Abstract
Throughout this Reflections article, I have tried to follow up on the genesis in the 1960s and subsequent evolution of the concept of allosteric interaction and to examine its consequences within the past decades, essentially in the field of the neuroscience. The main conclusion is that allosteric mechanisms built on similar structural principles operate in bacterial regulatory enzymes, gene repressors (and the related nuclear receptors), rhodopsin, G-protein-coupled receptors, neurotransmitter receptors, ion channels, and so on from prokaryotes up to the human brain yet with important features of their own. Thus, future research on these basic cybernetic sensors is expected to develop in two major directions: at the elementary level, toward the atomic structure and molecular dynamics of the conformational changes involved in signal recognition and transduction, but also at a higher level of organization, the contribution of allosteric mechanisms to the modulation of brain functions.
Keywords: Allosteric Regulation, Membrane Proteins, Neurons, Nicotinic Acetylcholine Receptors, Synaptic Plasticity
Theory in Biology
In the physical sciences, there is a long tradition of theory and model building; the advancement of the discipline has relied and still relies on an active interplay between theory and experimental studies and between the development of novel technologies and conceptual invention. In the biological sciences, a similar tradition exists, but it is not as systematic. The frequent enthusiasm for new techniques without explicit theoretical perspectives often hides an impoverished theoretical landscape where original hypotheses or ideas are rare. In my view, the theoretical objectives should precede, or be concomitant with, the technological and experimental means. According to Claude Bernard, “the experimenter who does not know what he is looking for will not understand what he finds” (1).1 The reasons for the difficulties encountered with the biological sciences possibly lie in the fact that the objects of biology are highly differentiated with multiple, intricate levels of organization. The identification of common conceptual rules further hinges on the considerable structural and functional diversity of the biological objects. Thus, there is the constant dilemma, often split between scientists, about the universality or the singularity of the objects they investigate. In this context, I have selected as a tentative common rule the concept of allosteric interaction and its consequences on the molecular chemistry of proteins, going from bacterial regulatory enzymes up to the nervous system and, tentatively, the higher functions of the brain.
The circumstances of my personal life have been such that as an adolescent, I was already exposed to theory through the teaching of an exceptional professor of natural sciences, Jean Bathellier, who introduced me to the idea of biological evolution. This experience deeply influenced my scientific career to the extent that until now, as we shall see, my thinking has been framed within the Darwinian evolutionary paradigm. My first laboratory work was in marine biology (2), but I felt rapidly unsatisfied by perspectives essentially limited to descriptive morphology. In the fall of 1958, I visited Jean Brachet's laboratory in Brussels, where I also attended Christian de Duve's lectures and became fascinated by their theoretical perspective that the solution of the great problems of biology had ultimately to be found in elementary biochemical mechanisms. My interest shifted to the more explanatory chemistry of embryonic development. In this context, I imagined a theory in which the enzyme activations that follow the entry of the spermatozoon in the egg were due to a release of enzymes hidden in subcellular particles referred to as lysosomes by Christian de Duve. Back in Paris, I tried to test this hypothesis. Confronted by concrete technical difficulties, I met, by chance, Jacques Monod, who offered to let me enter his laboratory at the Institut Pasteur on the strict condition I abandon my ideas on embryonic development and work with Escherichia coli. I drudgingly accepted his demand (3), keeping my theoretical interest in animal biology for the future!
My fascination for theory once again was stimulated in the spring of 1959, when the moment came for the selection of the topic for my Ph.D. thesis. I met Jacques Monod and François Jacob (Fig. 1), who suggested several themes for my research, most of them dealing with their ongoing work on the regulation of gene expression and the operon model. They did not suit me since the theoretical insights and outcomes were already in their hands. An alternative, original topic, mentioned by François Jacob, held my attention. He had heard a talk by Edwin Umbarger, who had shown that in bacterial biosynthetic pathways, such as those for the biosynthesis of l-isoleucine or l-valine, the first enzyme is feedback-inhibited by the end product of the pathway (4). The issue was to understand the molecular mechanism of this “apparently competitive” regulatory interaction, a critical step in the cybernetics of the cell. This topic fitted with my first interest about enzyme activations during fertilization but with a much broader biological perspective. I adopted the theme for my thesis work.
FIGURE 1.
The author reading a poem in 1960 at Jacques Monod's 50th birthday. From left to right: Sarah Rapkine, Jacques Monod, François Jacob, the author, and François Gros. The photo was provided by the Fonds d'Archives Madeleine Brunerie at the Institut Pasteur.
The Concept of Allosteric Interaction (1961–1963)
My first naive idea was that if a special molecular device mediated the feedback inhibition by the enzyme, one should be able to find a way to identify its molecular constituents, for instance, by dissociating the regulatory interaction from the catalytic activity in vitro. I first confirmed Umbarger's in vitro observations of the apparent competitive inhibition of l-threonine deaminase by l-isoleucine and its “bimolecular” cooperative kinetics toward both the substrate and the feedback inhibitor. I noticed that the sensitivity of enzyme preparations to l-isoleucine changed with the time of storage, purification, heating, and exposure to reagents for –SH groups, resulting in a loss of response to l-isoleucine without a significant decline in enzyme activity. Interestingly, the loss of l-isoleucine feedback inhibition was also accompanied by the abolition of the bimolecular kinetics of the enzyme toward its substrate. The paper I presented at the 26th Cold Spring Harbor Symposium on Quantitative Biology entitled “Cellular Regulatory Mechanisms” (5), at the initiative of Jacques Monod, gave me the stimulating opportunity to theorize. I briefly discussed the two plausible models that might account for the apparently competitive antagonism between the feedback inhibitor l-isoleucine and the substrate l-threonine. According to the first model, the binding sites for the substrate and regulatory inhibitor are partially overlapping, so the interaction is a classical competition by steric hindrance. In the second “new” model, referred to as “no-overlapping,” the two sites are separated from each other, and the interaction between ligands takes place between topographically distinct sites. I favored the second model, in which the substrate and regulatory effector were to bind topographically distinct sites, specifically on the basis of the argument that loss of feedback inhibition was accompanied by a normalization of the kinetics (5). Following my presentation, the distinguished bacteriologist Bernard Davis mentioned the possible analogy between the properties of hemoglobin and those of threonine deaminase. As we shall see, it was a highly relevant and inspiring comment (6).
In the oral presentation of the “General Conclusions” of the symposium, Jacques Monod reported my results and interpretation in the section dealing with the regulation of enzyme activity. He also wrote, “Closely similar observations have been made independently and simultaneously by Pardee (private communication) on another enzyme sensitive to end-product (aspartate-carbamyl-transferase)” (7). In Monod and Jacob's subsequently written “General Conclusions,” the word “allosteric” appears for the first time. It is composed of two Greek roots expressing the difference (allo-) in (stereo-) specificity of the two binding sites to qualify and generalize the no-overlapping sites mechanism of indirect interaction between stereospecifically distinct sites mediated by a conformational change of the protein (8).
This was the birth of the word allosteric and of its general definition, which is nowadays widely accepted (8). The introduction of the concept created a major landmark in classical enzymology and the ill-defined notions of “un-competitive” or “non-competitive” inhibition (9) most often (but not always) assumed to take place in the neighborhood (or even at the level) of the active site.
At this stage, the conformational change linking the topographically distinct sites was interpreted by us in terms of the “induced-fit” theory of Daniel Koshland (10, 11) in the sense that the ligand “instructs” rather than “selects” the structural change (12). At the time, Koshland's concern was not the regulation of enzyme activity by a metabolic signal but the specificity of enzyme action. His theory (11, 13) was that a local steric fit seemed essential for the reaction to occur “only after a change in shape of the enzyme molecule had been induced by the substrate” (13). We suggested the extension of the idea to a higher level long-range and distant allosteric interaction between active and regulatory sites (12). Without being aware of it, we were following the widely accepted tradition of Karl Landsteiner and Linus Pauling's empiricist ideology, viewing the local environment as directly instructing structural changes within biological organisms.
The Monod-Wyman-Changeux Model (1965)
A paradigmatic change from instruction to selection occurred with the Monod-Wyman-Changeux (MWC) model (14). Quite surprisingly, in my opinion, it did not emerge from a deliberate shift of theoretical position by any one of us, but from experimental observations. At the end of 1963, I handed Jacques Monod the first typed version of my thesis work (15, 73, 251–254). Of the many observations I had made, he became especially interested by the experiments I did in 1962 on the effects of urea (16). At an adequate concentration, urea reversibly inactivates l-threonine deaminase, an inactivation interpreted as a split of the enzyme into subunits. Interestingly, in this system, allosteric activators, such as l-norleucine, l-valine, and l-allo-threonine, facilitated inactivation and thus subunit dissociation; feedback inhibitors, such as isoleucine, protected against inactivation and thus strengthened the assembly of the subunits in the protein. To formally account for the observed effects, I mentioned the possibility of three possible conformational states, one for each experimental condition (presence of activator, presence of inhibitor, and no effector). Jacques Monod pressed me to systematically adopt in my theoretical reasoning the rule to reduce the number of hypotheses to the strict minimum. Two states should suffice! I immediately agreed and further documented the experimental consequences of the two states with a tight (T) or relaxed (R) mode of packing of the subunits, each state pre-existing the binding of ligand and exhibiting different intrinsic affinities for both substrate and allosteric effectors. In my opinion, this was the birth of the two-state mechanism of pre-existing conformational states (R ↔ T), a consequence of a selective, rather than an instructive, effect of the ligands. These views were the start of many debates and theoretical developments that, after many successive writings of the paper by Jacques Monod (17, 18), led to the final version of the MWC model (14).
The model was originally planned to conclude my thesis work with the aim to formally establish a causal link between the structural organization of the known regulatory proteins and their signal transduction properties (Fig. 2). The problem was risky because at that time, the only known three-dimensional structures of proteins were those of hemoglobin (19) and myoglobin (20). Emphasis was placed on the cooperative binding of ligands, a property found frequently associated with the ability to transduce regulatory signals. An essential postulate for us was that cooperativity between ligand-binding sites relies upon the structural cooperativity existing between several subunits in a regulatory protein. In other words, the quaternary assembly of the subunits into a cooperative symmetrical oligomers is critical (although exceptions exist where single folded polypeptides that form several binding sites for different ligands may display allosteric interactions) (21). Max Perutz's structural model of hemoglobin (22, 23) revealed the symmetries of the α2β2-tetramer and the 25–36-Å distance between hemes, together with the evidence that the conformational change that takes place upon O2 binding primarily concerns the quaternary organization of the molecule. Through several visits, Jeffries Wyman made us aware about his views that the symmetry properties of the O2 saturation function for hemoglobin reveal the existence of elements of structural symmetry (24). Additional analysis of the assembly of protein oligomers and their symmetry properties was carried out in parallel by Jacques Monod himself and was included in the text.
FIGURE 2.
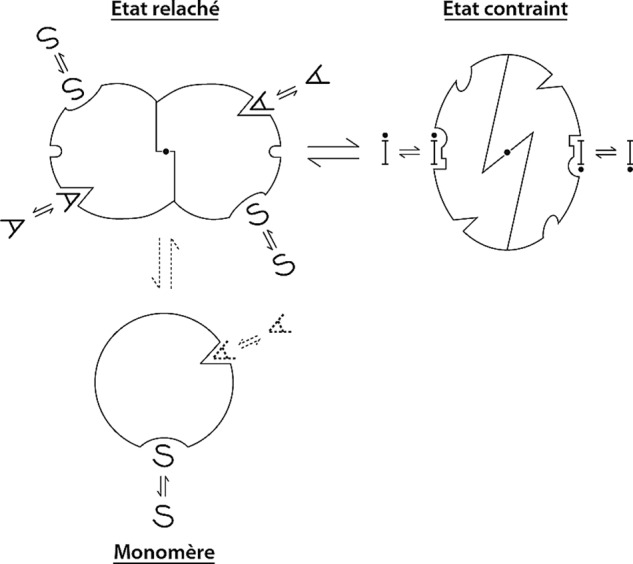
The two-state concerted Monod-Wyman-Changeux model (1965) as originally drawn by the author (Ph.D. thesis, 1964) (73). Etat relaché, relaxed state; Etat contraint, constrained state; Monomère, monomer.
Along these lines, the MWC model introduces the important notion that signal transduction requires a particular flexibility of protein structure associated with a quaternary constraint established between the subunits within the oligomer, which affects their tertiary organization (and vice versa) (14, 21, 25). Its principal consequence is the all-or-none character of the molecular switch between discrete conformational states, without intermediate hybrid states, associated with cooperative ligand binding. The statement then enunciated put forward that regulatory proteins spontaneously form closed and symmetrical oligomers (or microcrystals) that exist in a few discrete (all-or-none) and symmetrical (R, T…) conformations in thermal equilibrium in the absence of a regulatory signal. The regulatory ligands do not instruct any new adaptative conformations, but merely shift the spontaneous equilibrium between the conformations, selectively stabilizing the one that displays the biological activity and the highest affinity. The model fundamentally distinguishes a function of state “R,” which describes the spontaneous conformational equilibrium (defined by its intrinsic equilibrium constant L0), and a binding function, “Y,” which describes the occupation of the binding sites and distinctly evolves as a function of ligand concentration. Several of these statements have since then been reformulated and discussed by several groups in terms of “conformational shift” or “shape shifting” (21, 26–29). They created a theoretical landmark in biochemical thinking by opposing a selectionist model to the traditional induced-fit scheme.
