Abstract
Background
Major stroke clinical trials have failed during the past decades. The failures suggest the presence of heterogeneity among stroke patients. Biomarkers refer to indicators found in the blood, other body fluids or tissues that predicts physiologic or disease states, increased disease risk, or pharmacologic responses to a therapeutic intervention. Stroke biomarkers could be used as a guiding tool for more effective personalized therapy.
Main Contents
Three aspects of stroke biomarkers are explored in detail. First, the possible role of biomarkers in patients with stroke is discussed. Second, the limitations of conventional biomarkers (especially protein biomarkers) in the area of stroke research are presented with the reasons. Lastly, various types of biomarkers including traditional and novel genetic, microvesicle, and metabolomics-associated biomarkers are introduced with their advantages and disadvantages. We especially focus on the importance of comprehensive approaches using a variety of stroke biomarkers.
Conclusion
Although biomarkers are not recommended in practice guidelines for use in the diagnosis or treatment of stroke, many efforts have been made to overcome the limitations of biomarkers. The studies reviewed herein suggest that comprehensive analysis of different types of stroke biomarkers will improve the understanding of individual pathophysiologies and further promote the development of screening tools for of high-risk patients, and predicting models of stroke outcome and rational stroke therapy tailored to the characteristics of each case.
Keywords: Stroke, Ischemic; Biomarker; Personalized medicine; Risk factor; Treatment
Discovering biomarkers for stroke
In a narrow sense, biomarkers refer to indicators measured by chemical or biologic tests using blood or urine that predicts physiologic or disease states, or increased disease risk. Biomarkers are also a valuable tool in drug development, providing more accurate and complete information regarding drug performance, disease progression, or response to a specific drug therapy. In the research field of myocardial infarction, the role of biomarkers has been emphasized over a long period of time. Treatment according to the biomarkers has also been investigated in various diseases including diabetes or immunological disorders. On the contrary, there has been a relative dearth of biomarker research in cerebrovascular disease. Herein, we review the role of current and new stroke biomarkers with their strengths and weaknesses, focusing on the importance of comprehensive approaches.
Learning from the failure of recent clinical trials
Over the recent 10 years, numerous large multicenter randomized clinical trials (RCTs) on stroke patients have been performed in the stroke research field. However, almost all major studies including RCTs regarding secondary prevention of stroke,1-3 MR-based thrombolytic therapy,4,5 and STAIR (Stroke Treatment Academic Industry Roundtable) criteria-guided neuroprotection6 have failed to show meaningful clinical benefits. In this regard, several issues have been suggested to explain and overcome these failures.
First, it is warranted for more larger and methodologically sound RCTs which meet the STAIR7 and CONSORT8 (CON-solidated Standards Of Reporting Trials) criteria. These may enhance the success rate and reliability of the study. It is clear that findings derived from large-scale intervention trials have provided the impetus to change guidelines for stroke treatment. Nonetheless, direct application of RCTs results to daily clinical practice is dubious, because patients enrolled in large RCTs may not be representative of patients in our clinical practice.9
Second, there has been increasing interest in new statistical approaches to end-point analysis in RCTs (from dichotomized outcome scales to global statistics, responder, or shift analysis).10 These are mainly used to reduce sample size or enhance trial efficiency.11 Unfortunately, however, researchers cannot draw any new findings from RCTs through the novel statistical methods.
Third, the importance of considering heterogeneity among stroke patients has emerged. Unlike coronary heart disease, stroke has heterogeneous pathophysiologies and mechanisms. Moreover, individual patients with stroke have different features even among subjects with same stroke mechanisms. These aspects enhance the need for development of personalized medicine based on the characteristics of each patient rather than performing large RCTs. Stroke biomarkers may provide the information on the heterogeneity and could be a guiding tool for more effective personalized therapy among patients with ischemic cerebrovascular disease.
Role of biomarkers in stroke research
Emerging roles of stroke biomarkers are summarized in Table 1. Although the roles of biomarkers are basically diagnosing the disease and predicting the outcome, biomarkers in patients with stroke can also provide a large variety of other information about the risk of future stroke, possible stroke mechanisms for biomarker-guided treatment, or drug response. In addition, they can be used as surrogate endpoints in clinical trials.
Table 1.
Emerging roles of stroke biomarkers
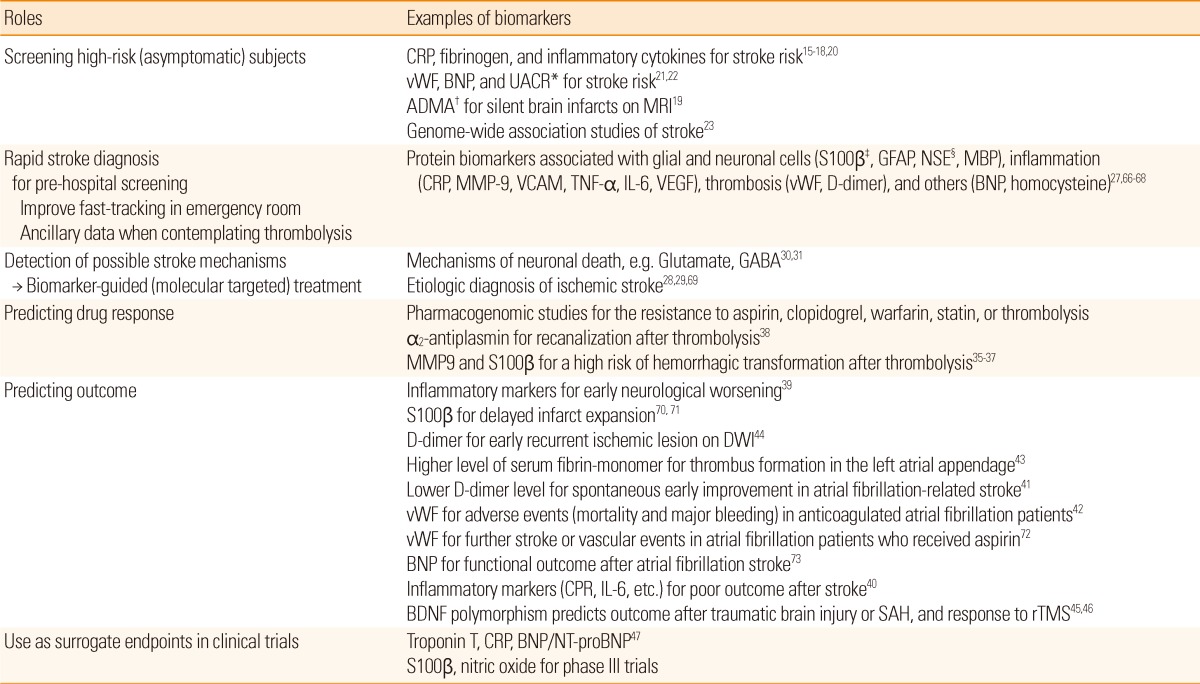
*Urinary albumin/creatinine ratio, a marker of endothelial dysfunction; †Asymmetrical dimethylarginine, an inhibitor of eNOS, a marker of endothelial dysfunction; ‡Protein S100β, homologue for CK-MB in coronary heart disease; §Neuronal specific enolase, homologue for troponin in coronary heart disease.
CRP, C-reactive protein; vWF, von Willebrand factor; BNP, B-type natriuretic peptide; GFAP, Glial fibrillary acidic protein; MBP, Myelin basic protein; MMP, Matrix metalloproteinase; VCAM, Vascular cell adhesion molecule; TNF, Tumor necrosis factor; IL, Interleukin; VEGF, Vascular endothelial growth factor; GABA, Gamma aminobutyric acid; DWI, Diffusion-weighted imaging; BDNF, Brain-derived neurotrophic factor; SAH, Subarachnoid hemorrhage; rTMS, Repetitive transcranial magnetic stimulation; NT-proBNP, N-terminal probrain natriuretic peptide.
