Abstract
Introduction
We conducted a study to test the hypothesis that systemic dysregulation of Th1/ Th2 cytokine levels was associated with detection of carcinogenic or overall human papilloma virus (HPV) at the cervix among 964 women residing in the rural village in Nigeria.
Methods
Levels in plasma were measured for 19 cytokines, including Th1-like cytokines IL-2, IL-12 (p40), TNF-a, IFN-g; Th2-like cytokines IL-4, IL-5, IL-6, IL-10, IL-13; innate/inflammation cytokines IL-1a, IL-1b,IL-8, Eotaxin, MCP-1, MIP-1a, and IL-7; and cell development cytokines G-CSF, VEGF, and IL-17. Analysis was restricted to 5 cytokines, TNF-α (Th1), IL-8 (Th2), eotaxin and MCP-1 (innate/inflammation), and G-CSF (cell development), whose levels were detected in 80% or more of the samples measured as well as had a coefficient of variation of <30%.
Results
Strong correlations were noted between levels of eotaxin and TNF-α (r=0.75), IL-8 and MCP-1 (r=0.60), eotaxin and G-CSF (r=0.44), and G-CSF and IFN-γ (r=0.43). Detection of carcinogenic or non-carcinogenic HPV DNA was unrelated to cytokine levels, except for levels of eotaxin and TNF-α, which were inversely correlated, albeit weakly, with detection of any carcinogenic HPV (P=0.048 and P=0.067, respectively). In analyses stratified by age group, levels of eotaxin were inversely correlated with detection of any HPV DNA (P=0.026) and carcinogenic HPV (P=0.042) in older, but not younger, women.
Conclusions
Our results do not support the hypothesis of systemic cytokine dysregulation and HPV at the cervix in Nigerian women, but subgroup analyses raise questions about inverse associations between eotaxin and TNF-α in older women.
Keywords: cytokines, cervical cancer, inflammation, Plasmodium falciparum malaria
1. Introduction
Squamous carcinoma of the cervix (SCC) is the first or second leading cancer and cause of cancer death in many African countries[1], where some of the world's highest incidence rates are observed [2]. This high incidence and mortality impact is due to high prevalence of human papillomaviruses (HPV) [3, 4], specifically of several of the 13 HPV genotypes considered carcinogenic [3], combined with lack of basic cervical cancer screening and early treatment services. In the West, the age-specific prevalence of HPV infection falls with age as the corresponding probabilities of new sexual partners and of encountering new HPV infections decrease [5, 6]. However, in contrast to patterns observed in women in developed countries [5–7], HPV prevalence does not decrease with age in women in most sub-Saharan. HPV prevalence appears to persist at high levels among older African women [5, 7, 8]. For example, HPV prevalence was 14.7% among 1282 women from households selected at random in a rural village in Ile-Ife in Nigeria[9].
The reasons for HPV persistence in women in sub-Saharan Africa are not well understood, but they include a high rate of acquisition of new infections (incidence) and low rate of clearance of acquired infections (duration). The risk factors for prevalent HPV infection include early age at first pregnancy, more than 2 lifetime sex partners, and oral contraceptive use [10]. Surprisingly, being in a multiple wife household, which may be a surrogate for HPV transmission in a married setting, was marginally associated with detection of carcinogenic HPV in older women [10]. While sexual contact is a necessary driver of acquisition of new HPV infections, acquisition of effective immune response to HPV proteins is necessary for clearance and protection against new HPV infections [11, 12]. One study, which noted a geographical correlation between the distribution of high grade of cervical cancer and malaria endemicity in Uganda, hypothesized that relative immunosuppression from lifelong malaria infection may contribute [13]. Plausibly, co-infection malaria or stool parasites [14] may modulate influence T helper (TH) 1/2 immunity [15], and, though that mechanism, persistence of cervical HPV prevalence at the cervix or progression to cancer. We therefore conducted a study to evaluate the hypothesis that HPV prevalence at the cervix, particularly among older women, in Nigeria may be associated with self-reported malaria or plasma levels of inflammatory cytokines.