A year following the MWC publication, Koshland, Némethy, and Filmer attempted to rehabilitate the Pauling scheme for O2 cooperativity in hemoglobin (30) by proposing an instructive model (KNF) (31) that implied a gradual induced change of biophysical parameters with abundant intermediate hybrid states accompanied by the superimposition of the state and binding functions. I took the opportunity to test this prediction during a postdoctoral position with John Gerhart and Howard Schachman. First, I extended the formulation of the MWC model to account for nonexclusive binding of regulatory ligands to both R and T states with the help of Merry Rubin, a computational scientist from the laboratory (32). Then making use of the high amounts of purified aspartate transcarbamylase (7, 33) available for binding experiments (34), together with simultaneously collected conformational data (35), we were able to demonstrate unambiguous differences between the state and binding functions. Moreover, the aspartate transcarbamylase data could be fitted with the general equation of the MWC model (Ref. 36; also see Ref. 37 with phosphofructokinase).
Since the 1960s, abundant structural studies have confirmed the MWC statement that many regulatory devices possess a symmetrical oligomeric structure and undergo the predicted conformational changes (21). The MWC model has also elicited new structural and molecular dynamics investigations in hemoglobin (38–43) and was extended to gene repressors (44, 45), together with the superfamily of nuclear receptors (46–48). We shall see that it was also extended to ion channel-linked (49) and G-protein-linked (50) membrane receptors.
As any scientific theory, the MWC model did not aim to give an exhaustive description of physical reality. It has intrinsic limitations introduced, in particular, by its founding postulates. The formal model was limited to a minimum of two main conformational states, which, as we shall see, are often more numerous (51, 52) and did not include either kinetics and/or molecular dynamics, which was invented after that (29). Also, it did not mention the possible occurrence of regulatory proteins undergoing allosteric transitions as single monomers (21, 29, 52) and the case of some G-protein-coupled receptors (GPCRs) (see “The Nicotinic Receptor: An Authentic Allosteric Membrane Protein”); yet, in my opinion, the major outcomes of the MWC theory are 2-fold. First, it implements a paradigmatic shift from the cybernetics of biological systems (53, 54) to the molecular mechanism of signal transduction. Second, it has been and still is a strong incentive for empirical research on regulatory proteins using the broad diversity of biophysical methods available.
Cooperativity of Membrane Receptors and the MWC Model
In the conclusion of my Ph.D. thesis in 1964 (15, 73, 251–254), I considered the possibility of extending the MWC model to the “membrane phenomena involved in the recognition of communication signals and their transmission (for example, synaptic transmission)” (73). A few years later, I met Max Delbrück at the California Institute of Technology (Caltech), and he gave me the opportunity to move a step forward in my theoretical reflections and to jump to the level of biological membranes. In September 1966, Max invited me to give a series of three lectures in October at Caltech. Commenting on my last lecture, he was struck by a slide on which I had shown where nearest-neighbor cooperative interactions between allosteric proteins were suggested to take place in a biological membrane. I said that I wanted to develop a mathematical model of such an interaction, and Max suggested that I meet the solid-state physicist Charles Kittel when back in Berkeley. Together with him and a French colleague, Jean Thiéry, the MWC model was re-examined and reformulated. In addition to the classical oligomeric case of membrane receptors, the model was applied to larger, unlimited cooperative assemblies of membrane proteins in a two-dimensional lattice (57). At variance with the MWC model, the thermodynamic formulation was based on the conformational transition of single units (or protomers) between a minimum of two states modulated (or not) by the interaction with other protomers. Depending on the value of the free energy of the interaction between protomers, the model predicts the existence of various classes of responses exhibited by biological systems: from a graded response of a single-receptor protomer or of an oligomeric receptor (MWC model) to an all-or-none phase transition response in large and periodic protein assemblies (56, 57).
The lattice model was rediscovered and successfully developed thirty years later by Dennis Bray and colleagues with the experimental system of bacterial chemoreceptors (58, 59); yet, to date, such cooperative interactions have not been identified in synaptic membranes, only in reconstituted artificial membranes. In contrast, with GPCRs (60–62), the assemblies of homo- and hetero-oligomers of variable sizes as well as between GPCRs and ion channels or ligand-gated ion channels (also referred to as receptor-receptor interactions) have been abundantly documented (63–66).
Other kinds of supramolecular assemblies might possibly be interpreted in terms of adapted versions of the MWC (14) and Changeux et al. (57) models such as the chaperonins (67, 68) and possibly the gene transcription complex (69). These models of allosteric interactions have still largely unexplored consequences of the structural and functional organization of supramolecular structures, where the interactions not only take place between ligand-binding sites but also mobilize multiple protein interfaces. In any case, it was an opportunity to reframe the original model in a new biological context.
The Identification of the Nicotinic Acetylcholine Receptor
The MWC model was a fertile inspiration for deeper research into the structural chemistry of proteins, protein design of new functions, and new modulators (21), but I preferred to extend my reflections about membrane cooperativity to carrying the concept of allostery into neuroscience and, to begin with, test the available models on a neural receptor. The John Newport Langley (70) concept of “receptive substance,” or receptor theory, was a major landmark in the history of biochemical pharmacology in 1905, yet it was soon criticized and even found “unnecessary” (71) by many experimentalists. Also, for decades, it was believed that neuronal communications in the brain were electrical rather than chemical! No neurotransmitter receptor was biochemically identified until nearly 60 years later (72).
Before leaving the Institut Pasteur in mid-1965, I decided to experimentally challenge the plausible extension of the MWC model to a synaptic protein, the enzyme that degrades the neurotransmitter acetylcholine (ACh), acetylcholinesterase (AChE). I discovered that some of the compounds designed by Daniel Bovet when he was working at the Institut Pasteur (in particular, derivatives of Flaxedil) did not behave as steric competitors but as allosteric modulators of AChE activity (55, 74–76), suggesting a possible but superficial analogy with the authentic receptor.
The time had come to test the MWC model with the true physiological receptor for ACh. A prerequisite was to identify it as a protein! It was achieved by starting from the Electrophorus electricus electric organ (72, 77) to use the deliberate strategy to follow the in vivo physiological and pharmacological response of a single electroplaque to nicotinic agents (77) and to keep these features unmodified in vitro at all stages of receptor isolation and chemical identification. I learned to dissect and record from the single electroplaque during a visit of a few months to David Nachmansohn's laboratory at Columbia University in 1967. Back at the Institut Pasteur, I developed an important intermediate assay from purified membrane fragments that make closed microsacs from which radioactive Na+ (or K+) ion flux responses to nicotine agonists can be measured. The in vitro data displayed ligand specificities that closely resembled those recorded with single electroplaques (78, 79). I used the detergent deoxycholate to gently extract, without denaturation, a protein that reversibly bound the radiolabeled nicotinic agonist decamethonium, using the method of equilibrium dialysis formerly used by Walter Gilbert and Benno Müller-Hill (80) to identify the lac repressor. Last, the snake venom toxin α-bungarotoxin, discovered by the Taiwanese pharmacologist Chen-Yuan Lee, was discovered to specifically block the in vivo neuromuscular transmission in high vertebrates at the postsynaptic level without interacting with AChE (81). α-Bungarotoxin was found to block altogether the electroplaque's in vivo electrical response to nicotinic agonists, the microsac's ion flux response in vitro, and the binding of radioactive decamethonium to the detergent extract (82). The extract contained a high molecular weight hydrophobic protein that could then be physically separated from AChE (82) and purified to homogeneity (72, 83).
The Nicotinic Receptor: An Authentic Allosteric Membrane Protein
The secure identification of the nicotinic ACh receptor (nAChR) protein opened a new field of empirical biochemical research (72) motivated by the following question: is the newly identified receptor a bona fide allosteric protein? Examination by electron microscopy of the nAChR protein purified from E. electricus and from purified nAChR-rich membranes from Torpedo marmorata revealed ring-like particles (8–9 nm in diameter) with a hydrophilic core made up of several (five to six) subunits associated in a compact bundle (84–94). Cross-linking of E. electricus nAChR further suggested a pentameric organization (95) composed of four types of homologous subunits, 2α1, β1, γ1, and δ1 (96–100), all of which with important sequence identities (99, 101, 102). This finding suggested the possibility, consistent with the MWC model, of a pseudo-symmetrical organization of the nAChR oligomer. The complete sequence of the cDNAs from the several homologous nAChR subunits from the electric organs of Torpedo californica (103–106) and T. marmorata (107, 108), muscle (109), and brain (110–113) revealed a common organization of all subunits (104, 106, 108) that consisted of a large N-terminal hydrophilic domain, four hydrophobic segments (TM1–TM4), and a small hydrophilic domain. Affinity labeling and site-directed mutagenesis demonstrated that the large hydrophilic domain is extracellular and carries the ACh-binding site (114–118) and that the four hydrophobic segments traverse the transmembrane, with the TM2 segment lining the ion channel (Figs. 3 and 4) (119–122).
FIGURE 3.

The author working in his laboratory at the Institut Pasteur in 1987. This photo was provided by Martine Franck of Magnum Photos.
FIGURE 4.
The author (1987) discussing models on the themes of his teaching at the Collège de France (Chair of Cellular Communications). This photo was provided by Martine Franck of Magnum Photos.
The nAChR appeared to behave as an authentic allosteric protein but with particularities of its own (49, 123, 124). 1) It possesses a rather odd oligomeric structure of an homo- or heteropentameric organization, with a C5 axis of rotational symmetry (or quasi-symmetry) perpendicular to the plane of the membrane. 2) The topological distinction between the neurotransmitter (ACh) sites (separated by 40 Å between ACh sites) in the extracellular domain and the ion channel (separated by 60 Å from the ACh sites) in the transmembrane domain unambiguously demonstrates that the interaction between the neurotransmitter (ACh) and the channel is an indirect allosteric interaction. 3) Consistent with the MWC model, several discrete conformational changes spontaneously occur in the absence of ligand, but more than two mediate activation (channel opening). The fast and slow inactivations toward higher affinity closed state(s) are referred to as desensitized (49, 125–131). 4) Multiple allosteric “modulatory sites” are present in the synaptic (e.g. Ca2+) and transmembrane domain. The anthelmintic ivermectin, general anesthetics, and other molecules regulate these transitions (see below). 5) The conformational equilibrium is shifted by orthosteric and allosteric ligands in favor of the state(s) for which they exhibit a preferential affinity (124–127). Last, constitutive mutations stabilize any one of the accessible conformations (active versus inactive) of the nAChR (129, 132–136). Such mutations confer predispositions to human diseases such as congenital myasthenia (129, 133) and autosomal dominant frontal lobe epilepsy (134, 137) (Fig. 5).
FIGURE 5.
The author in his laboratory at the Institut Pasteur in 1995 with (from left to right) Alain Bessis, Anne Devillers-Thiéry, Clément Léna, Jean-Luc Eiselé, and Jean-Luc Galzi. This photo was provided by the Institut Pasteur.
The recent discovery that orthologs of nAChRs are present in prokaryotes (138) and behave as ligand-gated ion channels (139) has led to high resolution crystal structures of the receptors from two bacterial species, Gloeobacter violaceus (140) and Erwinia chrysanthemi (141, 142). They confirm a pentameric symmetrical structure that is strikingly conserved from prokaryotes to eukaryotes (49, 143), thereby opening the exploration of the allosteric transition at the atomic level. Comparison of the x-ray core structures of Erwinia and Gloeobacter receptors, which are present in closed versus open conformations (140–142), revealed a conformational change associated with channel gating manifested by a global quaternary twist around the 5-fold axis of rotational symmetry yet with additional tertiary deformations. An in silico normal mode analysis, initially applied to a model of α7-nAChR (144–146), accounts for at least 29% of the allosteric transition deduced from Erwinia versus Gloeobacter receptor structures (140–142). The latest ongoing structural developments disclose a novel locally closed structure of the Gloeobacter receptor (51), pointing to additional intermediate conformations possibly relevant to the multistep process of desensitization (147).
The isolation of the nicotinic receptor and the demonstration of its allosteric properties were followed by the identification of many members of the pentameric receptor family in the brain (including nAChRs; 5-hydroxytryptamine type 3, GABAA, GABAC, and glycine receptors; and some glutamate, histamine, and 5-hydroxytryptamine-activated anionic receptors) (49, 148–150), as well as other classes of receptors, including the GPCRs (151–153) and channelrhodopsin light-gated cation channels (154).
These studies also pioneered the elucidation at the atomic level of the signal transduction mechanism these receptors mediate and their molecular dynamics (155). In a general manner, the available experimental data favor, but with possible important exceptions, the MWC scheme of pre-existing conformational equilibria (21, 156, 157). However, in several instances such as bias agonism in GPCRs (158), equilibria between multiple conformations (rather than two) have to be taken into consideration (52).
