Abstract
We report a case of IgG4-related disease (IgG4-RD) diagnosed after 3 years of follow-up for idiopathic membranous nephropathy (MN). MN has been considered as glomerular lesion of IgG4-related kidney diseases in recent years and was diagnosed simultaneously with or after a diagnosis of IgG4-RD in previously reported cases. In the present case, IgG4-RD developed 3 years after the diagnosis of idiopathic MN, indicating a possible relationship between idiopathic MN and IgG4-RD through common underlying mechanisms of development.
Keywords: idiopathic membranous nephropathy, IgG4-related disease, membranous nephropathy associated with IgG4-RD
Background
IgG4-RD is a newly recognized fibroinflammatory condition of unknown etiology characterized by tumefactive lesions, dense lymphoplasmacytic infiltration rich in IgG4-positive plasma cells, storiform fibrosis and an elevated serum IgG4 level [1–3]. The kidney is one of the organs commonly affected by IgG4-RD, and tubulointerstitial nephritis (TIN) with infiltration of numerous IgG4-positive plasma cells is the predominant type of kidney lesion [4–7]. Clinically, elderly men are most frequently affected. High levels of IgG and IgG4 are striking and characteristic features of renal involvement such as multiple lesions with low attenuation demonstrated by contrast-enhanced computed tomography are often evident [4–7]. Although TIN is the main feature of the kidney lesion in IgG4-RD, some glomerular lesions have also been described, and membranous nephropathy (MN) has been recognized as the most common form [4–8]. In previously reported cases of MN associated with IgG4-RD, MN was diagnosed together with, or after the onset of IgG4-RD [9–12]. Here, we report a case of idiopathic MN as a preceding disease before the onset of IgG4-RD.
Case report
A 59-year-old male was hospitalized because of edema of the lower extremities. He had previously been well and had no significant medical history. His serum total protein level was 46 g/L (4.6 g/dL), serum albumin 17 g/L (1.7 g/dL), urinary protein excretion 4.3 g/day without occult blood and the 24-h creatinine clearance was normal at 1.51 mL/s (90.5 mL/min) (Table 1). The anti-nuclear antibody was negative, and serum immunoglobulins G, A and M were all within normal limits at 11.98 g/L (1198 mg/dL), 11.37 g/L (1137 mg/dL) and 0.48 g/L (48 mg/dL), respectively. Complement levels were also within normal limits: C3 1.12 g/L (112 mg/dL), C4 0.314 g/L (31.4 mg/dL) and CH50 30 U/mL. Hepatitis-B surface antigen, hepatitis C antibody and cryoglobulin were all negative. Contrast-enhanced computed tomography (CT) demonstrated small, non-specific nodules in the right lung, and a hemangioma of the liver.
Table 1.
Clinical course and laboratory findings in the present case
| May 2008 | January 2009 | July 2009 | July 2010 | December 2010 | March 2011 | August 2011 | |
|---|---|---|---|---|---|---|---|
| Event | Diagnosed as MN | Worsening of NS | Discontinuation of PSL | Admission to our hospital | |||
| Treatment | Diuretics | PSL, furosemide | Furosemide, olmesartan | Furosemide, olmesartan | Furosemide, olmesartan | Furosemide, olmesartan | Furosemide, olmesartan |
| TP (g/L) | 46 | 56 | 60 | 71 | 77 | 85 | 92 |
| Alb (g/L) | 17 | 15 | 29 | 31 | 30 | 31 | 24 |
| IgG (g/L) | 12 | NA | NA | NA | NA | NA | 39 |
| Urinalysis (protein) | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ |
| UP (g/day) | 4.3 | 3.0 | 3.1 | NA | NA | NA | 2.1 |
| 24 h Ccr (mL/s) | 1.51 | 1.12 | 1.37 | NA | NA | NA | 1 |
MN, membranous nephropathy; NS, nephritic syndrome; PSL, prednisolone; TP, total protein; Alb, albumin; UP, urinary protein excretion; Ccr, creatinine clearance; NA, data not available.