2. Material and Methods
2.1. Study population
Detailed methods of the study have been reported elsewhere [9, 10]. Briefly, 2,100 women residing in 439 households selected at random in a village in Ondo State in Nigeria were invited to participate in an HPV/SCC screening survey. Multiple-wife households were over-sampled to study intra-familial HPV transmission in that setting. In total, 1,420 of 2,091 eligible women (not pregnant, without a hysterectomy, 15+ years of age, lived in the house for more than three months) consented for cervical screening using acetic acid and visual inspection of the cervix. Cervical cell samples were taken and placed in liquid media for cytology. Venous blood samples were taken in EDTA tubes, separated within 2 hours of collection, and stored at −80°C until shipped to the National Cancer Institute Repository. Women answered an interviewer-administered questionnaire about established risk factors for HPV, cervical cancer and a history of malaria in the past 24 months. Exposure to other endemic parasites was not evaluated.
2.2. HPV testing
HPV DNA testing was performed in the U.S. using polymerase chain reaction (PCR) MY09-MY11 primers to detect HPV genotypes [16]. Samples were considered adequate when positive for β-globin. HPV status was scored categorized as positive when at least one of ~42 HPV genotypes was detected, otherwise as negative. Results were further categorized as “definitely or possibly carcinogenic HPV positive” when at least one of the 13 carcinogenic types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68 [9] were detected; otherwise as negative.
2.3. Cytokine testing
Levels were measured for 19 cytokines/chemokines on a 96-well plate format using a custom Milliplex bead assay (Millipore, Billerica, MA) according to the manufacturer's protocol [17]. . The cytokines includes were as follows: a) IL-2, IL-12 (p40), TNF-α, and IFN-g (Th1-like cytokines); b) IL-4, IL-5, IL-10, and IL-13 (Th2-like cytokines); c) IL-1a, IL-1b, IL-6, IL-8, Eotaxin, MCP-1, MIP-1a, and IL-7 (innate/inflammation cytokines); and d) G-CSF, VEGF (growth factors), and e) IL-17 (Th2-17 cytokine). All tests were performed in duplicate and the mean results usedfor analysis. The assay readings from the BioPlex 100 instrument (v6.0) were expressed as mean fluorescent index (MFI). MFI values were used extrapolated to cytokine levels based on a standard fitting curve derived from results of six serial dilutions of a recombinant cytokine standard in assay diluent. An additional standard point was added to extend the lower level of the standard curve to 0.64 pg/ml. Four replicate samples (2 within- and 2 between-batches from a woman whose cervical sample was HPV positive and a woman whose cervical sample was HPV negative) were included to assure assay reliability and guard against assay drift.
2.4. Statistical analysis
Statistical analyses were performed using STATA (version 11, STATA Corp, College Station, Texas, USA). The MFI results for cytokine were log-transformed to normalize the distribution of results. Reliability of cytokine assays was evaluated by calculating within- or between-plate coefficients of variation (CVs) by dividing the assay mean by the assay standard deviation of replicate samples. Only assays with CVs less than 30% and detected in more than 80% of samples passed and were included in analyses. Assays with CVs equal or higher than 30% were considered to have failed and were excluded in analyses. Spearman's correlation coefficient was calculated for each pair of cytokines to identify cytokines with strongly correlated levels. Analysis of continuous values was performed using the Student's t-test. Differences in the proportional distribution of tertile levels for cytokine levels according to self-reported malaria or HVP DNA detection at the cervix were evaluated using Chi-squared tests. Tertiles for each cytokine were calculated based on cutoff points obtained among the HPV negative women. The possible effect of age group on associations between cytokines and self-reported malaria or HPV persistence was evaluated in separate analyses for women aged 15–34 years and 35+ years. These age groups were selected a priori based on consideration that HPV DNA in women aged 15–34 years is more likely to be due to recently acquired HPV, whereas HPV DNA in women aged 35+ is more likely to be due to persistent HPV infections. Crude and adjusted odds ratios (OR) and 2-sided 95% confidence intervals (CI) were estimated using unconditional logistic regression to assess association of cytokines with HPV and past malaria infection. A two-sided P-value <0.05 was considered statistically significant.
3. Results
3.1. Study population
In total, 964 women were evaluated in the study. The median age of the women was 45 years (Inter-quartile range 30–60 years). HPV DNA results were missing for 34 (3.5%). Among those with results, overall HPV DNA prevalence was 25.8% and carcinogenic HPV prevalence was 14.9%, based on being positive for at least one of the established 13 carcinogenic types.
