Abstract
Autism together with Asperger syndrome and pervasive developmental disorder not otherwise specified form a spectrum of conditions (autism spectrum disorders or ASD) that is characterized by disturbances in social behavior, impaired communication and the presence of stereotyped behaviors or circumscribed interests. Recent estimates indicate a prevalence of ASD of 1 per 150 (Kuehn, 2007). The cause(s) of most cases of ASD are unknown but there is an emerging consensus that ASD have multiple etiologies. One proposed cause of ASD is exposure of the fetal brain to maternal autoantibodies during pregnancy [Dalton, P., Deacon, R., Blamire, A., Pike, M., McKinlay, I., Stein, J., Styles, P., Vincent, A., 2003. Maternal neuronal antibodies associated with autism and a language disorder. Ann. Neurol. 53, 533–537]. To provide evidence for this hypothesis, four rhesus monkeys were exposed prenatally to human IgG collected from mothers of multiple children diagnosed with ASD. Four control rhesus monkeys were exposed to human IgG collected from mothers of multiple typically developing children. Five additional monkeys were untreated controls. Monkeys were observed in a variety of behavioral paradigms involving unique social situations. Behaviors were scored by trained observers and overall activity was monitored with actimeters. Rhesus monkeys gestationally exposed to IgG class antibodies from mothers of children with ASD consistently demonstrated increased whole-body stereotypies across multiple testing paradigms. These monkeys were also hyperactive compared to controls. Treatment with IgG purified from mothers of typically developing children did not induce stereotypical or hyperactive behaviors. These findings support the potential for an autoimmune etiology in a subgroup of patients with neurodevelopmental disorders. This research raises the prospect of prenatal evaluation for neurodevelopmental risk factors and the potential for preventative therapeutics.
Keywords: Repetitive, Primate, Macaque, Macaca mulatta, Activity, Asperger syndrome
1. Introduction
Abnormalities in both adaptive and innate immune responses have been reported in subjects with autism for nearly 30 years. These include suppressed cell-mediated and humoral responses to pathogens (Stubbs and Crawford, 1977; Warren et al., 1987; Warren et al., 1986), and active inflammation and autoantibody reactions to brain tissue or proteins (Ashwood and Van de Water, 2004; Vargas et al., 2005; Weizman et al., 1982). Neural proteins reported to be the target of autoantibodies in children with autism include the serotonin receptor (Todd and Ciaranello, 1985), myelin basic protein (Singh et al., 1993), neuron axon filament protein (Singh et al., 1997), cerebellar neurofilaments (Plioplys et al., 1994), and α-2-adrenergic binding sites (Cook et al., 1993).
In addition to the presence of autoantibodies in children with ASD, antibodies from the serum of some mothers of ASD children have been shown to react to antigens on lymphocytes from their affected children (Warren et al., 1990). Moreover, Dalton et al. (2003) demonstrated the presence of antibodies against brain protein in the serum of a mother of a child with autism. The exposure of pregnant mice to this mother’s serum resulted in offspring with behavioral abnormalities. A more exhaustive evaluation of maternally derived serum has recently been published by Zimmerman et al. (2007). They found a unique pattern of serum reactivity to rat brain from gestational day 18 but not to postnatal day 8 in mothers of children with autism. Similarly, we have identified a common, characteristic pattern of autoantibody production to fetal brain protein in the serum of mothers who have had two or more children with ASD (Braunschweig et al., 2007). These findings raise the possibility that a subset of ASD cases are caused by an IgG antibody response directed against the fetal brain during gestation. To explore this maternal antibody hypothesis, we exposed pregnant rhesus monkeys to antibodies collected from mothers of children with ASD. We compared the behavior of the offspring from this group of monkeys to a control group of monkeys prenatally exposed to human IgG from mothers of multiple typically developing children and monkeys that were left untreated.
We chose to use the rhesus monkey as a model for the study of autism because it has several specific advantages over other animal model systems. In particular, the social repertoire of monkeys is much broader than that of rodents making it useful for the analysis of normal and pathological human social behavior (Deaner and Platt, 2003; Gothard et al., 2004). Over the past several years, we have developed an extensive battery of behavioral tests aimed at exploring the neural basis of social behavior in rhesus monkeys. This battery involves testing during highly controlled social interactions using a comprehensive and well-defined ethogram (catalogue of species-typical behaviors) to quantitatively assess behavior. This testing battery has proven to be sensitive at detecting subtle alterations in social and emotional behavior including increased affiliative behavior in adult monkeys with bilateral lesions of the amygdala and increased fear responses in infant monkeys with bilateral lesions of the amygdala during novel dyadic social interactions (Bauman et al., 2004a, 2004b; Emery et al., 2001).
2. Methods
2.1. Antibody acquisition
Human sera from 21 mothers of at least one child with autism and one or more additional children with autism spectrum disorders were purchased from the Autism Genetic Resource Exchange (Mothers of autistic children—MAC IgG). Human sera from 7 mothers of multiple typically developing children were collected locally (Mothers of typically developing children—MTDC IgG).
2.2. Western blots
All serum samples were screened for the presence of antibodies directed against fetal brain tissue using Western blots. Human fetal brain protein medley (300 μg/gel; Clontech Laboratories, Mountain View, CA) or neonatal monkey brain protein (acquired from the California National Primate Research Center) was prepared according to the protocol used for the human fetal brain as provided by Clontech Laboratories. The monkey brain protein extract was similarly used at 300 μg/gel. Protein samples were separated on a 4–15% gradient reducing gel using SDS–PAGE electrophoresis and transferred onto nitrocellulose paper. Antibody reactivity to the fetal brain extracts was then analyzed for all samples individually by Western blotting. The blots were incubated for 3 hin a 0.1Mphosphate buffered saline (PBS,pH7.4) solution containing serum from the above samples at a dilution of 1:400, with 5% milk and 0.3% Triton X-100. Following a series of rinses, the extracts were incubated for 1 h with a goat anti-human IgG peroxidase-conjugated antibody and visualized using chemiluminescence with a Fluorchem 8900 imager (Alpha Innotech, San Leandro, CA). IgG was purified and pooled from 12 maternal samples derived from mothers of autistic children that demonstrated one or more bands to human fetal brain proteins which were absent in serum from control mothers. IgG from the 7 control samples did not show the reactivity to the brain extracts and were also pooled.
2.3. Purification of IgG antibodies
Each collection of pooled sera was diluted with Immunopure (G) IgG binding buffer (Pierce Biotechnology, Inc., Rockford, IL) and IgG antibodies were purified on Ultralink Affinity Pack immobilized protein G columns (Pierce Biotechnology, Inc, Rockford, IL).Purified IgG was then eluted from columns with Immunopure IgG elution buffer (Pierce Biotechnology, Inc., Rockford, IL). This process resulted in approximately 3.3 mg of purified IgG per 1 ml serum. The purified serum was screened for the presence of HIV and Hepatitis B and C and finally sterile filtered with a 0.2 μm filter prior to injection.
2.4. Subjects and living conditions
All procedures carried out on animal subjects were approved by the UC Davis Institutional Animal Care and Use Committee. The subjects for this study were 13 naturally born rhesus monkeys (Macaca mulatta). The mothers of the subjects were selected based on their proven birth record and on their high quality maternal behavior and randomly assigned to one of three conditions.
Pregnant rhesus monkeys (n = 4) were exposed to purified IgG (the only antibodies that cross the placental barrier) pooled from the serum of a subset of mothers of children with ASD that could be distinguished by the presence of reactivity to fetal brain proteins by Western blot (Fig. 1). A separate group of pregnant monkeys (n = 4) were exposed to purified IgG pooled from the serum of mothers of typically developing children. In all cases, 15–20 mg of purified IgG diluted in 5 ml of sterile saline was delivered intravenously on three separate occasions: days 27, 41, and 55 of gestation. Rhesus monkey gestation is approximately 165 days. Additional pregnant rhesus monkeys (n = 5) comprised an untreated control group.