Allosteric Modulation: A New Receptor Pharmacology
Another important consequence of the idea of allostery was the recent re-evaluation of the ancient concept of allosteric modulation (21, 123). It opened new avenues in the pharmacology of the brain and resulted in important applications to drug discovery (158, 159). Early studies carried out in the 1970s in my laboratory with the aim to identify the binding specificity of various ligands for the nAChR already revealed that channel blockers, like local anesthetics, do not directly displace nicotinic ligands from the ACh-binding site but reversibly bind to a different allosteric site (160–162). The interaction between ACh and local anesthetic sites examined by fluorescence recording was shown to be positive and reciprocal, thus introducing the concept of an allosteric modulatory ligand. Since then, in addition to the main allosteric interaction between neurotransmitter (orthosteric) sites and the ion channel (which binds local anesthetics), multiple allosteric sites to which ligands bind and thus regulate signal transduction have been identified (Fig. 6). One of them was for external Ca2+ ions (163, 164), which bind to the extracellular domain of the α7-receptor (165).
FIGURE 6.
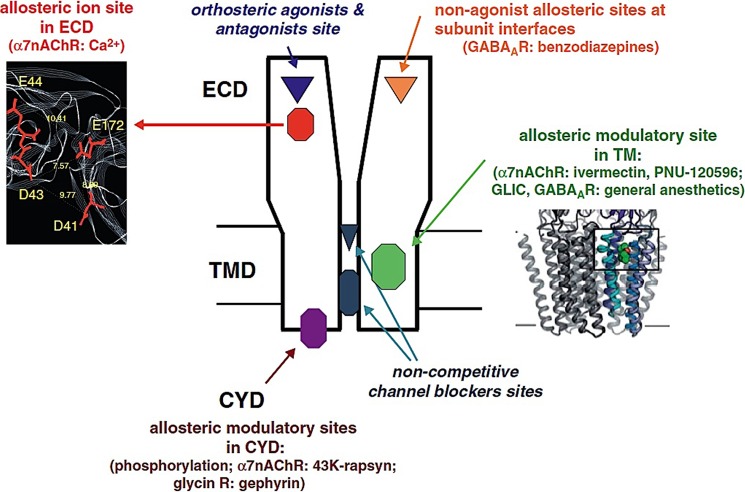
Schematic representation of the various classes of orthosteric and allosteric sites in pentameric ligand-gated ion channels. Orthosteric sites (shown in italic blue type) include the neurotransmitter-binding site and the ion channel. Allosteric sites (shown in various colors) are present in all three domains. Inset, upper left, model of the allosteric Ca2+ site in α-nAChR (from Ref. 146); lower right, x-ray structure of the Gloeobacter receptor intrasubunit site for general anesthetics (green) in the transmembrane domain (Ref. 169; from Ref. 187). ECD, extracellular domain; TMD, transmembrane domain; CYD, cytoplasmic domain; GABAAR, GABAA receptor; GLIC, Gloeobacter ligand-gated ion channel.
Another important type of site for allosteric modulation was discovered in a collaborative work carried out with Daniel Bertrand from the University Medical Center in Geneva (166) using ivermectin. In the micromolar range, this compound strongly enhances the ACh-evoked response of α7-receptors with increased apparent affinity, cooperativity, and maximal response. Based on the alteration of the ivermectin effect caused by mutations in the transmembrane domain (166), it was suggested that ivermectin, like PNU-120596, LY-2087101, and others, acts on α7-nAChRs at the level of a new type of allosteric modulatory site (167, 168), tentatively identified by mutagenesis within the transmembrane domain.
Amazingly, general anesthetics such as propofol and desflurane, which behave as negative modulators of the prokaryotic Gloeobacter receptor (169), possess a common binding site identified by x-ray analysis of the Gloeobacter receptor within the upper part of the transmembrane domain of each protomer inside a cavity delimited by TM1, TM3, and TM2 within each subunit (169). The cavity of the general anesthetics is accessible from the lipid bilayer, and its entrance is obstructed by a lipid alkyl chain that clashes with propofol binding. Thus, lipids might be the endogenous ligands of this membrane allosteric site (169). These sites appear to be homologous to important modulatory sites of pentameric receptors, such as for ethanol (170, 171), anticonvulsants, anesthetics, and diuretics acting on the glycine or GABAA receptor or on nAChR, as well present in the transmembrane domain both within and between subunits (172–174).
In homopentameric pentamers such as α7-nAChR, there are five active sites per pentamer located at subunit interfaces in the extracellular domain. In heteropentameric neuronal nAChRs such as α4β2-nAChR, not all five homologous sites bind ACh. The non-agonist-binding interface may accommodate, using ACh-binding protein as a model, galantamine, strychnine, cocaine, and morphine at micromolar concentrations (175–177). The possible binding of allosteric modulators at interfaces in nAChRs that do not normally bind nicotinic ligands might be homologous (178) to the important binding site through which benzodiazepines allosterically potentiate GABAA receptors. Considerable biochemical, pharmacological, and modeling evidence has since then demonstrated that benzodiazepine ligands bind to intersubunit sites in the extracellular domain of the GABAA receptor that are homologous to the GABA site but do not bind GABA (172–174). Benzodiazepines, after their discovery by Leo Sternbach in 1955, have become the most commonly prescribed psychopharmaceutical drug and the eleventh-most prescribed drug overall, with 46 million prescriptions written for them in 2010 in the United States! Other allosteric modulatory sites are present on the cytoplasmic domain and may play important roles in the clustering, stabilization, and modulation of receptor functions (21, 179, 180, 187).
Without a doubt, the concept of allosteric modulation has created a major landmark in the strategies of drug design for ligand-gated ion channels but also GPCRs, resulting in the successful development of new classes of drugs used in the clinic, such as Gleevec (allosteric inhibitor of Abl tyrosine kinase), cinacalcet (allosteric activator of the calcium-sensing receptor), and maraviroc (allosteric inhibitor of chemokine receptor) (158, 159).
Allosteric Models of Short- and Long-term Synaptic Plasticity
Since my early days in Jacques Monod's laboratory, where I was assigned to work on bacterial proteins, I faithfully remained attached to my adolescent wishes to explore the chemistry of higher organisms. I sought to exploit the knowledge acquired with the chemistry and allosteric properties of the nAChR to progress in the exploration of the well recognized plasticity of brain functions. I realized that to bridge the gap from molecules to cognition, multiple nested levels of organization had to be taken into consideration. Additional theoretical reflections on allosteric receptors were needed, which integrate the molecular, synaptic, neuronal assemblies and cognitive levels.
The first attempt dealt with the elementary process of synapse formation during the postnatal development period, which is especially long in humans (up to 15 years). In 1970, I had read Jacques Monod's Chance and Necessity with great interest but found Monod's position on brain development much too biased by innate influences. As a well informed admirer of Torsten Wiesel and David Hubel's work on the effects of experience on the postnatal development of the visual cortex, I did not share their functional validation hypothesis of preformed innate patterns of nerve connections. The proposal I suggested was that, as an alternative to the classical nativist versus empiricist instructive views, an epigenetic “Darwinian” mechanism of synapse selection took place (181–183). It was suggested that overproduced and variable distributions of connections would become transiently established in the course of synapse formation. Then, at a critical or sensitive period, a synapse selection would take place through some kind of trial-and-error process under the control of the electrical and chemical activity of the network (182, 183). As a consequence, some synapses would be stabilized, whereas others, in agreement with earlier observations (184), would regress. The model that was mathematically formalized together with Philippe Courrège and Antoine Danchin (182) had several major consequences. First, it predicted a phenotypic variability of the brain that is found in monozygotic twins. Also, it was an incentive for the search for synapse elimination (frequently called synaptic pruning) and its control by activity. It has since then been recognized in many systems (185, 186)2 and becomes of critical importance with human babies because of the extended interactions taking place with the social and cultural environment.
The model aroused many and constructive debates (188, 189) but also concrete biochemical investigations, for example, on the differentiation and plasticity of the postsynaptic domain (190, 191), and the discovery that distinct DNA elements (190) and transcription factors (192) control the targeting of receptor transcription under the neuromuscular synapse and its repression by electrical activity outside the end plate, followed by the identification of these various components (124). Finally, post-transcriptional mechanisms, including the assembly of the nAChR by the cytoplasmic 43K-rapsyn protein (87, 193) into supramolecular aggregates, revealed a progressive compartmentalization of the dispersed nAChR at the subjunctional level (194, 195). These studies unveiled the contribution of endogenous trophic factors and electrical activity in the assembly of the subsynaptic membrane (124) and offered a plausible mechanism of long-term synaptic plasticity at the gene expression level.
In the chapter “Mental Objects” of Neuronal Man: The Biology of Mind (196), inspired by the investigations of the allosteric modulation of the nAChR, I attempted to extend the theory of synapse selection to short-term learning in the brain. In 1982, Thierry Heidmann and I proposed a model for coincident reading of synaptic signals by an allosteric receptor and thus of short-term synaptic efficacy to be able to eventually link individual neurons into cooperative Hebbian assemblies (197). It is based upon the ability of postsynaptic receptors to integrate extra- and intracellular signals through their transmembrane organization via the allosteric modulatory sites and to shift the equilibrium between desensitized and activatable states. This would then determine the amplitude of the change of synaptic efficacy. This mechanism differs from that suggested for the glutamate NMDA receptor, which is based on the voltage-sensitive blocking of a “rigid” ion channel by Mg2+ ions; yet it strikingly resembles the proposal by Eric Kandel to account for data from Aplysia learning, although the allosteric mechanisms involved were not examined (198, 199).
This allosteric learning mechanism served as an elementary building block (200–202) in the subsequent modeling, realized together with Stanislas Dehaene, of cognitive functions such as the well known delayed-response tasks, which, in mammals, mobilize the prefrontal cortex (203). We proposed that the prefrontal cortex produces variable “anticipations,” or hypotheses, as transient patterns of spontaneous neuronal firings (200–202, 204–206). These firings could be selected (versus destabilized) at the level of allosteric receptors through the release of a positive (or negative) reward signal evoked by a successful (or unsuccessful) interaction with the outside world (generator of diversity). The neuronal systems specializing in reward and punishment are known to engage defined neurotransmitters and co-existing peptides, including dopamine and serotonin, or ACh/nicotine, together with their receptors, including the nAChR. More elaborate reward-learning algorithms based on predictive Hebbian learning were subsequently reported by other groups (207) and implemented both in silico and in vivo with monkeys (208, 209). An important consequence of these models, which rely on allosteric receptors, was to establish plausible biochemical links with concrete receptor pharmacology.
Nicotine Addiction, General Anesthesia, and Cognitive Functions
Smoking is the most important preventable cause of mortality and morbidity worldwide. Nicotine, the principal if not sole addictive component of tobacco smoke, exerts reinforcing effects through its action on brain nAChRs together with the laying down of long-term traces in the brain. Their identification and the eventual contribution of allosteric mechanisms to these alterations hinge upon the difficulty that nAChR oligomers are most often composed of several different subunits (sampled among the seventeen ones coded in the human genome) with distinct allosteric properties and are distributed over several brain areas. To identify the patterns of nAChR subunits that contribute to nicotine addiction, new strategies were developed in mice, including nAChR subunit gene deletions, targeted knock-in gene mutations, and re-expression of a deleted gene using stereotaxic injection of a lentiviral vector carrying the missing gene or the relevant siRNA (210–213). The data obtained in vivo with these genetically modified mice (214–217) provided evidence that within the dopaminergic neurons from the ventral tegmental area, nAChRs containing combinations of α4-, α3-, α5-, α6-, and β2-subunits mediate the rewarding effects of nicotine administration (215, 218–220). In all of these instances, the different kinds of oligomers involved display characteristic allosteric properties for channel activation, fast and slow desensitization, allosteric modulation, or up-regulation (221–224). These properties thus offer a novel panel of conformational targets for long-term smoking cessation therapies and possibly for alcohol consumption (225).
Addiction is viewed as the end point of a series of transitions from initial voluntary drug use to the “loss of control” over this behavior. Theoretical models (226, 227) and experimental approaches have been suggested for the neuronal bases of drug-elicited loss of control. Smokers with brain damage involving the insula are more likely to quit smoking than smokers with brain damage not involving the insula (228). Also, diffusion tensor imaging studies have revealed that prenatal and adolescent exposure to tobacco smoke alters the development of the microstructure of the white matter, with increased fractional anisotropy in the right and left frontal regions and in the genu of the corpus callosum (229). There is also electrophysiological evidence supporting a direct action of nicotine on axon conduction at the level of nAChRs present at the nodes of Ranvier (230) and at the white matter level (231). These observations support the proposal that nAChRs control a “gating circuit,” which itself modulates, in a top-down manner and through the long-range cortical connections of the “global neuronal workspace” (206, 232, 233), nicotine uptake and addiction (227, 234) (Fig. 7). In a general manner, they underline the contribution of ACh and more specifically nAChRs to global “conscious” control (232–235).
FIGURE 7.