3.2. Cytokine results and patterns
Of 19 cytokines measured, 5 cytokines passed quality control (Table 1), based on having CVs less than 30% and being detectable in 80% of the samples, including TNF-α (Th1), IL-8 (Th2), eotaxin and MCP-1 (innate/inflammation), and G-CSF (growth factors). The mean levels of the cytokines were variable ranging from 8.2 pg/ml (TNF-α) to 202.3 pg/ml (MCP-1). Four cytokines were detected in 40–80% of samples, IFN-γ (Th1), IL-10 (Th2), MIP-1a (innate/inflammation), and VEGF (growth factors), while 10 cytokines were detected in less than 25% of the samples, so their results were excluded from this analysis.
Table 1.
Cytokine results, including mean values and within and inter-batch coefficient of variation in rural women from Nigeria
| All Subjects | Number (%) | Detected | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Cytokine | All subjects | #Not detected* | # detected* | Mean§ | SD§ | C.V. (n/n)± |
| Th-1 | ||||||
| TNF-α | 964 | 3 (0.3) | 961 | 2.11 | 0.65 | 12/9 (62/62) |
| IFN-γ | 964 | 485 (50) | 479 | 3.05 | 1.44 | 32/34 (28/35) |
| Th-2 | ||||||
| IL-10 | 964 | 496 (51) | 468 | 2.42 | 1.25 | 27/26 (33/31) |
| Innate/Inflammatory | ||||||
| IL-8 | 964 | 9 (1.4) | 955 | 3.12 | 1.34 | 19/16 (62/62) |
| Eotaxin | 964 | 16 (1.7) | 948 | 4.00 | 0.64 | 15/12 (59/61) |
| MCP1 | 964 | 1 (0.1) | 963 | 5.31 | 0.55 | 13/9 (62/62) |
| MIP1a | 964 | 590 (61) | 374 | 4.08 | 1.54 | 22/23 (25/30) |
| Growth Factors | ||||||
| G-CSF | 964 | 173(18) | 791 | 3.53 | 0.79 | 15/25 (52/51) |
| VEGF | 964 | 351 (45) | 613 | 5.12 | 0.88 | 18/17 (37/39) |
Detection based on lower limit of detection for the assay (lower than the lowest standard deviation, see methods:
Mean and standard deviation (SD) based on log-transformed values;
within/between batch C.V.,
(n/n) number in parentheses indicate the number of samples tested in duplicate.
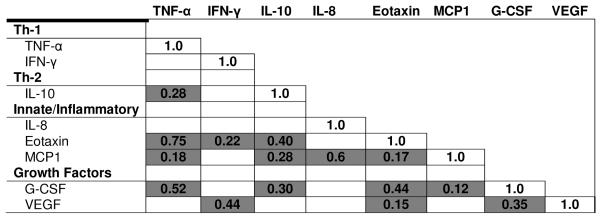
After adjusting for multiple comparisons with Bonferroni correction, strong correlations were observed between eotaxin and TNF-α (Spearman r=0.75: innate/inflammation-Th1), IL-8 and MCP-1 (Spearman r=0.60: Th2 and innate/inflammation), eotaxin and G-CSF (Spearman r=0.44: innate/inflammation and growth factors), and G-CSF and IFN-γ (Spearman r=0.43: growth factors and Th1) (Table 2). Cytokine levels were higher in women aged 35+ than women aged 15–34 years for eotaxin (4.09 versus 3.77, P<0.001), TNF-α (2.14 versus 2.0, P=0.003), IL-8 (3.20 versus 2.88, P=0.001), MCP-1 (5.35 versus 5.21, P=0.0006), but not for G-CSF (3.46 versus 3.55, P=0.14).
Table 2.
Pair wise Spearman rank correlation coefficients for plasma cytokines among rural women from Nigeria*.
|
Correlations performed for cytokines detected in ≥50% of samples and with a C.V. <30 using untransformed values. Only correlations that are significant at p<0.05 after Bonferroni correction are tabulated.