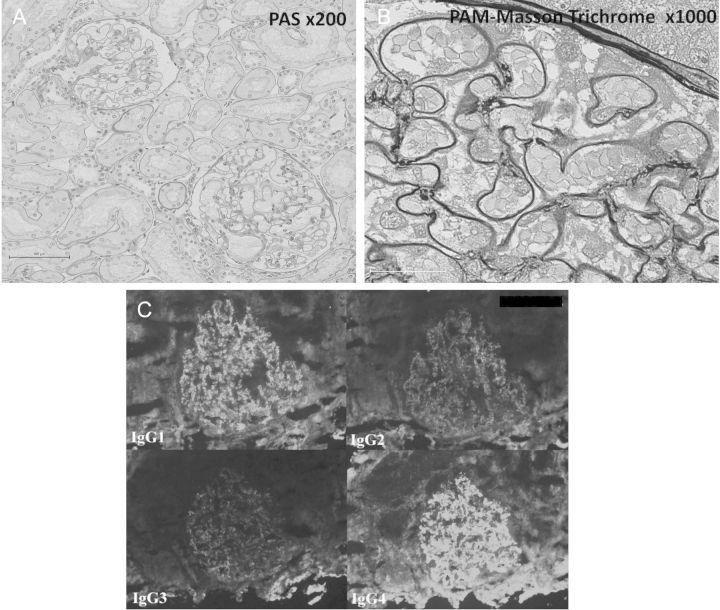
Percutaneous kidney biopsy was performed, and the histology revealed diffuse membranous changes on the glomerular basement membrane without tubulointerstitial lesions (Figure 1A and B). No mesangial hypercellularity, endocapillary proliferation or glomerular basement membrane duplication was observed. An immunofluorescence study showed diffuse, fine-granular staining of IgG, C3, fibrinogen, kappa- and lambda-chain in the glomerular capillary loops. There were no deposits in other areas including mesangeal matrix, subendothelial aspects and tubular basement membrane. An additional immunofluorescence study of IgG subclasses showed predominant positive staining for IgG4 and IgG1 and weak positive staining for IgG2 and IgG3 in the glomeruli (Figure 1C). An electron microscopy examination revealed diffuse, homogenous, small electron-dense deposits in the subepithelial aspects of the glomerular basement membrane, which corresponded to Ehrenreich and Churg stage 2. Subendothelial deposits were not present, and endothelial cells did not contain any tubuloreticular structure. The mesangial matrix and tubular basement membrane did not contain electron-dense deposits.
Fig. 1.
Histopathologic findings of kidney biopsy at the previous hospital at the time of onset of idiopathic MN. (A) Light microscopy shows no significant mesangial cell or matrix proliferation in glomeruli, and no interstitial lesions. Periodic acid-Schiff stain, ×200. (B) Diffuse, small deposits are evident in the subepithelial aspects of the glomerular basement membrane. Periodic acid-methenamine-Masson trichrome stain, ×1000. (C) An immunofluorescence study of IgG subclasses shows predominant positive staining for IgG1 and IgG4 and weak positive staining for IgG2 and IgG3 in glomeruli.
A systemic work-up revealed no other disease or malignancy, and the patient was diagnosed as having idiopathic MN. Prednisolone was administered at 40 mg daily, but the patient suffered repeated cerebral infarction attacks after starting the therapy. Prednisolone was tapered and discontinued 6 months after the start of the therapy, without any significant effect. The patient's proteinuria persisted at around 3 g/day and he was followed up while receiving diuretics and the angiotensin receptor blocker, olmesartan.
Two years after discontinuation of glucocorticoid therapy, the patient developed epigastric discomfort and was hospitalized again. Laboratory examinations revealed elevated serum levels of AST, ALT, ALP, total bilirubin and γ-GTP [64 IU/L, 106 IU/L, 1948 IU/L, 111 μmol/L (6.5 mg/dL) and 1544 IU/L, respectively] accompanied by a slight increase in serum creatinine at 100 μmol/L (1.13 mg/dL) and a normal serum amylase level of 111 IU/L. Abdominal CT revealed swelling of the pancreatic head, dilatation of the main pancreatic duct and stenosis of the common bile duct. Tumor markers including CEA and CA19-9 were all within the normal ranges, while hypergammaglobulinemia was observed and the serum IgG level was elevated at 39.21 g/dL (3921 mg/dL) with marked elevation of IgG4 at 19.2 g/L (1920 mg/dL). The patient was transferred to our hospital because of suspected type 1 autoimmune pancreatitis.
