Abstract
Background and Purpose
Health-related quality of life (HRQoL) is a multidimensional concept that signifies a subjective evaluation of perceived health; hence, it has gained wide acceptance in geriatrics. However, its application has not been tested in patients with post-stroke cognitive impairment with no dementia (PSCIND). We investigated whether PSCIND interferes with HRQoL measured by EQ-5D, compared the findings to those of healthy people with normal cognition, and evaluated the influence of each cognitive domain on this score.
Methods
In total, 1,528 subjects were identified who had undergone neuropsychological assessment using the 60-min protocol of the Korean version of Vascular Cognitive Impairment Harmonization Standards, EQ-5D, and magnetic resonance imaging at the stroke prevention clinic. Fifty PSCIND patients were matched to 50 post-stroke dementia (PSD) patients and 50 normal age- (±3 years) and sex-matched controls. The effects of PSCIND, PSD, and control groups upon the EQ-5Dindex score were tested by generalized estimating equation modeling.
Results
Estimated means±standard errors of EQ-5Dindex scores were as follows: 0.94±0.06 (control group), 0.86±0.08 (PSCIND group), and 0.61±0.32 (PSD group); and the difference among the three groups was statistically significant (P<0.0001). Pairwise comparisons showed that EQ-5Dindex scores in the PSCIND group differed from those in the PSD and control groups (both P<0.01). No cognitive domain was specifically associated with EQ-5Dindex scores after adjusting for functional status.
Conclusions
This study shows that PSCIND may interfere with the quality of life in stroke victims.
Keywords: Quality of life, Stroke, Dementia
Introduction
Health-related quality of life (HRQoL) is used to quantify patients' burden of disease.1 This measure focuses on the patient rather than the disease and is helpful to compare health status between different types of disease. HRQoL also allows direct comparisons of benefits from a variety of interventions on a variety of diseases.1,2 Recently, the US Food and Drug Administration announced to the pharmaceutical industry that HRQoL could be a possible endpoint in clinical trials.3 Therefore, HRQoL has begun to be used as an outcome measure in clinical trials on various diseases, including stroke and dementia.4,5
HRQoL was worse in people with Alzheimer's disease (AD) compared to those with mild cognitive impairment or normal cognition, but there was no difference between mild cognitive impairment and normal cognition.6 HRQoL of stroke survivors was lower than that of healthy elderly people living in the community.7 However, HRQoL has not been evaluated and reported in stroke victims with cognitive impairment but no dementia.
The utility weight of EQ-5D (EQ-5Dindex) in a representative sample of the Korean population was recently reported as a feasible and valid tool for measuring HRQoL in stroke survivors.8
The objectives of the present study were to compare patients with post-stroke cognitive impairment with no dementia (PSCIND) to those with post-stroke dementia (PSD) and healthy people with normal cognition with respect to HRQoL, measured by EQ-5Dindex, and to investigate the individual effect of each cognitive domain on EQ-5Dindex.
Methods
A series of 1,528 patients who visited the stroke prevention clinic at Seoul National University Bundang Hospital, underwent brain magnetic resonance imaging (MRI), and underwent the 60-min neuropsychological assessment protocol of the Korean version of Vascular Cognitive Impairment Harmonization Standards (K-VCIHS-NP) and EQ-5D between May 2007 and March 2009, were identified using the clinical data warehousing system embedded in our institution's electronic medical records system. This study protocol was reviewed and approved by your Institutional Review Board, and that informed consent was waived.
Of those 1,528, the PSD and PSCIND groups were selected from those who 1) had been hospitalized at our institution for acute stroke management and 2) were impaired in at least one of the frontal executive, language, visuospatial, or memory domains on the K-VCIHS-NP, and 3) in whom the K-VCIHS-NP might have been administered at least 90 days after stroke onset. Among them, those who did not meet the criteria of dementia were classified as the PSCIND group and those who met the criteria formed the PSD group. Dementia was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition9 criteria based on clinical interviews, cognitive functions assessed using the K-VCIHS-NP, and social functioning status assessed on the scale of Instrumental Activities of Daily Living. Patients who had depression were excluded. Stroke (ischemic or hemorrhagic) was defined using the World Health Organization's definition of "rapidly developed clinical signs of focal or global disturbance of cerebral function, lasting more than 24 hours or leading to death, with no apparent cause other than a vascular origin."
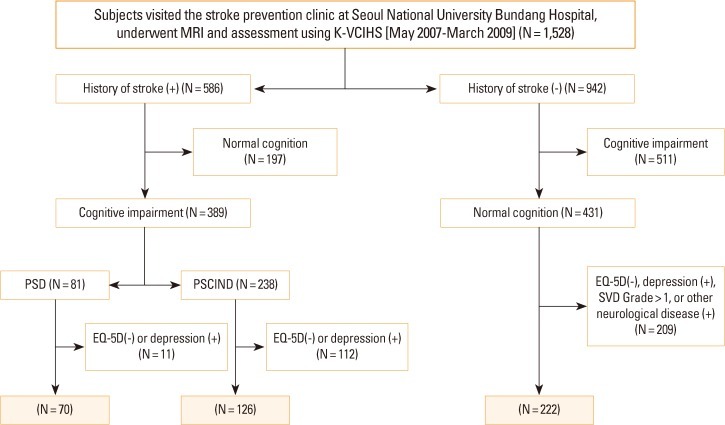
The control group was chosen from individuals who visited the stroke prevention clinic worrying about a risk of stroke, but who had no history of stroke and normal cognition on the 60-minutes protocol. Those who had moderate to severe white matter hyperintensities on the fluid-attenuated inversion recovery sequences of brain MRI, psychiatric illness, or other neurological disorders were excluded. We also excluded those who were not evaluated with EQ-5D. A flow chart for the identification of study subjects is displayed in Figure 1.
