Abstract
Background.
Seafood consumption may prevent age-related cognitive decline. However, benefits may vary by nutrient contents in different seafood types. We examined associations between total seafood consumption and cognitive decline and whether these associations differ by seafood types.
Methods.
We conducted a prospective cohort study of 5,988 women (mean age, 72 years) from the Women’s Health Study who self-reported seafood intake at Women’s Health Study baseline and also participated in telephone assessments of general cognition, verbal memory, and category fluency administered 5.6 years after Women’s Health Study baseline and 2 and 4 years thereafter. Primary outcomes were standardized composite scores of global cognition and verbal memory.
Results.
After adjusting for potential confounders, different amounts of total seafood consumption were not associated with changes in global cognition (p = .56) or verbal memory (p = .29). Considering seafood types, however, compared with women consuming less than once-weekly tuna or dark-meat finfish, those with once-weekly or higher consumption had significantly better verbal memory (0.079 standard units; p < .01) after 4 years—a difference comparable to that for women 2.1 years apart in age. There was also a statistically nonsignificant suggestion of better global cognition (p = .13) with once-weekly or higher tuna or dark-meat fish consumption. No significant associations were observed for light-meat finfish or shellfish.
Conclusions.
The relation of seafood to cognition may depend on the types consumed. Total consumption levels of seafood were unrelated to cognitive change. However, consumption of tuna and dark-meat fish once weekly or higher was associated with lower decline in verbal memory for a period of 4 years.
Key Words: Cognition, Epidemiology, Nutrition.
FISH and other seafood consumption may prevent age-related cognitive decline. Potential mechanisms involve neuro- and cardioprotective pathways (1–4), including specific benefits of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA)—long-chain omega-3 fatty acids that are crucial for brain structure and function. Several epidemiological studies found the association of seafood intake with lower risk of cognitive decline (5–8) and dementia (9–11), whereas some did not (12,13). These inconsistent results may be due to between-study differences in dietary assessment, outcome definitions, and cognitive tests (14). High-quality evidence for modifiable risk factors, including diet, of cognitive decline is urgently needed (14).
A key source of variation in findings is the different types of seafood consumed. For example, in cardiovascular disease (CVD) (15), the benefits of seafood consumption might be limited to fatty dark-meat fish intake; however, few large population–based studies of cognition have addressed specific seafood categories (dark-meat finfish, light-meat finfish, and shellfish). Consumption of dark-meat fish (e.g., salmon, mackerel, sardine, herring, trout, swordfish, and tuna) containing higher DHA and EPA (≈1 g/serving) may be associated with more favorable cognitive aging than light-meat fish (e.g., halibut, haddock, and cod) or shellfish (e.g., lobster, scallop, and shrimp) consumption (16). Moreover, light-meat fish are commonly fried, and excessive heat during preparation may further reduce the amount of DHA and EPA (17).
Thus, we examined relations of total and individual types of seafood consumption to cognitive change for a period of 4 years among almost 6,000 community-dwelling older women in the Women’s Health Study (WHS).
Method
WHS Cognitive Cohort
The WHS was a randomized, placebo-controlled, 2-×-2 factorial trial of low-dose aspirin (100 mg on alternate days) and vitamin E supplementation (600 IU on alternate days) for primary prevention of CVD and cancer in women. The design and main results of the trial have been published previously (18–21). Briefly, participants were female health professionals in the United States who were aged 45 and older; had no history of CVD, cancer, or other major chronic disease; and were not currently using the trial agents. All participants provided written informed consent, and 39,876 were randomized between 1992 and 1995. The trial continued until its scheduled end on March 31, 2004; follow-up was >99% (21).
In 1998, the cognitive substudy began to evaluate cognitive function in women aged 65 and older. Only 1.5% had died or been lost to follow-up by that time, leaving 7,175 eligible for the substudy. After excluding 296 (4.1%) who could not be reached by telephone and 502 (7.0%) who refused to participate, 6,377 (88.9%) completed the initial cognitive assessment, on average, 5.6 years after randomization. Two follow-up assessments were conducted approximately 2 and 4 years later. The current analysis includes 5,988 women who had complete seafood intake data on food-frequency questionnaires (FFQs) administered at WHS baseline (follow-up: 89.6% at 2 years and 82.2% at 4 years). The Institutional Review Board of Brigham and Women’s Hospital, Boston, MA, approved this study.
