Abstract
Stabilization of the hypoxia-inducible factor (HIF-1) protein extends longevity in Caenorhabditis elegans. However, stabilization of mammalian HIF-1α has been implicated in tumor growth and cancer development. Consequently, for the hypoxic response to benefit mammalian health, we must determine the components of the response that contribute to longevity, and separate them from those that cause harm in mammals. Here, we subject adult worms to low oxygen environments. We find that growth in hypoxia increases longevity in wild-type worms but not in animals lacking HIF-1 or DAF-16. Conversely, hypoxia shortens life span in combination with overexpression of the antioxidant stress response protein SKN-1. When combined with mutations in other longevity pathways or dietary restriction, hypoxia extends life span but to varying extents. Collectively, our results show that hypoxia modulates longevity in a complex manner, likely involving components in addition to HIF-1.
Key Words: Hypoxia, Longevity, HIF-1, DAF-16, Caenorhabditis elegans.
ADAPTING to stressful environments is important for the survival of cells and organisms. Through evolution and adaptation, organisms have developed sophisticated ways to respond to and survive during times of stress, and sometimes activating such responses can confer a longevity benefit (1). In Caenorhabditis elegans, both acute and chronic stress can extend healthspan and life span via several distinct pathways (2,3). Many stress response pathways present in C. elegans also exist in humans. By identifying mechanisms involved in stress-associated longevity, it may be possible to increase longevity without experiencing the stress itself.
The response to low oxygen (hypoxia) plays a major role in acute injury (eg, ischemia and reperfusion), tumor formation, and growth and has recently been implicated in aging (4–8). Previous work indicates that subjecting C. elegans to low oxygen conditions significantly increases worm life span (9,10). The life-span increase is observed when worms are exposed to hypoxia for life starting from a late larval stage or for just the first day of adulthood (10). The low oxygen environment activates the hypoxic response pathway, mediated by the hypoxia-inducible factor (HIF-1). Previous data also show genetic stabilization of the C. elegans HIF-1 protein (mammalian HIF-1α) under normoxic conditions is sufficient to extend life span (10–12). However, many aspects of this pathway remain unclear, including the mechanism of increased longevity, the difference between genetic and environmental manipulations of HIF-1, and the targets of HIF-1 that are relevant to aging.
The mechanism of HIF-1 stabilization under low oxygen has been extensively studied and appears to be highly conserved. When oxygen is abundant, HIF-1 is efficiently hydroxylated by the prolyl hydroxylase EGL-9 (ortholog to human PDH proteins). The hydroxylation allows HIF-1 to be recognized and ubiquitinated by the E3 ligase von Hippel–Lindau 1 (VHL-1) protein, leading to subsequent proteasomal degradation of HIF-1 (13). Hypoxia inhibits HIF-1 prolyl hydroxylation, leading to stabilization of the transcription factor and activation of its target genes. In mammals, this includes vascular endothelial growth factor, which activates a tyrosine kinase pathway, ultimately leading to angiogenesis (14–16). In humans, genetic activation of the hypoxic pathway has adverse effects. VHL disease is a recessive condition caused by deleterious mutations in the VHL-1 tumor suppressor that results in increased levels of HIF-1, giving rise to hemangioblastomas, typically found in the cerebellum, spinal cord, kidneys, and retina (17). However, in C. elegans, which lacks a circulatory system (and therefore, vascular endothelial growth factor), and, excluding the germ line, is postmitotic in adulthood, stabilization of HIF-1 does not appear to negatively affect the health of the worm and leads to a significant longevity benefit (10). Worms that have mutated vhl-1 have a 30%–50% increase of life span, depending on temperature, and have increased healthspan as measured by autofluorescence accumulation, vulval integrity, and protein homeostasis (18). Activation of the hypoxic pathway appears to extend life span and healthspan by a mechanism distinct from dietary restriction (DR) and insulin-like signaling (10).
Although it was unexpected that genetic stabilization of HIF-1 by vhl-1 mutation increased worm longevity, it was also surprising that subjecting adult worms to low levels of oxygen increased longevity without obvious health defects, except for decreased fecundity. However, the increase in longevity from hypoxia was reported to be considerably less than from vhl-1 mutation (10), and previous microarray studies suggest that hypoxia and vhl-1 mutation have overlapping but distinct transcriptional profiles (19). These data agree with the accepted mechanism of HIF-1 regulation because hypoxia prevents prolyl hydroxylation of HIF-1, whereas vhl-1 mutation only prevents proteosomal degradation. Thus, HIF-1 activation in hypoxia may more closely mirror egl-9 mutation rather than vhl-1 mutation. Interestingly, although RNAi knockdown of egl-9 can extend life span (10), some mutations in nematode egl-9 have no significant longevity effect (20).
In order to better understand the mechanisms of hypoxia-induced longevity, we explored the interaction between hypoxia and known pathways relevant to aging in C. elegans, many of which also affect resistance to diverse forms of stress. One pathway in C. elegans with the ability to respond to a wide variety of stresses is the insulin/IGF-1-like signaling (IIS) pathway, which was the first genetic pathway identified to influence longevity (21). Signaling through the insulin-like receptor DAF-2 acts through the worm phosphoinositide 3-kinase ortholog, AGE-1, to negatively regulate the activity of the forkhead transcription factor, DAF-16 (homolog to mammalian FOXO), resulting in its nuclear exclusion. DAF-16 is activated in response to multiple types of stress, and activating DAF-16 robustly increases the life span of C. elegans (22,23). Worms with a mutation to age 1 have increased activity of DAF-16, and their longevity is extended in 1% oxygen (9). We and others have shown that stabilization of HIF-1 extends life span independently of DAF-16, consistent with the model that HIF-1 promotes longevity by a mechanism distinct from IIS (7).
