Abstract
Background.
Chitotriosidase (ChT) is secreted by chronically activated macrophages in Gaucher’s disease. We hypothesize that circulating levels of ChT are altered with normal aging, reflecting age-related chronic macrophage activation. Potential sources that might contribute to altered levels were assessed by measuring systemic levels of ChT are α-naphthyl acetate esterase, a macrophage lysosomal enzyme; granulocyte-macrophage colony-stimulating factor (GM-CSF), which stimulates neutrophilic granule release of ChT; interleukin-6 (IL-6); and neopterin, a macrophage activation marker.
Methods.
Serum was obtained from 315 healthy participants whose age ranged from 18 to 92 years. Anthropometric measures included percent body fat and body mass index. ChT and α-naphthyl acetate esterase levels were measured by enzyme activity assays. GM-CSF, IL-6, and neopterin concentrations were measured by commercial enzyme-linked immunosorbent assays. Serum marker values were statistically analyzed using nonparametric tests.
Results.
Six percent of the participants had undetectable ChT levels. A positive association with age was observed for ChT and IL-6, whereas a negative correlation with age was seen for α-naphthyl acetate esterase and GM-CSF. ChT values were not associated with α-naphthyl acetate esterase or GM-CSF levels. ChT was independently associated with IL-6 and neopterin levels, but statistical significance was attenuated when controlled for age.
Conclusions.
The data are consistent with increased serum ChT activity not arising from altered macrophage lysosomal enzyme trafficking or GM-CSF-stimulated release of neutrophil granule stores. The association of ChT with age remains significant after controlling for neopterin and IL-6 changes with age, suggesting that ChT levels reflect a macrophage state distinct from acute macrophage activation or inflammatory state.
Key Words: Biomarkers, Inflammation, Blood, Macrophage
MACROPHAGES are a population of ubiquitously distributed mononuclear phagocytes responsible for numerous homeostatic, immunological, and inflammatory processes (1). Their wide tissue distribution and ability to participate in both specific immunity (via antigen presentation and interleukin-1 [IL-1] production) and nonspecific immunity (against bacterial, viral, fungal, and neoplastic pathogens) underlie their roles as first-line defensive agents (2). In addition to immune function, macrophages also play seminal roles in tissue remodeling and homeostasis (3,4). Understanding the status of resident and inflammatory macrophages may provide predictive information on host responses in human aging and disease. Aging is associated with an altered state of immune activity resulting in diminution of acquired immunity function (immunosenescence) and perturbation of cytokines and other innate immunity mediators (5). The resulting dysregulated inflammatory state underlies several diseases associated with aging such as atherosclerosis (6), autoimmune disease (7), cancer (8), obesity, and metabolic syndrome (9).
Chitotriosidase (ChT) is a secreted enzyme that catalyzes the hydrolysis of chitin and chitin-like substrates (10). ChT is considered part of innate immunity and involved in defense against chitin-containing pathogens such as fungi (11). ChT production is dramatically induced in disease states such as Gaucher’s where tissue-resident macrophages are chronically activated. The current study was undertaken to determine the distribution of the macrophage marker ChT across a wide-age spectrum and analyze for associations with (a) demographic and anthropometric variables (age, gender, body mass index, and percent body fat), (b) a housekeeping macrophage lysosomal enzyme α-naphthyl acetate esterase (ANAE), (c) the cytokines granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-6, and (d) neopterin.
Methods
Participants
Serum from 315 normal adults were obtained from a repository of cross-sectional samples derived from community-dwelling adults. Inclusion criteria as a healthy serum donor included measures within the normal range for fasting glucose (<100mg/dL) and thyroid-stimulating hormone (0.5–2.1 mIU/mL), as well as a clinical assessment by a physician. Patients with a history of smoking, angina, myocardial infarction, percutaneous transluminal coronary angioplasty, coronary artery bypass surgery, congestive heart failure, and stroke were ineligible. Other exclusionary criteria included a history of diabetes mellitus, pulmonary disease, uncompensated endocrine disorders, renal or hepatic dysfunction, dementia, cancer, any chronic inflammatory condition (eg, rheumatoid arthritis), and use of anti-inflammatory agents (eg, steroids). Approval for the study protocol was acquired from the local institutional review board, and informed consent was obtained from all patients.