Screening high-risk subjects
Although many attempts, including national publicity and various programs for health promotion, have been made to manage stroke risk factors, the prevalence of stroke has not been markedly reduced. This may be partially attributable to hidden risk factors of stroke. Interestingly, certain regions in the United States (Stroke Belt and Buckle) have an unusually high incidence and mortality of stroke and the phenomenon could not be explained by the differences of the conventional risk factors.12,13 The exact causes of the higher incidence and mortality of stroke in the regions have not been recognized. Therefore, many researchers have devoted themselves to find novel risk factors of stroke to explain it, whereupon numerous possible contributing factors have been identified, including obesity/metabolic syndrome, diet, sleep-related breathing disorders, air pollution, and cultural lifestyle.14
In addition to finding these new risk factors, a series of biomarkers reflecting inflammation, hemostasis, thrombosis, endothelial function, or neurohormonal activity have been evaluated as potential tools in an effort to improve risk prediction of future stroke, and thereby avert future events.15-22 For example, a recent investigation using data from the Framingham offspring study found that plasma asymmetrical dimethylarginine (ADMA) which is an inhibitor of endothelial nitric oxide synthase (eNOS) and a marker of endothelial dysfunction was associated with a prevalence of silent brain infarcts which is an important correlate of risk of future stroke.19 More recently, the same group published data on multiple biomarkers and identified that baseline B-type natriuretic peptide (BNP) having diuretic and vasodilatory activities and a urinary albumin/creatinine ratio indicating endothelial function were associated with the risk of incident stroke, and offered modest improvements in the accuracy of risk stratification.22 In the near future, a genome-wide association study may also greatly contribute to building risk stratification models by identifying genetic variants that confer susceptibility to cerebrovascular disease.23
Rapid stroke diagnosis
Although the diagnosis of acute stroke mostly relies on neuroimaging techniques, the evaluation of biomarkers of tissue injury would be an alternative strategy for rapid stroke assessment. This approach has already been successfully applied in the early management of other diseases including coronary heart disease (troponin, CK-MB), pulmonary embolism (D-dimer), and congestive heart failure (BNP).24-26 A rapid diagnosis of stroke based on biomarkers may be useful especially for pre-hospital screening, facilitating entry into a fast track care pathway, and for ancillary data when contemplating thrombolysis. However, a widely available, rapid, and sensitive diagnostic test for acute cerebral ischemia has not been available until now.
Recently, a biomarker panel rather than a single marker in isolation has been increasingly used to improve the diagnostic accuracy of suspected stroke. For instance, a diagnostic panel incorporating the levels of matrix metalloproteinase 9 (MMP-9), BNP, D-dimer, and S-100β into a composite score enhanced sensitivity of early noncontrast CT alone for acute stroke, although the diagnostic accuracy was clearly imperfect.27 Furthermore, the approach was feasible as a point-of-care test in the emergency setting.27 As the number of presumed biomarkers for stroke expands at an exponential rate, it would be expected to develop improved biomarker combinations for more accurate diagnosis of stroke.
Detection of possible stroke mechanisms
Several studies have focused on the use of biomarkers for detecting possible stroke mechanisms. A recent study investigated whether concentrations of von Willebrand factor (vWF) which plays crucial roles in thrombus formation differ depending on the etiologic subtypes of stroke, and found the highest levels in large artery disease and cardioembolic stroke.28 Recent data by our group also demonstrated that inflammatory markers, rather than traditional risk factors were associated with clinical and radiological differences among patients with atherosclerotic stroke.29 In addition, the molecular markers related to neuronal death can provide information about the presence of tissue at risk of infarction.30,31
Predicting drug response and outcome
It has been well known that different patients respond in different manners to the same medication. Among many factors that influence the effects of drugs, it is estimated that genetic factors can account for 20 to 95% of variability in drug disposition and effects.32 For example, previous studies revealed that CYP2C9 and VKORC1 genetic variants are associated with warfarin dose requirement and clinical outcomes.33,34 Besides pharmacogenetics, several biomarkers are also contributing to predicting drug responses in patients with stroke, particularly when thrombolysis is administered. Specifically, elevated S-100β and MMP-9 which were reported as serum markers of blood-brain barrier (BBB) dysfunction before thrombolysis could predict hemorrhagic transformation after thrombolysis,35-37 whereas baseline levels of α2-antiplasmin were predictive of recanalization in patients treated with rt-PA.38
There has been mounting evidence that a number of biomarkers can predict clinical or radiological outcomes from cerebral ischemic events. Inflammatory markers such as C-reactive protein (CRP) or proinflammatory cytokines are reportedly associated with early neurological worsening or poor functional outcome after stroke.39,40 Biomarkers related to coagulation/fibrinolysis system such as D-dimer or vWF may also have links with outcome prediction, especially in patients with cardioembolic stroke.41-44 Very recently, it was reported that genetic polymorphisms of brain-derived neurotrophic factor (BDNF) was associated with functional outcome after subarachnoid hemorrhage, and cortical plasticity.45,46
Surrogate endpoints in clinical trials
In cardiovascular diseases, many investigators have used biomarkers that correlate with clinical outcomes as surrogate endpoints, because event-driven clinical trials require much of the cost and time burden.47 In the area of stroke research, several studies has been started to use biomarkers to monitor the efficacy and safety of treatments in phase III clinical trials. However, changes detected in surrogate markers do not always translate into clinical endpoints, and may even be the opposite with clinical outcomes.48 Thus, biomarkers may be useful as a screening tool and secondary outcomes, but not primary outcome in clinical trials at the present time.
Type of biomarkers in stroke research
Stroke biomarkers include traditional protein biomarkers and novel genetic, microvesicle, and metabolomics-associated biomarkers (Table 2).
Table 2.
