Abstract
BACKGROUND:
Hypotension and a resultant decrease in cerebral blood flow have been implicated in the development of cognitive dysfunction. We tested the hypothesis that nimodipine (NIMO) administered at the onset of nitroglycerin (NTG)-induced hypotension would preserve long-term associative memory.
METHODS:
The passive avoidance (PA) paradigm was used to assess memory retention. For PA training, latencies (seconds) were recorded for entry from a suspended platform into a Plexiglas tube where a shock was automatically delivered. Latencies were recorded 48 h later for a testing trial. Ninety-six Swiss-Webster mice (30–35 g, 6–8 wk), were randomized into 6 groups 1) saline (control), 2) NTG immediately after learning, 3) NTG 3 h after learning, 4) NTG and NIMO, 5) vehicle, and 6) NIMO alone. The extent of hypotension and changes in brain tissue oxygenation (PbtO2) and in cerebral blood flow were studied in a separate group of animals.
RESULTS:
All groups exhibited similar training latencies (17.0 ± 4.6 s). Mice subjected to hypotensive episodes showed a significant decrease in latency time (178 ± 156 s) compared with those injected with saline, NTG + NIMO, or delayed NTG (580 ± 81 s, 557 ± 67 s, and 493 ± 146 s, respectively). A Kruskal-Wallis 1-way analysis of variance indicated a significant difference among the 4 treatment groups (H = 15.34; P < 0.001). In a separate group of mice not subjected to behavioral studies, the same dose of NTG (n = 3) and NTG + NIMO (n = 3) caused mean arterial blood pressure to decrease from 85.9 ± 3.8 mm Hg sem to 31.6 ± 0.8 mm Hg sem and from 86.2 ± 3.7 mm Hg sem to 32.6 ± 0.2 mm Hg sem, respectively. Mean arterial blood pressure in mice treated with NIMO alone decreased from 88.1 ± 3.8 mm Hg to 80.0 ± 2.9 mm Hg. The intergroup difference was statistically significant (P < 0.05). PbtO2 decreased from 51.7 ± 4.5 mm Hg sem to 33.8 ± 5.2 mm Hg sem in the NTG group and from 38.6 ± 6.1 mm Hg sem to 25.4 ± 2.0 mm Hg sem in the NTG + NIMO groups, respectively. There were no significant differences among groups.
CONCLUSION:
In a PA retention paradigm, the injection of NTG immediately after learning produced a significant impairment of long-term associative memory in mice, whereas delayed induced hypotension had no effect. NIMO attenuated the disruption in consolidation of long-term memory caused by NTG but did not improve latency in the absence of hypotension. The observed effect of NIMO may have been attributable to the preservation of calcium homeostasis during hypotension, because there were no differences in the PbtO2 indices among groups.
Numerous studies have demonstrated that arterial low blood pressure correlates with decreased cognitive performance in patients with chronic hypotension as well as in healthy individuals.1-3 The problem is especially pronounced in the elderly.4,5 The effect of transient hypotension on cognitive function is less evident. The International Study of Postoperative Cognitive Dysfunction, the largest investigation of postoperative cognitive dysfunction after noncardiac surgery, found no association between intraoperative hypotension and postoperative neurocognitive performance.6 The majority of studies that have assessed cognition after induced hypotension suggested no relationship between postoperative neuropsychological changes and the level of intraoperative arterial blood pressure.7-9 More recent investigations, however, have revealed a significant correlation between intraoperative mean arterial blood pressure (MAP) and postoperative cognitive performance in hypertensive patients undergoing spinal surgery.10 The negative effect of acute transient hypotension on cognitive recovery after mild traumatic brain injury has also been documented.11 However, it is difficult to compare the results of these studies because the authors have used varying definitions of hypotension and a variety of cognitive tests to assess changes in different cognitive domains.
