Background: Mechanisms of activation-induced PKC down-regulation are poorly understood. A characterized pathway involves priming site dephosphorylation and degradation of the dephosphorylated species.
Results: Mature, fully phosphorylated PKCα is a major target for activation-induced degradation, via mechanisms controlled by Hsps.
Conclusion: Hsps control phosphorylation and degradation of fully primed, activated PKC.
Significance: Findings from this study support a new model of PKC desensitization.
Keywords: Heat Shock Protein, Hsp90, Phosphorylation, Protein Degradation, Protein Kinase C (PKC), Hsp70/Hsc70, PKCα, Lysosomal Degradation, Proteasomal Degradation
Abstract
Although alterations in stimulus-induced degradation of PKC have been implicated in disease, mechanistic understanding of this process remains limited. Evidence supports the existence of both proteasomal and lysosomal mechanisms of PKC processing. An established pathway involves rate-limiting priming site dephosphorylation of the activated enzyme and proteasomal clearance of the dephosphorylated protein. However, here we show that agonists promote down-regulation of endogenous PKCα with minimal accumulation of a nonphosphorylated species in multiple cell types. Furthermore, proteasome and lysosome inhibitors predominantly protect fully phosphorylated PKCα, pointing to this form as a substrate for degradation. Failure to detect substantive dephosphorylation of activated PKCα was not due to rephosphorylation because inhibition of Hsp70/Hsc70, which is required for re-priming, had only a minor effect on agonist-induced accumulation of nonphosphorylated protein. Thus, PKC degradation can occur in the absence of dephosphorylation. Further analysis revealed novel functions for Hsp70/Hsc70 and Hsp90 in the control of agonist-induced PKCα processing. These chaperones help to maintain phosphorylation of activated PKCα but have opposing effects on degradation of the phosphorylated protein; Hsp90 is protective, whereas Hsp70/Hsc70 activity is required for proteasomal processing of this species. Notably, down-regulation of nonphosphorylated PKCα shows little Hsp70/Hsc70 dependence, arguing that phosphorylated and nonphosphorylated species are differentially targeted for proteasomal degradation. Finally, lysosomal processing of activated PKCα is not regulated by phosphorylation or Hsps. Collectively, these data demonstrate that phosphorylated PKCα is a direct target for agonist-induced proteasomal degradation via an Hsp-regulated mechanism, and highlight the existence of a novel pathway of PKC desensitization in cells.
Introduction
Signal desensitization plays a critical role in the maintenance of cellular homeostasis (1). Diminished responsiveness following prolonged or repeated cell stimulation is often achieved through modulation of intermediary kinases, including cell surface receptors and downstream intracellular effectors. Reduced output from these molecules can involve inhibition of their catalytic activity (e.g. by sequestration, phosphorylation, or dephosphorylation) and/or their down-regulation (e.g. by proteolytic processing).
PKC is a family of serine/threonine kinases that control fundamental cellular processes (e.g. cell growth, differentiation, survival, and migration) and are frequently dysregulated in disease, including cancer and neurodegenerative disorders (2, 3). PKC family members are divided into three classes based on differences in structure and cofactor requirements. The conventional PKCs (cPKCs3: PKCα, βI, βII, and γ) are calcium-dependent and are activated by the second messenger diacylglycerol (DAG). Novel PKCs (nPKCs: PKCδ, ϵ, θ, and η) are also activated by DAG but are calcium-independent. In contrast, atypical PKCs (PKCι/λ and ζ) are calcium- and DAG-independent. PKC function requires ordered phosphorylation at three priming sites (4). In the case of cPKCs, membrane-tethered, newly synthesized enzyme is phosphorylated on the activation loop by PDK1, which allows for subsequent mTORC2-dependent phosphorylation of the turn and hydrophobic motif sites. Following priming site phosphorylation, PKC is released into the cytosol, where it is maintained in an inactive state by a C-terminal pseudosubstrate domain that occupies the active site. The order and timing of these phosphorylation events are critically important, with mutation of any of the priming sites to nonphosphorylatable (alanine) or phosphomimetic (aspartate/glutamate) residues resulting in unstable/inactive protein (5–7). Notably, fully primed PKC adopts a conformation that is resistant to phosphatases (8, 9). As a result, priming site phosphorylation is highly stable, and no further phosphorylation is needed for activation of the enzyme following generation of appropriate second messengers (4).
Physiological stimulation of cPKCs and nPKCs occurs through receptor-mediated activation of phospholipase C, which generates DAG and inositol trisphosphate. DAG promotes PKC translocation to membranes (via interaction with the C1 domain of the enzyme), leading to a conformational change that releases the pseudosubstrate domain from the active site and enables substrate access. Pharmacological agonists such as phorbol esters (e.g. phorbol 12-myristate 13-acetate (PMA)) and macrolide lactones (e.g. bryostatin 1 (Bryo)) bind with high affinity to the C1 domain and promote sustained membrane association and activation of cPKCs and nPKCs. Based on the central role of PKCs in critical cellular processes, there is considerable interest in the potential of PKC agonists as therapeutic agents, with multiple recent and ongoing clinical trials examining the use of PMA (PD-616) or Bryo for the treatment of various cancers, HIV/AIDS, and Alzheimer disease (see clinicaltrials.gov).
PKC signal termination is mediated by acute inactivation and long term desensitization mechanisms (10, 11). For cPKCs and nPKCs, acute signal reversal is accomplished by rapid metabolism of DAG and “reverse translocation” of the enzyme to the cytoplasm, where it resumes an inactive conformation (12, 13). PKC signaling may also be terminated by priming site dephosphorylation, which inactivates the enzyme, and by protein degradation. These desensitization mechanisms, which are engaged following prolonged activation by physiological signals or pharmacological agonists (11, 14), play a critical role in the regulation of cellular responses. In this regard, alterations in degradation of activated PKC isozymes have been implicated in several pathological conditions, including drug-resistant cancer (15–18), neurodegenerative disorders such as Huntington (19) and Alzheimer disease (3, 20), and inflammatory bowel disease (22). As discussed above, PKC agonists are being investigated as therapeutic agents for these diseases, highlighting the need for a clear understanding of agonist-induced enzyme processing. However, despite their importance, the mechanisms underlying stimulus-induced PKC degradation remain poorly defined.
Based on evidence that (a) binding of lipid coactivators can sensitize PKC to phosphatases (23), (b) loss of phosphate at a single priming site predisposes the other sites to dephosphorylation (5, 24), (c) dephosphorylated PKC is highly unstable, and (d) the activated enzyme can be internalized, with dephosphorylation occurring on intracellular vesicles (4, 25–37), it has been proposed that agonist-induced down-regulation involves vesicle-mediated accumulation of the enzyme in a perinuclear compartment (38), followed by dephosphorylation of priming sites by a protein phosphatase 2A-like activity (27, 28) and/or PHLPP1/2 (26). The fully dephosphorylated protein is sequestered in a neutral detergent-insoluble fraction, where it is refractory to rephosphorylation (26, 27, 32), and is eventually degraded via a dephosphorylation-dependent, ubiquitin-mediated proteasomal mechanism (11, 14, 36).
Although this model reflects one pathway of PKC degradation, studies with endogenous PKCα indicate that PKC desensitization is considerably more complex. In IEC-18 intestinal epithelial cells, PKC agonists can induce both proteasomal and nonproteasomal degradation of the enzyme. Proteasomal degradation occurs primarily at the plasma membrane, whereas internalization targets the protein for endolysosomal processing (28, 39). Dephosphorylation may not be a prerequisite for down-regulation, because phosphorylated PKCα is ubiquitinated, and it is the phosphorylated form that is detected in early and late endosomal compartments following internalization (28, 39–41). Furthermore, physiological levels of nucleotides (or nucleotide analogs) maintain activated PKCs in a phosphatase-resistant conformation (42–45), indicating that activation may not directly induce PKC dephosphorylation within the cell. Thus, the role of dephosphorylation in desensitization of PKC signaling remains to be resolved.
Recent studies have identified important roles for Hsp70 and Hsp90 in regulation of PKC phosphorylation. Hsp70 proteins are members of a family of conserved molecular chaperones. The majority of Hsp70 activity in the cytoplasm is provided by two family members as follows: stress-inducible Hsp70 (Hsp72 or Hspa1A/Hspa1B) and constitutively expressed Hsc70 (Hsp73 or Hspa8) (46, 47). Based on their high sequence homology (86%) and overlapping functions (48), Hsp70 and Hsc70 are often referred to interchangeably. Hsp proteins assist with the folding of newly synthesized polypeptides and refolding of misfolded proteins, thus preventing the formation of protein aggregates (49, 50). They also control cellular localization of proteins, and their activities are closely coordinated with protein degradation systems, including autophagy-lysosomal and proteasomal degradation pathways (50–56). Hsp70 has been shown to extend the signaling lifetime of activated PKC by allowing reentry of the dephosphorylated protein into the activatable pool of cytosolic enzyme (57, 58). Importantly, rephosphorylation of dephosphorylated PKC requires Hsp70 activity (59). Dephosphorylated PKC is distinct from the never phosphorylated protein, and it is not a substrate for priming phosphorylation due to sequestration in the insoluble fraction and conformational changes. Hsp70 specifically recognizes PKC that has been dephosphorylated and enables its rephosphorylation by protecting it from sequestration and promoting a conformation that can be recognized by priming kinases (57–59).
Hsp90 is a ubiquitously expressed, highly conserved molecular chaperone that promotes protein folding, mediates protein quality control, and regulates protein turnover (60). It is involved in controlling the rate of the initial priming phosphorylation of PKCs (14, 61). Previous studies have demonstrated that Hsp90 stabilizes PKC mutants that lack turn motif phosphorylation (8, 9) and promotes efficient phosphorylation of the hydrophobic motif during maturation (61). As a result, inhibition of Hsp90 can result in a cell type-dependent reduction in steady-state levels of unstimulated enzyme via proteasomal degradation (8).
This study addresses the relationship between PKCα phosphorylation and stimulus-induced down-regulation of the enzyme. It shows that degradation of endogenous PKCα is associated with minimal, if any, accumulation of dephosphorylated protein and that the mature, fully phosphorylated PKCα represents a major target for activation-induced proteasomal and nonproteasomal degradation. It further identifies roles for chaperone proteins in agonist-induced processing of PKCα. Although both Hsp70/Hsc70 and Hsp90 help to maintain activated PKCα in the phosphorylated state, these molecules have opposing roles in regulating degradation of the fully primed, activated enzyme. Furthermore, proteasomal degradation of phosphorylated and nonphosphorylated PKCα species appears to be mediated by different mechanisms.
MATERIALS AND METHODS
Cell Culture and Drug Treatments
IEC-18 rat intestinal crypt-like cells (ATCC CRL-1589) were maintained in Dulbecco's modified Eagle's medium (DMEM), supplemented with 5% fetal bovine serum (FBS), 4 mm l-glutamine, and 5 μg/ml insulin (IEC-18 complete medium). Immortalized WT and Hsp70−/− (hspa1A and hspa1B knock-outs) mouse embryonic fibroblasts (MEFs), a kind gift from Dr. Donna George, University of Pennsylvania School of Medicine, were maintained in DMEM with 10% FBS and penicillin/streptomycin. Other cell lines were cultured in DMEM with 10% FBS and 4 mm l-glutamine (with penicillin/streptomycin for HeLa cells). PKC isozymes were activated by treating cells with 100 nm PMA (Sigma) or 100 nm Bryo (Biomol). Where indicated, cycloheximide (CHX), 2-phenylethynesulfonamide (PES), chloroquine, bafilomycin A1 (Sigma), 2-(3-chlorophenyl)ethynesulfonamide (PES-Cl), 17-N-allylamino-17-demethoxy-geldanamycin (17-AAG) (A.G. Scientific), bisindolylmaleimide I, N-acetyl-Leu-Leu-Nle-CHO, and/or MG132 (Calbiochem) were added 30 min prior to PKC agonist treatment. Leupeptin (A. G. Scientific) was added 24 h prior to addition of PKC agonists. Drugs were dissolved in DMSO except for Bryo and PMA, which were dissolved in ethanol, and leupeptin and chloroquine, which were dissolved in water. Equal volumes of the relevant solvent were used as vehicle controls (concentrations of individual solvents remained below 0.2% v/v and did not affect the localization or degradation of PKCα).
siRNA-mediated Silencing of Hsps
IEC-18 cells were transfected with 100 nm ON-TARGETplus SMARTpool siRNAs against rat Hspa1a (targeting Hsp70: Hspa1A and Hspa1B), rat Hspa8 (targeting Hsc70), or with ON-TARGETplus nontargeting pool siRNA (Thermo Scientific). Lipofectamine 2000 transfection reagent (Invitrogen) was used to transfect the cells according to the manufacturer's protocols (Invitrogen). Medium was changed, and transfection with Hspa8 and the nontargeting siRNAs was repeated after 48 h to ensure depletion of Hsc70. Only one 48-h Hspa1a siRNA treatment was required to achieve adequate knockdown of Hsp70 in IEC-18 cells. siRNA-treated cells were analyzed 48 h after their final transfection.