Figure 1.
Flow chart for identification of study subjects. PSCIND, post-stroke cognitive impairment with no dementia. PSD, post-stroke dementia; SVD, small vessel disease.
We selected cases and controls in the age band between 50 years (the lowest in PSD group) and 82 (the highest in control group). Each PSCIND patient (N=50) was matched to one PSD patient and one control according to age (±3 years) and sex, blinded to other clinical information.
Instruments and data collection
The K-VCIHS-NP was adapted from the 60-minutes neuropsychology protocol of the Vascular Cognitive Impairment Harmonization Standards (VCIHS) proposed by the National Institute of Neurological Disorders and Stroke and the Canadian Stroke Network.10 It comprises 4 cognitive domains and 8 cognitive tests: frontal executive function (animal naming test, phonemic fluency test, Digit Symbol Coding, Korean Trail-Making Test A and B); language/lexical retrieval (Korean-Boston Naming Test-short form); visuospatial (Rey Complex Figure Test: Copy); and memory (Seoul Verbal Learning Test). In addition, Korean Mini-Mental Status Examination for evaluating global cognitive dysfunctions and the Informant Questionnaire of Cognitive Decline in the Elderly for assessing the patients' pre-morbid history of cognitive dysfunctions, and Geriatric Depression Scale were also included. All tests and scales were validated and standardized in Korean subjects.11
Trained clinical psychometricians, blinded to the clinical and neuroradiological profiles of each patient, administered the series of tests. A score on each cognitive test was transformed into a standardized z-score (z-score=[individual score-mean score of the sample]/standard deviation of the sample). Cognitive impairments in language, visuospatial function, or memory domains were defined as a score of less than the 7th percentile (≈mean-1.5 standard deviation [SD]) in each domain-specific test. Cognitive impairment in the frontal executive domain was defined as a score of less than the 7th percentile in 2 or more of the 5 frontal domain-specific tests. Instrumental Activities of Daily Living score ≥0.43 was considered a significant impairment in everyday functioning,12 and Geriatric Depression Scale score of 18-30 as being depressive.13,14
HRQoL was assessed using the EQ-5D. Subjects were asked to rate their current health state across the five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression). Subjects were asked to choose one of three responses in each dimension. Trained psychometricians administered the EQ-5D questionnaire after the subjects finished the neuropsychological test series. The EQ-5D allows calculation of a preference-based summary index based on time trade-off techniques in which the value "0" represents death and "1" perfect health. We chose the utility measurement EQ-5Dindex score to identify the difference in HRQoL among the three groups because it is designed to compare HRQoL across different diseases as well as different populations. A utility was derived from a representative sample of the Korean population, and the use of the Korean version of EQ-5Dindex had been validated for the Korean general population and for stroke survivors.8,15
Baseline demographics and clinical characteristics of the study subjects were obtained from a prospective registry database of hospitalized stroke patients16 or by reviewing electronic medical records. Information on the following variables was collected: age, sex, years of education (categorized into 0-3, 4-9, 10-12, and ≥13 years of formal education), blood pressure, history of stroke, hypertension (previously diagnosed and treated, or systolic pressure >140 mmHg and/or diastolic pressure >90 mmHg), diabetes mellitus (previously diagnosed and treated, or fasting glucose 7 mmol/L, or postprandial 2 h blood glucose >11.1 mmol/L), hyperlipidemia (previously diagnosed and treated, or total cholesterol >6.0 mmol/L, or low density lipoprotein cholesterol >4.14 mmol/L), history of coronary artery disease and atrial fibrillation according to clinical diagnosis and electrocardiography, smoking (current smoker: smoked at least 1 cigarette during past 1 month), or alcohol intake (heavy alcoholics: >3 drinks on average/day).
Sample size calculation and statistical analysis
We adopted the generalized estimating equation (GEE) to assess the relationship between matched cases and controls. Because a statistical method to calculate sample size in the GEE model has not been developed yet, the sample size of this study was estimated by analysis of variance with a significance level of 0.05 and a power of 0.90. The mean±SD of EQ-5Dindex in the PSD group came from studies on AD and vascular or other kinds of dementias,17,18 and was assumed to be 0.64±0.21. Those of the control group were assumed to be 0.84±0.18, representative of the general population over 60 years in Korea and based on the Korean National Health and Nutrition Examination Survey between 2007 and 2009. The mean of EQ-5Dindex in the PSCIND group was estimated as that (≒0.77) of the upper two-thirds between the PSD and control groups. The calculated sample size was 40 for SD=0.21. The final sample size was determined to be 50 in each group, considering missing data that originated from the study's retrospective nature.