Assessment of Seafood Consumption and Covariates
Participants were asked how often they consumed canned tuna (3–4 oz), other dark-meat fish (e.g., mackerel, salmon, sardines, bluefish, and swordfish; 3–5 oz), other light-meat fish (e.g., cod, haddock, and halibut; 3–5 oz), and shellfish (shrimp, lobster, and scallops as a main dish) during the past year. Tuna and other dark-meat fish were combined, as in previous studies (9,15). Upper consumption categories were collapsed for individual seafood, rather than having all four categories (<1, 1, 1–2, or >2 servings/week) as in total seafood, due to small numbers in those categories: <1, 1, or >1 serving/week for tuna and other dark-meat fish and light-meat fish, and <1 or ≥1 serving/week for shellfish. Prior validation work in similar cohorts showed reproducibility (Spearman ρ, between two questionnaires administered 1 year apart: 0.63 for dark-meat fish; 0.54 for canned tuna; 0.48 for other light-meat fish; and 0.67 for shrimp, lobster, or scallops) and validity against two 1-week dietary records (ρ = 0.61) and subcutaneous fat content (ρ = 0.49 for EPA) (22–24). There was a moderate correlation (ρ = 0.52) between questionnaires administered 6 years apart (25), suggesting that FFQs can reflect long-term dietary exposures. Sociodemographic and lifestyle characteristics, chronic conditions, medications, and postmenopausal hormone use were self-reported by mailed questionnaires.
Assessment of Cognitive Function
There was a lag of 5.6 years between baseline FFQs and initial cognitive testing. This lag may have several advantages. First, because cognitive decline develops over many years, diet at earlier points may be more biologically relevant than later diet. Second, the lag may help reduce any bias due to dietary changes caused by underlying health conditions or cognitive changes.
Trained interviewers administered a telephone assessment that included the following: the Telephone Interview for Cognitive Status (TICS), an adaptation of the Mini-Mental State Examination (26); immediate and delayed recalls of the East Boston Memory Test (EBMT) paragraph (27); a delayed recall of the TICS 10-word list; and category fluency (naming as many animals as possible in 1 minute) (28). Primary outcomes were a composite score of global cognitive function, derived by averaging standardized z-scores from all individual tests, using the mean and standard deviation (SD) of the baseline score, and a verbal memory, derived by averaging z-scores from the immediate and delayed recalls of both the EBMT paragraph and the TICS 10-word list. Although we do not have clinical diagnosis of cognitive impairment or dementia in our study, we have shown high validity and reproducibility of this telephone battery against in-person interview (29,30); poor performance on the TICS and verbal memory was associated with 8-fold and 12-fold increased risk of dementia 3 years later, respectively (29,30).
Statistical Analysis
Our objective was to examine changes in global cognition and verbal memory for three assessments by levels of total and individual seafood consumption. Because the change in mean scores was nonlinear (“learning effect” between the first and second assessments), we carried out an analysis of response profiles using the MIXED procedure in SAS (SAS Institute Inc, Cary, NC). This approach incorporates within-person correlation and is robust to model misspecification because it imposes minimal restrictions on the shape of change patterns by modeling time as indicator variables (31). We examined whether the change patterns differed by levels of fish and seafood intake, by testing the intake category-by-time interactions using Wald tests, assuming unstructured covariance structures. In the first model, we adjusted for age (years), randomization assignment, and sociodemographic variables of race (white vs non-white), higher education (bachelor’s degree or higher [master’s or doctoral degree] vs others [licensed practical or vocational nurse, associate’s degree, or registered nurse]), and annual income (≥$50,000 vs <$50,000). In the second model, we further adjusted for alcohol intake (≥1 vs <1 drink/day), body mass index (<25, 25–29, and ≥30kg/m2), exercise (≥1 vs <1 occasion/week), current smoking, diabetes mellitus, hypertension, hypercholesterolemia, depression, total caloric intake, and current postmenopausal hormone use. We also tested models that adjusted for CVD as of the initial cognitive assessment and for saturated, trans, and monounsaturated fat; protein; and fruit and vegetable consumption. The results did not differ substantially and thus these variables were not included in the models. When we found differences in the overall change patterns according to fish intake, we did post hoc comparisons to determine whether relations were found for change during the first 2 years or the second 2-year follow-up period.
As an alternative approach to analyses of cognitive change, we utilized logistic regression to examine relations of fish and seafood consumption to odds of “worst cognitive change,” defined as the worst 10% of the distribution of change from the first to the third assessments. Such a population-based 10% cutoff enhances interpretability of findings and has high sensitivity and specificity for cognitive impairment (32). Logistic models adjusted for initial score, time between the assessments, and potential confounders listed previously.