Adenoviral Overexpression of PKCα
IEC-18 cells were infected with PKCα adenovirus at a multiplicity of infection of 5 for 16 h in IEC-18 minimal medium as follows: DMEM supplemented with 2% FBS, 4 mm l-glutamine, and 5 μg/ml insulin (62). The medium was then replaced with IEC-18 complete medium, and cells were subjected to the indicated treatments after an additional 24–48 h.
Antibodies
Primary antibodies for immunofluorescence and Western blotting were as follows: rabbit anti-C-terminal PKCα (Epitomics); rabbit anti-β-actin (Sigma); mouse anti-Hsp70 and rat anti-Hsc70 (Enzo), and mouse anti-LC3 (Nanotools). Secondary antibodies for immunofluorescence and Western blotting were as follows: TRITC-conjugated donkey anti-rabbit (Jackson ImmunoResearch), and horseradish peroxidase-conjugated goat secondary antibodies recognizing mouse, rat, or rabbit IgG (Millipore).
Immunoblot Analysis
Cells were lysed with 1% SDS, which extracts PKCs from the cytosol, membrane, and nonionic detergent-insoluble fractions, and immunoblot analysis of whole cell lysates was performed as we have described (28, 62) using SuperSignal West Pico ECL (ThermoScientific). The apparent molecular weight of bands was determined based on electrophoretic migration relative to prestained standard proteins (Bio-Rad/ThermoScientific). Nitrocellulose membranes were routinely stained with 0.1% Fast Green (Sigma) to confirm equal loading and even transfer. Antibody dilutions were as follows: anti-C-terminal PKCα (1:20,000), anti-actin (1:10,000), anti-Hsp70 (1:500), anti-Hsc70 (1:40,000), horseradish peroxidase-conjugated goat anti-mouse IgG (1:2,000), horseradish peroxidase-conjugated goat anti-rat IgG (1:40,000), and horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2,000). Relative band intensities were estimated using ImageJ software, and statistical analysis (Student's t test) was performed using Microsoft Excel software (p < 0.05 was considered statistically significant).
Immunofluorescence Analysis
Cells, grown on glass coverslips and treated as indicated, were fixed in 2% formaldehyde/PBS for 15 min at room temperature and processed for immunofluorescence microscopy as we have described previously, using 0.2% saponin for permeabilization (28). Antibodies were rabbit anti-C-terminal PKCα (1:500) and TRITC-conjugated anti-rabbit secondary antibody (1:100). Images were obtained using a Zeiss Axioskop epifluorescence microscope with a 63× Plan Apochromat (1.4 NA) objective lens and a Hamamatsu C7780 digital camera.
RESULTS
PKC Agonists Induce Minimal Accumulation of Dephosphorylated Endogenous PKCα in Multiple Cell Types
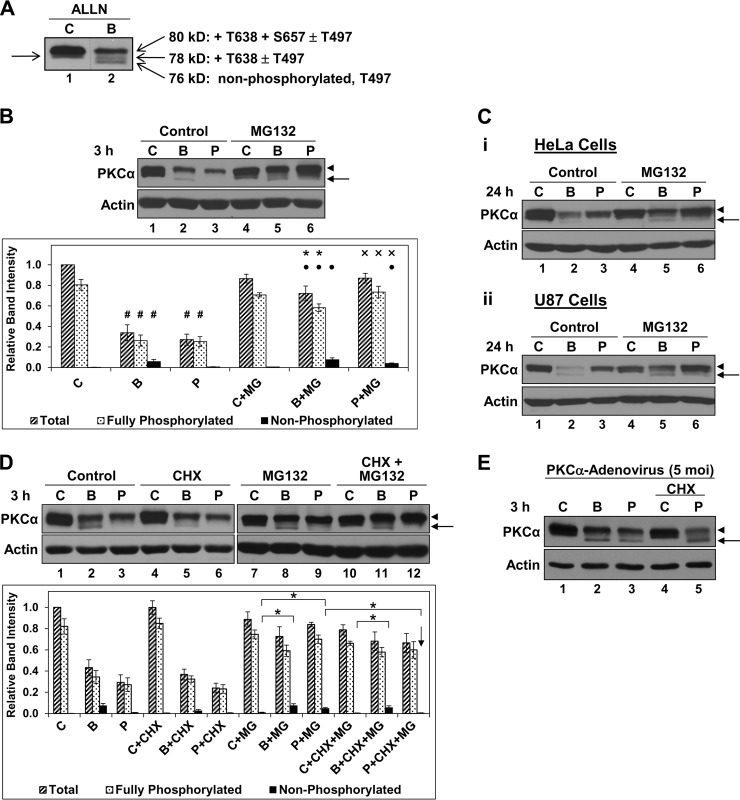
The prevailing model for agonist-induced long term PKC down-regulation involves proteasomal degradation of dephosphorylated protein following its accumulation in a detergent-insoluble compartment, where it is refractory to rephosphorylation (8, 9, 57–59). To explore the role of this model in down-regulation of endogenous PKCα, we monitored the ability of agonists to promote accumulation of dephosphorylated enzyme. Priming phosphorylation of PKCα at the activation loop (Thr497), turn motif (Thr638), and hydrophobic motif (Ser657) results in characteristic shifts in electrophoretic migration on SDS-polyacrylamide gels, as determined by extensive analysis by several groups using phosphosite-specific antibodies (63–65). As can be seen in Fig. 1A, these forms are readily visualized on Western blots of total PKCα; PKCα lacking phosphorylation on priming sites (nonphosphorylated PKCα) migrates at ∼76 kDa, with priming phosphorylation leading to progressively slower migration to yield a mature (fully phosphorylated) protein migrating at ∼80 kDa (63–65). In untreated IEC-18 cells, PKCα is primarily in the mature form, with variable amounts (up to ∼25%) of a species lacking Ser657 phosphorylation (Fig. 1A, lane 1, arrow on left) (28). The PKC agonists Bryo or PMA induce rapid down-regulation of endogenous PKCα (Fig. 1B, lanes 1–3) through a proteasomal mechanism (28), as indicated by the protective effects of the proteasome inhibitor MG132 (Fig. 1B, lanes 4–6). Bryo also elicits slower, nonproteasomal PKCα processing, as evident from the incomplete protection afforded by MG132 (Fig. 1B, lane 5) (28, 39). Notably, there is minimal accumulation of nonphosphorylated PKCα, even when proteasomal degradation of this unstable species is blocked (Fig. 1B, arrow and black bars in graph); instead, proteasome inhibition mainly protects the fully phosphorylated protein (Fig. 1B, arrowhead, speckled bars in graph). Thus, at most, minimal amounts of activated PKCα are sequestered in a dephosphorylated state prior to its degradation by the proteasome. This finding suggests that the prevailing model for agonist-induced targeting of dephosphorylated protein plays only a minor role in proteasomal processing of activated PKCα in IEC-18 cells and points to the mature species as a major substrate for proteasomal degradation.
FIGURE 1.
Bryo and PMA down-regulate endogenous PKCα with minimal or no accumulation of dephosphorylated enzyme in multiple cell types. A, electrophoretic migration of PKCα phosphoforms on SDS-polyacrylamide gels. IEC-18 cells were treated with vehicle (C) or 100 nm Bryo (B) for 2.5 h in the presence of 150 μm N-acetyl-Leu-Leu-Nle-CHO, and the relative electrophoretic migration of PKCα phosphoforms (open arrows) (63–65) was detected by immunoblot analysis of whole cell lysates. Note that, in untreated cells (lane 1), PKCα is primarily in the ∼80-kDa mature form, with variable detection of an ∼78-kDa species lacking Ser657 phosphorylation (arrow on left). Data are from a single blot; dashed line indicates rearrangement of lanes for clarity. B, Bryo and PMA promote proteasomal degradation of PKCα; predominant protection of the mature species by proteasome inhibition. IEC-18 cells were treated with vehicle (C), 100 nm Bryo (B), or 100 nm PMA (P), with or without 20 μm MG132 (MG), and subjected to immunoblot analysis for PKCα and β-actin. Graph, densitometric analysis of relative band intensities corresponding to total PKCα as well as the fully phosphorylated (indicated by the arrowhead next to the blot) and nonphosphorylated (indicated by the arrow next to the blot) forms under the indicated conditions from five independent experiments. #, statistically different from corresponding band in control; ●, statistically different from control + MG132; *, statistically different from Bryo alone; ×, statistically different from PMA alone. C, proteasome inhibitors predominantly protect the phosphorylated form of PKCα in multiple cell types. HeLa (panel i) and U87 (panel ii) cells were treated with vehicle (C), 100 nm Bryo (B), or 100 nm PMA (P), with or without 20 μm MG132, and subjected to immunoblot analysis for PKCα and β-actin. D, PMA does not promote the accumulation of dephosphorylated endogenous PKCα. Immunoblot analysis of IEC-18 cells treated with vehicle (C), 100 nm Bryo (B), 100 nm PMA (P), 200 μm CHX, and/or 20 μm MG132 (MG), as indicated. Graph, densitometric analysis of relative band intensities corresponding to total PKCα as well as the fully phosphorylated and nonphosphorylated forms under the indicated conditions from four independent experiments. * brackets indicate statistical significance between conditions. Note the lack of dephosphorylated PKCα detected following PMA treatment in the presence of MG132 and CHX (downward arrow). E, PMA induces dephosphorylation of exogenously expressed PKCα in IEC-18 cells. Cells were infected with PKCα-adenovirus (5 multiplicities of infection) and subjected to immunblot analysis following a 3-h treatment with vehicle (C), 100 nm Bryo (B), or 100 nm PMA (P) in the absence or presence of 200 μm CHX. Arrow, faster migrating, nonphosphorylated PKCα; arrowhead, mature phosphorylated PKCα. Data are representative of at least three independent experiments. Arrows, faster migrating, nonphosphorylated PKCα; arrowheads, mature phosphorylated PKCα. Data are representative of at least three independent experiments.
To determine whether the inability of PKC agonists to induce substantial accumulation of nonphosphorylated endogenous PKCα is unique to IEC-18 cells, we examined the effects of MG132 in other cell types. Although the kinetics varied, both Bryo and PMA induced proteasomal degradation of PKCα in HeLa cervical cancer cells and U87 glioblastoma cells, as indicated by the protective effects of MG132 (Fig. 1C, panels i and ii). As in IEC-18 cells, proteasome inhibition mainly protected the fully primed enzyme (Fig. 1C, arrowheads), with minimal accumulation (less than ∼10%) of the nonphosphorylated species even when proteasomal degradation was blocked with MG132 (arrows). Similar effects were seen in a panel of additional cell lines (data not shown). Thus, in multiple cell types, agonist-induced proteasomal degradation of endogenous PKCα occurs with minimal accumulation of nonphosphorylated enzyme, arguing that proteasomal processing of the mature form may be a common response to sustained PKCα activation.