Clinical studies are inherently limited in their ability to control for many variables and, consequently, to separate the effects of various factors. From this point of view, experimental studies can prove helpful because they could use “pure” models of various degrees or duration of hypotension with or without anesthetics. Surprisingly, the effect of transient hypo-tension on cognitive function has received little attention in anesthesia research. These issues have never been studied systematically despite numerous investigations addressing various aspects of complete cerebral ischemia. The aim of our study was to investigate whether transient hypotension induces cognitive changes in adult mice. Moreover, we were interested in examining whether nimodipine (NIMO), an L-type calcium channel blocker that crosses the blood-brain barrier, would reduce the hypotension-induced impairment of learning and memory. Our previous investigation demonstrated that NIMO reversed transient cognitive dysfunction caused by moderate hypoxia in adult mice.12 In this study, we examined cognitive changes in animals subjected to nitroglycerin (NTG)-induced hypotension using a passive avoidance (PA) memory retention paradigm.
METHODS
The protocol was approved by the Institutional Animal Care and Use Committee of the New York University Medical Center. Swiss-Webster adult male mice (30–35 g, 6–8 wk old) were used in all experiments. Mice were housed in groups of 5 per cage with 12-h light/dark cycles. They were tested in the daylight phase. Food and water were available ad libitum.
The PA learning paradigm was implemented as described elsewhere.13,14 PA performance is an adaptive response to a stressful experience that serves as a measure of learning and long-term memory. The 1-trial PA test is a learning paradigm that has provided much of the experimental data on contextual and emotional memory consolidation in animals. The 2-compartment step-through PA apparatus was manufactured on site and consisted of 1) an entry platform (15 cm long and 2.75 cm wide) suspended 1 m above floor level with no exit except for the entrance to a shock compartment through a guillotine door, and 2) a transparent Plexiglas shock compartment (a tube measuring 13 cm long, 9 cm wide, and 13 cm high) set on the end of the table with a floor constructed of parallel bands of stainless steel. Foot shock was delivered to the steel bands from a custom-built Colbourn constant-current generator. After each session, animals were returned to their housing cages, and the apparatus was cleaned with a 50% ethanol/50% water solution.
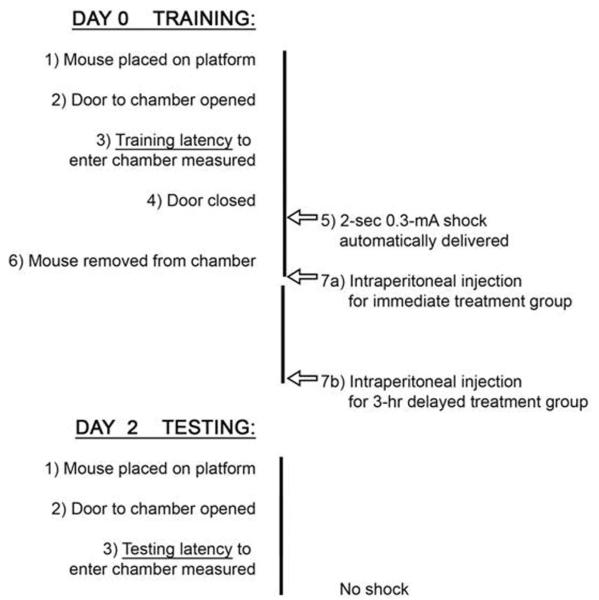
Figure 1 shows the sequence of events. On the acquisition day (training), mice were placed on the platform facing away from the door. The door was raised and training latencies (seconds) were automatically recorded for entry to the shock compartment. When the animal entered the compartment, the door was lowered and a 2-s 0.3-mA shock was automatically delivered. The initial latency to enter the dark compartment was recorded. Each mouse was trained only once. Mice had no other exposure to the entire apparatus before testing on Day 2. After training, animals had either immediate injection or delayed injection according to their randomized group. The memory retention test was performed 2 days later without any shock; the mice were again positioned on the raised platform facing away from the guillotine door and the time taken to enter the shock compartment was recorded as a measure of memory retention. A maximum retention latency of 600 s was given to mice that did not enter the shock compartment or that took longer than 600 s to enter it. Low latency is indicative of impairment of long-term associative memory, whereas high latency is suggestive of successful PA retention.
