Abstract
Preclinical reports support the concept of synergy between cancer vaccines and immune checkpoint blockade in non-immunogenic tumors. In particular, cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) antibodies have been successfully combined with GM-CSF cell-based vaccines (GVAX). Ipilimumab (anti-CTLA-4), has been tested as a single agent in patients with pancreatic ductal adenocarcinoma (PDA) resulting in one delayed response at a dose of 3mg/kg. Our study evaluated Ipilimumab 10mg/kg (arm 1) and Ipilimumab 10mg/kg + GVAX (arm 2). 30 patients with previously treated advanced PDA were randomized (1:1). Induction doses were administered every 3 weeks for a total of 4 doses followed by maintenance dosing every 12 weeks. Two patients in arm 1 showed evidence of stable disease (7 & 22 weeks) but none demonstrated CA19-9 biochemical responses. In contrast, 3 patients in arm 2 had evidence of prolonged disease stabilization (31, 71, & 81 weeks) and 7 patients experienced CA19-9 declines. In 2 of these patients, disease stabilization occurred after an initial period of progression. The median overall survival (OS) (3.6 vs 5.7 months, HR: 0.51, p=0.072) and 1 year OS (7 vs 27%) favored arm 2. Similar to prior Ipilimumab studies, 20% of patients in each arm had Grade 3/4 immune-related adverse events. Among patients with OS > 4.3 months, there was an increase in the peak mesothelin-specific T cells (p=0.014) and enhancement of the T cell repertoire (p=0.031). In conclusion, checkpoint blockade in combination with GVAX has the potential for clinical benefit and should be evaluated in a larger study.
Keywords: CTLA-4, GVAX, pancreatic cancer, vaccine, Ipilimumab
Introduction
Even with the recent progress in the treatment of metastatic pancreatic ductal adenocarcinoma (PDA), the median survival in the best performance status patients remains 11 months1,2. Progress has been made with immunotherapy for traditionally immunogenic cancers such as melanoma and even in some tolerogenic cancers such as lung and prostate cancer3-7. Despite the view that PDA is a particularly nonimmunogenic cancer, data suggest that immune responses and anti-tumor responses can be induced in PDA8-11.
Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) functions as a negative regulator of T cell activation. Ipilimumab, a CTLA-4 antagonist antibody, has been tested in patients with advanced PDA12. While single agent Ipilimumab at 3 mg/kg was minimally effective, a significant delayed response in one patient suggests that immunotherapy could play a role in PDA. Melanoma studies demonstrated a doseresponse relationship with Ipilimumab and the dose of 10mg/kg was selected from prior studies4,13. Furthermore, preclinical studies show synergy between anti-CTLA-4 antibodies and granulocyte macrophage colony stimulating factor (GM-CSF) cell-based vaccines14-16. The current PDA trial builds on these observations by evaluating Ipilimumab at 10mg/kg alone or in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene (GVAX) for the treatment of previously treated, locally advanced or metastatic PDA.
Patients and Methods
Patients
Study protocol (NCT00836407) was approved by the Johns Hopkins institutional review board, institutional biosafety committee, the FDA and the NIH Recombinant DNA Advisory Committee. Participating patients signed informed consent.
Patients were eligible for enrollment if they had previously treated, locally advanced or metastatic histologically proven PDA, were >18 years, had received gemcitabine-based chemotherapy, had Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1 with normal hematologic and renal function, AST/ALT/alkaline phosphatase < 2.5 × upper limit of normal (ULN) (<5 × ULN for patients with liver metastases), bilirubin <1.5 × ULN, and an expected survival of 9 weeks. Individuals were excluded if they had infection with HIV, hepatitis B or C, a history of brain metastases, autoimmune disease, prior CTLA-4 inhibitor or agonist use, surgery, radiation, chemotherapy, vaccination, or steroids within 28 days of study initiation.
Study design and treatment
This was a phase 1b, open-label, randomized study performed at Johns Hopkins University (JHU, Baltimore, MD). The primary objective of the study was to determine the safety profile of Ipilimumab alone or in combination with GVAX in patients with previously treated PDA. Secondary objectives included estimation of overall survival (OS), comparison of OS between treatment groups, measurement of CA19-9 kinetics, exploration of an association of mesothelin-specific T cell responses with OS, and estimation of overall response rate and immune-related response. Immune-related response criteria (irRC) account for the kinetics of both old and new lesions given the known for potential delayed responses with Ipilimumab17. Thirty patients with PDA were enrolled at JHU between March 11, 2009 and December 6, 2010 with follow-up censored as of January 27, 2013. All patients were included in the safety and efficacy analyses. Patients were randomized in a 1:1 fashion to Ipilimumab alone (arm 1) or Ipilimumab + GVAX (arm 2) using a randomized block design. In both arms, Ipilimumab 10mg/kg was administered intravenously (IV) over 90 minutes. In arm 2, prior to the Ipilimumab infusion, patients received GVAX, which consists of 2 pancreatic tumor cell lines (Panc 6.03 and Panc 10.05) which have been modified with a plasmid vector encoding the cDNA for human GM-CSF and subsequently cultured and irradiated8. The vaccine consists of Panc 6.03 and Panc 10.05 cells (2.5 × 108 cells each) combined into a single vaccine and administered as intradermal injections, 2 each in the right and left thighs and 2 in the non-dominant arm. Biosafety level 2 practices were employed for the containment of GVAX.