We conducted secondary analyses to address effect modification. Experimental data suggest that DHA is vulnerable to oxidative damage and may be more neuroprotective when combined with antioxidants (33). Thus, we took advantage of the study design to examine effect modification by vitamin E, an antioxidant, as half of the participants were randomly assigned to vitamin E. We also tested effect modification by randomization to aspirin, initial cognitive function (above vs below the mean), and education. Sensitivity analyses were performed to test the robustness of our results (Supplementary Methods). All analyses were conducted using SAS version 9.1, and a two-sided p value <.05 was considered statistically significant.
Results
Among 5,988 women (mean age [SD], 71.8 years [4.0]) included in our study, the median seafood consumption (interquartile range) was 1.5 servings/week (1.0, 2.5), and tuna and dark-meat fish made up to 54% of total seafood consumed (Supplementary Figure 1). Women who consumed more seafood tended to have fairly similar characteristics as those with lower intake, although education level and income were somewhat higher, as were alcohol intake and regular exercise (Table 1). Among chronic conditions, only hypercholesterolemia appeared slightly more prevalent with higher seafood intake.
Table 1.
Characteristics of Participants in the Women’s Health Study Cognitive Cohort According to Total Seafood Consumption*
| Characteristics | Servings per Week | ||||
|---|---|---|---|---|---|
| <1.0 (n = 1,378) | 1.0 (n = 1,131) | 1.1–2.0 (n = 1,949) | >2.0 (n = 1,530) | p | |
| Age at initial cognitive test, y | 72.1 (4.2) | 72.0 (4.2) | 71.7 (3.9) | 71.7 (3.9) | 0.01 |
| White race, n (%) | 95.5 | 96.6 | 96.3 | 94.6 | 0.03 |
| Bachelor’s degree or higher, n (%) | 28.9 | 30.9 | 35.7 | 38.2 | <0.001 |
| Annual income ≥$50,000, n (%) | 18.4 | 20.2 | 24.9 | 27.0 | <0.001 |
| Current smoking, n (%) | 10.7 | 10.3 | 10.8 | 7.3 | 0.002 |
| Alcohol intake ≥1 drink/d, n (%) | 8.9 | 11.2 | 13.6 | 14.7 | <0.001 |
| Exercise ≥1 occasion/wk, n (%) | 48.3 | 52.2 | 59.5 | 64.4 | <0.001 |
| Body mass index, kg/m2 | 25.6 (4.5) | 25.8 (4.6) | 25.6 (4.3) | 26.0 (4.5) | 0.03 |
| Hypertension, n (%) | 40.2 | 41.2 | 38.4 | 41.4 | 0.26 |
| Hypercholesterolemia, n (%) | 39.7 | 43.7 | 42.5 | 46.9 | 0.001 |
| Diabetes mellitus, n (%) | 3.4 | 3.5 | 3.6 | 3.9 | 0.93 |
| Cardiovascular disease,† n (%) | 2.5 | 2.1 | 1.8 | 1.9 | 0.56 |
| Depression, n (%) | 5.4 | 6.5 | 6.2 | 5.4 | 0.44 |
| Current hormone use, n (%) | 39.6 | 39.1 | 41.3 | 42.3 | 0.29 |
| Total caloric intake, kcal/d | 1748.6 (66.6) | 1737.0 (68.0) | 1731.7 (68.6) | 1731.8 (62.5) | <0.001 |
*All characteristics were assessed at the baseline of the Women’s Health Study. Continuous variables were presented in mean (standard deviation) and categorical variables were presented in percentages.
†Cardiovascular disease includes ascertained myocardial infarction and stroke that occurred between baseline and initial cognitive assessment.