Biomarkers for specific stroke subtypes
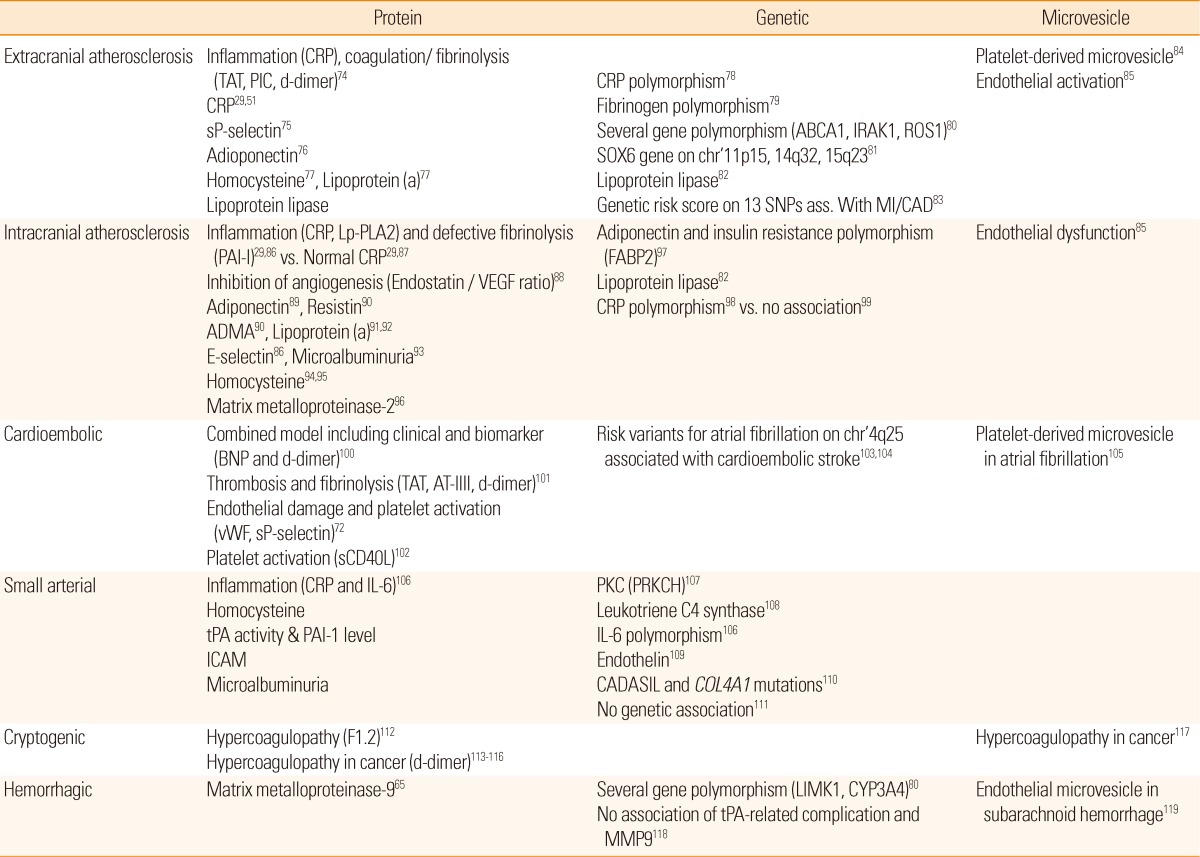
TAT, Thrombin-Antithrombin complex; PIC, Plasmin inhibitor complex; sP-selectin, Soluble P-selectin; ABCA1, ATP-binding cassette, subfamily A, member 1; IRAK1, Interleukin-1 receptor.associated kinase-1; ROS1, v-Ros avian UR2 sarcoma virus oncogene homolog-1; SOX6, sex-determining region Y-box 6; SNP, Single nucleotide polymorphism; MI, Myocardial infarction; CAD, Coronary artery disease; CRP, C-reactive protein; Lp-PLA2, Lipoprotein-associated phospholipase A2; PAI-1, Plasminogen activator inhibitor-1; VEGF, Vascular endothelial growth factor; ADMA, Asymmetrical dimethylarginine; FABP2, Fatty acid-binding protein 2; BNP, B-type natriuretic peptide; AT-III, Antithrombin-III; vWF, von Willebrand factor; sCD40L, Soluble CD40 ligand; tPA, Tissue plasminogen activator; ICAM, Intercellular adhesion molecule; PKC, Protein kinase C; PRKCH, Protein kinase C eta; CADASIL, Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; COL4A1, Collagen, type IV, alpha 1; F1.2, Prothrombin fragment 1.2; LIMK1, LIM domain kinase 1; CYP3A4, Cytochrome P450, subfamily IIIA, polypeptide 4.
Protein biomarkers
Research using protein biomarkers in patients with ischemic cerebrovascular disease have mainly focused on pathophysiology, diagnosis, prognostication, and neuronal death in stroke.49 A typical example of protein biomarkers is CRP.17,50,51 However, a recent study raised the possibility that the relation may result from various biases.52 Moreover, it has raised skepticism about the efficacy of biomarkers in predicting stroke risk because they provide only limited additional information compared to the well-known stroke risk factors.53,54 Further studies with more a systematic approach and analysis are needed in this area.
Genetic biomarkers
Many epidemiological studies suggested that stroke has genetic susceptibility, and various genetic factors were investigated.55 Among them, establishing the association of the 9p21.3 locus with risk of cerebral infarction is one of the biggest advances.56 It is estimated that the genetic influence is caused by enhanced platelet reactivity.56 However, many other genome-wide association studies failed to reproduce the positive results obtained from previous studies57 or have shown that the clinical usefulness was very low.58 For example, the hazard ratio and population attributable risk of hypertension to ischemic stroke is 2.0 and 26%, respectively. Conversely, the genetic influence on stroke was only 1.3-1.33 and 11-12%, respectively.58
Recently, studies in the pharmacogenomic area have been actively carried out. Among them, aspirin, clopidogrel, warfarin, statin, and thrombolytics-related genetic polymorphisms are particularly of interest. It is expected that selecting the type or dose of medication, avoiding side effects, or drug resistance may be guided by simple genetic tests in the near future.
Microvesicle
Microvesicles are defined as a heterogeneous population of small vesicles with a diameter of 0.1-1 µm. It was previously believed that microvesicles were artifacts generated by apoptotic cell death. However, this view has changed because the shedding of these small vesicles was recognized to result from an active process.59 Microvesicles may be a window for target cell/organs, and include genetic information as well as protein inside them.60 Moreover, it has been identified that microvesicles have their own function, revealing that microvesicles from ischemic tissue facilitated vasculogenesis in the ischemic limb model.61 In this regard, biomarker research using microvesicles is a prominent field.
Nevertheless, biomarker studies using microvesicles in stroke are mostly performed in small cohorts. As the methods for analyzing microvesicles are complicated and not unique mainly due to their very small size, investigations with microvesicles are currently at a rudimentary state of development. When seeing the results from a large-scale clinical study perspective for the prediction of the future risk of myocardial infarction, microvesicles could be a good candidate to compensate for the limitations of existing biomarker researches.62
Metabolomics
The assumption of metabolomics is that occurrence of the disease is directly related to the specific change of biochemical composition in the cell or biological fluid. Metabolomics-associated biomarker research analyzes profiles of fatty acids, amino acids, or polyamine in the blood or urine and determines normal or pathologic states. Furthermore, metabolomics-associated biomarkers can be applied to the monitoring recovery after treatment. Unfortunately, studies using metabolomics in the area of stroke is relatively lacking.
Limitations of stroke biomarkers
Currently, the application of biomarkers in the management of cerebral infarction has some limitations, despite their evolving role.
First, unlike myocardial infarction, changes in the brain are not sufficiently reflected by blood biomarkers due to the presence of the blood-brain barrier (low sensitivity and underpowered).
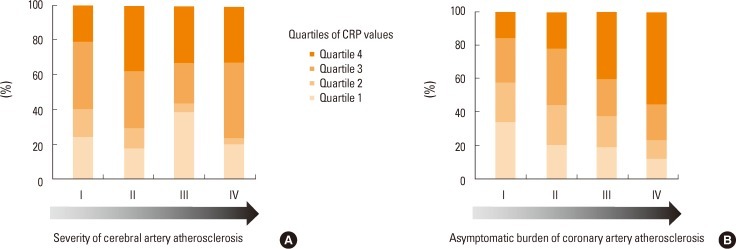
Second, biomarkers can change by a variety of comorbid conditions or brain damage itself (confounders and lack of specificities). As asymptomatic coronary atherosclerosis is frequently accompanied in patients with ischemic stroke,63 it may confound the levels of biomarkers. Indeed, our group recently found that the burden of asymptomatic coronary atherosclerosis was the most important factor for levels of C-reactive protein and homocysteine, regardless of vascular risk factors and the degree and distribution of cervicocephalic atherosclerosis (Figure 1).64 Furthermore, the direct role of biomarkers in the disease process may be hard to reveal. For example, matrix metalloproteinase-9 (MMP-9) which is known as a marker of hemorrhagic transformation after thrombolysis65 is significantly associated with the size of the cerebral infarction irrespective of hemorrhagic transformation.29 Therefore, it is difficult to establish the causal relationship between biomarkers and ischemic stroke in a real clinical setting.