Figure 1.
Experimental schedule.
Ninety-six animals were randomly assigned to 6 groups. Five groups had intraperitoneal (IP) injection immediately after training: Group 1, saline; Group 2, NTG (60 mg/kg); Group 4, NTG (60 mg/kg) and NIMO (0.1 mg/kg dissolved in a vehicle of 60% polyethylene glycol/40% methanol by volume) together; Group 5, vehicle; and Group 6, NIMO. Group 3 had NTG (60 mg/kg) injection after a 3-h delay. This additional experiment was performed to test whether retention is disrupted by hypotension after memory consolidation (approximately first 3 h) has taken place. Several studies suggest that memory consolidation, a process through which memories are transformed from a labile into more stable state, lasts from 1 to 5 h.15,16
Changes in systemic blood pressure, pulse oximetry, regional brain tissue oxygen tension, and cerebral blood flow (CBF) were measured in additional groups of mice treated with NTG, NIMO, or NTG + NIMO so that behavioral measurements would not be compromised. Animals were anesthetized with isoflurane 1.2% for insertion and externalization of a 32-gauge femoral arterial catheter and stereotactic placement (Kopf model 900 Small Animal Stereotaxic Instrument, Tujunga, CA) of the cerebral tissue monitoring probe. Anesthesia was discontinued after injection of NTG or NTG + NIMO as MAP decreased and animals exhibited no response to external stimuli such as prodding or repositioning. Those rodents tolerated placement of a femoral arterial line and OxyLite brain probe without the need for the additional anesthetics (NTG and NTG + NIMO groups). Isoflurane at 0.4% was used during physiologic measurements after NIMO injection because NIMO administration did not produce unresponsiveness similar to NTG or NTG + NIMO. The catheter was connected to a pressure transducer and an MC 110 bridge amplifier. Arterial blood pressure was measured continuously for approximately 5 h after injection and recorded on a Power-Lab/200 data acquisition system (ADInstruments, Sydney, Australia). A MouseOx pulse oximetry device (STARR Life Sciences, Allison Park, PA) was used to measure oxygen saturation and heart rate. Local parenchymal perfusion (perfusion index, PInd) and brain tissue oxygenation (PbtO2) were measured with penetrating probes using the OxyLite/OxyFlo system (Oxford Optronix, Oxford, UK). The fiberoptic probes (diameter 250 μm) were positioned approximately 2 mm lateral to the midline and 2 mm inferior to the bregma at a depth of 1 mm. Regional tissue oxygen tension was expressed in millimeters of mercury. Laser Doppler flowmetry was used to measure relative changes in CBF (PInd). The probes were inserted slowly under visual control using a surgical microscope, and special care was taken not to injure blood vessels. Animals were not used if superficial hemorrhages were seen. PInd was measured throughout the hypotension period. Data were expressed as mean percent of the baseline value.
The measure of memory retention was latency to reenter the shock compartment. A Kruskal-Wallis 1-way analysis of variance (ANOVA) was used to compare this dependent variable in 4 treatment groups: 1) saline injection immediately after training, 2) NTG injection immediately after training, 3) NTG injection 3 h after training, and 4) NTG + NIMO injection immediately after training. Post hoc comparisons were computed using the Dunn multiple comparison test. All latencies (nonparametric values) are expressed as median ± interquartile ranges. The statistical significance was set at P < 0.05 in all of the analyses. Physiologic results are reported as means of measurements obtained during 5 h (30-min intervals) of data collection. Statistical analysis of physiologic variables at different conditions (treatments) was performed using 1-way ANOVA followed by Mann-Whitney test for multiple comparisons (NTG versus NTG + NIMO versus NIMO). The data were analyzed using SPSS (version 12) package.
