Abstract
Purpose
Ethical and legal requirements for healthcare providers in the United States, stipulate that patients sign a consent form prior to undergoing medical treatment or participating in a research study. Currently, the majority of the hospitals obtain these consents using paper-based forms, which makes patient preference data cumbersome to store, search and retrieve. To address these issues, Health Sciences of South Carolina (HSSC), a collaborative of academic medical institutions and research universities in South Carolina, is developing an electronic consenting system, the Research Permissions Management System (RPMS). This article reports the findings of a study conducted to investigate the efficacy of the two proposed interfaces for this system – an iPad-based and touchscreen-based by comparing them to the paper-based and Topaz-based systems currently in use.
Methods
This study involved 50 participants: 10 hospital admission staff and 40 patients. The four systems were compared with respect to the time taken to complete the consenting process, the number of errors made by the patients, the workload experienced by the hospital staff and the subjective ratings of both patients and staff on post-test questionnaires.
Results
The results from the empirical study indicated no significant differences in the time taken to complete the tasks. More importantly, the participants found the new systems more usable than the conventional methods with the registration staff experiencing the least workload in the iPad and touchscreen-based conditions and the patients experiencing more privacy and control during the consenting process with the proposed electronic systems. In addition, they indicated better comprehension and awareness of what they were signing using the new interfaces.
Discussion
The results indicate the two methods proposed for capturing patient consents are at least as effective as the conventional methods, and superior in several important respects. While more research is needed, these findings suggest the viability of cautious adoption of electronic consenting systems, especially because these new systems appear to address the challenge of identifying the participants required for the complex research being conducted as the result of advances in the biomedical sciences.
Keywords: Informed consent, Mobile devices, Electronic consenting systems, iPad, Registration staff, Data collection, Informatics
1. Introduction
The informed consent process for medical treatment and clinical research is an essential component of the ethical and regulatory fabric in modern healthcare in the United States. This process involves providing a patient or research candidate with adequate information concerning the procedure, providing adequate opportunity for them to consider all options, responding to the questions they have and obtaining consent to undergo a treatment or participate in a research study [1,2]. Currently, hospital management systems still face challenges in effectively capturing and managing such permissions [3]. Many still use paper-based consent forms, which are typically scanned and stored in a document handling system with no mechanism for conducting systematic searches to identify potential candidates for research [4]. Specifically, such systems do not support retrieval of information about potential candidates using specific elements of the consent form such as consent to use tissues for research or other demographic characteristics as the search criteria. Another well-documented problem with current consenting mechanisms is patient comprehension [5–8], a topic many researchers have investigated given its implications in the consenting process [9,10].
Converting existing paper-based forms to an electronic format addresses both these issues by enabling (1) the indexing, search and retrieval of consent data, thus enhancing the ability to identify willing research participants and honor patient intent and (2) the inclusion of electronic educational media. The last decade has seen much research on the methods for capturing and managing these patient consent forms [11–16], a primary focus being on the next generation of electronic consenting infrastructure and its ability to address and balance privacy and compliance issues to make electronic consenting more viable in the current medical environment [17].
Health Sciences South Carolina (HSSC), a research collaborative of three principal research universities and four major health systems in South Carolina, received a research grant to develop a comprehensive infrastructure for managing informed consents and research permissions electronically called the Research Permissions Management System (RPMS). Since the general consent form used in hospitals upon admission for patient care may include statements pertaining to research and education, it has the potential to serve as a vehicle for obtaining patient permission for using excess tissues for research or for future contact for research participation, albeit within the constraints of the Common Rule which dictates federal policy for the protection of human subjects in research [18] and the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule [3,19]. A schematic diagram of RPMS is shown in Fig. 1.
Fig. 1.
A schematic diagram of the electronic consenting system in a multi-institutional setting. Research permissions and consent information are collected via iPad forms during patient registration and fed centrally to an HSSC-wide clinical data warehouse.
The development of an interface for such an electronic consenting system was a major challenge as it needed to address current issues with hospital staff and patients while providing a sound balance between usability and privacy and incorporating such features as electronic consent and signature capture, multilingual support, portability and the ability to be used in a wide variety of environments such as an emergency room. This development followed a user-centered design approach that involved user research, needs identification and analysis specification and concept development, and the development of a detailed design followed by iterative testing [27,28].
The first step in the development of the RPMS required an analysis of the existing flow of the admission and consenting process. To do so, the general registration and emergency room (ER) areas in one of the major hospitals in South Carolina were used to identify the issues and needs of both the patients and hospital registration staff, with a particular focus on determining their needs and the feasibility of adopting an electronic consent capture system. This activity involved five steps:
20 h of observational studies of the registration process to collect process flow data independently using shadowing and informal interview techniques.