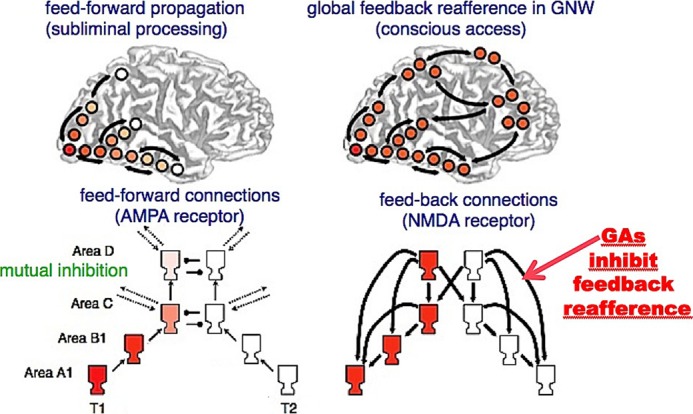
Schematic representation of the hypothesized events leading to conscious access according to the global neuronal workspace model and the action of general anesthetics on cortical reafference (232, 237). GNW, global neuronal workspace; GAs, general anesthetics.
In this framework, general anesthesia offers the most vivid evidence for an allosteric modulation of higher brain functions (236, 237). Administration of general anesthetics to patients causes a reversible loss of consciousness manifested, in particular, by a loss of response to oral commands. At appropriate levels of general anesthetics, a deeply unconscious state follows in which the electroencephalogram pattern resembles slow-wave sleep. Parallel biophysical studies on the detailed neural architecture accounting for conscious access called masking experiments (232, 238) suggested the contribution of two distinct sets of connections (239, 240). Bottom-up feedforward short connections link primary sensory areas up to the prefrontal cortex in a cascade. Complementary long-distance top-down connections project from the prefrontal cortex to all preceding areas, creating a top-down global “cortical reafference” (Fig. 7). Quantitative electroencephalogram analysis in humans under propofol anesthesia showed that upon induction, the dominant feedback connectivity of the base-line conscious state was differentially and reversibly inhibited in the frontoparietal network (241). Such selective inhibition would plausibly result from a positive allosteric modulation of the cortical GABAA receptor (among other molecular targets) (242). As mentioned above, these allosteric sites for general anesthetics have been identified in their transmembrane domain (Fig. 6) (169, 172–174). In other words, the loss of consciousness caused by general anesthetic infusion would ultimately result from a modulation of brain allosteric receptors and/or ion channels. The mode of action of general anesthetics on the brain may serve as a concrete illustration of how drugs may allosterically modulate higher brain functions.
Conclusion: The Brain as a Chemical Machine?
In Neuronal Man: The Biology of Mind (p. 114 of Ref. 196), I wrote “We must not underestimate the importance of the diversity of the neurons…the multiplicity of the chemical mediators… . (to the extent that) the chemical makeup of the cells that participate in a sensation…is closer to a canvas by Georges Seurat than a composition by Piet Mondrian.” This is still very true. The many neurotransmitters and coexisting neuropeptides (243, 244), their multiple receptors (49, 72, 196, 245), which may reach hundreds per cell (246), and their possible combinations are immensely rich and diverse. Also, any individual neuron differs from any other neuron within a given category by its connectivity, its biochemistry, and its physiology due to the developmental variability consecutive to the synapse selection mechanism (182, 196). Confronted with such a complexity, new ideas and modeling enterprises are needed (Fig. 8).
FIGURE 8.

The author at the 2009 meeting “Darwin: 200 Years” at Collège de France. This photo was provided by the Collège de France.
Such a multidisciplinary program is expected to contribute to our understanding of the brain but also to be an incentive for the design and development of technical applications. One of them is optogenetics, which allows the control of the activity of neuronal networks underlying behavior through the millisecond allosteric transition of channelrhodopsin or related photoactivatable molecules (247). Another one of biomedical importance is the design of drugs directed against brain diseases. For instance, it is now recognized that receptors may be present under diverse conformations that spontaneously exist in the absence of ligand. From a drug design perspective, the model suggests that the design of drugs should be targeted to site(s) present on these defined conformation(s) rather than to a single category of rigid binding sites, resulting in the production of agonistic versus antagonistic ligands. These studies have also led to the successful discovery of novel allosteric modulators that regulate the activity of ligand-gated ion channels, as well as of other receptors such as GPCRs and tyrosine kinase receptors. Among them are the benzodiazepines, which are the most commonly prescribed psychopharmaceutical drug in the world and which behave as allosteric modulators of GABAA receptors. The model also predicts that by altering the unliganded equilibrium between discrete conformational states, gene mutations may cause constitutive receptor activation (or inhibition) with important pathological consequences (124, 248). Also, these modeling approaches have enlarged the field of short- and long-term learning to still largely unexplored conformational mechanisms. The “molecular selectionist” schemes of short- and long-term learning, which take place within the genetic envelope of the adult and developing brain, are expected to have important consequences on higher brain functions and their pathologies. Last but not least, the analysis of the patterns of genes expressed in the course of brain development led to the suggestion of a new strategy for designing drugs as orthosteric and allosteric ligands, targeting the transcription factors regulating the “predisposition” genes rather than the proteins they encode (249). One should not forget in this respect that transcription factors are bona fide allosteric proteins.
These theoretical reasonings and data further document and enrich what may be referred to as a “chemical theory of higher brain functions” (196, 250). This creates a striking landmark in the thinking of brain sciences by causally and reciprocally linking the molecular to the cognitive levels both within the individual brain and between brains in the social and cultural environment, thus suggesting new bridges between brain sciences and humanities. From a philosophical point of view, it appears legitimate to say that the future understanding of the mind-brain relationships and the relevant mental processes is likely to rest upon the biochemical world of the allosteric transitions that mediate interneuronal communications through the multiple levels of organization spanning the human brain.
Acknowledgments
I gratefully thank Drs. Tamas Bartfai, Henri Korn, Uwe Maskos, and Ákos Nemecz for comments and suggestions.
Footnotes
The full quote (see Ref. 255) is, “Empiricism may serve to accumulate facts, but it will never build science. The experimenter who does not know what he is looking for will not understand what he finds.”
J.-P. Changeux, manuscript in preparation.
REFERENCES
- 1. Robin E. D. (1979) Claude Bernard. Pioneer of regulatory biology. JAMA 242, 1283–1284 [DOI] [PubMed] [Google Scholar]
- 2. Changeux J.-P. (1958) Some biological characters of a parasitic copepod from holothurians: allantogynus delamarei n.g.n.sp. C. R. Hebd. Seances Acad. Sci. 247, 961–964 [Google Scholar]
- 3. Changeux J.-P. (1960) On the biochemical expression of genetic determinants of Escherichia coli introduced to Salmonella typhimurium. C. R. Hebd. Seances Acad. Sci. 250, 1575–1577 [PubMed] [Google Scholar]
- 4. Umbarger H. E. (1956) Evidence for a negative-feedback mechanism in the biosynthesis of isoleucine. Science 123, 848. [DOI] [PubMed] [Google Scholar]
- 5. Changeux J.-P. (1961) The feedback control mechanisms of biosynthetic l-threonine deaminase by l-isoleucine. Cold Spring Harb. Symp. Quant. Biol. 26, 313–318 [DOI] [PubMed] [Google Scholar]
- 6. Davis B. (1961) Commentary to Changeux's presentation. Cold Spring Harbor Symp. Quant. Biol. 26, 318 [Google Scholar]
- 7. Gerhart J. C., Pardee A. B. (1962) The enzymology of control by feedback inhibition. J. Biol. Chem. 237, 891–896 [PubMed] [Google Scholar]
- 8. Changeux J.-P. (2011) 50th anniversary of the word “allosteric.” Protein Sci. 20, 1119–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haldane J. B. S. (1930) Enzymes, Longmans, Green and Co., London [Google Scholar]
- 10. Koshland D. E., Jr. (1956) Molecular geometry in enzyme action. J. Cell. Physiol. 47, 217–234 [DOI] [PubMed] [Google Scholar]
- 11. Koshland D. E., Jr. (1959) Enzyme flexibility and enzyme action. J. Cell. Comp. Physiol. 54, 245–258 [DOI] [PubMed] [Google Scholar]
- 12. Monod J., Changeux J.-P., Jacob F. (1963) Allosteric proteins and cellular control systems. J. Mol. Biol. 6, 306–329 [DOI] [PubMed] [Google Scholar]
- 13. Koshland D. E., Jr. (1963) The role of flexibility in enzyme action. Cold Spring Harbor Symp. Quant. Biol. 28, 473–482 [Google Scholar]
- 14. Monod J., Wyman J., Changeux J.-P. (1965) On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 12, 88–118 [DOI] [PubMed] [Google Scholar]
- 15. Changeux J.-P. (1964) On the allosteric properties of l-threonine deaminase. I. Methods of studying biosynthetic l-threonine deaminase. Bull. Soc. Chim. Biol. 46, 927–946 [PubMed] [Google Scholar]
- 16. Changeux J.-P. (1963) Allosteric interactions on biosynthetic l-threonine deaminase from E. coli K12. Cold Spring Harb. Symp. Quant. Biol. 28, 497–504 [Google Scholar]
- 17. Buc H. (2006) Interactions between Jacques Monod and Jeffries Wyman (or the burdens of co-authorship). Rendiconti Lincei 17, 31–49 [Google Scholar]
- 18. Buc H. (2013) The design of an enzyme: a chronology on the controversy. J. Mol. Biol. 425, 1407–1409 [DOI] [PubMed] [Google Scholar]
- 19. Perutz M. F., Rossmann M. G., Cullis A. F., Muirhead H., Will G., North A. C. T. (1960) Structure of haemoglobin: a three-dimensional Fourier synthesis at 5.5-Å resolution, obtained by x-ray analysis. Nature 185, 416–422 [DOI] [PubMed] [Google Scholar]
- 20. Kendrew J. C. (1959) Structure and function in myoglobin and other proteins. Fed. Proc. 18, 740–751 [PubMed] [Google Scholar]
- 21. Changeux J.-P. (2012) Allostery and the Monod-Wyman-Changeux model after 50 years. Annu. Rev. Biophys. 41, 103–133 [DOI] [PubMed] [Google Scholar]
- 22. Perutz M. F. (1963) X-ray analysis of hemoglobin. Science 140, 863–869 [DOI] [PubMed] [Google Scholar]
- 23. Muirhead H., Perutz M. F. (1963) Structure of haemoglobin. A three-dimensional Fourier synthesis of reduced human haemoglobin at 5–5 Å resolution. Nature 199, 633–638 [DOI] [PubMed] [Google Scholar]
- 24. Wyman J., Jr. (1948) Heme Proteins. Adv. Protein Chem. 4, 407–531 [DOI] [PubMed] [Google Scholar]
- 25. Changeux J.-P. (2013) The origins of allostery: from personal memories to material for the future. J. Mol. Biol. 425, 1396–1406 [DOI] [PubMed] [Google Scholar]
- 26. Boehr D. D., Nussinov R., Wright P. E. (2009) The role of dynamic conformational ensembles in biomolecular recognition. Nat. Chem. Biol. 5, 789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deupi X., Kobilka B. K. (2010) Energy landscapes as a tool to integrate GPCR structure, dynamics, and function. Physiology 25, 293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hammes G. G., Chang Y. C., Oas T. G. (2009) Conformational selection or induced fit: a flux description of reaction mechanism. Proc. Natl. Acad. Sci. U.S.A. 106, 13737–13741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cui Q., Karplus M. (2008) Allostery and cooperativity revisited. Protein Sci. 17, 1295–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pauling L. (1935) The oxygen equilibrium of hemoglobin and its structural interpretation. Proc. Natl. Acad. Sci. U.S.A. 21, 186–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koshland D. E., Jr., Némethy G., Filmer D. (1966) Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry 5, 365–385 [DOI] [PubMed] [Google Scholar]
- 32. Rubin M. M., Changeux J.-P. (1966) On the nature of allosteric transitions: implications of non-exclusive ligand binding. J. Mol. Biol. 21, 265–274 [DOI] [PubMed] [Google Scholar]
- 33. Gerhart J. C., Pardee A. B. (1964) Aspartate transcarbamylase, an enzyme designed for feedback inhibition. Fed. Proc. 23, 727–735 [PubMed] [Google Scholar]
- 34. Changeux J.-P., Gerhart J. C., Schachman H. K. (1968) Allosteric interactions in aspartate transcarbamylase. I. Binding of specific ligands to the native enzyme and its isolated subunits. Biochemistry 7, 531–538 [DOI] [PubMed] [Google Scholar]