RESULTS
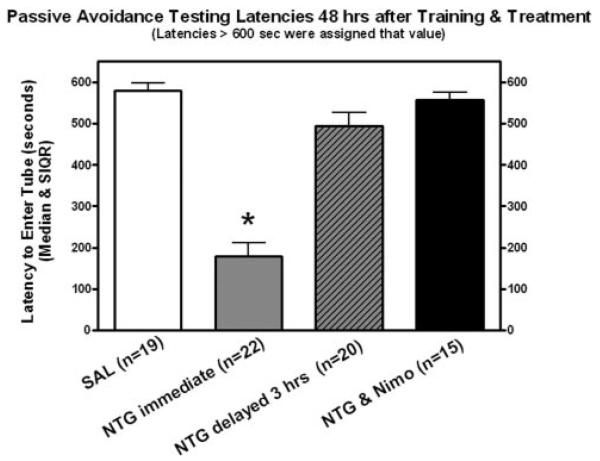
All groups exhibited similar training latencies (17.0 ± 4.6 s semiinterquartile range [SIQR]). Latencies to reenter the shock compartment during retention testing for treatment groups 1 (saline injection immediately after training), 2 (NTG injection immediately after training), 3 (NTG injection 3 h after training), and 4 (NTG + NIMO injection immediately after training) are shown in Figure 2. A Kruskal-Wallis 1-way ANOVA computed for these data indicated a significant difference among those 4 treatment groups (H = 15.34; P < 0.001). The NTG-treated group had significantly (P < 0.01) shorter latencies than the saline-treated control group thereby demonstrating that NTG induced retrograde amnesia for PA learning. The group treated with NTG 3 h after training exhibited significantly longer test latencies (P < 0.05) than the group treated with NTG immediately after learning. This finding indicates that the relationship between hypotension and amnesia is time dependent. Animals treated with NTG + NIMO simultaneously had significantly longer test latencies (P < 0.01) than mice given NTG alone. The results of this experiment suggest that a retrograde amnesia, which would generally follow NTG administration, can be prevented by coadministration of NIMO. The latencies for the other groups were as follows: Group 5 (vehicle alone, n = 10) 537 ± 94 s SIQR; Group 6 (NIMO alone, n = 10) 579 ± 110 s SIQR; mice were somnolent after injection of NTG. Animals did not respond to prodding and their righting reflex was absent. Animals began moving again after 5–6 h. They regained the righting reflex and resumed normal activity (i.e., moving around the cage and eating) after 12 h.
Figure 2.
Latencies to reenter the shock compartment on the retention test (passive avoidance paradigm). Latencies are reported as median ± semiinterquartile range. Latency decrease in nitroglycerin (immediate) group is statistically significant.
Table 1 shows changes in physiologic variables associated with a particular treatment. Baseline and post-treatment oxygen saturation in spontaneously breathing mice were >98% throughout the experiment. The temperature was maintained at 37°C ± 0.5°C with a warming blanket (Gaymar T/Pump, Orchard Park, NY) and did not vary significantly among groups during the course of the experiment. MAP in spontaneously breathing anesthetized animals was >85 mm Hg in all animals. MAP decreased by >60% after IP injection of NTG or NTG + NIMO, but only 10% after NIMO alone. There was a 65% decrease in PInd for all animals after NTG and NTG + NIMO injection. The initial PbtO2 values were 51.7 ± 4.5 mm Hg for the NTG group (after a 30-min stabilization period) and 38.6 ± 6.1 mm Hg for the NTG + NIMO group. The values decreased to 33.8 ± 5.2 mm Hg after NTG and to 25.4 ± 2 mm Hg after NTG + NIMO injections, but the differences did not reach statistical significance.
Table 1.