DR, defined as a reduction in nutrient availability without malnutrition, has been the most studied environmental intervention in aging research over the last 75 years (24). DR increases life span in many organisms, including yeast, C. elegans, mice, rats, and Rhesus monkeys (25,26). Although the mechanisms behind life-span extension by DR in C. elegans are not totally clear, there is evidence that DR acts through multiple longevity factors, including the mechanistic target of rapamycin pathway, AMP-activated protein kinase (AMPK), and the transcription factors HSF-1 and PHA-4 (27). Life-span extension from DR is distinct from stabilization of HIF-1, and HIF-1 is not required for DR to increase longevity (10).
In addition to IIS and DR, there are several factors that have been linked to cellular stress resistance or longevity that might be expected to interact with HIF-1. Among these are SKN-1, SIR-2.1, AAK-2, and CEP-1. The transcription factor, SKN-1 is an important developmental gene (28) that functions in adults to respond to oxidative stress, and overexpression of SKN-1 increases life span (28–30). Sirtuins are NAD-dependent protein deacetylases that promote longevity in Saccharomyces cerevisiae (31) and have been linked to multiple age-related diseases in mice (32). Overexpression of the worm sirtuin SIR-2.1 is reported to extend life span in C. elegans (33), although a recent report failed to replicate this result (34). Recent studies have linked sirtuins to HIF in mammals, showing that SIRT1 can deacetylate both HIF-1α and HIF-2α, and SIRT6 can regulate HIF-1α downstream genes (35–38). AMPK plays a central role maintaining cellular energy homeostasis and has been associated with life-span extension through both IIS and DR. Activating the worm AMPKα subunit, AAK-2, is sufficient to increase life span (39), and AMPK is required for worms to adapt to complete loss of oxygen (anoxia) (40). CEP-1 is the functional ortholog of the p53 tumor suppressor in C. elegans and mediates multiple stress responses. CEP-1 has been reported to act downstream of HIF-1 in protecting germ line cells from ionizing radiation (41). CEP-1 also may play a role in hypoxia-induced lethality and in longevity in response to starvation in worms (42).
In this study, we define the interaction between hypoxia and pathways thought to interact with the hypoxic response or the aging process to influence worm longevity. Collectively, the results confirm that hypoxic treatment increases life span in worms but show that the hypoxic pathway is intertwined with other stress response pathways in the context of aging.
Methods
Strains and Growth Conditions
Standard procedures for C. elegans strain maintenance and handling were used, as described previously (43–46). All experiments were performed on animals fed UV-killed Escherichia coli OP50 from egg. Experimental animals were maintained on solid nematode growth medium with 50 μg/mL ampicillin added. Experiments were performed on animals maintained at 20°C. Nematode strains used in this study are described in Supplementary Table 1.
Life-Span Analysis
Life-span analyses were carried out as described (47). Worms subjected to hypoxia were placed in an airtight chamber (Nalgene, Rochester, NY) filled with N2 gas to keep oxygen levels at 0.5%. This chamber was kept in the same incubator in which normoxia worms were stored. Worms undergoing bacterial deprivation treatment were transferred on Day 4 of adulthood to nematode growth medium without UV-killed OP50. Statistical analysis and replication of life-span experiments are shown in Supplementary Table 2. Ruptured animals were included in life-span experiments. Animals that left the surface of the plate and failed to return during the experiment were censored.
Quantification of DAF-16::GFP Puncta
Fluorescence microscopy was measured using a Zeiss SteREO Lumar.V12 (Thornwood, NY) microscope as previously described (10, 45). Eggs were prepared from gravid DAF-16::GFP adult worms and placed on nematode growth medium with empty vector control or RNAi expressing bacteria. Animals were grown at 20°C for 3 days and then treated with hypoxia or heat shock (37°C) for 2h, before being imaged using a Zeiss SteREO Lumar.V12 microscope as previously described (45). Animals were paralyzed with 25 mM sodium azide and placed on a Teflon printed 8-well glass slide with 6-mm well diameter (Electron Microscopy Sciences; Hatfield, PA). The GFP filter (470/40 excitation band-pass filter and 525/50 emission band-pass filter) was used to image the DAF-16::GFP worms at 150× magnification with exposure time of 40ms for bright field and 500ms for GFP filter. The Image J 1.341.5.0_07 software (48) was employed for inverting the images and converting them to 32-bit format and then manually counting the worms for the presence or absence of distinct GFP nuclear puncta. Worms were scored (+) or (–) for puncta, where (+) is defined as three or more puncta (worms on empty vector RNAi occasionally have a single punctum).