Treatments were administered at weeks 1, 4, 7, and 10. CT scans (MRI if CT contraindicated) were performed for tumor assessments (TA) at weeks 1, 7, 14, and 22. Patients with progressive disease (PD) without rapid clinical deterioration could continue on study treatment. At the week 22 evaluation, patients with evidence of a response or stable disease (SD) were offered maintenance dosing of the originally assigned treatment every 12 weeks. TAs were performed every 12 weeks during maintenance. Patients with early progression followed by SD or better between weeks 14 and 22 were also eligible for maintenance. Patients were followed by telephone contact every 12 weeks to evaluate survival, disease status, and adverse events (AE).
Patient monitoring and toxicity criteria
Adverse events were graded using the NCI Common Terminology Criteria for Adverse Events (CTCAE) v3.0. For purposes of determining unacceptable toxicity during the initial 22 week treatment phase, patients were followed for drug related >grade 4 AE or grade 3 AEs including immune-related adverse events (IRAE) not improving to <grade 2 under therapy within 2 weeks. In addition, >grade 2 eye pain or reduction of visual acuity that did not respond to topical therapy within 2 weeks was also an unacceptable toxicity. A 3+3 design was used to determine whether or not the toxicity was acceptable for the first 6 patients in each arm. If the toxicity rate was <33% then the remaining patients would be enrolled in that arm. The proportion of patients with unacceptable toxicities was continuously monitored. If the toxicity level in the combination arm was >2/6, then the dose of Ipilimumab could be reduced to 5mg/kg for the combination arm only. Intrapatient dose de-escalations were not permitted. Adverse events 70 days after the last dose were recorded if possibly-related to the investigational agents.
There were no protocol-defined unacceptable toxicities in the first 6 patients in either arm and enrollment continued to complete the goal of 15 patients per arm.
Immunologic assessments
Detection of mesothelin-specific CD8+ T cells by IFNγ-ELISPOT
Synthesis of peptides, ELISA assays for identifying mesothelin peptides, and ELISPOT assays have previously been described9-11. Peripheral blood lymphocytes (PBL) were collected at baseline, prior to each dose, 28 days after maintenance doses, and at the off study visit. PBLs from patients expressing HLA-A*0101 and/or HLA-A*0201 alleles were tested if pre- and post-treatment samples were available. T cell responses to mesothelin peptides were adjusted for background measured against irrelevant melanoma or renal cell carcinoma control peptides. Responses were measured to eight HLA-A*0101 and six HLA-A*0201 mesothelin peptides. The sum of the T cell responses to mesothelin peptides are reported. The size of the mesothelin-specific T cell repertoire was defined as the percentage of peptides for which an induction was measured. A response was considered to be induced when the frequency of specific T cells was > 5 in 1×105 CD8+ PBL and increased by ≥ 2-fold compared to baseline.
Clinical assessments
Radiographic imaging was obtained at the specified time points. Response was assessed by RECIST v1.0 and irRC. CA19-9 serum levels were measured at regular intervals. Overall survival was defined as the time from enrollment until death or loss to follow-up.
Statistical considerations
Fifteen patients in each arm were enrolled to refine estimates of toxicity and initial efficacy measurements. Comparisons of continuous and categorical characteristics were made using Wilcoxon rank-sum tests and Fisher's exact tests, respectively. For each arm, Kaplan Meier estimates of the survival curve were calculated and used to estimate median OS and the proportion of individuals alive at 1 year with 95% confidence intervals. Comparisons between groups were made using log-rank tests. Differences between pre and post-treatment immune responses were compared using Wilcoxon sign-rank tests.