Change in Cognitive Function by Total Seafood Consumption
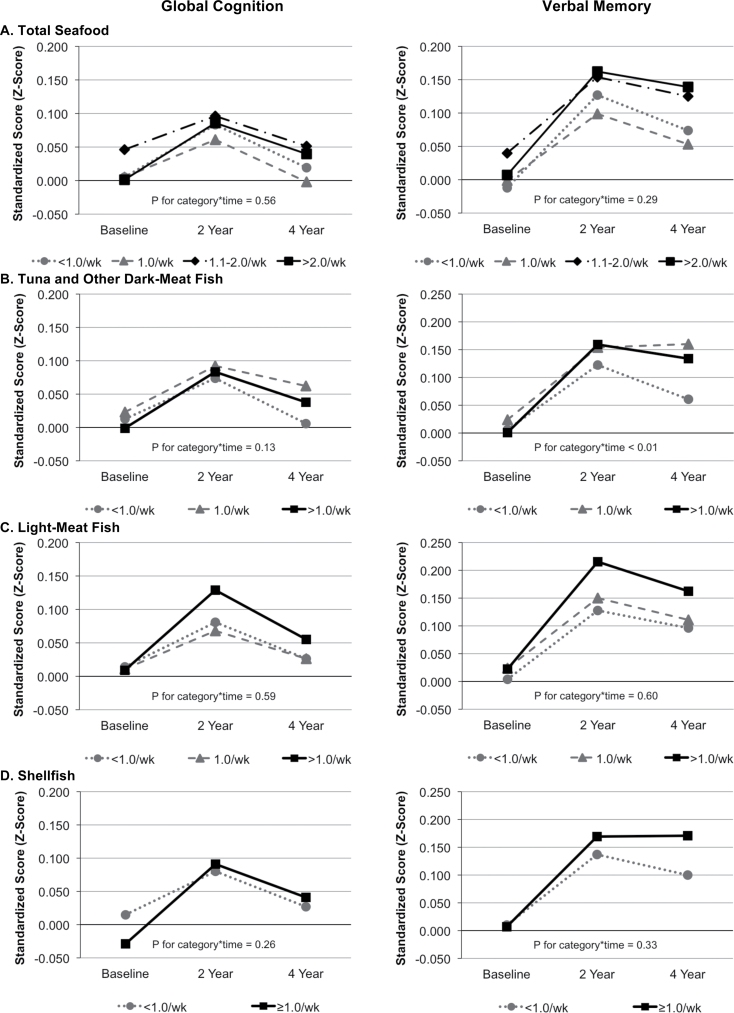
Mean scores of global cognitive function and verbal memory tended to increase from the first to the second assessments and then to decrease somewhat from the second to the third assessments: the mean [SD] values were 0.009 [0.639], 0.093 [0.710], and 0.065 [0.749] for global cognitive function scores and 0.006 [0.695], 0.147 [0.770], and 0.129 [0.799] for verbal memory. Women who consumed 1.1–2.0 servings/week of total seafood had higher cognitive scores than those who consumed <1.0 serving/week at the first assessment (p = .06 for global cognitive function and p = .03 for verbal memory), but there were no differences by seafood consumption categories in overall change for a period of 4 years (multivariable-adjusted p for seafood category-by-time interaction = 0.56 for global cognitive function and 0.29 for verbal memory; Figure 1 and Supplementary Table 1).
Figure 1.
Total and individual seafood consumption and cognitive function for a period of 4 y. Least-square mean cognitive scores shown, after adjusting for age (y), race (white vs non-white), education (bachelor’s degree or higher vs others), annual income (≥$50,000 vs <$50,000), randomization assignment, alcohol intake (≥1 vs <1 drink/d), body mass index (<25, 25–29, and ≥30kg/m2), exercise (≥1 vs <1 occasion/wk), current smoking, diabetes mellitus, hypertension, hypercholesterolemia, depression, total caloric intake, current hormone use, and all covariate-by-time interactions.
Change in Cognitive Function by Individual Types of Seafood Consumption
After adjusting for confounders, we found a significant difference in the trajectory of mean verbal memory scores with higher levels of tuna and dark-meat fish intake (p for fish category-by-time interaction <0.01; Figure 1), compared with the lowest intake. For global cognitive score, there were similar differences in mean trajectories with higher dark-meat fish consumption although this finding was not statistically significant (p for fish category-by-time interaction = 0.13). Post hoc analyses showed that the increase in verbal memory score from the first to the second assessments did not differ across fish consumption categories (p = .24), but the decline from the second to the third assessments did (p = .03), indicating that relations were stronger over later time periods. In addition, most benefit of tuna and dark-meat fish consumption was from increasing intake from <1 to 1 serving/week, with little additional gain beyond >1 serving/week. Specifically, for a period of 4 years, women who had tuna and dark-meat fish ≥1 serving/week had 0.079 standardized-unit higher verbal memory score than women who had <1 serving/week (p < .01). This difference was equivalent to the difference in verbal memory observed for women 2.1 years apart at the start of testing. Findings from these main analyses addressing trajectories of mean scores were supported by logistic regression results: tuna and dark-meat fish ≥1 serving/week vs <1 was associated with 20%–25% lower odds of worst cognitive change (Table 2).
Table 2.
