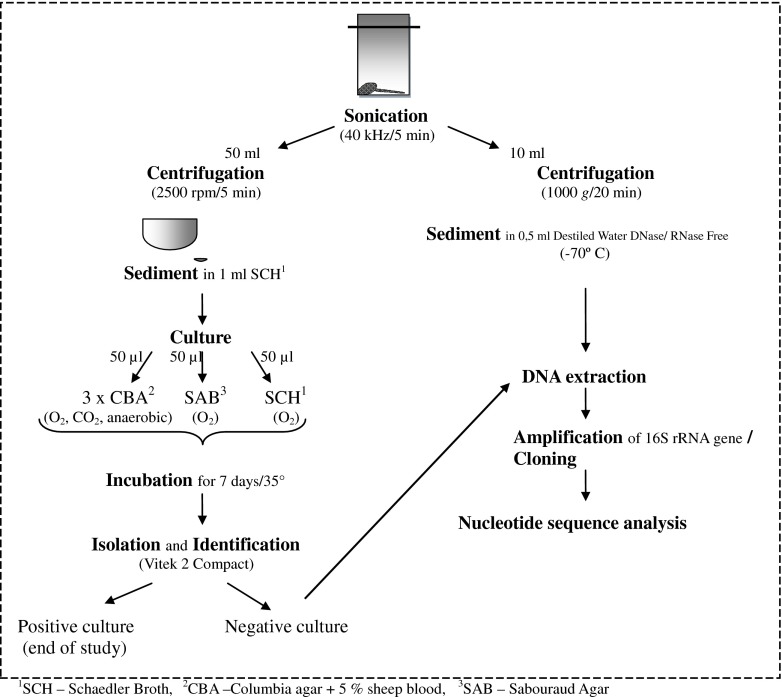
Aspiration of hip and knee joints was performed and the fluid from the aspirated joints was taken for culture. Directly after surgery the removed components of loosened prostheses were placed into sterile container with Ringer’s solution and immediately transported to the microbiological laboratory, where they were subjected to sonication in an ultrasonic bath (Sonic-6, SMS, Poland) for five minutes at a frequency of 40 kHz [4, 6]. Sediment received after vortexing (5 min/2,500 rpm) 50 ml of sonicated fluid was cultured. After rejection of the supernatant, one millilitre of liquid Schaedler medium was added to the sediment. A total of 50 μl of aliquots of sonicated fluid were inoculated onto Columbia agar with sheep blood (incubated aerobically, anaerobically and in high concentration of CO2), Sabouraud plate and liquid Schaedler medium. After at least seven days of incubation isolated bacteria were identified using the VITEK 2 Compact analyser (bioMérieux, Marcy-l’Étoile, France).
Molecular methods
Additional molecular detection was performed in the cases of aseptic loosening of the prosthesis. Sediment received after vortexing of ten millilitres of sonicated fluid (20 min/1,000 g) was suspended in 0.5 ml DNase/RNase-Free Distilled Water (Gibco, USA) and saved frozen at −70 °C for subsequent testing. DNA was isolated using GeneMATRIX Bacterial & Yeast Genomic DNA Purification Kit (EURx Ltd., Gdańsk, Poland) in accordance with the manufacturer’s instructions. The identification of clones was conducted, according to Dempsey et al. [7], amplification of 16S rRNA, cloning PCR products by using the pGEM-T Easy Vector System I Kit (Promega, Southampton, UK). DNA sequencing was conducted according to the manual provided by the DNA analyser 3130xl ABI PRISM, manufactured by Applied Biosystems. The sequences were compared to reference sequences in the BLAST programme (Database Name TL/16S ribosomal RNA Bacteria and Archaea). The differences were verified for final confirmation by analysis of the raw chromatogram sequencing data using Chromas Lite v.2.01 freeware (Fig. 1).
Fig. 1.
Diagnostic scheme for orthopaedic samples
Histopathological tests
Soft tissue surrounding the implants and periprosthetic interface membrane were taken for histopathological testing [8]. The outcomes were recorded according to the Krenn and Morawietz classification [9].