Quantification of DAF-16 Targets
Synchronized populations of N2 worms were generated by bleach prep (adapted from WormBook). At L4 stage, one third of the cohort was moved to hypoxia for 2h, one third to hypoxia for 6h, and one third remained in normoxia. Worms were harvested for mRNA using Trizol reagent (Invitrogen catalog no. 15596026) and TURBO DNA-free Kit treatment (Invitrogen catalog no. AM1907M) after these time points and subsequently used to produce cDNA (Superscript III, Invitrogen catalog no. 18080-0444) for qPCR amplification using a Rotor-Gene SYBR Green PCR Kit (Qiagen catalog no. 204076). Expression levels were normalized to the actin gene, act-3, and then to the normoxic controls. Primers used: act-3 F: CTCTTGCCCCATCAACCATG; act-3 R: CTTGC‑ TTGGAGATCCACATC; CC8.2 F: AAACCCTCCCATCA‑ GCTCTC; CC8.2 R: GCATTTGCCTTCCGAAAGTTTG; sod-3 F: ACACTATTAAGCGCGACTTCG; sod-3 R: AG‑ TTGGCAATCTTCCAAATAGC; mtl-1 F: ATGGCTTGCA‑ AGTGTGACTG; mtl-1 R: GCTTCTGCTCTGCACAATGA; sip-1 F: TTCGAGGACATGATGCCATA; sip-1 R: TGAA‑ CGATCTCTTGCTGAACC
Statistical Analysis
A Wilcoxon rank-sum test (MATLAB “ranksum” function) was used to calculate p values to define statistical significance for life-span assays. The mean life spans, number of animals, number of replicate experiments, and p values are shown in Supplementary Table 2. A two-tailed Student’s t test was done using the T-TEST function in Microsoft Excel to calculate p values for DAF-16::GFP positive worms.
Results
HIF-1 Is Required for Life-Span Extension by Hypoxia
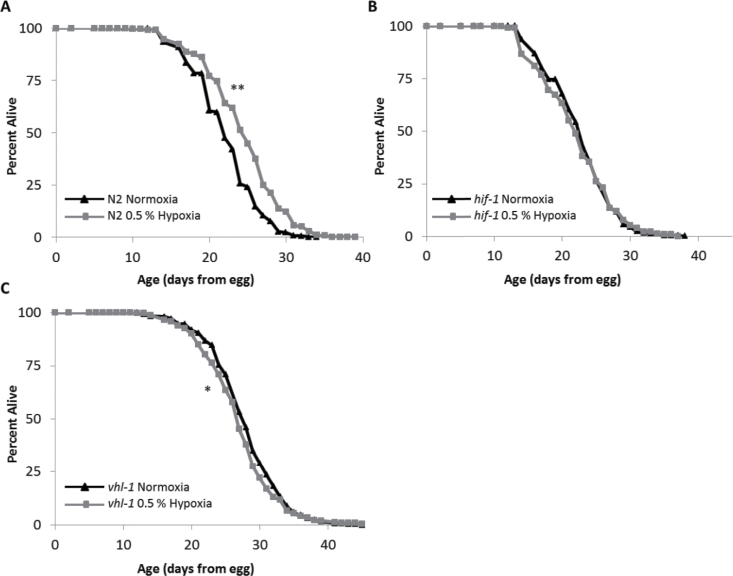
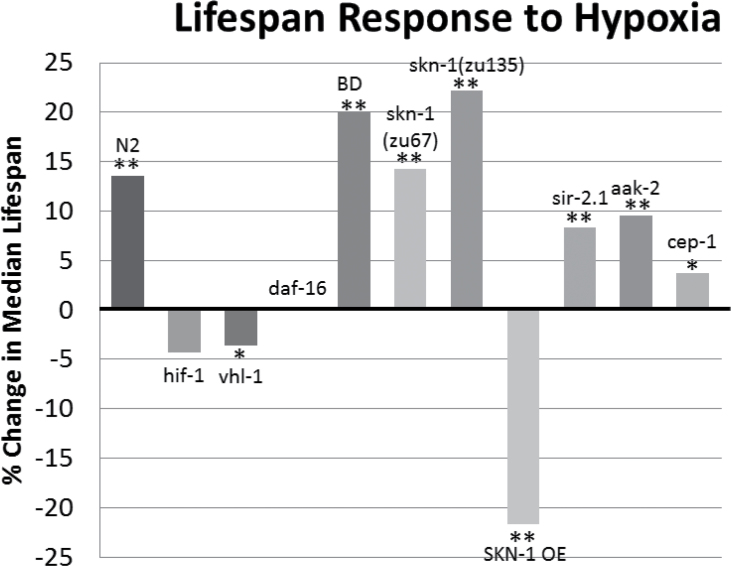
We first explored the role of HIF-1 in hypoxia-induced longevity by measuring life spans of well-characterized C. elegans strains with mutations in the hypoxic response pathway (Figure 1). In our standard laboratory conditions (20°C on solid agar plates containing UV-killed OP50 bacteria), hypoxia (0.5% O2) was introduced to one cohort of worms after development (L4 stage), whereas the other cohort remained in normoxia (21% O2). As expected, N2 (wild-type) worms in hypoxia had a statistically significant life-span increase when compared with control worms in normoxia (Figure 1A). Hif-1(ia4) mutants, which are null for HIF-1 protein (49), did not have a significant change in life span in hypoxia (Figure 1B). This indicates that although HIF-1 is required to extend longevity in hypoxia, hypoxic treatment during adulthood in the absence of HIF-1 does not decrease worm life span. To assess whether hypoxia could further increase the longevity of a strain with stabilized HIF-1, we measured life spans of vhl-1(ok161) mutants in normoxia and hypoxia. In normoxia, the stabilized HIF-1 causes these worms to be long-lived compared with N2 (10,12). However, compared with their normoxic counterparts, hypoxia decreased vhl-1 worm longevity slightly (−6.9%, Figure 1C), suggesting that hypoxic treatment could not further activate HIF-1 in a beneficial manner. The small decrease in longevity may indicate that further activation of HIF-1 by hypoxia in vhl-1 worms may be detrimental, possibly in a similar manner to previous work in egl-9 mutant worms in normoxia (13,20,50).
Figure 1.