Results
Patient Characteristics, Safety, and Tolerability
Patient characteristics are shown in Table 1. Baseline characteristics were similar among patients in each arm with the exception that arm 1 had fewer patients with > 2 prior therapies (60 vs 100%, p=0.017). The most common AEs reported for Ipilimumab therapy were IRAEs; the most common AEs reported for GVAX vaccines were localized vaccine reactions and self-limiting systemic rashes. Table 2 summarizes IRAEs observed during all treatment cycles by arm and CTCAE grade. The rate of IRAEs attributable to Ipilimumab was similar to what has been reported in other studies testing Ipilimumab at the 10mg/kg dose. 73% and 80% of patients in arm 1 and 2, respectively, experienced any grade IRAE and 20% of patients in both arms experienced Grade 3-4 IRAEs (colitis, Guillain-Barre Syndrome (GBS), nephritis in arm 1; colitis, rash, pneumonitis in arm 2). The case of nephritis was considered an unacceptable toxicity because while the patient died from progressive disease, he required hemodialysis. The case of pneumonitis was considered an unacceptable toxicity because it took 25 days to resolve to <grade 2. Arm 2 did have a higher rate of diffuse rashes (73% vs 53%, p=0.45). Although only one rash was classified as a grade 3, the rashes in these patients were more symptomatic but often lessened in severity with subsequent doses. Grade 1-2 fatigue was also frequently reported in arm 2. Seven patients (3 in arm 1, 4 in arm 2) with symptomatic diarrhea were diagnosed with diarrhea/colitis. The median time to onset to the highest grade of an IRAE was 53 days (range 15-221 days). Median time to resolution to grade 1 with steroids was 19 days with a range of 1 to 38 days. With the exception of the case of nephritis, the IRAEs did respond to steroids. The GBS case was also treated with intravenous immunoglobulin (IVIG) and technically resolved only to a grade 2 because the patient continued to use a cane.
Table 1.
Patient Characteristics
| Total (N=30) | Arm 1 (N=15) | Arm 2 (N=15) | P value | |
|---|---|---|---|---|
| Age, Median Year (range) | 0.77 | |||
| Median | 62 (44-77) | 63 (44-73) | 62 (49-77) | |
| Sex, N (%) | >0.99 | |||
| Male | 21 (70) | 11 (73) | 10 (67) | |
| Female | 9 (30) | 4 (27) | 5 (33) | |
| ECOG, N (%) | 0.70 | |||
| 0 | 10 (33) | 6 (40) | 4 (27) | |
| 1 | 20 (67) | 9 (60) | 11 (73) | |
| Prior Chemotherapy Regimens, N (%) | 0.017 | |||
| 1 | 6 (20) | 6 (40) | 0 (0) | |
| 2 | 10 (33) | 4 (27) | 6 (40) | |
| 3 | 12 (40) | 4 (27) | 8 (53) | |
| 4 | 2 (7) | 1 (7) | 1 (7) | |
| Metastatic Sites, N (%) | >0.99 | |||
| 0* | 4 (13) | 2 (13) | 2 (13) | |
| 1 | 6 (20) | 3 (20) | 3 (20) | |
| 2+ | 20 (67) | 10 (67) | 10 (67) |
(Locally Advanced, Recurrent)
Table 2.
Adverse Events
| Arm 1 N=15 | Arm 2 N=15 | Total N=30 | ||||
|---|---|---|---|---|---|---|
| Immune Related Adverse Events | Grade 1/2 | Grade 3/4 | Grade 1/2 | Grade 3/4 | Grade 1/2 | Grade 3/4 |
| Dermatological | ||||||
| Localized | 0 | 0 | 15 | 0 | 15 | 0 |
| Systemic* | 9 | 0 | 11 | 1 | 20 | 1 |
| Endocrine | ||||||
| Adrenal insufficiency | 0 | 0 | 1 | 0 | 1 | 0 |
| Hypophysitis | 2 | 0 | 1 | 0 | 3 | 0 |
| HEENT | ||||||
| Conjunctivitis | 1 | 0 | 0 | 0 | 1 | 0 |
| Gastrointestinal | ||||||
| Colitis | 2 | 1 | 3 | 1 | 5 | 2 |
| Neurological | ||||||
| Guillain-Barre Syndrome | 0 | 1 | 0 | 0 | 0 | 1 |
| Pulmonary | ||||||
| Pneumonitis | 0 | 0 | 0 | 1 | 0 | 1 |
| Renal | ||||||
| Nephritis | 0 | 1 | 0 | 0 | 0 | 1 |
| Other Related Adverse Events | ||||||
| Constitutional | ||||||
| Arthralgias | 0 | 0 | 1 | 0 | 1 | 0 |
| Anorexia | 0 | 0 | 2 | 0 | 2 | 0 |
| Cramps | 1 | 0 | 0 | 0 | 1 | 0 |
| Diarrhea | 0 | 0 | 2 | 0 | 2 | 0 |
| Dry eye | 1 | 0 | 0 | 0 | 1 | 0 |
| Dry skin | 1 | 0 | 0 | 0 | 1 | 0 |
| Fatigue | 0 | 0 | 6 | 0 | 6 | 0 |
| Fever | 3 | 0 | 5 | 0 | 8 | 0 |
| Flu-like symptoms | 2 | 0 | 4 | 0 | 6 | 0 |
| Headache | 0 | 0 | 3 | 0 | 3 | 0 |
| Nausea | 1 | 0 | 2 | 0 | 3 | 0 |
While there was only one grade 3 rash observed in arm 2, rashes were more severe/symptomatic than in arm 1.