Differences in baseline characteristics among the PSD, PSCIND, and control groups were examined using Pearson's chi-squared test or one-way analysis of variance as appropriate (Table 1). Results of neuropsychological tests are presented as mean±SD in z-scores and percentage of subjects with impairment in each domain (Table 2). Comparisons of z-scores between groups were performed using GEE adjusting for education. A partial correlation analysis adjusting for modified Rankin scale (mRS) measured at 3 months of stroke onset was performed to examine the relative contribution of each cognitive domain on the EQ-5Dindex with adjustment for physical disability. Finally, GEE adjusting for education was used to determine the significance of difference in EQ-5Dindex score among three groups.
Table 1.
Characteristics of the control, PSCIND, and PSD groups
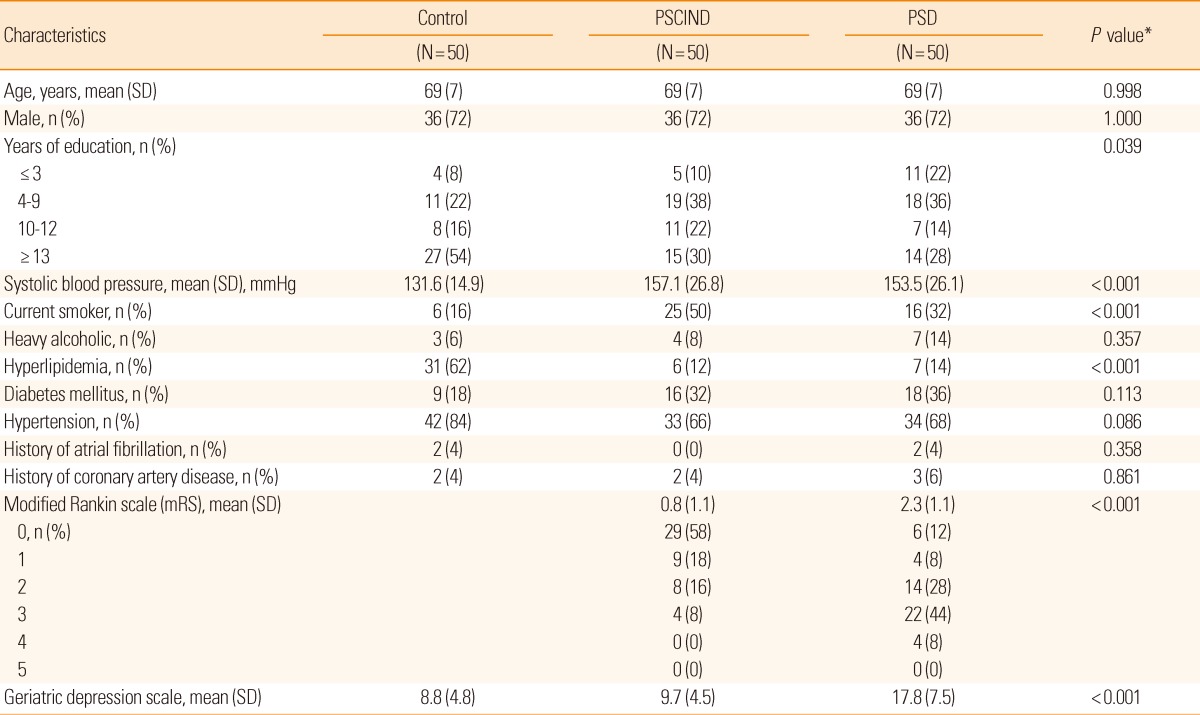
Values are number of patients (%) if not indicated. PSCIND indicates post-stroke cognitive impairment with no dementia.
PSD, post-stroke dementia; SD, standard deviation.
*P values were calculated by analysis of variance or Pearson's chi-squared test.
Table 2.
Mean (SD) of z-scores, frequency of impairment (%), and pairwise comparison of cognitive functions
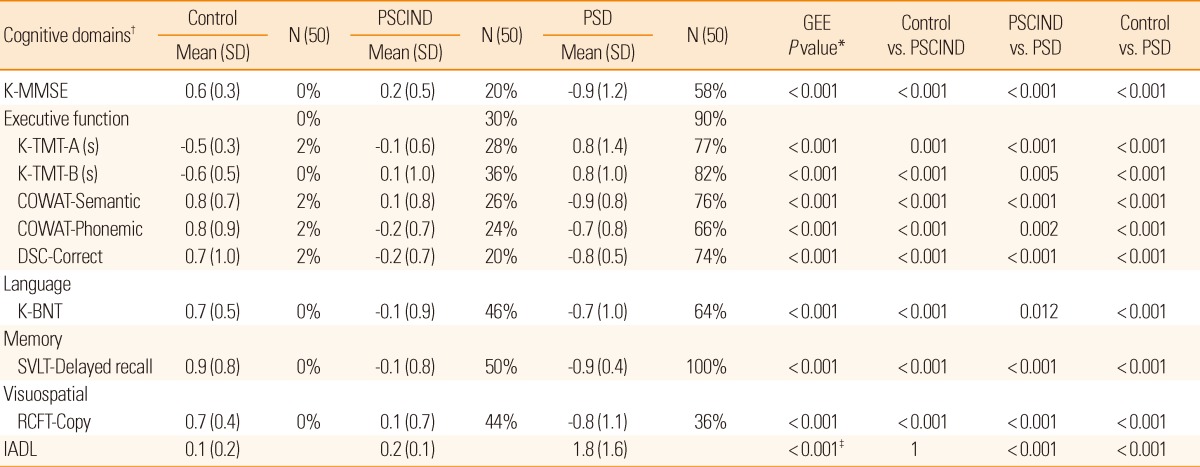
PSCIND indicates post-stroke cognitive impairment with no dementia.