3.3. Cytokine levels and self-reported malaria
Data on self-reported malaria was available on 889 women. Of these, 11%, 26% 28% 21% and 14% reported 0, 1, 2, 3, and 4–10 malaria episodes in the past 24 months, respectively. Reporting 4 or more malaria episodes in the past 24 months increased from 9% among women aged 15–24 years to 20% among women aged 65+ years (OR 2.5, 95% CI 1.1 −5.5). The distribution of tertile levels of cytokine was unrelated to the number of self-reported malaria episodes (Table 3), with the possible exception of IL8 (P=0.02) and eotaxin (P=0.08), whose distributions showed marginal statistical variation with the number of self-reported malaria episodes (Table 3).
Table 3.
Correlation between self-reported malaria episodes in the past two years and plasma cytokine levels among adult women from Nigeria
| Malaria episodes | 0 | 1 | 2 | 3 | 4–10 | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Cytokine | Titers | n | % | n | % | n | % | N | % | n | % | |
| Th-1 | ||||||||||||
| TNF-α | Lower third | 31 | 33.0 | 78 | 33.3 | 82 | 32.8 | 61 | 34.7 | 41 | 32.8 | 0.23 |
| Mid third | 34 | 36.2 | 65 | 27.8 | 84 | 33.6 | 66 | 37.5 | 43 | 34.4 | ||
| Upper third | 29 | 30.9 | 91 | 38.9 | 84 | 33.6 | 49 | 27.8 | 41 | 32.8 | ||
| IFN-γ | Lower third | 12 | 30.0 | 38 | 33.3 | 36 | 28.1 | 37 | 43.0 | 23 | 35.9 | 0.33 |
| Mid third | 17 | 42.5 | 40 | 35.1 | 42 | 32.8 | 27 | 31.4 | 19 | 29.7 | ||
| Upper third | 11 | 27.5 | 36 | 31.6 | 50 | 39.1 | 22 | 25.6 | 8.8 | 34.4 | ||
| Th-2 | ||||||||||||
| IL-10 | Lower third | 17 | 32.1 | 42 | 34.2 | 45 | 38.1 | 27 | 36.0 | 13 | 21.0 | 0.22 |
| Mid third | 14 | 26.4 | 37 | 30.1 | 44 | 37.3 | 20 | 26.7 | 25 | 40.3 | ||
| Upper third | 22 | 41.5 | 44 | 35.8 | 29 | 24.6 | 28 | 37.3 | 24 | 38.7 | ||
| Innate/Inflammatory | ||||||||||||
| IL-8 | Lower third | 25 | 26.6 | 80 | 34.5 | 75 | 30.1 | 75 | 43.1 | 33 | 26.4 | 0.02 |
| Mid third | 33 | 35.1 | 84 | 36.2 | 84 | 33.3 | 51 | 29.3 | 43 | 34.4 | ||
| Upper third | 36 | 38.3 | 68 | 29.3 | 91 | 36.6 | 48 | 27.6 | 49 | 39.2 | ||
| Eotaxin | Lower third | 32 | 34.0 | 81 | 35.5 | 68 | 27.8 | 66 | 37.9 | 32 | 25.6 | 0.07 |
| Mid third | 31 | 34.0 | 79 | 34.7 | 96 | 39.2 | 47 | 27.0 | 45 | 36.0 | ||
| Upper third | 31 | 33.0 | 68 | 29.8 | 81 | 33.1 | 61 | 35.1 | 48 | 38.4 | ||
| MCP1 | Lower third | 31 | 33.3 | 87 | 37.2 | 82 | 32.7 | 64 | 36.2 | 28 | 22.2 | 0.14 |
| Mid third | 28 | 30.1 | 79 | 33.8 | 89 | 35.5 | 49 | 27.7 | 53 | 42.1 | ||
| Upper third | 34 | 36.6 | 68 | 29.1 | 80 | 39.1 | 64 | 36.2 | 45 | 35.7 | ||
| MIP1a | Lower third | 11 | 32.4 | 30 | 37.0 | 31 | 27.9 | 20 | 28.2 | 16 | 34.8 | 0.79 |
| Mid third | 13 | 38.2 | 28 | 34.6 | 38 | 34.2 | 24 | 33.8 | 17 | 37.0 | ||
| Upper third | 10 | 29.4 | 23 | 28.4 | 42 | 37.8 | 27 | 38.0 | 13 | 28.3 | ||
| Cell Development | ||||||||||||
| G-CSF | Lower third | 34 | 42.0 | 67 | 35.6 | 69 | 32.2 | 47 | 33.8 | 29 | 27.1 | 0.46 |
| Mid third | 21 | 25.9 | 55 | 29.3 | 80 | 37.4 | 48 | 34.5 | 36 | 33.6 | ||
| Upper third | 26 | 32.1 | 66 | 35.1 | 65 | 30.4 | 44 | 31.7 | 42 | 39.3 | ||
| VEGF | Lower third | 22 | 34.9 | 50 | 33.1 | 57 | 36.1 | 32 | 30.8 | 24 | 28.6 | 0.85 |
| Mid third | 19 | 30.2 | 51 | 33.8 | 52 | 32.9 | 35 | 33.7 | 31 | 36.9 | ||
| Upper third | 22 | 34.9 | 50 | 33.1 | 49 | 31.0 | 37 | 35.6 | 29 | 34.5 | ||
