Abstract
Type I IFNs (IFN-α and IFN-β) and type II IFN (IFN-γ) mediate both regulation and inflammation in multiple sclerosis (MS), neuromyelitis optica (NMO) and in experimental autoimmune encephalomyelitis (EAE). However the underlying mechanism for these Janus-like activities of type I and II IFNs in neuroinflammation remains unclear. Although endogenous type I IFN-signaling provides a protective response in neuroinflammation, we find that when IFN-γ signaling is ablated, type I IFNs drive inflammation resulting in exacerbated EAE. IFN-γ has disease stage-specific opposing function in EAE. Treatment of mice with IFN-γ during initiation phase of EAE leads to enhanced severity of disease. In contrast, IFN-γ treatment during effector phase attenuated disease. This immunosuppressive activity of IFN-γ required functional type I IFN-signaling. In IFN-α/β receptor (IFNAR) deficient mice, IFN-γ treatment during effector phase of EAE exacerbated disease. Using an adoptive transfer-EAE model, we found that T-cell intrinsic type I and type II IFN signals are simultaneously required to establish chronic EAE by encephalitogenic Th1 cells. However in Th17 cells loss of either IFN signals leads to the development of a severe chronic disease. The data implies that type I and type II IFN signals have independent but non-redundant role in restraining encephalitogenic Th17 cells in vivo. Overall our data show that type I and type II IFNs function in an integrated manner to regulate the pathogenesis in EAE.
INTRODUCTION
Interferons (IFNs) are a family of related cytokines exerting an essential role in inflammation and autoimmunity. They are classified into two subtypes according to receptor specificity and sequence homology (1). Type I IFNs consist of IFN-β and several other members whereas type II IFN has only one single member, IFN-γ. Types I and II IFNs bind distinct cell surface receptor complexes: the IFNAR (IFN-α/β receptor) and the IFNGR (IFN-γ receptor), respectively. IFNAR and IFNGR are comprised of two transmembrane glycoproteins: IFNAR1 and IFNAR2, and IFNGR1 and IFNGR2 (2, 3). A common feature of both type I and II IFNs is the employment of the JAK-STAT signal transduction pathway to regulate nuclear gene expression (1).
Although types I and II IFNs bind distinctive receptors and differentially regulate the expression of a variety of other cytokines, they develop synergistic functions priming macrophages for tumor cell killing (4), enhancing CTL responses to melanoma cell vaccines (5), and inhibiting viral replication (6). Recruitment and modulation of STAT1 are central elements in the cross-talk between types I and II IFNs triggered in response to viral infection (7, 8). However, the cooperative action of types I and II IFNs and the pathophysiological significance of this interaction in autoimmunity have been less studied.
Multiple Sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS) and the leading cause of neurological disability in young adults (9). There is compelling evidence supporting the individual role of types I and II IFNs in the pathogenesis of MS and its animal model, experimental autoimmune encephalomyelitis (EAE3). The lack of either IFN-β or IFNAR results in a more severe and chronic EAE (10–12) and the systemic administration of IFN-β is, to date, the most commonly used therapy for MS providing longer periods of remission, reducing the severity of relapses, and decreasing the inflammatory lesion in the CNS (13–16). In contrast, controversial evidence has been reported in relation to the role of IFN-γ in MS and EAE. A positive association between increased levels of IFN-γ and clinical manifestations attributed a pathological role of IFN-γ in MS (17–20). Subsequent studies have challenged the notion that IFN-γ is pathogenic, and they have even suggested a protective role for IFN-γ in EAE, and perhaps in some forms of MS (21–27). Furthermore, EAE can be ameliorated after IFN-γ-treatment (24, 28–30), whereas MS patients treated with IFN-γ exhibited exacerbations of disease (31). Therefore, the role of IFN-γ in MS and EAE is still unresolved.
Th cells producing either IFN-γ (Th1) or IL-17 (Th17) have been shown to play a critical role in the immunopathogenesis of MS and EAE (32–35). Recently, we have demonstrated that IFN-β is very effective in the treatment of EAE induced by Th1 cells but it is ineffective and induces exacerbations when disease is led by Th17 lymphocytes (36). Remarkably, IFN-β treatment significantly worsened EAE in IFNGR deficient mice suggesting that immunosuppression by IFN-β required functional activity of IFN-γ (36). In this study we tested the hypothesis that IFN-β and IFN-γ act cooperatively to modulate autoimmune neuroinflammation. Our results revealed an intricate interaction between types I and II IFNs signaling in the pathogenesis of EAE regulating the threshold of EAE susceptibility, the effector function of Th1 and Th17 cells and the severity of disease. A functional and reciprocal interaction between types I and II IFNs signaling was indispensable to promote a protective response.
MATERIALS AND METHODS
Mice
C57BL/6 (45.1 and 24.2) mice were purchased from Frederick Cancer Research. B6.129S7-Ifngr1tm/Agt/J (Ifngr1−/−) mice were purchased from the Jackson Laboratory and backcrossed onto C57BL/6 (B6) background for 10–12 generations. The B6.Ifnar1−/− mouse (37) was obtained from Dr. Jocelyn Demengeot (Instituto Gulbenkian de Ciência, Oeiras, Portugal). Ifnagr1−/− mice were developed by crossing Ifnar1−/− and Ifngr1−/− mice. The Stat1−/− mice was provided to us by Dr. R. Lorenz (University of Alabama at Birmingham). All mice were maintained and bred at the UAB mouse facility and treated in accordance with National Institute of Health and the University of Alabama Animal Care and Use Committee guidelines.
Induction of EAE, scoring and treatment
Active EAE was induced by immunizing WT and knock-out mice 8 to 12 weeks of age with a s.c. injection of MOG35–55 peptide (MOGp) (CPC Scientific) at the dose indicated as described previously (38). For treatment with IFN-γ, either 400 ng or 1 μg of recombinant murine IFN-γ (Millipore) was administered i.p.. Onset and classical clinical progression of EAE symptoms were monitored daily using a standard scale of 0 to 6 as described previously (38). Atypical EAE symptoms were scored as follows: 0- no clinical signs, 1 - slight head tilt, 2 - severe head tilt, 3 - slight axial rotation/staggered walking, 4 - severe axial rotation/spinning, 5 - moribund, 6 - death.
CD4+ T cell differentiation and adoptive transfer of EAE
Single-cell suspensions were harvested from pooled spleens and lymph nodes (5 mice per group) 11 days after immunization with MOGp in CFA. The cells were restimulated with MOGp under non-polarizing (unpolarized) conditions or under Th1 or Th17 polarizing conditions as described previously (36, 39). The cell populations were characterized by flow cytometry for cellular populations expressing CD4, CD8, B220, CD11b, CD11c, CD25, FoxP3, IFN-γ and IL-17 as described reviously with antibodies obtained from BioLegend (San Diego, CA) (39). For induction of adoptive transfer EAE, unpolarized, Th1 1 polarized or Th17 polarized cells injected i.v. (6×106/mouse) into irradiated naïve WT mice. On the day of transfer and 48 hours later, each mouse received 200 ng of pertussis toxin (List Biologicals). To determine fate of Ifngr1−/− Th1 cells, 5 × 106 WT or Ifngr1−/− (CD45.2) Th1 polarized encephalitogenic cells were transferred into naïve WT CD45.1 mice. Donor and recipient infiltrating CD4+ T-cells were evaluated in spinal cords on day 14 and day 25 post transfer.
Cell proliferation
Encephalitogenic cells (5×105) were cultured in triplicate in 96-well U-bottomed plates in the presence or absence of 10 μg/ml of MOGp and under either Th1 or Th17 polarizing conditions at 37°C, 5% CO2 for 72 hours. Cells were pulsed with 1 μCi [3H]–thymidine/well during the last 18 hours of culture and then [3H]–thymidine incorporation was measured.
Protein extraction and cytokine quantification
Brain tissues (brain stem and cerebellum) and SC were quickly removed from mice after 20 days of EAE induction, snap-frozen and stored at −80°C until processing. For the extraction of total proteins, samples were homogenized in lysis buffer (20 mM Tris-HCl, pH=7.5; 150 mM NaCl; 1 mM PMSF; 0.05% Tween-20) containing proteinase inhibitors (Roche) and frozen at −80°C. After thawing, samples were sonicated on ice and centrifuged at 20,000 x g for 45 minutes at 4°C. The protein concentration in the supernatants was determined using the bicinchronic acid (BCA) assay (Pierce). Samples were analyzed for protein levels of a panel of 31 cytokines and chemokines (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-15, IL-17, IFN-γ, TNF-α, CCL2, CCL3, CCL4, CCL5, CCL11, CXCL1, CXCL2, CXCL9, CXCL10, G-CSF, GM-CSF, M-CSF, VEGF, LIF) using the Luminex-based Bio-Plex multiplex suspension protein array (Millipore), according to the manufacturer’s instructions. 100 μg and 50 μg of protein from each brain (brain stem and cerebellum) tissue and spinal cord (SC) were assayed, respectively. Concentrations of each cytokine and chemokine were determined using Bio-Plex Manager version 4.1.1 software. Cytokine concentration was normalized by the total protein concentration and expressed as picograms per milligram total protein.
Intracellular cytokine staining
Encephalitogenic cells were incubated with 50 ng/ml PMA, 500 ng/ml ionomycin and 10 μg/ml brefeldin A (all from Sigma-Aldrich) for the last 4 hours of culture. Cells were then surface stained with PerCp-conjugated anti-mouse CD4 (BioLegend), washed, fixed and permeabilized with Cytofix/Cytoperm kit (BD Biosciences-Pharmingen) according to the manufacturer’s directions. Finally, cells were stained intracellularly with PE-conjugated anti-mouse IFN-γ (BioLegend) and Alexa488-conjugated anti-mouse IL-17 (BioLegend). Samples were acquired on a FACSCalibur and data analyzed with CellQuest Pro Software (BD Bioscience).
Histology and immunohistochemistry
Cross sections of brain stem, cerebellum and spinal columns were obtained from mice at 20 days after EAE induction and were immersion-fixed in Bouin’s fixative. Histochemical and immunohistological analysis were performed as described previously (40). Inflammatory infiltrates and demyelination were analyzed histochemically by H&E and Luxol fast blue staining, respectively. Neutrophils (anti-myeloperoxidase, Lab Vision), CD4+ T-cells (goat anti-CD4, R&D Systems) and activated microglia (anti-GS-I-B4) were evaluated by immunohistological staining. Images were captured using a Zeiss LSM 710 Confocal Scanning microscope and Zen 2008 4.7.2 software (Carl Zeiss, Inc.)
