Abstract
Mice are increasingly used for investigation of the pathophysiology of osteoporosis because their genome is easily manipulated, and their skeleton is similar to that of humans. Unlike the human skeleton, however, the murine skeleton continues to grow slowly after puberty and lacks osteonal remodeling of cortical bone. Yet, like humans, mice exhibit loss of cancellous bone, thinning of cortical bone, and increased cortical porosity with advancing age. Histologic evidence in mice and humans alike indicates that inadequate osteoblast-mediated refilling of resorption cavities created during bone remodeling is responsible. Mouse models of progeria also show bone loss and skeletal defects associated with senescence of early osteoblast progenitors. Additionally, mouse models of atherosclerosis, which often occurs in osteoporotic participants, also suffer bone loss, suggesting that common diseases of aging share pathophysiological pathways. Knowledge of the causes of skeletal fragility in mice should therefore be applicable to humans if inherent limitations are recognized.
Key Words: Bones, Aging, Osteoporosis
OSTEOPOROSIS was not considered a medical problem until modern times when advances in working conditions, sanitation, and medical care extended life span well past the reproductive years. Once only seen in the few surviving elderly adults, the deterioration of the skeleton with advancing age affects an increasingly larger proportion of the population and contributes greatly to the associated epidemic of nontraumatic fractures. The consequent hospitalization, rehabilitation, and frequent loss of independence lead to substantial social and financial costs. Thus, there is a pressing need to understand the underlying pathophysiology of osteoporosis so that the condition can be managed more effectively.
Since the 1970s, animal models of postmenopausal osteoporosis have been used to advance knowledge of the cellular and molecular basis of osteoporosis and to develop preclinical models for testing therapeutic approaches (1,2). However, it is now evident that substantial skeletal deterioration occurs via mechanisms that are independent of the loss of sex steroids (3). Thus, loss of cancellous (also known as trabecular) bone begins during the third decade of life in both men and women (4) and is accelerated at the menopause. On the other hand, most of the cortical bone loss occurs 10 years after the menopause and is due to cortical thinning and increased cortical porosity (5,6). Though often called senile, or type II, osteoporosis, sex steroid–independent bone loss actually begins in early adulthood. Hence, this type of bone loss will be called age-related osteoporosis in this article.
It is thought that most mammals exhibit skeletal deterioration with advancing age, but among the animals commonly used for biological research, age-related bone loss has been well documented only in cynomolgus monkeys (7), rats (8), and mice (9–12). The laboratory mouse (Mus musculus) is the most highly utilized animal for studying human physiology and disease at the cellular, molecular, and genetic level due to its short 3-week gestation period, high reproductive capacity (~5–6 animals/litter), similarity to human physiology, and ease of genetic manipulation. Indeed, the ability to manipulate gene expression in a cell-specific fashion in mice has allowed scientists to move from the tissue culture dish to the in vivo situation to address fundamental questions underlying the regulation of bone cell formation and function, as well as bone remodeling. After briefly discussing current theories of how aging influences cell function and the potential impact of these mechanisms on bone homeostasis, this review will consider the advantages and limitations of mice for the study of age-related bone loss.
Aging and Bone Homeostasis
Studies with fruit flies, nematodes, rodents, and nonhuman primates have revealed the existence of several inter-related age-associated changes that negatively affect cell function and life span. These include excessive levels of reactive oxygen species due to dysfunctional mitochondria, increased DNA damage leading to chromosome instability, and chronic inflammation (13–16). Studies in mice with loss or gain of function of antioxidant enzymes have challenged the notion that oxidative stress negatively affects life span (17). However, the more urgent question concerns the identity of factors that determine health span—the age of onset of diseases associated with aging. Cells possess a variety of adaptive responses to reduce oxidative stress, repair DNA damage, and recycle damaged proteins and organelles. The failure to adapt and repair, whether due to excessive damage or damage to the repair system itself, leads to aberrant cell behavior, replicative senescence, and/or activation of cell death pathways. Such changes may underlie the age-related loss of normal tissue function, including skeletal integrity.
The complex interplay among stem cells, bone-resorbing osteoclasts, bone-forming osteoblasts, and osteocytes gives ample opportunity for cellular dysfunction to disturb skeletal homeostasis leading to bone loss. Bone undergoes constant renewal, or remodeling, to maintain its essential functions of providing anchorage for tendons and muscle, a site of hematopoiesis, a means of locomotion, and the protection of internal organs. Bone remodeling is performed by teams of osteoclasts and osteoblasts collectively known as the bone multicellular unit. The process is initiated by osteoclasts that excavate old bone (18,19). Osteoblasts are then recruited to the site of bone resorption by growth factors released during bone resorption (20), and the osteoblasts then refill the resorption cavity with new bone. Some of the osteoblasts become entombed within the bone matrix as osteocytes, but most die by apoptosis (21).
Osteoclasts and osteoblasts are short lived and develop from hematopoietic and mesenchymal stem cells, respectively, in response to locally produced cytokines and growth factors, as well as systemic endocrine factors like parathyroid hormone (18). Osteocytes are long-lived cells and are replaced when the bone is remodeled. They are connected with each other and with cells on the cell surface via projections called canaliculi. Because of their location, the network of osteocytes is capable of sensing the need for bone repair and to orchestrate the local development of the osteoclasts and osteoblasts that replace the old bone with new (22). Recent work on mice has demonstrated that osteocytes are the principal source of receptor activator of NfκB ligand—the essential pro-osteoclastogenic cytokine needed for activation of bone remodeling (23,24). Moreover, osteocytes stimulated to undergo apoptosis in response to fatigue damage release factors that stimulate neighboring viable osteocytes to synthesize receptor activator of NfκB ligand, as well as vascular endothelial growth factor that helps orchestrate the formation of vessels that act as a conduit for the delivery of circulating osteoclast progenitors to bone (25).
Bone loss occurs when the bone removed by osteoclasts is not fully replaced by osteoblasts. Accordingly, investigation of the pathophysiology of osteoporosis centers on elucidation of the mechanisms that control the balance between bone resorption and formation and on detection of age-associated changes in cell function (26). The long-lived stem cell progenitors of osteoclasts and osteoblasts and the long-lived osteocytes are the most likely to be damaged by the ravages of time. However, damage to stem cells may also affect the function of their fully differentiated progeny.