Figure 1.
Distribution of CRP according to the (A) severity of cerebral atherosclerosis or (B) asymptomatic burden of coronary atherosclerosis in patients with stroke. CRP values are proportional to the increase of coronary (P=0.010 for trends), but not cerebral atherosclerosis (P=0.131 for trends). Severity of cerebral or coronary atherosclerosis. I: No atherosclerosis or 1 segment with <50% stenosis, II: ≥2 segments with <50% stenosis, III: 1 segment with ≥50% stenosis, IV: ≥2 segments with ≥50% stenosis.
Third, there is no sufficiently robust single marker for stroke. As shown in Table 2, ischemic stroke is a complex process that includes various etiologies. In addition, the brain consists of many different cells with vessels having distinct anatomical characteristics.
Future direction and summary: need of comprehensive approach
Each biomarker has different aspects, and its own advantages and drawbacks (Table 3). Therefore, developing an integrated panel of biomarkers for specific stroke subtypes is needed. A recent study reported that multiple microparticle biomarkers in addition to existing protein biomarkers are valuable for predicting future cardio- and cerebrovascular events.62 Hence, a comprehensive approach to using a variety of biomarkers is warranted to overcome the limitations. In addition, multidisciplinary approaches including neuroimaging biomarkers are needed.
Table 3.
Strengths and weaknesses of various biomarkers
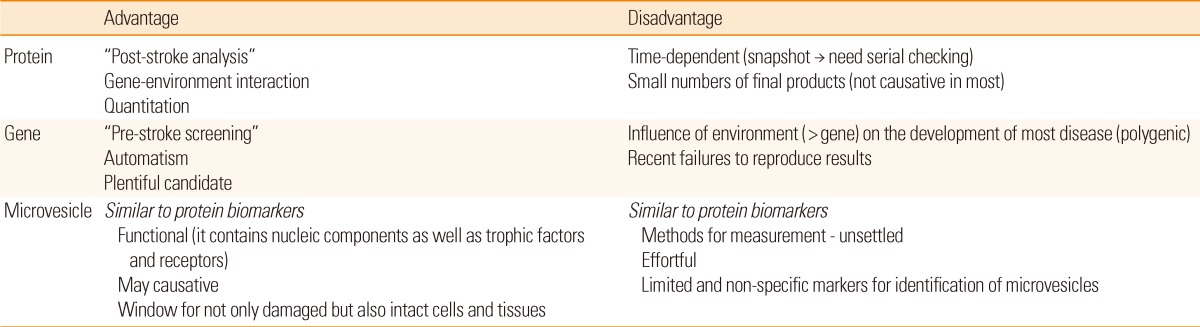
A number of biomarkers are under investigation in patients with ischemic stroke. Research about biomarkers can be helpful especially in predicting stroke and monitoring therapeutic effects. Currently, however, the application of biomarkers is only recommended for research purposes. Monitoring traditional risk factors or vessel status is more efficacious than measuring biomarkers in clinical practice. Considering the advantages and disadvantages of each biomarker is important for future study, and a comprehensive approach using multiple biomarkers is needed. It is strongly anticipated that the biomarkers can provide us a turning point for investigating pathophysiology and therapeutic mechanisms of ischemic stroke.
Footnotes
This study was supported by the Korean Healthcare Technology R&D Project, Ministry of Health & Welfare (A110208) and the Bio & Medical Technology Developmental Program of the National Research Foundation of Korea, Ministry of Education, Science and Technology (2011-0019389).
The authors have no financial conflicts of interest.
References
- 1.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 2.Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 4.Hacke W, Furlan AJ, Al-Rawi Y, Davalos A, Fiebach JB, Gruber F, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009;8:141–150. doi: 10.1016/S1474-4422(08)70267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 6.Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, et al. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 7.Albers GW, Goldstein LB, Hess DC, Wechsler LR, Furie KL, Gorelick PB, et al. Stroke Treatment Academic Industry Roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke. 2011;42:2645–2650. doi: 10.1161/STROKEAHA.111.618850. [DOI] [PubMed] [Google Scholar]
- 8.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7:e1000251. doi: 10.1371/journal.pmed.1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maasland L, van Oostenbrugge RJ, Franke CF, Scholte Op Reimer WJ, Koudstaal PJ, Dippel DW. Patients enrolled in large randomized clinical trials of antiplatelet treatment for prevention after transient ischemic attack or ischemic stroke are not representative of patients in clinical practice: the Netherlands Stroke Survey. Stroke. 2009;40:2662–2668. doi: 10.1161/STROKEAHA.109.551812. [DOI] [PubMed] [Google Scholar]
- 10.Saver JL. Novel end point analytic techniques and interpreting shifts across the entire range of outcome scales in acute stroke trials. Stroke. 2007;38:3055–3062. doi: 10.1161/STROKEAHA.107.488536. [DOI] [PubMed] [Google Scholar]
- 11.Saver JL, Gornbein J. Treatment effects for which shift or binary analyses are advantageous in acute stroke trials. Neurology. 2009;72:1310–1315. doi: 10.1212/01.wnl.0000341308.73506.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casper ML, Wing S, Anda RF, Knowles M, Pollard RA. The shifting stroke belt. Changes in the geographic pattern of stroke mortality in the United States, 1962 to 1988. Stroke. 1995;26:755–760. doi: 10.1161/01.str.26.5.755. [DOI] [PubMed] [Google Scholar]
- 13.El-Saed A, Kuller LH, Newman AB, Lopez O, Costantino J, McTigue K, et al. Geographic variations in stroke incidence and mortality among older populations in four US communities. Stroke. 2006;37:1975–1979. doi: 10.1161/01.STR.0000231453.98473.67. [DOI] [PubMed] [Google Scholar]
- 14.Howard G, Prineas R, Moy C, Cushman M, Kellum M, Temple E, et al. Racial and geographic differences in awareness, treatment, and control of hypertension: the REasons for Geographic And Racial Differences in Stroke study. Stroke. 2006;37:1171–1178. doi: 10.1161/01.STR.0000217222.09978.ce. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Brown NJ, Vaughan DE, Harrison DG, Mehta JL. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004;109:IV6–IV19. doi: 10.1161/01.CIR.0000133444.17867.56. [DOI] [PubMed] [Google Scholar]
- 16.Woodward M, Lowe GD, Campbell DJ, Colman S, Rumley A, Chalmers J, et al. Associations of inflammatory and hemostatic variables with the risk of recurrent stroke. Stroke. 2005;36:2143–2147. doi: 10.1161/01.STR.0000181754.38408.4c. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 18.Welsh P, Lowe GD, Chalmers J, Campbell DJ, Rumley A, Neal BC, et al. Associations of proinflammatory cytokines with the risk of recurrent stroke. Stroke. 2008;39:2226–2230. doi: 10.1161/STROKEAHA.107.504498. [DOI] [PubMed] [Google Scholar]
- 19.Pikula A, Boger RH, Beiser AS, Maas R, DeCarli C, Schwedhelm E, et al. Association of plasma ADMA levels with MRI markers of vascular brain injury: Framingham offspring study. Stroke. 2009;40:2959–2964. doi: 10.1161/STROKEAHA.109.557116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park CS, Ihm SH, Yoo KD, Kim DB, Lee JM, Kim HY, et al. Relation between C-reactive protein, homocysteine levels, fibrinogen, and lipoprotein levels and leukocyte and platelet counts, and 10-year risk for cardiovascular disease among healthy adults in the USA. Am J Cardiol. 2010;105:1284–1288. doi: 10.1016/j.amjcard.2009.12.045. [DOI] [PubMed] [Google Scholar]