Statistical analysis
All EAE disease scores were analyzed using Mann-Whitney non-parametric test or one-way ANOVA Kruskall-Wallis with Dunns post-test using Prism 5 software (GraphPad Software). Mann-Whitney (with Tukey’s multiple comparisons) was used for all other analyses.
RESULTS
Type I interferon signals exacerbate the effector phase of EAE in IFNGR deficient mice
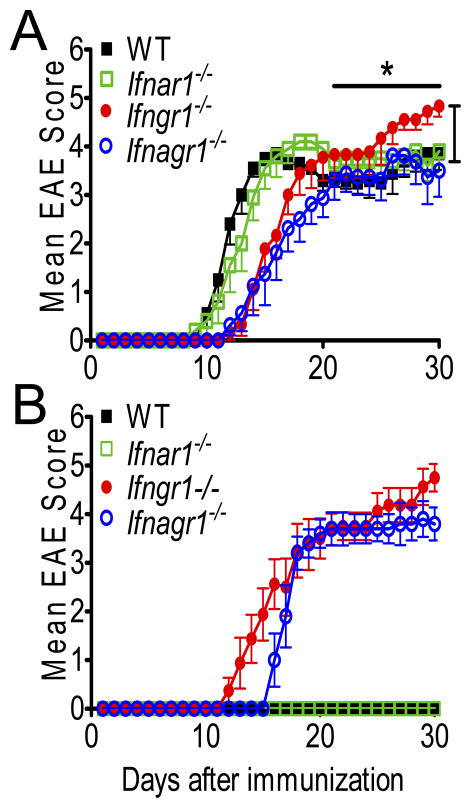
We reported that functional IFN-γ signaling is necessary for IFN-β to be therapeutically effective in EAE (36). Thus, in order to test the hypothesis that type I and type II IFNs cooperatively modulate pathogenesis in EAE, we examined the development and progression of EAE in Ifnar1−/−Ifngr1−/− mice (Ifnagr1−/−) and compared it to that in Ifngr1−/−, Ifnar1−/− and WT mice. EAE in Ifngr1−/− mice was delayed in onset but more severe during the effector phase than that in WT and Ifnar1−/− mice (Fig. 1A, Table I). In comparison, EAE in Ifnagr1−/− mice was less severe than that in Ifngr1−/− mice but just as delayed in onset. In fact, the overall severity of disease in Ifnagr1−/− mice was slightly and significantly lower than in Ifnar1−/− and WT mice (Fig. 1A). From these data we infer that (i) IFN-γ has a dual activity in the pathogenesis of EAE: pathogenic during the inductive phase, but protective during the effector phase; (ii) type I IFN signals exacerbate EAE in the absence of IFN-γ signals as suggested by the lowest EAE severity observed in Ifnagr1−/− mice; (iii) during the initiation phase of disease type II IFN signals are dominant over type I IFN.
FIGURE 1.
Type I and II IFN signaling determine severity of disease. (A) Classical and (B) atypical clinical scores from WT, Ifnar1−/−, Ifngr1−/− and Ifnagr1−/−mice induced with 150 μg of MOGp per mouse. Error bars represent means ± s.e.m., n=8–14 mice per group. Results are pooled from three experiments. *P < 0.05 for comparison between Ifngr1−/− vs WT, Ifnar1−/−and Ifnagr1−/− mice between days 21–30 and for Ifnagr1−/− vs WT, Ifnar1−/−mice and Ifngr1−/− mice between days 1–30.
Table I.
Classical EAE severity in type I and II interferon deficient mice is not sensitive to changes in inducing antigen dosea
| Group of mice | Day of Onset (mean±SD) | Max Score (mean±SD) | Time to Peak (days) (mean±SD) | Accumulative Score (mean±SD) | Mortality (%) |
|---|---|---|---|---|---|
| Standard MOG dose
|
|||||
| WT | 11.3 ± 1.0 | 4.3 ± 0.7 | 14 ± 1.5 | 67.6 ± 14.4 | 1/10 (10%) |
| Ifnar1−/− | 12.6 ± 2.3 | 4.2 ± 0.5 | 15.7 ± 1.6 | 69 ± 15.8 | 0/10 (0%) |
| Ifngr1−/− | 15.6 ± 4.1b | 4.8 ± 0.7b | 23.6 ± 4.3c | 62.1 ± 17.7 | 2/9 (22.2%) |
| Ifnagr1−/− | 14.9 ± 2.0c | 3.9 ± 1.0 | 24 ± 2.9c | 50.8 ± 22.1 | 1/8 (12.5%) |
| High MOG dose
|
|||||
| WT | 12.6 ± 2.0 | 3.8 ± 0.6 | 15.9 ± 2.7 | 53.2 ± 12.6d | 0/11 (0%) |
| Ifnar1−/− | 12.4 ± 1.7 | 4.2 ± 0.7 | 16.4 ± 2.7 | 64.2 ± 13.4 | 1/9 (11.1%) |
| Ifngr1−/− | 13.4 ± 1.1 | 5.1 ± 0.6c | 19.2 ± 1.5b,d | 72.7 ± 10.1b | 6e/10 (60%)b |
| Ifnagr1−/− | 14.5 ± 0.9b | 4.3 ± 1.3 | 18.5 ± 1.8b,d | 56.1 ± 17 | 2/8 (25%) |
| Low MOG dose
|
|||||
| WT | 14.6 ± 2.7d | 3.6 ± 0.5d | 18.1 ± 3.6d | 46.5 ± 14.4d | 0/14 (0%) |
| Ifnar1−/− | 14 ± 1.6 | 4.3 ± 0.3c | 18.1 ± 1.7d | 60.6 ± 11.6 | 0/13 (0%) |
| Ifngr1−/− | 15.9 ± 1.6 | 4.3 ± 1.0b | 22.4 ± 3.0b | 53.7 ± 17.5 | 2/14 (14.3%) |
| Ifnagr1−/− | 16.7 ± 3.1 | 3.8 ± 0.8 | 22.1 ± 3.2b | 48.7 ± 20.2 | 1/11 (9.1%) |
MOG dose were 150 μg/mouse (standard), 50 μg/mouse (low) and 300 μg/mouse (high), respectively.
p <0.05;
p <0.001. Comparisons between each IFNR KO mice vs WT mice within an inducing MOG dose.
p <0.05. Comparisons between high or low dose MOG vs standard dose MOG within a mouse line.
Includes 3 moribund mice that were sacrificed
In addition to classical EAE, mice with loss of IFNGR signals also develop an atypical form of disease (41, 42). Ifngr1−/− mice developed atypical EAE at an incidence greater than 85% and additional loss of IFNAR signaling, unlike classical EAE, had no effect in this form of disease (Fig. 1B, Table II).
Table II.
Incidence of atypical EAE in IFNGR deficient mice
| Group of mice | Incidence (%) |
|---|---|
| Standard MOG dose
|
|
| WT | 0/10 (0 %) |
| Ifnar1−/− | 0/10 (0 %) |
| Ifngr1−/− | 8/9 (89 %) |
| Ifnagr1−/− | 5/8 (63 %) |
| High MOG dose
|
|
| WT | 0/11 (0 %) |
| Ifnar1−/− | 0/9 (0 %) |
| Ifngr1−/− | 4/10 (40 %)a |
| Ifnagr1−/− | 6/8 (65 %) |
| Low MOG dose
|
|
| WT | 0/14 (0 %) |
| Ifnar1−/− | 0/13 (0 %) |
| Ifngr1−/− | 12/14 (86 %) |
| Ifnagr1−/− | 8/11 (73 %) |
These mice rapidly developed severe EAE, complicating the ability to resolve atypical from classical EAE.
The percent of atypical EAE is a conservative estimate; however, the actual incidence is most likely greater than 90 %.
Opposing activity of IFN-γ in EAE
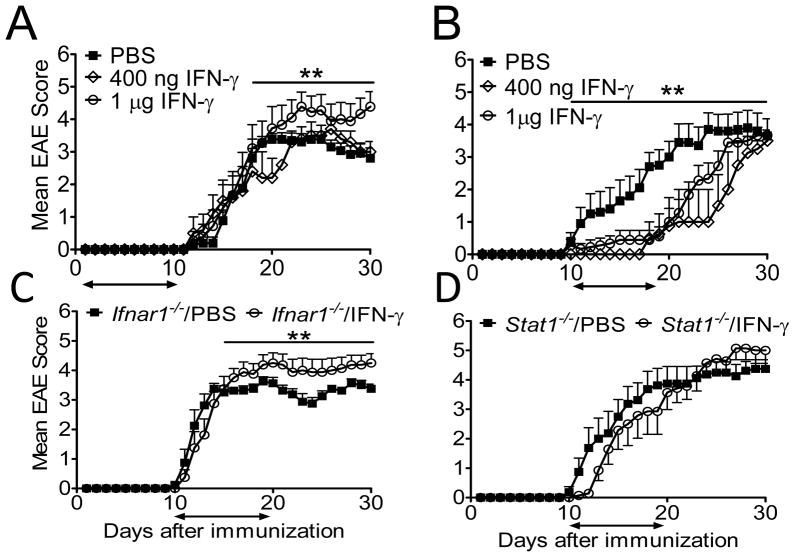
To test the prediction that IFN-γ has disease stage-specific opposing activity in EAE, we treated mice daily with IFN-γ beginning at the time of immunization (day 1–9) or after onset of clinical symptoms (day 10–19). Mice treated with 1 μg rIFN-γ beginning on the day of immunization developed exacerbated EAE with greater mortality than those treated with PBS (Fig. 2A, Table III). rIFN-γ treatment did not alter the mean day of onset (Table III). A lower daily dose of rIFN-γ (400 ng) had no effect on disease severity or mortality. Most remarkably, initiation of treatment with either 1 μg or 400 ng of rIFN-γ after first sign of clinical symptoms (day 10–19) suppressed disease progression for the course of the treatment (Fig. 2B). Following cessation of treatment, EAE severity bounced to levels equivalent to that of PBS treated mice. Re-initiation of rIFN-γ therapy moderately induced recovery (data not shown). The mean day of onset was also significantly delayed by both doses of rIFN-γ treatment (Table III).
FIGURE 2.