Fig. 1.
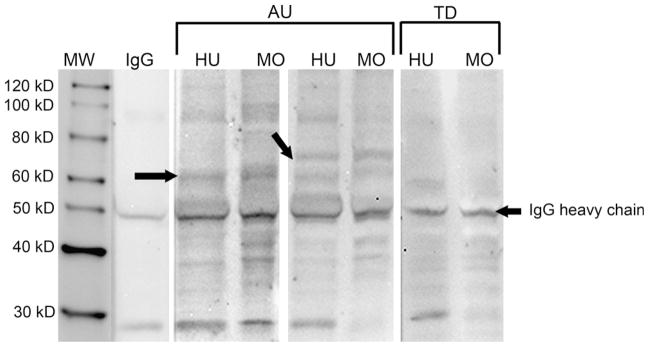
Western blot demonstrating reactivity of maternal serum against both human (HU) and monkey (MO) fetal brain proteins. Depicted are two representative samples from the mothers of multiple children with autism (AU) demonstrating the typical patterns of reactivity noted in the samples used for the IV injection of maternal IgG. Note the reactivity in the first AU sample to a band at approximately 60 kDa (left arrow), while the second AU sample reacts to the 60 kDa band as well as a band at 73 kDa (right arrow). Plasma from a representative mother of two typically developing children (TD) lacks a response to either of these two bands. Note that the patterns of reactivity for the human and monkey brain blots are quite similar.
All infants were born and raised in standard home cages (61 × 66 × 81 cm). Each mother–infant pair was assigned to one of three socialization cohorts consisting of 6 mother–infant pairs and 1 adult male. There were 2 male and4 female infants in each cohort. Mother–infant pairs from each study group were distributed across the socialization cohorts so that there was at least 1 MAC IgG treated monkey, 1 MTDC IgG control monkey and 1 untreated control monkey in each cohort. In addition to the 13monkeys in this study, the socialization cohorts included 5 other mother–infant pairs that were not part of this study. Offspring were thus raised with their mothers and were socialized for 3 h daily with 5 other mother–infant pairs and 1 adult male in large group cages (2.13 × 3.35 × 2.44 m). Formal assessments of dominance within each socialization cohort indicated that the average dominance rankings of the mothers from each study group were roughly equivalent (MAC IgG treated = 4.25/6, MTDC IgG control = 3/6, Untreated control = 4/6). When the youngest subject within each socialization cohort reached ~6 months of age, all of the infants within that cohort were permanently separated from their mothers (weaned), a standard practice at the primate center, and permanently moved to large group cages. The adult males remained with each cohort and a novel adult female was added to each cohort for a period of 1 month following weaning to promote group stability.
As anticipated, behavioral data from the control IgG monkeys and the untreated control monkeys were very similar and did not approach significance. These two groups were therefore pooled to form a single control group (n = 9) for comparisons with the MAC IgG treated monkeys.
2.5. Behavioral observations
Behavioral data were collected with The Observer software (Noldus, Sterling, VA; (Noldus, 1991) by three trained observers, demonstrating an inter-observer reliability of at least 85% (agreements/[agreements + disagreements] × 100). All observers had previous experience using Noldus software for the collection of rhesus macaque behavioral data (9 months, 2 years and 3 years, respectively). Reliability was evaluated using the most experienced behavioral observer as the reliability standard. Inter-rater reliability was attained for each behavioral task, with observers meeting at least 85% reliability over two consecutive days. All observers were blind to the experimental status of all subjects.
2.6. Preweaning social group and dyad observations
Beginning at approximately one month of age, the infants were observed in their socialization cohorts. Each subject was observed for 5 min twice per week during weeks in which no other testing took place. One month prior to weaning, two mother–infant pairs were placed together in the large testing enclosures for a 20 min tetradic social interaction (Bauman et al., 2004a). Each mother–infant pair was observed in pairings with every other mother–infant pair in its own socialization cohort. For both the social group and mother–infant pairings, trained observers blind to the condition of the animals used the Observer software program on laptop computers to score the behavior of each subject in real time during 5 min focused observations (ethograms of behaviors recorded in the social groups and the familiar dyads have been provided as supplementary material).
2.6.1. Mother preference test
On the first 4 days immediately following weaning, each infant was observed in a test designed to evaluate one aspect of mother–infant attachment, the infant’s preference for its mother over another familiar adult female (Bauman et al., 2004a). Five daily 2 min trials were conducted, with each trial consisting of a choice between the infant’s mother and one of the five other adult females from the infant’s socialization group (the stimulus female). A different stimulus female was used for each trial in a predetermined pseudo-random order. Before each trial, the test subject was hand-caught by a technician and placed in a plastic release box in the center of an unfamiliar chain link testing enclosure (5.56 × 1.91 × 2.13 m). The front of the subject’s release box was transparent and the remaining three sides were opaque allowing the test subject to view only the observers until released. The subject’s mother was placed in one of two holding cages, located at either end of the testing enclosure, and the other female was placed in the opposite holding cage (holding cage assignments were balanced across trials). For the safety of the infant, transparent plastic panels prevented physical contact between the test subject and the adult females. At the onset of the trial, the subject’s release box and the opaque panels in front of the holding cages were raised simultaneously, allowing the test subject to freely move around the testing enclosure and see both its mother and the other female. During each 2 min trial, trained observers recorded the behaviors exhibited by the test subject, including which adult was first approached (scored when the subject moved within a 1 m half-circle painted on the floor in front of each holding cage. An ethogram of behaviors for the maternal preference testing has been provided as supplementary material).
2.6.2. Solo and familiar dyad observations
One month following weaning (when the animals were on average 8.5 months old), each subject was observed in a test setting designed to study the behavior of the subject alone and during interactions with familiar peers (Bauman et al 2004b; Emery et al 2001). Subjects were removed from their socialization cohorts and placed alone in a large testing enclosure similar to their home environment and observed for two consecutive 5 min sessions. Immediately following these initial solo observations, the first day of familiar social dyad observations began. Subjects were placed into the testing enclosures in pairs to form social dyads. Each social dyad consisted of two subjects from the same socialization cohort. Social dyad sessions were 20 min in duration with the focal subject (the subject for whom data were collected) alternating every 5 min. Each subject met with each other subject in their socialization cohort on two occasions separated by at least one day. Social dyads were spread out over 5 consecutive testing days with each subject participating in 2 separate 20 min dyads each day in a predetermined pseudo-random order. On the final day of familiar social dyad testing, each subject was again observed alone for two consecutive 5 min sessions. Trained observers blind to the condition of the animals used the Observer software program on laptop computers to score the behavior of each focal subject in real time from a behavioral ethogram of approximately 50 normal and abnormal rhesus monkey behaviors (ethogram of behaviors has been provided as supplementary material). Behaviors included whole-body stereotypies such as pacing, back-flipping, twirling, and swinging. When a subject was engaged in a particular whole-body stereotypy for longer than 6 s, an extended stereotypy was scored.
2.6.3. Unfamiliar dyad observations
One month following the solo and familiar dyad observations (when the animals were on average 9.5 months old), each subject was observed in a test setting designed to study interactions with unfamiliar peers. Four age-appropriate monkeys (2 males and 2 females) served as unfamiliar peers (stimulus monkeys). These stimulus monkeys were maternally reared and socialized in group housing prior to their temporary assignment to this study. Subjects were again removed from their socialization cohorts and placed in individual holding cages. Subjects were then paired with one of the four stimulus monkeys in the same testing enclosures used for solo and familiar dyad observations. These unfamiliar dyad sessions were also 20 min in duration with the focal subject alternating every 5 min. Each subject met with each of the stimulus monkeys on two occasions separated by at least one day. The unfamiliar social dyads were therefore spread out over 4 consecutive testing days, with each subject participating in 2 separate 20 min dyads each day, again in a predetermined pseudo-random order.