Results
CRP results
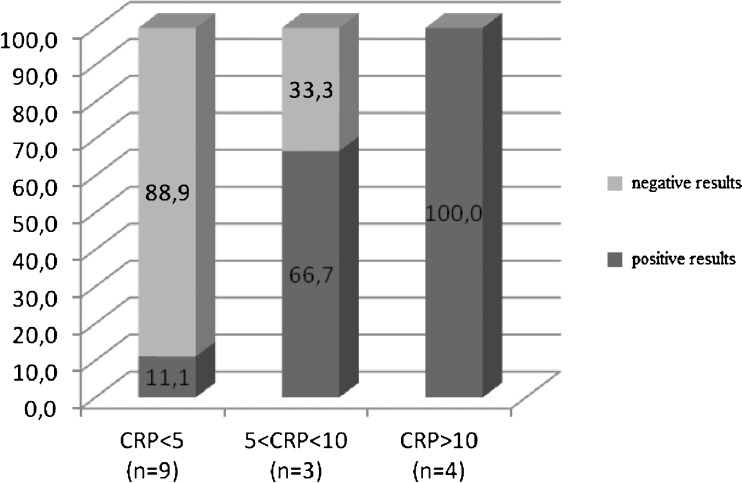
CRP was elevated (>5 mg/l) in seven of 16 cases. The number of positive cultures rises with a higher level of CRP. In the group of patients with CRP <5 mg/l culture results were positive in 11.1 % cases; however, in all cases with CRP > 10 mg/l PJI was recognised (Fig. 2).
Fig. 2.
Results of cultures and CRP (mg/l)
Microbiological results
Joint fluid culture
A negative joint fluid culture was observed in nine cases in the group of patients with CRP <5 mg/l and in one case of a patient with CRP = 6 mg/l (mean CRP value 5.1 mg/l). In all cases of positive joint fluid culture CRP was elevated >5 mg/l (mean value 32.8 mg/l). The number of positive joint fluid cultures rises with a higher level of CRP. In the group of patients with CRP <5 mg/l all results were negative; however, in 85.7 % patients with CRP > 5 mg/l PJI was recognised.
Sonicate culture
Negative sonicate cultures were demonstrated in eight cases in the group of patients with CRP <5 mg/l and in three cases with CRP > 5 mg/l (mean value 5.4 mg/l). Among five positive sonicate cultures four cases were obtained in patients with CRP > 5 mg/l, and one case of positive sonicate culture was obtained from a patient with CRP <5 mg/l (mean value 37.7 mg/l).
The number of positive sonicate culture rises with a higher level of CRP. In the group of patients with CRP <5 mg/l the results were positive only in 11.1 % cases; however, in all cases with CRP > 10 mg/l PJI was recognised (Fig. 2).
A 75 % compatibility (12 cases) was found between culture results of synovial fluid obtained through the joint aspiration and sonicate fluid obtained from components of the prosthesis. In two cases (patients 9 and 15) sonicate cultures additionally revealed a growth of Ralstonia pickettii (Table 1).
Molecular detection
The presence of bacteria was confirmed with molecular testing in 90 % of patients with negative results of synovial fluid cultures of aspirated joints (patients 1 to 10) and in 88.9 % patients with negative sonicate culture results. In three of ten cases (patients 7, 9 and 10) molecular techniques revealed coagulase-negative staphylococci (CNS), a typical aetiological agent of PJI. Positive PCR results revealed a presence of two or more different bacterial species, however, frequently of the some genus (Table 1).
Histopathological test
The results of histopathological tests revealed the presence of features of infection in all cases, with two cases combined with the wear particle-induced type (Table 1).
Discussion
This prospective study was designed to detect bacterial species in non-infected prosthetic joint loosening using ultrasound and 16S rRNA gene sequencing. We compared the results of bacterial culture of joint fluid and sonicate with sequencing results.