Enzyme Assays
ChT enzyme activity was measured by incubating 5 µL of serum with 100 µL of 22 µmol/L 4-methylumbelliferyl-β-d-N′,N′,N″-triacetylchitotrioside in McIlvain’s phosphate–citrate buffer (pH 5.2). Fluorescence of the enzymatic product 4-methylumbelliferone was read by a fluorimeter (excitation 360nm, emission 450nm). Duplicate samples were analyzed and averaged for ChT activity. Standard curves of 4-methylumbelliferone, spanning 1 and 100nM, were included to convert fluorescent emission values to nanomoles of product. Fluorescence was measured every 20 seconds, and the slope over 6 minutes was used to calculate enzyme activity expressed as nmol/min/mL. Assay performance characteristics for ChT were an interassay coefficient of variance (CV) was 12% and intraassay CV of 11%.
The level of ANAE present in serum was determined by incubating serum with reaction solution containing 100 µM α-naphthyl acetate in 0.1M Tris buffer, pH 7.4 (12). The standards, spanning 2.44 and 156.25 µM, were made from dilutions of a stock solution of 100mM 1-naphthol in ethanol. Evolution of fluorescent product over the time was measured at 355nm excitation and 460nm emission. Enzyme activity was calculated as µmol product/min/mL. The interassay CV was 13% and intraassay CV was 8%.
Immunossays
Neopterin was measured as previously described using a commercially available competitive enzyme-linked immunosorbent assay (13). Performance characteristics in the laboratory yielded an interassay CV of 6.0% and an intraassay CV of 5.4%. Serum GM-CSF and IL-6 were measured using a commercial sandwich ELISA kits (Mesoscale Discovery, Inc.) following the manufacturer’s protocol with the following modifications. The two highest standards were omitted, two additional 1:4 dilutions of the lowest standard were added, and the initial incubation of serum in 96-well plates was carried out overnight at 4°C. The sensitivity of the assays was less than 0.03 pg/mL, the interassay CVs were less than 6%, and the intraassay CVs were less than 5%.
Statistical Analysis
The distribution of serum markers was assessed by the D’Agostino–Pearson omnibus normality test. Comparisons of serum marker levels by gender were investigated using a Mann–Whitney t test. Correlations between serum markers and age, gender, body mass index, and percent body fat were assessed using Spearman rank correlations. To assess the contribution of specific age ranges, serum marker values were logarithmically transformed, segregated by gender and age group, and the mean and 95% confidence intervals were calculated and fit to a general linear model of mean log values versus age group.
Results
An initial group of serum samples from 315 participants recruited as part of a cross-sectional study of biomarkers and aging were measured for ChT activity. Of the total screened, samples from eight female and nine male Caucasians and one female African American had undetectable enzyme activity. The mean values ± standard deviations of these 18 samples for age, body mass index, and percent body fat were 44±12 years, 30.6±15.3kg/m2, and 32.6% ± 9.9%, respectively. The characteristics of the 297 ChT positive participants are listed in Table 1. Age and body composition were not significantly different between participants with unmeasurable enzyme activity and those with detectable ChT activity.
Table 1.
Chitotriosidase Positive Female and Male Participant Characteristics
| All | Female | Male | Gender | ||
|---|---|---|---|---|---|
| N | 297 | 141 | 156 | ||
| Age (y) | 50.2±18.9 | 54.9±20.8 | 46.0±16.1 | <.001 | |
| BMI (kg/m2) | 27.2±7.1 | 27.1±7.9 | 27.38±6.5 | ns | |
| % Body fat | 26.9±9.9 | 32.4±8.6 | 23.0±8.8 | <.0001 | |
| Marker | All | Female | Male | Gender | Normality |
| ChT (nmol/min/mL) | 0.870 (0.050–10.05) | 0.93 (0.87–7.58) | 0.85 (0.11–10.05) | ns | 169.4; p < .0001 |
| ANAE (µmol/min/mL) | 16.69 (3.98–67.20) | 15.85 (4.35–67.17) | 17.49 (3.98–54.56) | ns | 879.8; p < .0001 |
| GM-CSF (pg/mL) | 0.22 (0.18–0.28) | 0.21 (0.18–0.27) | 0.22 (0.18–0.28) | ns | 48.7; p < .0001 |
| IL-6 (pg/mL) | 1.50 (0.35–4.78) | 1.80 (0.25–5.05) | 1.49 (0.25–4.78) | ns | 71.3; p < .0001 |
| Neopterin (nM) | 6.18 (2.59–12.81) | 6.83 (2.81–13.43) | 6.09 (2.59–12.81) | p < .05 | 16.2; p < .0001 |
Notes: Values for age, body mass index (BMI), and % body fat reflect the mean ± standard deviation. For chitotriosidase (ChT), α-napthyl acetate esterase (ANAE), granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-6 (IL-6), and neopterin, the median values with range (in parenthesis) are given. Comparisons between genders were performed by Mann–Whitney U test. Markers were assessed by D’Agostino–Pearson omnibus normality test where the K 2 parameter and associated p value identify significant deviation from a Gaussian distribution. ns = not significant.