PMA-induced Accumulation of Nonphosphorylated PKCα Results from Delayed Maturation Rather than Dephosphorylation
Although proteasome inhibition primarily protects fully phosphorylated PKCα (Fig. 1B, arrowhead), minor amounts of the nonphosphorylated species also accumulate in the presence of MG132 (Fig. 1B, arrows) (28). PKC agonists have been reported to delay priming phosphorylation of PKC isozymes (66) as well as to promote dephosphorylation of the mature form (32); thus, the nonphosphorylated species seen with agonist treatment could arise from delayed processing of newly synthesized PKCα and/or from agonist-induced dephosphorylation of mature enzyme. We have previously shown that the nonphosphorylated PKCα species seen in Bryo-treated IEC-18 cells (e.g. Fig. 1B, lane 2) derives from both delayed maturation and dephosphorylation (28). These two sources of nonphosphorylated PKCα can be differentiated using the protein synthesis inhibitor CHX; in the presence of CHX, nonphosphorylated PKCα resulting from delayed maturation will fail to accumulate, whereas species derived from dephosphorylation will be retained. The incomplete loss of nonphosphorylated PKCα in the presence of CHX confirms the two sources for this species in Bryo-treated cells (Fig. 1D, compare lanes 2 and 5, and corresponding black bars in graph). In contrast, our previous studies failed to detect dephosphorylation of endogenous PKCα in PMA-treated cells, with the higher mobility species seen in some experiments attributed to delayed maturation because it could be blocked by CHX (28). However, as mentioned above, nonphosphorylated PKC is highly susceptible to proteasomal degradation (8); thus, it remained possible that the failure to accumulate dephosphorylated protein reflected its rapid degradation. Indeed, the failure to detect a nonphosphorylated intermediate of endogenous PKCα in PMA-treated COS cells has been attributed to hydrophobic motif dephosphorylation being the rate-limiting step in PKCα down-regulation, with subsequent rapid degradation of the dephosphorylated species (23). This possibility was tested by examining the combined effects of CHX and MG132 on PKCα expression in PMA-treated IEC-18 cells. Using a highly sensitive anti-PKCα antibody (Epitomics), nonphosphorylated enzyme was detected in PMA-treated cells in the presence of MG132, confirming that this species is cleared by the proteasome (Fig. 1, B, lane 6, and D, lane 9). However, the nonphosphorylated species can be attributed solely to newly synthesized, unprimed protein because its accumulation was completely abrogated by CHX (Fig. 1D, compare lanes 9 and 12, and corresponding black bars in graph, arrow). Thus, although PMA delays maturation of newly synthesized protein, it potently down-regulates endogenous PKCα in the absence of detectable dephosphorylation. Together, the data indicate that, in IEC-18 cells, PMA-induced proteasomal degradation of activated PKCα does not involve prior sequestration of dephosphorylated protein in a detergent-insoluble compartment where it is refractory to rephosphorylation.
Overexpression Can Alter Agonist-induced Processing of PKCα
In contrast to our findings with endogenous protein, studies using overexpressed PKC have readily detected accumulation of dephosphorylated enzyme in response to both PMA and Bryo. Therefore, we examined the effects of PKC agonists on adenovirally expressed PKCα in IEC-18 cells. Notably, PMA promoted accumulation of dephosphorylated forms in PKCα-overexpressing cells, as confirmed by retention of faster migrating species in the presence of CHX (Fig. 1E, lanes 3 and 5, arrow). These findings exclude technical limitations as the basis for our inability to detect dephosphorylated endogenous PKCα in response to PMA. Furthermore, they demonstrate that overexpression has the potential to alter agonist-induced processing of PKCs.
Hsp70/Hsc70 Protects Activated PKCα from Dephosphorylation but Is Required for Proteasomal Degradation of the Mature Form
Although experiments using CHX and MG132 argued that PMA promotes down-regulation of PKCα without the generation of a dephosphorylated intermediate, it remained formally possible that the failure to detect the dephosphorylated species in PMA-treated cells reflected its subsequent rapid rephosphorylation. Phosphorylation site mutants cannot be used to examine the role of dephosphorylation in agonist-induced degradation of PKCα because overexpression alters processing of the protein (e.g. Fig. 1E) and mutation of these sites leads to unstable/inactive enzyme (5–7). However, recent studies by several groups have determined that rephosphorylation of activated, dephosphorylated PKCs requires Hsp70 activity (58, 59, 67). Thus, Hsp70/Hsc70 inhibition can be used to assess the extent to which rephosphorylation accounts for the failure to detect substantive amounts of nonphosphorylated PKCα in agonist-treated cells. We therefore used pharmacological, molecular, and genetic approaches to inhibit Hsp70/Hsc70 activity, and examined the effects of these manipulations on PKC phosphorylation and degradation.
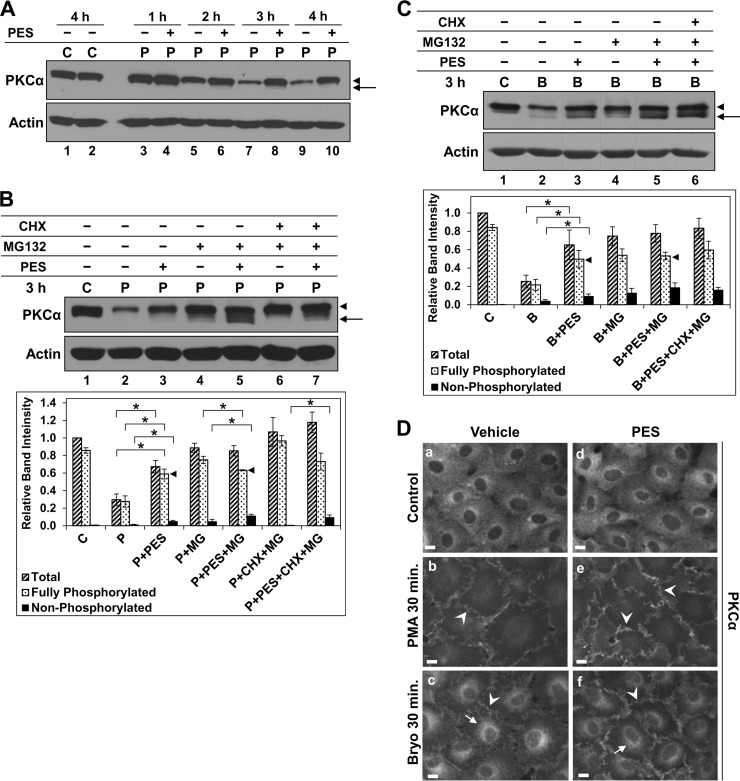
First, we tested the effects of the Hsp70/Hsc70 inhibitor 2-phenylethynesulfonamide (PES, also known as pifithrin-μ). PES has been shown to bind Hsp70 and Hsc70 and to block their chaperone function by disrupting their interaction with co-chaperones and client proteins (68, 69). Inhibition of Hsp70/Hsc70 activity with PES (up to 80 μm) did not affect steady-state levels or phosphorylation of unstimulated PKCα (Fig. 2A, lanes 1 and 2, and data not shown). Unexpectedly, PES (10–80 μm, with maximal effects seen at 20 μm) dramatically slowed the rate of down-regulation of the fully phosphorylated form of PKCα in cells treated with PMA (Fig. 2A, arrowheads, and data not shown), pointing to an unrecognized role for Hsp70/Hsc70 in promoting degradation of the activated protein. In addition, consistent with the known role of Hsp70/Hsc70 activity in facilitating rephosphorylation of activated PKCs (58, 59, 67), PES induced accumulation of faster migrating dephosphorylated forms of PKCα (Fig. 2A, arrow). Although PES stimulated some accumulation of the dephosphorylated protein by 1–2 h of PMA treatment (Fig. 2A, compare lane 3 with lanes 4, 5, and 6), the activated kinase remained predominantly in the fully phosphorylated form, even at 4 h when extensive down-regulation had occurred in nonPES-treated cells (lanes 9 and 10).
FIGURE 2.
Hsp70/Hsc70 inhibitor PES promotes dephosphorylation of activated PKCα but protects the mature enzyme from agonist-induced proteasomal degradation. A, effect of PES on steady-state levels and PMA-induced degradation of PKCα. IEC-18 cells were treated with vehicle (C) or 100 nm PMA (P) for the indicated times in the presence (+) or absence (−) of 20 μm PES and subjected to immunoblot analysis for PKCα and β-actin. Arrow, faster migrating, nonphosphorylated PKCα; arrowhead, mature phosphorylated PKCα. B, effects of PES on phosphorylation and degradation of PKCα activated by PMA. Immunoblot analysis of cells treated with vehicle (C) or 100 nm PMA (P) for 3 h in the absence or presence of 20 μm PES, 20 μm MG132, and/or 200 μm CHX, as indicated. Arrow and arrowhead are as in A. Graph, quantification of relative band intensities corresponding to total, fully phosphorylated, and nonphosphorylated PKCα under the indicated conditions (averages of five independent experiments). * brackets indicate statistical significance between conditions. Note the similar levels of fully phosphorylated PKCα following PMA treatment in the presence of PES or PES+MG132 (arrowheads). C, effects of PES on phosphorylation and degradation of PKCα activated by Bryo. Data are as in B except that cells were treated with 100 nm Bryo (B) rather than PMA, and the graph shows the averages of four experiments. Panels show data from a single blot; dashed lines indicate rearrangement of lanes for clarity. D, immunofluorescence analysis of PKCα in cells treated with vehicle (panels a–c) or 20 μm PES (panels d–f) before addition of vehicle (Control), 100 nm Bryo or 100 nm PMA for a further 30 min. Images were exposed and processed identically. Arrows, perinuclear staining; arrowheads, membrane staining. Magnification bars, 10 μm. Data are representative of at least three independent experiments.
Further analysis revealed that the faster migrating forms of PKCα seen following activation in the presence of PES (a) were protected by MG132, and are thus substrates for the proteasome (Fig. 2B, compare lane 3 with 5, arrows), and (b) largely reflect dephosphorylation of the mature protein because they were detected in the presence of CHX (Fig. 2B, compare lane 5 with 7). Together, these data confirm that Hsp70/Hsc70 helps to maintain activated PKCα in the phosphorylated form by facilitating its rephosphorylation after it has been dephosphorylated in response to activation, and/or by protecting it from agonist-induced dephosphorylation. However, even when rephosphorylation was blocked through Hsp70/Hsc70 inhibition, the extent of activation-induced PKCα dephosphorylation was relatively modest, with the level of fully phosphorylated protein decreasing from ∼85% of total PKCα in control cells to ∼65% of the total in cells treated with PMA in the presence of PES and MG132 (Fig. 2B, lanes 1 and 5 and corresponding speckled bars in graph); thus, rephosphorylation of dephosphorylated enzyme can make only a minor contribution to the fully phosphorylated form of activated PKCα observed in proteasome inhibitor-treated cells (Fig. 2B, graph). Furthermore, the extent of PMA-induced PKCα dephosphorylation (<∼25%) is insufficient to account for the degree of PKCα down-regulation observed following activation (∼70% at 3 h; Fig. 2B, compare lane 2 with 5). Thus, dephosphorylation-dependent mechanisms cannot account for the majority of agonist-induced PKCα down-regulation in IEC-18 cells, and fully phosphorylated PKCα is directly targeted for proteasomal degradation without the need for prior dephosphorylation.
PES also stabilized the mature form of PKCα and enhanced its dephosphorylation in Bryo-treated cells (Fig. 2C, arrowhead and arrow, respectively), indicating that the effects of Hsc70/Hsc70 inhibition were not selective for PMA-activated PKCα. Consistent with the inherent ability of Bryo to induce some dephosphorylation of PKCα (see Fig. 1D), the level of dephosphorylation in cells treated with Bryo and PES was greater than that seen with PMA and PES (∼35% compared with <∼25%). However, even with Bryo, the extent of dephosphorylation seen in the presence of PES and MG132 was insufficient to account for the level of activation-induced PKCα down-regulation (Fig. 2C, compare lane 2 with 5, and corresponding speckled bars in the graph), confirming that agonists directly target the phosphorylated species of PKCα for proteasomal degradation. The stabilization of the mature form of PKCα by PES in agonist-treated cells can be attributed to blockade of proteasomal degradation of phosphorylated PKCα because MG132 provided little additional protection of this species (Fig. 2, B and C, compare lane 3 with 5, and speckled bars in corresponding graphs, arrowheads). In contrast, proteasomal degradation of nonphosphorylated PKCα was relatively insensitive to Hsp70/Hsc70 inhibition, as revealed by the robust protection of this species by MG132 in PES-treated cells (Fig. 2, B and C, compare lane 3 with 5, arrows, and black bars in corresponding graphs). Importantly, the ability of PKC agonists to induce proteasomal degradation of dephosphorylated PKCα in the presence of PES excluded saturation of the proteasome machinery as the basis for stabilization of the mature form (68, 69).
