Background: Notch-1 plays a critical role in cell fate decisions by modulating cellular processes under irradiation.
Results: Irradiation-induced Notch-1 overexpression promoted survival and EMT in NSCLC, whereas rhamnetin and cirsiliol inhibited these effects via miR-34a-mediated Notch-1 down-regulation.
Conclusion: Rhamnetin and cirsiliol suppress Notch-1-mediated radioresistance and EMT phenotypes in NSCLC.
Significance: Rhamnetin and cirsiliol can act as novel radiosensitizers by inhibiting radiation-induced Notch-1 signaling.
Keywords: Cancer Therapy, EMT, Lung Cancer, Natural Products, Radiation Biology, Cirsiliol, Notch-1, Rhamnetin, miR-34a, Radiosensitization
Abstract
Radioresistance is a major cause of decreasing the efficiency of radiotherapy for non-small cell lung cancer (NSCLC). To understand the radioresistance mechanisms in NSCLC, we focused on the radiation-induced Notch-1 signaling pathway involved in critical cell fate decisions by modulating cell proliferation. In this study, we investigated the use of Notch-1-regulating flavonoid compounds as novel therapeutic drugs to regulate radiosensitivity in NSCLC cells, NCI-H1299 and NCI-H460, with different levels of radioresistance. Rhamnetin and cirsiliol were selected as candidate Notch-1-regulating radiosensitizers based on the results of assay screening for activity and pharmacological properties. Treatment with rhamnetin or cirsiliol reduced the proliferation of NSCLC cells through the suppression of radiation-induced Notch-1 expression. Indeed, rhamnetin and cirsiliol increased the expression of tumor-suppressive microRNA, miR-34a, in a p53-dependent manner, leading to inhibition of Notch-1 expression. Consequently, reduced Notch-1 expression promoted apoptosis through significant down-regulation of the nuclear factor-κB pathway, resulting in a radiosensitizing effect on NSCLC cells. Irradiation-induced epithelial-mesenchymal transition was also notably attenuated in the presence of rhamnetin and cirsiliol. Moreover, an in vivo xenograft mouse model confirmed the radiosensitizing and epithelial-mesenchymal transition inhibition effects of rhamnetin and cirsiliol we observed in vitro. In these mice, tumor volume was significantly reduced by combinational treatment with irradiation and rhamnetin or cirsiliol compared with irradiation alone. Taken together, our findings provided evidence that rhamnetin and cirsiliol can act as promising radiosensitizers that enhance the radiotherapeutic efficacy by inhibiting radiation-induced Notch-1 signaling associated with radioresistance possibly via miR-34a-mediated pathways.
Introduction
Lung cancer is not only the leading cause of cancer death but is the most commonly diagnosed cancer type worldwide. This disease can be categorized into two morphological types as follows: non-small cell lung cancer (NSCLC),2 which represents the majority of lung cancer cases, and small cell lung cancer. Most patients with lung cancer are diagnosed with inoperable and advanced stage disease (1). Radiotherapy plays a critical role in curative management of patients with inoperable cases of NSCLC (2). However, therapeutic outcomes of radiotherapy are often not fully satisfactory, and radioresistance of cancer cells is considered to be a main factor that prevents successful treatment (3). Although many efforts have been made to regulate radioresistance and develop potent radiosensitizers to establish better treatment strategies, there is currently no molecular targeted therapy that can be well combined with irradiation for treating lung cancer. Thus, the identification of molecular biomarker(s) responsible for emergence of radioresistance and modification of the radiation response by radiosensitizers would greatly advance the development of drugs that increase the sensitivity of NSCLC cells to irradiation (4–6).
Clinical data have demonstrated that 30% of NSCLC cases have increased Notch-1 activity, whereas 10% have a gain-of-function mutation in the NOTCH-1 gene (7). After a series of proteolytic cleavages, the active form of Notch-1 translocates from the cell membrane into the nucleus and subsequently regulates the expression of target genes, such as CCND1, Bcl-2, and Survivin (8–10). Because Notch-1 influences critical cell fate decisions, alterations in Notch-1 signaling are associated with tumorigenesis (7). Overexpression of Notch-1 has been shown to inhibit apoptosis in many human cancers, suggesting its potential as a therapeutic target (11, 12). Recently, Notch-1 has been reported to increase the survival of NSCLC cells under hypoxic conditions by activating the insulin-like growth factor pathway (13). The expression of cyclin D1 (encoded by CCND1), a downstream target of Notch-1, is another indicator of poor prognosis for resectable NSCLC (14).