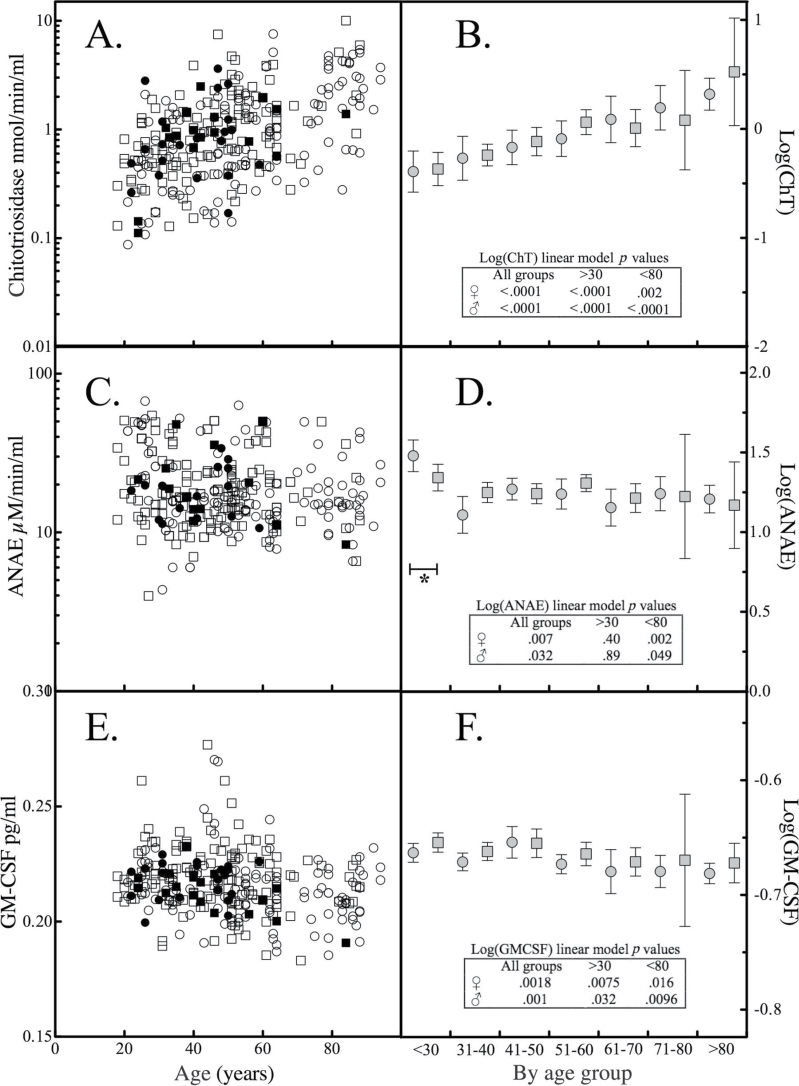
The median of ChT activity (Table 1) converts to nmol/h/mL values that are similar to published values for normal participants (14). The distribution of enzyme activity for ChT departed from normality and there was no difference in ChT activity by gender (Table 1). ChT activity increased with increasing age and was significantly correlated; Spearman r = .50, p < .0001 (Figure 1A). Profiling the mean values of logarithmically transformed ChT activity by age group revealed that the increase in ChT activity was gradual throughout the age span studied and was not sensitive to omitting age groups at either extreme (Figure 1B).