Use of Mice in Biological Research
Standardized outbred and inbred strains of laboratory mice have been used extensively for biological research for almost 100 years. Outbred strains were established in the early 20th century by interbreeding a small number of unrelated mice in a manner that maintained maximum genetic heterozygosity. The advantage of outbred strains is their genetic heterogeneity, analogous to the genetic variation seen in closely related human populations (27). There are presently 34 well-defined outbred lines. Each mouse of an outbred strain comprises a unique constellation of alleles, depending on the original stocks used for establishment of the outbred colony. Outbred mice are frequently used in genetic studies and as the starting point for developing models of human disease. A disadvantage of outbred mice is that genetic variability contributes to differences in phenotype and response to treatment among individuals. This requires the use of a large number of animals to achieve appropriate statistical power. Another disadvantage is that extensive breeding is required to introduce a specific genetic change—that is, gene knockout, knockin of a mutant gene, or overexpression of a particular gene—while maintaining the same degree of heterozygosity among individuals.
Inbred strains of mice were derived from outbred strains by intensive brother–sister mating to produce mice with practically identical genomes. There were 450 known inbred strains in 2000 (28). In the past, there were few criteria for choosing the appropriate inbred strain for study. The mouse phenome database has alleviated this problem to some extent by compiling morphological, physiological, and biochemical data for commonly used inbred strains (29). The genetic uniformity of each animal within an inbred strain enables reproducible results because the contribution of genetic differences to a particular outcome is nil. However, it has been argued that studies utilizing a single inbred strain can potentially give a result that is not applicable to the population as a whole because it might depend on a unique constellation of genes (30). This notion has not been accepted by other investigators (31). Indeed, reproduction of a specific human pathology in an inbred mouse strain gives confidence that this risk is minimal in at least some situations, for example, age-related bone loss in C57BL/6 mice as discussed below. Nevertheless, an outcross among four inbred strains (C57BL/6, C3H, Balb/cNia, and DBA/2), designated as UM-HET3 mice, has been generated to at least partially alleviate problems arising from genetic uniformity (32). These mice essentially represent a well-defined outbred strain.
The median life span among 30 inbred mice ranges from 476 to 964 days, excluding 2 strains that die before 1 year of age from lymphoma or sarcoma (33). The median life span of C57BL/6 mice is 866 days for females and 901 days for males (33), similar to the 880-day median life span of UM-HET3 mice. Short-lived mice are not useful for studies of normal aging because the development of age-related pathologies, as well as their demise, is often due to susceptibility to carcinogenesis or due to metabolic, immunologic, or other abnormalities. Thus, the relatively long life span of C57BL/6 mice makes them attractive for aging studies. It should be recognized, however, that intensive use of this strain also reflects “mob psychology”: the more information, and previous work done with, a particular model, the more valuable it becomes from the perspective of building upon previous results. Moreover, it is often considered too costly and time consuming to invest in a second model whether inbred or outbred.
Mice have been successfully used to model aspects of many human diseases, but there are limitations imposed by genetic and physiologic differences between these species. A recent example is the lack of similarity in the inflammatory response of mice and humans to sepsis (34). This may be due to the fact that mice are relatively resistant to sepsis compared with humans. In the skeletal biology arena, the importance of bone-derived undercarboxylated osteocalcin for the regulation of insulin sensitivity and secretion in mice (35) was not evident in humans (36)—perhaps because glucose utilization and hepatic production of glucose are much higher in mice than humans. Rather than casting doubt on the utility of mouse models for investigating human disease, however, these examples emphasize the need for a greater appreciation of the genetic and physiologic differences between laboratory mice and humans.
Comparison of Murine and Human Skeletal Physiology
In most mouse strains, peak bone mass is achieved at 4–6 months of age, as determined by dual-energy X-ray absorptiometry to quantify the calcium content of the entire skeleton (minus the head). Thus, unlike humans, bone acquisition and longitudinal bone growth continue in mice after sexual maturity, which occurs at 6–8 weeks of age. Bone mass increases further between 6 and 12 months of age in females of half of the 34 strains examined in the mouse phenome database and in males in 2/3 of these strains, with the magnitude of the increase ranging from 5% to 10% (37).
Longitudinal bone growth does not cease at sexual maturity in mice, instead slowing to very low levels. In growing long bones, the epiphysis adjacent to the articular cartilage is separated from the metaphysis and diaphysis by a cartilaginous disk called the growth plate. At the epiphyseal side of the growth plate, new cartilage is produced, while at the metaphyseal side, the previously made cartilage is replaced by new bone. Thus, bone lengthens because both processes occur at the same rate and the width of the growth plate does not change. With the onset of puberty in humans, the deposition of cartilage ceases and longitudinal appositional growth stops. The metaphysis then fuses with the epiphysis and the growth plate disappears (38). In mice, longitudinal growth slows dramatically at puberty, but the growth plates do not completely fuse and disappear. Growth continues at a slow rate that varies depending on the strain. In 129/Balb/c hybrid mice, femoral length in females increased by 9% between 8 and 30 months of age, but there was no detectable change in males (39). In UM-HET3 female mice, femoral length increased by 4% between 8 and 24 months of age (40); however, values for males were not reported. In outbred CD-1 mice, femoral length does not significantly change between 8 and 18 months of age in females or males (41). In C57BL/6 mice, there was no change between 6 and 12 months of age in either sex (11), despite the fact that the growth plate has not closed. Measurements of body length, an index of bone growth in the axial skeleton, in 32 inbred strains showed a 3%–8% increase between 6 and 12 months of age. However, a further increase was seen in only four of these strains between 12 and 20 months of age (37). Body length of C57BL/6 mice does not change between 12 and 24 months of age (42).
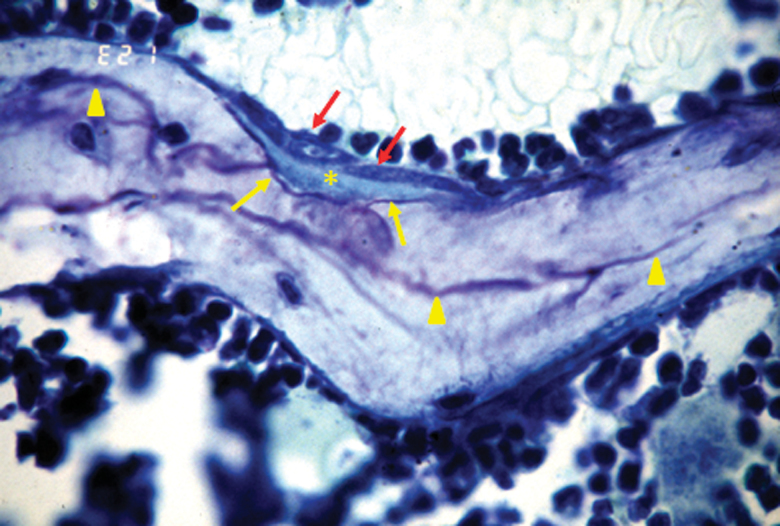
As in humans, murine cancellous bone undergoes remodeling. The remodeling process leaves a histologic trace in the form of uneven “cement lines” visualized by toluidine blue staining (Figure 1). The cement line comprises glycosaminoglycans that are deposited onto the bone surface by undefined cells just before osteoblasts are recruited to the resorption site. Thus, cement lines represent the boundary between episodes of bone resorption and bone formation (19). They are uneven because of the scalloped nature of the resorption pits created by osteoclasts.