Dual role of IFN-γ in EAE. Clinical scores from EAE in C57BL/6 mice that were treated with PBS, 400 ng or 1 μg IFN-γ daily from (A) day 1 to day 10 or (B) day 10 to day 19 after EAE induction. n=9–13 mice/group. (C) Clinical scores from EAE in WT and Ifnar1−/− mice that were treated with PBS or 1 μg IFN-γ daily from day 10 to day 19 after EAE-induction. n=8 mice/group. (D) Clinical scores from EAE in WT and Stat1−/− mice treated with PBS or 1 μg IFN-γ daily from day 10 to day 19 after EAE-induction. n=7–12 mice/group. Results are pooled from two or three experiments. **P < 0.001 for comparisons between (A) 1 μg IFN-γ-treated WT mice versus PBS-treated WT and 400 ng IFN-γ-treated WT between days 16–30; (B) PBS-treated WT versus 400 ng and 1 μg IFN-γ-treated WT mice between days 10–30; (C) PBS-treated Ifnar1−/− mice versus IFN-γ-treated Ifnar1−/− between days 16–30.
Table III.
Disease stage specific activity of IFN-γ in EAE
| Treatment | Day of Onset (mean±SD) | Max Score (mean±SD) | Time to Peak (days) (mean±SD) | Accumulative Score (mean±SD) | Incidence (%) | Mortality (%) |
|---|---|---|---|---|---|---|
| Before onset
|
||||||
| WT PBS | 16.1 ± 1.9 | 3.8 ± 0.7 | 19.5 ± 2.9 | 46.1 ± 7.0 | 13/13 (100%) | 1a/13 (7.7%) |
| WT 400 ng IFN-γ | 15.8 ± 4.0 | 4.1 ± 0.2 | 21.8 ± 4.5 | 46.6 ± 11.5 | 5/5 (100%) | 0/5 (0%) |
| WT 1 μg IFN-γ | 16.4 ± 2.7 | 4.7 ± 1.1 | 20.6 ± 3.0 | 58.1 ± 26.6 | 9/9 (100%) | 3/9 (33.3%) |
|
| ||||||
| After onset
|
||||||
| WT PBS | 14.5 ± 3.4 | 4.4 ± 1.2 | 21.6 ± 4.9 | 56.2 ± 26.9 | 10/10 (100%) | 3/10 (30%)b |
| WT 400 ng IFN-γ | 24.3 ± 4.3c | 3.6 ± 0.5 | 26.5 ± 4.0 | 22.1 ± 17.2c | 4/4 (100%) | 0/4 (0%) |
| WT 1 μg IFN-γ | ± 5.8c | 3.9 ± 0.5 | 27 ± 2.4c | 32.8 ± 12.1d | 9/9 (100%) | 0/9 (0%) |
Moribund mouse that had to be sacrificed
A higher mortality was observed within the PBS treated group, perhaps an outcome of the stress induced by daily injections
p <0.05. Comparison between IFN-γ treatment and PBS treatment
Our results from active EAE (Fig. 1A) suggested that IFN-α/β signaling work in cooperation with IFN-γ signaling to modulate EAE severity. Therefore, we interrogated if type I IFN signals participates in the disease amelioration induced by IFN-γ treatment. To test this hypothesis we induced EAE in Ifnar1−/− mice and on day 10 we initiated treatment with IFN-γ or PBS. Remarkably, EAE in Ifnar1−/− treated with IFN-γ was slight by significantly more severe than in PBS treated mice (Fig. 2C, Table IV). This leads us to infer that type I IFN signals cooperate with IFN-γ signals to attenuate disease. Not surprisingly, IFN-γ treatment has no effect on EAE in mice lacking STAT1, the major STAT activated in response to engagement of both IFN-γ and IFN-α/IFN-β receptors (Fig. 2D, Table IV) (1).
Table IV.
Activity of IFN-γ is dependent of type I IFN signaling and Stat1
| Treatment | Day of Onset (mean±SD) | Max Score (mean±SD) | Time to Peak (days) (mean±SD) | Accumulative Score (mean±SD) | Incidence (%) | Mortality (%) |
|---|---|---|---|---|---|---|
| WT PBS | 12.4 ± 2.7 | 4.3 ± 0.7 | 14.9 ± 3.8a | 65.9 ± 20.4 | 8/8 (100%) | 1/8 (12.5%) |
| WT IFN-γ | 15.6 ± 3.2 | 4.2 ± 0.4 | 22.3 ± 3.2 | 46.8 ± 15 | 8/8 (100%) | 0/8 (0%) |
| Ifnar1−/− PBS | 11.5 ± 0.9 | 3.8 ± 0.3 | 14.1 ± 1.9 | 62.3 ± 7.0 | 8/8 (100%) | 0/8 (0%) |
| Ifnar1−/− IFN-γ | 12.1 ± 1.0 | 4.4 ± 0.7 | 16.3 ± 2.4 | 70.5 ± 16.5 | 8/8 (100%) | 0/8 (0%) |
|
| ||||||
| WT PBS | 11.7 ± 2.6a | 3.8 ± 1.1 | 13.7 ± 2.9a | 55.7 ± 25.5a | 10/10 (100%) | 1/10 (30%) |
| WT IFN-γ | 18.9 ± 3.5 | 4.4 ± 0.8 | 25 ± 4.0 | 34.1 ± 12 | 7/7 (100%) | 1/7 (14.2%) |
| Stat1−/− PBS | 13.6 ± 4.2 | 4.6 ± 0.5 | 18.9 ± 5.4 | 69.3 ± 25.4 | 8/8 (100%) | 0/8 (0%) |
| Stat1−/− IFN-γ | 14.9 ± 3.6 | 5.1 ± 0.9 | 21.9 ± 3.5 | 65.5 ± 20.5 | 7/7 (100%) | 3/7 (42.9%) |
P <0.05. Comparison between treatments in WT mice
Type I and II IFN signals are required to sustain Th1 EAE but not Th17 EAE
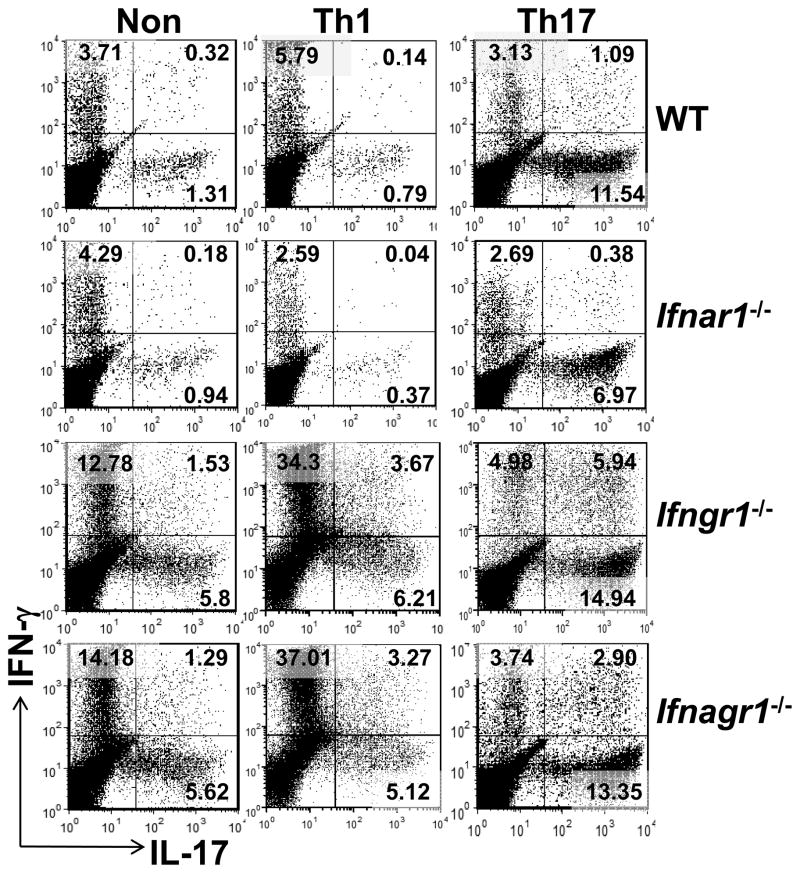
Both Th1 and Th17 cells contribute to the pathogenesis of EAE, albeit through different mechanisms (32, 43). To determine the T-cell intrinsic role of type I and II IFN signals in Th1 versus Th17 EAE, we generated non-polarized, Th1 or Th17 polarized encephalitogenic T-cells from WT and the different IFNR-deficient mice and transferred them into WT naïve recipients. The donor cell populations from all groups of mice contained equal proportion of CD4, CD8 and B220+ cells, Treg (CD4+CD25+FoxP3+) and no CD11b+ or CD11c+ cells at the time of transfer (Supplemental Fig. 1A, 1B and data not shown). Irrespective of the genotype of donor mice, restimulation cultures under non-polarizing conditions contained Th1 cells, Th17 cells and a small proportion of CD4+ T cells that co-expressed IFN-γ and IL-17 (ThIFN-γ+IL-17+) (Fig. 3). The proportion of Th1, Th17 and ThIFN-γ+IL-17+ cells in cultures from WT and Ifnar1−/− mice was similar. Likewise, non-polarized cultures from Ifngr1−/− and Ifnagr1−/− mice contained equal proportion of the three Th effector subpopulations with respect to each other, but greater than Th effector subpopulations from WT or Ifnar1−/− mice (Fig. 3). The elevated numbers of Th17 cells in non-polarized and Th17 polarized cultures from Ifngr1−/− mice is expected and reflects inability of IFN-γ to suppress Th17 differentiation (44, 45). However, the finding that lack of IFNGR signaling led several fold greater numbers of Th1 cells is unexpected since it opposes the dogma that IFN-γ is a feed forward signal for Th1 differentiation (46).
FIGURE 3.
Encephalitogenic Th1 and Th17 cell differentiation in the presence or absence of IFN receptors. Percentage of IFN-γ+CD4+ and IL-17+CD4+ T-cells in mononuclear cells isolated from pooled spleen and lymph nodes (5 mice per group) of WT, Ifnar1−/−, Ifngr1−/− and Ifnagr1−/−mice 11 days after EAE induction. Cells were re-stimulated in vitro with MOG35–55 peptide in nonpolarizing (Non), Th1-polarizing (Th1) or Th17-polarizing (Th17) conditions. After 72 hours, intracellular cytokine production was determined by flow cytometry. The data shown are representative of four independent experiments.
Cells cultured under Th1 or Th17 polarizing conditions predominantly contained IFN-γexpressing cells or IL-17-expressing cells, respectively, independent of the genotype of the donor (Fig. 3). This reflects a good efficiency in the generation of encephalitogenic Th1 cells or Th17 cells.