2.6.4. Social group observations
In addition to the acquisition of behavioral data in novel testing environments, each subject was also observed within their home cage socialization cohorts. Each subject was observed for 5 min twice per week during weeks in which no other testing took place. A total of 30 observations were conducted on each subject, with all testing taking place in the weeks following the unfamiliar dyads.
2.6.5. Activity monitoring
When the monkeys were approximately 1 year old, activity was measured with an actimeter (Actiwatch-64; MiniMitter, Bend, OR) housed in a metal casing (40 × 32 × 13 mm) attached to a nylon primate collar (Primate Products, Woodside, CA). The Actiwatch-64 is a small device (17 g) capable of detecting the degree and speed of omnidirectional motion with an accelerometer. Changes in the degree and speed of motion produce changes in voltage that are stored as activity counts. For this study, each actimeter was programmed to sample activity at a frequency of 32 Hz and record activity counts in 30 s intervals. Each monkey was fitted with the primate collars with the attached actimeters and allowed to acclimate to the collars for 1 week before monitoring began.
The activity of each monkey was monitored in two separate conditions. In the first, the activity of each monkey was monitored for 7 consecutive days while the monkeys remained in their routine social housing situation. In the second condition, the monkeys were removed from their social groups and their activity was monitored while they were individually housed in standard home cages (61 × 66 × 81 cm) over two separate 24 h periods. During each 24 h period, each monkey was observed over four separate 10 min sessions, two between 8 a.m. and 12 p.m. and two between 3 p.m. and 6 p.m. Behaviors were scored in real time using the same ethogram as the solo and dyadic observations. In between each individual housing period, the monkeys were returned to their social groups for 24 h. During both monitoring conditions, special precautions were made to minimize disturbances to the monkeys.
2.7. Statistical analyses
Due to the large number of zero values scored for some of the behavioral observations, the data were not normally distributed and the variance was not homogenous. We therefore used nonparametric Mann– Whitney tests with a .05 alpha level to determine between-groups differences in these data. For all data in which parametric test assumptions of normality and homogeneity of variance were not violated, Independent t-tests with a .05 alpha level were used. All statistical procedures were carried out using SPSS 14.0 statistical software.
3. Results
3.1. Social behaviors
Formal assessments of social behavior during preweaning and postweaning observation periods yielded very few differences between treated and control animals. In fact, the frequencies of social and nonsocial events were similar in the MAC IgG and control groups. When activities characterized as states (extending beyond 3 s) were analyzed, there were again only a few differences in social states. As summarized in Table 1, important features of the maternal infant interaction, such as the amount of ventral– ventral contact was also similar in both groups. Nonetheless, the MAC IgG treated monkeys did demonstrate a few differences in social behavior states during this period. For example, compared to controls, they demonstrated significantly more contact with their mother and peers prior to weaning (with the exception of ventral contact). However, they demonstrated less contact with familiar peers in the months following the removal of their mothers. During this period they also spent significantly more time engaged in nonsocial active behavior than controls. It is possible that the MAC IgG treatment was causally related to these changes in social behavior and we will explore this relationship during future replication studies with larger cohorts of treated animals. The most profound observations during the course of this study, however, were of the high level of clearly abnormal stereotypic behaviors exhibited by the MAC IgG monkeys. The remainder of this report highlights these differences in stereotypic behaviors observed across multiple behavioral settings as well as the increased activity demonstrated by these same monkeys.
Table 1.
Mean duration (s) of social states for MAC IgG and control monkeys across social testing paradigms
| Behavioral State | Prewean Mother–infant dyads
|
Prewean social groups
|
Postwean familiar dyads
|
Postwean unfamiliar dyads
|
Postwean social groups
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAC IgG
|
Control
|
MAC IgG
|
Control
|
MAC IgG
|
Control
|
MAC IgG
|
Control
|
MAC IgG
|
Control
|
|||||||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Breast contact | 619.53 | 217.13 | 312.04 | 73.02 | 682.95 | 258.57 | 446.94 | 183.37 | * | * | * | * | * | * | * | * | * | * | * | * |
| Ventral contact | 1109.88 | 318.96 | 1293.52 | 147.08 | 327.65 | 286.77 | 461.24 | 194.48 | * | * | * | * | * | * | * | * | * | * | * | * |
| Other contact | 1482.92 | 148.02 | 1170.40 | 106.93 | 1585.67 | 124.37 | 1162.91 | 102.18 | * | * | * | * | * | * | * | * | * | * | * | * |
| Extended play | 26.25 | 11.59 | 103.89 | 29.06 | 167.26 | 82.73 | 365.39 | 94.59 | 3.73 | 3.73 | 11.00 | 3.75 | 44.42 | 32.65 | 33.54 | 14.64 | 161.87 | 30.62 | 339.01 | 74.71 |
| Nonsocial active | 1451.23 | 402.39 | 1932.41 | 232.81 | 1687.75 | 476.09 | 2592.41 | 284.81 | 4235.61 | 186.10 | 3430.68 | 239.00 | 4055.24 | 139.54 | 4088.95 | 116.86 | 4357.64 | 90.52 | 3658.96 | 195.98 |
| Proximity | 1272.67 | 176.71 | 1142.05 | 67.08 | 1276.85 | 381.07 | 1495.93 | 115.51 | 832.30 | 180.92 | 1078.92 | 82.42 | 313.65 | 68.61 | 369.08 | 67.47 | 821.30 | 83.69 | 983.78 | 55.49 |
| Extended groom | * | * | * | * | 0.00 | 0.00 | 4.53 | 2.62 | 36.93 | 36.93 | 46.30 | 26.00 | 6.44 | 4.84 | 67.66 | 36.54 | 173.39 | 49.31 | 294.28 | 85.06 |
| Extended toy play | ** | ** | ** | ** | 15.70 | 6.41 | 11.91 | 3.51 | 7.12 | 0.36 | 77.62 | 24.11 | 3.76 | 3.76 | 19.84 | 7.57 | 105.69 | 49.23 | 158.89 | 39.37 |
| Extended contact | * | * | * | * | * | * | * | * | 353.19 | 125.09 | 811.80 | 113.14 | 161.29 | 39.05 | 135.42 | 35.98 | 1018.86 | 105.89 | 1071.86 | 122.10 |
| Sleep | * | * | * | * | * | * | * | * | 22.28 | 20.61 | 23.34 | 9.91 | ** | ** | ** | ** | 470.60 | 163.41 | 551.42 | 86.97 |
There were no differences in important maternal–infant interactions such as ventral–ventral contact. During the preweaning social groups, MAC IgG treated infants spent significantly more time in contact with their mother and peers than control infants (t(11) = 2.410, p = .035). During the postweaning familiar dyads, MAC IgG treated monkeys spent significantly less time in the extended contact state with their peers (t(11) = 2.403, p = .035) and showed a trend towards more time in a nonsocial active state than control monkeys (t(11) = 2.088, p = .061). The increase in nonsocial active behavior was also observed in postweaning social groups (t(11) = 2.279, p = .044).
Indicates that the social state was not applicable to the testing paradigm.
Indicates insufficient data due to the rare occurrence of the social state within the testing paradigm. Bold font indicates significant differences between control and MAC IgG monkeys.