- 35. Gerhart J. C., Schachman H. K. (1968) Allosteric interactions in aspartate transcarbamylase. II. Evidence for different conformational states of the protein in the presence and absence of specific ligands. Biochemistry 7, 538–552 [DOI] [PubMed] [Google Scholar]
- 36. Changeux J.-P., Rubin M. M. (1968) Allosteric interactions in aspartate transcarbamylase. III. Interpretations of experimental data in terms of the model of Monod, Wyman, and Changeux. Biochemistry 7, 553–561 [DOI] [PubMed] [Google Scholar]
- 37. Blangy D., Buc H., Monod J. (1968) Kinetics of the allosteric interactions of phosphofructokinase from Escherichia coli. J. Mol. Biol. 31, 13–35 [DOI] [PubMed] [Google Scholar]
- 38. Perutz M. F., Wilkinson A. J., Paoli M., Dodson G. G. (1998) The stereochemical mechanism of the cooperative effects in hemoglobin revisited. Annu. Rev. Biophys. Biomol. Struct. 27, 1–34 [DOI] [PubMed] [Google Scholar]
- 39. Edelstein S. J. (1971) Extensions of the allosteric model for haemoglobin. Nature 230, 224–227 [DOI] [PubMed] [Google Scholar]
- 40. Cammarata M., Levantino M., Wulff M., Cupane A. (2010) Unveiling the timescale of the R-T transition in human hemoglobin. J. Mol. Biol. 400, 951–962 [DOI] [PubMed] [Google Scholar]
- 41. Fischer S., Olsen K. W., Nam K., Karplus M. (2011) Unsuspected pathway of the allosteric transition in hemoglobin. Proc. Natl. Acad. Sci. U.S.A. 108, 5608–5613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Levantino M., Spilotros A., Cammarata M., Schirò G., Ardiccioni C., Vallone B., Brunori M., Cupane A. (2012) The Monod-Wyman-Changeux allosteric model accounts for the quaternary transition dynamics in wild type and a recombinant mutant human hemoglobin. Proc. Natl. Acad. Sci. U.S.A. 109, 14894–14899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tekpinar M., Zheng W. (2013) Coarse-grained and all-atom modeling of structural states and transitions in hemoglobin. Proteins 81, 240–252 [DOI] [PubMed] [Google Scholar]
- 44. Lewis M. (2005) The lac repressor. C. R. Biol. 328, 521–548 [DOI] [PubMed] [Google Scholar]
- 45. Lewis M. (2011) A tale of two repressors. J. Mol. Biol. 409, 14–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gronemeyer H., Gustafsson J. A., Laudet V. (2004) Principles for modulation of the nuclear receptor superfamily. Nat. Rev. Drug Discov. 3, 950–964 [DOI] [PubMed] [Google Scholar]
- 47. Chandra V., Huang P., Hamuro Y., Raghuram S., Wang Y., Burris T. P., Rastinejad F. (2008) Structure of the intact PPAR-γ-RXR-α nuclear receptor complex on DNA. Nature 456, 350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang P., Chandra V., Rastinejad F. (2010) Structural overview of the nuclear receptor superfamily: insights into physiology and therapeutics. Annu. Rev. Physiol. 72, 247–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Corringer P. J., Poitevin F., Prevost M. S., Sauguet L., Delarue M., Changeux J.-P. (2012) Structure and pharmacology of pentameric receptor channels: from bacteria to brain. Structure 20, 941–956 [DOI] [PubMed] [Google Scholar]
- 50. Canals M., Lane JR, Wen A., Scammells P. J., Sexton P. M., Christopoulos A. (2012) A Monod-Wyman-Changeux mechanism can explain G protein-coupled receptor (GPCR) allosteric modulation. J. Biol. Chem. 287, 650–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Prevost M. S., Sauguet L., Nury H., Van Renterghem C., Huon C., Poitevin F., Baaden M., Delarue M., Corringer P. J. (2012) A locally closed conformation of a bacterial pentameric proton-gated ion channel. Nat. Struct Mol. Biol. 19, 642–649 [DOI] [PubMed] [Google Scholar]
- 52. Nygaard R., Zou Y., Dror R. O., Mildorf T. J., Arlow D. H., Manglik A., Pan A. C., Liu C. W., Fung J. J., Bokoch M. P., Thian F. S., Kobilka T. S., Shaw D. E., Mueller L., Prosser R. S., Kobilka B. K. (2013) The dynamic process of β2-adrenergic receptor activation. Cell 152, 532–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Novick A., Szilard L. (1954) Dynamics of Growth Processes, pp. 21–32, Princeton University Press, Princeton, NJ [Google Scholar]
- 54. Liu Y.-Y., Slotine J.-J., Barabási A.-L. (2013) Observability of complex systems. Proc. Natl. Acad. Sci. U.S.A. 110, 2460–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Changeux J.-P. (1966) Responses of acetylcholinesterase from Torpedo marmorata to salts and curarizing drugs. Mol. Pharmacol. 2, 369–392 [PubMed] [Google Scholar]
- 56. Changeux J.-P. (1969) Symmetry and cooperative properties of biological membranes. in Nobel Symposium 11: Symmetry and Function of Biological Systems at the Macromolecular Level (Engström A., Strandberg B., eds) pp. 235–256, Almqvist & Wiksell, Stockholm [Google Scholar]
- 57. Changeux J.-P., Thiéry J., Tung Y., Kittel C. (1967) On the cooperativity of biological membranes. Proc. Natl. Acad. Sci. U.S.A. 57, 335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Duke T. A., Bray D. (1999) Heightened sensitivity of a lattice of membrane receptors. Proc. Natl. Acad. Sci. U.S.A. 96, 10104–10108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shimizu T. S., Le Novère N., Levin M. D., Beavil A. J., Sutton B. J., Bray D. (2000) Molecular model of a lattice of signalling proteins involved in bacterial chemotaxis. Nat. Cell Biol. 2, 792–796 [DOI] [PubMed] [Google Scholar]
- 60. Gurevich V. V, Gurevich E. V. (2008) How and why do GCPRs dimerize? Trends Pharmacol. Sci. 29, 234–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Breitwieser G. E. (2004) G protein-coupled receptor oligomerization: implications for G protein activation and cell signaling. Circ. Res. 94, 17–27 [DOI] [PubMed] [Google Scholar]
- 62. Pin J.-P., Comps-Agrar L., Maurel D., Monnier C., Rives M. L., Trinquet E., Kniazeff J., Rondard P., Prézeau L. (2009) G-protein-coupled receptor oligomers: two or more for what? Lessons from mGlu and GABAB receptors. J. Physiol. 587, 5337–5344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ferré S., Fredholm B. B., Morelli M., Popoli P., Fuxe K. (1997) Adenosine dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 20, 482–487 [DOI] [PubMed] [Google Scholar]
- 64. Agnati L. F., Fuxe K., Zini I., Lenzi P., Hökfelt T. (1980) Aspects on receptor regulation and isoreceptor identification. Med. Biol. 58, 182–187 [PubMed] [Google Scholar]
- 65. Ferré S., Baler R., Bouvier M., Caron M. G., Devi L. A., Durroux T., Fuxe K., George S. R., Javitch J. A., Lohse M. J., Mackie K., Milligan G., Pfleger K. D. G., Pin J.-P., Volkow N. D., Waldhoer M., Woods A. S., Franco R. (2009) Building a new conceptual framework for receptor heteromers. Nat. Chem. Biol. 5, 131–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fuxe K., Canals M., Torvinen M., Marcellino D., Terasmaa A., Genedani S., Leo G., Guidolin D., Diaz-Cabiale Z., Rivera A., Lundstrom L., Langel U., Narvaez J., Tanganelli S., Lluis C., Ferré S., Woods A., Franco R., Agnati L. F. (2007) Intramembrane receptor-receptor interactions: a novel principle in molecular medicine. J. Neural Transm. 114, 49–75 [DOI] [PubMed] [Google Scholar]
- 67. Horwich A. L., Fenton W. A., Chapman E., Farr G. W. (2007) Two families of chaperonin: physiology and mechanism. Annu. Rev. Cell Dev. Biol. 23, 115–145 [DOI] [PubMed] [Google Scholar]
- 68. Bai F., Branch R. W., Nicolau D. V., Jr., Pilizota T., Steel B. C., Maini P. K., Berry R. M. (2010) Conformational spread as a mechanism for cooperativity in the bacterial flagellar switch. Science 327, 685–689 [DOI] [PubMed] [Google Scholar]
- 69. Garcia H. G., Kondev J., Orme N., Theriot J. A., Phillips R. (2011) Thermodynamics of biological processes. Methods Enzymol. 492, 27–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Langley J. N. (1905) On the reaction of cells and of nerve-endings to certain poisons, chiefly as regards the reaction of striated muscle to nicotine and to curari. J. Physiol. 33, 374–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dale H. H. (1942) Wartime arrangements for international biological standards. Br. Med. J. 2, 385–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Changeux J.-P. (2012) The nicotinic acetylcholine receptor: the founding father of the pentameric ligand-gated ion channel superfamily. J. Biol. Chem. 287, 40207–40215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Changeux J.-P. (1965) On the allosteric properties of biosynthetic l-threonine deaminase. VI. General discussion. Bull. Soc. Chim. Biol. 47, 281–300 [PubMed] [Google Scholar]
- 74. Morel N., Bon S., Greenblatt H. M., Van Belle D., Wodak S. J., Sussman J. L., Massoulié J., Silman I. (1999) Effect of mutations within the peripheral anionic site on the stability of acetylcholinesterase. Mol. Pharmacol. 55, 982–992 [DOI] [PubMed] [Google Scholar]
- 75. Rosenberry T. L., Johnson J. L., Cusack B., Thomas J. L., Emani S., Venkatasubban K. S. (2005) Interactions between the peripheral site and the acylation site in acetylcholinesterase. Chem. Biol. Interact. 157–158, 181–189 [DOI] [PubMed] [Google Scholar]
- 76. Silman I., Sussman J. L. (2008) Acetylcholinesterase: how is structure related to function? Chem. Biol. Interact. 175, 3–10 [DOI] [PubMed] [Google Scholar]
- 77. Schoffeniels E., Nachmansohn D. (1957) An isolated single electroplax preparation. I. New data on the effect of acetylcholine and related compounds. Biochim. Biophys. Acta 26, 1–15 [DOI] [PubMed] [Google Scholar]
- 78. Kasai M., Changeux J.-P. (1970) Demonstration of the excitation by cholinergic agonists from fractions of purified membranes, in vitro. C. R. Acad. Sci. Hebd. Seances Acad. Sci. D 270, 1400–1403 [PubMed] [Google Scholar]
- 79. Kasai M., Changeux J.-P. (1971) In vitro excitation of purified membrane fragments by cholinergic agonists. J. Memb. Biol. 6, 1–23 [DOI] [PubMed] [Google Scholar]
- 80. Gilbert W., Müller-Hill B. (1966) Isolation of the lac repressor. Proc. Natl. Acad. Sci. U.S.A. 56, 1891–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lee C.-Y., Chang C. (1966) Modes of actions of purified toxins from elapid venoms on neuromuscular transmission. Mem. Inst. Butantan 33, 555–572 [PubMed] [Google Scholar]
- 82. Changeux J.-P., Kasai M., Lee C.-Y. (1970) Use of a snake venom toxin to characterize the cholinergic receptor protein. Proc. Natl. Acad. Sci. U.S.A. 67, 1241–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Olsen R. W., Meunier J.-C., Changeux J.-P. (1972) Progress in purification of the cholinergic receptor protein from Electrophorus electricus by affinity chromatography. FEBS Lett. 28, 96–100 [DOI] [PubMed] [Google Scholar]
- 84. Cartaud J., Benedetti E. L., Cohen J. B., Meunier J.-C., Changeux J.-P. (1973) Presence of a lattice structure in membrane fragments rich in nicotinic receptor protein from the electric organ of Torpedo marmorata. FEBS Lett. 33, 109–113 [DOI] [PubMed] [Google Scholar]
- 85. Nickel E., Potter L. T. (1973) Ultrastructure of isolated membranes of Torpedo electric tissue. Brain Res. 57, 508–517 [DOI] [PubMed] [Google Scholar]
- 86. Cartaud J., Bon S., Massoulié J. (1978) Electrophorus acetylcholinesterase. Biochemical and electron microscope characterization of low ionic strength aggregates. J. Cell Biol. 7, 315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cartaud J., Sobel A., Rousselet A., Devaux P. F., Changeux J.-P. (1981) Consequences of alkaline treatment for the ultrastructure of the acetylcholine-receptor-rich membranes from Torpedo marmorata electric organ. J. Cell Biol. 90, 418–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ross M. J., Klymkowsky M. W., Agard D. A., Stroud R. M. (1977) Structural studies of a membrane-bound acetylcholine receptor from Torpedo californica. J. Mol. Biol. 116, 635–659 [DOI] [PubMed] [Google Scholar]
- 89. Kistler J., Stroud R. M., Klymkowsky M. W., Lalancette R. A., Fairclough R. H. (1982) Structure and function of an acetylcholine receptor. Biophys. J. 37, 371–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kistler J., Stroud R. M. (1981) Crystalline arrays of membrane-bound acetylcholine receptor. Proc. Natl. Acad. Sci. U.S.A. 78, 3678–3682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bon F., Lebrun E., Gomel J., van Rapenbusch R., Cartaud J., Popot J. L., Changeux J.-P. (1982) Relative orientation of the two oligomers composing the heavy form of the acetylcholine receptor from Torpedo marmorata. C. R. Seances Acad. Sci. III 295, 199–204 [PubMed] [Google Scholar]