Figure 1.
Morphological evidence for remodeling in murine cancellous bone. Unmineralized osteoid (asterisk) is being deposited by osteoblasts (dark gray arrows) onto a previously site of bone resorption, marked by the scalloped purple cement line (light gray arrows). Scalloped cement lines from previous episodes of bone remodeling are evident (light gray arrowheads). Wall width is the distance from a cement line to a completely quiescent perimeter and is an index of the amount of bone made by the previous team of osteoblasts. Photomicrograph is from Weinstein and coworkers (77), original magnification, ×630.
The suppressive effect of the antiosteoclastogenic agent osteoprotegerin on the number of osteoblasts in murine cancellous bone provides additional evidence for remodeling (43). Administration of osteoprotegerin to mice depletes the bone of osteoclasts, and consequently removes a source of pro-osteoblastogenic factors that are critical for the coupling of bone formation to bone resorption, including transforming growth factor-ß(20). As a result, osteoblast number slowly declines in osteoprotegerin-treated mice over a 2-week period, consistent with the 10- to 14-day life span of murine osteoblasts (44,45). The effects of osteoprotegerin on osteoblast number are best explained by the coupling of bone formation to bone resorption during remodeling. Such coupling does not occur during modeling, wherein osteoblasts and osteoclasts operate independently to sculpt bone during growth.
Cancellous bone turnover in mice is approximately 0.7% per day as measured in the distal femur; each episode of remodeling takes about 2 weeks to complete (44). In humans, turnover is about 0.1% per day as measured in the iliac crest (46), and each remodeling event takes 6–9 months to complete (47). The more rapid pace of events in the murine skeleton is in keeping with the higher metabolic rate of small animals. Metabolic rate among animals varies in proportion to the ¾ power of body mass (48). This difference explains, in part, the need to administer higher concentrations of biological agents to mice than to humans to achieve an equivalent physiologic effect.
The most conspicuous structural difference between the human and murine skeleton is that the later lacks osteons, also known as Haversian systems, in cortical bone (49). Thus, in humans, but not mice, remodeling of cortical bone is carried out within the bone interior by teams of osteoclasts and osteoblasts surrounding a blood vessel. The blood vessels serve as a conduit for the delivery of osteoclast, and perhaps osteoblast, progenitors to the bone interior. Nevertheless, blood vessels are present in murine cortical bone (50).
Comparison of Age-Related Bone Loss in Mice and Humans
The age-related loss of cancellous and cortical bone in mice bears a remarkable resemblance to the phenomenon in humans. Loss of bone strength precedes loss of bone mineral density (BMD), determined by dual-energy X-ray absorptiometry, in both humans and C57BL/6 mice (12,51). Loss of BMD in C57BL/6 mice occurs between 16 and 25 months of age, that is, well before reaching their median life span of 29 months of age (12,33). Thus, the metabolic disorders, illnesses, and tumors leading to death are unlikely to be involved in the loss of bone mass. Longitudinal measurements of BMD in 26 inbred mouse strains indicate considerable variability in age-related bone loss (37). For example, BMD of DBA/2 females declined 4% between 12 and 20 months of age and in NZO/HILtJ females by 8% during this period. On the other hand, BMD of RIIIS/J females increased by 10%. A significant change in BMD in male or female C57BL/6 mice was not detected between 12 and 20 months of age in this study, which contrasts with the findings of Almeida and coworkers (12), who reported identical rates of bone loss in male and female mice up to 30 months of age. This discrepancy may be explained by the use of more mice and therefore greater statistical power in the Almeida and coworkers study.
Among 26 strains examined, bone loss between 12 and 20 months of age occurred in female but not male mice in 5 of the strains, and in males but not females in 4 strains (29). On the other hand, the bone loss is similar in male and female Balb/cByJ and SWR/J mice between 12 and 20 months of age, as is the rate of bone loss and in male and female C57BL/6 mice between 16 and 30 months of age (12). These strain differences indicate that genetic factors dominate sex steroid–dependent factors as determinants of bone loss in mice. Moreover, a significant difference between mice and humans is that mice do not undergo a true menopause. Instead they exhibit irregular cycling beginning at 8–12 months of age as exemplified in C57BL/6 mice (52,53). Nevertheless, at this stage, estrogen levels are still maintained. Thereafter, they decline by 45%–80% but still remain at detectable levels. Uterine weight, a sensitive indicator of physiological levels of estrogens, is maintained at normal levels up to at least 31 months of age (12). Males maintain testosterone levels (54). Hence, age-related changes in bone mass in mice are dominated by age-related factors rather than sex steroid deficiency. This contention is supported by evidence that, despite the bone loss, the rate of cancellous bone remodeling declines with age in mice, which contrasts with the stimulating effect of sex steroid deficiency on remodeling in both humans and rodents (12,55).
As in the case of humans (4), cancellous bone loss begins in early adulthood in C57BL/6 mice, that is, much earlier than indicated by BMD determinations (9,11,12). Femoral cancellous bone mass declines beginning at about 3 months of age, and it practically disappears by 8–12 months of age in both male and female animals, albeit the magnitude of this decline is greater in females (9,11). Vertebral trabecular bone measured in the lumbar spine also declines during this period but at a slower rate (11,12).
Age-related changes in cancellous architecture in humans are characterized by decreased trabecular number, thickness, and connectivity; but there is a larger decrease in thickness in men than in women (56). In contrast, only trabecular number and connectivity decline with age in male and female C57BL/6 mice, with no changes in trabecular thickness (9,11). The failure to observe decreased trabecular thickness with age may be explained by the fact that murine trabeculae are thinner than in humans (40–50 μm vs. 150 μm, respectively) and thus are more likely to suffer penetration during unbalanced bone remodeling, with a consequent decrease in number and connectivity. Moreover, trabecular thinning may be a comparatively fleeting event in mice versus humans because of the higher rate of bone turnover in the former.