3.2. Appearance of stereotypies during the mother preference task
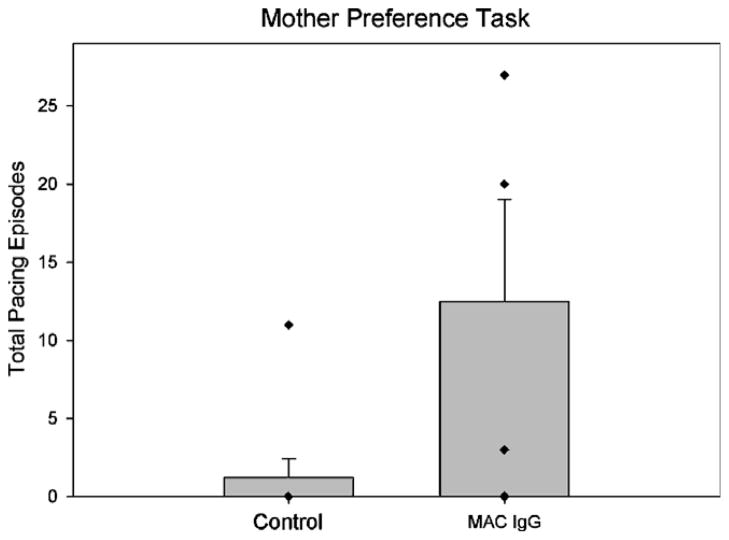
Results from the mother preference task showed no significant differences between the infant monkeys in their initial approach towards their mother or a familiar adult female (Mother: t(11) = .423, p = ns; Familiar Adult Female: t(11) = .107, p = ns). As with control monkeys, the MAC IgG treated monkeys tended to approach their own mother first. However, a unique pattern of pacing across the length of the testing cage was observed in infants exposed to IgG from mothers of children with autism (See Supplementary Video 1). The Mann–Whitney test revealed that the number of pacing episodes was significantly higher for these monkeys compared to controls (U = 6.00, p = .024, Fig. 2). This behavior stood out to our trained observers as being highly unusual for socially reared monkeys and prompted a more intensive analysis of motor activity and stereotyped behaviors in other testing conditions.
Fig. 2.
Mean episodes of pacing behavior observed during the mother preference task in control monkeys (both untreated controls and animals treated with IgG from mothers of typically developing children) compared to MAC IgG treated monkeys. The MAC IgG treated monkeys demonstrated significantly more pacing episodes than controls (U = 6.00, p = .024). Error bars represent SEM. Diamonds here, and in Figs. 3–5 and 7–8, represent scores of individual animals in each group. In some cases, scores are so similar that symbols overlap.
3.3. Presence of stereotypies during solo observations and familiar pairings
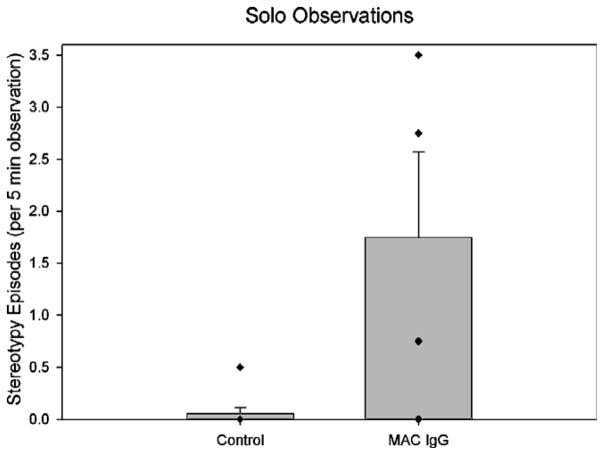
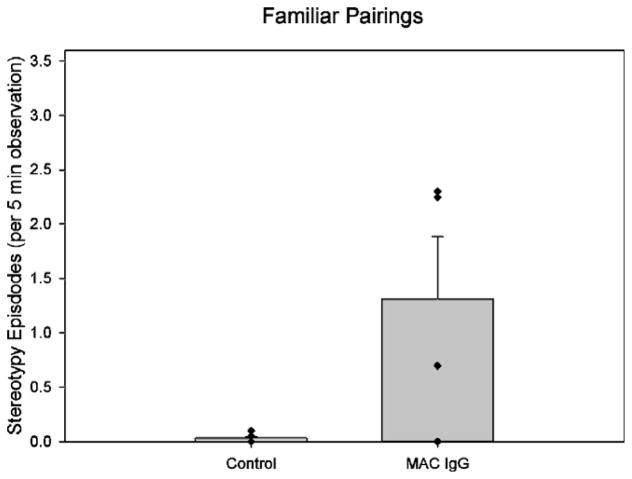
One month following the mother preference task, each subject was removed from their social group and observed either alone or with a familiar partner in a large novel enclosure that was similar to their home environment. Nonparametric statistics demonstrated significant differences in the frequency of whole-body stereotypies between groups when the animals were alone (U = 5.00, p = .014). Fig. 3 shows that the MAC IgG treated monkeys showed elevated frequencies of whole-body stereotypies compared to the control monkeys. A similar pattern of elevated stereotypies was also apparent when the monkeys were paired with a familiar partner (Fig. 4). In this case, the frequency of stereotypies demonstrated a tendency to be higher in the MAC IgG treated animals (U = 7.00, p = .076). However, both the frequency and duration of bouts of extended stereotypy were clearly significantly higher in this paired condition (Frequency: U = 5.00, p = .014; Duration: U = 5.00, p = .014). Supplementary Videos 2 and 3 demonstrate the pacing and back-flipping behavior observed during the pairing with a familiar partner.
Fig. 3.
Mean episodes of whole-body stereotypies observed in control (both untreated controls and animals treated with IgG from mothers of typically developing children) and MAC IgG treated monkeys during solo observations. The MAC IgG treated monkeys displayed significantly more episodes of whole-body stereotypies than controls (U = 5.00, p = .014). Error bars represent SEM.
Fig. 4.
Mean episodes of whole-body stereotypies observed in control (both untreated controls and animals treated with IgG from mothers of typically developing children) and MAC IgG treated monkeys during observations with a familiar partner. The MAC IgG treated monkeys displayed more episodes of whole-body stereotypies than control monkeys, although the results only approached significance (U = 7.00, p = .076). Data analysis of the number and duration of extended stereotypy bouts were significant during this task (frequency: U = 5.00, p = .014; Duration: U = 5.00, p = .014). Error bars represent SEM.
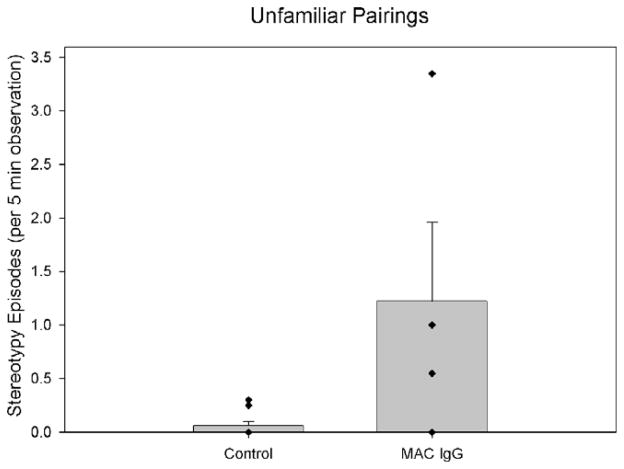
3.4. Presence of stereotypies during unfamiliar pairings
Monkeys were also observed for behavioral differences when paired with unfamiliar peers in dyadic situations. One month following the pairings with familiar peers, monkeys were removed from their social groups and placed with one of four unfamiliar “stimulus” monkeys in the same large testing enclosures. Once again, we observed a significant difference in the frequency of whole-body stereotypies between the groups (U = 5.50, p = .028). The elevated stereotypies of the MAC IgG treated monkeys compared to control monkeys are illustrated in Fig. 5.