Figure 1.
Chitotriosidase (ChT), α-naphthyl acetate esterase (ANAE), and granulocyte-macrophage colony-stimulating factor (GM-CSF) levels and association with age. The levels of (A) ChT, (C) ANAE, and (E) GM-CSF in normal participants were segregated by race (solid symbols: African American; open symbols: Caucasian) and gender (circles: female; squares: male) and plotted as a function of donor age. The serum levels of the (B) ChT, (D) ANAE, and (F) GM-CSF were logarithmically transformed, segregated by gender (circles: female; squares: male) and by age group, and the mean and 95% confidence intervals were calculated. The mean values were fit to a general linear model. Asterisk and bracket mark age group whose omission attenuated statistical significance of the general linear model. The inset table reports p values associated with a general linear model of mean values of Log(serum marker) vs age group for all ages, or omitting participants <30 or >80 y of age.
When ChT is synthesized as a mature 50kDa protein, it is either directed to secretory vesicles or processed to a approximately 39kDa variant that accumulates in lysosomes (15). Thus, age-related changes in ChT activity could reflect increased gene translation, synthesis and secretion, and/or altered lysosomal component trafficking. The effect of donor age on a housekeeping lysosomal enzyme, ANAE, was studied to assess whether changes in ChT systemic levels reflect altered lysosomal enzyme trafficking. The distribution of ANAE enzyme activity in the 297 ChT positive samples was found to depart from normality and there was no difference in ANAE activity by gender (Table 1). The relationship between ANAE activity and age (Figure 1C) exhibited a significant negative association (r = −.17, p < .05). Segregating logarithmically transformed ANAE activities by age group revealed that the change was greatest with the less than 30 years of age group and when this group was omitted from a linear model of log-transformed values, statistical significance was lost (Figure 1D).
ChT is also produced by neutrophils where it is stored in granules and released upon stimulation with GM-CSF (11). Neutrophil number does not change with age in normal older individuals (16). If neutrophils were the source of the increase in serum ChT, it would be reasonable to expect that GM-CSF levels increase with age. Serum GM-CSF distribution departed from normality and was not different by gender. Serum GM-CSF possessed a negative correlation (r = −.14, p < .05) with age (Figure 1E). Segregation by age group revealed no significant gender differences, and exclusion of age groups (<30 and/or >80) did not abrogate the linear model (Figure 1F).
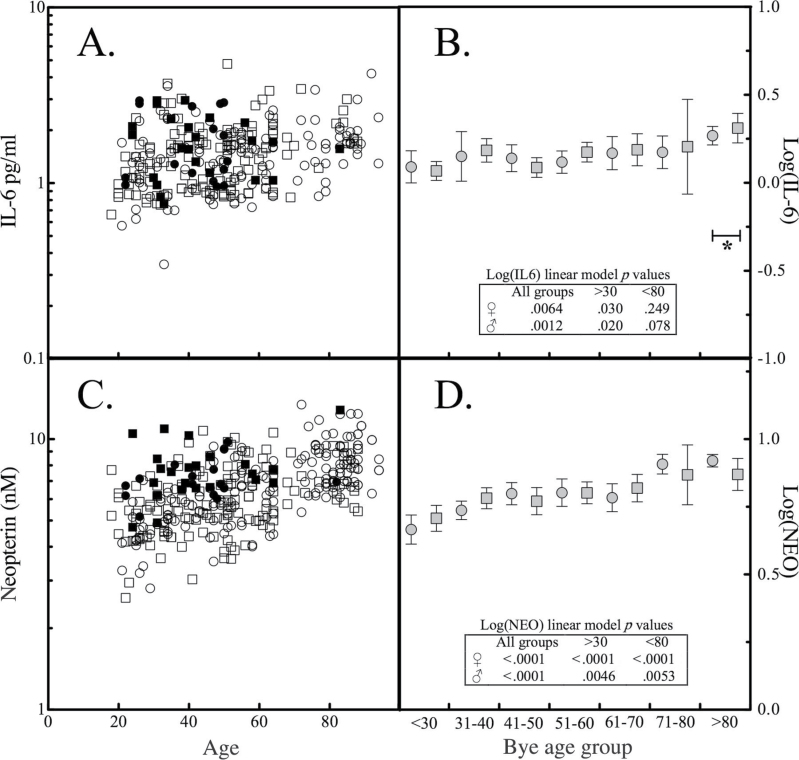
The pro-inflammatory cytokine IL-6 is produced by many cell types including macrophages and has been implicated in modulating macrophage function with increasing age (17). IL-6 can modulate the transition from acute to chronic inflammation by changing the leukocyte infiltrate from polymorphonuclear neutrophils to monocyte and macrophages (18). As observed with the other serum markers, IL-6 level distribution departed significantly from normality and was not different by gender (Table 1). A significant positive correlation (r = .34, p < .0001) was observed between IL-6 and age (Figure 2A). Segregation by age group revealed that a linear model of log-transformed IL-6 values and age group was no longer statistically significant if samples from participants more than 80 years of age were omitted (Figure 2B).