Overall survival
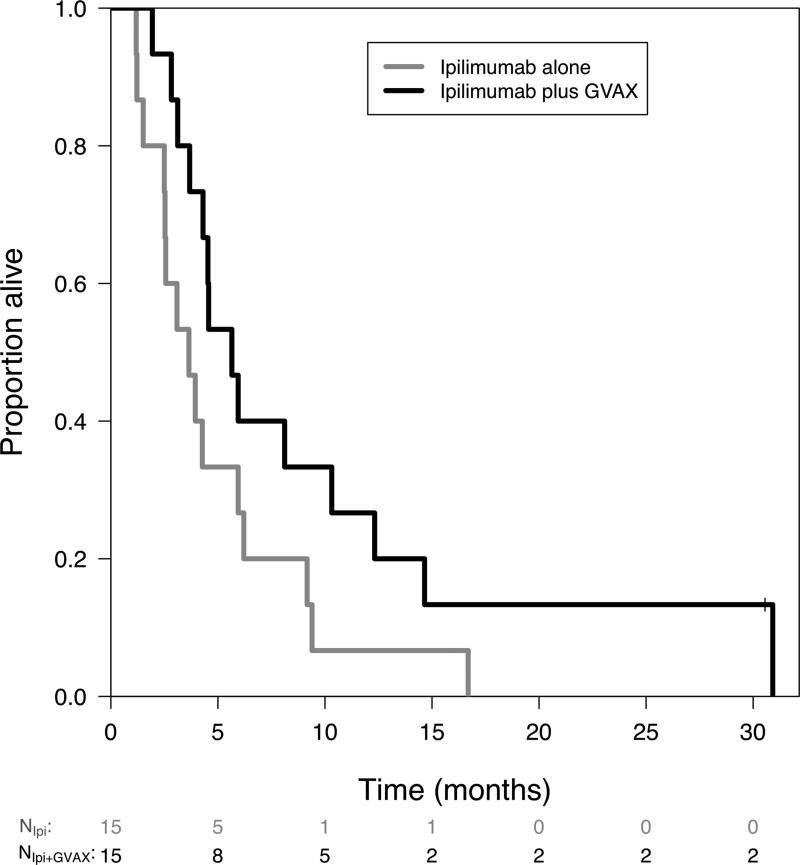
Median OS for the all patients was 4.3 months (95% CI: 3.65 to 8.11). Median OS was 3.6 months (95% CI: 2.5 to 9.2) for arm 1 and 5.7 months (95% CI: 4.3 to 14.7) for arm 2 (HR: 0.51, 95% CI: 0.23 to 1.08, p=0.072). The percent alive after one year also favored the combination arm (7% vs 27%) (95% CI: 1 to 45% vs 11 to 62%) (Figure 1).
Figure 1. Survival.
Kaplan-Meier overall survival curve as of 1/27/2013. One patient in arm 2 (Ipilimumab + GVAX) is still alive.
Mesothelin-specific T cell responses
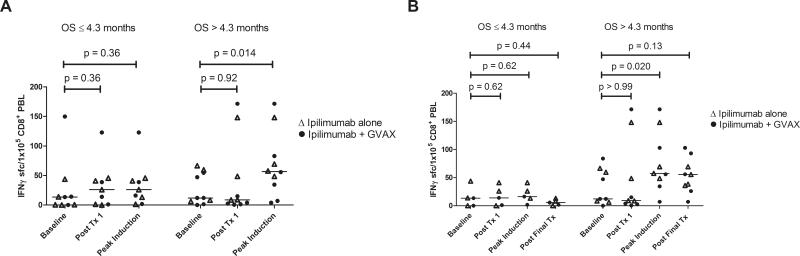
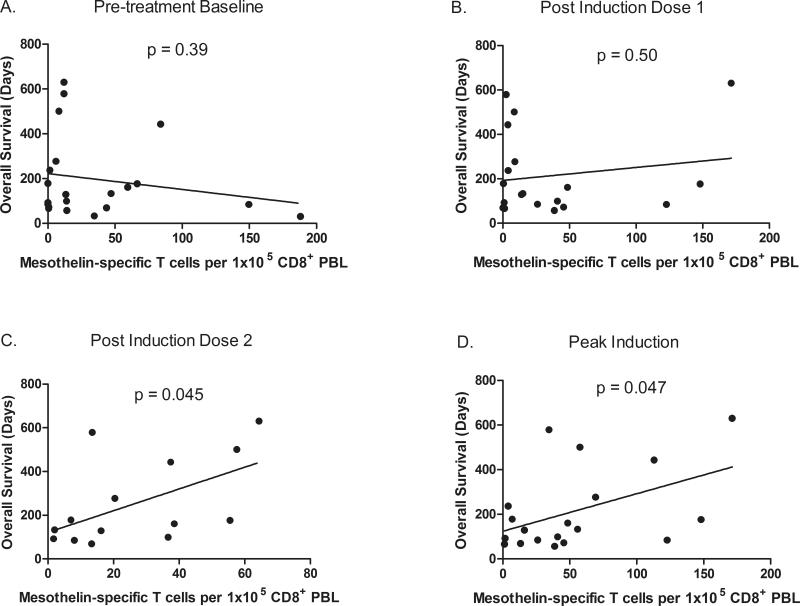
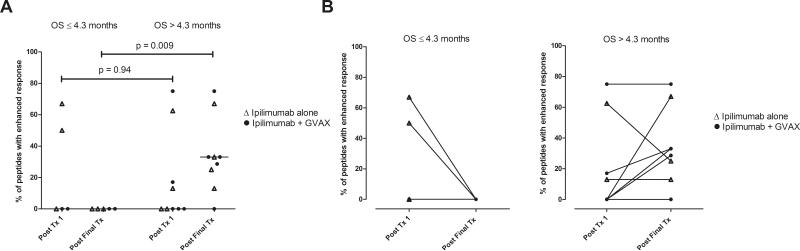
Enhanced post-vaccination mesothelin-specific T cell responses were associated with increased disease-free survival (DFS) and OS in prior GVAX studies8-11. Mesothelin-specific T cell responses were analyzed in PBL from 19 HLA-A1 and/or HLA-A2 positive patients with at least one post-treatment PBL sample. Baseline, posttreatment 1, and peak-induction T cell responses are shown in Figure 2A as a correlate of OS for the combined treatment arms. Although mesothelin-specific T cell responses were not enhanced following the first treatment in either group, there was a significant induction of peak post-treatment responses among patients with OS > 4.3 months (p=0.014). Mesothelin-specific T cell responses were measured following 2 or more treatments in 14 of the 19 patients and are shown in Figure 2B. Similar to the analysis for all 19 patients evaluated, T cell responses were not enhanced following the first treatment for either group, and peak post-treatment responses were enhanced only in patients with OS > 4.3 months (p=0.020) and remained elevated throughout treatment, albeit not significantly (p=0.13). Similarly, when levels of circulating mesothelin-specific T cells were compared to OS, significant correlations were only seen following the second treatment (p=0.045) and at the time of peak induction (p=0.047), but not at baseline (p=0.39) or following the first treatment (p=0.50) (Figures 3A-D). Comparisons following the third and fourth induction