- 92. Bon F., Lebrun E., Gomel J., Van Rapenbusch R., Cartaud J., Popot J. L., Changeux J.-P. (1984) Image analysis of the heavy form of the acetylcholine receptor from Torpedo marmorata. J. Mol. Biol. 176, 205–237 [DOI] [PubMed] [Google Scholar]
- 93. Brisson A., Unwin P. N. (1985) Quaternary structure of the acetylcholine receptor. Nature 315, 474–477 [DOI] [PubMed] [Google Scholar]
- 94. Unwin N. (2005) Refined structure of the nicotinic acetylcholine receptor at 4 Å resolution. J. Mol. Biol. 346, 967–989 [DOI] [PubMed] [Google Scholar]
- 95. Hucho F., Changeux J.-P. (1973) Molecular weight and quaternary structure of the cholinergic receptor protein extracted by detergents from Electrophorus electricus electric tissue. FEBS Lett. 38, 11–15 [DOI] [PubMed] [Google Scholar]
- 96. Weill C. L., McNamee M. G., Karlin A. (1974) Affinity labeling of purified acetylcholine receptor from Torpedo californica. Biochem. Biophys. Res. Commun. 61, 997–1003 [DOI] [PubMed] [Google Scholar]
- 97. Karlin A. (1980) Molecular properties of nicotinic acetylcholine receptors. in Cell Surface Reviews (Poste G., Nicolson G. L, Cotman C. W., eds) pp. 191–260, Elsevier Science Ltd., Amsterdam [Google Scholar]
- 98. Raftery M. A., Vandlen R., Michaelson D., Bode J., Moody T., Chao Y., Reed K., Deutsch J., Duguid J. (1974) The biochemistry of an acetylcholine receptor. J. Supramol. Struct. 2, 582–592 [DOI] [PubMed] [Google Scholar]
- 99. Raftery M. A., Hunkapiller M. W., Strader C. D., Hood L. E. (1980) Acetylcholine receptor: complex of homologous subunits. Science 208, 1454–1456 [DOI] [PubMed] [Google Scholar]
- 100. Lindstrom J., Merlie J., Yogeeswaran G. (1979) Biochemical properties of acetylcholine receptor subunits from Torpedo californica. Biochemistry 18, 4465–4470 [DOI] [PubMed] [Google Scholar]
- 101. Devillers-Thiery A., Changeux J.-P., Paroutaud P., Strosberg A. D. (1979) The amino-terminal sequence of the 40,000 molecular weight subunit of the acetylcholine receptor protein from Torpedo marmorata. FEBS Lett. 104, 99–105 [DOI] [PubMed] [Google Scholar]
- 102. Hunkapiller M. W., Strader C. D., Hood L., Raftery M. A. (1979) Amino terminal amino acid sequence of the major polypeptide subunit of Torpedo californica acetylcholine receptor. Biochem. Biophys. Res. Commun. 91, 164–169 [DOI] [PubMed] [Google Scholar]
- 103. Noda M., Takahashi H., Tanabe T., Toyosato M., Furutani Y., Hirose T., Asai M., Inayama S., Miyata T., Numa S. (1982) Primary structure of α-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature 299, 793–797 [DOI] [PubMed] [Google Scholar]
- 104. Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Furutani Y., Hirose T., Takashima H., Inayama S., Miyata T., Numa S. (1983) Structural homology of Torpedo californica acetylcholine receptor subunits. Nature 302, 528–532 [DOI] [PubMed] [Google Scholar]
- 105. Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Hirose T., Asai M., Takashima H., Inayama S., Miyata T., Numa S. (1983) Primary structures of β- and γ-subunit precursors of Torpedo californica acetylcholine receptor deduced from cDNA sequences. Nature 301, 251–255 [DOI] [PubMed] [Google Scholar]
- 106. Claudio T., Ballivet M., Patrick J., Heinemann S. (1983) Nucleotide and deduced amino acid sequences of Torpedo californica acetylcholine receptor γ-subunit. Proc. Natl. Acad. Sci. U.S.A. 80, 1111–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sumikawa K., Houghton M., Smith J. C., Bell L., Richards B. M., Barnard E. A. (1982) The molecular cloning and characterisation of cDNA coding for the α-subunit of the acetylcholine receptor. Nucleic Acids Res. 10, 5809–5822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Devillers-Thiéry A., Giraudat J., Bentaboulet M., Changeux J.-P. (1983) Complete mRNA coding sequence of the acetylcholine binding α-subunit of Torpedo marmorata acetylcholine receptor: a model for the transmembrane organization of the polypeptide chain. Proc. Natl. Acad. Sci. U.S.A. 80, 2067–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Boulter J., Evans K., Goldman D., Martin G., Treco D., Heinemann S., Patrick J. (1986) Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor α-subunit. Nature 319, 368–374 [DOI] [PubMed] [Google Scholar]
- 110. Boulter J., Evans K., Martin G., Mason P., Stengelin S., Goldman D., Heinemann S., Patrick J. (1986) Isolation and sequence of cDNA clones coding for the precursor to the γ subunit of mouse muscle nicotinic acetylcholine receptor. J. Neurosci. Res. 16, 37–49 [DOI] [PubMed] [Google Scholar]
- 111. Boulter J., O'Shea-Greenfield A., Duvoisin R. M., Connolly J. G., Wada E., Jensen A., Gardner P. D., Ballivet M., Deneris E. S., McKinnon D. (1990) α3, α5, and β4: three members of the rat neuronal nicotinic acetylcholine receptor-related gene family form a gene cluster. J. Biol. Chem. 265, 4472–4482 [PubMed] [Google Scholar]
- 112. Couturier S., Erkman L., Valera S., Rungger D., Bertrand S., Boulter J., Ballivet M., Bertrand D. (1990) α5, α3, and non-α3: three clustered avian genes encoding neuronal nicotinic acetylcholine receptor-related subunits. J. Biol. Chem. 265, 17560–17567 [PubMed] [Google Scholar]
- 113. Couturier S., Bertrand D., Matter J.-M., Hernandez M.-C., Bertrand S., Millar N., Valera S., Barkas T., Ballivet M. (1990) A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-Btx. Neuron 5, 847–856 [DOI] [PubMed] [Google Scholar]
- 114. Kao P. N., Dwork A. J., Kaldany R. R., Silver M. L., Wideman J., Stein S., Karlin A. (1984) Identification of the α-subunit half-cystine specifically labeled by an affinity reagent for the acetylcholine receptor binding site. J. Biol. Chem. 259, 11662–11665 [PubMed] [Google Scholar]
- 115. Langenbuch-Cachat J., Bon C., Mulle C., Goeldner M., Hirth C., Changeux J.-P. (1988) Photoaffinity labeling of the acetylcholine binding sites on the nicotinic receptor by an aryldiazonium derivative. Biochemistry 27, 2337–2345 [DOI] [PubMed] [Google Scholar]
- 116. Dennis M., Giraudat J., Kotzyba-Hibert F., Goeldner M., Hirth C., Chang J.-Y., Changeux J.-P. (1986) A photoaffinity ligand of the acetylcholine-binding site predominantly labels the region 179–207 of the α-subunit on native acetylcholine receptor from Torpedo marmorata. FEBS Lett. 207, 243–249 [Google Scholar]
- 117. Dennis M., Giraudat J., Kotzyba-Hibert F., Goeldner M., Hirth C., Chang J.-Y., Lazure C., Chrétien M., Changeux J.-P. (1988) Amino acids of the Torpedo marmorata acetylcholine receptor subunit labeled by a photoaffinity ligand for the acetylcholine binding site. Biochemistry 27, 2346–2357 [DOI] [PubMed] [Google Scholar]
- 118. Galzi J.-L., Revah F., Black D., Goeldner M., Hirth C., Changeux J.-P. (1990) Identification of a novel amino acid α-tyrosine 93 within the cholinergic ligands-binding sites of the acetylcholine receptor by photoaffinity labeling. Additional evidence for a three-loop model of the cholinergic ligands-binding sites. J. Biol. Chem. 265, 10430–10437 [PubMed] [Google Scholar]
- 119. Giraudat J., Dennis M., Heidmann T., Chang J.-Y., Changeux J.-P. (1986) Structure of the high affinity site for noncompetitive blockers of the acetylcholine receptor: serine 262 of the δ-subunit is labeled by [3H]chlorpromazine. Proc. Natl. Acad. Sci. U.S.A. 83, 2719–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Giraudat J., Dennis M., Heidmann T., Haumont P. Y., Lederer F., Changeux J.-P. (1987) Structure of the high affinity binding site for noncompetitive blockers of the acetylcholine receptor: [3H]chlorpromazine labels homologous residues in the β and δ chains. Biochemistry 26, 2410–2418 [DOI] [PubMed] [Google Scholar]
- 121. Hucho F., Oberthür W., Lottspeich F. (1986) The ion channel of the nicotinic acetylcholine receptor is formed by the homologous helices M II of the receptor subunits. FEBS Lett. 205, 137–142 [DOI] [PubMed] [Google Scholar]
- 122. Imoto K., Busch C., Sakmann B., Mishina M., Konno T., Nakai J., Bujo H., Mori Y., Fukuda K., Numa S. (1988) Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature 335, 645–648 [DOI] [PubMed] [Google Scholar]
- 123. Changeux J.-P. (1990) The TiPS lecture. The nicotinic acetylcholine receptor: an allosteric protein prototype of ligand-gated ion channels. Trends Pharmacol. Sci. 11, 485–492 [DOI] [PubMed] [Google Scholar]
- 124. Changeux J.-P., Edelstein S. (2005) Nicotinic Acetylcholine Receptors: From Molecular Biology to Cognition, Odile Jacob, New York [Google Scholar]
- 125. Heidmann T., Changeux J.-P. (1979) Fast kinetic studies on the interaction of a fluorescent agonist with the membrane-bound acetylcholine receptor from Torpedo marmorata. Eur. J. Biochem. 94, 255–279 [DOI] [PubMed] [Google Scholar]
- 126. Heidmann T., Changeux J.-P. (1980) Interaction of a fluorescent agonist with the membrane-bound acetylcholine receptor from Torpedo marmorata in the millisecond time range: resolution of an “intermediate” conformational transition and evidence for positive cooperative effects. Biochem. Biophys. Res. Commun. 97, 889–896 [DOI] [PubMed] [Google Scholar]
- 127. Neubig R. R., Cohen J. B. (1980) Permeability control by cholinergic receptors in Torpedo postsynaptic membranes: agonist dose-response relations measured at second and millisecond times. Biochemistry 19, 2770–2779 [DOI] [PubMed] [Google Scholar]
- 128. Edelstein S. J., Schaad O., Henry E., Bertrand D., Changeux J.-P. (1996) A kinetic mechanism for nicotinic acetylcholine receptors based on multiple allosteric transitions. Biol. Cybern. 75, 361–379 [DOI] [PubMed] [Google Scholar]
- 129. Edelstein S. J., Schaad O., Changeux J.-P. (1997) Myasthenic nicotinic receptor mutant interpreted in terms of the allosteric model. C. R. Acad. Sci. III 320, 953–961 [DOI] [PubMed] [Google Scholar]
- 130. Edelstein S. J., Schaad O., Changeux J.-P. (1997) Single binding versus single channel recordings: a new approach to study ionotropic receptors. Biochemistry 36, 13755–13760 [DOI] [PubMed] [Google Scholar]
- 131. Purohit P., Auerbach A. (2009) Unliganded gating of acetylcholine receptor channels. Proc. Natl. Acad. Sci. U.S.A. 106, 115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Revah F., Bertrand D., Galzi J.-L., Devillers-Thiéry A., Mulle C., Hussy N., Bertrand S., Ballivet M., Changeux J.-P. (1991) Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature 353, 846–849 [DOI] [PubMed] [Google Scholar]
- 133. Ohno K., Engel A. G. (1998) Congenital myasthenic syndromes: gene mutation. Neuromuscul. Disord. 8, XII–XIII [PubMed] [Google Scholar]
- 134. Steinlein O. K., Mulley J. C., Propping P., Wallace R. H., Phillips H. A., Sutherland G. R., Scheffer I. E., Berkovic S. F. (1995) A missense mutation in the neuronal nicotinic acetylcholine receptor α4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat. Genet. 11, 201–203 [DOI] [PubMed] [Google Scholar]
- 135. Jadey S. V., Purohit P., Bruhova I., Gregg T. M., Auerbach A. (2011) Design and control of acetylcholine receptor conformational change. Proc. Natl. Acad. Sci. U.S.A. 108, 4328–4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Auerbach A. (2012) Thinking in cycles: MWC is a good model for acetylcholine receptor-channels. J. Physiol. 590, 93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Steinlein O. K. (2010) Animal models for autosomal dominant frontal lobe epilepsy: on the origin of seizures. Expert Rev. Neurother. 10, 1859–1867 [DOI] [PubMed] [Google Scholar]
- 138. Tasneem A., Iyer L. M., Jakobsson E., Aravind L. (2005) Identification of the prokaryotic ligand-gated ion channels and their implications for the mechanisms and origins of animal Cys-loop ion channels. Genome Biol. 6, R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Bocquet N., Prado de Carvalho L., Cartaud J., Neyton J., Le Poupon C., Taly A., Grutter T., Changeux J.-P., Corringer P. J. (2007) A prokaryotic proton-gated ion channel from the nicotinic acetylcholine receptor family. Nature 445, 116–119 [DOI] [PubMed] [Google Scholar]
- 140. Bocquet N., Nury H., Baaden M., Le Poupon C., Changeux J.-P., Delarue M., Corringer P. J. (2009) X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 457, 111–114 [DOI] [PubMed] [Google Scholar]
- 141. Hilf R. J., Dutzler R. (2008) X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature 452, 375–379 [DOI] [PubMed] [Google Scholar]