A role for Hsp70/Hsc70 in mediating proteasomal degradation of mature PKCα is further supported by the ability of PES to increase levels of the enzyme at the plasma membrane (the major site for proteasomal processing of PKC; Refs. 28, 39) in PMA and Bryo-treated cells, as shown by immunofluorescence microscopy (Fig. 2D, panels b and e and c and f, arrowheads). Notably, Hsp70/Hsc70 inhibition does not interfere with PKCα activation because PES did not prevent agonist-induced membrane translocation of the enzyme (Fig. 2D), and agonist-induced PKCα dephosphorylation, an effect that requires PKC activity (37), occurred in the presence of PES (Fig. 2, B and C). Together, these findings pointed to a previously unidentified role of Hsp70/Hsc70 in promoting activation-dependent desensitization of PKC signaling.
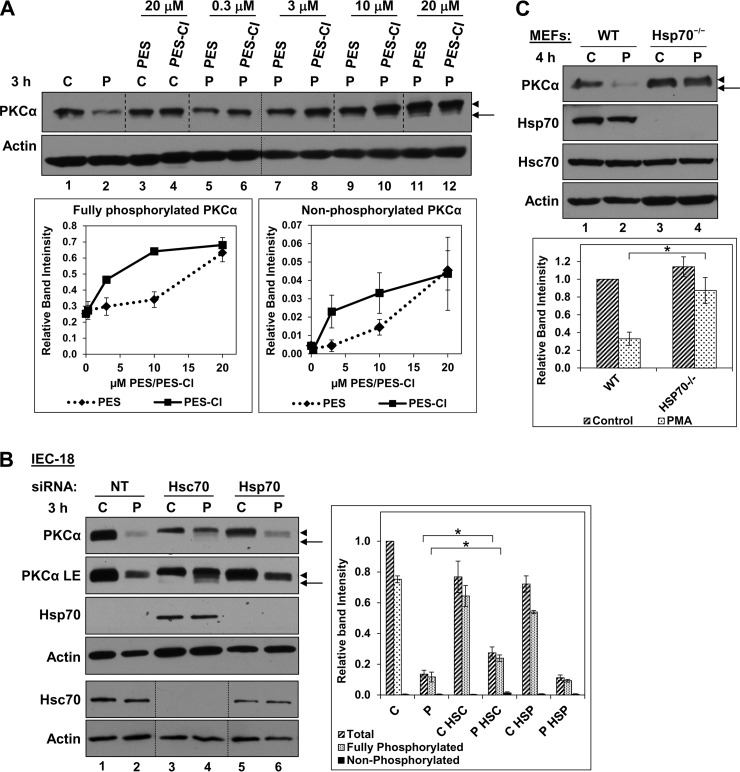
Three approaches were used to confirm the involvement of Hsp70/Hsc70 in the effects of PES on PKCα. The first approach used a novel PES analog, PES-Cl, which is a more potent inhibitor of Hsp70/Hsc70 that shows a 2–8-fold increased potency in cell-based assays (70, 71). PES-Cl was also more effective (∼3-fold) in protecting mature PKCα from proteasomal degradation and in enhancing accumulation of dephosphorylated species (Fig. 3A, compare lanes 6, 8, and 10 with lanes 5, 7, and 9); thus, the potency of PES analogs in modulating PKCα corresponds to their relative activity against Hsp70/Hsc70.
FIGURE 3.
Effects of Hsp70/Hsc70 deficiency on processing of activated PKCα. A, comparison of the effects of PES and PES-Cl on PMA-induced PKCα processing. Immunoblot analysis of IEC-18 cells treated with vehicle (C) or 100 nm PMA (P) for 3 h in the absence or presence of PES or PES-Cl at the indicated concentrations. Panels show data from a single blot; small dashed lines indicate rearrangement of lanes for clarity. Large dashed lines are included for clarity only. The graph shows the relative band intensities (averages of three experiments) for fully phosphorylated and nonphosphorylated PKCα seen following PMA treatment in the presence of PES (♦) or PES-Cl (■). B, effects of Hsp70 or Hsc70 deficiency on activated PKCα in IEC-18 cells. Cells were incubated with nontargeting siRNA (NT), Hspa8 (Hsc70), or Hspa1a (Hsp70) siRNA. After 48 h, Hspa8 (Hsc70) siRNA-treated cells were re-treated with the same siRNA for an additional 48 h. Hspa1a (Hsp70) siRNA was added to cells for 48 h only. Cells were then treated with vehicle (C) or 100 nm PMA (P) for 3 h and subjected to immunoblotting for the indicated proteins. Different exposures are shown for PKCα to facilitate visualization of faster migrating species (LE, longer exposure). Lines indicate rearrangement of lanes for clarity. The graph shows average relative band intensities for the indicated forms of PKCα from four independent experiments. * brackets indicate statistical significance between conditions. C, comparison of PMA-induced PKCα down-regulation in wild-type and Hsp70−/− MEFs. Immunoblot analysis of immortalized WT and Hsp70−/− MEFs treated with vehicle (C) or 100 nm PMA (P) for 4 h. The graph shows average relative band intensities of total PKCα from three independent experiments. * brackets indicate statistical significance between conditions. Arrows, nonphosphorylated PKCα; arrowheads, mature phosphorylated PKCα.
The second approach involved siRNA-mediated silencing of Hsps. IEC-18 cells express readily detectable Hsc70 and barely detectable levels of Hsp70. Hsc70 knockdown led to induction of Hsp70 (as seen in other systems; Ref. 72), and combined silencing of Hsp70 and Hsc70 was highly cytotoxic (Fig. 3B, lanes 3 and 4, and data not shown), indicating that chaperone function was limiting in cells treated with Hsc70 siRNA. Notably, PMA induced significantly less down-regulation of the mature form of PKCα in cells where Hsc70 had been knocked down compared with cells transfected with nonsilencing siRNA (Fig. 3B, lanes 2 and 4). Furthermore, longer exposures revealed that knockdown of Hsc70 consistently promoted accumulation of dephosphorylated species in PMA-treated cells (Fig. 3B; PKCα LE, compare lane 2 with 4). Thus, Hsc70 knockdown paralleled the effects of PES (Fig. 2B, arrowhead and arrow). In keeping with its barely detectable levels in IEC-18 cells, Hsp70 silencing did not appreciably alter the effects of agonists on PKCα (Fig. 3B, lanes 2 and 6).
Finally, PKCα processing was compared in immortalized wild-type and Hsp70−/− MEFs. Wild-type MEFs express readily detectable levels of Hsp70 (Fig. 3C), indicating that Hsc70 is not sufficient for full chaperone function in these cells. PMA-induced down-regulation of mature PKCα was consistently inhibited in Hsp70−/− cells compared with wild-type MEFs (Fig. 3C, arrowheads, compare lane 2 with 4), indicating that Hsp70/Hsc70 activity was insufficient to support efficient proteasomal degradation of the enzyme in these cells. Interestingly, dephosphorylated PKCα was not detected in PMA-treated Hsp70−/− MEFs, perhaps reflecting rapid proteasomal clearance of this unstable species. Alternatively, maintenance of phosphorylation on activated PKCα may be less sensitive to loss of Hsp70 than proteasomal degradation of the mature species in these cells.
Collectively, the data indicate the following: (a) the Hsp70/Hsc70 chaperone system plays a role in enhancing rephosphorylation and/or preventing dephosphorylation of PKCα in agonist-treated cells; (b) upon activation, mature PKCα undergoes proteasomal degradation via an Hsp70/Hsc70-dependent pathway; and (c) degradation of dephosphorylated PKCα is proteasome-dependent but relatively independent of Hsp70/Hsc70 activity. These findings point to the intriguing possibility that fully primed and dephosphorylated species of PKCα are targeted for proteasomal degradation via different mechanisms.
Hsp90 Protects Activated PKCα from Dephosphorylation and Reduces Agonist-induced Degradation of the Mature Enzyme
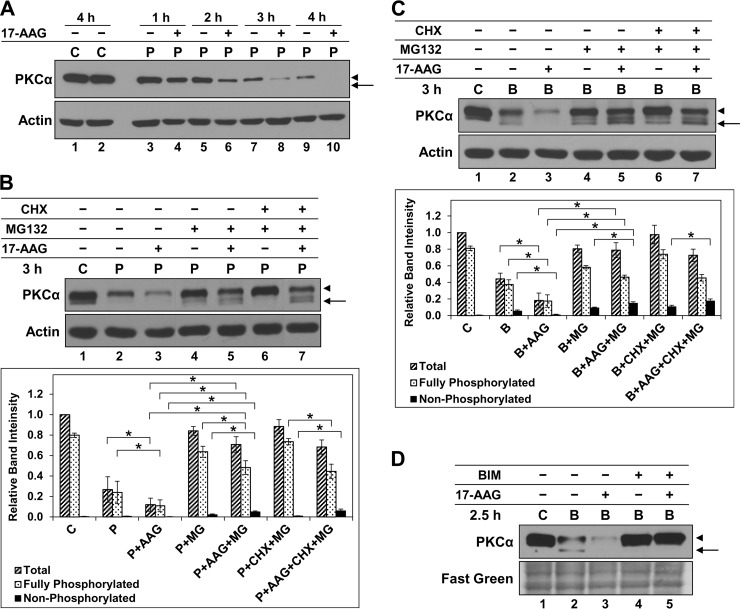
To gain further insight into the mechanisms underlying maintenance and degradation of mature activated PKCα, we explored the role of Hsp90 using 17-AAG (8, 73). This well characterized inhibitor has proved highly useful in studies of Hsp90 function because of its exquisite specificity, which derives from recognition of the unique structure of the ATP binding pocket in this chaperone (73). 17-AAG did not appreciably affect steady-state levels or phosphorylation of unstimulated PKCα in IEC-18 cells over a 4-h time frame (Fig. 4A, lanes 1 and 2); however, Hsp90 inhibition accelerated PMA-induced down-regulation of the protein over this time period (Fig. 4A, compare odd with even lanes). A similar ability of 17-AAG to destabilize PKCα was seen in both PMA- and Bryo-treated cells (Fig. 4, B and C, lanes 1–3), indicating that Hsp90 protects activated PKCα from degradation. Addition of MG132 to prevent degradation of unstable species revealed that Hsp90 inhibition promotes significant accumulation of faster migrating forms of PKCα in PMA- and Bryo-treated cells, which are degraded by the proteasome (Fig. 4, B and C, compare lanes 3, 4, and 5, arrows). These PKCα forms were largely the result of dephosphorylation because they were retained in the presence of CHX (Fig. 4, B and C, lanes 5 and 7). The effects of 17-AAG required PKC catalytic activity, as demonstrated by the ability of the PKC inhibitor bisindolylmaleimide I to block 17-AAG-induced enhanced degradation and dephosphorylation of the enzyme (Fig. 4D; data not shown for PMA). This finding excludes the possibility that the observed effects of 17-AAG are due to alterations in PKC maturation (8, 61) and confirms that Hsp90 modulates the effect of activation on the mature enzyme.
FIGURE 4.
Hsp90 protects activated PKCα from dephosphorylation and degradation in IEC-18 cells. A, effect of Hsp90 inhibition on steady-state levels and PMA-induced degradation of PKCα. IEC-18 cells were treated with vehicle (C) or 100 nm PMA (P) for the indicated times in the presence (+) or absence (−) of 1 μm 17-AAG and subjected to immunoblot analysis for PKCα and β-actin. B, effects of Hsp90 inhibition on phosphorylation and degradation of PKCα activated by PMA. Immunoblot analysis of cells treated with vehicle (C), 100 nm PMA (P), 1 μm 17-AAG, 20 μm MG132, and/or 200 μm CHX, as indicated. Graph shows average relative band intensity from four independent experiments. * brackets indicate statistical significance between conditions. C, effects of Hsp90 inhibition on phosphorylation and degradation of PKCα activated by Bryo. As in B except that cells were treated with 100 nm Bryo rather than PMA. D, effects of 17-AAG require PKC enzyme activity. Immunoblot analysis of cells treated with vehicle (C), 100 nm Bryo (B), 1 μm 17-AAG, and/or 5 μm bisindolylmaleimide I (BIM), as indicated. Fast green staining of the immunoblot membrane is shown as a loading control. Arrows, nonphosphorylated PKCα; arrowheads, mature phosphorylated PKCα. Panels show data from a single blot; dashed lines indicate rearrangement of lanes for clarity. Data are representative of at least three independent experiments.