treatments were not performed because too few PBL samples were analyzed at these later time points. The size of the mesothelinspecific T cell repertoire measured following the first and final treatments in the 14 patients who received 2 or more treatments are shown in Figure 4A. T cell repertoire size was similar following the initial treatment, but significantly larger following multiple treatments among patients with OS > 4.3 months (p=0.009). Furthermore, expansion in the repertoire was observed in six of nine patients with OS > 4.3 months but not in any of the five patients with OS ≤ 4.3 months (Figure 4B) and was associated with longer OS (p=0.031).
Figure 2. Longer survival is associated with an induction of CD8+mesothelinspecific T cell responses.
Mesothelin peptide-specific CD8+ T cells were quantitated in pre- and post-treatment PBL using IFNγ ELISPOT assays. A) Mesothelin-specific T cell frequencies measured at baseline, following the first treatment, and at the peak of induction in 19 HLA-A1+ and/or HLA-A2+ patients receiving at least one treatment. B) Mesothelin-specific T cell frequencies measured at baseline, following the first treatment, at the peak of induction, and following the final treatment in 14 of the 19 patients who received at least two treatments. Patients in both treatment arms (Ipilimumab alone = open triangles; Ipilimumab + GVAX = solid circles) were grouped together based on survival of greater than or less than 4.3 months. Post-treatment T cell levels were compared to baseline levels using Wilcoxon sign-rank tests.
Figure 3. Increased levels of peak and post-induction dose 2 mesothelin-specific T cells are associated with longer survival.
Frequencies of mesothelin-specific T cells measured by IFNγ ELISPOT in PBL isolated A) prior to treatment, B) following the initial treatment, C) following the second treatment and D) at the time of peak induction are plotted against overall survival. Linear regressions were performed and respective p values are shown for each timepoint.
Figure 4. Longer survival is associated with post-treatment expansion of the mesothelin-specific CD8+ T cell repertoire.