PSD, post-stroke dementia; SD, standard deviation; K-MMSE, Korean-Mini Mental State Examination; K-TMT, Korean Trail-Making Test; COWAT, Controlled Oral Word Association Test; DSC, Digit Symbol Coding; K-BNT, Short version of the Korean Boston Naming Test; SVLT, Seoul Verbal Learning Test; RCFT, Rey Complex Figure Test; IADL, Instrumental Activity of Daily Living.
*P values were calculated by Generalized Estimating Equation (GEE) adjusting for education. All the mean difference is significant at the 0.05 level; †The values for the cognitive-domain measures are standardized composite z-scores; ‡The value was calculated by analysis of variance.
Results
Of 1,528 patients who visited the stroke prevention clinic and underwent brain MRI and the K-VCIHS-NP during the study period, 586 had a history of stroke and 942 had not (Figure 1). Among those 586 with stroke, we identified 70 PSD and 126 PSCIND patients, after excluding those who had depression or in whom the EQ-5D was not available. Among 942 without stroke, 222 were suitable for the control group. Lastly, 150 subjects, 50 for each group matched for age (±3 years) and sex, were enrolled in the study.
The mean age was 69 years and 72.0% of subjects were male. The clinical characteristics of the study subjects are presented in Table 1. The level of education was significantly different among the three groups. The PSD and PSCIND groups had higher systolic blood pressure, were more likely to be current smokers, and were less likely to have hyperlipidemia compared to the control group. Three-month mRS score was different between the PSD and PSCIND groups and significantly correlated with the EQ-5Dindex at 3 months of stroke onset in the PSD group (-0.38, P=0.007), but not in the PSCIND group (-0.06, P=0.70).
Comparisons between the 3 groups were made with respect to the z-scores of the individual neuropsychological tests, adjusted for education (Table 2). The z-scores of all the cognitive tests of the PSD group were significantly lower than those of the PSCIND and control groups, and those of the PSCIND group were lower than those of the control group. The most frequently impaired cognitive domain was memory, and the most frequently impaired frontal executive function test was the Korean Trail Making Test B (K-TMT-B), in both the PSCIND and PSD groups. With respect to Instrumental Activities of Daily Living scores, the PSCIND group was not different from the control group, while the PSD group had significantly lower scores than the other groups. The frequency of pre-stroke cognitive decline evaluated using Korean-IQCODE (IQCODE ≥3.6) was 35% (137 of 389) among stroke survivors.
Correlations between EQ-5Dindex and neuropsychological tests in the three groups are presented in Table 3, with and without adjustment for 3-month mRS in stroke survivors. In the PSCIND group, EQ-5Dindex correlated only with visuospatial function before adjustment, but with none after adjustment. In the PSD group, EQ-5Dindex correlated with Korean Mini-Mental Status Examination and visuospatial function with and without adjustment, and with Digit Symbol Coding-correct and language, with adjustment.
Table 3.