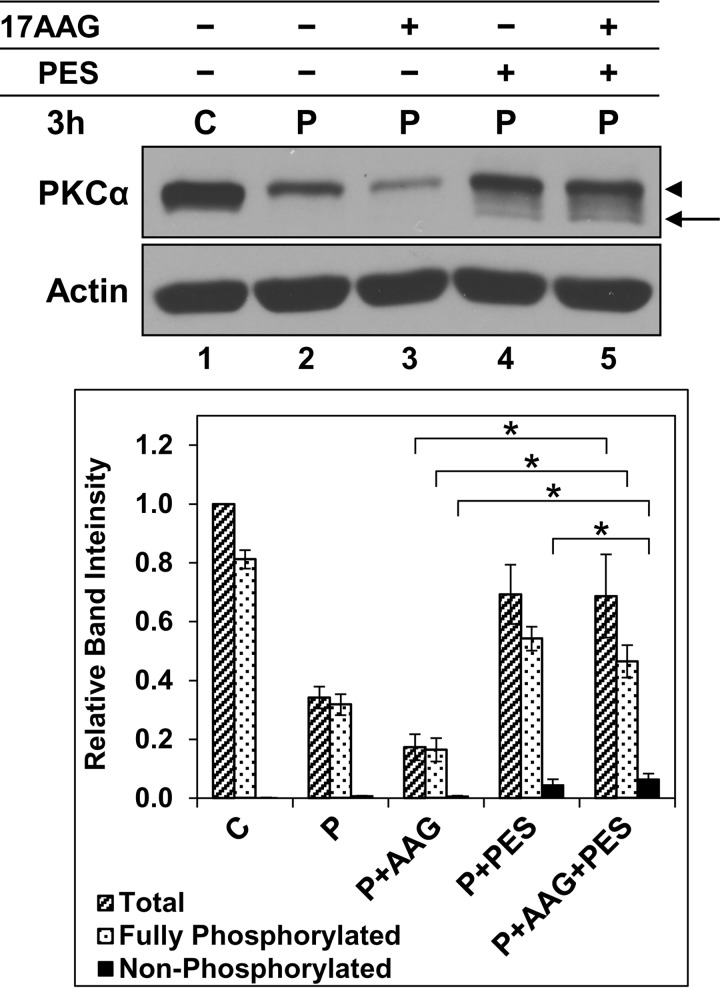
Notably, MG132 also strongly protected the mature form of activated PKCα in 17-AAG-treated cells (Fig. 4, B and C, compare lane 3 with 5), pointing to a role for Hsp90 in stabilizing the fully phosphorylated enzyme. This possibility was tested using PES because Hsp70/Hsc70 inhibition (a) protects phosphorylated PKCα, but not dephosphorylated PKCα, from proteasomal degradation, and (b) prevents rephosphorylation of the dephosphorylated protein (see above). Addition of PES to cells treated with 17-AAG markedly inhibited PMA-induced down-regulation of PKCα (Fig. 5, compare lane 3 with 5). This effect was mainly due to protection of the fully phosphorylated protein (Fig. 5, arrowhead), providing further evidence that Hsp90 directly protects mature PKCα from agonist-induced degradation. Although the majority of PKCα was in the fully phosphorylated form under these conditions, 17-AAG retained the ability to enhance dephosphorylation of activated PKCα in the presence of PES (Fig. 5, compare lane 4 with 5), indicating (a) that Hsp70/Hsc70 and Hsp90 independently protect activated PKCα from dephosphorylation, and (b) that the ability of PES to protect the mature form of activated PKCα in 17-AAG-treated cells is not due to inhibition of its dephosphorylation but rather to an effect on degradation of the phosphorylated protein. Thus, Hsp90 acted on activated PKCα in two ways as follows: it directly protects the phosphorylated form of the protein from proteasomal processing, and it also protects it from dephosphorylation. Although a role for Hsp90 in facilitating priming phosphorylation of newly synthesized PKCs has been described (8, 9, 14, 61), our findings identify novel functions of this chaperone in regulating agonist-induced processing of mature PKCα. In so doing, they provide further support for direct targeting of the phosphorylated species for agonist-induced proteasomal degradation.
FIGURE 5.
Hsp70/Hsc70 and Hsp90 protect mature and activated PKCα from dephosphorylation but play opposing roles in control of proteasomal processing of the phosphorylated enzyme. Immunoblot analysis of PKCα and β-actin in IEC-18 cells treated with vehicle (C), 100 nm PMA (P), 1 μm 17-AAG, and/or 20 μm PES, as indicated. Arrow, nonphosphorylated PKCα; arrowhead, mature phosphorylated PKCα. The graph shows average band intensities from five independent experiments. * brackets indicate statistical significance between conditions.
Nonproteasomal Degradation of Endogenous PKCα Is Not Affected by Hsp Inhibition
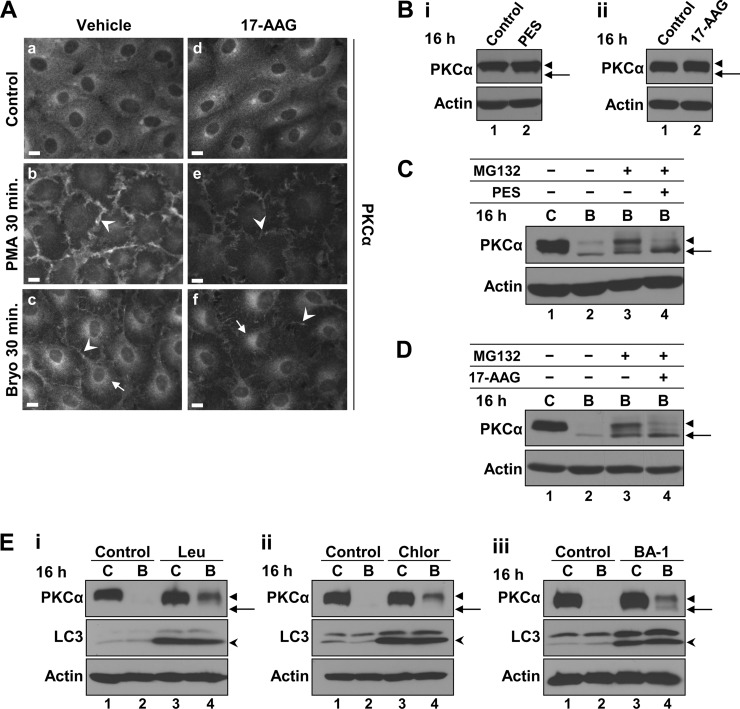
We have recently demonstrated that, in addition to rapid proteasomal degradation of plasma membrane-associated PKCα, Bryo induces slower lysosomal processing of internalized enzyme (Fig. 1B) (28, 39). Because Hsps can regulate endolysosomal trafficking (53, 69), we examined the effects of PES and 17-AAG on Bryo-induced lysosomal processing of internalized PKCα. As seen with PES (Fig. 2D), 17-AAG did not prevent agonist-induced translocation of PKCα to the plasma membrane (Fig. 6A, panels e and f, arrowheads). The diminished levels of membrane-associated PKCα seen in the presence of 17-AAG can be attributed to the increased proteasomal degradation of phosphorylated enzyme under these conditions (Fig. 4). Neither PES nor 17-AAG affected Bryo-induced intracellular accumulation of the enzyme (Figs. 2C and 6A, panels c and f, arrows), indicating that PKCα internalization is independent of Hsp70/Hsc70 and Hsp90 function.
FIGURE 6.
Inhibition of Hsp function does not affect nonproteasomal degradation of activated PKCα in IEC-18 cells. A, immunofluorescence detection of PKCα in cells treated with vehicle (panels a–c) or 1 μm 17-AAG (panels d–f) for 30 min before addition of vehicle (Control), 100 nm Bryo, or 100 nm PMA for a further 30 min. Images were exposed and processed identically. Arrows, perinuclear staining; arrowheads, membrane staining. Magnification bars, 10 μm. B, effects of long term Hsp inhibition on steady-state levels of PKCα. Immunoblot analysis of PKCα and β-actin in cells treated with vehicle (Control), 20 μm PES (panel i) or 1 μm 17-AAG (panel ii) for 16 h. Arrows, nonphosphorylated PKCα; arrowheads, mature phosphorylated PKCα. C and D, effects of PES and 17-AAG on nonproteasomal degradation of activated PKCα. Immunoblot analysis of cells treated with vehicle (C), 100 nm Bryo (B), 20 μm PES, 1 μm 17-AAG, and/or 20 μm MG132, as indicated. Arrows and arrowheads, as in B. E, blockade of lysosomal function protects the phosphorylated form of activated PKCα. Cells treated with vehicle (C) or 100 nm Bryo for 16 h with or without 200 μm leupeptin (Leu, panel i), 50 μm chloroquine (Chlor, panel ii) or 100 nm bafilomycin A1 (BA-1, panel iii) were subjected to immunoblot analysis for PKCα, LC3, and actin. Arrows, nonphosphorylated PKCα; arrowheads, mature phosphorylated PKCα; open arrowheads, faster migrating, lipidated form of LC3 (LC3-II). Data are representative of at least three independent experiments.
Because nonproteasomal degradation of PKCα is most readily observed after prolonged Bryo exposure in the presence of proteasome inhibitors (28, 39), the role of chaperones in this process was examined in cells treated with Bryo for 16 h. As seen at 4 h (Figs. 2A and 4A), a 16-h treatment with either PES or 17-AAG did not appreciably affect steady-state levels or phosphorylation of PKCα in unstimulated IEC-18 cells (Fig. 6B). Nonproteasomal down-regulation of PKCα was clearly evident following 16 h of Bryo treatment in the presence of MG132, with the remaining protein detected in both phosphorylated and nonphosphorylated forms (Fig. 6, C and D, lane 3). Consistent with the role of Hsp70/Hsc70 and Hsp90 in protecting phosphorylation of activated PKCα, a 16-h treatment with Bryo and MG132 in the presence of either PES or 17-AAG led to loss of phosphorylated PKCα, with a concomitant increase in the nonphosphorylated species (Fig. 6, C and D, compare lanes 3 and 4). Fig. 6, C and D, also clearly shows that neither PES nor 17-AAG inhibited the degradation of PKCα following 16 h of Bryo treatment in the presence of MG132, indicating that Hsp function is not required for nonproteasomal/lysosomal degradation of activated PKCα and that changes in phosphorylation state are not a major factor in nonproteasomal processing of the activated enzyme. Lysosomal degradation of phosphorylated PKCα in Bryo-treated cells was further examined by disrupting lysosomal function with the lysosomal protease inhibitor, leupeptin, or by blocking lysosomal acidification with chloroquine or bafilomycin A. The efficacy of these agents was confirmed by their ability to induce accumulation of the lipidated form of microtubule-associated protein light chain 3 (LC3), LC3-II (Fig. 6E, open arrowheads), which is a well characterized target of lysosomal proteases (75). Notably, all three lysosome-disrupting agents mainly protected the fully phosphorylated form of PKCα (Fig. 6E) (39), indicating that, as with the proteasomal pathway, it is the mature form of the protein that represents the major substrate for lysosomal degradation.