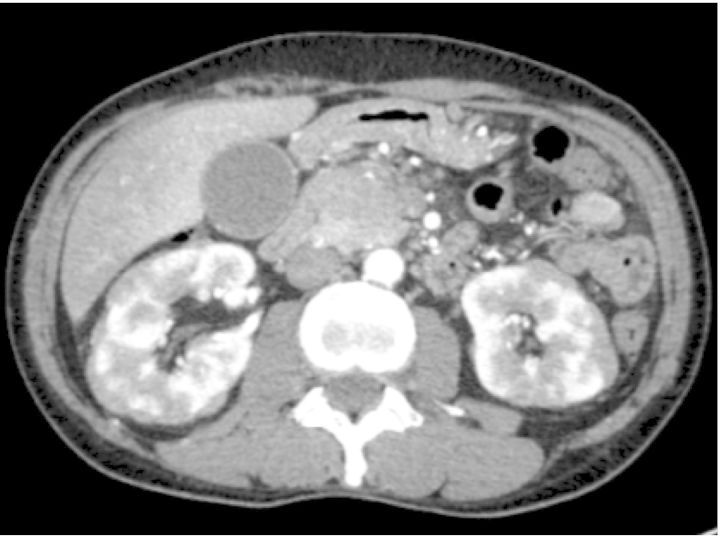
A physical examination demonstrated jaundice and upper abdominal tenderness. Swelling of the bilateral lacrimal and parotid glands was observed. Hypergammaglobulinemia persisted, and serum IgA, IgM and complement studies showed that all were within normal limits. Serum anti-nuclear antibody was negative, whereas the rheumatoid factor was positive at 120.6 IU/mL. Urinary protein excretion was 2.1 g/day without occult blood and 24-h creatinine clearance was mildly deteriorated at 1 mL/s (60.0 mL/min) (Table 1). Excretion of urinary β2-microglobulin (β2-MG) and N-acetyl-β-d-glucosaminidase was markedly elevated at 11 636 μg/day and 25.0 IU/day, respectively. Contrast-enhanced CT showed bronchial thickening in the bilateral lungs, pancreatic swelling with stenosis of the main pancreatic duct and bile duct, bilateral swollen kidneys with multiple lesions exhibiting low attenuation, periaortic and periarterial mass lesions, diffuse swelling of the prostate gland and systemic lymphadenopathy, compatible with the radiologic abnormalities characteristic of IgG4-RD (Figure 2).
Fig. 2.
Contrast-enhanced CT at our hospital at the time of onset of IgG4-RD. Marked swelling of pancreatic head and bilateral swollen kidneys with multiple lesions exhibiting low attenuation were observed.
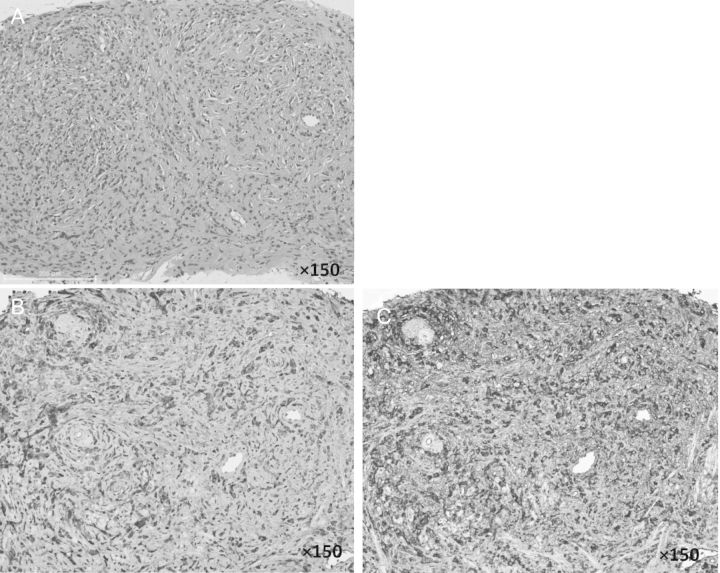
Percutaneous kidney biopsy was not performed since we could not obtain the patient's agreement. Instead, percutaneous needle biopsies from the pancreas and prostate gland were performed to rule out malignancies and confirm the diagnosis. Specimens obtained by biopsy from the prostate gland and pancreas revealed dense lymphoplasmacytic infiltration with storiform fibrosis and infiltration of numerous IgG4-positive plasma cells (IgG4/ IgG-positive plasma cell ratio >50%) without malignancy (Figure 3).
Fig. 3.