Most importantly, from the pathophysiological perspective, the cancellous bone of both aging mice and humans is characterized by a decline in wall thickness—a histologic measure of the amount of bone made by each team of osteoblasts during remodeling as explained in Figure 1 (12,57). This observation indicates that the amount of bone replaced by osteoblasts during each remodeling transaction is slightly less than needed to refill the resorption cavity in both mice and humans. Defects in osteoblast differentiation and survival may thus account for age-related bone loss. Studies of aging mice have identified several underlying mechanisms, including oxidative stress and consequent activation of FoxO transcription factors (12,58), relative hyperglucocorticoidism (59), and increased lipid oxidation (60).
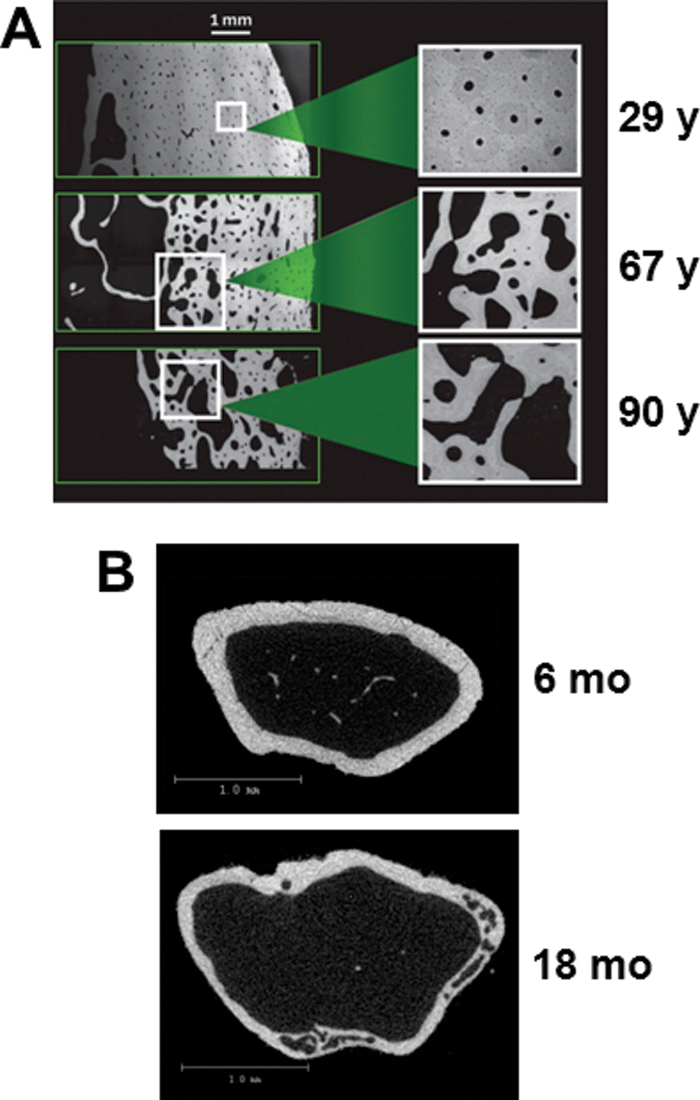
Loss of cortical bone with advancing age also occurs in both mice and humans. In aging humans, individual pores are distributed throughout the cortex and their size increases with age, resulting in a cancellous-like architecture near the endosteal surface (Figure 2A) (5). Thus, human cortical bone becomes thinner and more porous with age (11,61). Cortical porosity also increases with advancing age in the femurs of various mouse strains (10,42,62,63). Micro-computed tomography imaging of aging C57BL/6 mice reveals that the pores frequently occupy a large part of the intracortical area of femoral metaphysis of 18-month-old mice but are not evident in 6- month-old mice (Figure 2B). More detailed studies are needed to quantify and characterize the development of cortical porosity in mice with advancing age and to establish whether the severity of cortical porosity varies depending on the mouse strain examined.
Figure 2.
Comparison of cortical porosity in mice and humans. (A) Backscattered scanning electron microscope images of cross-sections of femoral bone from women at 29, 67, or 90 y of age. Reproduced with permission from Zebaze and coworkers (5). (B) Cross-sectional micro-CT images of distal metaphyseal femoral bone from 6- or 18-month-old female C57BL/6 mice (R. L. Jilka, unpublished data).
Synchrotron imaging studies in humans indicates that large cortical pores result from coalescence of enlarged osteonal canals (64). Analogous to the situation in cancellous bone, enlargement of osteon canals is likely due to incomplete replacement of bone during remodeling because of reduced osteoblastogenesis. The cortical voids of aged mice also contains teams of osteoclasts and osteoblasts, as well as blood vessels (42,65). Thus, as in humans, intracortical remodeling in mice may be initiated by extravasation of osteoclast progenitors from vessels that exist within the cortex of murine bone (50). Evidence in support of this notion has been provided by our recent studies showing that long-term prevention of osteocyte apoptosis by deletion of the proapoptotic proteins Bak and Bax magnifies the age-related increase in femoral cortical porosity (65). Importantly, the increased porosity in mice lacking Bak and Bax was associated with increased production of receptor activator of NfκB ligand and vascular endothelial growth factor by osteocytes and the presence of blood vessels within the cortical pores. This finding suggests that osteocytes in aged murine bone activate intracortical remodeling by producing cytokines needed for osteoclast differentiation and for the development of blood vessels that deliver osteoclast progenitors to the interior of the cortex. In the case of mice lacking Bak and Bax, prevention of apoptosis likely increased the life span of osteocytes beyond their intrinsic limit, making them dysfunctional and causing them to produce increased levels of pro-osteoclastogenic cytokines.
Low Bone Mass in Mouse Models of Premature Aging
Low bone mass and skeletal abnormalities are among the many disorders seen in human diseases of premature aging, including Werner’s syndrome and Hutchinson Gilford Progeria syndrome (HGPS) (66,67). Werner’s syndrome is caused by loss of Wrn, the gene encoding an enzyme involved in DNA repair and telomere maintenance. Mutations in the gene encoding lamin A, a nuclear matrix protein, cause the development of HGPS. Investigation of these rare genetic diseases may shed light on mechanisms that underlie diseases of normal aging. In support of this approach, cell nuclei from elderly humans exhibit some of the same histone modifications and DNA damage as nuclei from HGPS participants (68). Low bone mass has been noted in mouse models of both diseases. Both Wrn and telomerase must be deleted to obtain mice with Werner’s syndrome, reflecting species differences in telomere maintenance (69,70). A reduction in spinal and femoral cancellous bone mass was noted at 4 and 15 months of age in these mice. This phenotype is associated with reduced numbers of mesenchymal stem cells and increased replicative senescence of marrow progenitors (70). Reduced trabecular and cortical bone was also evident at 4 weeks of age in mice lacking lamin A, the model used to investigate HGPS (71). Bone formation rate was reduced by 10-fold and was associated with a 3-fold reduction in marrow-derived osteoblast progenitors. Both osteoclast and osteoblast numbers were lower in Lamin A null mice, but the reduction in osteoblasts was greater.