20 formal structured contextual interviews with the registration staff and patients to obtain detailed information from each perspective concerning issues involved in the current consenting process, conducted using a prospective think-aloud protocol.
Mapping and development of a hierarchical task analysis modeling the patient's journey through the registration process, focusing on the consenting procedure.
Refinement of the process flow to ensure an accurate representation of the registration process both in the ER and general registration areas.
One of the key resulting observations was that the general consent for treatment and the consents for specific procedures included requests for permissions in various sections about future contact for research or the use of excess blood or tissues collected during regular patient care for research purposes. These are particularly important because patients are increasingly able to indicate their desire to participate in various aspects of research, in part because of new technologies and experimental procedures. For example, registering as a volunteer for clinical research and obtaining the consent to be contacted about specific research areas during the general consenting process are relatively new, both of which bring legal, ethical, social and informatics challenges to institutions as they begin to collate clinical data with genomic and bio-specimen data for research purposes. Managing research permissions and privacy authorizations becomes even more complicated for distributed clinical trials networks as they are often loose affiliations between collaborating institutions that may not be covered entities under the privacy laws as many fully integrated healthcare delivery networks are. Addressing these issues is a primary need for any electronic consenting system being considered; it should provide for an integrated collection and management mechanism for the consents and privacy authorizations that will accrue for individuals through their direct and indirect interactions with the hospital system.
In addition, the results of this study suggest several requirements needed by an electronic consenting system. For example, observation indicated the advantage of a mobile system over a stationary one, allowing the capturing of the patient consents in areas other than the registration desk, particularly in an ER setting where this process may take place at the patient's bedside [4]. Other requirements include the provision of the signed electronic consent form for the patient to review and modify at any point in time; allowing for authorized signatory, as in the case of the guardian for a minor or a relative for a mentally incapacitated patient; and the capability to capture the patient's specific wishes, such as the refusal to accept blood or blood products for a Jehovah Witness [4]. Finally, the user interface of the electronic consenting system should be easy-to-read and navigate even for patients with limited exposure to electronic devices and computers and for those who may have difficulty using modern electronic systems due to poor eye sight or age-related deterioration of motor skills. Similar studies evaluating the efficacy of touchscreen-based systems have been conducted on electronic voting systems [25,26].
Based on these findings, product specifications were created, and multiple concepts were generated to accommodate the needs of the stakeholders. Two types of interfaces were proposed for investigation for the RPMS: a stationary touch screen interface to be used in regular patient registration areas, and a tablet-based system presented on an iPad to be used primarily in mobile situations such as an ER. The empirical study reported here investigated the efficacy of these interfaces, comparing them with the current paper- and Topaz-based systems in order to explore how the two concepts were perceived and experienced by the patients and the registration staff. We hypothesized that these proposed systems are at least as good as the conventional systems in terms of the dependent measures of time, errors made, workload, satisfaction and patient comprehension.
2. Methods
2.1. Participants
Forty patients between the ages of 18 and 77 and ten hospital registration staff between 23 and 74 were recruited for this study between September and October 2010. The sample size was determined based on the findings of Nielsen et al. [20] and Faulkner [21] for conducting empirical research. The hospital staff had at least one year of experience working in the registration area. All participants were screened for physical disabilities limiting their mobility, motor or visual functions. The 40 patients were equally divided among the four environments, 10 in the paper-based, 10 in the Topaz-based, 10 in the touchscreen-based and the remaining 10 in the iPad-based. The 10 registration staff were paired with one participant in each of these environments; thus, each registration staff monitored four sessions, one for each consenting system.
2.2. Testing conditions
The independent variable for this study was the consenting method, examined at four levels: the paper-based method; an existing electronic signature capture method using Topaz; and the two new systems, one using a touchscreen and the other using an iPad. The user interface design was informed by the formative usability studies conducted at the initial phase of the application development [22]. The paper-based system used here was the existing consent form used by one of the major hospitals in South Carolina. In addition, a few registration areas in the hospital serving as the research sites currently use the Topaz electronic signature capture system (Fig. 2) based on a web application developed using Forms onDemand investigated here. The touchscreen-based system (Fig. 3) and the iPad-based system (Fig. 4) employed web applications developed using HTML5/CSS3 and JavaScript. Based on the results from concept evaluation studies [22], only the pagination variants of these interfaces were tested.
Fig. 2.
Topaz-based electronic signature system.
Fig. 3.
Touchscreen-based electronic consenting system.
Fig. 4.
iPad-based electronic consenting system.
2.3. Tasks
The tasks in this study involved the hospital staff interacting with the participants as they read and signed each type of consent form. For the paper-based condition, the hospital staff went through each section of the consent form with the patients, asking them to sign at marked locations. For the Topaz-based condition, the hospital staff explained the sections orally, and the patients signed using a Topaz signature capture device. For the touchscreen-based condition, patients were asked to read and sign a web-based application displayed on a touch sensitive screen using a stylus. The iPad-based condition used a web-based application on an iPad, with the patients again signing with a stylus. For each condition, the hospital staff signed as the witness.