The ultrasound procedure followed by PCR improves bacterial identification in non-infected prosthetic joint loosening. Our study suggests that the diagnosis of aseptic loosening does not exclude the presence of bacteria on the surface of the prosthesis. The ultrasound procedure releases active bacteria from removed parts of prostheses, promotes culture growth and also facilitates identification of pathogens. This process considerably enhances culture sensitivity [6, 7]. The ultrasound procedure may become one of the standard methods facilitating the diagnosis of PJI. Our study demonstrates a non-significant increase in positive results after sonication from 37.5 to 43.75 %. We revealed that a positive culture correlates with a higher value of CRP. This finding is in agreement with outcomes published by Gomez et al., who reported the mean value of CRP of 50.2 mg/l in the group of PJI and 11.8 mg/l in the group of aseptic loosening [6]. We diagnosed PJI in all of the patients with CRP > 10 mg/l. In our study we demonstrated bacterial growth in six of 16 cases, viz. Staphylococcus aureus, CNS and Gram-negative bacilli, i.e. typical aetiological agents of periprosthetic infection [10]. Moran et al. isolated strains of S. aureus and CNS, respectively, in 52 and 47 % of patients with PJI [11]. These species were also predominant in other studies [6, 12]. In our study, we confirmed the infectious process by histopathological testing. Gomez et al. stated that synovial culture, tissue culture and sonicate liquid culture in comparison with PCR of sonicate fluid demonstrate similar sensitivity and specificity. On the other hand, combined sonicate liquid culture and PCR of sonicates have a higher sensitivity than a single test [6]. However, in our study, ultrasound did not confirm two positive synovial cultures. False-positive outcomes are predominantly attributed to contamination by patient microflora and staff hands. Contamination is also possible in the region of the surgical site, during the collection of specimens, and rarely during specimen processing in the laboratory.
We confirmed the growth of R. pickettii in two cultures obtained from sonicates—in one case of aseptic loosening and the second of PJI with identified S. hominis. R. pickettii is described as a non-fermenting Gram-negative bacillus with low virulence which lives in a wet environment (water, skin disinfectants, care products) and can colonise humans without clear symptoms. It has been emphasised lately that microbes that live in the natural environment could become very serious pathogens, especially in patients with immunodeficiency and many co-morbidities.
The alternative to culture methods for the detection of pathogens based on isolation is amplification and DNA sequencing [6, 13, 14]. In our study we identified various species of bacteria in aseptic cases of prosthesis loosening, which occur in the human environment, e.g. R. pickettii, Pseudomonas spp., Brevibacterium spp. (in food), Lactobacillus spp., Propionibacterium spp. and Staphylococcus spp. (on skin). These micro-organisms are described as aetiological agents of infection in patients with immunodeficiency [15–19]. Using 16S rRNA we detected two or more pathogens in one sample, in some cases from the same genera. The analysis of these outcomes is very difficult. In the studies of other researchers who used DNA sequencing to identify bacteria, mixed, polymicrobial infection has been noted, both in PJI [6, 7, 16] and in other clinical cases [13].
Studies which are trying to establish the diagnostic criteria of PJI are still in progress [2, 3]. The latest report was presented by Parvizi et al. [20]. The diagnosis of periprosthetic infection should be based on the medical history and clinical examination of the patient, and the culture considered as a “gold standard” by some of the researchers still is insufficient in sensitivity and specificity [9, 21]. Aseptic loosening of a prosthesis is caused by a lack of stability of the prosthesis and proper integration with the bone and excludes an infectious process. This concept is changing, as the presence of pathogens on the surface of implants has been confirmed [5, 22, 23].
In our study the histopathological tests of periprosthetic tissues revealed the infectious type (type II) in every patient, and only in two cases did the results show the presence of wear particles, which coexisted with type II (Table 1). These results were particularly associated with PJI. Many studies showed that the periprosthetic membrane is an ideal sample material for characterising the type of inflammation by histology, thus providing valuable evidence for the underlying cause of implant loosening [8, 24].
Limitations
Our study was performed over a short period of time (January–June 2012). Therefore, the number of patients studied was small. However, we obtained very promising results, as ultrasound and PCR followed by sequencing yielded positive results in 90 % of cases. Further studies are necessary to obtain more information about the role of bacterial species in the aetiology of clinically silent PJI.
The knowledge about the new aetiological agents, and their role in initiating or activating infections, is the main clinical interest of this study. New findings from theoretical studies could change the management of silent periprosthetic infection, including antibiotic therapy and surgery. From the economic point of view modification of the treatment regime could reduce the high costs of revision surgery. In addition, the rarely isolated and environmental bacteria isolated from the surface of implants possibly could be the centre of interest for future studies.