Progeria was also seen in mice with collagen-expressing cells that lack the mitochondrial form of superoxide dismutase, which plays a major role in cellular defense against oxidative stress. The median life span of these mice, which have a mixed C57BL/6/Sv129 background, is 444 days—about half that of most laboratory strains. Besides skin atrophy and increased oxidative damage in connective tissues, similar to that seen in aging mice, they have low BMD at 5 months of age (72). The cellular basis of the low BMD remains unknown.
Specific genetic manipulations were used to generate the above mouse models of early senescence. In contrast, the senescence-prone (SAMP) and senescence-resistant (SAMR) mouse strains were derived from AKR/J inbred mice in the 1970s (73). Inbred mice are genetically identical, raising the question of how such new phenotypes arose. Subsequent genetic evidence established that an accidental outcross with another strain had occurred (74). Thus, subsequent brother–sister mating of senescence-prone and senescence-resistant progeny led to the generation of the SAMP and SAMR strains. Early occurrence of tibial fractures heralded the osteopenic phenotype of the SAMP6 strain. These mice exhibit a reduction in the thickness and strength of the femoral diaphysis that was manifest at 3–4 months of age compared with the SAMR1 control strain (75). Cancellous bone of the femur and vertebrae was also reduced. SAMP6 mice also suffer an age-dependent decline in spinal and hind-limb BMD as determined at 15–16 months of age, but this does not occur in the SAMR1 control strain (76). The low trabecular bone mass was associated with reduced bone formation rate, reduced wall thickness, and reduced osteoblast number (76,77). The number of mesenchymal stem cells in the femur was reduced, as determined by the number of colony-forming cells capable of generating osteoblastic cells (76). Moreover, SAMP6 mesenchymal stem cells tended to differentiate into adipocytes, and these mice exhibited increased marrow fat (78), analogous to the situation in aging humans.
Most of the models of progeria do not exhibit bone loss but rather fail to achieve normal peak bone mass. Because the congenital defect has obviously interfered with normal skeletal development, one might question whether the information gained from these mouse models of premature aging has relevance to mechanisms of age-related bone loss. However, the number of mesenchymal stem cells, as well as their ability to differentiate into osteoblasts, is compromised in each of these models, similar to the situation in both mice and humans during aging (79–81). The same population of stem cells probably serves as a reservoir of osteoblast progenitors needed for the growth of the skeleton and its maintenance by bone remodeling during adulthood. If so, these models of early aging may indeed help identify mechanisms that compromise mesenchymal stem cell function with advancing age.
Closely related inbred strains, like SAMP6 and SAMR1, that display differences in bone mass are ideal for mapping genes that contribute to the phenotypic difference. Multiple loci have been identified using these mice (82–86). The existence of many BMD-determining loci reflects the complex nature of skeletal physiology, similar to that in humans. At last count, genome-wide association studies in humans had identified 62 loci (87). All loci had small effect sizes, some with known roles in bone metabolism and others with no previous association. Because of the small effect size, large-scale efforts combining high throughput sequencing and bioinformatics may be needed to harness the advantages of mice for identification of genes that contribute to age-related bone loss.
Atherosclerosis and Age-Related Bone Loss
Age-related bone loss in humans often occurs in the context of other diseases of aging, including sarcopenia, insulin resistance, Alzheimer’s disease, and atherosclerosis. Extensive epidemiological evidence links osteoporosis and atherosclerosis in humans (88–91), raising the possibility that these diseases share common pathogenetic factors. Atherosclerosis does not occur with advancing age in mice because the rapid clearance of low-density lipoprotein in the murine liver keeps total serum cholesterol levels very low. To overcome this limitation, mouse models of atherosclerosis have been developed by deleting ApoE or the low-density lipoprotein receptor. The ApoE protein is a constituent of plasma lipoproteins and serves as a ligand for the cell-surface lipoprotein receptor, thereby promoting the uptake of low-density lipoprotein. When mice lacking ApoE or the low-density lipoprotein receptor are fed a high fat diet, the level of low-density lipoprotein rises substantially because it cannot be cleared from the circulation. This leads to the development of hypercholesterolemia, atherosclerosis, and vascular calcification (92). Thus, even though some aspects of murine and human lipid metabolism are different, it is possible to generate a relevant mouse model by manipulating its genome.
Examination of bone from low-density lipoprotein receptor null mice aged 4–5 months (93), as well as the ApoE mice (R. L. Jilka, unpublished data), fed a high fat diet for 2–3 months revealed loss of femoral cancellous bone, decreased cortical thickness, and increased cortical porosity, recapitulating the osteoporotic features of aged mice. Numerous studies in mice and humans have shown that lipid oxidation plays a pathological role in atherosclerosis (94,95). Work with the atherosclerosis-prone mouse models indicates that lipid oxidation is mediated at least in part by 15-lipoxygenase (Alox15) (96,97). Moreover, our recent studies have demonstrated increased Alox15 expression and levels of oxidized lipids, in skeletal tissue of aged mice, leading to diminished pro-osteogenic Wnt signaling (60). Thus, studies of skeletal homeostasis in mouse models of age-related disease may uncover pathways that link osteoporosis with other diseases of aging.
Summary and Future Directions
Research conducted with mouse models sometimes gives results that challenge conventional wisdom regarding normal and pathological bone remodeling. When faced with such contradictions, it is often pointed out that, after all, mice are not humans. To distinguish clinically relevant findings from mouse-specific findings requires full appreciation of the differences in the physiology of the human and murine skeletons (Table 1) as well as differences among various mouse strains. As summarized in this review, evidence obtained to date indicates that the characteristics and timing of age-related bone loss in mice are quite similar to that of humans. Moreover, there is no evidence to suggest that slow continued growth of the murine skeleton affects the characteristics of age-related bone loss. Indeed, most bone loss in C57BL/6 mice occurs when such growth can no longer be detected, despite the failure of the growth plate to close. Although mice lack osteonal cortical remodeling, they clearly exhibit an age-related increase in cortical porosity that may be informative for the situation in humans. Thus, studies using mice should be able to identify at least some of the factors that contribute to age-related bone loss in humans.