2.4. Experimental design
The study used a mixed experimental design, with the hospital staff facilitating the consenting process in a within-subjects design and the patients experiencing the consenting condition in a between-subjects design. The within-subject experimental design involved collecting data from the hospital staff for each of the four conditions. The between-subjects experimental design involved collecting data from patients in one test condition and comparing these data with those from the patients in the other conditions, with the constraint that data from any one patient were collected for only one test condition. To minimize order effects, each hospital staff received the consenting conditions in a different order randomly generated by a computer program.
2.5. Procedure
The patients and hospital staff followed the same procedure for all four test conditions. At the beginning of the session, the researcher greeted the hospital staff and the patient separately, providing each with an overview of the study. If they agreed to participate, they were asked to read and sign a consent form and to complete a brief pre-test questionnaire, asking for their demographic information. Next, the hospital staff and the patient were taken to the registration office to complete the consenting process for the first condition. The researcher was colocated with the participants and recorded both the time taken to complete this process using a stop watch and the errors made by the participants. After the completion of the consenting process, both the patient and the hospital staff were asked to detail their concerns while interacting with the interface in a retrospective think-aloud session. The researchers recorded the issues raised.
Upon completing the consenting process under the first condition, both the patient and the hospital staff were asked to complete the IBM Computer Systems Usability Questionnaire (IBM-CSUQ) [23]. Then a semantic questionnaire was administered to the patients to evaluate their perceived level of understanding of the general consent form. This questionnaire included seven questions, each measuring the level of understanding of one section of the consent form as shown in Table 1.
Table 1. Questions in the semantic questionnaire.
| Semantic Questionnaire |
|---|
|
Three options, yes, no and unsure, were provided for each question. Once finished, the participants were de-briefed by the researcher. The hospital staff then completed the NASA-TLX workload assessment instrument [24]. The hospital staff followed the same process with different patients in the other three conditions. Once the hospital staff completed the consenting process under all the four conditions, they completed a final post-test questionnaire, ranking each system based on their preference. The time taken for the entire four-condition session was approximately 80 min.
2.6. Dependent variables
The four consenting systems were compared based on objective and subjective measures. The objective measures consisted of the task completion time and the number of errors made by the participants. The subjective measures consisted of the data from the NASA-TLX completed by the hospital staff and the post-test questionnaires completed by the participants and the hospital staff. The data for the number of errors were obtained from the observation of the consenting process by the researcher. The data collected were classified into the following:
Dataset of patients – 40 datasets, 10 for each condition.
Dataset of hospital staff – 10 datasets, each across all four conditions.
The subjective evaluation of each dataset was conducted by analyzing the NASA-TLX indices, the results of the retrospective think-aloud sessions and the post-test subjective questionnaires.
3. Results
SPSS 18.0 was used for analysis. Initially, a normality test was conducted to determine whether the data followed a normal distribution. The data were then analyzed using a one-way ANOVA with a 95% confidence interval to determine the presence of significant differences, if any, among the test conditions. If these results permitted rejection of the null hypothesis, they were then subjected to a post hoc LSD test (see Table 2) to determine the locus of the significant differences. Because of the ordinal nature of the ranked preference data, the Friedman test was used to identify the significant effects for this metric. The four consenting processes were compared with respect to the time taken to complete the tasks and the number of errors made by the participants, and the subjective ratings from the NASA-TLX, the CSUQ and the post-test subjective questionnaires.
Table 2.
Post hoc LSD comparisons (significantly different at p = 0.05).