Life-span extension by hypoxia requires HIF-1. This figure shows life spans of Caenorhabditis elegans cultured at 20°C on UV-killed OP50 bacteria in both normoxia (21% O2) and hypoxia (0.5% O2) throughout adulthood. (A) N2 (wild-type) worms have increased longevity in hypoxia (median life-span increase of 12.5%, p value 2.3E-4). (B) hif-1 mutant worm life span is unaffected by hypoxia (−4.3% decrease in median life span, p value .18). (C) vhl-1 mutant worm (stabilized HIF-1) life span is decreased by hypoxia (median life-span decrease of −6.9%, p value 1.2E-4). Composite of all trials are shown. Significance: *p < .01, **p < .001.
DAF-16 Is Required for Lifespan Extension in Hypoxia
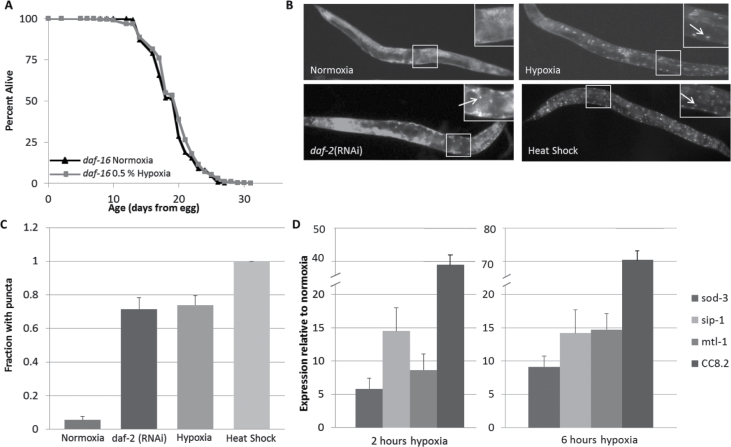
We next studied if hypoxia interacts with the IIS pathway. Using daf-16(mu86) mutant worms lacking functional DAF-16/FOXO protein (23), we found that hypoxia did not increase the longevity of these animals (Figure 2A). Thus, DAF-16 is required for hypoxia-induced longevity. To determine whether DAF-16 is also activated by hypoxia, we observed DAF-16 localization as an indicator of function. Using DAF-16::GFP transgenic worms, we assayed the subcellular localization of the DAF-16::GFP fusion protein. As expected, in normoxia, the worms had diffuse cytoplasmic DAF-16 localization (Figure 2B). However, when exposed to hypoxia, DAF-16 translocated to the nucleus, which indicates activation (Figure 2B and C) (51). Thus, DAF-16 is not only necessary to extend life span by hypoxia but is relocalized to the nucleus by hypoxia.
Figure 2.
DAF-16 is required for life-span extension in hypoxia. (A) daf-16 mutant worm life span is not extended by hypoxia (change in median life span is 0%). Composite of all four trials are shown. (B) Hypoxic conditions cause DAF-16 to localize to the nucleus, approximately to the frequency of daf-2 (RNAi), as visualized by DAF-16::GFP transgenic worms with nuclear puncta when imaged. (C) Quantification of the fraction of animals with nuclear puncta. (D) Quantification of DAF-16 target mRNA levels by qPCR.
We further explored the effect of hypoxia on DAF-16 activity by measuring the expression of previously reported (52–54) DAF-16 targets by quantitative PCR in worms maintained under normoxic and hypoxic conditions (Figure 2D). In agreement with the nuclear localization data, we found that mRNA levels DAF-16 targets including sod-3, sip-1, mtl-1, and CC8.2 were all increased in worms exposed to a low oxygen environment for either 2 or 6 hours. These data support the life span and protein localization findings, and suggest that hypoxia causes DAF-16 to activate at least a subset of its targets.
DR and Hypoxia Do Not Interact to Extend Life Span
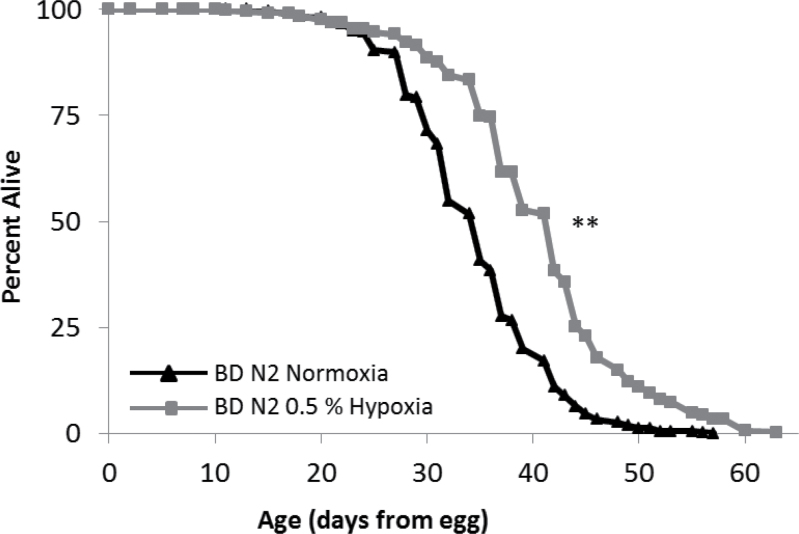
DR is the most reproducible environmental method for increasing longevity across multiple species. There are several published DR protocols in C. elegans (55). We have extensively utilized the bacterial deprivation method of DR, where animals are kept on nematode growth medium plates without bacterial food during adulthood (43–46). We previously reported that deletion of HIF-1 does not prevent life-span extension from bacterial deprivation, suggesting that HIF-1 and DR act in genetically distinct longevity pathways (10). Consistent with this model, hypoxia further extended the life span of dietary-restricted animals that were already long lived relative to control animals (Figure 3).