Table 1.
Comparison Between Human and Murine Skeletal Physiology
| Parameter | Humans | Mice |
|---|---|---|
| Longitudinal bone growth | Ceases at sexual maturity | Slow growth after sexual maturity |
| Cancellous bone turnover | ~0.1% per day (iliac crest) | ~0.7% per day (distal femur) |
| Life span of bone multicellular unit | 6–9 mo | ~2 wk |
| Osteonal bone remodeling | Present | Absent |
| Age-related cancellous bone loss | Begins between 20 and 30 y of age | Begins at ~3 mo of age |
| Pathophysiology of cancellous bone loss | Decline in wall width, indicating inadequate osteoblasts | Decline in wall width, indicating inadequate osteoblasts |
| Age-related cortical bone loss | Increased porosity leading to trabecularization and cortical thinning | Increased porosity, cortical thinning |
| Pathophysiology of cortical bone loss | Enlargement and coalescence of osteonal canals | Development of intracortical remodeling associated with blood vessels |
Funding
The author is the recipient of a Research Career Scientist award from the Department of Veterans Affairs (VA), and he is supported by the Biomedical Laboratory Research and Development Service of the VA Office of Research and Development (I01 BX000514), the National Institutes of Health (P01 AG13918), the University of Arkansas for Medical Sciences Translational Research Institute (UL1 RR029884), and Tobacco Settlement funds.
Acknowledgments
The author is indebted to Charles A. O’Brien, Maria Almeida, Robert S. Weinstein, and Stavros C. Manolagas for helpful discussions.
References
- 1. Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991; 15: 175–191 [DOI] [PubMed] [Google Scholar]
- 2. Jerome CP. Primate models of osteoporosis. Lab Anim Sci. 1998; 48: 618–622 [PubMed] [Google Scholar]
- 3. Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010; 31: 266–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khosla S. Pathogenesis of Age-Related Bone Loss in Humans J Gerontol A Biol Sci Med Sci. 2012.10.1093/gerona/gls163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zebaze RM, Ghasem-Zadeh A, Bohte A, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010; 375: 1729–1736 [DOI] [PubMed] [Google Scholar]
- 6. Nicks KM, Amin S, Atkinson EJ, Riggs BL, Melton LJ, 3rd, Khosla S. Relationship of age to bone microstructure independent of areal bone mineral density. J Bone Miner Res. 2012; 27: 637–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colman RJ, Lane MA, Binkley N, Wegner FH, Kemnitz JW. Skeletal effects of aging in male rhesus monkeys. Bone. 1999; 24: 17–23 [DOI] [PubMed] [Google Scholar]
- 8. Duque G, Rivas D, Li W, et al. Age-related bone loss in the LOU/c rat model of healthy ageing. Exp Gerontol. 2009; 44: 183–189 [DOI] [PubMed] [Google Scholar]
- 9. Halloran BP, Ferguson VL, Simske SJ, Burghardt A, Venton LL, Majumdar S. Changes in bone structure and mass with advancing age in the male C57BL/6J mouse. J Bone Miner Res. 2002; 17: 1044–1050 [DOI] [PubMed] [Google Scholar]
- 10. Ferguson VL, Ayers RA, Bateman TA, Simske SJ. Bone development and age-related bone loss in male C57BL/6J mice. Bone. 2003; 33: 387–398 [DOI] [PubMed] [Google Scholar]
- 11. Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res. 2007; 22: 1197–1207 [DOI] [PubMed] [Google Scholar]
- 12. Almeida M, Han L, Martin-Millan M, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007; 282: 27285–27297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011; 333: 1109–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011; 192: 547–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee HY, Choi CS, Birkenfeld AL, et al. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab. 2010; 12: 668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Storz P. Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid Redox Signal. 2011; 14: 593–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pérez VI, Bokov A, Van Remmen H, et al. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009; 1790: 1005–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000; 21: 115–137 [DOI] [PubMed] [Google Scholar]
- 19. Seeman E, Delmas PD. Bone quality–the material and structural basis of bone strength and fragility. N Engl J Med. 2006; 354: 2250–2261 [DOI] [PubMed] [Google Scholar]
- 20. Tang Y, Wu X, Lei W, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009; 15: 757–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jilka RL, Weinstein RS, Parfitt AM, Manolagas SC. Quantifying osteoblast and osteocyte apoptosis: challenges and rewards. J Bone Miner Res. 2007; 22: 1492–1501 [DOI] [PubMed] [Google Scholar]
- 22. Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011; 26: 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakashima T, Hayashi M, Fukunaga T, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011; 17: 1231–1234 [DOI] [PubMed] [Google Scholar]
- 24. Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011; 17: 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kennedy OD, Herman BC, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone. 2012; 50: 1115–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Almeida M, O’Brien CA. Basic biology of skeletal aging J Gerontol A Biol Sci Med Sci. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chia R, Achilli F, Festing MF, Fisher EM. The origins and uses of mouse outbred stocks. Nat Genet. 2005; 37: 1181–1186 [DOI] [PubMed] [Google Scholar]
- 28. Beck JA, Lloyd S, Hafezparast M, et al. Genealogies of mouse inbred strains. Nat Genet. 2000; 24: 23–25 [DOI] [PubMed] [Google Scholar]
- 29. Maddatu TP, Grubb SC, Bult CJ, Bogue MA. Mouse Phenome Database (MPD). Nucleic Acids Res. 2012; 40(Database issue):D887–D894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miller RA, Austad S, Burke D, et al. Exotic mice as models for aging research: polemic and prospectus. Neurobiol Aging. 1999; 20: 217–231 [DOI] [PubMed] [Google Scholar]
- 31. Festing MF. Warning: the use of heterogeneous mice may seriously damage your research. Neurobiol Aging. 1999; 20: 237–44; discussion 245. [DOI] [PubMed] [Google Scholar]
- 32. Miller RA, Harper JM, Galecki A, Burke DT. Big mice die young: early life body weight predicts longevity in genetically heterogeneous mice. Aging Cell. 2002; 1: 22–29 [DOI] [PubMed] [Google Scholar]