Seafood Consumption and Worst Cognitive Change for a Period of 4 y
| Seafood Types | Frequency per Week | N (%) | Global Cognition | Verbal Memory | ||||
|---|---|---|---|---|---|---|---|---|
| OR* | 95% CI | p | OR* | 95% CI | p | |||
| Total seafood | <1.0 | 1,378 (23.0) | 1 | 1 | ||||
| 1.0 | 1,131 (18.9) | 0.98 | 0.73–1.31 | 0.87 | 1.24 | 0.92–1.67 | 0.16 | |
| 1.1–2.0 | 1,949 (32.5) | 0.96 | 0.74–1.24 | 0.76 | 1.02 | 0.78–1.33 | 0.90 | |
| >2.0 | 1,530 (25.6) | 0.80 | 0.60–1.06 | 0.12 | 0.96 | 0.72–1.28 | 0.77 | |
| p trend | 0.14 | 0.50 | ||||||
| Tuna and other dark-meat fish | <1.0 | 3,068 (51.2) | 1 | 1 | ||||
| 1.0 | 1,567 (26.2) | 0.76 | 0.60–0.96 | 0.02 | 0.76 | 0.60–0.96 | 0.02 | |
| >1.0 | 1,353 (22.6) | 0.79 | 0.61–1.01 | 0.06 | 0.84 | 0.65–1.08 | 0.18 | |
| p trend | 0.02 | 0.08 | ||||||
| Light-meat fish | <1.0 | 4241 (70.8) | 1 | 1 | ||||
| 1.0 | 1,380 (23.1) | 0.86 | 0.68–1.09 | 0.22 | 0.95 | 0.75–1.20 | 0.68 | |
| >1.0 | 367 (6.1) | 0.81 | 0.52–1.25 | 0.33 | 1.03 | 0.68–1.56 | 0.87 | |
| p trend | 0.15 | 0.89 | ||||||
| Shellfish | <1.0 | 5,674 (94.8) | 1 | 1 | ||||
| ≥1.0 | 314 (5.2) | 0.88 | 0.55–1.41 | 0.61 | 1.02 | 0.65–1.59 | 0.95 | |
Note. CI = confidence interval; OR = odds ratio.
*Adjusted for age (y), race (white vs non-white), education (bachelor’s degree or higher vs others), annual income (≥$50,000 vs <$50,000), randomization assignment, alcohol intake (≥1 vs <1 drink/d), body mass index (<25, 25–29, and ≥30kg/m2), exercise (≥1 vs <1 occasion/wk), current smoking, diabetes mellitus, hypertension, hypercholesterolemia, depression, total caloric intake, current hormone use, initial cognitive score, and time interval between the assessments.
There were no significant differences in the change in global cognitive score or verbal memory by levels of light-meat fish or shellfish intake (Figure 1). The results were similar when individual seafood types were simultaneously adjusted for each other (data not shown).
Assessment of Effect Modification
The association of total and individual types of seafood consumption to cognition was not different in the vitamin E or aspirin group compared with placebo group (data not shown). There was also no evidence of effect modification by the first cognitive scores (Supplementary Figure 2) or education (data not shown).
Sensitivity Analyses
Our main findings were consistent in sensitivity analyses in which we accounted for the possibility of regression-to-the-mean and assessed potential impacts of censoring (Supplementary Results and Supplementary Table 2).
Discussion
In this large cohort of women, tuna and dark-meat fish consumption of at least 1 serving/week was related to better performance in verbal memory for a period of 4 years. There were no clear associations with light-meat fish or shellfish. Total seafood consumption as a whole was not associated with the changes in cognitive function, which suggests that benefits of seafood may come mainly from tuna and dark-meat fish.
Several biological mechanisms explain how seafood may prevent age-related cognitive decline: their omega-3 fatty acids reduce the accumulation of amyloid β protein, decrease inflammation, increase neurotrophic factors, and improve synaptic membrane fluidity (1–4). The favorable associations observed for tuna and dark-meat fish may be explained by difference in DHA and EPA contents and in preparation methods. Tuna and dark-meat fish contain a higher amount of DHA and EPA per serving (0.2–1.8 g) than light-meat finfish (0.1–0.2 g) and shellfish (0.1–0.4 g) (16). Moreover, dark-meat fish are usually baked or broiled, whereas light-meat fish are often fried. Free radicals generated during cooking may variably oxidize DHA (17).
Several epidemiological studies have examined relations of fish and seafood consumption to cognitive outcomes and found inconsistent results (5–13). Few studies investigated the association by different seafood types. In the Cardiovascular Health Study, fatty fish intake was inversely associated with dementia and Alzheimer’s disease (AD), whereas fried lean fish intake was not (9). A similar, but statistically nonsignificant trend was observed in the Rancho Bernardo Study (10). The Rotterdam Study did not show differences in dementia risk by fish types, but very few people were consuming fatty fish (13). In the Veterans Affairs Normative Aging Study, there was no difference in cognitive scores for a period of 6 years across quartiles of fatty fish intake, but loss to follow-up was substantial (12). Thus, our study substantially adds to this scant literature by providing data on longer term cognitive trajectories from nearly 6,000 women by commonly consumed fish and seafood types. In addition, our data provide insights into the dose-response relationship: most of the apparent benefit of tuna and dark-meat fish was observed with increasing intake from <1 to 1 serving/week.