Fig. 5.
Mean episodes of whole-body stereotypies observed in control (both untreated controls and animals treated with IgG from mothers of typically developing children) and MAC IgG treated monkeys during observations with an unfamiliar partner. The MAC IgG treated monkeys displayed significantly more episodes of whole-body stereotypies than control monkeys (U = 5.50, p = .028). Error bars represent SEM.
3.5. Elevated levels of activity during solo housing
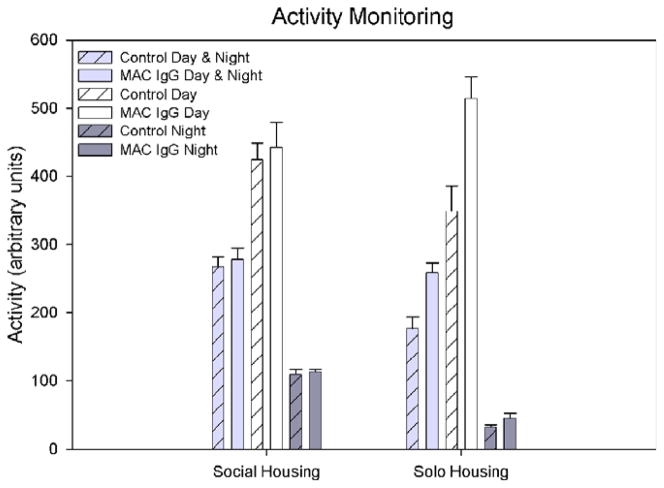
Independent t-tests did not reveal any significant differences in activity between control and MAC IgG monkeys over the 7 days of monitoring during routine social housing (Fig. 6). However, during the individual housing condition, MAC IgG monkeys demonstrated significantly more activity than controls (t(11) = 2.954, p = .013; Fig. 6). Further analysis showed that this increase in activity was primarily caused by higher activity levels during the day (light cycle; t(11) = 2.758, p = .019) rather than at night (dark cycle; t(11) = 1.951, p = .077).
Fig. 6.
Mean number of activity counts (arbitrary units) per 30 s of monitoring for control (both untreated controls and animals treated with IgG from mothers of typically developing children) and MAC IgG monkeys during day and night combined, day only, and night only, in both social housing and individual housing conditions. There were no differences in activity demonstrated during the social housing condition. When the monkeys were removed from their social cages and placed into individual housing, significantly higher activity counts were recorded for the MAC IgG treated monkeys (day and night combined: t(11) = 2.954, p = .013). Error bars represent SEM.
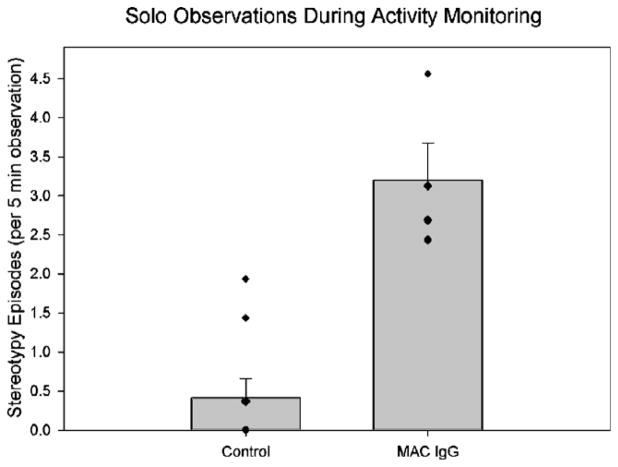
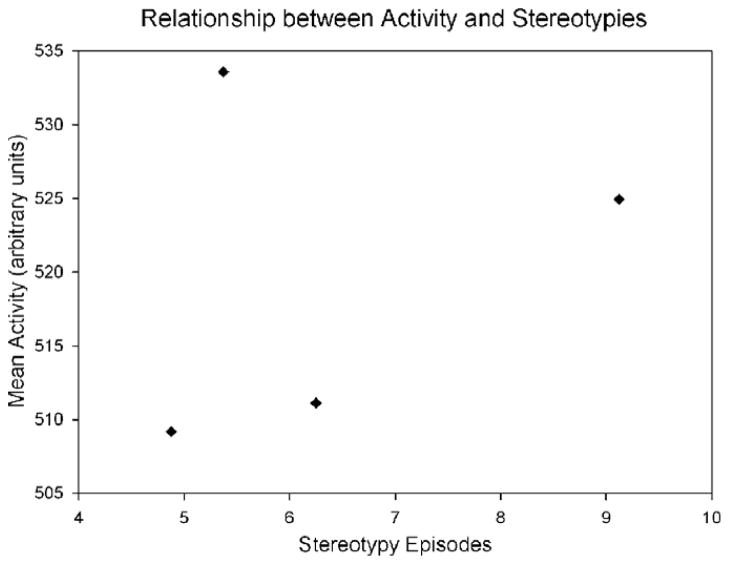
Behavioral observations during solo housing again revealed significantly more whole-body stereotypies in MAC IgG monkeys compared to controls (U = 0.00, p = .003; Fig. 7). As it is reasonable to assume that the elevated activity of the MAC IgG monkeys is related to their whole-body stereotypies, we compared their activity counts to their whole-body stereotypies recorded during eight 10 min observations. The monkey with the highest number of activity counts actually had the second lowest number of stereotypies (Fig. 8). In addition, there was less than 5% difference in the activity counts of all of the MAC IgG monkeys, but there was a 187% increase in the number of stereotypies from the lowest count to the highest. Therefore, there did not appear to be any causal relationship between the whole-body stereotypies and elevated activity.
Fig. 7.
Mean episodes of whole-body stereotypies observed in control (both untreated controls and animals treated with IgG from mothers of typically developing children) and MAC IgG treated monkeys over eight 10 min observation periods occurring during solo activity monitoring. The data are shown per 5 min of observation for comparison with previous figures. The MAC IgG treated monkeys displayed significantly more episodes of whole-body stereotypies than control monkeys (U = 0.00, p = .003). Error bars represent SEM.
Fig. 8.
Scatterplot of the whole-body stereotypy and activity count data of the 4 MAC IgG treated monkeys collected during eight 10 min observation periods. The numbers of stereotypies do not appear to be closely related to the activity counts indicating that the stereotypic movements were largely independent of generalized activity.
4. Discussion
To summarize, the group of monkeys exposed prenatally to IgG from mothers of children with ASD demonstrated significantly more stereotypies and higher levels of motor activity than control monkeys. The elevation in stereotypies was first evident during the mother preference task that was conducted in the first 4 days following the permanent removal of the subjects’ mothers from the socialization groups. The increase in episodes of stereotyped behavior persisted and strengthened in the six months following weaning. Importantly, significantly increased stereotypies were observed consistently across five different testing paradigms: mother preference testing, solo observations, familiar dyadic interactions, unfamiliar dyadic interactions, and during solo activity monitoring.
In addition to quantifying stereotyped behaviors, we also used actimiters to monitor the general activity of the animals at approximately one year of age. Significant elevation of activity was evident when the monkeys were individually housed, but not in their normal social environment. It is important to point out that the rise in activity did not appear to be caused by the presence of stereotypies. A scatterplot of stereotypy and activity data collected over eight 10 min blocks indicated that the contribution of the stereotypies to the overall measure of activity was minimal (Fig. 8) and thus the presence of stereotypies could not account for the 1.47 fold increase in activity observed between the MAC IgG treated and control monkeys (Fig. 6).