Figure 3.
Dietary restriction (DR) and hypoxia do not interact to extend life span. Worms under DR (bacterial deprivation [BD] after Day 4 of adulthood) have extended longevity in hypoxia (20.0% median life-span increase, p value 1.5E-31). Composite of all trials are shown. Significance: **p < .001.
SKN-1 Interactions With Hypoxia
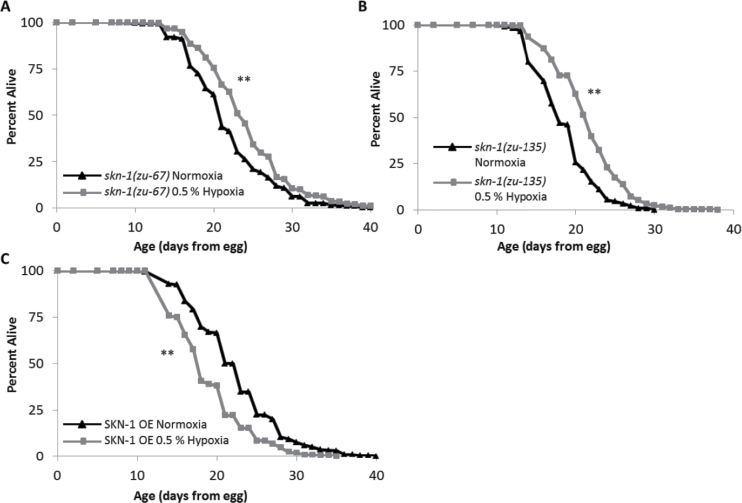
SKN-1 is a transcription factor involved in response to oxidative stress and is the ortholog to mammalian Nrf2. We found that the life spans of loss-of-function alleles skn-1(zu67) and skn-1(zu135) mutants were extended in hypoxia to an extent comparable to or greater than that of N2 (Figure 4). This suggests that SKN-1 is not necessary for hypoxia-induced life-span extension. A previous study reported that worms that overexpress SKN-1 are long lived when compared with controls in normal conditions (30). Surprisingly, we find that worms overexpressing SKN-1 (ls007 skn-1b/c::gfp) have shorter life spans in hypoxia than in normoxia (Figure 4C). The detrimental effect of hypoxia on these worms indicates that these two pathways are not independent, despite SKN-1 not being a required for the longevity effects of hypoxia.
Figure 4.
SKN-1 and the hypoxic response. This figure shows life spans of Caenorhabditis elegans cultured at 20°C on UV-killed OP50 bacteria in both normoxia (21% O2) and hypoxia (0.5% O2) throughout adulthood. (A) In hypoxic conditions, skn-1(zu-67) mutants increase median life span 14.3% and (B) skn-1(zu135) mutant worms increase median life span by 22.2% (p values 9.0E-13 and 5.8E-16, respectively). (C) ls007 (skn-1b/c::gfp) worms (overexpressing SKN-1) have a median life-span decrease of −21.7% (p value 8.0E-6). Composite of all trials are shown. Significance: **p < .001.
Life-Span Extension From Hypoxia Does Not Require SIR-2.1, AAK-2, or CEP-1
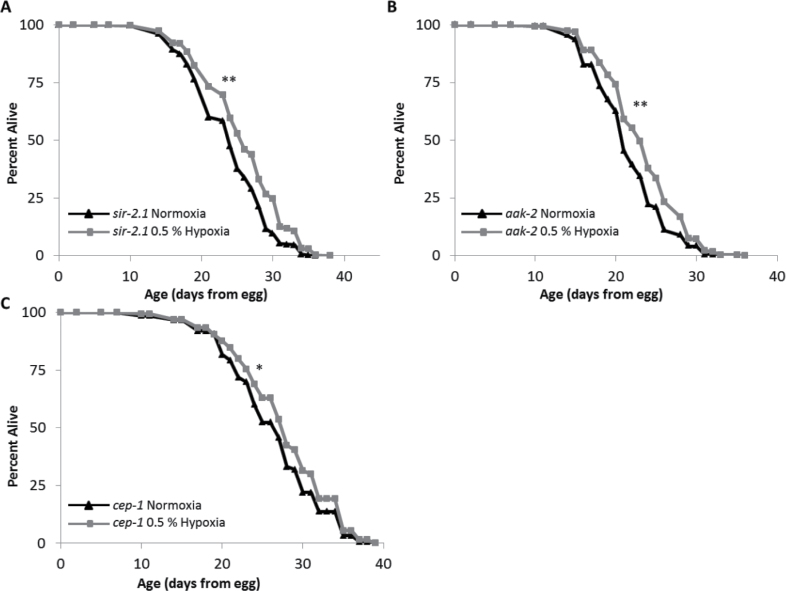
We also tested several additional genetic backgrounds known to influence longevity for interactions with hypoxia-mediated longevity (Figure 5, summarized in Figure 6). Sirtuins are a well-studied group of proteins that have recently been linked to the hypoxic response in mammals (35–38). However, using the null sir-2.1(ok434) worm strain, we found that sir-2.1(ok434) worm life spans were extended by hypoxia, albeit by a slightly lower percentage than control worms (Figure 5A). This likely indicates little or no interaction between hypoxia and SIR-2.1 for worm longevity. Similarly, aak-2, encoding the catalytic alpha subunit of AMPK, is not required for life-span extension by hypoxia (Figure 5B). Aak-2(ok524) mutant worms responded in a manner similar to that of sir-2.1(ok434) worms with increased longevity in hypoxia, but not quite at the same level as the wild type. Thus, the AAK-2 catalytic subunit of AMPK, which acts in response to environmental stresses, insulin-like signaling, and cellular energy status, is not required for life-span extension by hypoxia. Finally, to determine whether hypoxia interacts with the worm p53 ortholog, we tested the effect of hypoxia on cep-1(gk138) mutants. Similar to the case for SIR-2.1 and AAK-2, hypoxia was able to extend the life span of cep-1 mutant animals (Figure 5C).