Effect of Treatments on Physiologic Variables
| NTG |
NTG + NIMO |
NIMO |
||||
|---|---|---|---|---|---|---|
| Dependent variables | Baseline | 5-h average | Baseline | 5-h average | Baseline | 5-h average |
| MAP (mm Hg) | 85.9 ± 3.8 | 31.6 ± 0.8* | 86.2 ± 3.7 | 32.6 ± 0.2* | 88.1 ± 3.8 | 80.0 ± 2.9 |
| % change from baseline | 36.8 ± 0.9* | 37.8 ± 0.9* | 90.7 ± 3.3 | |||
| PInd, % change from baseline | 100 | 30.4 ± 5.0* | 100 | 31.6 ± 0.8* | 100 | 89.8 ± 5.3 |
| PbtO2 (mm Hg) | 51.7 ± 4.5 | 33.8 ± 5.2 | 38.6 ± 6.1 | 25.4 ± 2.0 | 24.3 ± 4.8 | 23.2 ± 2.3 |
| % change from baseline | 65.3 ± 9.1 | 65.9 ± 5.3 | 95.2 ± 9.4 | |||
| O2 saturation | 99.0 ± 0.1 | 98.2 ± 0.1 | 98.9 ± 0.2 | 97.8 ± 0.7 | 98.5 ± 0.3 | 98.7 ± 0.1 |
| HR (bpm) | 463.7 ± 12.3 | 465.8 ± 7.2 | 424.0 ± 10.3 | 360.8 ± 17.9 | 431.7 ± 10.2 | 398.0 ± 8.5 |
All data are presented as mean ± sem. Change in mean arterial blood pressure (MAP) and perfusion index (PInd) is significantly larger in animals treated with nitroglycerin (NTG) or NTG + nimodipine (NIMO) than in the NIMO group (Mann-Whitney test).
PbtO2 = brain tissue oxygenation; HR = heart rate.
Statistical significance (P < 0.05).
DISCUSSION
Our results suggest that mice subjected to transient hypotension (approximately 60% of baseline) for 5 h immediately after learning develop a significant impairment of long-term associative memory as measured by the PA learning paradigm. Animals were somnolent during the hypotensive period but behaved normally after arousal. When the onset of hypo-tension was delayed (3 h after training), there was no effect on performance, suggesting that animals retain aversive behavior if hypotension is induced after memory consolidation. Associative memory for aversive stimuli was preserved in mice treated with NIMO at the time of NTG injection. PbtO2 and PInd were significantly decreased in all hypotensive animals compared with the baseline values. NIMO (when administered with NTG) administration did not change the degree of cerebral oxygenation or hypotension.
The hemorrhagic shock model has been used to examine neuronal damage after brief episodes (up to 3 min) of systemic hypotension in the rat, where MAP was maintained at about 25 mm Hg. Significant neuronal loss was observed after 3 min of hypotension in the hippocampal CA1 region17,18 as well as in the cortex.19 Neither PbtO2 nor PInd was measured in those experiments, but it is likely that both variables were below normal. Mild to moderate blood loss normally causes a decrease of resistance in the brain as part of the autoregulatory response. However, acute hemorrhage and hypotension cause an increase in cerebrovascular resistance and a disproportionate decrease in CBF.20 Both PbtO2 and local CBF are decreased profoundly in these circumstances,21 which, in turn, may lead to cell necrosis and apoptosis. Contrary to the vasoconstriction associated with severe hemorrhage, NTG dilates intracranial vessels.22 Although PbtO2 and PInd were decreased compared with baseline, they remained at a level that is not considered ischemic. Limitation of oxygen supply to the brain reduces cellular adenosine triphosphate (ATP) generation and leads to a collapse of ion gradients. This, in turn, leads to initiation of multiple intracellular processes that ultimately may result in neuronal injury (ischemia). The so-called “critical oxygen tension” is defined as PbtO2 at which the first results of decreased ATP production begin to be seen. An occurrence of neuronal injury is unlikely as long as PbtO2 is above that critical level. Rolett et al.23 measured both the brain tissue oxygen tension and the high-energy phosphate compounds simultaneously. They suggested that the initial signs of cell failure decrease in pH and in the creatinine/[Pi] appear at cortical PbtO2 of 6.8–8.8 mm Hg. Folbergrová et al.24 observed a decrease in ATP and increases in ADP and AMP at PbtO2 <6 mm Hg. It is unlikely that cellular integrity will be compromised at PbtO2 >20 mm Hg. Thus, we think that NTG-induced hypotension does not lead to neuronal damage (although only a histological investigation can address this issue directly) but to metabolic changes involving the synthesis and turnover of neurotransmitters that are essential to memory and cognition. Indirect support for the assertion that hypotension impairs only memory consolidation is provided by the presence of normal locomotion and normal performance on the object recognition test of short-term working memory 24 and 48 h after a hypotensive episode.25 In addition, mice injected with NTG 3 h after the aversive stimuli (presumably after the memory was formed) behaved similar to untreated animals. These observations in conjunction with the available data suggest that impairment in memory consolidation is transient and probably not caused by a permanent neuronal injury.