Pearson correlation coefficients and partial correlation coefficients adjusted for 3-month mRS between EQ-5Dindex and neuropsychological tests
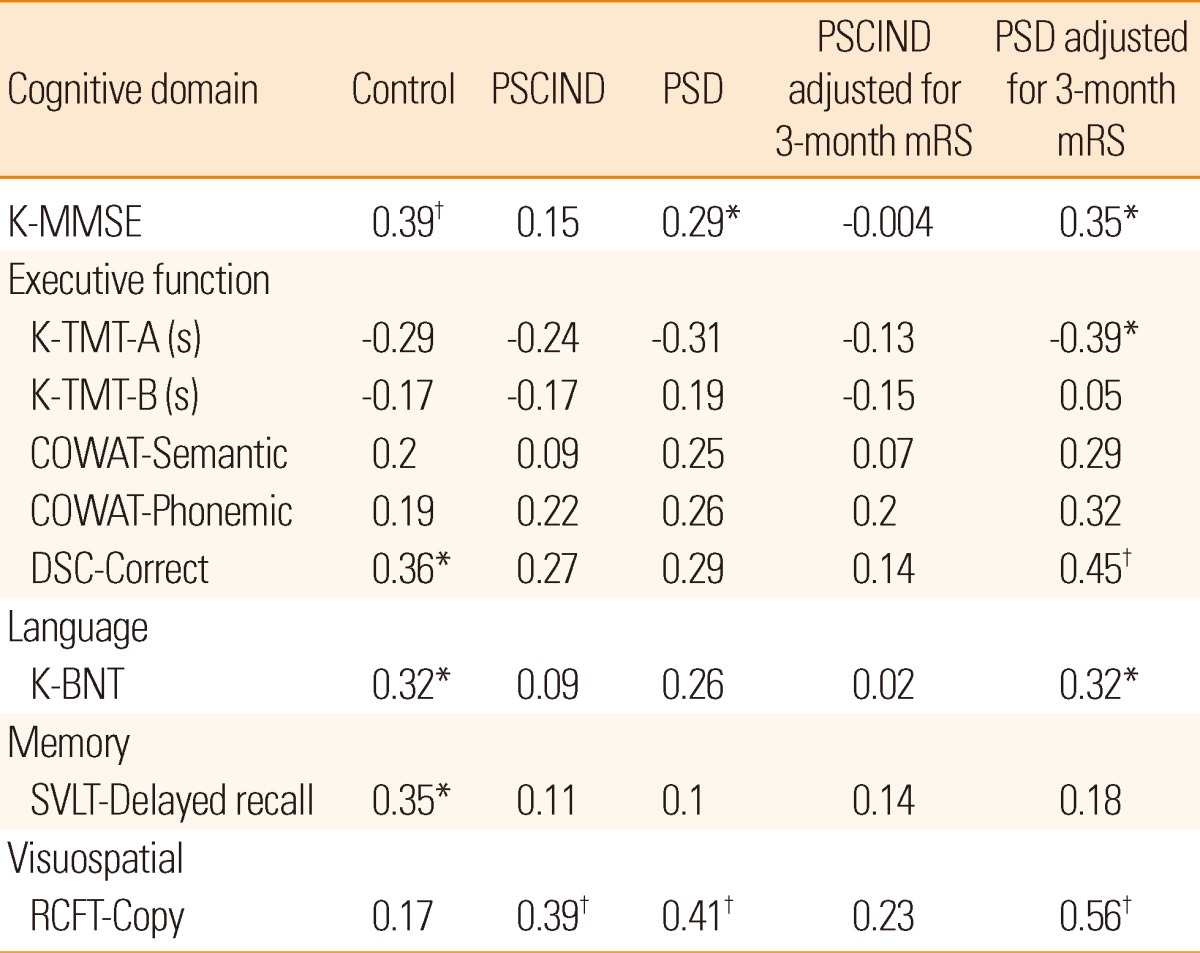
K-MMSE, Korean-Mini Mental State Examination; K-TMT, Korean Trail-Making Test; COWAT, Controlled Oral Word Association Test; DSC, Digit Symbol Coding; K-BNT, Short version of the Korean Boston Naming Test; SVLT, Seoul Verbal Learning Test; RCFT, Rey Complex Figure Test.
*Correlation is significant at the 0.05 level (2-tailed); †Correlation is significant at the 0.01 level (2-tailed).
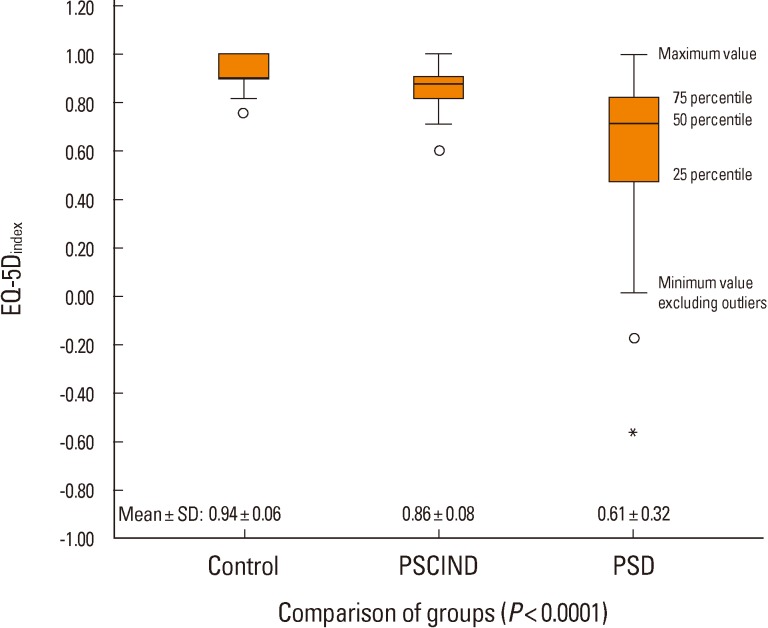
There was a significant difference in the mean EQ-5Dindex among the three groups, and pairwise comparisons between the PSCIND and control groups and between the PSCIND and PSD groups showed similar results even after adjustment for education and depression (Figure 2, Table 4).
Figure 2.
Distribution of subjects according to the EQ-5Dindex values and mean±SD of EQ-5Dindex in the PSD, PSCIND, and control groups.
Table 4.