3.4. Cytokine levels and HPV DNA detection at the cervix
Detection of HPV (overall, carcinogenic or possibly carcinogenic) DNA at the cervix was unrelated to cytokine levels, with the possible exceptions of eotaxin for which detection of any HPV (P=0.048) as well as detection of any carcinogenic HPV (P=0.067) was inversely associated with eotaxin tertiles (Table 4). Similar, but less strong patterns were observed for tertiles of TNF-α for detection of any carcinogenic, but not non-carcinogenic HPV. When mean levels were examined according to HPV status, mean eotaxin level was 0.10 log lower in the women positive for any HPV (3.94 versus 4.04 log, P=0.03) and 0.14 log lower in women positive for any carcinogenic HPV (3.89 versus 4.03 log, P=0.015). The corresponding results for TNF-α were as follows: mean level was 0.06 log lower in women positive for any HPV (2.06 versus 2.12 log, P=0.24) and 0.12 log lower in women positive for any carcinogenic HPV was considered (2.00 versus 2.12 log P=0.03) when compared to those who were negative.
Table 4.
Detection of human papilloma virus (any and carcinogenic) at the cervix and cytokines in rural women from Nigeria
| All subjects | Any HPV | Any carcinogenic HPV | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Cytokine | Levels | n | (%) | n/N | (%) | P | n/N | % | P |
| Th-1 | |||||||||
| TNF-α | Lower | 320 | 33.3 | 84/311 | 27.0 | 0.568 | 60/311 | 19.3 | 0.077 |
| Middle | 321 | 33.4 | 87/305 | 28.5 | 43/305 | 14.1 | |||
| Upper | 320 | 33.3 | 77/311 | 24.8 | 41/311 | 13.2 | |||
| Innate/Inflammatory | |||||||||
| IL-8 | Lower | 319 | 33.4 | 83/302 | 27.5 | 0.946 | 49/302 | 16.2 | 0.830 |
| Middle | 318 | 33.3 | 82/310 | 27.5 | 49/310 | 15.8 | |||
| Upper | 318 | 32.3 | 82/310 | 26.5 | 45/310 | 14.5 | |||
| Eotaxin | Lower | 316 | 33.3 | 86/295 | 29.2 | 0.048 | 55/295 | 18.4 | 0.067 |
| Middle | 316 | 33.3 | 92/309 | 29.8 | 50/309 | 16.2 | |||
| Upper | 316 | 33.3 | 68/311 | 22.9 | 37/311 | 11.9 | |||
| MCP-1 | Lower | 321 | 33.3 | 73/309 | 23.6 | 0.272 | 45/309 | 14.6 | 0.855 |
| Middle | 321 | 33.3 | 90/308 | 29.2 | 45/309 | 15.9 | |||
| Upper | 321 | 33.3 | 86/312 | 27.6 | 50/312 | 16.0 | |||
| Growth Factors | |||||||||
| G-CSF | Lower | 263 | 33.3 | 72/257 | 28.0 | 0.566 | 43/257 | 16.7 | 0.860 |
| Middle | 265 | 33.3 | 62/254 | 24.4 | 38/254 | 15.0 | |||
| Upper | 263 | 33.3 | 71/253 | 28.1 | 40/253 | 15.8 | |||
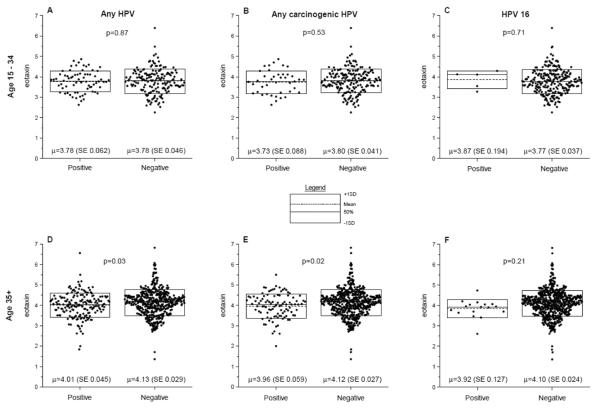
Because we observed that mean eotaxin level was 0.32 log higher in women aged 35+ years than those aged 20–34 years (P<0.001), we therefore repeated the analysis of mean level of eotaxin and TNF-α in HPV positive versus negative women stratified according to age group 20–34 years or 35+ years. Among women aged 15–34 years, no difference was noted in mean eotaxin level in women who were positive for any HPV (P=0.87), any carcinogenic HPV (P=0.53), or HPV 16 (P=0.71) compared to those who were negative (Figure 1 A, B and C). The results were similar for TNF- α (supplementary Figure 1 A, B, and C). Among women aged 35+ years, mean eotaxin level was 0.12 log lower in women who were positive for any HPV (P=0.03), 0.16 log lower in those who were positive for any carcinogenic HPV (P=0.02), and 0.17 log lower in those positive to HPV16 (P=0.21) compared to those who were negative (Figure 1 D, E, and F). The last result was based on small numbers (HPV16 accounted for 13% of all carcinogenic HPV) and was not statically significant. Levels for TNF-α were depressed in positive women, but the result was not statistically significant for any HPV (P=0.30), marginally significant for any carcinogenic HPV DNA detection (P=0.08), and not significant for HPV16 (P=0.8) (supplementary Figure 1 D, E and F).