- 142. Hilf R. J., Dutzler R. (2009) A prokaryotic perspective on pentameric ligand-gated ion channel structure. Curr. Opin. Struct. Biol. 19, 418–424 [DOI] [PubMed] [Google Scholar]
- 143. Hibbs R. E., Gouaux E. (2011) Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 474, 54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Taly A., Delarue M., Grutter T., Nilges M., Le Novère N., Corringer P. J., Changeux J.-P. (2005) Normal mode analysis suggests a quaternary twist model for the nicotinic receptor gating mechanism. Biophys. J. 88, 3954–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Taly A., Corringer P. J., Grutter T., Prado de Carvalho L., Karplus M., Changeux J.-P. (2006) Implications of the quaternary twist allosteric model for the physiology and pathology of nicotinic acetylcholine receptors. Proc. Natl. Acad. Sci. U.S.A. 103, 16965–16970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Le Novère N., Grutter T., Changeux J.-P. (2002) Models of the extracellular domain of the nicotinic receptors and of agonist- and Ca2+-binding sites. Proc. Natl. Acad. Sci. U.S.A. 99, 3210–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Heidmann T., Bernhardt J., Neumann E., Changeux J.-P. (1983) Rapid kinetics of agonist binding and permeability response analyzed in parallel on acetylcholine receptor-rich membranes from Torpedo marmorata. Biochemistry 22, 5452–5459 [DOI] [PubMed] [Google Scholar]
- 148. Pfeiffer F., Graham D., Betz H. (1982) Purification by affinity chromatography of the glycine receptor of rat spinal cord. J. Biol. Chem. 257, 9389–9393 [PubMed] [Google Scholar]
- 149. Sigel E., Stephenson F. A., Mamalaki C., Barnard E. A. (1983) γ-Aminobutyric acid/benzodiazepine receptor complex of bovine cerebral cortex. J. Biol. Chem. 258, 6965–6971 [PubMed] [Google Scholar]
- 150. Stephenson F. A., Mukhopadhyay R. (2012) How the glycine and GABA receptors were purified. J. Biol. Chem. 287, 40835–40837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Levitzki A., Atlas D., Steer M. L. (1974) The binding characteristics and number of β-adrenergic receptors on the turkey erythrocyte. Proc. Natl. Acad. Sci. U.S.A. 71, 2773–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Bartfai T., Anner J., Schultzberg M., Montelius J. (1974) Partial purification and characterization of a muscarinic acetylcholine receptor from rat cerebral cortex. Biochem. Biophys. Res. Commun. 59, 725–733 [DOI] [PubMed] [Google Scholar]
- 153. Cherezov V., Rosenbaum D. M., Hanson M. A., Rasmussen S. G., Thian F. S., Kobilka T. S., Choi H. J., Kuhn P., Weis W. I., Kobilka B. K., Stevens R. C. (2007) High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science 318, 1258–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kato H. E., Zhang F., Yizhar O., Ramakrishnan C., Nishizawa T., Hirata K., Ito J., Aita Y., Tsukazaki T., Hayashi S., Hegemann P., Maturana A. D., Ishitani R., Deisseroth K., Nureki O. (2012) Crystal structure of the channelrhodopsin light-gated cation channel. Nature 482, 369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Nury H., Poitevin F., Van Renterghem C., Changeux J.-P., Corringer P. J., Delarue M., Baaden M. (2010) One-microsecond molecular dynamics simulation of channel gating in a nicotinic receptor homologue. Proc. Natl. Acad. Sci. U.S.A. 107, 6275–6280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Xu Y., Colletier J. P., Weik M., Jiang H., Moult J., Silman I., Sussman J. L. (2008) Flexibility of aromatic residues in the active-site gorge of acetylcholinesterase: X-ray versus molecular dynamics. Biophys. J. 95, 2500–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Changeux J.-P., Edelstein S. (2011) Conformational selection or induced fit? Fifty years of debate resolved. F1000 Biol. Rep. 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kenakin T., Miller L. J. (2010) Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol. Rev. 62, 265–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Melancon B. J., Hopkins C. R., Wood M. R., Emmitte K. A., Niswender C. M., Christopoulos A., Conn P. J., Lindsley C. W. (2012) Allosteric modulation of seven transmembrane spanning receptors: theory, practice, and opportunities for central nervous system drug discovery. J. Med. Chem. 55, 1445–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Weber M., Changeux J.-P. (1974) Binding of Naja nigricollis [3H]α-toxin to membrane fragments from Electrophorus and Torpedo electric organs. III. Effects of local anaesthetics on the binding of the tritiated α-neurotoxin. Mol. Pharmacol. 10, 35–40 [PubMed] [Google Scholar]
- 161. Cohen J. B., Weber M., Changeux J.-P. (1974) Effects of local anesthetics and calcium on the interaction of cholinergic ligands with the nicotinic receptor protein from Torpedo marmorata. Mol. Pharmacol. 10, 904–932 [PubMed] [Google Scholar]
- 162. Grünhagen H. H., Changeux J.-P. (1976) Studies on the electrogenic action of acetylcholine with Torpedo marmorata electric organ. Quinacrine: a fluorescent probe for the conformational transitions of the cholinergic receptor protein in its membrane bound state. J. Mol. Biol. 106, 497–516 [DOI] [PubMed] [Google Scholar]
- 163. Mulle C., Léna C., Changeux J.-P. (1992) Potentiation of nicotinic receptor response by external calcium in rat central neurons. Neuron 8, 937–945 [DOI] [PubMed] [Google Scholar]
- 164. Vernino S., Amador M., Luetje C. W., Patrick J., Dani J. A. (1992) Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron 8, 127–134 [DOI] [PubMed] [Google Scholar]
- 165. Galzi J.-L., Bertrand S., Corringer P. J., Changeux J.-P., Bertrand D. (1996) Identification of calcium binding sites that regulate potentiation of a neuronal nicotinic acetylcholine receptor. EMBO J. 15, 5824–5832 [PMC free article] [PubMed] [Google Scholar]
- 166. Krause R. M., Buisson B., Bertrand S., Corringer P. J., Galzi J.-L., Changeux J.-P., Bertrand D. (1998) Ivermectin: a positive allosteric effector of the α7 neuronal nicotinic acetylcholine receptor. Mol. Pharmacol. 53, 283–294 [DOI] [PubMed] [Google Scholar]
- 167. Bertrand D., Gopalakrishnan M. (2007) Allosteric modulation of nicotinic acetylcholine receptors. Biochem. Pharmacol. 74, 1155–1163 [DOI] [PubMed] [Google Scholar]
- 168. Gill J. K., Chatzidaki A., Ursu D., Sher E., Millar N. S. (2013) Contrasting properties of α7-selective orthosteric and allosteric agonists examined on native nicotinic acetylcholine receptors. PLoS ONE 8, e55047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Nury H., Van Renterghem C., Weng Y., Tran A., Baaden M., Dufresne V., Changeux J.-P., Sonner J. M., Delarue M., Corringer P. J. (2011) X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature 469, 428–431 [DOI] [PubMed] [Google Scholar]
- 170. Murail S., Wallner B., Trudell J. R., Bertaccini E., Lindahl E. (2011) Microsecond simulations indicate that ethanol binds between subunits and could stabilize an open-state model of a glycine receptor. Biophys. J. 100, 1642–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Sauguet L., Howard R. J., Malherbe L., Lee U. S., Corringer P. J., Harris R. A., Delarue M. (2013) Structural basis for potentiation by alcohols and anaesthetics in a ligand-gated ion channel. Nat. Commun. 4, 1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Li G.-D., Chiara D. C., Cohen J. B., Olsen R. W. (2010) Numerous classes of general anesthetics inhibit etomidate binding to γ-aminobutyric acid type A (GABAA) receptors. J. Biol. Chem. 285, 8615–8620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Forman S. A. (2012) Monod-Wyman-Changeux allosteric mechanisms of action and the pharmacology of etomidate. Curr. Opin. Anaesthesiol. 25, 411–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Forman S. A. (2013) A paradigm shift from biophysical to neurobiological: the fading influence of Claude Bernard's ideas about general anesthesia. Anesthesiology 118, 984–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Hansen S. B., Taylor P. (2007) Galanthamine and non-competitive inhibitor binding to ACh-binding protein: evidence for a binding site on non-α-subunit interfaces of heteromeric neuronal nicotinic receptors. J. Mol. Biol. 369, 895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Nemecz Á., Taylor P. (2011) Creating an α7 nicotinic acetylcholine recognition domain from the acetylcholine-binding protein. Crystallographic and ligand selectivity analyses. J. Biol. Chem. 286, 42555–42565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Hamouda A. K., Kimm T., Cohen J. B. (2013) Physostigmine and galanthamine bind in the presence of agonist at the canonical and noncanonical subunit interfaces of a nicotinic acetylcholine receptor. J. Neurosci. 33, 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Galzi J.-L., Changeux J.-P. (1994) Neurotransmitter-gated ion channels as unconventional allosteric proteins. Curr. Opin. Cell Biol. 4, 554–565 [Google Scholar]
- 179. Triller A., Choquet D. (2008) New concepts in synaptic biology derived from single-molecule imaging. Neuron 59, 359–374 [DOI] [PubMed] [Google Scholar]
- 180. Zhang H., Etherington L. A., Hafner A. S., Belelli D., Coussen F., Delagrange P., Chaouloff F., Spedding M., Lambert J. J., Choquet D., Groc L. (2013) Regulation of AMPA receptor surface trafficking and synaptic plasticity by a cognitive enhancer and antidepressant molecule. Mol. Psychiatry 18, 471–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Changeux J.-P. (1972) The brain and the event. in Communications, pp. 18–37, Ecole des Hautes Etudes en Sciences Sociales, Paris [Google Scholar]
- 182. Changeux J.-P., Courrège P., Danchin A. (1973) A theory of the epigenesis of neuronal networks by selective stabilization of synapses. Proc. Natl. Acad. Sci. U.S.A. 70, 2974–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Changeux J.-P., Danchin A. (1976) Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature 264, 705–712 [DOI] [PubMed] [Google Scholar]
- 184. Redfern P. A. (1970) Neuromuscular transmission in new-born rats. J. Physiol. 209, 701–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Luo L., O'Leary D. D. (2005) Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 28, 127–156 [DOI] [PubMed] [Google Scholar]
- 186. Wu X., Fu Y., Knott G., Lu J., Di Cristo G., Huang Z. J. (2012) GABA signaling promotes synapse elimination and axon pruning in developing cortical inhibitory interneurons. J. Neurosci. 32, 331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Changeux J.-P. (2013) The concept of allosteric modulation: an overview. Drug Discov. Today Technol. 10, e223–e228 [DOI] [PubMed] [Google Scholar]
- 188. Edelman G. M. (1987) Neural Darwinism: The Theory of Neural Group Selection, Basic Books, New York: [DOI] [PubMed] [Google Scholar]
- 189. Quartz S. R., Sejnowski T. J. (1997) The neural basis of cognitive development: a constructivist manifesto. Behav. Brain Sci. 20, 537–556 [DOI] [PubMed] [Google Scholar]
- 190. Duclert A., Changeux J.-P. (1995) Acetylcholine receptor gene expression at the developing neuromuscular junction. Physiol. Rev. 75, 339–368 [DOI] [PubMed] [Google Scholar]
- 191. Sanes J. R., Lichtman J. W. (2001) Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2, 791–805 [DOI] [PubMed] [Google Scholar]
- 192. Schaeffer L., Duclert N., Huchet-Dymanus M., Changeux J.-P. (1998) Implication of a multisubunit Ets-related transcription factor in synaptic expression of the nicotinic acetylcholine receptor. EMBO J. 17, 3078–3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Rousselet A., Cartaud J., Devaux P. F., Changeux J.-P. (1982) The rotational diffusion of the acetylcholine receptor in Torpeda marmorata membrane fragments studied with a spin-labelled α-toxin: importance of the 43,000 protein(s). EMBO J. 1, 439–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Cartaud J., Changeux J.-P. (1993) Post-transcriptional compartmentalization of acetylcholine receptor biosynthesis in the subneural domain of muscle and electrocyte junctions. Eur. J. Neurosci. 5, 191–202 [DOI] [PubMed] [Google Scholar]
- 195. Marchand S., Cartaud J. (2002) Targeted trafficking of neurotransmitter receptors to synaptic sites. Mol. Neurobiol. 26, 117–135 [DOI] [PubMed] [Google Scholar]
- 196. Changeux J.-P. (1983) Neuronal Man: The Biology of Mind, Fayard, Paris [Google Scholar]