The percentage of mesothelin peptides for which enhanced T cell responses were measured following the first (Post Tx1) and final treatments (Post Final Tx) are shown for 14 patients receiving two or more treatments with Ipilimumab alone (open triangles) or Ipilimumab + GVAX (solid circles). A) T cell repertoires in patients grouped based on survival of less than or greater than 4.3 months. Comparisons between group T cell repertoires were made using Mann Whitney tests. B) Changes in mesothelin-specific T cell repertoires in patients surviving 4.3 months or less (left) and greater than 4.3 months (right).
Antitumor activity
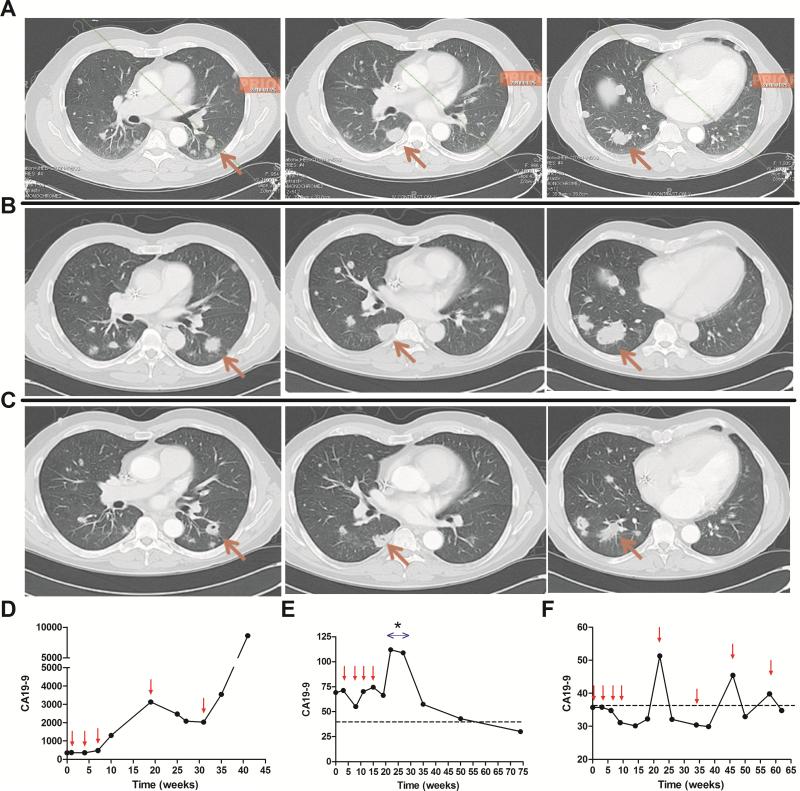
The best RECIST response was SD in two patients in arm 1 and two patients in arm 2. Using the irRC, arm 2 had an additional patient with SD until week 81. The quality of the responses in the two arms was different. Patients with SD on arm 1 had continuous disease progression that did not reach the 20% growth cutoff for 7 and 22 weeks. Arm 2 had three SD (1 regression starting at week 14 and maintained until week 31, one stabilization starting at week 22 and maintained until week 81, one SD lasting until week 71). Disease stabilization occurred by the week 22 scan in all of the patients. Maintenance phase imaging documented disease stability but no further regressions. Figure 5A-C demonstrates CT findings of early tumor progression followed by regression starting at week 14 and the corresponding CA19-9 responses (Figure 5D). Figure 5E demonstrates interesting CA19-9 kinetics in a patient who had stable local disease lasting 71 weeks. The baseline elevated CA 19-9 increased further during high dose steroid treatment for hypophysitis and then showed a gradual delayed normalization. Figure 5F shows the CA 19-9 kinetics of a patient with early local progression and a new omental lesion at week 7 followed by disease stabilization starting at week 22 and lasting until week 81. The CA19-9 rise between the 12 week maintenance doses declined in response to treatments. CA19-9 declines in association with Ipilimumab + GVAX treatment were seen for 7/15 patients. In contrast, 0/15 patients receiving Ipilimumab alone had CA19-9 declines.
Figure 5. Delayed tumor responses both radiographically and by CA 19-9 tumor marker kinetics in arm 2 only.
A) Baseline scan. B) Week 7 scan shows growth from baseline. C) Week 14 (4 weeks post dose 3) scan shows minor response maintained until week 31. D) CA 19-9 responses paralleled clinical response in the same patient. Small arrows denote treatment administration. E) Delayed CA 19-9 response in a patient with localized disease who received steroids (*) for hypophysitis. F) Patient with localized disease that was progressive on CT at week 7 (local progression and new omental lesion) and 14 and then stabilized from week 22 to 81.