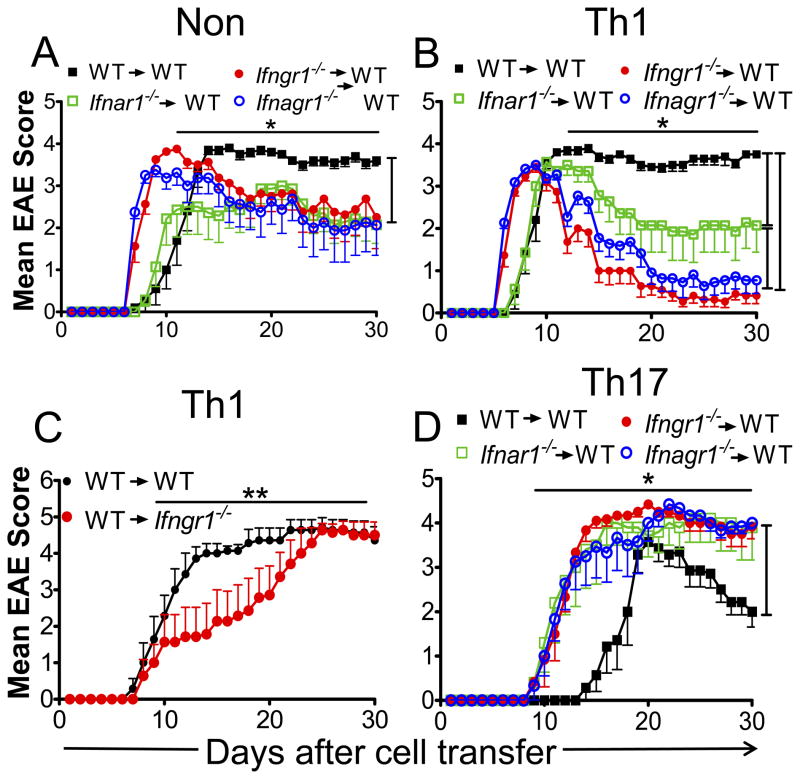
We found that WT encephalitogenic T-cells induced a more severe disease in naïve recipients than Ifnar1−/− donor T-cells, but with similar onset (11 days) (Fig. 4A, Table V). Ifngr1−/− or Ifnagr1−/− donor encephalitogenic T-cells induced disease with earlier onset and peak of severity than disease induced by WT or Ifnar1−/− encephalitogenic cells (Table V). A feature common to recipients of Ifngr1−/− or Ifnagr1−/− cells is the partial remission of disease to a severity less than that induced by WT donors and equivalent to that induced by Ifnar1−/− donors (Fig. 4A).
FIGURE 4.
Type I and II IFN signals are required to sustain Th1 EAE but not Th17 EAE. (A, B and D) Clinical scores of WT mice with EAE induced by adoptive transfer of (A) nonpolarized cells (NON), (B) Th1 cells or (D) Th17 cells isolated from MOGp-induced WT, Ifnar1−/−, Ifngr1−/− or Ifnagr1−/− mice. (C) Clinical scores of EAE in WT or Ifngr1−/− mice induced by adoptive transfer of WT Th1 cells. Error bars represent means ± SEM (n= 7–11 mice/group). Results are pooled from three experiments. *P < 0.05: (A) WT versus Ifnar1−/−, Ifngr1−/− and Ifnagr1−/− mice between days 11–30; (B) WT versus Ifnar1−/−, Ifngr1−/− and Ifnagr1−/− mice between days 12–30 and Ifnar1−/− mice vs Ifngr1−/− and Ifnagr1−/− mice between days 12–30; (D) WT versus Ifnar1−/−, Ifngr1−/− and Ifnagr1−/− mice between days 10–30. **P < 0.001.
Table V.
Type I and II interferons are required to sustain Th1 EAE and restrain Th17 EAE
| Group of mice | Day of Onset (mean±SD) | Max Score (mean±SD) | Time to Peak (days) (mean±SD) | Accumulative Score (mean±SD) | Incidence (%) | Mortality (%) |
|---|---|---|---|---|---|---|
| Non polarized T cells
|
||||||
| WT | 10.8 ± 2.2 | 4.0 ± 0.0 | 13 ± 1.7 | 72.3 ± 5.2 | 10/10 (100%) | 0/10 (0%) |
| Ifnar1−/− | 11.1 ± 4.5 | 3.8 ± 0.4 | 13.7 ± 4.8 | 52.8 ± 19.5 | 7/7 (100%) | 0/7 (0%) |
| Ifngr1−/− | 7.2 ± 0.5a | 4.4 ± 0.7 | 10.1 ± 0.8a | 68.6 ± 21.9 | 8/8 (100%) | 1/8 (12.5%) |
| Ifnagr1−/− | 7.0 ± 0.0a | 3.8 ± 0.7 | 9.5 ± 1.7a | 62.1 ± 31.8 | 8/8 (100%) | 0/8 (0%) |
| Th1 cells
|
||||||
| WT | 8.5 ± 1.1 | 4 ± 0.0 | 10.8 ± 1.7 | 80.9 ± 4.9 | 10/10 (100%) | 0/10 (0%) |
| Ifnar1−/− | 8 ± 0.8 | 3.9 ± 0.2 | 9.7 ± 1.1 | 56.6 ± 26 | 7/7 (100%) | 0/7 (0%) |
| Ifngr1−/− | 6.2 ± 0.4b | 3.5 ± 0.4a | 8.5 ± 0.7a | 31.6 ± 14.3b | 11/11 (100%) | 0/11 (0%) |
| Ifnagr1−/− | 6 ± 0.0b | 3.5 ± 0.2b | 8.1 ± 0.7b | 43.1 ± 17.3b | 11/11 (100%) | 0/11 (0%) |
| Th17 cells
|
||||||
| WT | 17.1 ± 2.1 | 3.6 ± 0.9 | 19.7 ± 1.6 | 40 ± 15 | 7/7 (100%) | 0/7 (0%) |
| Ifnar1−/− | 11.4 ± 2.8a | 4.0 ± 1.1 | 14.4 ± 1.5a | 75.8 ± 28 | 5/5 (100%) | 1c/5 (20%) |
| Ifngr1−/− | 10.8 ± 1.1a | 4.4 ± 0.2a | 14.5 ± 1.1a | 77.5 ± 8.2a | 6/6 (100%) | 0/6 (0%) |
| Ifnagr1−/− | 8.8 ± 3.4a | 4.5 ± 0.0a | 17.2 ± 2.5a | 74.7 ± 14.5a | 6/6 (100%) | 0/6 (0%) |
P <0.05;
P <0.001. Comparison of each IFNR-deficient mice vs WT mice with a similar experimental paradigm.
1 moribund mouse was sacrificed.
Th1 polarized encephalitogenic T-cells from Ifngr1−/− and Ifnagr1−/− mice induced a very rapid and acute disease followed by a near complete remission (Fig. 4B, Table V). In contrast, WT Th1 polarized cells induced a slightly delayed but a non-remitting chronic disease. This rapid remission in the recipients that received IFNGR-deficient Th1 polarized encephalitogenic T-cells was impressive considering that the transferred cell population contained up to 6 fold greater IFN-γ expressing CD4+ T-cells than in the WT Th1 polarized population. We observed that Ifnar1−/− encephalitogenic Th1 polarized cells induced EAE with onset and peak of severity equivalent to WT Th1 polarized cells, but this was followed by a partial remission of disease (Fig. 4B). The results suggest that whereas there is a T-cell intrinsic requirement for type I IFN signals to maintain severity of Th1 EAE, type II IFN signals are essential for sustaining disease. The rapid recovery in recipients of IFNGR-deficient Th1 polarized cells does not represent increased numbers of Treg cells (Supplemental Fig. 1B), but may reflect the ability of the innate cells to respond to IFN-γ stimulation. IFN-γ induces inducible nitric oxide synthase in innate cells and production of NO leading their death and consequently disease attenuation (47). This property is likely to be more pronounced in recipients of Ifngr1−/− Th1 cells since the donor populations contain greater numbers of Th1 cells expressing high levels of IFN-γ (Fig. 3). To test this possibility, we performed a converse experiment in which WT encephalitogenic Th1 cells were transferred into naïve WT and Ifngr1−/− recipients. We observed that WT and Ifngr1−/− recipients of encephalitogenic WT Th1 cells induced non-remitting EAE that ultimately reached equal severity (Fig. 4C). The Ifngr1−/− recipients did exhibit a delay to reach peak of disease. We suggest that this delay in reaching peak of disease mechanistically reflects the delayed onset of active EAE in Ifngr1−/− mice (Fig. 1A), however this requires additional investigation.
In opposition to Th1 EAE, recipients of Th17 polarized encephalitogenic T-cells from all IFN receptor deficient mice developed EAE that was significantly rapid in onset followed by a chronic phase showing no recovery over the 30 days of observation (Fig. 4D, Table V). The development of Th17 EAE with WT donor cells was delayed and followed by a limited recovery. A previous study showed that transfer of IFN-γ-deficient Th1 cells induced atypical EAE in recipients (47). None of the recipient mice transferred with type II IFN receptor-deficient Th1 or Th17 polarized cells developed atypical EAE. This supports the premise that IFN-γ inducible factors, such as CXCL9 and CXC10 (47, 48), in the host and not donor T cells are necessary to restrain encephalitogenic Th1 cells from entering the brain.