- 33. Yuan R, Tsaih SW, Petkova SB, et al. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell. 2009; 8: 277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013; 110: 3507–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferron M, Wei J, Yoshizawa T, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010; 142: 296–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwartz AV Schafer AL Grey A et al. Effects of antiresorptive therapies on glucose metabolism: results from the FIT, HORIZON-PFT and FREEDOM trials J Bone Miner Res. 2013. 10.1002/jbmr.1865. [DOI] [PubMed] [Google Scholar]
- 37. Ackert-Bicknell C, Beamer WG, Rosen CJ, Sundberg JP. Aging Study: Bone Mineral Density and Body Composition of 32 Inbred Strains of Mice. MPD;Ackert1. Mouse Phenome Database Web Site. Bar Harbor, ME: The Jackson Laboratory; http://phenome.jax.org [Google Scholar]
- 38. Parfitt AM. Misconceptions (1): epiphyseal fusion causes cessation of growth. Bone. 2002; 30: 337–339 [DOI] [PubMed] [Google Scholar]
- 39. Bonkowski MS, Pamenter RW, Rocha JS, Masternak MM, Panici JA, Bartke A. Long-lived growth hormone receptor knockout mice show a delay in age-related changes of body composition and bone characteristics. J Gerontol A Biol Sci Med Sci. 2006; 61: 562–567 [DOI] [PubMed] [Google Scholar]
- 40. Miller RA, Kreider J, Galecki A, Goldstein SA. Preservation of femoral bone thickness in middle age predicts survival in genetically heterogeneous mice. Aging Cell. 2011; 10: 383–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. He J, Rosen CJ, Adams DJ, Kream BE. Postnatal growth and bone mass in mice with IGF-I haploinsufficiency. Bone. 2006; 38: 826–835 [DOI] [PubMed] [Google Scholar]
- 42. Courtland HW Kennedy OD Wu Y et al. Low levels of plasma IGF-1 inhibit intracortical bone remodeling during aging. Age (Dordr). 2012.10.1007/s11357-012-9469-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jilka RL, O’Brien CA, Bartell SM, Weinstein RS, Manolagas SC. Continuous elevation of PTH increases the number of osteoblasts via both osteoclast-dependent and -independent mechanisms. J Bone Miner Res. 2010; 25: 2427–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998; 102: 274–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jilka RL, O’Brien CA, Ali AA, Roberson PK, Weinstein RS, Manolagas SC. Intermittent PTH stimulates periosteal bone formation by actions on post-mitotic preosteoblasts. Bone. 2009; 44: 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parfitt AM. Misconceptions (2): turnover is always higher in cancellous than in cortical bone. Bone. 2002; 30: 807–809 [DOI] [PubMed] [Google Scholar]
- 47. Parfitt AM, Han ZH, Palnitkar S, Rao DS, Shih MS, Nelson D. Effects of ethnicity and age or menopause on osteoblast function, bone mineralization, and osteoid accumulation in iliac bone. J Bone Miner Res. 1997; 12: 1864–1873 [DOI] [PubMed] [Google Scholar]
- 48. Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. Effects of size and temperature on metabolic rate. Science. 2001; 293: 2248–2251 [DOI] [PubMed] [Google Scholar]
- 49. Parfitt AM. Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem. 1994; 55: 273–286 [DOI] [PubMed] [Google Scholar]
- 50. Schneider P, Krucker T, Meyer E, et al. Simultaneous 3D visualization and quantification of murine bone and bone vasculature using micro-computed tomography and vascular replica. Microsc Res Tech. 2009; 72: 690–701 [DOI] [PubMed] [Google Scholar]
- 51. Hui SL, Slemenda CW, Johnston CC., Jr Age and bone mass as predictors of fracture in a prospective study. J Clin Invest. 1988; 81: 1804–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nelson JF, Felicio LS, Osterburg HH, Finch CE. Differential contributions of ovarian and extraovarian factors to age-related reductions in plasma estradiol and progesterone during the estrous cycle of C57BL/6J mice. Endocrinology. 1992; 130: 805–810 [DOI] [PubMed] [Google Scholar]
- 53. Mobbs CV, Cheyney D, Sinha YN, Finch CE. Age-correlated and ovary-dependent changes in relationships between plasma estradiol and luteinizing hormone, prolactin, and growth hormone in female C57BL/6J mice. Endocrinology. 1985; 116: 813–820 [DOI] [PubMed] [Google Scholar]
- 54. Finch CE, Jonec V, Wisner JR, Jr, Sinha YN, de Vellis JS, Swerdloff RS. Hormone production by the pituitary and testes of male C57BL/6J mice during aging. Endocrinology. 1977; 101: 1310–1317 [DOI] [PubMed] [Google Scholar]
- 55. Recker R, Lappe J, Davies KM, Heaney R. Bone remodeling increases substantially in the years after menopause and remains increased in older osteoporosis patients. J Bone Miner Res. 2004; 19: 1628–1633 [DOI] [PubMed] [Google Scholar]
- 56. Seeman E. Pathogenesis of bone fragility in women and men. Lancet. 2002; 359: 1841–1850 [DOI] [PubMed] [Google Scholar]
- 57. Parfitt AM. Bone-forming cells in clinical conditions. In: Hall BK, ed. Bone: A Treatise. Volume 1. The Osteoblast and Osteocyte. Boca Raton, FL: Telford Press; and CRC Press; 1990; 351–429 [Google Scholar]
- 58. Ambrogini E, Almeida M, Martin-Millan M, et al. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab. 2010; 11: 136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Weinstein RS, Wan C, Liu Q, et al. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell. 2010; 9: 147–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Almeida M, Ambrogini E, Han L, Manolagas SC, Jilka RL. Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic Wnt signaling in the skeleton. J Biol Chem. 2009; 284: 27438–27448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Han ZH, Palnitkar S, Rao DS, Nelson D, Parfitt AM. Effect of ethnicity and age or menopause on the structure and geometry of iliac bone. J Bone Miner Res. 1996; 11: 1967–1975 [DOI] [PubMed] [Google Scholar]
- 62. Weiss A, Arbell I, Steinhagen-Thiessen E, Silbermann M. Structural changes in aging bone: osteopenia in the proximal femurs of female mice. Bone. 1991; 12: 165–172 [DOI] [PubMed] [Google Scholar]
- 63. Silbermann M, Weiss A, Reznick AZ, Eilam Y, Szydel N, Gershon D. Age-related trend for osteopenia in femurs of female C57BL/6 mice. Compr Gerontol A. 1987; 1: 45–51 [PubMed] [Google Scholar]
- 64. Raum K, Leguerney I, Chandelier F, et al. Bone microstructure and elastic tissue properties are reflected in QUS axial transmission measurements. Ultrasound Med Biol. 2005; 31: 1225–1235 [DOI] [PubMed] [Google Scholar]