Figure 1. Dot plot showing of log-transformed (base 2) eotaxin levels for women aged 20–34 years or 35+ years who were positive or negative for any human papillomavirus (HPV), any carcinogenic HPV, or HPV 16 by age group.
Each dot represents a single subject. The dotted line inside the box represents the mean and the solid line inside the box the median and the outer lines of the boxes represent values equal to the mean plus or minus one standard deviation. The P values are the probability that the mean results in the controls are equal to the mean results in the cases, based on the Student's t test.
In analyses stratified according to age and adjusting for each other, unit increase in log eotaxin, but not TNF-α, was inversely associated with detection of any HPV (OR 0.73, 95% CI 0.54–0.99), any carcinogenic HPV (OR 0.72, 95% CI 0.5–1.05), and HPV16 (OR 0.57, 95% CI 0.27–1.21) in women aged 35+ years. Levels of eotaxin and TNF-α were unrelated to detection of any HPV, of any carcinogenic HPV, or HPV16 in women aged 15–34 years (data not shown).
5. Discussion
To our knowledge, we present the first data to test the hypothesis whether systemic immune dysregulation, as measured by plasma cytokine levels, is related to HPV detection at the cervix. In addition, we also investigated correlation between a set of systemic cytokine levels with reported malaria. We did not find evidence for a correlation between plasma Th1/Th2 cytokines and HPV detection at the cervix or with self-reported malaria. As a possible exception, assuming that our findings did not occur by chance related to multiple comparisons, we found an association between low levels of eotaxin and increased prevalence of carcinogenic HPV at the cervix, particularly among older women. The inverse associations remained when analyses were restricted to HPV16, although the sample size was small and the result was not statistically significant. We also found an inverse, but less strong, association between levels of TNF-α with carcinogenic, but not possibly carcinogenic HPV genotypes. Given our finding that the of eotaxin and TNF-α generally increased with age, the inverse associations with HPV observed in older women may be specific. TNF-α is involved in Th1 immunity and HPV was correlated with eotaxin levels in our data, providing internal consistency in our data. Possibly, eotaxin and TNF-α levels in rural women in Nigeria might be associated with higher point prevalence of HPV infection at the cervix. Given that persistence of high-risk HPV, particularly HPV16, is associated with higher risk of cancer progression, the inverse association of lower mean levels of eotaxin and HPV detection among women aged 35+ years may be a clue about cytokines linked to persistence of HPV infection and risk for progression to cancer of the cervix. Future work is needed to evaluate the possible relationship between age, eotaxin and TNF-α level and HPV infection at the cervix in rural women in Africa.