Histopathologic features of a specimen obtained from the prostate gland by percutaneous needle biopsy at our hospital at the time of onset of IgG4-RD. (A) A needle biopsy specimen from the prostate gland. Hematoxylin–eosin stain, ×150. There is no evidence of cancer cells and there is a typical swirling pattern of fibrosclerosing inflammation with infiltration of numerous lymphocytes, the so-called ‘storiform fibrosis’. (B) Immunohistochemical staining for IgG in a needle biopsy specimen from the prostate gland, ×150. (C). Immunohistochemical staining for IgG4 in a needle biopsy specimen from the prostate gland, ×150. The IgG4-positive plasma cell/IgG-positive plasma cell ratio was over 50% in tissue from the prostate gland.
Based on these findings, the patient was definitively diagnosed with IgG4-RD [1]. Western blot analysis of the patient's serum for the detection of serum autoantibodies against the M-type phospholipase A2 receptor (anti-PLA2R) was performed, as described previously [13], and was negative. Oral administration of prednisolone was initiated at 40 mg daily, and the patient's clinical symptoms including jaundice and anorexia improved rapidly. The laboratory data and multiple organ lesions also improved, while proteinuria persisted at around 3 g/day.
Discussion
It has been reported that MN is present in ∼7% of kidney biopsy specimens in IgG4-RD [7]. Most cases of MN associated with IgG4-RD are accompanied by IgG4-related TIN [4–6, 9, 10], although recent reports have also documented MN without TIN but with other features of IgG4-RD (8, 11, 12). In MN associated with IgG4-RD, positive deposition of IgG4 in the glomerular basement membrane has been observed, either predominantly or together with other IgG subclasses [8–12].
Originally, the immunofluorescent examination of IgG subclasses was known as a very useful tool to distinguish the primary MN from the secondary MN. Predominant deposition of IgG4, often accompanied by other subclasses such as IgG1, was one of the well-known features of idiopathic MN [14]. Moreover, idiopathic MN and MN associated with IgG4-RD share an essential immunologic feature known as ‘Th2 cytokine-mediated’ immune reaction [14, 15], suggesting that these two disorders share common pathophysiologic processes for increased production of Th2 cytokines and subsequent increase of IgG4. On the other hand, these two disorders differ in many other features, including clinical symptoms, laboratory parameters and radiographic findings. An anti-PLA2R antibody, which has been described as a major target antigen involved in idiopathic MN and seen in ∼70–80% of patients with idiopathic MN [13, 16–18], has been reported to be negative in all cases of IgG4-RD [19] and also cases of MN associated with IgG4-RD [8, 10, 12], suggesting no involvement of this antibody in the development of IgG4-RD.
In the present case, there was no evidence of any association of IgG4-RD based on the clinical symptoms, serological data and imaging findings when the patient developed nephrotic syndrome. Therefore, it was reasonable to have diagnosed the present case as idiopathic MN at the time of onset of nephrotic syndrome. The anti-PLA2R antibody is described as negative in all cases evaluated in MN associated with IgG4-RKD [8] and was also negative in this patient, although the result was obtained from the serum 3 years after the diagnosis of idiopathic MN and after corticosteroid therapy. However, 20–25% of patients with idiopathic MN are reported to be negative for the antibody [16–18]. The absence of the anti-PLA2R antibody is thus only partly helpful to distinguish from idiopathic MN. Interestingly, the patient's total serum protein level increased gradually without any significant improvement of proteinuria or elevation of the serum albumin level (Table 1), and eventually the clinical symptoms and laboratory abnormalities of IgG4-RD became evident 3 years after the onset of idiopathic MN. Accordingly, it is possible that some conditions underlying the persistent predominance of Th2-type immune responses during the 3 years of follow-up might have led to the development of IgG4-RD.
Currently, idiopathic MN is defined as MN without any other disorder that could cause secondary MN, with predominant deposition of IgG4 in the glomerular basement membrane. However, MN associated with IgG4-RD has also been revealed to be IgG4-predominant and, interestingly, the IgG subclass of the anti-PLA2R antibody seen in idiopathic MN is predominantly IgG4. Although the pathogenesis of IgG4-RD is poorly understood, it may share some common mechanisms with idiopathic MN, and thus a comparative study of the two diseases might be helpful for clarifying their pathogenesis. Consideration for IgG4-RD in MN, including systemic evaluation or measurement of serum IgG4 level, would be necessary in addition to the evaluation of the anti-PLA2R antibody and IgG subclasses in the immune complex deposited on the glomerular basement membrane.
Acknowledgements
We are grateful to Dr David Salant for his helpful advice on the manuscript and anti-PLA2R assay.
This work was supported in part by research grant DK090029 from the United States National Institutes of Health to Dr David Salant and a research fellowship grant from the NephCure Foundation to Dr Ayalon.