DISCUSSION
The activity of PKC isozymes is under tight control. Extensive evidence points to proteolytic degradation as a major mechanism for desensitization of PKC signaling following prolonged activation. A widely accepted desensitization pathway involves proteasomal degradation of dephosphorylated protein that has accumulated in an intracellular detergent-insoluble compartment, with priming site dephosphorylation being the rate-limiting step for degradation (4, 11, 14, 26, 30, 31, 36, 38, 40). Although our findings confirm the existence of this pathway for endogenous PKCα, the data point to the fully phosphorylated form of the activated protein as a major target for degradation in multiple cell types. Agonist-induced down-regulation is accompanied by at most minimal accumulation of nonphosphorylated PKCα, with all or most of the faster migrating species detected coming from delayed maturation rather than dephosphorylation (Fig. 1). This is the case even when degradation of nonphosphorylated PKCα is blocked, with proteasome inhibitors predominantly protecting the mature form of the activated enzyme (Fig. 1) (28). Finally, blockade of rephosphorylation through inhibition of Hsp70/Hsc70 activity confirmed that dephosphorylation of the endogenous protein is a relatively minor response to stimulation, which cannot account for the majority of degradation of the activated species (Fig. 2). The finding that dephosphorylation of PKCα is a minor response to agonist treatment in cells is consistent with evidence that physiological nucleotide occupancy of the active site confers phosphatase resistance to activated PKCs (and other AGC kinases) (42–45). Spatial considerations also argue for direct targeting of the phosphorylated form. Although the major site for proteasomal degradation of activated PKCα is the plasma membrane (Fig. 2D) (28, 39), the enzyme is dephosphorylated in an intracellular compartment (28, 32). Taken together with the fact that activation induces ubiquitination of fully primed PKCα and that phosphatase inhibitors accelerate agonist-induced PKCα ubiquitination and degradation (28), these findings demonstrate that dephosphorylation is not a prerequisite for agonist-induced degradation of conventional PKCs and that targeting of the fully phosphorylated mature protein is a major mechanism for down-regulation of endogenous PKCα.
It has been proposed that priming phosphorylation is required for degradation of PKCδ (41), and phosphorylated PKCβII has been shown to be a substrate for the HOIL-1L/HOIL-1L-interacting protein E3 ubiquitin ligase complex (LUBAC) in vitro (76), indicating that direct targeting of the mature species may be a general mechanism for agonist-induced down-regulation of PKC isozymes. Direct targeting of the activated, phosphorylated protein has also been noted for other AGC kinases (77). For example, although catalytically active AKT can return to an inactive pool via PHLPP/protein phosphatase 2A-mediated dephosphorylation of Thr308 and Ser473 (25, 78), the phosphorylated kinase is also targeted for degradation by a number of ubiquitin ligases, including C-terminal Hsp70-interacting protein (CHIP), tetratrico-peptide repeat domain 3 (TTC3), and BRCA1 (79–81). Thus, engagement of mechanisms that involve either dephosphorylation or direct targeting of the active phosphorylated protein is likely a characteristic of signal termination for the AGC kinase family in general.
In addition to identifying the mature form of PKCα as a major target for agonist-induced down-regulation, our studies unveiled a role for Hsp90 in helping to preserve priming site phosphorylation on activated PKCα (Fig. 4). Previous studies have established that Hsp90 promotes hydrophobic motif phosphorylation during maturation (8, 61); thus, the effects of Hsp90 inhibition on phosphorylation of activated PKCα may, in part, reflect an ability of this chaperone to cooperate with Hsp70/Hsc70 in rephosphorylation of the dephosphorylated protein. However, because 17-AAG also enhanced PKCα dephosphorylation when its rephosphorylation was prevented by Hsp70/Hsc70 inhibition (Fig. 5), it is clear that Hsp90 has protective effects of its own. To our knowledge, these findings provide the first demonstration that Hsp90 activity plays a role in maintenance of phosphorylation on activated PKCα.
Our studies also identify distinct roles for Hsp70/Hsc70 and Hsp90 in regulating the degradation of mature, fully phosphorylated PKCα. Notably, reducing Hsp70/Hsc70 activity was found to protect the mature form from agonist-induced degradation, whereas Hsp90 inhibition accelerated its down-regulation. These different outcomes indicate that the results of disrupting the activity of either chaperone were not due to a general effect of limiting overall cellular chaperone activity, but rather they reflected specific activities of Hsp70/Hsc70 and Hsp90.
Hsp70/Hsc70 was found to be a critical mediator of agonist-induced proteasomal degradation of the primed species (Figs. 2 and 3), with loss of Hsp70/Hsc70 activity stabilizing the mature protein to the same extent as proteasome inhibitors in PKC agonist-treated cells (Fig. 2). In contrast, proteasomal degradation of dephosphorylated PKCα persisted in the presence of Hsp70/Hsc70 inhibition (Fig. 2, B and C), indicating that processing of this species is relatively independent of Hsp70/Hsc70 activity. These findings provide the first evidence for the existence of distinct pathways targeting fully phosphorylated and dephosphorylated PKC to the proteasome. Taken together, the data point to dual opposing roles for Hsp70/Hsc70 activity in regulation of the signaling life cycle of PKCα. On the one hand, the Hsp70 chaperone system stabilizes the active enzyme by facilitating its rephosphorylation and/or inhibiting its dephosphorylation (Figs. 2 and 3) (61), and on the other hand, it is required for proteasomal targeting of mature PKCα after prolonged activation. A dual role of Hsp70 has been reported for other client proteins, including HIF1α and AKT. Under conditions of hypoxia, HIF1α is initially stabilized and subsequently degraded in an Hsp70-dependent manner (82). In the case of AKT, Hsp70 can either promote refolding or degradation of the enzyme depending on the cellular environment (83).
In contrast to Hsp70/Hsc70, Hsp90 plays a major role in protecting phosphorylated PKCα from activation-induced degradation (Fig. 4). It has been proposed that Hsp90 protects client enzymes by binding and stabilizing the ligand binding cleft (52); thus, adoption of an active conformation and exposure of the active site by removal of the pseudosubstrate domain is likely to provide a binding site for Hsp90 on PKCα. This idea is consistent with the binding of Hsp90 to PKCs during maturation when the active site is also not occupied by the pseudosubstrate domain (61). Because activation sensitizes PKCs to both proteasomal degradation and dephosphorylation (44), stabilization of the active site could account for both functions of Hsp90 observed here.
The effects of the combined inhibition of Hsp90 and Hsp70/Hsc70 provide further support for antagonistic roles of these chaperones in the maintenance of the signaling lifetime of activated PKCα. Inhibition of Hsp70/Hsc70 rescued the mature form from the rapid degradation induced by impaired Hsp90 function (Fig. 5), indicating that Hsp90 protects PKCα from engagement by the Hsp70/Hsc70-dependent proteasomal degradation pathway. Although such interplay between Hsp90 and Hsp70 functions has been seen with other molecules, including neuronal NOS (85), ErbB2 (86), and the mineralocorticoid receptor (87), to our knowledge, this is the first time that it has been described for the PKC family. As discussed above, PKC isozymes have been identified as clients for both Hsp70 and Hsp90, arguing that the effects seen here are likely the consequence of direct binding of Hsps to PKCα. However, it is also possible that some or all of the observed effects of these chaperones are mediated by other client proteins.
Our demonstration that endogenous and exogenous PKCα can be processed differently in response to activation (Fig. 1E) brings to mind the differences in regulation of phosphorylation noted for endogenous and overexpressed PKCδ (88), and it highlights the need to study endogenous proteins to understand the regulation of cellular signaling. The ability of PMA to induce dephosphorylation of overexpressed PKCα is reminiscent of its effects on the endogenous protein in the context of Hsp70/Hsc70 and/or Hsp90 inhibition (Figs. 2 and 4). Transfection of cells can elicit a heat-shock response (e.g. 89), raising the possibility that chaperone proteins become limiting under these conditions. Thus, a role for chaperones in protecting activated PKCs from dephosphorylation together with the requirement for Hsp70/Hsc70 for degradation of the mature form may explain why the dephosphorylation → proteasome pathway is often more prominent in studies utilizing overexpressed PKCs.
In contrast to their involvement in proteasomal degradation of PKCα, neither Hsp90 nor Hsp70/Hsc70 appear to play a significant role in nonproteasomal/lysosomal processing of the enzyme (Fig. 6). This finding is in keeping with our demonstration that the two pathways are independent and subject to differential regulation (Fig. 7) (28, 39).
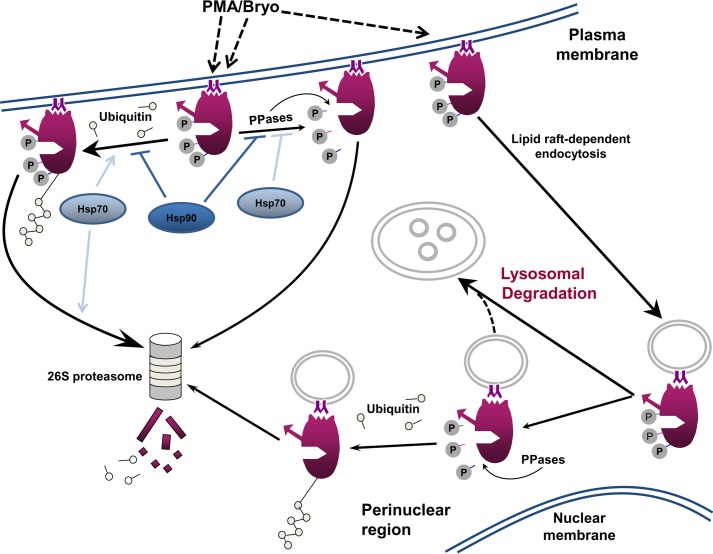
FIGURE 7.
Mechanisms of agonist-induced processing of PKCα. PKC agonists (e.g. PMA and Bryo) promote plasma membrane translocation and activation of mature phosphorylated endogenous PKCα (dashed arrows). Priming phosphorylation of membrane-associated, catalytically active enzyme is maintained by the actions of Hsp70 (light blue) and Hsp90 (dark blue) (PPases: phosphatases). The main pathway for PKCα desensitization at the plasma membrane involves ubiquitination and proteasomal degradation of the mature, fully phosphorylated protein. Hsp90 protects phosphorylated PKCα from proteasomal degradation, whereas Hsp70 activity is required for proteasomal processing of this species. Bryo also targets a pool of mature phosphorylated PKCα for endocytosis in an Hsp-independent manner. Internalized protein is translocated to the perinuclear region, where it is ultimately degraded via a lysosomal pathway. In addition, a small proportion of the internalized protein undergoes dephosphorylation and proteasomal degradation (some of this dephosphorylated protein may also be degraded by the lysosome, dotted line).
Our data, in combination with previous findings (8, 28, 58, 61), suggest a model for agonist-induced desensitization of PKCα signaling (Fig. 7). Activation-induced down-regulation of PKCα can involve proteasomal degradation of both the phosphorylated and dephosphorylated protein, as well as phosphorylation-independent lysosomal degradation of internalized protein. Under normal conditions, activation elicits at most minimal dephosphorylation of the endogenous protein, and the main mechanism of proteasomal degradation involves direct targeting of phosphorylated PKCα at the plasma membrane. We propose that Hsp90 and Hsp70/Hsc70 activities combine to preserve phosphorylation of activated, membrane-associated protein by protecting it from dephosphorylation and by facilitating its rephosphorylation. In addition, Hsp90 directly protects fully phosphorylated PKCα from being targeted for proteasomal degradation, whereas Hsp70/Hsc70 opposes this activity by promoting proteasomal processing of this species (Fig. 7). The ability of PKC agonists to induce proteasomal degradation of PKCα indicates that the destabilizing effects of Hsp70/Hsc70 eventually override the protective effects of these chaperones, even in the presence of Hsp90 activity. The interaction between Hsp and PKC families described here is likely to have important implications for disorders such as cancer, HIV/AIDS, neurodegenerative diseases, and inflammatory bowel disease, which (a) have been linked to aberrant chaperone expression/function (90–92), (b) show altered degradation of activated PKCα and other PKC family members (3, 15–20, 22), and (c) are currently being evaluated as candidates for PKC agonist-based therapies (21, 74, 84, 93).
Acknowledgments
We thank Kathryn J. Curry for expert technical assistance and Drs. Xinjiang Wang and Srikumar M. Raja for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants DK60632 (to J. D. B.) and DK54909 (to J. D. B.), and Cancer Center Grants CA16056 and CA036727. This work was also supported by the Head and Neck Cancer Keystone at the Fox Chase Cancer Center (to G. M. B.).
- cPKC
- conventional PKC
- 17-AAG
- 17-(allylamino)-17-demethoxygeldanamycin
- Bryo
- bryostatin 1
- CHX
- cycloheximide
- DAG
- diacylglycerol
- Hsp
- heat shock protein
- LC3
- microtubule-associated protein light chain 3
- MEF
- mouse embryonic fibroblast
- m.o.i.