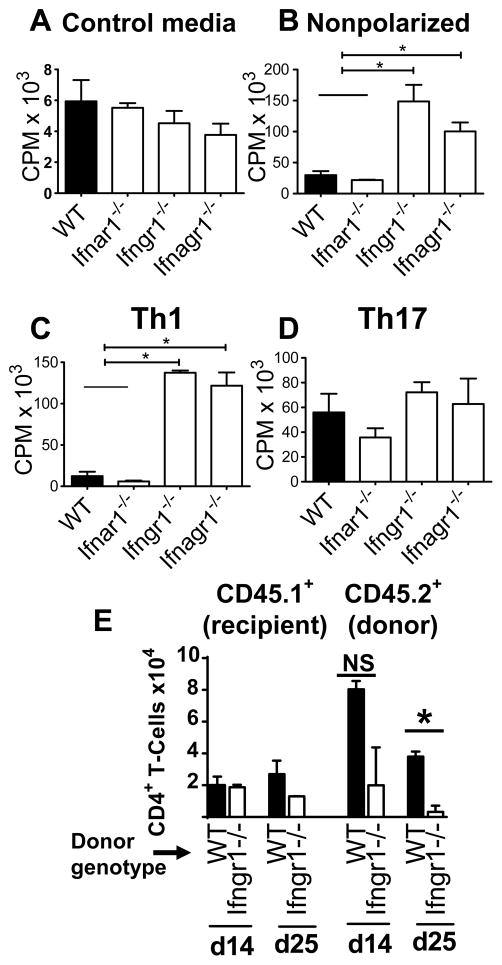
Th1 cells and not Th17 cells from mice lacking IFNGR hyperproliferate in response to antigen
The rapid remission or development of chronicity may also reflect the differential ability of Th1 and Th17 cells from the IFNR deficient mice to proliferate in response to antigen restimulation. To test for this possibility, we determined the proliferation of encephalitogenic T cells stimulated with MOGp and cultured under non-polarizing, Th1 polarizing or Th17 polarizing conditions. In absence of stimulation or under Th17 polarizing condition, the proliferation was similar in T-cells from all mice (Fig. 5A, 5B). In contrast, T cells from Ifngr1−/− and Ifnagr1−/− mice cultured under non-polarizing or Th1 polarizing conditions significantly hyperproliferated in response to MOGp stimulation compared to WT and Ifnar1−/− cells (Fig. 5C, 5D). Thus the failure of type II IFN receptor-deficient Th1 cells to sustain disease is not due to T-cell intrinsic inability to proliferate in the recipient mice. We next determined if Ifngr1−/− Th1 cells are unable to persist in recipients. We transferred equivalent numbers of WT or Ifngr1−/− Th1 polarized cells (CD45.2+) into naïve WT CD45.1 mice and the number of CNS (SC) infiltrating recipient and donor CD4+ T-cells were determined 14 and 25 days following transfer. The number of infiltrating recipient CD4+ T-cells were equivalent in the recipients of WT or Ifngr1−/− Th1 polarized cells. In contrast, the SC of recipients that received IFNGR-deficient cells contained fewer donor cells than that of WT recipients on day 14 (Fig. 5 E). By day 28, less than 3500 IFNGR-deficient donor cells were recovered from the recipients. From these results we infer that the near complete remission of EAE in recipient mice that received IFNGR-deficient Th1 cells is, at least in part, due to a T-cell intrinsic requirement for IFNGR-signaling for the persistence of encephalitogenic Th1 cells.
FIGURE 5.
IFNGR deficient encephalitogenic Th1 cells but not Th17 cells hyperproliferate but don’t persist in vivo. [3H]-Thy-cell proliferation of donor cells re-stimulated in vitro in (A) absence (control media) or (B–D) presence of 10 μg/ml MOGp in (B) nonpolarizing (Non), (C) Th1-polarizing (Th1) or (D) Th17-polarizing (Th17) conditions. Results are expressed in counts per minute (CPM). Error bars represent mean ± SEM values from three independent experiments. * P < 0.05. (E) IFNGR1-deficient Th1 polarized encephalitogenic T-cells do not persist. CD45.2 WT or Ifngr1−/− Th1 polarized encephalitogenic cells were transferred into naïve WT CD45.1 recipients. On days 14 and 25 post transfer, the number of infiltrating recipient (CD45.1+) CD4+ T-cells or donor (CD45.2+) CD4+ T-cells was determined by flow cytometry. Cell number±SD at each time point represents mean from two pools of three mice each.
IFNAR signaling does not affect IFN-γ regulated migration to brain
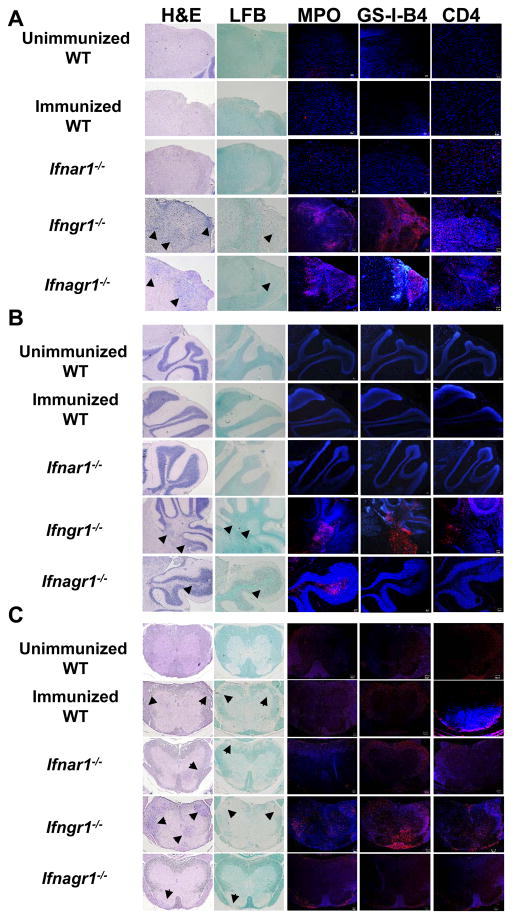
The infiltration of mononuclear cells into the CNS is dependent on Th1:Th17 encephalitogenic T-cell ration and is regulated by IFN-γ (47, 49). To determine whether IFNAR signaling altered IFN-γ-regulated migration of leucocytes into the CNS, we evaluated infiltration and demyelination in brain stem, cerebellum and SC at the peak of disease (day 20). We observed detectible mononuclear infiltration and demyelination of brain stem and cerebellum only in sections from Ifngr1−/− and Ifnagr1−/− mice (Fig. 6A, 6B). The levels of activated microglia/macrophages and the extent infiltration of neutrophils and CD4+ T-cells were similar in the CNS of Ifngr1−/− mice and Ifnagr1−/− mice. The results show that type I IFN signaling does not alter IFN-γ-dependent entry of inflammatory cells into the brain.
FIGURE 6.
IFN-γ signaling determines the distribution of CNS cell-infiltration independent of type I IFN signaling. Histology of (A) brain stem, (B) cerebellum and(C) SC sections from uninduced wild type (WT) mice and EAE-induced wild type (WT), Ifnar1−/−, Ifngr1−/−and Ifnagr1−/− mice after 20 days of induction. Tissue sections were stained to evaluate cell infiltration (H&E), demyelination (luxol fast blue, LFB), neutrophil infiltration (myeloperoxidase, MPO), microglia/macrophages activation (GS-l-B4) and CD4+ T-cells infiltration. Arrows mark areas of cell infiltration or demyelination. Representative sections from two serial sections per mouse from two mice per group are shown.
Although the clinical score was similar to WT (3.5) and Ifnar1−/− (3.5) mice, the SC sections from Ifngr1−/− mice (3.0) showed the greatest extent of mononuclear cell infiltration, demyelination, presence of neutrophils, CD4+ T-cells and activated microglia/macrophages (Fig. 6C). There was no difference between WT and Ifnar1−/− for all parameters evaluated. The extent of mononuclear cell infiltration and demyelination in SC from Ifnagr1−/− mouse was the least and this correlated with the lower classical EAE score of 1.0. However, this mouse had severe atypical EAE (3.5).
Contribution of combined absence of IFNGR and IFNAR in modulating expression of cytokines and chemokines in the spinal cord and brain
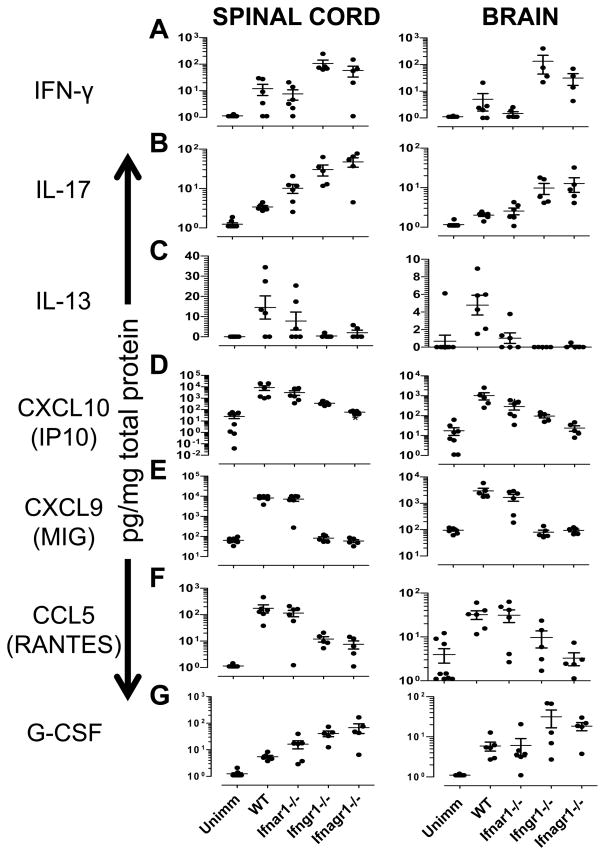
In order to determine the effect of the cooperative IFNAR and IFNGR signaling on the expression of cytokines and chemokines in the CNS, we evaluated the expression of 31 cytokines and chemokines at the peak of disease (day 20) in brain and SC. IFN-γ expression was elevated to equivalent levels in SC of WT and IFNAR-deficient mice (Fig. 7A). Small amounts of IFN-γ were detected in brains of some WT and IFNAR-deficient mice, but overall the levels were not significantly different from unimmunized mice (Supplemental Table I). Notably, IFN-γ was expressed at significantly higher levels in brain and SC of Ifngr1−/− and Ifnagr1−/− mice than in WT and Ifnar1−/− mice (Fig 7A, Supplemental Table I). In both CNS tissues, there was a trend for lower IFN-γ in mice with combined lack of IFNAR and IFNGR compared to IFNGR-only deficient mice; however, the difference was not statistically significant within this sample size. This elevated level of IFN-γ in the absence of IFNGR-signaling was not associated with increased expression of Tbet as determined by real-time PCR or IL-12 p70 (data not shown). IL-17 was detected in all mice with EAE in both SC and brain (Fig. 7B). We observed significantly higher levels of IL-17 in the SC, but not in the brain of Ifnar1−/− mice, compared to WT mice. The CNS of IFNGR-deficient− mice contained greatly elevated levels of IL-17 that was not altered by the co-deletion of IFNAR (Fig. 7B). WT mice had the highest levels of IL-13 in comparison to any of the IFNR-deficient mice (Fig 7C). IL-13 was essentially absent when IFNGR signaling was ablated.
FIGURE 7. Cytokine and chemokine expression in the CNS of mice lacking type I and/or type II IFN receptors.
Expression of (A) IFN-γ (B) IL-17, (C) IL-13 (D) CXCL10 (IP10), (E) CXCL9 (F) CCL5 (RANTES), and (G) G-CSF in spinal cord and brain isolated from unimmunized (unimm, n=9) and EAE-induced wild type (WT, n=6), Ifnar1−/− (n=5), Ifngr1−/− (n=5) and Ifnagr1−/− (n=5) mice at day 20 after EAE induction. Data represent means ± SEM of cytokine/chemokine concentration expressed as pg/mg total protein. The statistical comparisons are presented in Table S1.