Conflict of interest statement. None declared.
References
- 1.Umehara H, Okazaki K, Masaki Y, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD) Mod Rheumatol. 2012;22:21–30. doi: 10.1007/s10165-011-0571-z. [DOI] [PubMed] [Google Scholar]
- 2.Stone JH, Khosroshahi A, Deshpande V, et al. Recommendation for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012;64:2061–3067. doi: 10.1002/art.34593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone JH, Zen Y, Deshpande V. IgG4-related disease. New Engl J Med. 2012;366:539–551. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 4.Kawano M, Saeki T, Nakashima H, et al. Proposal for diagnostic criteria for IgG4-related kidney disease. Clin Exp Nephrol. 2011;15:615–626. doi: 10.1007/s10157-011-0521-2. [DOI] [PubMed] [Google Scholar]
- 5.Saeki T, Nishi S, Imai N, et al. Clinicopathological characteristics of patients with IgG4-related tubulointerstitial nephritis. Kidney Int. 2010;78:1016–1023. doi: 10.1038/ki.2010.271. [DOI] [PubMed] [Google Scholar]
- 6.Nishi S, Imai N, Yoshita K, et al. Clinicopathological findings of immunoglobulin G4-related kidney disease. Clin Exp Nephrol. 2011;15:810–819. doi: 10.1007/s10157-011-0526-x. [DOI] [PubMed] [Google Scholar]
- 7.Cornell LD. IgG4-related kidney disease. Curr Opin Nephrol Hypertens. 2012;21:279–288. doi: 10.1097/MNH.0b013e32835265ac. [DOI] [PubMed] [Google Scholar]
- 8.Alexander MP, Larsen CP, Gibson LW, et al. Membranous glomerulonephritis is a manifestation of IgG4-related disease. Kidney Int. 2013;83:455–462. doi: 10.1038/ki.2012.382. [DOI] [PubMed] [Google Scholar]
- 9.Saeki T, Imai N, Ito T, et al. Membranous nephropathy associated with IgG4-related systemic disease and without autoimmune pancreatitis. Clin Nephrol. 2009;71:173–178. doi: 10.5414/cnp71173. [DOI] [PubMed] [Google Scholar]
- 10.Fervenza FC, Downer G, Beck LH, et al. IgG4-related tubulointerstitial nephritis with membranous nephropathy. Am J Kidney Dis. 2011;58:320–324. doi: 10.1053/j.ajkd.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Palmisano A, Corradi D, Carnevali ML, et al. Chronic periaortitis associated with membranous nephropathy: clues to common pathogenetic mechanisms. Clin Nephrol. 2010;74:485–490. doi: 10.5414/cnp74485. [DOI] [PubMed] [Google Scholar]
- 12.Cravedi P, Abbate M, Gagliardini E, et al. Membranous nephropathy associated with IgG4-related disease. Am J Kidney Dis. 2011;58:272–275. doi: 10.1053/j.ajkd.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Beck LH, Bonegio RGB, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Eng J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuroki A, Iyoda M, Shibata T, et al. Th2 cytokines increase and stimulate B cells to produce IgG4 in idiopathic membranous nephropathy. Kidney Int. 2005;68:302–310. doi: 10.1111/j.1523-1755.2005.00415.x. [DOI] [PubMed] [Google Scholar]
- 15.Nakashima H, Miyake K, Moriyama M, et al. An amplification of IL-10 and TGF-beta in patients with IgG4-related tubulointerstitial nephritis. Clin Nephrol. 2010;73:385–391. doi: 10.5414/cnp73385. [DOI] [PubMed] [Google Scholar]
- 16.Beck LH, Salant DJ. Membranous nephropathy: recent travels and new roads ahead. Kidney Int. 2010;77:765–770. doi: 10.1038/ki.2010.34. [DOI] [PubMed] [Google Scholar]
- 17.Hofstra JM, Beck LH, Beck DM, et al. Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2011;6:1286–1291. doi: 10.2215/CJN.07210810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin W, Beck LH, Zeng C, et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol. 2011;22:1137–1143. doi: 10.1681/ASN.2010090967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khosroshahi A, Ayalon R, Beck LH, et al. IgG4-related disease is not associated with antibody to the phospholipase A2 receptor. Int J Rheumatol. 2012 doi: 10.1155/2012/139409. doi:10.1155/2012/139409. [DOI] [PMC free article] [PubMed] [Google Scholar]