In contrast to suggested cognitive benefits of fish intake from observational studies, randomized controlled trials (RCTs) of fish oil supplementation in cognitively intact older adults were largely negative (34). Dose of marine omega-3 supplement was 400–1800 mg per day, and duration of supplementation was up to 48 months but typically less than that. However, participants in the control group had little cognitive decline, suggesting that the follow-up was not long enough to observe meaningful changes in cognitive function. Additionally, the effect of fish oil might depend on the duration of supplementation, stage of cognitive decline (e.g., mild impairment) (35,36), or specific target populations (37,38). Therefore, the null findings from RCTs do not exclude modest benefits of long-term fish intake, and trials with a longer follow-up are needed. Finally, this discrepancy between observational studies and RCTs may reflect the importance of nutrients in fatty fish other than omega-3, including vitamin A (which may inhibit amyloid β formation) (39), vitamin D (which has anti-inflammatory and antioxidative effects) (40), and selenium (a strong antioxidant) (41).
Interestingly, we found no evidence that seafood consumption was more strongly related to cognition among vitamin E users. DHA is vulnerable to oxidative damage and may need to be combined with antioxidants to show neuroprotective effects (33,42). Vitamin E supplements are commonly used by many as a means to prevent cognitive decline and dementia, despite lack of benefits in RCTs, including WHS (29,43). Regardless, it remains of interest whether the cognitive benefit of seafood consumption is larger among vitamin E users, as reported in an animal study (44) and human studies of schizophrenia (45,46). In the WHS, in which 50% of women were randomized to vitamin E 600 IU on alternate days for 10 years, we had an excellent setting for evaluating this interaction in the context of cognition. Nonetheless, our findings do not exclude a possible benefit with other vitamin E doses.
Strengths of our study include prospective assessment of seafood intake 5.6 years before cognitive testing; the use of a well-validated FFQ and cognitive battery; large sample size; repeated assessments of cognitive function over the relatively long duration of 4 years; and consistent findings in several sensitivity analyses. Limitations must also be considered. Our sample included generally healthy, mostly white, female health care professionals in their early 70s and with high educational levels. Thus, findings may not be generalizable to men or populations with different age (e.g., oldest-old), race, or educational levels. Recent evidence suggests that relations of dietary fat intake to AD risk may differ by apolipoprotein E (APOE) gene polymorphism (47). Because we did not have data on APOE, we were not able to assess seafood–cognition associations by APOE ε4 status. Nonetheless, our results can be viewed as a weighted average of the association among APOE ε4 carriers and among noncarriers in our WHS cohort. Therefore, although our results may not generalize to other populations with different proportions of APOE ε4 carriers, they are internally valid. Also, despite statistical adjustment for a wide variety of potential confounders, residual confounding is possible, and the data should be interpreted with appropriate caution. Finally, fish and seafood consumption was assessed only at baseline. Participants might have changed their diet because their entry into a clinical trial, which may introduce misclassification bias of recent fish consumption. If such change is related to participation in a clinical trial, the change should be random across all participants, leading to bias toward the null. Any association between fish consumption and cognition that we found would be an underestimation. However, if recent fish consumption is biologically less relevant to cognitive decline that develops over years, such misclassification may not affect our results substantially.
In summary, we found a moderate association between at least once-weekly consumption of tuna and dark-meat fish and lower verbal memory decline, but no association with light-meat fish or shellfish consumption. Although, to date, RCTs of fish oil supplements have failed to show reductions in cognitive decline, benefits for cognition may come from consuming fish as food, not individual nutrients. Tuna and dark-meat fish consumption already has known advantages for cardiovascular health (48); thus, further rigorous study will be important to inform public health recommendations regarding its potential to ameliorate age-related cognitive decline.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/.
Funding
This study was supported by the National Institute on Aging (R01 AG-015933 to FG and K08 AG-029813 to OIO), the National Heart, Lung, and Blood Institute (R01 HL-043851 to JEB and R01 HL-080467 to JEB), and the National Cancer Institute (R01 CA-047988 to JEB) at the National Institute of Health. The funding sources had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Acknowledgment
We are indebted to the participants in the Women’s Health Study for their continuing outstanding support and our many colleagues working in the Study for their valuable help.