Accumulating data have demonstrated that miRNAs play important roles in cancer development by regulating the expression of various oncogenes and tumor suppressor genes (15). Various miRNAs are reported to affect tumor development at multiple stages and tumor radiosensitivity by regulating DNA damage response, cell cycle arrest, apoptosis, radiation-induced signaling, and tumor microenvironment (16). Recently, the transcription factor encoded by the tumor suppressor gene p53 was shown to regulate the expression of miRNA in response to DNA-damaging stimuli (17, 18). The most significant level of expression induced by p53 was observed for the miR-34a, a direct target of p53 (19). Ectopic miR-34a expression induces apoptosis, cell cycle arrest, or senescence (17). Furthermore, the loss of miR-34a expression has been linked to resistance to apoptosis induced by p53-activating agents used in chemotherapy (20).
Epithelial-mesenchymal transition (EMT) is a process by which epithelial cells undergo phenotypic transition into mesenchymal cells (21). During cancer progression, tumor cells become more invasive after undergoing EMT and gain access to blood vessels through intravasation resulting in distant metastasis, the major cause of death from cancer (22). Several factors have been shown to induce EMT in vitro and in vivo, including hypoxia, reactive oxygen species, transforming growth factor β1, and radiation (23–25). Radiation elicits cell behaviors that are strongly associated with epithelial cancer such as differentiation dysregulation, genomic instability, phenotypic transition such as EMT, and aberrant multicellular organization (26, 27). Interestingly, several lines of evidence indicate that Notch-1 signaling is critical for EMT (23, 28). It has been demonstrated that blocking Notch-1 signaling by Hey-1 or Jagged1 knockdown or a γ-secretase inhibitor (GSI) attenuates EMT (29).
Chemotherapy alone or in combination with radiation provides new paradigms for various methods of disease management (30–32). There have been many investigations for an application of flavonoids as anti-carcinogenic and radiosensitizing agents, because flavonoids display anti-inflammatory, anti-oxidant, anti-proliferative, and anti-bacterial activities (33, 34). Some of the flavonoids, such as genistein, quercetin, baicalin, baicalein, and niclosamide, have been reported to regulate the Notch-1 pathway in various cancer cell lines (35–38). Thus, we performed flavonoid screening and selected rhamnetin and cirsiliol as Notch-1-regulating candidates, which have been poorly studied in cancer.
To better understand the pharmacological regulation of radioresistance, we initiated a project to identify potent and selective radiosensitizers for overcoming the radioresistance of NSCLC cells. Here, we report on the ability of two flavonoids (rhamnetin and cirsiliol) to induce radiosensitization and EMT inhibition through miR-34a-mediated Notch-1 suppression in NSCLC cells. The lead compounds may show therapeutic effects for treating radioresistant tumors as well as provide the basis for developing specific inhibitors.
EXPERIMENTAL PROCEDURES
Chemicals and Reagents
Rhamnetin and cirsiliol were obtained from Sigma and Lancaster (Heysham, UK), respectively. Cell culture media (RPMI 1640), FBS, penicillin, streptomycin, and TRIzol® were acquired from Invitrogen. miR-34a mimics (sequences for hsa-miR-34a, 5′-UGG CAG UGU CUU AGC UGG UUG U-3′) and a negative control miRNA mimic were obtained from Dharmacon (Chicago, IL). Control siRNA, Notch-1 siRNA, and p53 siRNA were purchased from Dharmacon. The NOTCH-1 cDNA expression vector pCMV6-Entry/Notch-1 was from OriGene Technologies, Inc. (Rockville, MD).
Cell Lines, Cell Culture, Irradiation, and Drug Treatment
Two human NSCLC cell lines, NCI-H1299 and NCI-H460, and two normal human lung cell lines, WI-26 VA4 and MRC-5, were acquired from the American Type Culture Collection (ATCC, Manassas, VA). Cells were exposed to a single dose of γ-rays using a Gamma Cell 40 Exactor (Nordion International, Inc., Kanata, Ontario, Canada) at a dose rate of 0.81 Gy/min. After 6 h, the cells were subjected to further analyses, including biochemical studies. Flasks containing the control cells were placed in the irradiation chamber but were not exposed to radiation. Cells were treated with rhamnetin and cirsiliol dissolved in DMSO for 4 h.