The observed salutary effect of NIMO on preservation (and improvement in some circumstances) of memory under physiologic stress is in agreement with previous investigations. NIMO preserves performance in PA tasks, the radial maze, and the object recognition test after memory disruption induced by exposure to hypoxia.12,26
Although the final neurochemical pathway leading to impairment of memory formation may be similar to the one observed in hypoxia, this lasting effect of transient hypotension and subsequent reversal of memory impairment has not been reported.
NIMO (a cerebral vasodilator) increases CBF after ischemia. Increasing blood flow could increase oxygen and glucose delivery to the brain, which may potentially prevent changes in synthesis of oxygen/glucose-dependent neurotransmitters. However, our data indicate that administration of NIMO did not affect MAP, PbtO2, or the cerebral PInd. Thus, it is likely that the observed effect is related to the restoration of neurotransmitter concentrations via stabilization of the cellular membrane rather than an increase in CBF.
NTG may act both directly and indirectly on the central nervous system.27 The vasodilatory effect of NTG is well documented and depends on the formation of nitric oxygen (NO) in blood vessels. The resulting hypotension causes a massive release of monoaminergic neurotransmitters. More recent data suggest that NTG (via release of NO) also directly activates brain structures that are involved in the control of sympathetic, nociceptive, and behavioral functions.28 Activation of the monoaminergic system may enhance cognition and memory formation. In addition, NO is an intracellular messenger in the brain. Smith et al.29 found that NO-stimulated guanylyl cyclase activation and cyclic guanosine monophosphate formation in the brain improve task acquisition in cognitively impaired animals. The authors observed the improvement in task learning with 2,3-dinitrooxy-2, 3-bis-nitrrxypropyldisulfanyl)-propane (effective NO donor) but not with the low concentration of NTG (500 mmol/kg). We report impairment rather than enhancement of cognitive function after administration of substantially higher doses of NTG. It is likely that the negative effect of profound hypotension on memory formation overwhelms any direct positive effect of NO on cognition.
This study has several important limitations. First, the design of the present experiments did not include histo-logical assessment of neuronal necrosis and/or apoptosis. Although this would have been of great interest considering the paucity of these types of studies (such is in our plans), this information is beyond the scope of this study. Our main goal in this investigation was to examine whether transient hypotension affected memory processes during insult. Second, the animals were not tested beyond the second day. It is possible that the NIMO-associated improvement in performance could well be transient and would not persist beyond the second day. Third, our physiologic data may have been affected by the effect of isoflurane on cerebral vasculature and respiration, especially in the NIMO group. Isoflurane was used only during instrumentation in the NTG and NTG + NIMO groups. Although the effect of isoflurane on physiologic measurement cannot be excluded, it is likely that a residual concentration of isoflurane would have only minimally affected the results. Finally, we did not measure concentrations of neuro-transmitters in the brain. Although a significant body of literature on this subject is available, the proposed mechanism of cognitive changes caused by hypotension remains speculative.
We conclude that memory retention as measured by the PA learning paradigm is impaired in adult mice exposed to profound hypotension caused by NTG. Administration of NIMO simultaneously with NTG preserves the memory consolidation process.
Acknowledgments
Supported in part by National Institute of Aging/NYU Alzheimer's Disease Core Center Grant AG09051-19.
Footnotes
Presented, in part, at the Annual American Society of Anesthesiologists Meeting, October, 2008.