Figure 2.
Interleukin-6 (IL-6) and neopterin levels and association with age. The levels of (A) IL-6 and (C) neopterin in normal participants were segregated by race and gender as a function of age. The mean and 95% confidence intervals were determined for logarithmically transformed (B) IL-6 and (D) neopterin values segregated by gender and age group. Asterisk and bracket mark age group whose omission attenuated statistical significance of a general linear model. The inset table reports p values associated with a general linear model of mean values of Log(serum marker) vs age group for all ages, or omitting participants <30 or >80 y of age.
Neopterin is produced by monocytes and macrophages in response to interferon-γ-stimulated activation. Neopterin levels in the 297 ChT positive samples also did not have a normal distribution, but did exhibit significant gender differences (Table 1). Spearman rank order correlation analysis of neopterin levels and participant age yielded a correlation coefficient of .45 with p < .0001 (Figure 2C). Exclusion of age groups (<30 and/or >80) did not abrogate a linear model of logarithmically transformed neopterin values versus age (Figure 2D).
Potential correlations between the macrophage markers were investigated. ChT was significantly correlated with neopterin (Spearman r = .25, p < .0001) and IL-6 (Spearman r = .13, p < .05), but not ANAE or GM-CSF. When logarithmically transformed values of ChT were modeled by multiple regression including covariates for logarithmically tranformed serum markers and age, the association of ChT with IL-6 and neopterin was no longer significant. A Mann–Whitney U test revealed no significant difference in any of the serum markers between participants with detectable versus undetectable ChT activity.
Discussion
Chitin is a structural component of cell walls and coatings of many organisms. The physiological role of ChT in innate immunity is unclear, though it has been proposed to be a biostatic agent targeting chitin-containing pathogens such as yeast and other fungi (10). One in every three individuals from various ethnic groups carries an abnormal ChT gene with a 24-bp duplication that prevents production of enzyme (19). The frequency of homozygosity for this mutant allele has been estimated as between 4% and 7% depending on the population (19). In our current study, 6% of the participants exhibited no ChT activity. There were no significant differences in the age, body mass index, percent body fat, ANAE activity, GM-CSF, IL-6, and neopterin levels between participants with undetectable ChT and those with measureable enzyme levels.
ChT activity has long been used as a marker of disease activity and therapeutic response in Gaucher’s disease (20). In this disease, abnormalities are caused by the presence of Gaucher cells, chronically activated tissue-resident macrophage cells engorged by the presence of incompletely degraded lipid glucocerebroside in lysosomes (20). Accumulation of Gaucher cells in the liver and spleen results in hepatosplenomegaly and an associated anemia and thrombocytopenia. Gaucher cells in the bone marrow lead to infarction, ischemia, necrosis, and cortical bone destruction. In bone, remodeling is defective resulting in osteonecrosis, avascular infarction, and vertebral fractures. In more severe forms of the disease, turnover of complex lipids during brain development is impaired leading to cognitive impairment and dementia. In addition to lipid storage disorders (21), elevation of circulating ChT has been observed in a number of diseases where macrophages are chronically challenged with plaques, tissue debris, or intercellular bacteria: coronary artery disease (22), multiple sclerosis (23), Alzheimer’s (24), Wegener’s granulomatosis (25), and sarcoidosis (26).