- 197. Heidmann T., Changeux J.-P. (1982) Molecular model of the regulation of chemical synapse efficiency at the postsynaptic level. C. R. Seances Acad. Sci. III 295, 665–670 [PubMed] [Google Scholar]
- 198. Hawkins R. D., Abrams T. W., Carew T. J., Kandel E. R. (1983) A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science 219, 400–405 [DOI] [PubMed] [Google Scholar]
- 199. Goelet P., Castellucci V. F., Schacher S., Kandel E. R. (1986) The long and the short of long-term memory: a molecular framework. Nature 322, 419–422 [DOI] [PubMed] [Google Scholar]
- 200. Dehaene S., Changeux J.-P., Nadal J. P. (1987) Neural networks that learn temporal sequences by selection. Proc. Natl. Acad. Sci. U.S.A. 84, 2727–2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Dehaene S., Changeux J.-P. (1989) A simple model of prefrontal cortex. Function in delayed-response tasks. J. Cogn. Neurosci. 1, 244–261 [DOI] [PubMed] [Google Scholar]
- 202. Dehaene S., Changeux J.-P. (1991) The Wisconsin Card Sorting Test: theoretical analysis and modelling in a neuronal network. Cereb. Cortex 1, 62–79 [DOI] [PubMed] [Google Scholar]
- 203. Fuster J. M. (2008) The Prefrontal Cortex, 4th Ed, Academic Press, London [Google Scholar]
- 204. Dehaene S., Changeux J.-P. (1997) A hierarchical neuronal network for planning behaviour. Proc. Natl. Acad. Sci. U.S.A. 94, 13293–13298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Dehaene S., Changeux J.-P. (2000) Reward-dependent learning in neuronal networks for planning and decision making. Prog. Brain Res. 126, 217–229 [DOI] [PubMed] [Google Scholar]
- 206. Dehaene S., Kerszberg M., Changeux J.-P. (1998) A neuronal model of a global workspace in effortful cognitive tasks. Proc. Natl. Acad. Sci. U.S.A. 95, 14529–14534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Montague P. R., Dayan P., Sejnowski T. J. (1996) A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J. Neurosci. 16, 1936–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Schultz W., Dayan P., Montague P. R. (1997) A neural substrate of prediction and reward. Science 275, 1593–1599 [DOI] [PubMed] [Google Scholar]
- 209. Schultz W. (2012) Risky dopamine. Biol. Psychiatry 71, 180–181 [DOI] [PubMed] [Google Scholar]
- 210. Picciotto M. R., Zoli M., Léna C., Bessis A., Lallemand Y., Le Novère N., Vincent P., Pich E. M., Brûlet P., Changeux J.-P. (1995) Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature 374, 65–67 [DOI] [PubMed] [Google Scholar]
- 211. Picciotto M. R., Zoli M., Rimondini R., Léna C., Marubio L. M., Pich E. M., Fuxe K., Changeux J.-P. (1998) Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature 391, 173–177 [DOI] [PubMed] [Google Scholar]
- 212. Maskos U., Molles B. E., Pons S., Besson M., Guiard B. P., Guilloux J. P., Evrard A., Cazala P., Cormier A., Mameli-Engvall M., Dufour N., Cloëz-Tayarani I., Bemelmans A. P., Mallet J., Gardier A. M., David V., Faure P., Granon S., Changeux J.-P. (2005) Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436, 103–107 [DOI] [PubMed] [Google Scholar]
- 213. Avale M. E., Faure P., Pons S., Robledo P., Deltheil T., David D. J., Gardier A. M., Maldonado R., Granon S., Changeux J.-P., Maskos U. (2008) Interplay of β2* nicotinic receptors and dopamine pathways in the control of spontaneous locomotion. Proc. Natl. Acad. Sci. U.S.A. 105, 15991–15996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Mameli-Engvall M., Evrard A., Pons S., Maskos U., Svensson T. H., Changeux J.-P., Faure P. (2006) Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron 50, 911–921 [DOI] [PubMed] [Google Scholar]
- 215. Tolu S., Eddine R., Marti F., David V., Graupner M., Pons S., Baudonnat M., Husson M., Besson M., Reperant C., Zemdegs J., Pagès C., Hay Y. A., Lambolez B., Caboche J., Gutkin B., Gardier A. M., Changeux J.-P., Faure P., Maskos U. (2013) Co-activation of VTA DA and GABA neurons mediates nicotine reinforcement. Mol. Psychiatry 18, 382–393 [DOI] [PubMed] [Google Scholar]
- 216. Granon S., Faure P., Changeux J.-P. (2003) Executive and social behaviors under nicotinic receptor regulation. Proc. Natl. Acad. Sci. U.S.A. 100, 9596–9601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Maubourguet N., Lesne A., Changeux J.-P., Maskos U., Faure P. (2008) Behavioral sequence analysis reveals a novel role for β2* nicotinic receptors in exploration. PLoS Comput. Biol. 4, e1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Changeux J.-P. (2010) Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat. Rev. Neurosci. 11, 389–401 [DOI] [PubMed] [Google Scholar]
- 219. Jackson K. J., Imad Damaj M. (2013) β2-containing nicotinic acetylcholine receptors mediate calcium/calmodulin-dependent protein kinase-II and synapsin I protein levels in the nucleus accumbens after nicotine withdrawal in mice. Eur. J Pharmacol. 701, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Picciotto M. R., Kenny P. J. (2013) Molecular mechanisms underlying behaviors related to nicotine addiction. Cold Spring Harb. Perspect. Med. 3, a012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Sallette J., Bohler S., Benoit P., Soudant M., Pons S., Le Novère N., Changeux J.-P., Corringer P. J. (2004) An extracellular microdomain controls up-regulation of neuronal nicotinic acetylcholine receptors by nicotine. J. Biol. Chem. 279, 18767–18775 [DOI] [PubMed] [Google Scholar]
- 222. Sallette J., Pons S., Devillers-Thiery A., Soudant M., Prado de Carvalho L., Changeux J.-P., Corringer P. J. (2005) Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron 46, 595–607 [DOI] [PubMed] [Google Scholar]
- 223. Govind A. P., Walsh H., Green W. N. (2012) Nicotine-induced upregulation of native neuronal nicotinic receptors is caused by multiple mechanisms. J. Neurosci. 32, 2227–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Baker L. K., Mao D., Chi H., Govind A. P., Vallejo Y. F., Iacoviello M., Herrera S., Cortright J. J., Green W. N., McGehee D. S., Vezina P. (2013) Intermittent nicotine exposure upregulates nAChRs in VTA dopamine neurons and sensitizes locomotor responding to the drug. Eur. J. Neurosci. 37, 1004–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Liu L., Hendrickson L. M., Guildford M. J., Zhao-Shea R., Gardner P. D., Tapper A. R. (2013) Nicotinic acetylcholine receptors containing the α4 subunit modulate alcohol reward. Biol. Psychiatry 73, 738–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Bechara A., Tranel D., Damasio H. (2000) Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain 123, 2189–2202 [DOI] [PubMed] [Google Scholar]
- 227. Changeux J.-P., Lou H. C. (2011) Emergent pharmacology of conscious experience: new perspectives in substance addiction. FASEB J. 25, 2098–2108 [DOI] [PubMed] [Google Scholar]
- 228. Naqvi N. H., Rudrauf D., Damasio H., Bechara A. (2007) Damage to the insula disrupts addiction to cigarette smoking. Science 315, 531–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Jacobsen L. K., Picciotto M. R., Heath C. J., Frost S. J., Tsou K. A., Dwan R. A., Jackowski M. P., Constable R. T., Mencl W. E. (2007) Prenatal and adolescent exposure to tobacco smoke modulates the development of white matter microstructure. J. Neurosci. 27, 13491–13498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Léna C., Changeux J.-P., Mulle C. (1993) Evidence for “preterminal” nicotinic receptors on GABAergic axons in the rat interpeduncular nucleus. J. Neurosci. 13, 2680–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Kawai H., Lazar R., Metherate R. (2007) Nicotinic control of axon excitability regulates thalamocortical transmission. Nat. Neurosci. 10, 1168–1175 [DOI] [PubMed] [Google Scholar]
- 232. Dehaene S., Changeux J.-P. (2011) Experimental and theoretical approaches to conscious processing. Neuron 70, 200–227 [DOI] [PubMed] [Google Scholar]
- 233. Avale M. E., Chabout J., Pons S., Serreau P., De Chaumont F., Olivo-Marin J. C., Bourgeois J. P., Maskos U., Changeux J.-P., Granon S. (2011) Prefrontal nicotinic receptors control novel social interaction between mice. FASEB J. 25, 2145–2155 [DOI] [PubMed] [Google Scholar]
- 234. Rømer Thomsen K., Joensson M., Lou H. C., Møller A., Gross J., Kringelbach M. L., Changeux J.-P. (2013) Altered paralimbic interaction in behavioral addiction. Proc. Natl. Acad. Sci. U.S.A. 110, 4744–4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Koch C. (2004) Thinking about the conscious mind. Science 306, 979–980 [Google Scholar]
- 236. Franks N. P. (2008) General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci. 9, 370–386 [DOI] [PubMed] [Google Scholar]
- 237. Changeux J.-P. (2012) Conscious processing: implications for general anesthesia. Curr. Opin. Anaesthesiol. 25, 397–404 [DOI] [PubMed] [Google Scholar]
- 238. Breitmeyer B. (2006) Visual Masking: Time Slices through Conscious and Unconscious Vision, Oxford University Press, New York [Google Scholar]
- 239. Dehaene S., Sergent C., Changeux J.-P. (2003) A neuronal network model linking subjective reports and objective physiological data during conscious perception. Proc. Natl. Acad. Sci. U.S.A. 100, 8520–8525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Lamme V. A., Roelfsema P. R. (2000) The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 23, 571–579 [DOI] [PubMed] [Google Scholar]
- 241. Ku S. W., Lee U., Noh G. J., Jun I. G., Mashour G. A. (2011) Preferential inhibition of frontal-to-parietal feedback connectivity is a neurophysiologic correlate of general anesthesia in surgical patients. PLoS ONE 6, e25155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. van Loon A. M., Scholte H. S., van Gaal S., van der Hoort B. J., Lamme V. A. (2012) GABAA agonist reduces visual awareness: a masking-EEG experiment. J. Cogn. Neurosci. 24, 965–974 [DOI] [PubMed] [Google Scholar]
- 243. Hökfelt T., Rehfeld J. F., Skirboll L., Ivemark B., Goldstein M., Markey K. (1980) Evidence for coexistence of dopamine and CCK in meso-limbic neurones. Nature 285, 476–478 [DOI] [PubMed] [Google Scholar]
- 244. Agnati L. F., Benfenati F., Solfrini V., Biagini G., Fuxe K., Guidolin D., Carani C., Zini I. (1992) Brain aging and neuronal plasticity. Ann. N.Y. Acad. Sci. 673, 180–186 [DOI] [PubMed] [Google Scholar]
- 245. Amunts K., Lenzen M., Friederici A. D., Schleicher A., Morosan P., Palomero-Gallagher N., Zilles K. (2010) Broca's region: novel organizational principles and multiple receptor mapping. PLoS Biol. 8, e1000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Bartfai T., Buckley P. T., Eberwine J. (2012) Drug targets: single-cell transcriptomics hastens unbiased discovery. Trends Pharmacol. Sci. 33, 9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Pastrana E. (2010) Optogenetics: controlling cell function with light. Nat. Methods 8, 24–25 [Google Scholar]
- 248. Changeux J.-P., Edelstein S. J. (1998) Allosteric receptors after 30 years. Neuron 21, 959–980 [DOI] [PubMed] [Google Scholar]
- 249. Tsigelny I. F., Kouznetsova V. L., Baitaluk M., Changeux J.-P. (2013) A hierarchical coherent-gene-group model for brain development. Genes Brain Behav. 12, 147–165 [DOI] [PubMed] [Google Scholar]
- 250. Changeux J.-P. (2012) The Good, the True and the Beautiful: A Neuronal Approach, Odile Jacob/Yale University Press, New Haven, CT [Google Scholar]
- 251. Changeux J.-P. (1964) On the allosteric properties of biosynthetic l-threonine deaminase. II. Kinetics of action of biosynthetic l-threonine deaminase with respect to the natural substrate and inhibitor. Bull. Soc. Chim. Biol. 46, 947–961 [PubMed] [Google Scholar]
- 252. Changeux J.-P. (1964) On the allosteric properties of biosynthetic l-threonine deaminase. III. Interpretation of the inhibitory effect of l-isoleucine: steric hindrance or allosteric effect. Bull. Soc. Chim. Biol. 46, 1151–1173 [PubMed] [Google Scholar]
- 253. Changeux J.-P. (1965) On the allosteric properties of biosynthetic l-threonine deaminase. IV. The desensitization phenomenon. Bull. Soc. Chim. Biol. 47, 113–139 [PubMed] [Google Scholar]
- 254. Changeux J.-P. (1965) On the allosteric properties of biosynthetic l-threonine deaminase. V. The allosteric transition. Bull. Soc. Chim. Biol. 47, 267–280 [PubMed] [Google Scholar]
- 255. Grande F., Visscher M. B. (eds) (1967) Introduction. in Claude Bernard and Experimental Medicine, Schenkman Publishing Co., Cambridge, MA [Google Scholar]