Discussion
The data from this phase 1b trial testing Ipilimumab alone or in combination with GVAX in PDA patients report three new findings. First, the safety profile for Ipilimumab alone or the combination is similar for patients with PDA when compared with reported studies testing Ipilimumab in patients with melanoma. Second, immune responses can be induced even in patients with advanced PDA, and these responses correlate with clinical activity. Third, clinical responses to immunotherapy in PDA patients with advanced disease require prolonged treatment, similar to what has been observed in melanoma patients treated successfully with immunotherapy. Taken together, this study provides strong support for further testing of Ipilimumab and other immunotherapies in patients with PDA.
This study demonstrates that the toxicity spectrum and rates observed in PDA patients are similar to what has been reported for melanoma patients treated with Ipilimumab. IRAEs were evident even in advanced PDA patients, which supports that these patients do have reactive immune systems. In addition, the non-dermatologic toxicities were often limited to one organ system in patients receiving the combination of Ipilimumab + GVAX (7/7) when compared to those receiving Ipilimumab alone (1/4). This raises the question of whether the use of a vaccine can skew the immune response. Toxicities are likely to differ depending on the Ipilimumab combination. Ipilimumab in combination with dacarbazine resulted in higher than previously reported immune-related hepatitis4. However, prior studies have not reported on the number of organ systems affected in an individual patient. Further studies are needed to better characterize this observation.
We observed an improvement in OS that was associated with clinical activity in the combination arm (p=0.07). Ipilimumab was previously tested as a single agent in advanced PDA patients12. Only 1 patient demonstrated clinical activity and this was at the lower dose level of 3mg/kg. The higher rate of clinical activity observed in our study may be due to differences in Ipilimumab dose or more likely, due to the need of combining this T cell activating agent with T cell inducing agents (vaccines). Ipilimumab was previously combined with prostate GVAX in a phase 1 dose-escalation trial for the treatment of castrate-resistant prostate cancer18. The dose selected for expansion in that study was 3 mg/kg because of signs of clinical activity at that dose level. 50% or greater declines from baseline in prostate-specific antigen levels were observed in 25% of patients. All of these patients received either 3 or 5 mg/kg of Ipilimumab. HLA-DR, a marker of T cell activation, was only upregulated at the higher dose levels. Induction of PSMA-specific antibody responses was associated with improved OS. Studies of immunotherapy-induced antibody responses are ongoing for our PDA study.
This is the first study to evaluate Ipilimumab-induced mesothelin-specific T cell responses either alone or in combination with a vaccine. Indeed, mesothelin-specific T cell responses were measured in patients following treatment with Ipilimumab alone and with the combination. T cell responses measured in both arms were analyzed together because the pattern of induction and association with survival were similar between the two arms and also because of the small sample size. The induction of mesothelinspecific responses in the Ipilimumab alone arm support the concept that non-antigenspecific agents such as Ipilimumab act by enhancing pre-existing endogenous tumorspecific T cells. A correlation between the magnitude of the mesothelin-specific T cell response and OS was only seen for post induction 2 and peak responses. This suggests that T cell levels at baseline and following an initial treatment do not predict who will respond to therapy, and that multiple doses are required to induce T cell responses in most patients. Similar to prior studies7,8, these data also suggest that the maintenance of an enhanced T cell response may better predict which patients are more likely to benefit from treatment. Interestingly, diversification of the mesothelinspecific T cell repertoire with additional treatments was seen in both arms. However, a greater number of patients in the combination arm exhibited these responses (4/7) compared to the Ipilimumab alone arm (2/7) suggesting that the frequency of preexisting mesothelin-specific T cells are low and require a vaccine to induce larger pools of precursor T cells. Larger clinical trials comparing the induction of T cell responses between these two arms should further clarify this issue. Consistent with a prior study10, post-treatment expansion of the mesothelin-specific T cell repertoire was associated with longer OS. In this study, median survival for the six patients demonstrating an expansion in their mesothelin-specific T cell repertoires was 15.7 months compared to 4.1 months for the 8 patients whose repertoires were unchanged following treatment. When expansions in the T cell repertoire were seen, they were detected by the end of induction suggesting the possibility that this measure could potentially be used to predict who might benefit from maintenance treatments.