4.1. Exposure to MAC IgG appears to cause the stereotypical behavior observed in the experimental monkeys
Stereotypical behavior in monkeys is a sign of pathology. It can be brought on by, among other things, long-term individual housing conditions or environmental impoverishment (Lutz et al., 2003; Mason, 1991). Neither of these conditions applies to our experimental subjects. The monkeys in this study were maternally reared and socialized with other mother–infant pairs on a daily basis prior to weaning, and permanently socialized with 5 other monkeys following weaning. It is important to point out that the much more invasive procedure of producing neo-natal lesions of the amygdala or hippocampal formation (Bauman et al 2004a; Bauman et al 2004b) did not result in the production of increased whole body stereotypies during the first year of life. As an added precaution in the current study, each social cohort included representation from each treatment group so it is unlikely that the increased stereotypies were learned through social transmission. Therefore, given that these stereotypies were only observed in the MAC IgG treatment group, they appear to be attributable to that particular form of IgG exposure. It is also important to note that no differences were observed between untreated control animals and animals treated with IgG from mothers of typically developing children.
4.2. Potential exacerbation of stereotypies and hyperactivity by novel social and environmental conditions
The stereotypies observed in the MAC IgG monkeys were not as apparent prior to weaning, and, while occasionally observed in their home cages, were not consistently present in their routine living condition. Rather, the stereotypies emerged when the monkeys were removed from their normal environment and placed in a novel social setting. It is important to emphasize that the control monkeys had precisely the same rearing conditions as the MAC IgG group.
Stereotypies have been considered one of the defining features of autism since the earliest descriptive account (Kanner, 1943). Along with the presence of stereotypies, individuals with autism are often described as having an “insistence on sameness” in their environment. A few studies have explored the effects of the environment on the rates of stereotyped behavior in autism. One study reported a progressive increase in stereotypies as unfamiliar toys followed by an unfamiliar passive adult were introduced into an empty room (Hutt, 1965). Another study demonstrated a significant increase in stereotypies with an unfamiliar versus a familiar therapist (Runco et al., 1986). Given these observations of children with autism, it is noteworthy that the treated monkeys in our study did not demonstrate increased stereotypical behavior in their long-term, stable social groups, but only in novel environmental or social situations.
Interestingly, the significantly elevated levels of activity found in the treated monkeys were also only present after they were removed from their normal social environment and housed individually in a novel environment. Although hyperactivity is not a defining feature of autism, it is one of the most frequently reported problems in ASD (Lecavalier, 2006). In fact, recent studies have indicated that approximately 50% of children with ASD meet DSM-IV symptom criteria for ADHD (Gadow and DeVincent, 2005; Gadow et al., 2004). It is likely that changes in social environments exacerbate these symptoms.
4.3. Caveats of the current model
While the neurodevelopmental alterations that have been produced in this population of animals are striking, it is premature to conclude that this is an animal model of autism. We have thus far not found any profound differences in social behavior between groups as one might predict in a monkey model of autism. We believe that this may be due to experimental limitations of the model. First, due to limited availability of IgG, we were only able to expose the pregnant females to IgG for approximately 25% of the duration of the pregnancy. Second, the time during pregnancy when the females were exposed may determine the type of behavioral pathology that arises. Our maternal antibody hypothesis of human autism is based on the premise that before or during pregnancy, the maternal immune system recognizes one or more unidentified fetal brain proteins as foreign and mounts an antibody response against it. This, therefore, involves an active immune process with a constant source of the pathologic antibody. In other words, it is likely that the donor mothers had titre of these autoantibodies during most of their pregnancy. Interestingly, of the 12 mothers who contributed serum to the MAC IgG group, there were no typical children born after their first child with ASD, potentially indicative of a persistent pathological immune response. We chose to deliver the IgG in three different doses spanning the end of the first trimester of pregnancy based upon the demonstrated sensitivity of the nervous system during this period of development in the monkey (Hendrickx, 1973; Hendrickx et al., 1988), and during the equivalent time of neural development in rodent models (Rodier et al., 1997; Shi et al., 2003). However, we were limited from producing a longer exposure by the quantity of available IgG and the half-life of human IgG in the rhesus monkey (Hinton et al., 2004). We would predict that a longer exposure of the fetus to the IgG may either increase the severity of the stereotypical and hyperactive behaviors or produce impairments of social behavior that are characteristic of autism. This will be addressed in future studies.
4.4. Precedents for neonatal disorders caused through placental transfer of IgG
There are a number of examples in the literature of pediatric disorders caused by transplacental transfer of maternally derived IgG. Transplacental transfer of anti-acetylcholine receptor antibody, for example, can produce symptoms of myasthenia gravis in the newborn even if the mother is in remission during pregnancy (Elias et al., 1979). Some mothers with myasthenia gravis who carry highly specific antibodies to a fetal isoform of the nicotinic acetylcholine receptor give birth to children with arthrogryposis multiplex congenital (AMC; Brueton et al., 2000; Vincent et al., 1995). AMC is a severe developmental condition involving fixed joint contractures and other deformities that results from lack of movement. In anti-AChR antibody-associated AMC, fetal or neonatal death is common and the condition usually recurs in the mother’s subsequent pregnancies (Polizzi et al., 2000). Administration of serum from such mothers to pregnant mice results in offspring with fixed joint contractures and other developmental abnormalities (Jacobson et al., 1999).
Another example, Graves’ disorder, involves the production of antibodies against the thyroid stimulating hormone receptor (TSHr) in the thyroid gland. The antibodies appear to act as a receptor agonist since symptoms of Graves’ disease are identical to the symptoms of hyperthyroidism and include an enlarged thyroid, nervousness, heat intolerance, weight loss, sweating, tremors, heart palpitations and exophthalmos. During pregnancy, antibodies to TSHr cross the placenta and affect the thyroid gland of the fetus (Radetti et al., 1999; Volpe et al., 1984). Infants born to mothers with Graves’ disease often show signs similar to those of the mother; the symptoms disappear as the maternal antibodies are eliminated. There are a number of other transient neonatal autoimmune diseases (reviewed in Giacoia and Azubuike, 1991) which effect the central or periphereral nervous system. Thus, there is adequate precedent for the hypothesis that maternally derived antibodies to fetal brain protein may cross the placenta, interact with the developing fetal brain and affect the outcome of the developmental process.
4.5. Evidence from experimental animal and clinical research for immune modulation of cognitive and motor function
Systemic lupus erythematosous (LSE) is an autoimmune disorder that affects multiple body systems. In an elegant series of studies, Diamond, Volpe and colleagues have examined the role of serum antibodies specific for DNA and for the N-methly D-aspartate receptor (NMDAr) in the neuropsychiatric and cognitive symptoms associated with LSE (Diamond et al., 2006; Kowal et al., 2006). They have injected serum from patients containing these antibodies into mature mice who are also treated with lipopolysacharide to cause a breach of the blood brain barrier. They find that the brains of treated animals show neuronal pathology in the CA1 region of the hippocampus and are impaired on hippocampal-dependent memory tasks. They have raised the hypothesis that lupus neuropsychiatric symptoms are caused by a direct action of the antibodies on brain function. Consistent with this, they found that the postmortem brains of patients with SLE demonstrate the anti NMDAr and anti DNA antibodies. Interestingly, if the breach of the blood brain barrier leads to the antibodies interacting with a specific brain region such as the amygdala, then the resulting behavioral pathology can be quite different (Emmer et al., 2006).
There is also substantial support for the idea that immune modulation of the brain can lead to pathological motor behaviors. An excellent example of this is the work of Zalcman et al. (1999), Zalcman (2001). They have found, for example, that a single intraperitoneal injection of IL-2 produces significantly increased locomotor and exploratory behavior in mice in a novel environment. Interestingly, IL-2 treatment also significantly increased sensitivity to the behavioral stimulating properties of GBR 12909 which is a highly selective dopamine uptake inhibitor. This raises the possibility that IL-2 acts to increase locomotor activity through a dopaminergic mechanism.