Conclusion
Based on the results of our study and outcomes of other authors, the lack of clinical signs of infection, negative culture of preoperative joint aspiration and intraoperative specimens do not exclude the presence of bacteria on the implants in cases of aseptic loosening of a prosthesis. There is a need for subsequent studies using ultrasound/sonication and molecular biology techniques. It could be especially helpful to understand the role of rarely isolated bacteria from the biological materials in periprosthetic infections.
Acknowledgments
This study has received funding from the Medical University of Silesia in Katowice (KNW-1-067/P/1/0; 2011r. and KNW-1-044/P/2/0; 2012). Equipment for molecular analysis was purchased using Silesian Bio-Farma Center for Biotechnology, Bioengineering and Bioinformatics Project no. POIG.02.01.00-00-166/08 THE OPERATIONAL PROGRAMME INNOVATIVE ECONOMY FOR 2007–2013. Priority Axis 2.
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Przemysław L. Bereza, Phone: +48-32-3598270, FAX: +48-32-2029932, Email: pberez@o2.pl
Alicja Ekiel, Email: aekiel@sum.edu.pl.
Aleksandra Auguściak-Duma, Email: aaugusciak@sum.edu.pl.
References
- 1.AAOS Guideline on the Diagnosis of Periprosthetic Joint Infection of the Hip and Knee, Guideline and Evidence Report adopted by American Academy of Orthopaedic Surgeons, Board of Directors, 2010. Available via http://www.aaos.org/
- 2.Parvizi J, Walinchus L, Adeli B. Molecular diagnostics in periprosthetic joint infection. Int J Artif Organs. 2011;34(9):847–855. doi: 10.5301/ijao.5000054. [DOI] [PubMed] [Google Scholar]
- 3.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469(11):2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357(7):654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 5.Tunney MM, Patrick S, Curran MD, Ramage G, Hanna D, Nixon JR, Gorman SP, Davis RI, Anderson N. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J Clin Microbiol. 1999;37(10):3281–3290. doi: 10.1128/jcm.37.10.3281-3290.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez E, Cazanave C, Cunningham SA, Greenwood-Quaintance KE, Steckelberg JM, Uhl JR, Hanssen AD, Karau MJ, Schmidt SM, Osmon DR, Berbari EF, Mandrekar J, Patel R. Prosthetic joint infection diagnosis using broad-range PCR of biofilms dislodged from knee and hip arthroplasty surfaces using sonication. J Clin Microbiol. 2012;50:3501–3508. doi: 10.1128/JCM.00834-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dempsey KE, Riggio MP, Lennon A, Hannah VE, Ramage G, Allan D, Bagg J. Identification of bacteria on the surface of clinically infected and non-infected prosthetic hip joints removed during revision arthroplasties by 16S rRNA gene sequencing and by microbiological culture. Arthritis Res Ther. 2007;9:R46. doi: 10.1186/ar2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müller M, Morawietz L, Hasart O, Strube P, Perka C, Tohtz S. Histopathological diagnosis of periprosthetic joint infection following total hip arthroplasty: use of a standardized classification system of the periprosthetic interface membrane. Orthopade. 2009;38(11):1087–1096. doi: 10.1007/s00132-009-1471-1. [DOI] [PubMed] [Google Scholar]
- 9.Morawietz L, Classen RA, Schröder JH, Dynybil C, Perka C, Skwara A, Neidel J, Gehrke T, Frommelt L, Hansen T, Otto M, Barden B, Aigner T, Stiehl P, Schubert T, Meyer-Scholten C, König A, Ströbel P, Rader CP, Kirschner S, Lintner F, Rüther W, Bos I, Hendrich C, Kriegsmann J, Krenn V. Proposal for a histopathological consensus classification of the periprosthetic interface membrane. J Clin Pathol. 2006;59(6):591–597. doi: 10.1136/jcp.2005.027458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bozic KJ, Katz P, Cisternas M, Ono L, Ries MD, Showstack J. Hospital resource utilization for primary and revision total hip arthroplasty. J Bone Joint Surg Am. 2005;87:570–576. doi: 10.2106/JBJS.D.02121. [DOI] [PubMed] [Google Scholar]