- 21.Wieberdink RG, van Schie MC, Koudstaal PJ, Hofman A, Witteman JC, de Maat MP, et al. High von Willebrand factor levels increase the risk of stroke: the Rotterdam study. Stroke. 2010;41:2151–2156. doi: 10.1161/STROKEAHA.110.586289. [DOI] [PubMed] [Google Scholar]
- 22.Pikula A, Beiser AS, DeCarli C, Himali JJ, Debette S, Au R, et al. Multiple biomarkers and risk of clinical and subclinical vascular brain injury: the Framingham Offspring Study. Circulation. 2012;125:2100–2107. doi: 10.1161/CIRCULATIONAHA.110.989145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada Y, Fuku N, Tanaka M, Aoyagi Y, Sawabe M, Metoki N, et al. Identification of CELSR1 as a susceptibility gene for ischemic stroke in Japanese individuals by a genome-wide association study. Atherosclerosis. 2009;207:144–149. doi: 10.1016/j.atherosclerosis.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 24.Penttila K, Koukkunen H, Halinen M, Rantanen T, Pyorala K, Punnonen K, et al. Myoglobin, creatine kinase MB isoforms and creatine kinase MB mass in early diagnosis of myocardial infarction in patients with acute chest pain. Clin Biochem. 2002;35:647–653. doi: 10.1016/s0009-9120(02)00385-5. [DOI] [PubMed] [Google Scholar]
- 25.Gibler WB, Blomkalns AL, Collins SP. Evaluation of chest pain and heart failure in the emergency department: impact of multimarker strategies and b-type natriuretic peptide. Rev Cardiovasc Med. 2003;4(Suppl 4):S47–S55. [PubMed] [Google Scholar]
- 26.Stein PD, Hull RD, Patel KC, Olson RE, Ghali WA, Brant R, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med. 2004;140:589–602. doi: 10.7326/0003-4819-140-8-200404200-00005. [DOI] [PubMed] [Google Scholar]
- 27.Laskowitz DT, Kasner SE, Saver J, Remmel KS, Jauch EC. Clinical usefulness of a biomarker-based diagnostic test for acute stroke: the Biomarker Rapid Assessment in Ischemic Injury (BRAIN) study. Stroke. 2009;40:77–85. doi: 10.1161/STROKEAHA.108.516377. [DOI] [PubMed] [Google Scholar]
- 28.Hanson E, Jood K, Karlsson S, Nilsson S, Blomstrand C, Jern C. Plasma levels of von Willebrand factor in the etiologic subtypes of ischemic stroke. J Thromb Haemost. 2011;9:275–281. doi: 10.1111/j.1538-7836.2010.04134.x. [DOI] [PubMed] [Google Scholar]
- 29.Bang OY, Lee PH, Yoon SR, Lee MA, Joo IS, Huh K. Inflammatory markers, rather than conventional risk factors, are different between carotid and MCA atherosclerosis. J Neurol Neurosurg Psychiatry. 2005;76:1128–1134. doi: 10.1136/jnnp.2004.054403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez-Yanez M, Sobrino T, Arias S, Vazquez-Herrero F, Brea D, Blanco M, et al. Early biomarkers of clinical-diffusion mismatch in acute ischemic stroke. Stroke. 2011;42:2813–2818. doi: 10.1161/STROKEAHA.111.614503. [DOI] [PubMed] [Google Scholar]
- 31.Kernagis DN, Laskowitz DT. Evolving role of biomarkers in acute cerebrovascular disease. Ann Neurol. 2012;71:289–303. doi: 10.1002/ana.22553. [DOI] [PubMed] [Google Scholar]
- 32.Kalow W, Tang BK, Endrenyi L. Hypothesis: comparisons of inter- and intra-individual variations can substitute for twin studies in drug research. Pharmacogenetics. 1998;8:283–289. doi: 10.1097/00008571-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Higashi MK, Veenstra DL, Kondo LM, Wittkowsky AK, Srinouanprachanh SL, Farin FM, et al. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA. 2002;287:1690–1698. doi: 10.1001/jama.287.13.1690. [DOI] [PubMed] [Google Scholar]
- 34.Sconce EA, Khan TI, Wynne HA, Avery P, Monkhouse L, King BP, et al. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood. 2005;106:2329–2333. doi: 10.1182/blood-2005-03-1108. [DOI] [PubMed] [Google Scholar]
- 35.Heo JH, Kim SH, Lee KY, Kim EH, Chu CK, Nam JM. Increase in plasma matrix metalloproteinase-9 in acute stroke patients with thrombolysis failure. Stroke. 2003;34:e48–e50. doi: 10.1161/01.STR.0000073788.81170.1C. [DOI] [PubMed] [Google Scholar]
- 36.Montaner J, Molina CA, Monasterio J, Abilleira S, Arenillas JF, Ribo M, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107:598–603. doi: 10.1161/01.cir.0000046451.38849.90. [DOI] [PubMed] [Google Scholar]
- 37.Foerch C, Wunderlich MT, Dvorak F, Humpich M, Kahles T, Goertler M, et al. Elevated serum S100B levels indicate a higher risk of hemorrhagic transformation after thrombolytic therapy in acute stroke. Stroke. 2007;38:2491–2495. doi: 10.1161/STROKEAHA.106.480111. [DOI] [PubMed] [Google Scholar]
- 38.Marti-Fabregas J, Borrell M, Cocho D, Belvis R, Castellanos M, Montaner J, et al. Hemostatic markers of recanalization in patients with ischemic stroke treated with rt-PA. Neurology. 2005;65:366–370. doi: 10.1212/01.wnl.0000171704.50395.ba. [DOI] [PubMed] [Google Scholar]
- 39.Vila N, Castillo J, Davalos A, Chamorro A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke. 2000;31:2325–2329. doi: 10.1161/01.str.31.10.2325. [DOI] [PubMed] [Google Scholar]
- 40.Whiteley W, Jackson C, Lewis S, Lowe G, Rumley A, Sandercock P, et al. Inflammatory markers and poor outcome after stroke: a prospective cohort study and systematic review of interleukin-6. PLoS Med. 2009;6:e1000145. doi: 10.1371/journal.pmed.1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon CW, Kim SJ, Bang OY, Chung CS, Lee KH, Kim GM. Premorbid warfarin use and lower D-dimer levels are associated with spontaneous early improvement in atrial fibrillation-related stroke. J Thromb Haemost. 2012 doi: 10.1111/j.1538-7836.2012.04909.x. (doi: 10.1111/j.1538-7836.2012.04909.x.) [DOI] [PubMed] [Google Scholar]
- 42.Roldan V, Marin F, Muina B, Torregrosa JM, Hernandez-Romero D, Valdes M, et al. Plasma von Willebrand factor levels are an independent risk factor for adverse events including mortality and major bleeding in anticoagulated atrial fibrillation patients. J Am Coll Cardiol. 2011;57:2496–2504. doi: 10.1016/j.jacc.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 43.Motoki H, Tomita T, Aizawa K, Kasai H, Izawa A, Kumazaki S, et al. Coagulation activity is increased in the left atria of patients with paroxysmal atrial fibrillation during the non-paroxysmal period. Comparison with chronic atrial fibrillation. Circ J. 2009;73:1403–1407. doi: 10.1253/circj.cj-09-0008. [DOI] [PubMed] [Google Scholar]