Since this was a pilot study, there is insufficient data to conclude that Ipilimumab alone or the combination of Ipilimumab + GVAX leads to better clinical outcomes. However, the clinical data in this study is provocative. Despite having a small sample size, the survival benefit (HR: 0.51, p=0.072) has meaning in the context of other signals of activity. Delayed SD and CT scan responses in association with declines in CA19-9 biomarker levels (a measure of tumor burden), were only observed in patients receiving the combination therapy. Notably, of the 3 patients with radiographic and CA19-9 changes, 2 had localized disease and 1 had lung only metastases. Additionally, two patients with liver metastases survived 10 and 15 months. The groups were balanced as the Ipilimumab alone group also had 2 patients with localized disease and 3 patients with lung only metastases. The fact that most patients demonstrating a response required at least 12 weeks of treatment suggests that this form of immunotherapy should be initiated earlier in the treatment course of PDA patients. In fact, this study may have underestimated the activity of both the Ipilimumab alone and the Ipilimumab + GVAX combination since the majority of enrolled patients were treated with 2 or more prior chemotherapies and were expected to live no more than 3 months on average. Studying patients with locally advanced or resected disease will allow more time to induce immune responses and also provide a less immune tolerant host given the lower disease burden. However, limiting studies in metastatic disease to patients with better prognosis (e.g. no ascites, lower tumor burden) and less exposure to immunosuppressive chemotherapy regimens may also be considered given signals of activity in the patients with metastatic disease. An additional benefit of selecting patients with more time to develop a delayed immune response is that these patients will also have better reserve and more time to recover from grade 2 or less IRAEs and still be eligible for retreatment. With the intention of introducing immunotherapy to a more stable patient population and at an earlier time in the disease process, the next study proposal will be to test Ipilimumab + GVAX in patients with metastatic disease who have achieved disease stability after upfront chemotherapy. This experimental paradigm is now possible with the newer treatment regimens such as FOLFIRINOX (5-fluorouracil/irinotecan/oxaliplatin) inducing disease stability in up to 70% of patients1. Proposals for additional combinatorial strategies are on the horizon. Preclinical studies support the combination of GVAX with a number of immune modulating techniques, including OX-40 and 4-1BB costimulation and programmed death-1 (PD-1) blockade19-21. As more experience is obtained with these agents in the clinics, the armamentarium for translation of combination strategies continues to expand.
In conclusion, immunotherapy shows promise in the treatment of PDA. Rational combination strategies and better patient selection should greatly improve chances for success.
Acknowledgments
Financial support:
Dung Le: NIH/R21 (CA1266058), NIH/GI SPORE (2P50 CA062924), and The Viragh Family Foundation.
Elizabeth Jaffee: NIH/GI SPORE (2P50 CA062924), and The Viragh Family Foundation.
Daniel Laheru: NIH/R21 (CA1266058), NIH/GI SPORE (2P50 CA062924), and The Viragh Family Foundation.
Footnotes
Financial Disclosure: Elizabeth Jaffee has the potential to receive royalties for a patent from Aduro BioTech, Inc. All other authors have declared there are no conflicts of interest in regards to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 2.De Jesus-Acosta A, Oliver GR, Blackford A, et al. A multicenter analysis of GTX chemotherapy in patients with locally advanced and metastatic pancreatic adenocarcinoma. Cancer chemotherapy and pharmacology. 2012;69:415–24. doi: 10.1007/s00280-011-1704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 5.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaffee EMHR, Biedrzycki B. Novel allogeniec granulocyte-macrophage colonystimulating factor-secreting tumor vaccine for pancreatic cancer: A phase I trial of safety an immune activation. J Clinical Oncol. 2001;19(1):145–56. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 9.Laheru D, Lutz E, Burke J, et al. Allogeneic granulocyte macrophage colonystimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–63. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lutz E, Yeo CJ, Lillemoe KD, et al. A lethally irradiated allogeneic granulocytemacrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–35. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–33. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712–7. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 14.Van Elsa A HA, Allison JP. Combination immunotherapy of B16 Melanoma using anti-cytotoxic T lymphocyte-associated antigen (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF) producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190(3):355–66. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurwitz AAYT, Leach DR, Allison JP. CTLA-4 blockade synergizes with tumorderived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci USA. 1998;95(17):10067–71. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurwitz AAFB, Kwon ED. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60(9):2444–8. [PubMed] [Google Scholar]
- 17.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 18.van den Eertwegh AJ, Versluis J, van den Berg HP, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castrationresistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:509–17. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 19.Murata S, Ladle BH, Kim PS, et al. OX40 costimulation synergizes with GM-CSF whole-cell vaccination to overcome established CD8+ T cell tolerance to an endogenous tumor antigen. J Immunol. 2006;176:974–83. doi: 10.4049/jimmunol.176.2.974. [DOI] [PubMed] [Google Scholar]
- 20.Li B, VanRoey M, Wang C, Chen TH, Korman A, Jooss K. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor--secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clin Cancer Res. 2009;15:1623–34. doi: 10.1158/1078-0432.CCR-08-1825. [DOI] [PubMed] [Google Scholar]
- 21.Li B, Lin J, Vanroey M, Jure-Kunkel M, Jooss K. Established B16 tumors are rejected following treatment with GM-CSF-secreting tumor cell immunotherapy in combination with anti-4-1BB mAb. Clin Immunol. 2007;125:76–87. doi: 10.1016/j.clim.2007.07.005. [DOI] [PubMed] [Google Scholar]