In both the SC and brain the levels of CXCL10 (IP10) was lower in IFNAR-deficient mice than in WT mice, however, this difference was significant only in the brain (Fig. 7D). IP10 levels were further significantly reduced in CNS of IFNGR-deficient mice and mice lacking both IFN receptors had the lowest levels of the chemokine. In fact, the expression of CXCL10 in Ifnagr1−/− mice was comparable to that in unimmunized mice. The results indicate that both IFNAR and IFNGR independently induce CXCL10 and combined loss of both IFN receptors has an additive effect. Mice lacking only IFNAR had levels of CXCL9 (MIG) or CCL5 (RANTES) similar to WT mice (Fig. 7E, 7F). However, ablation of IFNGR resulted in complete absence of CXCL9. IFNGR-deficient mice also had dramatically reduced levels of CCL5 that was decreased to levels equivalent to that in unimmunized mice when both IFNGR and IFNAR was absent (Fig. 7F). IFNAR-signaling dependent expression of CCL5 has been observed previously (50) and our data suggest that does so only in the absence of IFN-γ signaling.
Higher levels of G-CSF were observed in SC and brains of mice lacking IFNGR compared to IFNAR-deficient or WT mice (Fig. 7G). This observation is consistent with the elevated infiltration of neutrophils in the CNS of Ifngr1−/− mice (Fig. 6). In the SC, the levels of G-CSF were significantly higher in IFNAR-deficient mice compared to WT mice but this was not associated with any significant increase in neutrophil infiltration (Fig 7G). We found no significant effect of IFN receptor deletion on the remaining 24 cytokines (see Materials and Methods and data not shown).
Type I and II interferon signals regulate threshold of response to development of EAE
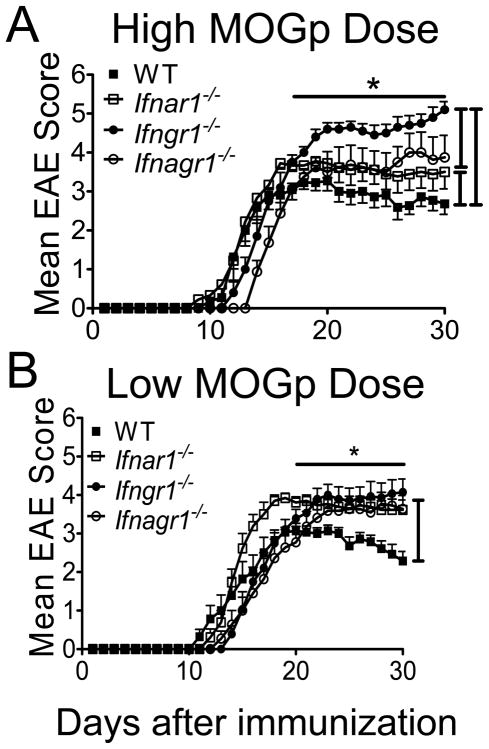
Two previous studies using the same Ifnar1−/− mouse as this study showed that loss of IFNAR signaling exacerbates EAE, a finding that differs from our result (Fig. 1A) (10, 11). We noticed that in both these reports EAE was induced with greater amount of MOGp than our protocol. To determine if the difference in antigen dose explains the divergence of results, we immunized the different IFN-receptor deficient mice and WT mice with 300 μg of MOGp (high dose) and evaluated the development and progression of EAE. At this higher immunization dose of MOGp, EAE in Ifnar1−/− mice as well as in Ifngr1−/− and Ifnagr1−/− mice was more severe that in WT mice, thus reproducing the published reports (Fig. 8A, Table I) (10, 11). However, increasing the MOGp immunization dose from 150 μg to 300 μg led to a decrease in disease severity in WT mice (Table I). This decrease in disease severity with increase in immunization dose might reflect the phenomenon of high-dose tolerance (51, 52). Such decrease in disease severity was not observed in Ifnar1−/− mice. In Ifngr1−/− mice the increase in peptide immunization dose did lead to significant increase in disease severity and mortality (Supplemental Fig. 2D, Table I).
FIGURE 8.
Type I and II IFN signaling determine severity of disease and the threshold for EAE susceptibility. Classical clinical scores from WT, Ifnar1−/−, Ifngr1−/− and Ifnagr1−/− mice induced with (A) 300 μg (high dose) or (B) 50 μg (low dose) of MOGp per mouse. Error bars represent mean ± SEM, n=8–14 mice/group. Results are pooled from three similar experiments. *P < 0.05 for groups of mice compared in the figure between days (A) 17–30 and (B) 20–30.
The above results suggest that type I and II IFNs have a role in regulating the threshold or response to immunization dose and consequently affecting EAE disease course. We therefore examined the effect of low dose MOGp (50 μg) immunization on development of EAE in all groups of mice. The severity of EAE in all of the IFN-receptor deficient mice was similar to each other but greater than that in WT mice (Fig. 8B, Table I). Importantly, immunization with lower amounts of peptide did not significantly alter onset, incidence or severity of disease in any of the IFNR deficient mice. In contrast, in WT mice immunized with 50 μg of MOGp EAE was significantly less severe than mice immunized with 150 μg of MOGp (Supplemental Fig. 2, Table I). The severity of atypical EAE in Ifngr1−/− mice and Ifnagr1−/− was not significantly altered with 300 μg peptide immunization (Supplemental Fig. 3). However, in mice immunized with 50 μg peptide, Ifnagr1−/− mice exhibited lower atypical disease severity than Ifngr1−/− mice. Overall the results show that type I (IFNα and/or IFN-β) and IFN-γ signals are each independently involved in setting the thresholds for susceptibility to EAE.
DISCUSSION
This study reveals a remarkable cooperative relationship between type I and II IFN signaling in regulating pathogenesis of EAE. We show that the exacerbated disease observed in IFNGR-deficient mice is reversed to levels observed in WT mice when there is concomitant ablation of type I IFN signaling. IFN-β is highly expressed by brain-resident glial cells and possibly infiltrating DC subsets in the CNS of mice during active EAE (11, 53–55). In fact, we recently showed that endogenous IFN-β continues to be highly expressed in the CNS of mice lacking IFNGR (56). This local expression of IFN-β is a protective response in EAE and is the principle behind using it as therapy for the treatment of MS (10–12, 57). Our results now show that in the absence of IFN-γ co-signaling endogenous IFN-α/β drives inflammation rather than immunosuppression. This is consistent with our recent finding that administration of IFN-β to IFNGR deficient mice with EAE further exacerbates disease (36). This Janus like property of IFNAR signaling is also evident for IFNGR signaling. During the initiation phase of EAE, IFN-γ drives pathogenesis as suggested by the delayed onset of disease in IFNGR-deficient mice. Indeed, we found that administration of IFN-γ during this period leads to exacerbation of disease. In contrast, type I IFN signaling seems to have limited role in disease initiation. In comparison to EAE in WT mice, the lack of IFNAR or IFN-β does not alter disease onset (10–12). The disease onset in mice with combined absence of IFNAR and IFNGR was not different from those lacking only IFNGR, indicating that type I IFN signaling begins to participate in EAE at a time point later than IFN-γ signaling. In fact, IFN-β in EAE is first detected in the CNS only after onset of clinical symptoms (11).
IFN-γ treatment controls the progression of experimental autoimmune myocarditis (58). In rodent, IFN-γ treatment ameliorated EAE in some studies but induced encephalomyelitis in others (24, 28–31, 59, 60). In MS, IFN-γ treatment led to exacerbations in some patients and no benefit in others, an outcome recapitulated to some extent in a novel marmoset model of EAE (31, 61). Overall these studies led to ambiguity regarding the role of IFN-γ in EAE and MS (17–26). Our current study contributes to clarify this paradoxical evidence and provides insight into the complex role of IFN-γ during EAE pathogenesis. Our results indicate that the previous mixed outcome likely reflects disease-stage specific Janus-like function of IFN-γ. We found that IFN-γ treatment during initiation phase promoted pathogenesis; in contrast such treatment during the effector phase was immunosuppressive in EAE. However, this immunosuppressive activity of IFN-γ required functional type I IFN signaling. The composite developing picture reinforces the model that integration of type I and II IFN signaling pathways are necessary to regulate pathogenesis in EAE. Imbalance of either pathway drives pathogenesis.
We found that encephalitogenic Th1 cells require type II IFN signals for inducing persistent and chronic EAE. In comparison, IFN-α/β signals are not necessary for chronicity but important for disease severity. As reported here and shown previously, MOGp induced EAE in IFN-γ signaling deficient mice is severe and chronic and this seems to conflict with the self-limiting EAE induced by IFNGR-deficient Th1 cells (21–23, 27). We suggest this difference reflects role of IFNGR signaling in Th1 cells versus other effector cells. IFN-γ signaling is needed (1) to restrain Th17 differentiation and expansion (44, 45), (2) for efficient IL-27 induction by dendritic cells and macrophages (62, 63), and (3) for induction of inducible nitric oxide synthase and consequently production of nitric oxide (23, 28). Loss of these key protective mechanisms would lead to exacerbation of disease.
The absence of persistent EAE induced by IFN-γ signaling-deficient Th1 donor cells can also be attributed to the protective effects of IFN-γ. We find that Th1 polarized cell cultures from IFNGR deficient mice contain several fold greater proportion of IFN-γ expressing cells and these cells also express higher levels of IFN-γ compared to cells from WT or IFNAR deficient mice. The high levels of IFN-γ can initially promote pathogenesis by promoting activation of antigen presenting cells and other accessory cells such as upregulation of MHC class II, co-stimulatory molecules, and adhesion molecules (64). Subsequently this higher level of IFN-γ can suppress CNS inflammation by inducing expression of NO in activated macrophages (65), inducing apoptosis of CNS-infiltrating lymphocytes and other mononuclear cells (28) and/or production of anti-inflammatory or neuroprotective cytokines/chemokines such as IL-13 and LIF(66–69). Indeed, we did observe that both IL-13 and LIF were barely induced in the CNS of IFNGR deficient mice with EAE.
In opposition to Th1 cells, type I and II IFN signals restrain encephalitogenic Th17 cells and do so independently of each other. These observations recapitulate in vivo our previous in vitro finding of the ability of IFN-β to directly suppress Th17 cells but not Th1 cells (36). The direct suppression of Th17 cell differentiation by IFN-γ or IFN-β in vitro has been reported in several studies (10, 44, 45, 70).