Finally, the PANDAS (Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infections) provide another good example of how an immune response can produce antibodies that interact with brain and result in abnormal behaviors, including motor and vocal tics. Through what appears to be a molecular mimicry mechanism, strep infections lead to the production of autoantibodies that particularly affect the basal ganglia (Snider and Swedo, 2004). Interestingly, antibodies associated with PANDAS have been demonstrated to react with neurons from the caudate-putamen and induce calcium– calmodulin dependent protein kinase II (CaM kinase II) activity. Depletion of serum IgG reduced CaM kinase II cell signaling and reactivity was blocked by streptococcal antigen N-acetyl-β-D-glucosamine (Kirvan et al., 2006b). A very similar mechanism has been proposed for the development of Syndeham’s chorea (Kirvan et al., 2006a).
While this has not been an exhaustive review, there is substantial evidence to conclude that an autoimmune mechanism can play a role in the development of pediatric disorders and disorders that manifest with cognitive and behavioral alterations. Given the demonstration of unusual fetal brain-directed autoantibodies in the serum of some women who give birth to children with autism (Braunschweig et al., 2007; Zimmerman et al., 2007) and the current data of abnormal motor behaviors in rhesus monkeys treated during gestation with IgG from mothers of children with autism, the hypothesis of an autoimmune etiology for at least some cases of autism appears reasonable.
4.6. Clinical implications of this research
Mindful of the caveats described above, the present study nonetheless raises several important clinical implications. The evaluation of the occurrence of brain-directed autoantibodies could be a useful indication of risk factors for autism or other neurodevelopmental disorders in women who intend to become pregnant. This would be particularly relevant to mothers who have already had a child with autism since the risk of a second child with autism increases substantially over that of the general population. Moreover, by determining that brain-directed antibodies are present, appropriate therapeutic interventions could be implemented to lessen the risk of producing a child with a neurodevelopmental disorder. This raises the optimistic prospect that some future cases of autism or related neurodevelopmental disorders may be prevented through prenatal diagnostic screening.
Supplementary Material
Acknowledgments
We thank Jennifer Forcier, Jessica Toscano, Melissa Marcucci, and Hannah Cuthbert for technical support. This research was supported by grants from the U.S. EPA through the Science to Achieve Results (STAR) program (Grant R829388) and NIEHS 1P01 ES11269-01 (to D.G.A. and J.V.), from the NIMH R37 MH057502 and MH41479 (to D.G.A.), by an NRSA grant from NIMH (to L.A.M.) and by grants from Visceral and the Ted Lindsay Foundation (to P.A.). In addition, we acknowledge support from the Autism Genetic Resource Exchange (AGRE) and Cure Autism Now for the resources provided by the AGRE consortium and the participating AGRE families. The Autism Genetic Resource Exchange (AGRE) is a program of Cure Autism Now and is supported, in part, by a grant from the NIMH to Daniel H. Geschwind (PI). This research was conducted, in part, as a component of the UC Davis Children’s Center for Environmental Health and was supported, in part, by the M.I.N.D. Institute.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bbi.2007.12.007.
Footnotes
Please see Brief Commentary by Robert Dantzer and Keith W. Kelley in this issue.
References
- Ashwood P, Van de Water J. A review of autism and the immune response. Clin Dev Immunol. 2004;11:165–174. doi: 10.1080/10446670410001722096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of mother–infant interactions after neonatal amygdala lesions in rhesus monkeys. J Neurosci. 2004a;24:711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. J Cogn Neurosci. 2004b;16:1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- Braunschweig D, Ashwood P, Krakowiak P, Hertz-Piciotto I, Hansen R, Croen L, Pessah IN, Van de Water J. Matenal plasma antibodies to fetal brain in autism. J Neurotoxicol. 2007 doi: 10.1016/j.neuro.2007.10.010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueton LA, Huson SM, Cox PM, Shirley I, Thompson EM, Barnes PR, Price J, Newsom-Davis J, Vincent A. Asymptomatic maternal myasthenia as a cause of the Pena-Shokeir phenotype. Am J Med Genet. 2000;92:1–6. doi: 10.1002/(sici)1096-8628(20000501)92:1<1::aid-ajmg1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr, Perry BD, Dawson G, Wainwright MS, Leventhal BL. Receptor inhibition by immunoglobulins: specific inhibition by autistic children, their relatives, and control subjects. J Autism Dev Disord. 1993;23:67–78. doi: 10.1007/BF01066419. [DOI] [PubMed] [Google Scholar]
- Dalton P, Deacon R, Blamire A, Pike M, McKinlay I, Stein J, Styles P, Vincent A. Maternal neuronal antibodies associated with autism and a language disorder. Ann Neurol. 2003;53:533–537. doi: 10.1002/ana.10557. [DOI] [PubMed] [Google Scholar]
- Deaner RO, Platt ML. Reflexive social attention in monkeys and humans. Curr Biol. 2003;13:1609–1613. doi: 10.1016/j.cub.2003.08.025. [DOI] [PubMed] [Google Scholar]
- Diamond B, Kowal C, Huerta PT, Aranow C, Mackay M, DeGiorgio LA, Lee J, Triantafyllopoulou A, Cohen-Solal J, Volpe BT. Immunity and acquired alterations in cognition and emotion: lessons from SLE. Adv Immunol. 2006;89:289–320. doi: 10.1016/S0065-2776(05)89007-8. [DOI] [PubMed] [Google Scholar]
- Elias SB, Butler I, Appel SH. Neonatal myasthenia gravis in the infant of a myasthenic mother in remission. Ann Neurol. 1979;6:72–75. doi: 10.1002/ana.410060119. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2001;115:515–544. [PubMed] [Google Scholar]
- Emmer BJ, van der Grond J, Steup-Beekman GM, Huizinga TW, van Buchem MA. Selective involvement of the amygdala in systemic lupus erythematosus. PLoS Med. 2006;3:e499. doi: 10.1371/journal.pmed.0030499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, DeVincent CJ. Clinical significance of tics and attention-deficit hyperactivity disorder (ADHD) in children with pervasive developmental disorder. J Child Neurol. 2005;20:481–488. doi: 10.1177/08830738050200060301. [DOI] [PubMed] [Google Scholar]
- Gadow KD, DeVincent CJ, Pomeroy J, Azizian A. Psychiatric symptoms in preschool children with PDD and clinic and comparison samples. J Autism Dev Disord. 2004;34:379–393. doi: 10.1023/b:jadd.0000037415.21458.93. [DOI] [PubMed] [Google Scholar]
- Giacoia GP, Azubuike K. Autoimmune diseases in pregnancy: their effect on the fetus and newborn. Obstet Gynecol Surv. 1991;46:723–732. [PubMed] [Google Scholar]
- Gothard KM, Erickson CA, Amaral DG. How do rhesus monkeys (Macaca mulatta) scan faces in a visual paired comparison task? Anim Cogn. 2004;7:25–36. doi: 10.1007/s10071-003-0179-6. [DOI] [PubMed] [Google Scholar]