| Consenting System | Mean | SD | Consenting system | Mean | SD |
|---|---|---|---|---|---|
| Number of errors (n= 40) | |||||
| Paper-based | 0 | 0 | Touchscreen-based | 0.7 | 0.82 |
| Paper-based | 0 | 0 | iPad-based | 0.7 | 0.67 |
| Overall satisfaction (patients) (n= 40) | |||||
| Paper-based | 3.08 | 0.55 | Touchscreen-based | 4.2 | 0.56 |
| Paper-based | 3.08 | 0.55 | iPad-based | 4.41 | 0.48 |
| Topaz-based | 3.29 | 0.49 | Touchscreen-based | 4.2 | 0.56 |
| Topaz-based | 3.29 | 0.49 | iPad-based | 4.41 | 0.48 |
| System usefulness (patients) (n= 40) | |||||
| Paper-based | 3.2 | 0.54 | Touchscreen-based | 4.21 | 0.78 |
| Paper-based | 3.2 | 0.54 | iPad-based | 4.49 | 0.45 |
| Topaz-based | 3.64 | 0.79 | Touchscreen-based | 4.21 | 0.78 |
| Topaz-based | 3.64 | 0.79 | iPad-based | 4.49 | 0.45 |
| Interface quality (patients) (n= 40) | |||||
| Paper-based | 3.1 | 0.55 | Touchscreen-based | 4.4 | 0.47 |
| Paper-based | 3.1 | 0.55 | iPad-based | 4.53 | 0.61 |
| Topaz-based | 3.03 | 0.73 | Touchscreen-based | 4.4 | 0.47 |
| Topaz-based | 3.03 | 0.73 | iPad-based | 4.53 | 0.61 |
| Information quality (patients) (n= 40) | |||||
| Paper-based | 2.91 | 0.72 | Touchscreen-based | 4.1 | 0.57 |
| Paper-based | 2.91 | 0.72 | iPad-based | 4.2 | 0.58 |
| Topaz-based | 3.08 | 0.69 | Touchscreen-based | 4.1 | 0.57 |
| Topaz-based | 3.08 | 0.69 | iPad-based | 4.2 | 0.58 |
| Workload (hospital staff) (n= 10) | |||||
| Paper-based | 57.26 | 25.69 | Touchscreen-based | 34.86 | 22.88 |
| Paper-based | 57.26 | 25.69 | iPad-based | 33.06 | 25.63 |
| Topaz-based | 50.23 | 26.63 | iPad-based | 33.06 | 25.63 |
| Physical demand (hospital staff) (n= 10) | |||||
| Paper-based | 9.96 | 5.70 | iPad-based | 4.53 | 2.93 |
| Effort (hospital staff) (n= 10) | |||||
| Paper-based | 12.03 | 6.63 | Topaz-based | 5.86 | 5.02 |
| Paper-based | 12.03 | 6.63 | Touchscreen-based | 2.93 | 2.98 |
| Paper-based | 12.03 | 6.63 | iPad-based | 5.66 | 7.58 |
| Frustration (hospital staff) (n= 10) | |||||
| Paper-based | 10.86 | 7.50 | iPad-based | 1.80 | 3.32 |
| Topaz-based | 10.03 | 7.00 | Touchscreen-based | 4.49 | 8.23 |
| Topaz-based | 10.03 | 7.00 | iPad-based | 1.80 | 3.32 |
Time taken to complete the task
The time taken to complete the paper-based task was measured from the time the consent form was handed to the participant to the time the registration staff signed as the witness. For the Topaz-based system, the time taken was measured from the time the registration staff began explaining the consent to the patient to the time the staff signed as the witness. For the touch-screen and iPad-based systems, the time taken was measured from the time the consent form was shown to the participant until the staff signed as a witness. The results of this analysis indicated that the effect of the consenting condition on the time taken to complete the task was not significant, F(3,36) = 0.483, p = 0.696, as shown in Fig. 5.
Fig. 5.
Mean time taken to complete the tasks.
Number of errors
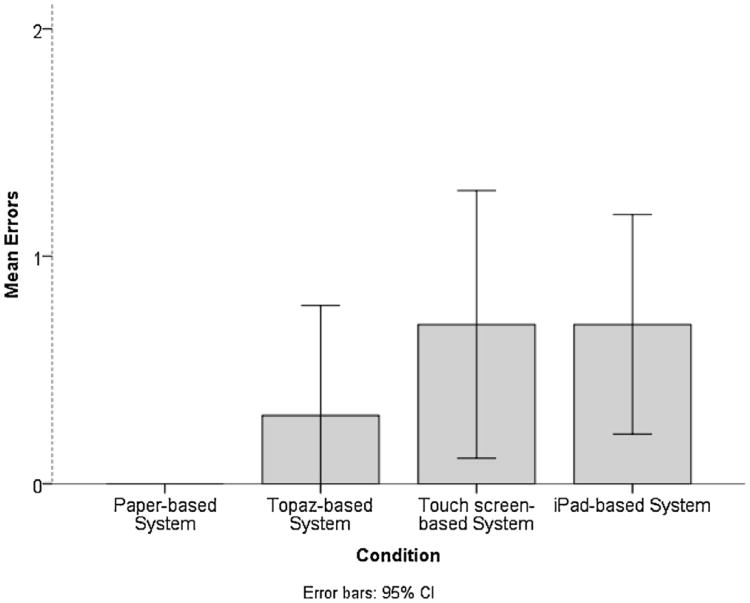
The events listed in Table 3 indicate the types of error for each condition. The mean number of errors made by the participants differed significantly among the consenting conditions, F(3,36) = 2.916, p = 0.046 as shown in Fig. 6. Participants made no errors in the paper-based condition; however, a higher number of errors was found for the Topaz-based and iPad-based conditions. Clearing the autograph from the signature box contributed to the majority of the errors in the iPad and touch screen-based conditions.
Table 3.