Comparisons of EQ-5Dindex among the Control, PSCIND, and PSD groups
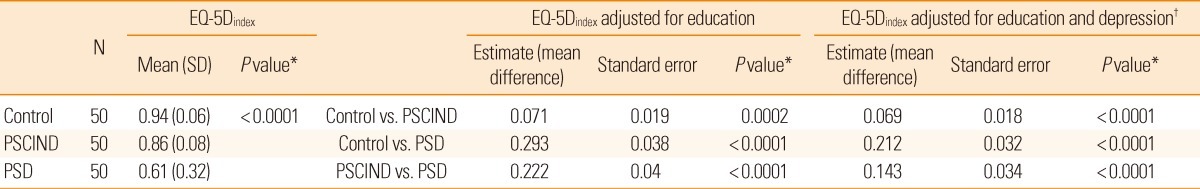
PSCIND indicates post-stroke cognitive impairment with no dementia. PSD, post-stroke dementia; SD: standard deviation.
*P value is for Generalized Estimating Equation (GEE); †Depression is continuous variable.
Discussion
This study finds that the HRQoL of PSCIND is intermediate between that of PSD and normal cognition and that no particular cognitive domain is more influential on the HRQoL of PSCIND. To the best of our knowledge, this study is the first to directly compare PSD, PSCIND, and normal cognition with respect to HRQoL.
The frequency of cognitive impairment in post-stroke (66.4%) was higher than that among previous studies.19-21 A recent report from a multicenter prospective stroke cohort in Korea appeared to be similar with our study in frequency of cognitive impairment after stroke (62.6%) even though it was a bit of difference in study setting such as cutoff value of cognitive impairment (<10 percentile).11 The higher frequency of cognitive impairment in our study might be partially explained by discrepancy with pre-stroke cognitive dysfunction (7.2%) in recent study.11
A previous study reported that the mean EQ-5Dindex was 0.60 in all types of dementia, which was similar to that in the PSD group of the present study, 0.61.17 In another study on AD, the mean EQ-5Dindex score was 0.62 when rated by proxy, but 0.86 when self-rated.22 The HRQoL may be lower in those with PSD than in those with AD; this needs to be confirmed in another study. For determinants of HRQoL in dementia, there is a clear and consistent association with depression (the more severe the depression, the lower the HRQoL), but there have been inconsistent reports on other factors, such as age, gender, education, ethnicity, and activity limitation.23,24 Subjects who had a history of depression (previously diagnosed and treated) was excluded, despite our findings might be partially biased by the inclusion of healthier controls. With high frequency of depression in PSD evaluated by a geriatric depression scale, depression was included as confounder in final model, but the impact of PSCIND on HRQoL was not different from the unadjusted result in statistical significance. Low HRQoL in PSD could be explained by medical comorbidities such as diabetes or coronary heart disease, which are known to be related to HRQoL25,26 and more prevalent in PSD than in AD.
The mean EQ-5Dindex of PSCIND (0.86) was analogous to that of chronic obstructive pulmonary disease (≒0.81) in Korea.27 The reported mean EQ-5Dindex of minor stroke (mRS≤3) was between 0.6 and 0.7, which was similar to that of PSD and lower than that of PSCIND.28 A few previous studies on stroke survivors have shown no influence of vascular cognitive impairment on HRQoL.7,29 From a viewpoint of HRQoL after stroke, the contribution of mRS to HRQoL should be emphasized.7 We found that 3-month mRS was associated with 3-month EQ-5Dindex in PSD, but the difference in EQ-5Dindex between PSD and PSCIND was still significant after adjusting for 3-month mRS. A positive association between cognition and HRQoL has been reported among functionally independent stroke survivors.30 We cautiously suggest that cognitive impairment may further impact the HRQoL in stroke victims.
EQ-5Dindex of PSCIND was significantly lower than that of normal cognition in this study. This result is different from previous ones in which there was no significant difference between patients with mild cognitive impairment and controls.4,31 Between the PSCIND and control group, a difference existed not only in cognition but also in stroke history, which had an impact on HRQoL in Koreans,32 and both of them might lead to a difference in EQ-5Dindex. A further study comparing those with PSCIND and stroke survivors with normal cognition can address the question of whether cognitive impairment that is not enough to be diagnosed as dementia, truly impacts HRQoL in stroke survivors.
EQ-5Dindex in the PSCIND group did not correlate with impairment in any specific cognitive domain. A relationship between poor TMT-B performance and poor perceived quality of life has been reported in stroke patients.33 This study showed that impairment in the K-TMT-B, one of the executive function tests, was observed most frequently in both PCIND and PSD groups, but the K-TMT-B scores did not correlate with EQ-5Dindex. However, it should be noted that 48% in the PSD group and 20% in the PSCIND group were unable to complete the K-TMT-B; this was the worst outcome among all cognitive tests.
This study has some limitations. First, the eligible stroke patients were not representative of the general stroke population in Korea, or even hospitalized stroke patients in our institution. Inevitably, patients who were unable to complete neuropsychological tests, did not undergo MRI, and were not followed up at the outpatient clinic after discharge were excluded from this study. Second, as mentioned above, this study did not include stroke survivors with normal cognition. A further study including all cognitive spectrums of stroke survivors is therefore warranted.