References
- 1. Cole GM, Ma QL, Frautschy SA. Omega-3 fatty acids and dementia. Prostaglandins Leukot Essent Fatty Acids. 2009;81:213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freemantle E, Vandal M, Tremblay-Mercier J, et al. Omega-3 fatty acids, energy substrates, and brain function during aging. Prostaglandins Leukot Essent Fatty Acids. 2006;75:213–220 [DOI] [PubMed] [Google Scholar]
- 3. Youdim KA, Martin A, Joseph JA. Essential fatty acids and the brain: possible health implications. Int J Dev Neurosci. 2000;18:383–399 [DOI] [PubMed] [Google Scholar]
- 4. Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959 [DOI] [PubMed] [Google Scholar]
- 5. Kalmijn S, van Boxtel MP, Ocké M, et al. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology. 2004;62:275–280 [DOI] [PubMed] [Google Scholar]
- 6. Morris MC, Evans DA, Tangney CC, et al. Fish consumption and cognitive decline with age in a large community study. Arch Neurol. 2005;62:1849–1853 [DOI] [PubMed] [Google Scholar]
- 7. Nurk E, Drevon CA, Refsum H, et al. Cognitive performance among the elderly and dietary fish intake: the Hordaland Health Study. Am J Clin Nutr. 2007;86:1470–1478 [DOI] [PubMed] [Google Scholar]
- 8. van Gelder BM, Tijhuis M, Kalmijn S, et al. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen Elderly Study. Am J Clin Nutr. 2007;85:1142–1147 [DOI] [PubMed] [Google Scholar]
- 9. Huang TL, Zandi PP, Tucker KL, et al. Benefits of fatty fish on dementia risk are stronger for those without APOE epsilon4. Neurology. 2005;65:1409–1414 [DOI] [PubMed] [Google Scholar]
- 10. Lopez LB, Kritz-Silverstein D, Barrett Connor E. High dietary and plasma levels of the omega-3 fatty acid docosahexaenoic acid are associated with decreased dementia risk: the Rancho Bernardo study. J Nutr Health Aging. 2011;15:25–31 [DOI] [PubMed] [Google Scholar]
- 11. Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60:940–946 [DOI] [PubMed] [Google Scholar]
- 12. van de Rest O, Spiro A, 3rd, Krall-Kaye E, et al. Intakes of (n-3) fatty acids and fatty fish are not associated with cognitive performance and 6-year cognitive change in men participating in the Veterans Affairs Normative Aging Study. J Nutr. 2009;139(12):2329–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Devore EE, Grodstein F, van Rooij FJ, et al. Dietary intake of fish and omega-3 fatty acids in relation to long-term dementia risk. Am J Clin Nutr. 2009;90:170–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Daviglus ML, Bell CC, Berrettini W, et al. National Institutes of Health State-of-the-Science Conference statement: preventing Alzheimer disease and cognitive decline. Ann Intern Med. 2010;153:176–181 [DOI] [PubMed] [Google Scholar]
- 15. Mozaffarian D, Longstreth WT, Jr, Lemaitre RN, et al. Fish consumption and stroke risk in elderly individuals: the cardiovascular health study. Arch Intern Med. 2005;165:200–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kris-Etherton PM Harris WS Appel LJ Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757 [DOI] [PubMed] [Google Scholar]
- 17. Candela M, Astiasaran I, Bello J. Deep-fat frying modifies high-fat fish lipid fraction. J Agric Food Chem. 1998;46:2793–2796 [Google Scholar]
- 18. Buring JE, Hennekens CH. The Women’s Health Study: summary of the design. J Myocardial Ischemia. 1992;4:27–29 [Google Scholar]
- 19. Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:47–55 [DOI] [PubMed] [Google Scholar]
- 20. Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:56–65 [DOI] [PubMed] [Google Scholar]
- 21. Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304 [DOI] [PubMed] [Google Scholar]
- 22. Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–11126 [DOI] [PubMed] [Google Scholar]
- 23. Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69:243–249 [DOI] [PubMed] [Google Scholar]
- 24. Hunter DJ, Rimm EB, Sacks FM, et al. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol. 1992;135:418–427 [DOI] [PubMed] [Google Scholar]
- 25. Nagel G, Zoller D, Ruf T, et al. Long-term reproducibility of a food-frequency questionnaire and dietary changes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Heidelberg cohort. Br J Nutr. 2007;98:194–200 [DOI] [PubMed] [Google Scholar]
- 26. Brandt J, Folstein MF. Telephone Interview for Cognitive Status: Professional Manual. Lutz, FL: Psychological Assessment Resources; 2003. [Google Scholar]