Figure 5.
Summary of hypoxia’s effect upon median life span. The percentage of change in median life span of worms in hypoxia compared with their normoxic counterparts. Composite of all trials are shown. Significance: *p < .01, **p < .001.
Figure 6.
Genes independent of the HIF-1 pathway. This figure shows life spans of Caenorhabditis elegans cultured at 20°C on UV-killed OP50 bacteria in both normoxia (21% O2) and hypoxia (0.5% O2) throughout adulthood. (A) sir-2.1, (B) aak-2, and (C) cep-1 worms in hypoxia have increased longevity (median life-span increases of 8.3%, p value 9.6E-6; 9.5%, p value 4.6E-8; and 3.7%, p value 5.1E-3, respectively). Composite of all trials are shown. Significance: *p < .01, **p < .001.
Discussion
The hypoxic response pathway is critical for adapting to low oxygen environments and plays an important role in promoting longevity in C. elegans. Here, we have identified HIF-1 as a key factor involved in mediating life-span extension from hypoxia in worms. We have also identified unexpected interactions between hypoxia-induced longevity and two additional transcription factors: DAF-16 and SKN-1. These observations suggest that the longevity effects of hypoxia are interwoven with several longevity pathways. They also suggest that hypoxia extends life span by mechanisms that are overlapping but distinct from stabilization of HIF-1 under normoxic conditions.
The fact that HIF-1 is required for life-span extension by hypoxia is not surprising, given that HIF-1 is known to be stabilized and activated in hypoxia. It is somewhat surprising, however, that hypoxia does not have a detrimental effect on the longevity of hif-1 mutants. This suggests that HIF-1 may be more important during development in hypoxia, at least at 0.5% oxygen, and is largely dispensable for longevity during adulthood. We also observed that vhl-1 mutants, which have constitutively active HIF-1 and are long lived in normoxia (10), do not further benefit from low oxygen conditions. Recall that HIF-1 in vhl-1 mutants is still hydroxylated, and consequently, it has lower transcriptional activity (50). Therefore, hypoxia does not further enhance HIF-1 activity in these animals in a way that increases life span, perhaps suggesting a threshold beyond which further activation of HIF-1 does not equate with additional longevity.
The requirement of DAF-16 for life-span extension from hypoxia was unanticipated. Our results agree with a previous study reporting that some DAF-16 targets are activated by hypoxia and there is evidence that FOXO3a is a HIF-1α target gene in mammalian cells (19,56,57). Also, several daf-2 mutants confer resistance to hypoxia (58), and have been reported to act partially through a DAF-16-dependent mechanism (59). Our results support the model that DAF-16 is not only required for life-span extension from hypoxia but is also activated by hypoxia. Hypoxia is sufficient to cause DAF-16 to relocalize to the nucleus and also induces transcriptional changes in known DAF-16 target genes consistent with activation of DAF-16. Interestingly, stabilization of HIF-1 by mutation of vhl-1 does not cause DAF-16 to localize to the nucleus (18), and life-span extension in vhl-1 mutants is independent of DAF-16 (10–12). This suggests that hypoxia-induced longevity may involve factors other than HIF-1. Thus, it seems unlikely that activation of DAF-16 by hypoxia is solely the result of HIF-1 stabilization. In fact, there is evidence that HIF-1 can act as a negative regulator of DAF-16. RNAi knockdown of hif-1 causes DAF-16 to relocalize to the nucleus, and DAF-16 is required for life-span extension from hif-1 deletion or knockdown at 25°C (18). Together with the data presented here, this may suggest that knockdown of hif-1 under normoxic conditions is sufficient to trigger the “hypoxia signal” that DAF-16 is responding to when worms are subjected to hypoxia. It will be of interest in future studies to determine how DAF-16 is regulated under each of these conditions.
The observation that hypoxia confers an additive life-span increase when combined with DR by bacterial deprivation is consistent with prior data indicating that both HIF-1 and DAF-16 promote longevity by mechanisms that are distinct from bacterial deprivation (10,43). Likewise, the ability of hypoxia to extend life span in animals with mutations in sir-2.1 and aak-2 indicates that the mechanism of life-span extension is at least partially distinct from these factors as well. These data should be considered in light of potential redundancy of function with other sirtuin family members or the aak-2 paralog aak-1. Thus, it is not possible to completely rule out a role for sirtuins or AMPK in the life-span extension under hypoxic conditions.
Our rationale for examining the role of CEP-1 in hypoxia-mediated life-span extension was based on a recent study reporting that HIF-1 expression in neurons antagonizes CEP-1-mediated germ line apoptosis (41). Reduced expression of cep-1 is also reported to extend life span (60). Thus, we considered the possibility that hypoxia might extend life span by inhibition of CEP-1. This does not appear to be the case, however, because hypoxia was still able to increase life span in cep-1 mutant animals.