Eotaxin is chemokine that modulates the recruitment and regulation of eosinophils and basophils through signaling of chemokine receptor-3 (CCR3) in the innate immunity/inflammation pathway [18]. Eosinophils, basophils, and mast cells are involved in innate immunity [19]. Our results are different from those reported by Marks et al [20] among women aged 30–60 years in Baltimore. In this study, eotaxin levels were higher in HPV-positive women and eotaxin levels were strongly and significantly correlated with T cell lineage molecules, such as IL-5, IL-, IL-9, IL-12, IL-15, and TNF-α. Our results may not be comparable to those reported by Marks et al because of different epidemiological setting, high proportion of undetectable cytokines in our study, and different body fluid assessed. Nonetheless, if our findings are confirmed, they could shed light on systemic dysregulation that might be correlated with epithelial control of HPV infection at the cervix.
Our study has limitations. We measured cytokines in plasma, yet cytokines act locally and they are degraded quickly through dilution and metabolism to minimize danger of undesirable systemic effects [17, 21]. Assuming detectable and physiologically relevant levels can be measured in plasma, delay in processing of samples, which was on average about two hours in our study, may have attenuated measurable signal. Second, we did not assess co-infections, such as hepatitis B and C viruses and human immunodeficiency virus, which may alter plasma levels of these cytokines. Third, we measured malaria infection using questionnaire, thus the null results might be due to gross misclassification of malaria exposure. Future work using malaria antibody tests to classify exposure could provide critical information about the role of malaria in HPV persistence. Finally, while cytological and colposcopic data were collected in the field and read in the U.S., variability in sample quality was observed, thereby reducing the value of the data to inform on the relationship between cytokine levels and dysplastic lesions. The strengths of our study include use of samples from a well characterized epidemiological study, use of high throughput technology to examine multiple markers from multiple immunological pathways, and inclusion of cytokines with high reproducibility.
To conclude, HPV DNA detection at the cervix in Nigerian women was unrelated to systemic cytokine levels. An inverse correlation with eotaxin was observed, especially in older women. Self-reported malaria was unrelated to HPV detection or systemic cytokines, perhaps because the exposure is grossly misclassified by interview.
Supplementary Material
Highlights
Cytokines were reliably measured in plasma in Nigerian women using Luminex assays
Cytokine levels were not associated with HPV detection at the cervix
A possible exception is inverse associations between eotaxin and carcinogenic HPV
Eotaxin findings suggest innate immunity may play a role in HPV persistence
Acknowledgement
We would like to thank Mr. David Check at the Biostatistics Branch at the National Cancer Institute (Rockville, Maryland) for help drawing the graphs. The study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services NIH grant # 5U01CA078527-13 & NIH contract HHSN261200900303P. The CareHPV equipment and supplies used in this study were donated by Qiagen Corporation (Gaithersburg, MD); CareHPV is not the subject of this report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
6. References
- [1].Sylla BS, Wild CP. A million africans a year dying from cancer by 2030: what can cancer research and control offer to the continent? International journal of cancer Journal international du cancer. 2012;130:245–50. doi: 10.1002/ijc.26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Parkin DM, Sitas F, Chirenje M, Stein L, Abratt R, Wabinga H. Part I: Cancer in Indigenous Africans--burden, distribution, and trends. Lancet Oncol. 2008;9:683–92. doi: 10.1016/S1470-2045(08)70175-X. [DOI] [PubMed] [Google Scholar]