Events contributing to the number of errors.
| Event | Paper-based | Topaz-based | Touchscreen-based | iPad-based |
|---|---|---|---|---|
| Clearing signature from the signature box | ✓ | ✓ | ✓ | |
| User not able to complete the task | ✓ | ✓ | ✓ | ✓ |
| Crossing out the signature after signing | ✓ | |||
| Users completing the task without signing in the required fields | ✓ | ✓ | ✓ | ✓ |
Fig. 6.
Mean number of errors made by the participant.
3.1. The patient experience
The nineteen questions in the CSUQ measured both overall satisfaction as well as decomposing it into the subscales of system usefulness, information quality and interface quality.
Overall satisfaction
The overall satisfaction of the patients differed significantly, F (3,36) = 15.630, p<0.05, as shown in Fig. 7. Higher numeric scores indicate a greater level of satisfaction.
Fig. 7.
Subjective satisfaction of the patients from IBM CSUQ.
System usefulness
The patients' perceptions of system usefulness differed significantly, F(3,36) = 8.48, p < 0.05, as shown in Fig. 7.
Interface quality
The interface quality differed significantly, F(3,36) = 18.51, p < 0.05, as shown in Fig. 8.
Fig. 8.
NASA-TLX workload ratings for the hospital staff.
Information quality
The information quality differed significantly, F(3,36) = 10.90, p<0.05, as shown in Fig. 7.
3.2. The hospital staff experience
Overall satisfaction
The effect of the consenting condition on the overall satisfaction for the hospital staff was not significant, F (3, 27) = 1.789, p = 0.237.
System usefulness
The effect of the consenting condition on system usefulness was not significant, F (3, 27) = 3.826, p = 0.06.
Interface quality
The effect of the consenting condition on interface quality was not significant, F (3, 27) = 2.587, p = 0.136.
Information quality
The effect of the consenting condition on information quality was not significant, F (3,27) = 4.046, p = 0.058.
3.3. Workload measures
The NASA-TLX derives the total workload based on the weighted average ratings of the six subscales of mental demand, physical demand, temporal demand, effort, performance and frustration. Fig. 8 shows these data for the hospital staff.
Total workload
The effect of consenting condition on the total workload experienced by the hospital staff was significant, F (3,27) = 6.778, p = 0.018.
Mental demand
The effect of consenting condition on mental demand was not significant, F (3, 27) = 1.610, p = 0.271.
Physical demand
The effect of consenting condition on the physical demand experienced by the hospital staff was significant, F (3,27) = 32.158, p < 0.001.
Temporal demand
The effect of consenting condition on temporal demand was not significant, F (3,27) = 2.689, p = 0.127.
Effort
The effect of consenting condition on the effort experienced by the hospital staff was significant, F (3,27) = 8.503, p = 0.010.
Performance
The effect of consenting condition on performance was not significant, F (3, 27) = 0.064, p = 0.977.
Frustration
The effect of consenting condition on the frustration experienced by the hospital staff was significant, F (3,27) = 6.158, p = 0.022.
3.4. Preference rankings
Preference data were obtained from the hospital staff through a ranking scale, with a lower score indicating greater preference. The analysis of these data using the Friedman test indicated significant differences in preference among the four systems, χ2 (3, 10) = 25.680, p<0.005. The Wilcoxon-signed rank test found that hospital staff preferred the iPad-based system (M = 1.2) to the touchscreen-based (M = 2.0), the Topaz-based (M = 2.8) and the paper-based (M = 4.0) systems.
3.5. Semantic questionnaire
A semantic questionnaire was developed by the researchers to assess how well the patients understood the different sections of the general consent, in particular the use of their discarded tissues for research, under the four consenting conditions. These results suggest that the participants' level of understanding was greater for the touchscreen-based and iPad-based interfaces. When asked if they remembered signing a document indicating that they understood their rights as a patient, the participants who used the paper-based, touchscreen-based and iPad-based systems responded yes. However, 10% of the participants using the Topaz-based system indicated that they did not recollect signing such a document, perhaps because for this process, the questions on the different elements were asked on the consent form and participants signed on a signature pad. For this study, we were particularly interested in the patient responses to questions 1, 3 and 4.
For the third question pertaining to the use of their unused tissues for research, the majority of the participants in the touch-screen and iPad-based conditions (70 and 80% respectively) answered positively. However, the majority in the paper-based system and Topaz-based systems suggested that they were unsure. For the question on the consent to draw blood to test for infectious diseases including HIV and hepatitis, the majority in touchscreen and iPad-based systems answered positively. More importantly, 80–90% of the participants in the paper-based system and Topaz-based system gave a negative answer.
The subjective feedback provided by the participants indicates the need for a standard procedure for administering the general consent for the paper-based and Topaz-based systems. Frequently, the participants did not read the information in those modalities, suggesting that the information should be presented to the patient so that it is easily comprehended and the hospital staff should take necessary steps to ensure the patient has understood it.