Animal Maintenance
Six-week-old male BALB/c athymic nude mice (Central Lab Animals Inc., Seoul, South Korea) were used for the in vivo experiments. The protocols used were approved by the Institutional Animal Care and Use Committee of Pusan National University (Busan, South Korea) and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animals were housed individually or in groups of up to five in sterile cages. They were maintained in animal care facilities in a temperature-regulated room (23 ± 1 °C) with a 12-h light/dark cycle and were quarantined for 1 week prior to the study. They were fed water and a standard mouse chow diet ad libitum.
Flow Cytometry, Immunofluorescence (IF), and Immunohistochemistry (IHC)
For flow cytometry, cells were harvested after the experimental treatments. The cells were then incubated with the appropriate dilution of anti-Notch-1 antibody (Cell Signaling Technology). After washing with PBS, the cells were incubated with DyLight 488-conjugated secondary antibodies (Thermo Scientific, Rockford, IL). The cells were then washed again before analysis using a FACSVerse flow cytometer (BD Biosciences).
For IF, cells were grown on glass slides. Following the experimental treatments, the cells were fixed and permeabilized in cold acetone and then washed with cold PBS. After blocking with 1% bovine serum albumin/PBS, the cells were incubated overnight with anti-Notch-1 antibody at 4 °C. Next, the cells were washed three times with cold PBS and incubated with DyLight 488-conjugated secondary antibodies (Thermo Scientific). After washing and counterstaining with DAPI (Sigma), the glass slides were mounted with VECTASHIELD Hard-Set Mounting Medium (Vector Laboratories, Burlingame, CA) and visualized with an Olympus IX71 fluorescence microscope (Olympus Optical Co. Ltd., Tokyo, Japan).
For IHC, the mice were sacrificed after the experimental treatments. Sections 4-mm thick were cut, fixed in formalin, and embedded in paraffin before being dewaxed and rehydrated. The sections were then incubated in 3% hydrogen peroxide/methanol. Antigen retrieval was performed by incubation with 0.25% pepsin (Dako, Carpinteria, CA). The sections were blocked in blocking solution (Dako) and then incubated overnight with anti-Notch-1 antibody (AbCAM, Cambridge, UK) at 4 °C. After washing with TBST, the sections were incubated with polymer-HRP-conjugated secondary antibody (Dako). A 3,3′-diaminobenzidine substrate chromogen system (Dako) was used to detect antibody binding. The stained sections were examined with an Olympus IX71 inverted microscope (Olympus Optical Co. Ltd.).
Thiazolyl Blue Tetrazolium Bromide Assay (Cell Viability Assay)
Cells were seeded and cultured in a 24-well plate with or without different concentrations of rhamnetin or cirsiliol for 4 h. The media were removed; a 0.05% thiazolyl blue tetrazolium bromide solution (Sigma) was added, and the cells were incubated at 37 °C for 2 h. Next, the thiazolyl blue tetrazolium bromide solution was replaced with DMSO, and the plates were incubated for 10 min. After incubation, the solution was transferred to a 96-well plate in duplicate, and the absorbance was measured.
Colony Forming Assay
Cells were plated at a density of 300 cells in 6-well dishes. After 24 h, the cells were treated with the indicated drugs, exposed to a specific dose of radiation, and subsequently grown for 14 days. Next, the cells were fixed with 10% methanol, 10% acetic acid and stained with 1% crystal violet. Colonies containing more than 50 cells were identified using densitometric software and scored as survivors (39).
Northern Blot Analysis
Following the experimental treatments, total cellular RNA was isolated from cells (3 × 106 cells) using TRIzol® (Invitrogen). The RNA was separated in a 1.2% agarose gel containing formaldehyde and transferred to nylon membranes. The membranes were UV cross-linked, prehybridized for 30 min in ExpressHyb hybridization solution, and hybridized for 4 h at 65 °C with radiolabeled DNA probes specific for human NOTCH-1 cDNA (forward oligonucleotide, 5′-AGC TCT GGT TCC CTG AGG GCT T-3′, and reverse oligonucleotide, 5′-ATG CAG TCG GCG TCA ACC TCA C-3′). The NOTCH-1 probes were labeled with [α-32P]CTP using a random priming kit. Following hybridization, the membranes were washed twice (first in 1× SSC and then 0.1% SDS). The washed membranes were then subjected to autoradiography.