A shared biological feature of both aging and Gaucher’s disease is an accumulation of lipid material in the lysosomal apparatus. Lipofuscin, a nondegradable intralysosomal substance arising from oxidation/polymerization of protein and lipid components, accumulates with age. It has been proposed that lipofuscin accumulation, which inversely correlates with longevity, interferes with cellular self-renewal through disrupting macromolecular and organelle autophagy and lysosomal protein degradation (27). Lipofuscin accumulation may further compromise cellular functional capacity by sequestering transition metals that can exacerbate free-radical generation and by diverting lysosomal hydrolases and lipases away from their normal substrates (28). The observed increase in circulating ChT with increasing age is consistent with an age-associated chronic macrophage activation, perhaps in response to accumulating lipofuscin.
The enzyme ANAE belongs to a family of cellular carboxylesterases that act on short-chain esters during lysosomal-mediated catabolism. Although ANAE is detected primarily in monocytes, macrophages, and histocytes and is normally absent in granulocytes (29), lymphocytes may occasionally exhibit enzyme activity (30). Esterases released from macrophages are thought to be involved in the clearance of apoptotic cellular debris (31). The absence of an age-related increase in ANAE activity in serum suggests that the increase in serum ChT activity observed throughout the age span studied is not accounted for by altered lysosomal trafficking. In the current study, the negative association between age and circulating ANAE activity was driven by high levels at less than 30 years of age. Serum levels of lysosomal enzymes exhibit peaks of activity from infancy to early adulthood (32).
Neutrophils are relatively short-lived rapid responders to pathogen challenge/inflammation. Though their cell number in the absence of infection remains stable throughout life (16), an age-related decline in neutrophil chemotaxis and phagocytosis has been described (33). Neutrophils are a potential source of serum ChT activity, releasing it from granules when stimulated with GM-CSF (11). The current observation of an age-related decline in serum GM-CSF levels is consistent with a recent study of young and old (<45 or >65 years) groups (34). Given observations of unchanging number of neutrophils and the decrease in GM-CSF levels with age, neutrophil-derived ChT as a source of the age-related increase in ChT activity, although still possible, is less likely.
IL-6 is a pro-inflammatory cytokine and is produced in response to infection and tissue injury. IL-6 exerts its effects on multiple cell types and can act systemically. IL-6 modulates leukocyte recruitment transition from an acute primarily neutrophilic infiltrate to a chronic primarily macrophage infiltrate (35). Recent studies employing strict criteria for good health have observed attenuation of the association between circulating levels of IL-6 and increasing age when adjusted for cardiovascular risk factors and morbidity (36), or when only participants less than 75 years of age were analyzed (34). In the current study, the correlation of IL-6 with age was attenuated by excluding participants more than 80 years of age.
There are a number of limitations to the current study. The research protocol for collecting serum samples did not include DNA sampling and storage. Thus, genotyping of ChT allelic status could not be carried out to confirm the cause of the null ChT activity observed in approximately 6% of the samples. Although the inclusion/exclusion criteria for the study were designed to identify normal healthy participants, it is possible that some participants may have had occult pathologies operant that could perturb ChT levels. This is unlikely to account for the consistent pattern of change across all ages and participants. Another limitation is that the study design involved a cross-sectional analysis, whereas longitudinal samples might provide a clearer profile of individual and aggregate changes with age. A further limitation is that measuring circulating levels of biomarkers secreted by cells does not identify the specific cells involved. Although it is likely, given what is known about Gaucher’s disease, macrophage pathology, and ChT biology, that macrophages are the primary source, it is possible that another cell type may be contributing to the increase in circulating levels with age. Finally, the possibility that the increase in circulating ChT activity reflects an age-associated change in the number of macrophages is difficult to assess given their wide distribution in various tissues, organs, and systems. Detailed phenotyping of monocytes has indicated that although the total number of monocytes did not change dependent on age, specific subsets of monocytes do change in abundance in healthy adults with increasing age (37).