REFERENCES
- 1.Duschek S, Schandry R. Reduced brain perfusion and cognitive performance due to constitutional hypotension. Clin Auton Res. 2007;17:69–76. doi: 10.1007/s10286-006-0379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuccala G, Onder G, Pedone C, Carosella L, Pahor M, Bernabei R, Cocchi A. Hypotension and cognitive impairment: Selective association in patients with heart failure. Neurology. 2001;57:1986–92. doi: 10.1212/wnl.57.11.1986. [DOI] [PubMed] [Google Scholar]
- 3.Wharton W, Hirshman E, Marritt P, Stangl B, Scanlin K, Krieger L. Lower blood pressure correlates with poorer performance on visuospatial attention task in younger individuals. Biolog Physiol. 2006;73:227–34. doi: 10.1016/j.biopsycho.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Yap PL, Niti M, Yap KB, Ng TP. Orthostatic hypotension, hypotension and cognitive status: early comorbid markers of primary dementia? Dement Geriatr Cogn Disord. 2008;26:239–46. doi: 10.1159/000160955. [DOI] [PubMed] [Google Scholar]
- 5.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–99. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 6.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Joilles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–61. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 7.Williams-Russo P, Sharrock NE, Mattis S, Liguori GA, Mancuso C, Peterson MG, Hollenberg J, Ranawat C, Salvati E, Sculco T. Randomized trial of hypotensive epidural anesthesia in older adults. Anesthesiology. 1999;91:926–35. doi: 10.1097/00000542-199910000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Choi SH, Lee SJ, Jung YS, Shin YS, Jun DB, Hwang KH, Liu J, Kim KI. Nitroglycerin- and nicardipine-induced hypotension does not affect cerebral oxygen saturation and postoperative cognitive function in patients undergoing orthognatic surgery. J Oral Maxillofac Surg. 2008:2104–9. doi: 10.1016/j.joms.2008.06.041. [DOI] [PubMed] [Google Scholar]
- 9.Townes BD, Dikmen SS, Bledsoe SW, Hornbein TF, Martin DC, Janesheski JA. Neurophsychological changes in a young, healthy population after controlled hypotensive anesthesia. Anesth Analg. 1986;65:955–9. [PubMed] [Google Scholar]
- 10.Yocum GT, Gaudet JG, Teverbaugh LA, Quest DO, McCormick PC, Connolly ES, Heyer EJ. Neurocognitive performance in hypertensive patients after spine surgery. Anesthesiology. 2009;110:254–61. doi: 10.1097/ALN.0b013e3181942c7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schutz C, Stover JF, Thomson HJ, Hoover RC, Morales DM, Schouten JW, McMillan A, Soltesz K, Molta M, Spangler Z, Neugebauer E, McIntosh TK. Acute transient hemorrhagic hypotension does not aggravate structural damage or neurologic motor deficits but delays the long-term cognitive recovery following mild to moderate traumatic brain injury. Crit Care Med. 2006;34:492–501. doi: 10.1097/01.ccm.0000198326.32049.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haile M, Limson F, Gingrich K, Li Y-S, Quartermain D, Blanck T, Bekker A. Nimodipine prevents transient cognitive dysfunction after moderate hypoxia in adult mice. J Neurosurg Anesth. 2009;21:140–4. doi: 10.1097/ANA.0b013e3181920d28. [DOI] [PubMed] [Google Scholar]
- 13.Quartermain D, deSoria VG, Kwan A. Calcium channel antagonists enhance retention of passive avoidance and maze learning in mice. Neurobiol Learn Mem. 2001;75:77–90. doi: 10.1006/nlme.1999.3958. [DOI] [PubMed] [Google Scholar]