Summary
This study shows that among stroke patients surviving more than 3 months, even mild cognitive impairment may interfere with a sense of well-being. We cautiously suggest that cognitive impairment may be an important determinant lowering HRQoL. Interventions aimed at enhancing cognition may improve HRQoL even in PSCIND. This is inferred from a randomized controlled trial in dementia, which proved that improvement in cognition is associated with improvement in HRQoL.5
Acknowledgements
H.-J.B is involved as the principal investigator, a member of the steering committee, and/or a site investigator of multicenter clinical trials or clinical studies sponsored by Otsuka Korea, Bayer Korea, Handok Pahrmaceutical Company, SK Chemicals, ESAI-Korea, Daewoong Pharmaceutical Co. Ltd, Daichi Sankyo, Pfizer, Sanofi-Aventis Korea, and Yuhan Corporation; has served as the consultant or on the scientific advisory board for Bayer Korea, Boehringer Ingelheim Korea, YuYu Pharmaceutical Company, and BMS Korea; and has received lecture honoraria from MSD Korea, AstraZeneca Korea, BMS Korea, Novartis Korea, Ostuka Korea, Pfizer Korea, Daichi Sankyo Korea, and Handok Pharmaceutical Company (modest).
Footnotes
This study was partly supported by a grant from the Korea Health 21 R&D project, Ministry of Health and Welfare, Korea (A102065) and by a grant from Esai Korea Inc.
The authors have no financial conflicts of interest.
References
- 1.Carod-Artal FJ, Egido JA. Quality of life after stroke: the importance of a good recovery. Cerebrovasc Dis. 2009;27(Suppl 1):204–214. doi: 10.1159/000200461. [DOI] [PubMed] [Google Scholar]
- 2.The WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med. 1998;28:551–558. doi: 10.1017/s0033291798006667. [DOI] [PubMed] [Google Scholar]
- 3.Arpinelli F, Bamfi F. The FDA guidance for industry on PROs: the point of view of a pharmaceutical company. Health Qual Life Outcomes. 2006;4:85. doi: 10.1186/1477-7525-4-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lees KR, Zivin JA, Ashwood T, Davalos A, Davis SM, Diener HC, et al. NXY-059 for acute ischemic stroke. N Engl J Med. 2006;354:588–600. doi: 10.1056/NEJMoa052980. [DOI] [PubMed] [Google Scholar]
- 5.Woods B, Thorgrimsen L, Spector A, Royan L, Orrell M. Improved quality of life and cognitive stimulation therapy in dementia. Aging Ment Health. 2006;10:219–226. doi: 10.1080/13607860500431652. [DOI] [PubMed] [Google Scholar]
- 6.Missotten P, Squelard G, Ylieff M, Notte DD, Paquay L, Lepeleire JD, et al. Quality of life in older Belgian people: comparison between people with dementia, mild cognitive impairment, and controls. Int J Geriatr Psychiatry. 2008;23:1103–1109. doi: 10.1002/gps.1981. [DOI] [PubMed] [Google Scholar]
- 7.Sturm JW, Donnan GA, Dewey HM, Macdonell RA, Gilligan AK, Srikanth V, et al. Quality of life after stroke: the North East Melbourne Stroke Incidence Study (NEMESIS) Stroke. 2004;35:2340–2345. doi: 10.1161/01.STR.0000141977.18520.3b. [DOI] [PubMed] [Google Scholar]
- 8.Jo MW, Bae HJ. One-year health related quality of life and its comparison with various clinical and functional scale in hospitalized patients with acute ischemic stroke: Seoul National University Bundang Stroke Registry Study. J Korean Neurol Assoc. 2009;27:28–35. [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 10.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 11.Yu KH, Cho SJ, Oh MS, Jung S, Lee JH, Shin JH, et al. Cognitive impairment evaluated with Vascular Cognitive Impairment Harmonization Standards in a multicenter prospective stroke cohort in Korea. Stroke. 2012 doi: 10.1161/STROKEAHA.112.668343. doi: 10.1161/STROKEAHA.112.668343. [DOI] [PubMed] [Google Scholar]
- 12.Kang SJ, Choi SH, Lee BH, Kwon JC, Na DL, Han SH, et al. The reliability and validity of the Korean Instrumental Activities of Daily Living (K-IADL) J Korean Neurol Assoc. 2002;20:8–14. [Google Scholar]
- 13.Jung IK, Kwak DI, Joe SH, Lee HS. A study of standardization of Korean form of Geriatric Depression Scale (KGDS) J Korean Geriatr Psychiatry. 1997;1:61–72. [Google Scholar]
- 14.Jung IK, Kwak DI, Shin DK, Lee MS, Lee HS, Kim JY. A reliability and validity study of Geriatric Depression Scale. J Korean Neuropsychiatr Assoc. 1997;36:103–110. [Google Scholar]
- 15.Jo MW, Yun SC, Lee SI. Estimating quality weights for EQ-5D health states with the Time Trade-off method in South Korea. Value Health. 2008;11:1186–1189. doi: 10.1111/j.1524-4733.2008.00348.x. [DOI] [PubMed] [Google Scholar]