Our data illustrate an age-associated increase in ChT activity. The increase is unlikely to be due to altered lysosomal trafficking or GM-CSF-mediated neutrophil release of ChT given our observations on ANAE and GM-CSF levels. Age-related change in ChT has been reported by comparing discrete (young vs old) age groups, though this study lacked detail on inclusion criteria as “healthy,” the age distribution within the groups, assumed a normal distribution to ChT, and reported no p values (38). The current study is the first to demonstrate across a wide-age spectrum, a continuous interaction of age with serum ChT activity that is independent of IL-6 (an inflammation marker) and neopterin (an acute macrophage activation marker). Further research with a longitudinal design and involving pathological states that are associated with chronic inflammation (such as frailty) may provide additional evidence as to the potential utility of ChT as a biomarker.
Funding
This research was supported by National Institutes of Health grants CA 113865 (N.S.F.), UL1 RR 025005, T35 AG026758 (R.R. and A.K.).
References
- 1. Rees AJ. Monocyte and macrophage biology: an overview. Semin Nephrol 2010; 30: 216–233 [DOI] [PubMed] [Google Scholar]
- 2. Sheikh SZ, Plevy SE. The role of the macrophage in sentinel responses in intestinal immunity. Curr Opin Gastroenterol 2010; 26: 578–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghosh S. Macrophage cholesterol homeostasis and metabolic diseases: critical role of cholesteryl ester mobilization. Expert Rev Cardiovasc Ther 2011; 9: 329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Surmi BK, Hasty AH. Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future Lipidol 2008; 3: 545–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gomez CR, Nomellini V, Faunce DE, Kovacs EJ. Innate immunity and aging. Exp Gerontol 2008; 43: 718–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blasi C. The autoimmune origin of atherosclerosis. Atherosclerosis 2008; 201: 17–32 [DOI] [PubMed] [Google Scholar]
- 7. Vasto S, Candore G, Balistreri CR, et al. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev 2007; 128: 83–91 [DOI] [PubMed] [Google Scholar]
- 8. Dalgleish AG, O’Byrne KJ. Chronic immune activation and inflammation in the pathogenesis of AIDS and cancer. Adv Cancer Res 2002; 84: 231–276 [DOI] [PubMed] [Google Scholar]
- 9. Beavers KM, Hsu FC, Houston DK, et al. The role of metabolic syndrome, adiposity, and inflammation in physical performance in the Health ABC Study. J Gerontol A Biol Sci Med Sci 2012. 10.1093/gerona/gls213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Renkema GH, Boot RG, Muijsers AO, Donker-Koopman WE, Aerts JM. Purification and characterization of human chitotriosidase, a novel member of the chitinase family of proteins. J Biol Chem 1995; 270: 2198–2202 [DOI] [PubMed] [Google Scholar]
- 11. van Eijk M, van Roomen CP, Renkema GH, et al. Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity. Int Immunol 2005; 17: 1505–1512 [DOI] [PubMed] [Google Scholar]
- 12. Kolios G, Valatas V, Psilopoulos D, Petraki K, Kouroumalis E. Depletion of non specific esterase activity in the colonic mucosa of patients with ulcerative colitis. Eur J Clin Invest 2002; 32: 265–273 [DOI] [PubMed] [Google Scholar]
- 13. Spencer ME, Jain A, Matteini A, et al. Serum levels of the immune activation marker neopterin change with age and gender and are modified by race, BMI, and percentage of body fat. J Gerontol A Biol Sci Med Sci 2010; 65: 858–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Artieda M, Cenarro A, Gañán A, et al. Serum chitotriosidase activity is increased in subjects with atherosclerosis disease. Arterioscler Thromb Vasc Biol 2003; 23: 1645–1652 [DOI] [PubMed] [Google Scholar]
- 15. Renkema GH, Boot RG, Strijland A, et al. Synthesis, sorting, and processing into distinct isoforms of human macrophage chitotriosidase. Eur J Biochem 1997; 244: 279–285 [DOI] [PubMed] [Google Scholar]