- multiplicity of infection
- nPKC
- novel PKC
- PES
- 2-phenylethynesulfonamide
- PES-Cl
- 2-(3-chlorophenyl)ethynesulfonamide
- PHLPP
- pleckstrin homology domain leucine-rich repeat protein phosphatase
- PMA
- phorbol 12-myristate 13-acetate
- TRITC
- tetramethylrhodamine isothiocyanate.
REFERENCES
- 1. Ligeti E., Csépányi-Kömi R., Hunyady L. (2012) Physiological mechanisms of signal termination in biological systems. Acta Physiol. 204, 469–478 [DOI] [PubMed] [Google Scholar]
- 2. Black J. D. (2001) Protein kinase C isozymes in colon carcinogenesis: guilt by omission. Gastroenterology 120, 1868–1872 [DOI] [PubMed] [Google Scholar]
- 3. Alkon D. L., Sun M. K., Nelson T. J. (2007) PKC signaling deficits: a mechanistic hypothesis for the origins of Alzheimer's disease. Trends Pharmacol. Sci. 28, 51–60 [DOI] [PubMed] [Google Scholar]
- 4. Newton A. C. (2010) Protein kinase C: poised to signal. Am. J. Physiol. Endocrinol. Metab. 298, E395–E402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bornancin F., Parker P. J. (1997) Phosphorylation of protein kinase C-α on serine 657 controls the accumulation of active enzyme and contributes to its phosphatase-resistant state. J. Biol. Chem. 272, 3544–3549 [DOI] [PubMed] [Google Scholar]
- 6. Keranen L. M., Dutil E. M., Newton A. C. (1995) Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr. Biol. 5, 1394–1403 [DOI] [PubMed] [Google Scholar]
- 7. Gysin S., Imber R. (1996) Replacement of Ser657 of protein kinase C-α by alanine leads to premature down regulation after phorbol ester-induced translocation to the membrane. Eur. J. Biochem. 240, 747–750 [DOI] [PubMed] [Google Scholar]
- 8. Facchinetti V., Ouyang W., Wei H., Soto N., Lazorchak A., Gould C., Lowry C., Newton A. C., Mao Y., Miao R. Q., Sessa W. C., Qin J., Zhang P., Su B., Jacinto E. (2008) The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 27, 1932–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ikenoue T., Inoki K., Yang Q., Zhou X., Guan K. L. (2008) Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation, and signalling. EMBO J. 27, 1919–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alvi F., Idkowiak-Baldys J., Baldys A., Raymond J. R., Hannun Y. A. (2007) Regulation of membrane trafficking and endocytosis by protein kinase C: emerging role of the pericentrion, a novel protein kinase C-dependent subset of recycling endosomes. Cell. Mol. Life Sci. 64, 263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carmena D., Sardini A. (2007) Lifespan regulation of conventional protein kinase C isotypes. Biochem. Soc. Trans. 35, 1043–1045 [DOI] [PubMed] [Google Scholar]
- 12. Feng X., Hannun Y. A. (1998) An essential role for autophosphorylation in the dissociation of activated protein kinase C from the plasma membrane. J. Biol. Chem. 273, 26870–26874 [DOI] [PubMed] [Google Scholar]
- 13. Feng X., Zhang J., Barak L. S., Meyer T., Caron M. G., Hannun Y. A. (1998) Visualization of dynamic trafficking of a protein kinase C βII/green fluorescent protein conjugate reveals differences in G protein-coupled receptor activation and desensitization. J. Biol. Chem. 273, 10755–10762 [DOI] [PubMed] [Google Scholar]
- 14. Gould C. M., Newton A. C. (2008) The life and death of protein kinase C. Curr. Drug Targets 9, 614–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Basu A. (2010) in Protein Kinase C in Cancer Signaling and Therapy (Kazanietz M. G., ed) pp. 409–429, Springer-Verlag Inc., New York [Google Scholar]
- 16. Huang J., Mohanty S., Basu A. (2004) Cisplatin resistance is associated with deregulation in protein kinase C-δ. Biochem. Biophys. Res. Commun. 316, 1002–1008 [DOI] [PubMed] [Google Scholar]
- 17. Ward N. E., O'Brian C. A. (1991) Distinct patterns of phorbol ester-induced downregulation of protein kinase C activity in adriamycin-selected multidrug resistant and parental murine fibrosarcoma cells. Cancer Lett. 58, 189–193 [DOI] [PubMed] [Google Scholar]
- 18. Cloud-Heflin B. A., McMasters R. A., Osborn M. T., Chambers T. C. (1996) Expression, subcellular distribution and response to phorbol esters of protein kinase C (PKC) isozymes in drug-sensitive and multidrug-resistant KB cells evidence for altered regulation of PKC-α. Eur. J. Biochem. 239, 796–804 [DOI] [PubMed] [Google Scholar]
- 19. Zemskov E. A., Nukina N. (2003) Impaired degradation of PKCα by proteasome in a cellular model of Huntington's disease. Neuroreport 14, 1435–1438 [DOI] [PubMed] [Google Scholar]
- 20. Favit A., Grimaldi M., Nelson T. J., Alkon D. L. (1998) Alzheimer's-specific effects of soluble β-amyloid on protein kinase C-α and -γ degradation in human fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 95, 5562–5567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeChristopher B. A., Loy B. A., Marsden M. D., Schrier A. J., Zack J. A., Wender P. A. (2012) Designed, synthetically accessible bryostatin analogues potently induce activation of latent HIV reservoirs in vitro. Nat. Chem. 4, 705–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mashukova A., Wald F. A., Salas P. J. (2011) Tumor necrosis factor α and inflammation disrupt the polarity complex in intestinal epithelial cells by a posttranslational mechanism. Mol. Cell. Biol. 31, 756–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dutil E. M., Keranen L. M., DePaoli-Roach A. A., Newton A. C. (1994) In vivo regulation of protein kinase C by trans-phosphorylation followed by autophosphorylation. J. Biol. Chem. 269, 29359–29362 [PubMed] [Google Scholar]
- 24. Bornancin F., Parker P. J. (1996) Phosphorylation of threonine 638 critically controls the dephosphorylation and inactivation of protein kinase Cα. Curr. Biol. 6, 1114–1123 [DOI] [PubMed] [Google Scholar]
- 25. Brognard J., Newton A. C. (2008) PHLiPPing the switch on Akt and protein kinase C signaling. Trends Endocrinol. Metab. 19, 223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gao T., Brognard J., Newton A. C. (2008) The phosphatase PHLPP controls the cellular levels of protein kinase C. J. Biol. Chem. 283, 6300–6311 [DOI] [PubMed] [Google Scholar]
- 27. Hansra G., Bornancin F., Whelan R., Hemmings B. A., Parker P. J. (1996) 12-O-Tetradecanoylphorbol-13-acetate-induced dephosphorylation of protein kinase Cα correlates with the presence of a membrane-associated protein phosphatase 2A heterotrimer. J. Biol. Chem. 271, 32785–32788 [DOI] [PubMed] [Google Scholar]
- 28. Leontieva O. V., Black J. D. (2004) Identification of two distinct pathways of protein kinase Cα down-regulation in intestinal epithelial cells. J. Biol. Chem. 279, 5788–5801 [DOI] [PubMed] [Google Scholar]
- 29. Parker P. J., Bosca L., Dekker L., Goode N. T., Hajibagheri N., Hansra G. (1995) Protein kinase C (PKC)-induced PKC degradation: a model for down-regulation. Biochem. Soc. Trans. 23, 153–155 [DOI] [PubMed] [Google Scholar]
- 30. Lee H. W., Smith L., Pettit G. R., Bingham Smith J. (1996) Dephosphorylation of activated protein kinase C contributes to downregulation by bryostatin. Am. J. Physiol. 271, C304–C311 [DOI] [PubMed] [Google Scholar]
- 31. Lee H. W., Smith L., Pettit G. R., Smith J. B. (1997) Bryostatin 1 and phorbol ester down-modulate protein kinase C-α and -ϵ via the ubiquitin/proteasome pathway in human fibroblasts. Mol. Pharmacol. 51, 439–447 [PubMed] [Google Scholar]
- 32. Hansra G., Garcia-Paramio P., Prevostel C., Whelan R. D., Bornancin F., Parker P. J. (1999) Multisite dephosphorylation and desensitization of conventional protein kinase C isotypes. Biochem. J. 342, 337–344 [PMC free article] [PubMed] [Google Scholar]
- 33. Junoy B., Maccario H., Mas J. L., Enjalbert A., Drouva S. V. (2002) Proteasome implication in phorbol ester- and GnRH-induced selective down-regulation of PKC (α, ϵ, ζ) in α T(3)-1 and L β T(2) gonadotrope cell lines. Endocrinology 143, 1386–1403 [DOI] [PubMed] [Google Scholar]
- 34. Faghiri Z., Bazan N. G. (2006) Selective relocalization and proteasomal downregulation of PKCα induced by platelet-activating factor in retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 47, 397–404 [DOI] [PubMed] [Google Scholar]
- 35. Olivier A. R., Parker P. J. (1994) Bombesin, platelet-derived growth factor, and diacylglycerol induce selective membrane association and down-regulation of protein kinase C isotypes in Swiss 3T3 cells. J. Biol. Chem. 269, 2758–2763 [PubMed] [Google Scholar]
- 36. Lee H. W., Smith L., Pettit G. R., Vinitsky A., Smith J. B. (1996) Ubiquitination of protein kinase C-α and degradation by the proteasome. J. Biol. Chem. 271, 20973–20976 [PubMed] [Google Scholar]
- 37. Lu Z., Liu D., Hornia A., Devonish W., Pagano M., Foster D. A. (1998) Activation of protein kinase C triggers its ubiquitination and degradation. Mol. Cell. Biol. 18, 839–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prevostel C., Alice V., Joubert D., Parker P. J. (2000) Protein kinase Cα actively downregulates through caveolae-dependent traffic to an endosomal compartment. J. Cell Sci. 113, 2575–2584 [DOI] [PubMed] [Google Scholar]
- 39. Lum M. A., Pundt K. E., Paluch B. E., Black A. R., Black J. D. (2013) Agonist-induced down-regulation of endogenous protein kinase Cα through an endolysosomal mechanism. J. Biol. Chem. 288, 13093–13109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Melnikov S., Sagi-Eisenberg R. (2009) Down-regulating protein kinase Cα: functional cooperation between the proteasome and the endocytic system. Cell. Signal. 21, 1607–1619 [DOI] [PubMed] [Google Scholar]
- 41. Srivastava J., Procyk K. J., Iturrioz X., Parker P. J. (2002) Phosphorylation is required for PMA- and cell cycle-induced degradation of protein kinase Cδ. Biochem. J. 368, 349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chan T. O., Pascal J. M., Armen R. S., Rodeck U. (2012) Autoregulation of kinase dephosphorylation by ATP binding in AGC protein kinases. Cell Cycle 11, 475–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Srivastava J., Goris J., Dilworth S. M., Parker P. J. (2002) Dephosphorylation of PKCδ by protein phosphatase 2Ac and its inhibition by nucleotides. FEBS Lett. 516, 265–269 [DOI] [PubMed] [Google Scholar]
- 44. Gould C. M., Antal C. E., Reyes G., Kunkel M. T., Adams R. A., Ziyar A., Riveros T., Newton A. C. (2011) Active site inhibitors protect protein kinase C from dephosphorylation and stabilize its mature form. J. Biol. Chem. 286, 28922–28930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cameron A. J., Escribano C., Saurin A. T., Kostelecky B., Parker P. J. (2009) PKC maturation is promoted by nucleotide pocket occupation independently of intrinsic kinase activity. Nat. Struct. Mol. Biol. 16, 624–630 [DOI] [PubMed] [Google Scholar]
- 46. Brocchieri L., Conway de Macario E., Macario A. J. (2008) hsp70 genes in the human genome: Conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol. Biol. 8, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Black A. R., Subjeck J. R. (1991) The biology and physiology of the heat shock and glucose-regulated stress protein systems. Methods Achiev. Exp. Pathol. 15, 126–166 [PubMed] [Google Scholar]