- 16.Yu KH, Bae HJ, Kwon SU, Kang DW, Hong KS, Lee YS, et al. Analysis of 10,811 Cases with acute ischemic stroke from Korean Stroke Registry: hospital-based multicenter prospective registration study. J Korean Neurol Assoc. 2006;24:535–543. [Google Scholar]
- 17.Andersen CK, Wittrup-Jensen KU, Lolk A, Andersen K, Kragh-Sørensen P. Ability to perform activities of daily living is the main factor affecting quality of life in patients with dementia. Health Qual Life Outcomes. 2004;2:52. doi: 10.1186/1477-7525-2-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jönsson L, Andreasen N, Kilander L, Soininen H, Waldemar G, Nygaard H, et al. Patient- and proxy-reported utility in Alzheimer Disease using the EuroQoL. Alzheimer Dis Assoc Disord. 2006;20:49–55. doi: 10.1097/01.wad.0000201851.52707.c9. [DOI] [PubMed] [Google Scholar]
- 19.Leys D, Hénon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. Lancet Neurol. 2005;4:752–759. doi: 10.1016/S1474-4422(05)70221-0. [DOI] [PubMed] [Google Scholar]
- 20.Pohjasvaara T, Mäntylä R, Aronen HJ, Leskelä M, Salonen O, Kaste M, et al. Clinical and radiological determinants of prestroke cognitive decline in a stroke cohort. J Neurol Neurosurg Psychiatry. 1999;67:742–748. doi: 10.1136/jnnp.67.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barba R, Castro MD, del Mar Morín M, Rodriguez-Romero R, Rodríguez-García E, Cantón R, et al. Prestroke dementia. Cerebrovasc Dis. 2001;11:216–224. doi: 10.1159/000047642. [DOI] [PubMed] [Google Scholar]
- 22.Naglie G, Tomlinson G, Tansey C, Irvine J, Ritvo P, Black SE, et al. Utility-based quality of life measures in Alzheimer's Disease. Qual Life Res. 2006;15:631–643. doi: 10.1007/s11136-005-4364-8. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee S, Samsi K, Petrie CD, Alvir J, Treglia M, Schwam EM, et al. What do we know about quality of life in dementia? A review of the emerging evidence on the predictive and explanatory value of disease specific measures of health related quality of life in people with dementia. Int J Geriatr Psychiatry. 2009;24:15–24. doi: 10.1002/gps.2090. [DOI] [PubMed] [Google Scholar]
- 24.Banerjee S, Smith SC, Lamping DL, Harwood RH, Foley B, Smith P, et al. Quality of life in dementia: more than just cognition. An analysis of associations with quality of life in dementia. J Neurol Neurosurg Psychiatry. 2006;77:146–148. doi: 10.1136/jnnp.2005.072983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan PW, Ghushchyan VH, Bayliss E. The impact of comorbidity burden on preference-based health-related quality of life in the United States. Pharmacoeconomics. 2012;30:431–442. doi: 10.2165/11586840-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Solli O, Stavem K, Kristiansen IS. Health-related quality of life in Diabetes: the associations of complications with EQ-5D scores. Health Qual Life Outcomes. 2010;8:18. doi: 10.1186/1477-7525-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korea Centers for Disease Control and Prevention. The Fourth Korea Natioanal Health and Nutrition Examination Survey; 2007-2009. [Accessed May 3, 2012]. http://knhanes.cdc.go.kr/
- 28.Post PN, Stiggelbout AM, Wakker PP. The utility of health states after stroke: a systematic review of the literature. Stroke. 2001;32:1425–1429. doi: 10.1161/01.str.32.6.1425. [DOI] [PubMed] [Google Scholar]
- 29.Kwa VI, Limburg M, de Haan RJ. The role of cognitive impairment in the quality of life after ischaemic stroke. J Neurol. 1996;243:599–604. doi: 10.1007/BF00900948. [DOI] [PubMed] [Google Scholar]
- 30.Dhamoon MS, Moon YP, Paik MC, Boden-Albala B, Rundek T, Sacco RL, et al. Quality of life declines after first ischemic stroke: the Northern Manhattan study. Neurology. 2010;75:328–334. doi: 10.1212/WNL.0b013e3181ea9f03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ready RE, Ott BR, Grace J. Patient versus informant perspectives of quality of life in mild cognitive impairment and Alzheimer's Disease. Int J Geriatr Psychiatry. 2004;19:256–265. doi: 10.1002/gps.1075. [DOI] [PubMed] [Google Scholar]
- 32.Kil SR, Lee SI, Yun SC, An HM, Jo MW. The decline of health-related quality of life associated with some diseases in Korean adults. J Prev Med Public Health. 2008;41:434–441. doi: 10.3961/jpmph.2008.41.6.434. [DOI] [PubMed] [Google Scholar]
- 33.Hochstenbach JB, Anderson PG, van Limbeek J, Mulder TT. Is there a relation between neuropsychologic variables and quality of life after stroke? Arch Phys Med Rehabil. 2001;82:1360–1366. doi: 10.1053/apmr.2001.25970. [DOI] [PubMed] [Google Scholar]