- 16. Angelis P, Scharf S, Christophidis N. Effects of age on neutrophil function and its relevance to bacterial infections in the elderly. J Clin Lab Immunol 1997; 49: 33–40 [PubMed] [Google Scholar]
- 17. Gomez CR, Karavitis J, Palmer JL, et al. Interleukin-6 contributes to age-related alteration of cytokine production by macrophages. Mediators Inflamm 2010; 2010: 475139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther 2006; 8(suppl 2):S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boot RG, Renkema GH, Verhoek M, et al. The human chitotriosidase gene. Nature of inherited enzyme deficiency. J Biol Chem 1998; 273: 25680–25685 [DOI] [PubMed] [Google Scholar]
- 20. Bussink AP, van Eijk M, Renkema GH, Aerts JM, Boot RG. The biology of the Gaucher cell: the cradle of human chitinases. Int Rev Cytol 2006; 252: 71–128 [DOI] [PubMed] [Google Scholar]
- 21. Isman F, Hobert JA, Thompson JN, Natowicz MR. Plasma chitotriosidase in lysosomal storage diseases. Clin Chim Acta 2008; 387: 165–167 [DOI] [PubMed] [Google Scholar]
- 22. Karadag B, Kucur M, Isman FK, Hacibekiroglu M, Vural VA. Serum chitotriosidase activity in patients with coronary artery disease. Circ J 2008; 72: 71–75 [DOI] [PubMed] [Google Scholar]
- 23. Sotgiu S, Musumeci S. Chitotriosidase in multiple sclerosis. Clin Immunol 2009; 133: 282–283 ; author reply 284 [DOI] [PubMed] [Google Scholar]
- 24. Sotgiu S, Piras MR, Barone R, et al. Chitotriosidase and Alzheimer’s disease. Curr Alzheimer Res 2007; 4: 295–296 [DOI] [PubMed] [Google Scholar]
- 25. Koening CL, Gota CE, Langford CA, Hoffman GS, Natowicz MR. Serum chitotriosidase activity and Wegener’s granulomatosis. Clin Biochem 2010; 43: 512–514 [DOI] [PubMed] [Google Scholar]
- 26. Tercelj M, Salobir B, Simcic S, Wraber B, Zupancic M, Rylander R. Chitotriosidase activity in sarcoidosis and some other pulmonary diseases. Scand J Clin Lab Invest 2009; 69: 575–578 [DOI] [PubMed] [Google Scholar]
- 27. Jung T, Bader N, Grune T. Lipofuscin: formation, distribution, and metabolic consequences. Ann N Y Acad Sci 2007; 1119: 97–111 [DOI] [PubMed] [Google Scholar]
- 28. Fedarko NS. The biology of aging and frailty. Clin Geriatr Med 2011; 27: 27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sestini P. Usefulness of non-specific esterase stain for the morphometric evaluation of alveolar macrophage heterogeneity in human lung diseases. Boll Soc Ital Biol Sper 1984; 60: 1045–1050 [PubMed] [Google Scholar]
- 30. Facchini A, Mariani AR, Papa S, Mariani E, Manzoli FA. Distribution of acid alpha-naphthyl acetate esterase among human T lymphocyte subsets. Immunol Lett 1984; 8: 207–210 [DOI] [PubMed] [Google Scholar]
- 31. Feng JM, Wu JS, Campagnoni AT, Chen WF. Nonspecific esterase released from thymic macrophages accumulates in the apoptotic thymocytes: an indication for this enzyme participating in the clearance of apoptotic thymocytes. Eur J Immunol 2002; 32: 1386–1392 [DOI] [PubMed] [Google Scholar]
- 32. Lombardo A, Goi GC, Marchesini S, Caimi L, Moro M, Tettamanti G. Influence of age and sex on five human plasma lysosomal enzymes assayed by automated procedures. Clin Chim Acta 1981; 113: 141–152 [DOI] [PubMed] [Google Scholar]
- 33. Wenisch C, Patruta S, Daxböck F, Krause R, Hörl W. Effect of age on human neutrophil function. J Leukoc Biol 2000; 67: 40–45 [DOI] [PubMed] [Google Scholar]
- 34. Kim HO, Kim HS, Youn JC, Shin EC, Park S. Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead-based immunoassays. J Transl Med 2011; 9: 113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol 2003; 24: 25–29 [DOI] [PubMed] [Google Scholar]
- 36. Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related proinflammatory state. Blood 2005; 105: 2294–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seidler S, Zimmermann HW, Bartneck M, Trautwein C, Tacke F. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol 2010; 11: 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kurt I, Abasli D, Cihan M, et al. Chitotriosidase levels in healthy elderly subjects. Ann N Y Acad Sci 2007; 1100: 185–188 [DOI] [PubMed] [Google Scholar]