In contrast to HIF-1 and DAF-16, the SKN-1 transcription factor appears to antagonize the effect of hypoxia on longevity. Animals with reduced SKN-1 function show a robust life-span extension from hypoxia, whereas animals with increased skn-1 expression have their life spans shortened by hypoxia. These data suggest that hypoxia is not acting through SKN-1 to promote longevity, but instead is acting despite SKN-1. One possibility is that SKN-1 impairs the hypoxic response itself; however, this seems unlikely as hif-1 mutants are not short-lived in hypoxia. Alternatively, activating the SKN-1 antioxidant response may create a buffer against reactive oxygen species, and in combination with the decrease in oxygen during hypoxia, this may create a reducing environment that is toxic to worms. It is also possible that overexpressed SKN-1 competes with HIF-1 for binding sites or other cofactors in a way that disrupts the normal response to hypoxia. Regardless, it is interesting that the action of a stress response pathway is able to not only preclude the longevity benefit of hypoxia but to cause a longevity deficit.
The role of hypoxia in mammals has mainly been studied in the context of ischemia and reperfusion injury and tumor cell growth. Because of this, the positive effects of HIF-1 stabilization in mammals are less understood, but there are data that suggest HIF-1 plays a protective role in brain aging (61). It is clear that increased HIF-1 activity in an organism such as C. elegans that is not susceptible to cancer leads to a significant life-span benefit. HIF-1 activation is not always beneficial to C. elegans (as supported by work with egl-9 mutants), however, and it is likely that the prolongevity effects of HIF-1 arise due to the action of specific HIF-1 target genes in a subset of cells. In order to gain insight into potential benefits of HIF-1 activity in mammals, it is necessary to explore the HIF-1 pathway in greater detail, including the downstream genes, tissues, and pathways involved in HIF-1-mediated longevity in C. elegans. By defining conserved longevity pathways and key regulatory factors for these pathways, it may be possible to enhance longevity and healthspan in people.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported by the National Institutes of Health (R01AG031108 and R01AG038518 to M.K.). S.F.L. was supported by National Institutes of Health training (T32AG000057) and by a postdoctoral fellowship from the Ellison Medical Foundation and the American Federation for Aging Research.
Acknowledgments
We thank Dr. Keith Blackwell for providing the SKN-1 overexpressing strain. Some nematode strains used in this study were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR).
References
- 1. Hochachka PW, Somero GN. Biochemical Adaptation: Mechanism and Process in Physiological Evolution. New York: Oxford University Press; 2002. [Google Scholar]
- 2. Lithgow GJ, Walker GA. Stress resistance as a determinate of C. elegans lifespan. Mech Ageing Dev. 2002; 123: 765–771 [DOI] [PubMed] [Google Scholar]
- 3. Cypser JR, Tedesco P, Johnson TE. Hormesis and aging in Caenorhabditis elegans. Exp Gerontol. 2006; 41: 935–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loor G, Schumacker PT. Role of hypoxia-inducible factor in cell survival during myocardial ischemia-reperfusion. Cell Death Differ. 2008; 15: 686–690 [DOI] [PubMed] [Google Scholar]
- 5. Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007; 129: 465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010; 29: 625–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leiser SF, Kaeberlein M. The hypoxia-inducible factor HIF-1 functions as both a positive and negative modulator of aging. Biol Chem. 2010; 391: 1131–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaeberlein M, Kapahi P. The hypoxic response and aging. Cell Cycle. 2009; 8: 2324 [DOI] [PubMed] [Google Scholar]
- 9. Adachi H, Fujiwara Y, Ishii N. Effects of oxygen on protein carbonyl and aging in Caenorhabditis elegans mutants with long (age-1) and short (mev-1) life spans. J Gerontol A Biol Sci Med Sci. 1998; 53: B240–B244 [DOI] [PubMed] [Google Scholar]
- 10. Mehta R, Steinkraus KA, Sutphin GL, et al. Proteasomal regulation of the hypoxic response modulates aging in C. elegans. Science. 2009; 324: 1196–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Y, Shao Z, Zhai Z, Shen C, Powell-Coffman JA. The HIF-1 hypoxia-inducible factor modulates lifespan in C. elegans. PLoS ONE. 2009; 4: e6348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Müller RU, Fabretti F, Zank S, Burst V, Benzing T, Schermer B. The von Hippel Lindau tumor suppressor limits longevity. J Am Soc Nephrol. 2009; 20: 2513–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Epstein AC, Gleadle JM, McNeill LA, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001; 107: 43–54 [DOI] [PubMed] [Google Scholar]
- 14. Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996; 16: 4604–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Terman BI, Dougher-Vermazen M, Carrion ME, et al. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992; 187: 1579–1586 [DOI] [PubMed] [Google Scholar]
- 16. Connolly DT, Heuvelman DM, Nelson R, et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989; 84: 1470–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaelin WG. Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2002; 2: 673–682 [DOI] [PubMed] [Google Scholar]
- 18. Leiser SF, Begun A, Kaeberlein M. HIF-1 modulates longevity and healthspan in a temperature-dependent manner. Aging Cell. 2011; 10: 318–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shen C, Nettleton D, Jiang M, Kim SK, Powell-Coffman JA. Roles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegans. J Biol Chem. 2005; 280: 20580–20588 [DOI] [PubMed] [Google Scholar]
- 20. Chen D, Thomas EL, Kapahi P. HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet. 2009; 5: e1000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993; 366: 461–464 [DOI] [PubMed] [Google Scholar]