- 44.Kang DW, Yoo SH, Chun S, Kwon KY, Kwon SU, Koh JY, et al. Inflammatory and hemostatic biomarkers associated with early recurrent ischemic lesions in acute ischemic stroke. Stroke. 2009;40:1653–1658. doi: 10.1161/STROKEAHA.108.539429. [DOI] [PubMed] [Google Scholar]
- 45.Siironen J, Juvela S, Kanarek K, Vilkki J, Hernesniemi J, Lappalainen J. The Met allele of the BDNF Val66Met polymorphism predicts poor outcome among survivors of aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:2858–2860. doi: 10.1161/STROKEAHA.107.485441. [DOI] [PubMed] [Google Scholar]
- 46.Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586:5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halim SA, Newby LK, Ohman EM. Biomarkers in cardiovascular clinical trials: past, present, future. Clin Chem. 2012;58:45–53. doi: 10.1373/clinchem.2011.165787. [DOI] [PubMed] [Google Scholar]
- 48.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 49.Lynch JR, Blessing R, White WD, Grocott HP, Newman MF, Laskowitz DT. Novel diagnostic test for acute stroke. Stroke. 2004;35:57–63. doi: 10.1161/01.STR.0000105927.62344.4C. [DOI] [PubMed] [Google Scholar]
- 50.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 51.Van Der Meer IM, De Maat MP, Hak AE, Kiliaan AJ, Del Sol AI, Van Der Kuip DA, et al. C-reactive protein predicts progression of atherosclerosis measured at various sites in the arterial tree: the Rotterdam study. Stroke. 2002;33:2750–2755. doi: 10.1161/01.str.0000044168.00485.02. [DOI] [PubMed] [Google Scholar]
- 52.Hemingway H, Philipson P, Chen R, Fitzpatrick NK, Damant J, Shipley M, et al. Evaluating the quality of research into a single prognostic biomarker: a systematic review and meta-analysis of 83 studies of C-reactive protein in stable coronary artery disease. PLoS Med. 2010;7:e1000286. doi: 10.1371/journal.pmed.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hankey GJ. Potential new risk factors for ischemic stroke: what is their potential? Stroke. 2006;37:2181–2188. doi: 10.1161/01.STR.0000229883.72010.e4. [DOI] [PubMed] [Google Scholar]
- 54.O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 55.Dichgans M. Genetics of ischaemic stroke. Lancet Neurol. 2007;6:149–161. doi: 10.1016/S1474-4422(07)70028-5. [DOI] [PubMed] [Google Scholar]
- 56.Meschia JF. Advances in genetics 2010. Stroke. 2011;42:285–287. doi: 10.1161/STROKEAHA.110.605089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lanktree MB, Dichgans M, Hegele RA. Advances in genomic analysis of stroke: what have we learned and where are we headed? Stroke. 2010;41:825–832. doi: 10.1161/STROKEAHA.109.570523. [DOI] [PubMed] [Google Scholar]
- 58.Ikram MA, Seshadri S, Bis JC, Fornage M, DeStefano AL, Aulchenko YS, et al. Genomewide association studies of stroke. N Engl J Med. 2009;360:1718–1728. doi: 10.1056/NEJMoa0900094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Kim SJ, Moon GJ, Cho YH, Kang HY, Hyung NK, Kim D, et al. Circulating mesenchymal stem cells microparticles in patients with cerebrovascular disease. PLoS One. 2012;7:e37036. doi: 10.1371/journal.pone.0037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leroyer AS, Ebrahimian TG, Cochain C, Recalde A, Blanc-Brude O, Mees B, et al. Microparticles from ischemic muscle promotes postnatal vasculogenesis. Circulation. 2009;119:2808–2817. doi: 10.1161/CIRCULATIONAHA.108.816710. [DOI] [PubMed] [Google Scholar]
- 62.Nozaki T, Sugiyama S, Koga H, Sugamura K, Ohba K, Matsuzawa Y, et al. Significance of a multiple biomarkers strategy including endothelial dysfunction to improve risk stratification for cardiovascular events in patients at high risk for coronary heart disease. J Am Coll Cardiol. 2009;54:601–608. doi: 10.1016/j.jacc.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 63.Calvet D, Touze E, Varenne O, Sablayrolles JL, Weber S, Mas JL. Prevalence of asymptomatic coronary artery disease in ischemic stroke patients: the PRECORIS study. Circulation. 2010;121:1623–1629. doi: 10.1161/CIRCULATIONAHA.109.906958. [DOI] [PubMed] [Google Scholar]
- 64.Kim SJ, Choe YH, Bang OY. Are stroke biomarkers seeing brain vessels in patients with ischemic stroke? A C-reactive protein and homocysteine study. Stroke. 2011;42:1464–1468. doi: 10.1161/STROKEAHA.110.607432. [DOI] [PubMed] [Google Scholar]
- 65.Castellanos M, Sobrino T, Millan M, Garcia M, Arenillas J, Nombela F, et al. Serum cellular fibronectin and matrix metalloproteinase-9 as screening biomarkers for the prediction of parenchymal hematoma after thrombolytic therapy in acute ischemic stroke: a multicenter confirmatory study. Stroke. 2007;38:1855–1859. doi: 10.1161/STROKEAHA.106.481556. [DOI] [PubMed] [Google Scholar]
- 66.Reynolds MA, Kirchick HJ, Dahlen JR, Anderberg JM, McPherson PH, Nakamura KK, et al. Early biomarkers of stroke. Clin Chem. 2003;49:1733–1739. doi: 10.1373/49.10.1733. [DOI] [PubMed] [Google Scholar]
- 67.Jauch EC, Lindsell C, Broderick J, Fagan SC, Tilley BC, Levine SR. Association of serial biochemical markers with acute ischemic stroke: the National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator Stroke Study. Stroke. 2006;37:2508–2513. doi: 10.1161/01.STR.0000242290.01174.9e. [DOI] [PubMed] [Google Scholar]
- 68.Montaner J, Rovira A, Molina CA, Arenillas JF, Ribo M, Chacon P, et al. Plasmatic level of neuroinflammatory markers predict the extent of diffusion-weighted image lesions in hyperacute stroke. J Cereb Blood Flow Metab. 2003;23:1403–1407. doi: 10.1097/01.WCB.0000100044.07481.97. [DOI] [PubMed] [Google Scholar]
- 69.Iwamoto T, Kubo H, Takasaki M. Platelet activation in the cerebral circulation in different subtypes of ischemic stroke and Binswanger's disease. Stroke. 1995;26:52–56. doi: 10.1161/01.str.26.1.52. [DOI] [PubMed] [Google Scholar]
- 70.Matsui T, Mori T, Tateishi N, Kagamiishi Y, Satoh S, Katsube N, et al. Astrocytic activation and delayed infarct expansion after permanent focal ischemia in rats. Part I: enhanced astrocytic synthesis of s-100beta in the periinfarct area precedes delayed infarct expansion. J Cereb Blood Flow Metab. 2002;22:711–722. doi: 10.1097/00004647-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 71.Foerch C, du Mesnil de Rochemont R, Singer O, Neumann-Haefelin T, Buchkremer M, Zanella FE, et al. S100B as a surrogate marker for successful clot lysis in hyperacute middle cerebral artery occlusion. J Neurol Neurosurg Psychiatry. 2003;74:322–325. doi: 10.1136/jnnp.74.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Conway DS, Pearce LA, Chin BS, Hart RG, Lip GY. Prognostic value of plasma von Willebrand factor and soluble P-selectin as indices of endothelial damage and platelet activation in 994 patients with nonvalvular atrial fibrillation. Circulation. 2003;107:3141–3145. doi: 10.1161/01.CIR.0000077912.12202.FC. [DOI] [PubMed] [Google Scholar]