- Hendrickx AG. The sensitive period and malformation syndrome produced by thalidomide in crab-eating monkey (Macaca fascicularis) J Med Primatol. 1973;2:267–276. doi: 10.1159/000460334. [DOI] [PubMed] [Google Scholar]
- Hendrickx AG, Nau H, Binkerd P, Rowland JM, Rowland JR, Cukierski MJ, Cukierski MA. Valproic acid developmental toxicity and pharmacokinetics in the rhesus monkey: an interspecies comparison. Teratology. 1988;38:329–345. doi: 10.1002/tera.1420380405. [DOI] [PubMed] [Google Scholar]
- Hinton PR, Johlfs MG, Xiong JM, Hanestad K, Ong KC, Bullock C, Keller S, Tang MT, Tso JY, Vasquez M, Tsurushita N. Engineered human IgG antibodies with longer serum half-lives in primates. J Biol Chem. 2004;279:6213–6216. doi: 10.1074/jbc.C300470200. [DOI] [PubMed] [Google Scholar]
- Hutt CAHSJ. The effects of environmental complexity on the stereotyped behaviour of children. Anim Behav. 1965;13:1–4. [Google Scholar]
- Jacobson L, Polizzi A, Morriss-Kay G, Vincent A. Plasma from human mothers of fetuses with severe arthrogryposis multiplex congenita causes deformities in mice. J Clin Invest. 1999;103:1031–1038. doi: 10.1172/JCI5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Nervous Child. 1943;2:217–250. [Google Scholar]
- Kirvan CA, Swedo SE, Kurahara D, Cunningham MW. Streptococcal mimicry and antibody-mediated cell signaling in the pathogenesis of Sydenham’s chorea. Autoimmunity. 2006a;39:21–29. doi: 10.1080/08916930500484757. [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Snider LA, Cunningham MW. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol. 2006b;179:173–179. doi: 10.1016/j.jneuroim.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Kowal C, Degiorgio LA, Lee JY, Edgar MA, Huerta PT, Volpe BT, Diamond B. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc Natl Acad Sci USA. 2006;103:19854–19859. doi: 10.1073/pnas.0608397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn BM. CDC: autism spectrum disorders common. JAMA. 2007;297:940. doi: 10.1001/jama.297.9.940. [DOI] [PubMed] [Google Scholar]
- Lecavalier L. Behavioral and emotional problems in young people with pervasive developmental disorders: relative prevalence, effects of subject characteristics, and empirical classification. J Autism Dev Disord. 2006;36:1101–1114. doi: 10.1007/s10803-006-0147-5. [DOI] [PubMed] [Google Scholar]
- Lutz C, Well A, Novak M. Stereotypic and self-injurious behavior in rhesus macaques: a survey and retrospective analysis of environment and early experience. Am J Primatol. 2003;60:1–15. doi: 10.1002/ajp.10075. [DOI] [PubMed] [Google Scholar]
- Mason GJ. Stereotypies: a critical review. Anim Behav. 1991;41:1015–1037. [Google Scholar]
- Plioplys AV, Greaves A, Kazemi K, Silverman E. Lymphocyte function in autism and Rett syndrome. Neuropsychobiology. 1994;29:12–16. doi: 10.1159/000119056. [DOI] [PubMed] [Google Scholar]
- Polizzi A, Huson SM, Vincent A. Teratogen update: maternal myasthenia gravis as a cause of congenital arthrogryposis. Teratology. 2000;62:332–341. doi: 10.1002/1096-9926(200011)62:5<332::AID-TERA7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Radetti G, Persani L, Moroder W, Cortelazzi D, Gentili L, Beck-Peccoz P. Transplacental passage of anti-thyroid auto-antibodies in a pregnant woman with auto-immune thyroid disease. Prenat Diagn. 1999;19:468–471. [PubMed] [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B, Croog VJ. Linking etiologies in humans and animal models: studies of autism. Reprod Toxicol. 1997;11:417–422. doi: 10.1016/s0890-6238(97)80001-u. [DOI] [PubMed] [Google Scholar]
- Runco MA, Charlop MH, Schreibman L. The occurrence of autistic children’s self-stimulation as a function of familiar versus unfamiliar stimulus conditions. J Autism Dev Disord. 1986;16:31–44. doi: 10.1007/BF01531576. [DOI] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Warren R, Averett R, Ghaziuddin M. Circulating autoantibodies to neuronal and glial filament proteins in autism. Pediatr Neurol. 1997;17:88–90. doi: 10.1016/s0887-8994(97)00045-3. [DOI] [PubMed] [Google Scholar]
- Singh VK, Warren RP, Odell JD, Warren WL, Cole P. Antibodies to myelin basic protein in children with autistic behavior. Brain Behav Immun. 1993;7:97–103. doi: 10.1006/brbi.1993.1010. [DOI] [PubMed] [Google Scholar]
- Snider LA, Swedo SE. PANDAS: current status and directions for research. Mol Psychiatry. 2004;9:900–907. doi: 10.1038/sj.mp.4001542. [DOI] [PubMed] [Google Scholar]
- Stubbs EG, Crawford ML. Depressed lymphocyte responsiveness in autistic children. J Autism Child Schizophr. 1977;7:49–55. doi: 10.1007/BF01531114. [DOI] [PubMed] [Google Scholar]
- Todd RD, Ciaranello RD. Demonstration of inter- and intraspecies differences in serotonin binding sites by antibodies from an autistic child. Proc Natl Acad Sci USA. 1985;82:612–616. doi: 10.1073/pnas.82.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Vincent A, Newland C, Brueton L, Beeson D, Riemersma S, Huson SM, Newsom-Davis J. Arthrogryposis multiplex congenita with maternal autoantibodies specific for a fetal antigen. Lancet. 1995;346:24–25. doi: 10.1016/s0140-6736(95)92652-6. [DOI] [PubMed] [Google Scholar]
- Volpe R, Ehrlich R, Steiner G, Row VV. Graves’ disease in pregnancy years after hypothyroidism with recurrent passive-transfer neonatal Graves’ disease in offspring. Therapeutic considerations. Am J Med. 1984;77:572–578. doi: 10.1016/0002-9343(84)90125-6. [DOI] [PubMed] [Google Scholar]
- Warren RP, Cole P, Odell JD, Pingree CB, Warren WL, White E, Yonk J, Singh VK. Detection of maternal antibodies in infantile autism. J Am Acad Child Adolesc Psychiatry. 1990;29:873–877. doi: 10.1097/00004583-199011000-00005. [DOI] [PubMed] [Google Scholar]
- Warren RP, Foster A, Margaretten NC. Reduced natural killer cell activity in autism. J Am Acad Child Adolesc Psychiatry. 1987;26:333–335. doi: 10.1097/00004583-198705000-00008. [DOI] [PubMed] [Google Scholar]
- Warren RP, Margaretten NC, Pace NC, Foster A. Immune abnormalities in patients with autism. J Autism Dev Disord. 1986;16:189–197. doi: 10.1007/BF01531729. [DOI] [PubMed] [Google Scholar]
- Weizman A, Weizman R, Szekely GA, Wijsenbeek H, Livni E. Abnormal immune response to brain tissue antigen in the syndrome of autism. Am J Psychiatry. 1982;139:1462–1465. doi: 10.1176/ajp.139.11.1462. [DOI] [PubMed] [Google Scholar]
- Zalcman S, Savina I, Wise RA. Interleukin-6 increases sensitivity to the locomotor-stimulating effects of amphetamine in rats. Brain Res. 1999;847:276–283. doi: 10.1016/s0006-8993(99)02063-6. [DOI] [PubMed] [Google Scholar]
- Zalcman SS. Interleukin-2 potentiates novelty- and GBR 12909-induced exploratory activity. Brain Res. 2001;899:1–9. doi: 10.1016/s0006-8993(01)02090-x. [DOI] [PubMed] [Google Scholar]
- Zimmerman AW, Connors SL, Matteson KJ, Lee LC, Singer HS, Castaneda JA, Pearce DA. Maternal antibrain antibodies in autism. Brain Behav Immun. 2007;21:351–357. doi: 10.1016/j.bbi.2006.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.