Prinz et al. (11) using Ifnar1−/− conditional mice demonstrated that development and severity of MOGp induced EAE was not altered in mice with selective loss of IFNAR in CD4+ T cells. On first examination our result is inconsistent with their findings. However, it is important to note that the previous study represents active EAE and therefore the combined result of Th1 and Th17 cells. Our study dissects the relative role of IFNAR in Th1 cells versus Th17 cells. Since loss of IFNAR signaling has opposing outcomes in Th1 and Th17 EAE, the combined effect could be null and therefore it reconciles the inconsistencies between the two studies.
The higher levels of IFN-γ and IL-17 in brains and SC of IFNGR−/− mice is expected since IFN-γ signals inhibit IL-17 gene expression (44, 45). However, the expanded numbers of Th1 cells induced in the absence of IFNGR is counter-intuitive when considering the paradigm that IFN-γ provides feed-forward signals for the expression of Tbet (46). This suggests that IFN-γ may also have regulatory effects in Th1 cells. IFNAR-deficient mice showed significantly lower concentration of CXCL10 and IL-13 in brain whereas higher levels of G-CSF and CCL11 were detected in SC with respect to WT mice. Remarkably, the absence of IFN-γ signaling resulted in a similar effect on these cytokines. These results suggest that the expression of CXCL10, IL-13, G-CSF and CCL11 is independently regulated by both type I and II IFN signaling and in a tissue-specific manner. The CNS of mice lacking both IFNAR and IFNGR contained lower levels of CXCL10 and higher levels of IL-17 than the CNS of mice that only lacked IFNGR. This indicates modulation by type I IFN signaling on those IFN-γ-regulated cytokines. The brain and spinal cords of IFNGR-deficient mice with EAE contained high levels of G-CSF and very low expression of the IFN-γ regulated chemokines, CXCL9, CXCL10 and CCL5. The elevated G-CSF might contribute to the enhanced numbers of neutrophils detected in the CNS of IFN-γ signaling deficient mice. CXCL9 and CXCL10 are chemokines that restrain the entry of leukocytes into the brain and their down regulation is associated with atypical EAE (47, 48), whereas CCL5 is an important chemokine for firm adhesion of leukocytes to the vasculature in the brain (71). Collectively these results reveal the independent and dependent action of types I and II IFNs in the CNS-specific modulation of key cytokines and chemokines in the pathogenesis of EAE.
As previously reported, we found high levels of IFN-γ and IL-17 in the brains of IFNGR- deficient mice as they developed atypical EAE (35, 47). Mice with atypical EAE have much greater infiltration of encephalitogenic effector cells into the brain and brain stem than those with classical EAE (47). Our finding that IFNAR-deficient does not develop symptoms or pathological features of atypical EAE indicates that IFN-γ signaling, but not IFNAR signaling is necessary to restrain encephalitogenic cells from entering the brain. None of the mice receiving IFNGR-deficient Th1 or Th17 cells developed clinical symptoms of atypical EAE. This implies that loss of IFNGR signals in T-cells is insufficient to induce atypical EAE and suggest a role of other cells such as macrophages, epithelial cells, and glial cells in this process.
In summary our data reveal that cooperative signaling from types I and II IFNs are necessary to restrain the pathogenesis in EAE. Loss and perhaps imbalance of either IFN signal aggravates inflammation and results in exacerbated disease. This study also demonstrates that response to IFN signals by the different effector populations involved in the pathogenesis of EAE varies and can have opposite outcome. Elucidating these mechanisms for the different innate and adaptive effector cells that contribute to the pathogenesis of MS will enable the development of effective therapeutics that selectively target arms of the IFN signaling pathway.
Supplementary Material
Acknowledgments
We thank the University of Alabama Neuroscience Core Facilities (NS47466, NS57098) for assistance with histology and immunohistochemistry, X-radiation facility (1 G20RR022807-01), UAB CAMAC Comprehensive Flow Cytometry Core (P30 AR48311), Analytic imaging and immunoreagent core (P30 AR48311) and the UAB Animal Resources Program (G20 RR025858 and G20 RR022807-01) for technical support. We also thank Dr. Robin Lorenz (Department of Pathology, University of Alabama at Birmingham) for Stat1−/− mice, Allison E. Metz (Department of Medicine, University of Alabama at Birmingham) for assistance with the multiplex cytokine assay, PingAr Yang and Qi Wu (Department of Medicine, University of Alabama at Birmingham) for technical assistance with mice.
Footnotes
Supported by grants from NIH AI1076562 and National Multiple Sclerosis Society RG3891 to CR, NIH NS064261 to PD and FONDECYT 1110523 to RN. RN was supported by a postdoctoral fellowship from Beca Chile.
Abbreviations used in this paper: CNS, central nervous system; EAE, experimental autoimmune encephalomyelitis; MS, multiple sclerosis; MOG, myelin oligodendrocyte glycoprotein; wild type, WT; SC, spinal cord; MOGp, MOG35–55 peptide.
References
- 1.Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, Stark GR. Interferons at age 50: past, current and future impact on biomedicine. Nature reviews Drug discovery. 2007;6:975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farrar MA, Schreiber RD. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 3.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 4.Pace JL. Synergistic interactions between IFN-gamma and IFN-beta in priming murine macrophages for tumor cell killing. J Leukoc Biol. 1988;44:514–520. doi: 10.1002/jlb.44.6.514. [DOI] [PubMed] [Google Scholar]
- 5.Dezfouli S, Hatzinisiriou I, Ralph SJ. Enhancing CTL responses to melanoma cell vaccines in vivo: synergistic increases obtained using IFNgamma primed and IFNbeta treated B7–1+ B16-F10 melanoma cells. Immunology and cell biology. 2003;81:459–471. doi: 10.1046/j.0818-9641.2003.01189.x. [DOI] [PubMed] [Google Scholar]
- 6.Peng T, Zhu J, Hwangbo Y, Corey L, Bumgarner RE. Independent and cooperative antiviral actions of beta interferon and gamma interferon against herpes simplex virus replication in primary human fibroblasts. Journal of virology. 2008;82:1934–1945. doi: 10.1128/JVI.01649-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gough DJ, Messina NL, Hii L, Gould JA, Sabapathy K, Robertson AP, Trapani JA, Levy DE, Hertzog PJ, Clarke CJ, Johnstone RW. Functional crosstalk between type I and II interferon through the regulated expression of STAT1. PLoS biology. 2010;8:e1000361. doi: 10.1371/journal.pbio.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takaoka A, Mitani Y, Suemori H, Sato M, Yokochi T, Noguchi S, Tanaka N, Taniguchi T. Cross talk between interferon-gamma and -alpha/beta signaling components in caveolar membrane domains. Science. 2000;288:2357–2360. doi: 10.1126/science.288.5475.2357. [DOI] [PubMed] [Google Scholar]
- 9.Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- 10.Guo B, Chang EY, Cheng G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J Clin Invest. 2008;118:1680–1690. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prinz M, Schmidt H, Mildner A, Knobeloch KP, Hanisch UK, Raasch J, Merkler D, Detje C, Gutcher I, Mages J, Lang R, Martin R, Gold R, Becher B, Bruck W, Kalinke U. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity. 2008;28:675–686. doi: 10.1016/j.immuni.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Teige I, Treschow A, Teige A, Mattsson R, Navikas V, Leanderson T, Holmdahl R, Issazadeh-Navikas S. IFN-beta gene deletion leads to augmented and chronic demyelinating experimental autoimmune encephalomyelitis. J Immunol. 2003;170:4776–4784. doi: 10.4049/jimmunol.170.9.4776. [DOI] [PubMed] [Google Scholar]
- 13.Giovannoni G, Miller DH. Multiple sclerosis and its treatment. J R Coll Physicians Lond. 1999;33:315–322. [PMC free article] [PubMed] [Google Scholar]
- 14.Markowitz CE. Interferon-beta: mechanism of action and dosing issues. Neurology. 2007;68:S8–11. doi: 10.1212/01.wnl.0000277703.74115.d2. [DOI] [PubMed] [Google Scholar]
- 15.Panitch HS. Early treatment trials with interferon beta in multiple sclerosis. Mult Scler. 1995;1(Suppl 1):S17–21. [PubMed] [Google Scholar]
- 16.Tourbah A, Lyon-Caen O. Interferons in multiple sclerosis: ten years’ experience. Biochimie. 2007;89:899–902. doi: 10.1016/j.biochi.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Brosnan CF, Cannella B, Battistini L, Raine CS. Cytokine localization in multiple sclerosis lesions: correlation with adhesion molecule expression and reactive nitrogen species. Neurology. 1995;45:S16–21. doi: 10.1212/wnl.45.6_suppl_6.s16. [DOI] [PubMed] [Google Scholar]
- 18.Olsson T. Cytokines in neuroinflammatory disease: role of myelin autoreactive T cell production of interferon-gamma. J Neuroimmunol. 1992;40:211–218. doi: 10.1016/0165-5728(92)90135-8. [DOI] [PubMed] [Google Scholar]
- 19.Renno T, Lin JY, Piccirillo C, Antel J, Owens T. Cytokine production by cells in cerebrospinal fluid during experimental allergic encephalomyelitis in SJL/J mice. J Neuroimmunol. 1994;49:1–7. doi: 10.1016/0165-5728(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 20.Renno T, Taupin V, Bourbonniere L, Verge G, Tran E, De Simone R, Krakowski M, Rodriguez M, Peterson A, Owens T. Interferon-gamma in progression to chronic demyelination and neurological deficit following acute EAE. Molecular and cellular neurosciences. 1998;12:376–389. doi: 10.1006/mcne.1998.0725. [DOI] [PubMed] [Google Scholar]
- 21.Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 22.Krakowski M, Owens T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996;26:1641–1646. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- 23.Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- 24.Billiau A, Heremans H, Vandekerckhove F, Dijkmans R, Sobis H, Meulepas E, Carton H. Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J Immunol. 1988;140:1506–1510. [PubMed] [Google Scholar]
- 25.Duong TT, St Louis J, Gilbert JJ, Finkelman FD, Strejan GH. Effect of anti-interferon-gamma and anti-interleukin-2 monoclonal antibody treatment on the development of actively and passively induced experimental allergic encephalomyelitis in the SJL/J mouse. J Neuroimmunol. 1992;36:105–115. doi: 10.1016/0165-5728(92)90042-j. [DOI] [PubMed] [Google Scholar]