4. Discussion
One of the primary purposes of this study was to determine the differences, if any, in the effectiveness of the four consenting conditions, paper-based, Topaz-based, touchscreen-based and iPad-based, in capturing electronic consents. The results indicate no significant differences in the time taken to complete tasks, although the mean time appears to be lower for the paper-based approach, perhaps because of the learning curve involved in initial interaction with the electronic interfaces. It was also found that the participants made no errors in the paper-based condition, a finding probably attributable to the fact that the hospital staff marked an “X” where the patients needed to sign. Participants encounter Topaz-based systems (e.g., making a purchase at Walmart with a credit card which requires the customers to sign on an electronic pad) in their daily life. This could have been the reason for lower number of errors in this condition. For the Topaz-based system, the errors were primarily due to an improper signature. However, patients made fewer errors under this condition than for the touchscreen-based and iPad-based conditions. One major issue observed for the latter two conditions was that the participants rested their hands on the display while signing, invoking inadvertent actions such as zooming and selection of commands, resulting in a higher number of errors. As a result minor improvements to the interface are recommended to address these issues (see Table 4).
Table 4.
Interface design recommendations.
| Modality | Findings |
|---|---|
| iPad | A pagination interface is preferred to a scrolled interface containing text-based information This system is well suited to emergency rooms and scenarios involving mobile consenting Portrait mode is preferred when presenting text-based information Mechanisms need to be incorporated on the interface to address inadvertent inputs when users rest their hands on the display Participants preferred radio buttons to buttons emulating iPad-native applications. These buttons should be large enough to address accessibility concerns |
| A progress bar should be added at the top of the consent form, indicating the number of sections completed Feedback should be provided when the user hits the buttons on the iPad interface | |
| Buttons should be provided on the top of the signature box to avoid inadvertent invocation of the buttons while interacting with the signature box | |
| Based on observation, a thin stroke is recommended for capturing electronic signatures | |
| Touch screen | A pagination interface rather than a scrolled interface is recommended This system is well suited for registration offices |
| This study used a touchscreen based on a resistive touch technology, requiring participants to press hard to record their signatures. A touchscreen based on capacitive technology might be easier to use |
Significant differences in workload were identified for the four conditions, the NASA-TLX scales indicating that the hospital staff experienced the least workload for the iPad-based condition and the most in the paper-based. Further analysis of the workload subscales suggests that the physical demand was higher in the paper-based and Topaz-based conditions. Specifically, a few hospital staff indicated that obtaining paper-based consents from a patient in the ER is a cumbersome task. These results may have been due to the necessity of printing and scanning the consent form for the paper-based system. Significant differences were also found for the effort and the frustration subscales of the NASA-TLX. The hospital staff thought that the effort required was higher for the paper-based condition than for the three electronic consenting conditions, and the staff using the paper-based and Topaz-based systems appeared to be frustrated primarily due to the effort required to complete the task. In addition, one staff member reported that many times patients using the Topaz-based system complained that they were signing a document without seeing what they were agreeing to; in fact, one patient using the Topaz-based system refused to sign because of this issue.
The ratings provided by the patients in the final subjective questionnaire revealed significant differences in terms of overall satisfaction, system usefulness, interface quality and information quality. The iPad-based and touchscreen-based system were equally preferred by the patients, this high overall satisfaction perhaps resulting because the patients were able to see what they were signing, specifically the options available to them, in an appealing format, a conclusion supported by one patient's retrospective think-aloud session. Analyses of these sessions suggest that interface quality was an important factor affecting the level of satisfaction, while the semantic questionnaire indicated that the level of comprehension was better on the iPad and touchscreen-based systems. The subjective ratings of the hospital staff did not show significant differences for any of the subscales. However, the preference rankings suggest that they preferred the iPad-based system, followed by the touchscreen, the Topaz-based system, and finally the paper-based.
This study indicates that the two methods proposed for capturing patient consents, the iPad-based and touchscreen-based approaches, were at least as effective as the existing paper-based and Topaz-based systems, and more effective in certain areas. The results of this exploratory study suggest that it is feasible to replace the existing paper-based system with an electronic consent capture system. Table 4 provides a set of interface design recommendations.
One of the most important findings was that the time taken to complete the consenting process was essentially the same for the four conditions. Moreover, the participants indicated that they had more control over their privacy and that they could more easily move forward at their own pace using the new electronic systems. They also found the material more comprehensible and easier to follow on these two systems than on the traditional ones. Similarly, the hospital staff preferred the iPad and touchscreen-based approach over the Topaz-based and paper-based systems, indicating that they experienced lower workloads in particular with the iPad. The subjective feedback provided by the participants suggested the need for a standard procedure for administering general consent for treatment, particularly for the paper-based and Topaz-based systems. Since participants frequently did not read the information in these modalities before signing, hospital staff should take steps to present the information clearly to the patients and ensure they understand it, particularly the information pertaining to the use of patient tissue for research. However, for the two proposed consenting systems, the participants carefully read the consent information, which was divided into small sections, with one section per screen. Their increased focus on and, consequently, understanding of the information may have resulted from this format which reduced the information overload commonly associated with the more traditional systems. Thus, the results of this study suggest that the comprehension of consent information depends on its format as well as the mode of delivery.