- 11.Moran E, Masters S, Berendt AR, McLardy-Smith P, Byren I, Atkins BL. Guiding empirical antibiotic therapy in orthopaedics: the microbiology of prosthetic joint infection managed by debridement, irrigation and prosthesis retention. J Infect. 2007;55:1–7. doi: 10.1016/j.jinf.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Marín M, Garcia-Lechuz JM, Alonso P, Villanueva M, Alcalá L, Gimeno M, Cercenado E, Sánchez-Somolinos M, Radice C, Bouza E. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50:583–589. doi: 10.1128/JCM.00170-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marques da Silva R, Caugant DA, Eribe ER, Aas JA, Lingaas PS, Geiran O, Tronstad L, Olsen I. Bacterial diversity in aortic aneurysms determined by 16S ribosomal RNA gene analysis. J Vasc Surg. 2006;44:1055–1060. doi: 10.1016/j.jvs.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikulska M, Durando P, Pia Molinari M, Alberti M, Del Bono V, Dominietto A, Raiola AM, Van Lint MT, Bregante S, Orengo G, Bacigalupo A, Viscoli C. Outbreak of Ralstonia pickettii bacteraemia in patients with haematological malignancies and haematopoietic stem cell transplant recipients. J Hosp Infect. 2009;72(2):187–188. doi: 10.1016/j.jhin.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Makaritsis KP, Neocleous C, Gatselis N, Petinaki E, Dalekos GN. An immunocompetent patient presenting with severe septic arthritis due to Ralstonia pickettii identified by molecular-based assays: a case report. Cases J. 2009;2:8125. doi: 10.4076/1757-1626-2-8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aubin GG, Bémer P, Guillouzouic A, Crémet L, Touchais S, Fraquet N, Boutoille D, Reynaud A, Lepelletier D, Corvec S. First report of a hip prosthetic and joint infection caused by Lactococcus garvieae in a woman fishmonger. J Clin Microbiol. 2011;49:2074–2076. doi: 10.1128/JCM.00065-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talento AF, Malnick H, Cotter M, Brady A, McGowan D, Smyth E, Fitzpatrick F. Brevibacterium otitidis: an elusive cause of neurosurgical infection. J Med Microbiol. 2013;62(Pt 3):486–488. doi: 10.1099/jmm.0.043109-0. [DOI] [PubMed] [Google Scholar]
- 19.Orkaby AR, Chen B, Iliaki EF, Sulis CA, Oates DJ. A curious case of Lactobacillus casei in a prosthetic joint: was it the yogurt? J Am Geriatr Soc. 2012;60(6):1177–1178. doi: 10.1111/j.1532-5415.2012.03980.x. [DOI] [PubMed] [Google Scholar]
- 20.Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104. doi: 10.2106/JBJS.K.01417. [DOI] [PubMed] [Google Scholar]
- 21.Müller M, Morawietz L, Hasart O, Strube P, Perka C, Tohtz S. Diagnosis of periprosthetic infection following total hip arthroplasty–evaluation of the diagnostic values of pre- and intraoperative parameters and the associated strategy to preoperatively select patients with a high probability of joint infection. J Orthop Surg Res. 2008;21(3):31. doi: 10.1186/1749-799X-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moussa FW, Anglen JO, Gehrke JC, Christensen G, Simpson WA. The significance of positive cultures from orthopedic fixation devices in the absence of clinical infection. Am J Orthop (Belle Mead NJ) 1997;26(9):617–620. [PubMed] [Google Scholar]
- 23.Tunney MM, Patrick S, Curran MD, Ramage G, Anderson N, Davis RI, Gorman SP, Nixon JR. Detection of prosthetic joint biofilm infection using immunological and molecular techniques. Methods Enzymol. 1999;310:566–576. doi: 10.1016/S0076-6879(99)10044-2. [DOI] [PubMed] [Google Scholar]
- 24.Krenn V, Morawietz L, Jakobs M, Kienapfel H, Ascherl R, Bause L, Kuhn H, Matziolis G, Skutek M, Gehrke T. Joint endoprosthesis pathology: histopathological diagnostics and classification. Pathologe. 2011;32(3):210–219. doi: 10.1007/s00292-011-1418-2. [DOI] [PubMed] [Google Scholar]