- 22. Ogg S, Paradis S, Gottlieb S, et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997; 389: 994–999 [DOI] [PubMed] [Google Scholar]
- 23. Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: an HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997; 278: 1319–1322 [DOI] [PubMed] [Google Scholar]
- 24. Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005; 126: 913–922 [DOI] [PubMed] [Google Scholar]
- 25. Fontana L, Partridge L, Longo VD. Extending healthy life span–from yeast to humans. Science. 2010; 328: 321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kennedy BK, Steffen KK, Kaeberlein M. Ruminations on dietary restriction and aging. Cell Mol Life Sci. 2007; 64: 1323–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kenyon CJ. The genetics of ageing. Nature. 2010; 464: 504–512 [DOI] [PubMed] [Google Scholar]
- 28. Bowerman B, Eaton BA, Priess JR. skn-1, a maternally expressed gene required to specify the fate of ventral blastomeres in the early C. elegans embryo. Cell. 1992; 68: 1061–1075 [DOI] [PubMed] [Google Scholar]
- 29. An JH, Blackwell TK. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003; 17: 1882–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tullet JM, Hertweck M, An JH, et al. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008; 132: 1025–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999; 13: 2570–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009; 460: 587–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001; 410: 227–230 [DOI] [PubMed] [Google Scholar]
- 34. Burnett C, Valentini S, Cabreiro F, et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011; 477: 482–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dioum EM, Chen R, Alexander MS, et al. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009; 324: 1289–1293 [DOI] [PubMed] [Google Scholar]
- 36. Zhong L, D’Urso A, Toiber D, et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 2010; 140: 280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1a. Mol Cell. 2010; 38: : 864–878 [DOI] [PubMed] [Google Scholar]
- 38. Leiser SF, Kaeberlein M. A role for SIRT1 in the hypoxic response. Mol Cell. 2010; 38: 779–780 [DOI] [PubMed] [Google Scholar]
- 39. Apfeld J, O’Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004; 18: 3004–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. LaRue BL, Padilla PA. Environmental and genetic preconditioning for long-term anoxia responses requires AMPK in Caenorhabditis elegans . PLoS ONE. 2011; 6: e16790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sendoel A, Kohler I, Fellmann C, Lowe SW, Hengartner MO. HIF-1 antagonizes p53-mediated apoptosis through a secreted neuronal tyrosinase. Nature. 2010; 465: 577–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Derry WB, Putzke AP, Rothman JH. Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science. 2001; 294: 591–595 [DOI] [PubMed] [Google Scholar]
- 43. Kaeberlein TL, Smith ED, Tsuchiya M, et al. Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell. 2006; 5: 487–494 [DOI] [PubMed] [Google Scholar]
- 44. Smith ED, Kaeberlein TL, Lydum BT, et al. Age- and calorie-independent life span extension from dietary restriction by bacterial deprivation in Caenorhabditis elegans. BMC Dev Biol. 2008; 8: 49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Steinkraus KA, Smith ED, Davis C, et al. Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell. 2008; 7: 394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sutphin GL, Kaeberlein M. Dietary restriction by bacterial deprivation increases life span in wild-derived nematodes. Exp Gerontol. 2008; 43: 130–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sutphin GL, Kaeberlein M. Measuring Caenorhabditis elegans life span on solid media. J Vis Exp. 2009;(27). pii: 1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012; 9: 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jiang H, Guo R, Powell-Coffman JA. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proc Natl Acad Sci USA. 2001; 98: 7916–7921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shao Z, Zhang Y, Powell-Coffman JA. Two distinct roles for EGL-9 in the regulation of HIF-1-mediated gene expression in Caenorhabditis elegans. Genetics. 2009; 183: 821–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol. 2001; 11: 1975–1980 [DOI] [PubMed] [Google Scholar]
- 52. Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999; 13: 1385–1393 [PubMed] [Google Scholar]
- 53. Barsyte D, Lovejoy DA, Lithgow GJ. Longevity and heavy metal resistance in daf-2 and age-1 long-lived mutants of Caenorhabditis elegans. FASEB J. 2001; 15: 627–634 [DOI] [PubMed] [Google Scholar]
- 54. Murphy CT, McCarroll SA, Bargmann CI, et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003; 424: 277–283 [DOI] [PubMed] [Google Scholar]
- 55. Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009; 8: 113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Benita Y, Kikuchi H, Smith AD, Zhang MQ, Chung DC, Xavier RJ. An integrative genomics approach identifies hypoxia inducible factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res. 2009; 37: 4587–4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bakker WJ, Harris IS, Mak TW. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cell. 2007; 28: 941–953 [DOI] [PubMed] [Google Scholar]
- 58. Scott BA, Avidan MS, Crowder CM. Regulation of hypoxic death in C. elegans by the insulin/IGF receptor homolog DAF-2. Science. 2002; 296: 2388–2391 [DOI] [PubMed] [Google Scholar]
- 59. Mabon ME, Scott BA, Crowder CM. Divergent mechanisms controlling hypoxic sensitivity and lifespan by the DAF-2/insulin/IGF-receptor pathway. PLoS ONE. 2009; 4: e7937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Arum O, Johnson TE. Reduced expression of the Caenorhabditis elegans p53 ortholog cep-1 results in increased longevity. J Gerontol A Biol Sci Med Sci. 2007; 62: 951–959 [DOI] [PubMed] [Google Scholar]
- 61. Ogunshola OO, Antoniou X. Contribution of hypoxia to Alzheimer’s disease: is HIF-1alpha a mediator of neurodegeneration? Cell Mol Life Sci. 2009; 66: 3555–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]