5. Conclusion
Recent advances in biomedical sciences and the growing complexity of our understanding of the molecular basis of diseases necessitate more extensive clinical data and patient participation in research than in the past. However, identifying patients reflecting the specific criteria required by a particular clinical trial [2–4] remains a challenge. An application suite like RPMS that facilitates management of research permissions for a collaborative of hospitals with a statewide reach, creating common policies and functioning as an integrated research network, could enhance the patient recruitment process. While further work is needed to explore the use of electronic consent forms to address such issues as accessibility, portability and scalability for integration with existing systems, the analyses presented here suggest the viability of cautious adoption of electronic consenting systems.
Summary points.
What was already known on this topic
Current hospital management systems lack an effective way to capture and manage research permissions.
Many hospitals still use paper-based consent forms including questions pertaining to participating in research activities.
There is a need for a consenting infrastructure with the ability to address permissions management, privacy and compliance issues to make consenting more viable in the current medical environment.
What this study added to our knowledge
Converting existing paper-based forms to an electronic format enables the indexing, search and retrieval of consent data, thus enhancing the ability to identify willing research participants as well as honor patient intent.
Having a state-wide permissions management system enables researchers to contact patients for studies with specific inclusion criteria.
The registration staff experienced a lower workload with the electronic consenting systems.
Electronic consenting systems based on iPads and touch screens were preferred over the conventional systems by both the staff and the patients.
Patients seem to pay attention to the key statements in the consent form when presented on electronic devices developed using a user-centered design process.
Acknowledgments
This research was supported by the National Institutes of Health's and National Library of Medicine's GO (Grand Opportunities) Grant # RC2LM01079. None of the authors have any financial conflicts of interest. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Authors' contributions: All of the authors have contributed to all of the following: (1) the conception and design of the study or the acquisition of the data or the analysis and the interpretation of the data, (2) drafting the article or revising it critically for important intellectual content, and (3) giving final approval of the version to be submitted. All persons who qualify as authors have been listed in this manuscript.
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.Informed consent [Internet] 2012 Available from: http://www.ama-assn.org/ama/pub/physician-resources/legal-topics/patient-physician-relationship-topics/informed-consent.page.
- 2.Informed consent – FAQs [Internet] 2012 Available from: http://answers.hhs.gov/ohrp/categories/1566.
- 3.Ness RB. Joint Policy Committee, Societies of Epidemiology, Influence of the HIPAA privacy rule on health research. JAMA. 2007 Nov;298(18):2164–2170. doi: 10.1001/jama.298.18.2164. [DOI] [PubMed] [Google Scholar]
- 4.Chalil Madathil K, Koikkara R, Gramopadhye AK, Fryar K. An analysis of the general consenting process in an emergency department at a major hospital: challenges for migrating to an electronic health record; Proceedings of the 2011 Industrial Engineering Research Conference; Reno, NV. 2011. [Google Scholar]
- 5.Bergler JH, Pennington AC, Metcalfe M, Freis ED. Informed consent: how much does the patient understand? Clin Pharmacol Ther. 1980 Apr;27(4):435–440. doi: 10.1038/clpt.1980.60. [DOI] [PubMed] [Google Scholar]
- 6.Reidenberg MM. Informed consent or acknowledgment of disclosure. Clin Pharmacol Ther. 2005 Oct;78(4):439–440. doi: 10.1016/j.clpt.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Betti S, Sironi A, Saino G, Ricci C, Bonavina L. Effect of the informed consent process on anxiety and comprehension of patients undergoing esophageal and gastrointestinal surgery. J Gastrointest Surg. 2011 Jun;15(6):922–927. doi: 10.1007/s11605-011-1517-7. [DOI] [PubMed] [Google Scholar]
- 8.Chalil Madathil K, Koikkara R, Dorlette-Paul M, Ranganayakulu S, Greenstein JS, Gramopadhye AK. An investigation of format modifications on the comprehension of information in consent form when presented on mobile devices; Proceedings of the Human Factors and Ergonomics Society Annual Meeting, Sage Publications; 2012. [Google Scholar]