- 73.Rost NS, Biffi A, Cloonan L, Chorba J, Kelly P, Greer D, et al. Brain natriuretic peptide predicts functional outcome in ischemic stroke. Stroke. 2012;43:441–445. doi: 10.1161/STROKEAHA.111.629212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tohgi H, Konno S, Takahashi S, Koizumi D, Kondo R, Takahashi H. Activated coagulation/fibrinolysis system and platelet function in acute thrombotic stroke patients with increased C-reactive protein levels. Thromb Res. 2000;100:373–379. doi: 10.1016/s0049-3848(00)00356-x. [DOI] [PubMed] [Google Scholar]
- 75.Yip HK, Lu CH, Yang CH, Chang HW, Hung WC, Cheng CI, et al. Levels and value of platelet activity in patients with severe internal carotid artery stenosis. Neurology. 2006;66:804–808. doi: 10.1212/01.wnl.0000208220.04165.05. [DOI] [PubMed] [Google Scholar]
- 76.Barseghian A, Gawande D, Bajaj M. Adiponectin and vulnerable atherosclerotic plaques. J Am Coll Cardiol. 2011;57:761–770. doi: 10.1016/j.jacc.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 77.Kim SJ, Song P, Park JH, Lee YT, Kim WS, Park YG, et al. Biomarkers of asymptomatic carotid stenosis in patients undergoing coronary artery bypass grafting. Stroke. 2011;42:734–739. doi: 10.1161/STROKEAHA.110.595546. [DOI] [PubMed] [Google Scholar]
- 78.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359:1897–1908. doi: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- 79.Tosetto A, Prati P, Baracchini C, Manara R, Rodeghiero F. Association of plasma fibrinogen, C-reactive protein and G-455>A polymorphism with early atherosclerosis in the VITA Project cohort. Thromb Haemost. 2011;105:329–335. doi: 10.1160/TH10-08-0522. [DOI] [PubMed] [Google Scholar]
- 80.Yamada Y, Metoki N, Yoshida H, Satoh K, Kato K, Hibino T, et al. Genetic factors for ischemic and hemorrhagic stroke in Japanese individuals. Stroke. 2008;39:2211–2218. doi: 10.1161/STROKEAHA.107.507459. [DOI] [PubMed] [Google Scholar]
- 81.Dong C, Beecham A, Slifer S, Wang L, Blanton SH, Wright CB, et al. Genomewide linkage and peakwide association analyses of carotid plaque in Caribbean Hispanics. Stroke. 2010;41:2750–2756. doi: 10.1161/STROKEAHA.110.596981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang C, Sun T, Li H, Bai J, Li Y. Lipoprotein lipase Ser447Ter polymorphism associated with the risk of ischemic stroke: a meta-analysis. Thromb Res. 2011;128:e107–e112. doi: 10.1016/j.thromres.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 83.Hamrefors V, Hedblad B, Engstrom G, Almgren P, Sjogren M, Melander O. A myocardial infarction genetic risk score is associated with markers of carotid atherosclerosis. J Intern Med. 2012;271:271–281. doi: 10.1111/j.1365-2796.2011.02472.x. [DOI] [PubMed] [Google Scholar]
- 84.Kuriyama N, Nagakane Y, Hosomi A, Ohara T, Kasai T, Harada S, et al. Evaluation of factors associated with elevated levels of platelet-derived microparticles in the acute phase of cerebral infarction. Clin Appl Thromb Hemost. 2010;16:26–32. doi: 10.1177/1076029609338047. [DOI] [PubMed] [Google Scholar]
- 85.Jung KH, Chu K, Lee ST, Park HK, Bahn JJ, Kim DH, et al. Circulating endothelial microparticles as a marker of cerebrovascular disease. Ann Neurol. 2009;66:191–199. doi: 10.1002/ana.21681. [DOI] [PubMed] [Google Scholar]
- 86.Arenillas JF, Alvarez-Sabin J, Molina CA, Chacon P, Fernandez-Cadenas I, Ribo M, et al. Progression of symptomatic intracranial large artery atherosclerosis is associated with a proinflammatory state and impaired fibrinolysis. Stroke. 2008;39:1456–1463. doi: 10.1161/STROKEAHA.107.498600. [DOI] [PubMed] [Google Scholar]
- 87.Takahashi W, Ohnuki T, Ohnuki Y, Kawada S, Takagi S. The role of high-sensitivity C-reactive protein in asymptomatic intra- and extracranial large artery diseases. Cerebrovasc Dis. 2008;26:549–555. doi: 10.1159/000160212. [DOI] [PubMed] [Google Scholar]
- 88.Arenillas JF, Alvarez-Sabin J, Montaner J, Rosell A, Molina CA, Rovira A, et al. Angiogenesis in symptomatic intracranial atherosclerosis: predominance of the inhibitor endostatin is related to a greater extent and risk of recurrence. Stroke. 2005;36:92–97. doi: 10.1161/01.STR.0000149617.65372.5d. [DOI] [PubMed] [Google Scholar]
- 89.Bang OY, Saver JL, Ovbiagele B, Choi YJ, Yoon SR, Lee KH. Adiponectin levels in patients with intracranial atherosclerosis. Neurology. 2007;68:1931–1937. doi: 10.1212/01.wnl.0000263186.20988.9f. [DOI] [PubMed] [Google Scholar]
- 90.Lopez-Cancio E, Galan A, Dorado L, Jimenez M, Hernandez M, Millan M, et al. Biological Signatures of Asymptomatic Extra- and Intracranial Atherosclerosis: The Barcelona-AsIA (Asymptomatic Intracranial Atherosclerosis) Study. Stroke. 2012;43:2712–2719. doi: 10.1161/STROKEAHA.112.661702. [DOI] [PubMed] [Google Scholar]
- 91.Kim BS, Jung HS, Bang OY, Chung CS, Lee KH, Kim GM. Elevated serum lipoprotein(a) as a potential predictor for combined intracranial and extracranial artery stenosis in patients with ischemic stroke. Atherosclerosis. 2010;212:682–688. doi: 10.1016/j.atherosclerosis.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 92.Arenillas JF, Molina CA, Chacon P, Rovira A, Montaner J, Coscojuela P, et al. High lipoprotein (a), diabetes, and the extent of symptomatic intracranial atherosclerosis. Neurology. 2004;63:27–32. doi: 10.1212/01.wnl.0000132637.30287.b4. [DOI] [PubMed] [Google Scholar]
- 93.Bang OY. Intracranial atherosclerotic stroke: specific focus on the metabolic syndrome and inflammation. Curr Atheroscler Rep. 2006;8:330–336. doi: 10.1007/s11883-006-0012-1. [DOI] [PubMed] [Google Scholar]
- 94.Huang YC, Kuo YW, Lee TH, Lee M, Hsiao MC, Wang CL, et al. Hypoalbuminemia and not hyperhomocysteinemia as a risk factor for dementia in hemodialysis patients. J Ren Nutr. 2008;18:347–354. doi: 10.1053/j.jrn.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 95.Yoo JH, Chung CS, Kang SS. Relation of plasma homocyst(e)ine to cerebral infarction and cerebral atherosclerosis. Stroke. 1998;29:2478–2483. doi: 10.1161/01.str.29.12.2478. [DOI] [PubMed] [Google Scholar]