- 14.Quartermain D, Hawxhurst A, Ermita B, Puente J. Effect of the calcium channel blocker amlodipine on memory in mice. Behav Neural Biol. 1993;60:211–9. doi: 10.1016/0163-1047(93)90390-4. [DOI] [PubMed] [Google Scholar]
- 15.Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- 16.McGaugh JL. Memory—a century of consolidation. Science. 2000;287:248–51. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi Y, Kato H, Kogure K. Brain damage in a new hemorrhagic shock model in the rat using long-term recovery. J Cereb Blood Flow Metab. 1990;10:207–12. doi: 10.1038/jcbfm.1990.36. [DOI] [PubMed] [Google Scholar]
- 18.Yamauchi Y, Kato H, Kogure K. Hippocampal damage following repeated brief hypotensive episodes in the rat. J Cereb Blood Flow Metab. 1991;11:974–8. doi: 10.1038/jcbfm.1991.163. [DOI] [PubMed] [Google Scholar]
- 19.Chaparro RE, Quiroga CE, Karlnoski R, Mangar D, Camporesi EM. Brief systemic hypotension results in cortical neuronal loss in Sprague Dawley rats. Anesthesiology. 2008;109:A1517. [Google Scholar]
- 20.Lomas-Niera JL, Perl M, Chung CS. Shock and hemorrhage: an overview of animal models. Shock. 2005;24(suppl):33–9. doi: 10.1097/01.shk.0000191411.48719.ab. [DOI] [PubMed] [Google Scholar]
- 21.Cavus E, Meybolun P, Dorges V, Stadlbauer KH, Wenzel V, Weiss H, Scholz J, Bein B. Regional and local brain oxygenation during hemorrhagic shock: a prospective experimental study on the effects of small-volume resuscitation with norepinephrine. J Trauma. 2008;64:641–9. doi: 10.1097/TA.0b013e3181637a6c. [DOI] [PubMed] [Google Scholar]
- 22.Siepmann M, Kirch W. Effects of nitroglycerine on cerebral blood flow velocity, quantitative electroencephalogram and cognitive performance. Eur J Clin Invest. 2000;30:832–7. doi: 10.1046/j.1365-2362.2000.00713.x. [DOI] [PubMed] [Google Scholar]
- 23.Rolett E, Azzawi A, Liu KJ, Yongbi MN, Swartz HM, Dunn JF. Critical oxygen tension in rat brain: a combined 31P-NMR and EPR oximetry study. Am J Physiol. 2000;279:R9–16. doi: 10.1152/ajpregu.2000.279.1.R9. [DOI] [PubMed] [Google Scholar]
- 24.Folbergrová J, Minamisawa H, Ekholm A, Siesjö BK. Phosphorylase alpha and labile metabolites during anoxia: correlation to membrane fluxes of K+ and Ca2+ J Neurochem. 1990;55:1690–6. doi: 10.1111/j.1471-4159.1990.tb04957.x. [DOI] [PubMed] [Google Scholar]
- 25.Haile M, Rocco M, Li Y-S, Quartermain D, Blanck T, Bekker A. Nitroglycerin induced hypotension causes delayed transient cognitive dysfunction in adult mice. Anesth Analg. 2008;106(suppl):S–134. [Google Scholar]
- 26.Mrsic J, Zupan G, Erakovic V, Simonić A, Varljen J. The influence of nimodipine and MK-801 on the brain free arachidonic acid level and the learning ability in hypoxia-exposed rats. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:345–58. doi: 10.1016/s0278-5846(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 27.Tassorelli C, Joseph SA, Buzz MG, Nappi G. The effects on the central nervous system of nitroglycerin-putative mechanisms and mediators. Prog Neurobiol. 1999;57:607–24. doi: 10.1016/s0301-0082(98)00071-9. [DOI] [PubMed] [Google Scholar]
- 28.Tassorelli C, Blandini F, Costa A, Preza E, Nappi G. Nitroglycerin-induced activation of monoaminergic transmission in the rat. Cephalalgia. 2002;22:226–32. doi: 10.1046/j.1468-2982.2002.00355.x. [DOI] [PubMed] [Google Scholar]
- 29.Smith S, Dringenberg HC, Bennett BM, Thatcher GRJ, Reynolds JN. A novel nitrate ester reverses the cognitive impairment caused by scopolamine in the Morris water maze. Neuroreport. 2000;11:3883–6. doi: 10.1097/00001756-200011270-00055. [DOI] [PubMed] [Google Scholar]