- 26.Lublin FD, Knobler RL, Kalman B, Goldhaber M, Marini J, Perrault M, D’Imperio C, Joseph J, Alkan SS, Korngold R. Monoclonal anti-gamma interferon antibodies enhance experimental allergic encephalomyelitis. Autoimmunity. 1993;16:267–274. doi: 10.3109/08916939309014645. [DOI] [PubMed] [Google Scholar]
- 27.Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:123–128. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furlan R, Brambilla E, Ruffini F, Poliani PL, Bergami A, Marconi PC, Franciotta DM, Penna G, Comi G, Adorini L, Martino G. Intrathecal delivery of IFN-gamma protects C57BL/6 mice from chronic-progressive experimental autoimmune encephalomyelitis by increasing apoptosis of central nervous system-infiltrating lymphocytes. J Immunol. 2001;167:1821–1829. doi: 10.4049/jimmunol.167.3.1821. [DOI] [PubMed] [Google Scholar]
- 29.Heremans H, Dillen C, Groenen M, Martens E, Billiau A. Chronic relapsing experimental autoimmune encephalomyelitis (CREAE) in mice: enhancement by monoclonal antibodies against interferon-gamma. Eur J Immunol. 1996;26:2393–2398. doi: 10.1002/eji.1830261019. [DOI] [PubMed] [Google Scholar]
- 30.Voorthuis JA, Uitdehaag BM, De Groot CJ, Goede PH, van der Meide PH, Dijkstra CD. Suppression of experimental allergic encephalomyelitis by intraventricular administration of interferon-gamma in Lewis rats. Clin Exp Immunol. 1990;81:183–188. doi: 10.1111/j.1365-2249.1990.tb03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;1:893–895. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- 32.Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Connor RA, Malpass KH, Anderton SM. The inflamed central nervous system drives the activation and rapid proliferation of Foxp3+ regulatory T cells. J Immunol. 2007;179:958–966. doi: 10.4049/jimmunol.179.2.958. [DOI] [PubMed] [Google Scholar]
- 34.Steinman L. A rush to judgment on Th17. J Exp Med. 2008;205:1517–1522. doi: 10.1084/jem.20072066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P, Naves R, Han M, Zhong F, Castellanos JG, Mair R, Christakos A, Kolkowitz I, Katz L, Killestein J, Polman CH, de Waal Malefyt R, Steinman L, Raman C. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 38.Axtell RC, Webb MS, Barnum SR, Raman C. Cutting edge: critical role for CD5 in experimental autoimmune encephalomyelitis: inhibition of engagement reverses disease in mice. J Immunol. 2004;173:2928–2932. doi: 10.4049/jimmunol.173.5.2928. [DOI] [PubMed] [Google Scholar]
- 39.Sestero CM, McGuire DJ, De Sarno P, Brantley EC, Soldevila G, Axtell RC, Raman C. CD5-dependent CK2 activation pathway regulates threshold for T cell anergy. J Immunol. 2012;189:2918–2930. doi: 10.4049/jimmunol.1200065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Sarno P, Axtell RC, Raman C, Roth KA, Alessi DR, Jope RS. Lithium prevents and ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2008;181:338–345. doi: 10.4049/jimmunol.181.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abromson-Leeman S, Bronson R, Luo Y, Berman M, Leeman R, Leeman J, Dorf M. T-cell properties determine disease site, clinical presentation, and cellular pathology of experimental autoimmune encephalomyelitis. The American journal of pathology. 2004;165:1519–1533. doi: 10.1016/S0002-9440(10)63410-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wensky AK, Furtado GC, Marcondes MC, Chen S, Manfra D, Lira SA, Zagzag D, Lafaille JJ. IFN-gamma determines distinct clinical outcomes in autoimmune encephalomyelitis. J Immunol. 2005;174:1416–1423. doi: 10.4049/jimmunol.174.3.1416. [DOI] [PubMed] [Google Scholar]
- 43.Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 45.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 47.Lees JR, Golumbek PT, Sim J, Dorsey D, Russell JH. Regional CNS responses to IFN-gamma determine lesion localization patterns during EAE pathogenesis. J Exp Med. 2008;205:2633–2642. doi: 10.1084/jem.20080155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dev KK, Mullershausen F, Mattes H, Kuhn RR, Bilbe G, Hoyer D, Mir A. Brain sphingosine-1-phosphate receptors: implication for FTY720 in the treatment of multiple sclerosis. Pharmacol Ther. 2008;117:77–93. doi: 10.1016/j.pharmthera.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 49.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xin L, Vargas-Inchaustegui DA, Raimer SS, Kelly BC, Hu J, Zhu L, Sun J, Soong L. Type I IFN receptor regulates neutrophil functions and innate immunity to Leishmania parasites. J Immunol. 2010;184:7047–7056. doi: 10.4049/jimmunol.0903273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Critchfield JM, Racke MK, Zuniga-Pflucker JC, Cannella B, Raine CS, Goverman J, Lenardo MJ. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science. 1994;263:1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 52.Weishaupt A, Jander S, Bruck W, Kuhlmann T, Stienekemeier M, Hartung T, Toyka KV, Stoll G, Gold R. Molecular mechanisms of high-dose antigen therapy in experimental autoimmune encephalomyelitis: rapid induction of Th1-type cytokines and inducible nitric oxide synthase. J Immunol. 2000;165:7157–7163. doi: 10.4049/jimmunol.165.12.7157. [DOI] [PubMed] [Google Scholar]
- 53.Delhaye S, Paul S, Blakqori G, Minet M, Weber F, Staeheli P, Michiels T. Neurons produce type I interferon during viral encephalitis. Proc Natl Acad Sci U S A. 2006;103:7835–7840. doi: 10.1073/pnas.0602460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller SD, McMahon EJ, Schreiner B, Bailey SL. Antigen presentation in the CNS by myeloid dendritic cells drives progression of relapsing experimental autoimmune encephalomyelitis. Ann N Y Acad Sci. 2007;1103:179–191. doi: 10.1196/annals.1394.023. [DOI] [PubMed] [Google Scholar]
- 55.Town T, Jeng D, Alexopoulou L, Tan J, Flavell RA. Microglia recognize double-stranded RNA via TLR3. J Immunol. 2006;176:3804–3812. doi: 10.4049/jimmunol.176.6.3804. [DOI] [PubMed] [Google Scholar]
- 56.Rowse AL, Naves R, Cashman KS, McGuire DJ, Mbana T, Raman C, De Sarno P. Lithium Controls Central Nervous System Autoimmunity through Modulation of IFN-gamma Signaling. PLoS One. 2012;7:e52658. doi: 10.1371/journal.pone.0052658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Antel JP, V, Miron E. Central nervous system effects of current and emerging multiple sclerosis-directed immuno-therapies. Clinical neurology and neurosurgery. 2008;110:951–957. doi: 10.1016/j.clineuro.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 58.Barin JG, Talor MV, Baldeviano GC, Kimura M, Rose NR, Cihakova D. Mechanisms of IFNgamma regulation of autoimmune myocarditis. Experimental and molecular pathology. 2010;89:83–91. doi: 10.1016/j.yexmp.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sethna MP, Lampson LA. Immune modulation within the brain: recruitment of inflammatory cells and increased major histocompatibility antigen expression following intracerebral injection of interferon-gamma. J Neuroimmunol. 1991;34:121–132. doi: 10.1016/0165-5728(91)90121-m. [DOI] [PubMed] [Google Scholar]
- 60.Simmons RD, Willenborg DO. Direct injection of cytokines into the spinal cord causes autoimmune encephalomyelitis-like inflammation. J Neurol Sci. 1990;100:37–42. doi: 10.1016/0022-510x(90)90010-k. [DOI] [PubMed] [Google Scholar]
- 61.Jagessar SA, Gran B, Heijmans N, Bauer J, Laman JD, t Hart BA, Constantinescu CS. Discrepant effects of human interferon-gamma on clinical and immunological disease parameters in a novel marmoset model for multiple sclerosis. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2012;7:253–265. doi: 10.1007/s11481-011-9320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu J, Guan X, Ma X. Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-gamma-mediated pathways. J Exp Med. 2007;204:141–152. doi: 10.1084/jem.20061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murugaiyan G, Mittal A, Weiner HL. Identification of an IL-27/osteopontin axis in dendritic cells and its modulation by IFN-gamma limits IL-17-mediated autoimmune inflammation. Proc Natl Acad Sci U S A. 2010;107:11495–11500. doi: 10.1073/pnas.1002099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Willenborg DO, Fordham SA, Staykova MA, Ramshaw IA, Cowden WB. IFN-gamma is critical to the control of murine autoimmune encephalomyelitis and regulates both in the periphery and in the target tissue: a possible role for nitric oxide. J Immunol. 1999;163:5278–5286. [PubMed] [Google Scholar]
- 66.Butzkueven H, Zhang JG, Soilu-Hanninen M, Hochrein H, Chionh F, Shipham KA, Emery B, Turnley AM, Petratos S, Ernst M, Bartlett PF, Kilpatrick TJ. LIF receptor signaling limits immune-mediated demyelination by enhancing oligodendrocyte survival. Nat Med. 2002;8:613–619. doi: 10.1038/nm0602-613. [DOI] [PubMed] [Google Scholar]
- 67.Cash E, Minty A, Ferrara P, Caput D, Fradelizi D, Rott O. Macrophage-inactivating IL-13 suppresses experimental autoimmune encephalomyelitis in rats. J Immunol. 1994;153:4258–4267. [PubMed] [Google Scholar]
- 68.de Vries JE. The role of IL-13 and its receptor in allergy and inflammatory responses. The Journal of allergy and clinical immunology. 1998;102:165–169. doi: 10.1016/s0091-6749(98)70080-6. [DOI] [PubMed] [Google Scholar]
- 69.Linker RA, Kruse N, Israel S, Wei T, Seubert S, Hombach A, Holtmann B, Luhder F, Ransohoff RM, Sendtner M, Gold R. Leukemia inhibitory factor deficiency modulates the immune response and limits autoimmune demyelination: a new role for neurotrophic cytokines in neuroinflammation. J Immunol. 2008;180:2204–2213. doi: 10.4049/jimmunol.180.4.2204. [DOI] [PubMed] [Google Scholar]
- 70.Ramgolam VS, Sha Y, Jin J, Zhang X, Markovic-Plese S. IFN-beta inhibits human Th17 cell differentiation. J Immunol. 2009;183:5418–5427. doi: 10.4049/jimmunol.0803227. [DOI] [PubMed] [Google Scholar]
- 71.Vilela MC, Mansur DS, Lacerda-Queiroz N, Rodrigues DH, Lima GK, Arantes RM, Kroon EG, da Silva Campos MA, Teixeira MM, Teixeira AL. The chemokine CCL5 is essential for leukocyte recruitment in a model of severe Herpes simplex encephalitis. Ann N Y Acad Sci. 2009;1153:256–263. doi: 10.1111/j.1749-6632.2008.03959.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.