- 27. Scherr PA, Albert MS, Funkenstein HH, et al. Correlates of cognitive function in an elderly community population. Am J Epidemiol. 1988;128:1084–1101 [DOI] [PubMed] [Google Scholar]
- 28. Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165 [DOI] [PubMed] [Google Scholar]
- 29. Kang JH, Cook N, Manson J, et al. A randomized trial of vitamin E supplementation and cognitive function in women. Arch Intern Med. 2006;166:2462–2468 [DOI] [PubMed] [Google Scholar]
- 30. Kang JH, Cook N, Manson J, et al. Low dose aspirin and cognitive function in the women’s health study cognitive cohort. BMJ. 2007;334:987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fitzmaurice GM, Laird NM, Ware JH. Modelling the mean: analyzing response profiles. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons; 2004:103–139 [Google Scholar]
- 32. Ganguli M, Belle S, Ratcliff G, et al. Sensitivity and specificity for dementia of population-based criteria for cognitive impairment: the MoVIES project. J Gerontol. 1993;48:M152–M161 [DOI] [PubMed] [Google Scholar]
- 33. Cole GM, Frautschy SA. Docosahexaenoic acid protects from amyloid and dendritic pathology in an Alzheimer’s disease mouse model. Nutr Health. 2006;18:249–259 [DOI] [PubMed] [Google Scholar]
- 34. Sydenham E, Dangour AD, Lim WS. Omega 3 fatty acid for the prevention of cognitive decline and dementia. Cochrane Database Syst Rev. 2012;6:CD005379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006;63:1402–1408 [DOI] [PubMed] [Google Scholar]
- 36. Yurko-Mauro K, McCarthy D, Rom D, et al. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010;6:456–464 [DOI] [PubMed] [Google Scholar]
- 37. Andreeva VA, Kesse-Guyot E, Barberger-Gateau P, et al. Cognitive function after supplementation with B vitamins and long-chain omega-3 fatty acids: ancillary findings from the SU.FOL.OM3 randomized trial. Am J Clin Nutr. 2011;94:278–286 [DOI] [PubMed] [Google Scholar]
- 38. Geleijnse JM, Giltay EJ, Kromhout D. Effects of n-3 fatty acids on cognitive decline: a randomized, double-blind, placebo-controlled trial in stable myocardial infarction patients. Alzheimers Dement. 2012;8:278–287 [DOI] [PubMed] [Google Scholar]
- 39. Obulesu M, Dowlathabad MR, Bramhachari PV. Carotenoids and Alzheimer’s disease: an insight into therapeutic role of retinoids in animal models. Neurochem Int. 2011;59:535–541 [DOI] [PubMed] [Google Scholar]
- 40. Annweiler C, Rolland Y, Schott AM, et al. Higher vitamin D dietary intake is associated with lower risk of A lzheimer’s disease: a 7-year follow-up. J Gerontol A Biol Sci Med Sci. 2012;67:1205–1211 [DOI] [PubMed] [Google Scholar]
- 41. Berr C, Arnaud J, Akbaraly TN. Selenium and cognitive impairment: a brief-review based on results from the EVA study. Biofactors. 2012;38:139–144 [DOI] [PubMed] [Google Scholar]
- 42. Calon F, Lim GP, Yang F, et al. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer’s disease mouse model. Neuron. 2004;43:633–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388 [DOI] [PubMed] [Google Scholar]
- 44. Suchy J, Chan A, Shea TB. Dietary supplementation with a combination of alpha-lipoic acid, acetyl-L-carnitine, glycerophosphocoline, docosahexaenoic acid, and phosphatidylserine reduces oxidative damage to murine brain and improves cognitive performance. Nutr Res. 2009;29:70–74 [DOI] [PubMed] [Google Scholar]
- 45. Mahadik SP, Pillai A, Joshi S, et al. Prevention of oxidative stress-mediated neuropathology and improved clinical outcome by adjunctive use of a combination of antioxidants and omega-3 fatty acids in schizophrenia. Int Rev Psychiatry. 2006;18:119–131 [DOI] [PubMed] [Google Scholar]
- 46. Sivrioglu EY, Kirli S, Sipahioglu D, et al. The impact of omega-3 fatty acids, vitamins E and C supplementation on treatment outcome and side effects in schizophrenia patients treated with haloperidol: an open-label pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1493–1499 [DOI] [PubMed] [Google Scholar]
- 47. Barberger-Gateau P, Samieri C, Féart C, et al. Dietary omega 3 polyunsaturated fatty acids and Alzheimer’s disease: interaction with apolipoprotein E genotype. Curr Alzheimer Res. 2011;8: 479–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899 [DOI] [PubMed] [Google Scholar]