- [3].Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- [4].Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. International journal of cancer Journal international du cancer. 2007;121:621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- [5].Thomas JO, Herrero R, Omigbodun AA, Ojemakinde K, Ajayi IO, Fawole A, et al. Prevalence of papillomavirus infection in women in Ibadan, Nigeria: a population-based study. Br J Cancer. 2004;90:638–45. doi: 10.1038/sj.bjc.6601515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–8. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- [7].Keita N, Clifford GM, Koulibaly M, Douno K, Kabba I, Haba M, et al. HPV infection in women with and without cervical cancer in Conakry, Guinea. British journal of cancer. 2009;101:202–8. doi: 10.1038/sj.bjc.6605140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wall SR, Scherf CF, Morison L, Hart KW, West B, Ekpo G, et al. Cervical human papillomavirus infection and squamous intraepithelial lesions in rural Gambia, West Africa: viral sequence analysis and epidemiology. Br J Cancer. 2005;93:1068–76. doi: 10.1038/sj.bjc.6602736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gage JC, Ajenifuja KO, Wentzensen NA, Adepiti AC, Eklund C, Reilly M, et al. The age-specific prevalence of human papillomavirus and risk of cytologic abnormalities in rural Nigeria: implications for screen-and-treat strategies. International journal of cancer Journal international du cancer. 2012;130:2111–7. doi: 10.1002/ijc.26211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Clarke MA, Gage JC, Ajenifuja KO, Wentzensen NA, Adepiti AC, Wacholder S, et al. A population-based, cross-sectional study of age-specific risk factors for high risk human papillomavirus prevalence in rural Nigeria. Infect Agent Cancer. 6:12. doi: 10.1186/1750-9378-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 103:1444–51. doi: 10.1093/jnci/djr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Safaeian M, Porras C, Schiffman M, Rodriguez AC, Wacholder S, Gonzalez P, et al. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and −18 infections. J Natl Cancer Inst. 102:1653–62. doi: 10.1093/jnci/djq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Odida M, Schmauz R, Lwanga SK. Grade of malignancy of cervical cancer in regions of Uganda with varying malarial endemicity. International journal of cancer Journal international du cancer. 2002;99:737–41. doi: 10.1002/ijc.10384. [DOI] [PubMed] [Google Scholar]
- [14].Brooker S, Kabatereine NB, Gyapong JO, Stothard JR, Utzinger J. Rapid mapping of schistosomiasis and other neglected tropical diseases in the context of integrated control programmes in Africa. Parasitology. 2009:1–12. doi: 10.1017/S0031182009005940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nature reviews Immunology. 2003;3:733–44. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- [16].Castle PE, Schiffman M, Gravitt PE, Kendall H, Fishman S, Dong H, et al. Comparisons of HPV DNA detection by MY09/11 PCR methods. J Med Virol. 2002;68:417–23. doi: 10.1002/jmv.10220. [DOI] [PubMed] [Google Scholar]
- [17].Chaturvedi A, Kemp TJ, Pfeiffer RM, Biancotto A, Williams MC, Munuo S, et al. Evaluation of multiplexed cytokine and inflammation marker measurements: A methodologic study. Cancer Epidemiol Biomarkers Prev. doi: 10.1158/1055-9965.EPI-11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jose PJ, Griffiths-Johnson DA, Collins PD, Walsh DT, Moqbel R, Totty NF, et al. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J Exp Med. 1994;179:881–7. doi: 10.1084/jem.179.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shinkai A, Yoshisue H, Koike M, Shoji E, Nakagawa S, Saito A, et al. A novel human CC chemokine, eotaxin-3, which is expressed in IL-4-stimulated vascular endothelial cells, exhibits potent activity toward eosinophils. J Immunol. 1999;163:1602–10. [PubMed] [Google Scholar]
- [20].Marks MA, Viscidi RP, Chang K, Silver M, Burke A, Howard R, et al. Differences in the concentration and correlation of cervical immune markers among HPV positive and negative perimenopausal women. Cytokine. 2011;56:798–803. doi: 10.1016/j.cyto.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wong HL, Pfeiffer RM, Fears TR, Vermeulen R, Ji S, Rabkin CS. Reproducibility and correlations of multiplex cytokine levels in asymptomatic persons. Cancer Epidemiol Biomarkers Prev. 2008;17:3450–6. doi: 10.1158/1055-9965.EPI-08-0311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.