- 48. Daugaard M., Rohde M., Jäättelä M. (2007) The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 581, 3702–3710 [DOI] [PubMed] [Google Scholar]
- 49. Massey A. J., Williamson D. S., Browne H., Murray J. B., Dokurno P., Shaw T., Macias A. T., Daniels Z., Geoffroy S., Dopson M., Lavan P., Matassova N., Francis G. L., Graham C. J., Parsons R., Wang Y., Padfield A., Comer M., Drysdale M. J., Wood M. (2010) A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in HCT116 colon carcinoma cells. Cancer Chemother. Pharmacol. 66, 535–545 [DOI] [PubMed] [Google Scholar]
- 50. Schmitt E., Gehrmann M., Brunet M., Multhoff G., Garrido C. (2007) Intracellular and extracellular functions of heat shock proteins: Repercussions in cancer therapy. J. Leukocyte Biol. 81, 15–27 [DOI] [PubMed] [Google Scholar]
- 51. Grune T., Catalgol B., Licht A., Ermak G., Pickering A. M., Ngo J. K., Davies K. J. (2011) HSP70 mediates dissociation and reassociation of the 26S proteasome during adaptation to oxidative stress. Free Radic. Biol. Med. 51, 1355–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pratt W. B., Morishima Y., Peng H. M., Osawa Y. (2010) Proposal for a role of the Hsp90/Hsp70-based chaperone machinery in making triage decisions when proteins undergo oxidative and toxic damage. Exp. Biol. Med. 235, 278–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shin Y., Klucken J., Patterson C., Hyman B. T., McLean P. J. (2005) The co-chaperone carboxyl terminus of Hsp70-interacting protein (CHIP) mediates α-synuclein degradation decisions between proteasomal and lysosomal pathways. J. Biol. Chem. 280, 23727–23734 [DOI] [PubMed] [Google Scholar]
- 54. Lüders J., Demand J., Höhfeld J. (2000) The ubiquitin-related BAG-1 provides a link between the molecular chaperones Hsc70/Hsp70 and the proteasome. J. Biol. Chem. 275, 4613–4617 [DOI] [PubMed] [Google Scholar]
- 55. Luo W., Zhong J., Chang R., Hu H., Pandey A., Semenza G. L. (2010) Hsp70 and CHIP selectively mediate ubiquitination and degradation of hypoxia-inducible factor (HIF)-1α but not HIF-2α. J. Biol. Chem. 285, 3651–3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fisher E. A., Zhou M., Mitchell D. M., Wu X., Omura S., Wang H., Goldberg A. L., Ginsberg H. N. (1997) The degradation of apolipoprotein B100 is mediated by the ubiquitin-proteasome pathway and involves heat shock protein 70. J. Biol. Chem. 272, 20427–20434 [DOI] [PubMed] [Google Scholar]
- 57. Gao T., Newton A. C. (2002) The turn motif is a phosphorylation switch that regulates the binding of Hsp70 to protein kinase C. J. Biol. Chem. 277, 31585–31592 [DOI] [PubMed] [Google Scholar]
- 58. Gao T., Newton A. C. (2006) Invariant Leu preceding turn motif phosphorylation site controls the interaction of protein kinase C with Hsp70. J. Biol. Chem. 281, 32461–32468 [DOI] [PubMed] [Google Scholar]
- 59. Abrahamsen H., O'Neill A. K., Kannan N., Kruse N., Taylor S. S., Jennings P. A., Newton A. C. (2012) Peptidyl-prolyl isomerase Pin1 controls down-regulation of conventional protein kinase C isozymes. J. Biol. Chem. 287, 13262–13278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Whitesell L., Lindquist S. L. (2005) HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 5, 761–772 [DOI] [PubMed] [Google Scholar]
- 61. Gould C. M., Kannan N., Taylor S. S., Newton A. C. (2009) The chaperones Hsp90 and Cdc37 mediate the maturation and stabilization of protein kinase C through a conserved PXXP motif in the C-terminal tail. J. Biol. Chem. 284, 4921–4935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pysz M. A., Leontieva O. V., Bateman N. W., Uronis J. M., Curry K. J., Threadgill D. W., Janssen K. P., Robine S., Velcich A., Augenlicht L. H., Black A. R., Black J. D. (2009) PKCα tumor suppression in the intestine is associated with transcriptional and translational inhibition of cyclin D1. Exp. Cell Res. 315, 1415–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pears C., Stabel S., Cazaubon S., Parker P. J. (1992) Studies on the phosphorylation of protein kinase C-α. Biochem. J. 283, 515–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cazaubon S. M., Parker P. J. (1993) Identification of the phosphorylated region responsible for the permissive activation of protein kinase C. J. Biol. Chem. 268, 17559–17563 [PubMed] [Google Scholar]
- 65. Cazaubon S., Bornancin F., Parker P. J. (1994) Threonine-497 is a critical site for permissive activation of protein kinase Cα. Biochem. J. 301, 443–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Borner C., Filipuzzi I., Wartmann M., Eppenberger U., Fabbro D. (1989) Biosynthesis and posttranslational modifications of protein kinase C in human breast cancer cells. J. Biol. Chem. 264, 13902–13909 [PubMed] [Google Scholar]
- 67. Mashukova A., Oriolo A. S., Wald F. A., Casanova M. L., Kröger C., Magin T. M., Omary M. B., Salas P. J. (2009) Rescue of atypical protein kinase C in epithelia by the cytoskeleton and Hsp70 family chaperones. J. Cell Sci. 122, 2491–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Leu J. I., Pimkina J., Frank A., Murphy M. E., George D. L. (2009) A small molecule inhibitor of inducible heat shock protein 70. Mol. Cell 36, 15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Leu J. I., Pimkina J., Pandey P., Murphy M. E., George D. L. (2011) HSP70 inhibition by the small-molecule 2-phenylethynesulfonamide impairs protein clearance pathways in tumor cells. Mol. Cancer Res. 9, 936–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Balaburski G., Leu J. I., Andrake M., Hayik S., Beeharry N., Dunbrack R., Yen T., George D. L., Murphy M. E. (2012) Characterization of the mechanism of action of a novel small molecule inhibitor of HSP70. AACR Annual Meeting, Abstr. 3793 [Google Scholar]
- 71. Balaburski G., Leu J. I.-J., Beeharry N., Hayik S., Andrake M. D., Zhang G., Herlyn M., Villanueva J., Dunbrack R. L., Yen T., George D. L., Murphy M. E. (2013) A modified HSP70 inhibitor shows broad activity as an anticancer agent. Mol. Cancer Res. 11, 219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Powers M. V., Clarke P. A., Workman P. (2009) Death by chaperone: HSP90, HSP70 or both? Cell Cycle 8, 518–526 [DOI] [PubMed] [Google Scholar]
- 73. Chiosis G., Huezo H., Rosen N., Mimnaugh E., Whitesell L., Neckers L. (2003) 17AAG: low target binding affinity and potent cell activity–finding an explanation. Mol. Cancer Ther. 2, 123–129 [PubMed] [Google Scholar]
- 74. Irie K., Yanagita R. C., Nakagawa Y. (2012) Challenges to the development of bryostatin-type anticancer drugs based on the activation mechanism of protein kinase Cδ. Med. Res. Rev. 32, 518–535 [DOI] [PubMed] [Google Scholar]
- 75. Mizushima N., Yoshimori T., Levine B. (2010) Methods in mammalian autophagy research. Cell 140, 313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nakamura M., Tokunaga F., Sakata S., Iwai K. (2006) Mutual regulation of conventional protein kinase C and a ubiquitin ligase complex. Biochem. Biophys. Res. Commun. 351, 340–347 [DOI] [PubMed] [Google Scholar]
- 77. Pearce L. R., Komander D., Alessi D. R. (2010) The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 11, 9–22 [DOI] [PubMed] [Google Scholar]
- 78. Liao Y., Hung M. C. (2010) Physiological regulation of Akt activity and stability. Am. J. Transl. Res. 2, 19–42 [PMC free article] [PubMed] [Google Scholar]
- 79. Su C. H., Wang C. Y., Lan K. H., Li C. P., Chao Y., Lin H. C., Lee S. D., Lee W. P. (2011) Akt phosphorylation at Thr308 and Ser473 is required for CHIP-mediated ubiquitination of the kinase. Cell. Signal. 23, 1824–1830 [DOI] [PubMed] [Google Scholar]
- 80. Suizu F., Hiramuki Y., Okumura F., Matsuda M., Okumura A. J., Hirata N., Narita M., Kohno T., Yokota J., Bohgaki M., Obuse C., Hatakeyama S., Obata T., Noguchi M. (2009) The E3 ligase TTC3 facilitates ubiquitination and degradation of phosphorylated Akt. Dev. Cell 17, 800–810 [DOI] [PubMed] [Google Scholar]
- 81. Xiang T., Ohashi A., Huang Y., Pandita T. K., Ludwig T., Powell S. N., Yang Q. (2008) Negative regulation of AKT activation by BRCA1. Cancer Res. 68, 10040–10044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Guo W., Yang Z., Xia Q., Liu J., Yu Y., Li J., Zuo Z., Zhang D., Li X., Shi X., Huang C. (2011) Arsenite stabilizes HIF-1α protein through p85α-mediated up-regulation of inducible Hsp70 protein expression. Cell. Mol. Life Sci. 68, 475–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Koren J., 3rd, Jinwal U. K., Jin Y., O'Leary J., Jones J. R., Johnson A. G., Blair L. J., Abisambra J. F., Chang L., Miyata Y., Cheng A. M., Guo J., Cheng J. Q., Gestwicki J. E., Dickey C. A. (2010) Facilitating Akt clearance via manipulation of Hsp70 activity and levels. J. Biol. Chem. 285, 2498–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. McKernan L. N., Momjian D., Kulkosky J. (2012) Protein kinase C: One pathway towards the eradication of latent HIV-1 reservoirs. Adv. Virol. 2012, 805347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Peng H.-M., Morishima Y., Clapp K. M., Lau M., Pratt W. B., Osawa Y. (2009) Dynamic cycling with Hsp90 stabilizes neuronal nitric oxide synthase through calmodulin-dependent inhibition of ubiquitination. Biochemistry 48, 8483–8490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhou P., Fernandes N., Dodge I. L., Reddi A. L., Rao N., Safran H., DiPetrillo T. A., Wazer D. E., Band V., Band H. (2003) ErbB2 degradation mediated by the co-chaperone protein CHIP. J. Biol. Chem. 278, 13829–13837 [DOI] [PubMed] [Google Scholar]
- 87. Faresse N., Ruffieux-Daidie D., Salamin M., Gomez-Sanchez C. E., Staub O. (2010) Mineralocorticoid receptor degradation is promoted by Hsp90 inhibition and the ubiquitin-protein ligase CHIP. Am. J. Physiol. Renal Physiol. 299, F1462–F1472 [DOI] [PubMed] [Google Scholar]
- 88. Rybin V. O., Guo J., Gertsberg Z., Elouardighi H., Steinberg S. F. (2007) Protein kinase Cϵ (PKCδ) and Src control PKCδ activation loop phosphorylation in cardiomyocytes. J. Biol. Chem. 282, 23631–23638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fiszer-Kierzkowska A., Vydra N., Wysocka-Wycisk A., Kronekova Z., Jarząb M., Lisowska K. M., Krawczyk Z. (2011) Liposome-based DNA carriers may induce cellular stress response and change gene expression pattern in transfected cells. BMC Mol. Biol. 12, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Luo W., Sun W., Taldone T., Rodina A., Chiosis G. (2010) Heat shock protein 90 in neurodegenerative diseases. Mol. Neurodegen. 5, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Turturici G., Sconzo G., Geraci F. (2011) Hsp70 and its molecular role in nervous system diseases. Biochem. Res. Int. 2011, 618127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Calderwood S. K., Khaleque M. A., Sawyer D. B., Ciocca D. R. (2006) Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem. Sci. 31, 164–172 [DOI] [PubMed] [Google Scholar]
- 93. Corbett A., Smith J., Ballard C. (2012) New and emerging treatments for Alzheimer's disease. Expert. Rev. Neurother. 12, 535–543 [DOI] [PubMed] [Google Scholar]