- 96.Jeon SB, Chun S, Choi-Kwon S, Chi HS, Nah HW, Kwon SU, et al. Biomarkers and location of atherosclerosis: matrix metalloproteinase-2 may be related to intracranial atherosclerosis. Atherosclerosis. 2012;223:442–447. doi: 10.1016/j.atherosclerosis.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 97.Yamada Y, Kato K, Oguri M, Yoshida T, Yokoi K, Watanabe S, et al. Association of genetic variants with atherothrombotic cerebral infarction in Japanese individuals with metabolic syndrome. Int J Mol Med. 2008;21:801–808. [PubMed] [Google Scholar]
- 98.Arenillas JF, Massot A, Alvarez-Sabin J, Fernandez-Cadenas I, del Rio-Espinola A, Chacon P, et al. C-reactive protein gene C1444T polymorphism and risk of recurrent ischemic events in patients with symptomatic intracranial atherostenoses. Cerebrovasc Dis. 2009;28:95–102. doi: 10.1159/000222660. [DOI] [PubMed] [Google Scholar]
- 99.Liu ZZ, Lv H, Gao F, Liu G, Zheng HG, Zhou YL, et al. Polymorphism in the human C-reactive protein (CRP) gene, serum concentrations of CRP, and the difference between intracranial and extracranial atherosclerosis. Clin Chim Acta. 2008;389:40–44. doi: 10.1016/j.cca.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 100.Montaner J, Perea-Gainza M, Delgado P, Ribo M, Chacon P, Rosell A, et al. Etiologic diagnosis of ischemic stroke subtypes with plasma biomarkers. Stroke. 2008;39:2280–2287. doi: 10.1161/STROKEAHA.107.505354. [DOI] [PubMed] [Google Scholar]
- 101.Kataoka S, Hirose G, Hori A, Shirakawa T, Saigan T. Activation of thrombosis and fibrinolysis following brain infarction. J Neurol Sci. 2000;181:82–88. doi: 10.1016/s0022-510x(00)00435-4. [DOI] [PubMed] [Google Scholar]
- 102.Ferro D, Loffredo L, Polimeni L, Fimognari F, Villari P, Pignatelli P, et al. Soluble CD40 ligand predicts ischemic stroke and myocardial infarction in patients with nonvalvular atrial fibrillation. Arterioscler Thromb Vasc Biol. 2007;27:2763–2768. doi: 10.1161/ATVBAHA.107.152777. [DOI] [PubMed] [Google Scholar]
- 103.Gretarsdottir S, Thorleifsson G, Manolescu A, Styrkarsdottir U, Helgadottir A, Gschwendtner A, et al. Risk variants for atrial fibrillation on chromosome 4q25 associate with ischemic stroke. Ann Neurol. 2008;64:402–409. doi: 10.1002/ana.21480. [DOI] [PubMed] [Google Scholar]
- 104.Lemmens R, Buysschaert I, Geelen V, Fernandez-Cadenas I, Montaner J, Schmidt H, et al. The association of the 4q25 susceptibility variant for atrial fibrillation with stroke is limited to stroke of cardioembolic etiology. Stroke. 2010;41:1850–1857. doi: 10.1161/STROKEAHA.110.587980. [DOI] [PubMed] [Google Scholar]
- 105.Choudhury A, Chung I, Blann AD, Lip GY. Elevated platelet microparticle levels in nonvalvular atrial fibrillation: relationship to p-selectin and antithrombotic therapy. Chest. 2007;131:809–815. doi: 10.1378/chest.06-2039. [DOI] [PubMed] [Google Scholar]
- 106.Fornage M, Chiang YA, O'Meara ES, Psaty BM, Reiner AP, Siscovick DS, et al. Biomarkers of inflammation and MRI-defined small vessel disease of the brain: The Cardiovascular Health Study. Stroke. 2008;39:1952–1959. doi: 10.1161/STROKEAHA.107.508135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kubo M, Hata J, Ninomiya T, Matsuda K, Yonemoto K, Nakano T, et al. A nonsynonymous SNP in PRKCH (protein kinase C eta) increases the risk of cerebral infarction. Nat Genet. 2007;39:212–217. doi: 10.1038/ng1945. [DOI] [PubMed] [Google Scholar]
- 108.Bevan S, Dichgans M, Wiechmann HE, Gschwendtner A, Meitinger T, Markus HS. Genetic variation in members of the leukotriene biosynthesis pathway confer an increased risk of ischemic stroke: a replication study in two independent populations. Stroke. 2008;39:1109–1114. doi: 10.1161/STROKEAHA.107.491969. [DOI] [PubMed] [Google Scholar]
- 109.Gormley K, Bevan S, Hassan A, Markus HS. Polymorphisms in genes of the endothelin system and cerebral small-vessel disease. Stroke. 2005;36:1656–1660. doi: 10.1161/01.STR.0000173173.38289.69. [DOI] [PubMed] [Google Scholar]
- 110.Lanfranconi S, Markus HS. COL4A1 mutations as a monogenic cause of cerebral small vessel disease: a systematic review. Stroke. 2010;41:e513–e518. doi: 10.1161/STROKEAHA.110.581918. [DOI] [PubMed] [Google Scholar]
- 111.Paternoster L, Chen W, Sudlow CL. Genetic determinants of white matter hyperintensities on brain scans: a systematic assessment of 19 candidate gene polymorphisms in 46 studies in 19,000 subjects. Stroke. 2009;40:2020–2026. doi: 10.1161/STROKEAHA.108.542050. [DOI] [PubMed] [Google Scholar]
- 112.Di Tullio MR, Homma S, Jin Z, Sacco RL. Aortic atherosclerosis, hypercoagulability, and stroke the APRIS (Aortic Plaque and Risk of Ischemic Stroke) study. J Am Coll Cardiol. 2008;52:855–861. doi: 10.1016/j.jacc.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bang OY, Seok JM, Kim SG, Hong JM, Kim HY, Lee J, et al. Ischemic stroke and cancer: stroke severely impacts cancer patients, while cancer increases the number of strokes. J Clin Neurol. 2011;7:53–59. doi: 10.3988/jcn.2011.7.2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seok JM, Kim SG, Kim JW, Chung CS, Kim GM, Lee KH, et al. Coagulopathy and embolic signal in cancer patients with ischemic stroke. Ann Neurol. 2010;68:213–219. doi: 10.1002/ana.22050. [DOI] [PubMed] [Google Scholar]
- 115.Kim SG, Hong JM, Kim HY, Lee J, Chung PW, Park KY, et al. Ischemic stroke in cancer patients with and without conventional mechanisms: a multicenter study in Korea. Stroke. 2010;41:798–801. doi: 10.1161/STROKEAHA.109.571356. [DOI] [PubMed] [Google Scholar]
- 116.Kim SJ, Park JH, Lee MJ, Park YG, Ahn MJ, Bang OY. Clues to occult cancer in patients with ischemic stroke. PLoS One. 2012;7:e44959. doi: 10.1371/journal.pone.0044959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hron G, Kollars M, Weber H, Sagaster V, Quehenberger P, Eichinger S, et al. Tissue factor-positive microparticles: cellular origin and association with coagulation activation in patients with colorectal cancer. Thromb Haemost. 2007;97:119–123. [PubMed] [Google Scholar]
- 118.Fernandez-Cadenas I, Del Rio-Espinola A, Carrera C, Domingues-Montanari S, Mendioroz M, Delgado P, et al. Role of the MMP9 gene in hemorrhagic transformations after tissue-type plasminogen activator treatment in stroke patients. Stroke. 2012;43:1398–1400. doi: 10.1161/STROKEAHA.111.639823. [DOI] [PubMed] [Google Scholar]
- 119.Lackner P, Dietmann A, Beer R, Fischer M, Broessner G, Helbok R, et al. Cellular microparticles as a marker for cerebral vasospasm in spontaneous subarachnoid hemorrhage. Stroke. 2010;41:2353–2357. doi: 10.1161/STROKEAHA.110.584995. [DOI] [PubMed] [Google Scholar]