- 65. Jilka R DeLoose A Climer L et al. Dysfunctional osteocytes increase RANKL and promote cortical pore formation in their vicinity: a mechanistic explanation for the development of cortical porosity with age J Bone Miner Res. 2012; 27: S348 [Google Scholar]
- 66. Mohaghegh P, Hickson ID. Premature aging in RecQ helicase-deficient human syndromes. Int J Biochem Cell Biol. 2002; 34: 1496–1501 [DOI] [PubMed] [Google Scholar]
- 67. Burtner CR, Kennedy BK. Progeria syndromes and ageing: what is the connection? Nat Rev Mol Cell Biol. 2010; 11: 567–578 [DOI] [PubMed] [Google Scholar]
- 68. Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006; 312: 1059–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Du X, Shen J, Kugan N, et al. Telomere shortening exposes functions for the mouse Werner and Bloom syndrome genes. Mol Cell Biol. 2004; 24: 8437–8446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pignolo RJ, Suda RK, McMillan EA, et al. Defects in telomere maintenance molecules impair osteoblast differentiation and promote osteoporosis. Aging Cell. 2008; 7: 23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li W, Yeo LS, Vidal C, et al. Decreased bone formation and osteopenia in lamin a/c-deficient mice. PLoS One. 2011; 6: e19313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Treiber N, Maity P, Singh K, et al. Accelerated aging phenotype in mice with conditional deficiency for mitochondrial superoxide dismutase in the connective tissue. Aging Cell. 2011; 10: 239–254 [DOI] [PubMed] [Google Scholar]
- 73. Takeda T, Matsushita T, Kurozumi M, Takemura K, Higuchi K, Hosokawa M. Pathobiology of the senescence-accelerated mouse (SAM). Exp Gerontol. 1997; 32: 117–127 [DOI] [PubMed] [Google Scholar]
- 74. Xia C, Higuchi K, Shimizu M, et al. Genetic typing of the senescence-accelerated mouse (SAM) strains with microsatellite markers. Mamm Genome. 1999; 10: 235–238 [DOI] [PubMed] [Google Scholar]
- 75. Silva MJ, Brodt MD, Ettner SL. Long bones from the senescence accelerated mouse SAMP6 have increased size but reduced whole-bone strength and resistance to fracture. J Bone Miner Res. 2002; 17: 1597–1603 [DOI] [PubMed] [Google Scholar]
- 76. Jilka RL, Weinstein RS, Takahashi K, Parfitt AM, Manolagas SC. Linkage of decreased bone mass with impaired osteoblastogenesis in a murine model of accelerated senescence. J Clin Invest. 1996; 97: 1732–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. The effects of androgen deficiency on murine bone remodeling and bone mineral density are mediated via cells of the osteoblastic lineage. Endocrinology. 1997; 138: 4013–4021 [DOI] [PubMed] [Google Scholar]
- 78. Kajkenova O, Lecka-Czernik B, Gubrij I, et al. Increased adipogenesis and myelopoiesis in the bone marrow of SAMP6, a murine model of defective osteoblastogenesis and low turnover osteopenia. J Bone Miner Res. 1997; 12: 1772–1779 [DOI] [PubMed] [Google Scholar]
- 79. Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004; 3: 379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Stolzing A, Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell. 2006; 5: 213–224 [DOI] [PubMed] [Google Scholar]
- 81. Zhou S, Greenberger JS, Epperly MW, et al. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008; 7: 335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shimizu M, Higuchi K, Bennett B, et al. Identification of peak bone mass QTL in a spontaneously osteoporotic mouse strain. Mamm Genome. 1999; 10: 81–87 [DOI] [PubMed] [Google Scholar]
- 83. Benes H, Weinstein RS, Zheng W, et al. Chromosomal mapping of osteopenia-associated quantitative trait loci using closely related mouse strains. J Bone Miner Res. 2000; 15: 626–633 [DOI] [PubMed] [Google Scholar]
- 84. Shimizu M, Higuchi K, Kasai S, et al. Chromosome 13 locus, Pbd2, regulates bone density in mice. J Bone Miner Res. 2001; 16: 1972–1982 [DOI] [PubMed] [Google Scholar]
- 85. Nakanishi R, Shimizu M, Mori M, et al. Secreted frizzled-related protein 4 is a negative regulator of peak BMD in SAMP6 mice. J Bone Miner Res. 2006; 21: 1713–1721 [DOI] [PubMed] [Google Scholar]
- 86. Szumska D, Benes H, Kang P, et al. A novel locus on the X chromosome regulates post-maturity bone density changes in mice. Bone. 2007; 40: 758–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Richards JB, Zheng HF, Spector TD. Genetics of osteoporosis from genome-wide association studies: advances and challenges. Nat Rev Genet. 2012; 13: 576–588 [DOI] [PubMed] [Google Scholar]
- 88. Farhat GN, Cauley JA, Matthews KA, et al. Volumetric BMD and vascular calcification in middle-aged women: the Study of Women’s Health Across the Nation. J Bone Miner Res. 2006; 21: 1839–1846 [DOI] [PubMed] [Google Scholar]
- 89. Hak AE, Pols HA, van Hemert AM, Hofman A, Witteman JC. Progression of aortic calcification is associated with metacarpal bone loss during menopause: a population-based longitudinal study. Arterioscler Thromb Vasc Biol. 2000; 20: 1926–1931 [DOI] [PubMed] [Google Scholar]
- 90. Vogt MT, Wolfson SK, Kuller LH. Lower extremity arterial disease and the aging process: a review. J Clin Epidemiol. 1992; 45: 529–542 [DOI] [PubMed] [Google Scholar]
- 91. Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004; 89: 4246–4253 [DOI] [PubMed] [Google Scholar]
- 92. Shao JS, Cheng SL, Sadhu J, Towler DA. Inflammation and the osteogenic regulation of vascular calcification: a review and perspective. Hypertension. 2010; 55: 579–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pirih F, Lu J, Ye F, et al. Adverse effects of hyperlipidemia on bone regeneration and strength. J Bone Miner Res. 2012; 27: 309–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Berliner JA, Watson AD. A role for oxidized phospholipids in atherosclerosis. N Engl J Med. 2005; 353: 9–11 [DOI] [PubMed] [Google Scholar]
- 95. Steinberg D. The LDL modification hypothesis of atherogenesis: an update. J Lipid Res. 2009; 50(suppl):S376–S381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. George J, Afek A, Shaish A, et al. 12/15-Lipoxygenase gene disruption attenuates atherogenesis in LDL receptor-deficient mice. Circulation. 2001; 104: 1646–1650 [DOI] [PubMed] [Google Scholar]
- 97. Cyrus T, Witztum JL, Rader DJ, et al. Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E-deficient mice. J Clin Invest. 1999; 103: 1597–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]