- 9.Flory J, Emanuel E. Interventions to improve research participants' understanding in informed consent for research: a systematic review. JAMA. 2004 Oct;292(13):1593–1601. doi: 10.1001/jama.292.13.1593. [DOI] [PubMed] [Google Scholar]
- 10.Chaisson LH, Kass NE, Chengeta B, Mathebula U, Samandari T. Repeated assessments of informed consent comprehension among HIV-infected participants of a three-year clinical trial in botswana. PLoS ONE. 2011;6(10):e22696. doi: 10.1371/journal.pone.0022696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lunshof J, Chadwick R, Vorhaus D, Church G. From genetic privacy to open consent. Nat Rev Genet. 2008 May;9(5):406–411. doi: 10.1038/nrg2360. [DOI] [PubMed] [Google Scholar]
- 12.HMK. Informed consent to promote patient-centered care. JAMA: J Am Med Assoc. 2010;303(12):1190–1191. doi: 10.1001/jama.2010.309. [DOI] [PubMed] [Google Scholar]
- 13.Paterick TJ, Paterick BB, Paterick TE. Expanding electronic transmissions in the practice of medicine and the role of electronic informed consent. J Patient Saf. 2008;4(4):217–220. http://dx.doi.org/10.1097/PTS.0b013e31818f35c2. [Google Scholar]
- 14.Kluge EHW. Informed consent and the security of the electronic health record (EHR): some policy considerations. Int J Med Inf. 2004;73(3):229–234. doi: 10.1016/j.ijmedinf.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Bergmann J, Bott OJ, Pretschner DP, Haux R. An e-consent-based shared EHR system architecture for integrated healthcare networks. Int J Med Inf. 2007;76(2–3):130–136. doi: 10.1016/j.ijmedinf.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Hakimzada AF, Green RA, Sayan OR, Zhang J, Patel VL. The nature and occurrence of registration errors in the emergency department. Int J Med Inf. 2008;77(3):169–175. doi: 10.1016/j.ijmedinf.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coiera E, Clarke R. e-consent: The design and implementation of consumer consent mechanisms in an electronic environment. J Am Med Inform Assoc. 2004;11(2):129–140. doi: 10.1197/jamia.M1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Federal policy for the protection of human subjects (‘common rule’) [Internet] 2009 Available from: http://www.hhs.gov/ohrp/humansubjects/commonrule/index.html.
- 19.Annas GJ. HIPAA regulations - a new era of medical-record privacy? N Engl J Med. 2003 Apr;348(15):1486–1490. doi: 10.1056/NEJMlim035027. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen J, Landauer TK. A mathematical model of the finding of usability problems; CHI'93: Proceedings of the INTERACT'93 and CHI'93 conference on human factors in computing systems, ACM; Amsterdam, The Netherlands; New York, NY, USA. 1993. [Google Scholar]
- 21.Faulkner L. Beyond the five-user assumption: benefits of increased sample sizes in usability testing. Behav Res Methods Instrum Comput. 2003 Aug;35(3):379–383. doi: 10.3758/bf03195514. [DOI] [PubMed] [Google Scholar]
- 22.Chalil Madathil K, Koikkara R, Gramopadhye AK, Greenstein JS. An empirical study of the usability of consenting systems: iPad, touchscreen and paper-based systems; Proceedings of the human factors and ergonomics society annual meeting; Las Vegas, NV. 2011. [Google Scholar]
- 23.Lewis JR. IBM computer usability satisfaction questionnaires: psychometric evaluation and instructions for use. Int J Hum Comput Interact. 1995;7(1):57–78. [Google Scholar]
- 24.Hart SG, Stavenland LE. Development of NASA-TLX (task load index): results of empirical and theoretical research. In: Hancock PA, Meshkati N, editors. Human Mental Workload. Elsevier; 1988. pp. 139–183. [Google Scholar]
- 25.Bederson BB, Lee B, Sherman RM, Herrnson PS, Niemi RG. Electronic voting system usability issues; Proceedings of the SIGCHI conference on human factors in computing systems, ACM; 2003. [Google Scholar]
- 26.Cross EV, II, McMillian Y, Gupta P, Williams P, Nobles K, Gilbert JE. CHI'07 Extended Abstracts on Human Factors in Computing Systems. ACM; 2007. Prime III: A user centered voting system. [Google Scholar]
- 27.Obeid J, Reilly K, Chalil Madathil K, Rugg D, Alstad C, Fryar K, et al. 2013 Summit on Clinical Research Informatics. San Francisco; 2013. Development of an electronic research permissions management system to enhance informed consents and capture research authorizations data. [PMC free article] [PubMed] [Google Scholar]
- 28.Sanderson I, Obeid J, Chalil Madathil K, Gerken K, Fryar K, Rugg D, Alstad C, Alexander R, Kathleen B, Gramopadhye A, Moskowitz J. Managing clinical research permissions electronically: a novel approach to enhancing recruitment and managing consents. Clin Trials J. 2013 doi: 10.1177/1740774513491338. forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]