Western Blot Analysis, Immunoprecipitation (IP), and Transient Transfection
Following the experimental treatment, Western blot analysis and IP studies were performed as described previously (40). For Western blot analysis or IP, all the antibodies were from Santa Cruz Biotechnology or Cell Signaling Technology. For transient transfection, cells were plated at a density of 5 × 105 cells in 6-well dishes and incubated for 4 h. The cells were transiently transfected with the indicated plasmid using Lipofectin (Invitrogen), the siRNA oligonucleotides targeting NOTCH-1 and p53 using DharmaFECT 1 (Dharmacon), and the miR-34a mimics using Lipofectamine 2000 transfection reagent (Invitrogen), respectively, according to the manufacturer's instructions.
Quantitative RT-PCR (qRT-PCR)
Six sets of primers (Table 1) were designed based on the primary precursor molecular sequences from a human miRNA database (41). The primers were first validated on human genomic DNA. Following the experimental treatments, total cellular RNA was isolated from 3 × 106 cells using TRIzol® (Invitrogen). cDNA was prepared using an ImProm-IITM reverse transcription system (Promega, Madison, WI) according to the manufacturer's instructions. Reverse transcription was then carried out in a mixture with each gene-specific primer and U6 RNA. Each RT product was used as a template for quantitative PCR performed with a thermal cycler (Eppendorf Mastercycler gradient, Hauppauge, NY).
TABLE 1.
Primers for determining levels of expression of human miRNA molecules, pro-survival genes, and EMT-related genes
| miRNA or gene name | Forward primer | Reverse primer |
|---|---|---|
| U6 (control) | 5′-CTC GCT TCG GCA GCA CA-3′ | 5′-AAC GCT TCA CGA ATT TGC GT-3′ |
| miR-92 | 5′-TCT ACA CAG GTT GGG ATC GG-3′ | 5′-CGG GAC AAG TGC AAT ACC ATA-3′ |
| miR-93 | 5′-AAG TGC TGT TCG TGC AGG T-3′ | 5′-CTC GGG AAG TGC TAG CTC A-3′ |
| miR-106b | 5′-TAA AGT GCT GAC AGT GCA GAT AGT G-3′ | 5′-CAA GTA CCC ACA GTG CGG T-3′ |
| miR-7-1 | 5′-TGG AAG ACT AGT GAT TTT GTT GT-3′ | 5′-AGA CTG TGA TTT GTT GTC GAT T-3′ |
| miR-34a | 5′-TGG CAG TGT CTT AGC TGG TTG T-3′ | 5′-GGC AGT ATA CTT GCT GAT TGC TT-3′ |
| miR-99a | 5′-TAA ACC CGT AGA TCC GAT CTT G-3′ | 5′-CCA CAG ACA CGA GCT TGT G-3′ |
| cIAP1 | 5′-CAG CCT GAG CAG CTT GCA A-3′ | 5′-CAA GCC ACC ATC ACA ACA AAA-3′ |
| cIAP2 | 5′-TCC GTC AAG TTC AAG CCA GTT-3′ | 5′-TCT CCT GGG CTG TCT GAT GTG-3′ |
| Survivin | 5′-TGC CTG GCA GCC CTT TC-3′ | 5′-CCT CCA AGA AGG GCC AGT TC-3′ |
| E-cadherin | 5′-GGA TTG CAA ATT CCT GCC ATT C-3′ | 5′-AAC GTT GTC CCG GGT GTC A-3′ |
| Vimentin | 5′-GAC AAT GCG TCT CTG GCA CGT CTT-3′ | 5′-TCC TCC GCC TCC TGC AGG TTC TT-3′ |
| Fibronectin | 5′-TGA CCT TTT CTG GCT CGT CT-3′ | 5′-GTT CAG CAC AAA GGG CTC TC-3′ |
Real Time Quantitative RT-PCR (Real Time qRT-PCR)
The levels of miR-34a, pro-survival genes, and EMT-related gene expression were measured using real time qRT-PCR as described previously (42) with several modifications. Briefly, aliquots of a master mix containing all of the reaction components with the primers were dispensed into a real time PCR plate (Applied Biosystems, Foster City, CA). All of the PCR reagents were from a SYBR Green core reagent kit (Applied Biosystems). The expression of miR-34a and all genes evaluated was measured in triplicate in the reaction plate. Real time qRT-PCR was performed with an Applied Biosystems 7900 HT real time qRT-PCR instrument. PCR was performed for 40 cycles of 95 °C for 15 s and 60 °C for 1 min followed by thermal denaturation. The expression of each miRNA relative to U6 RNA was determined using the 2−ΔCT method (43). To simplify the data presentation, values for the relative expression were multiplied by 102.
Luciferase Reporter Gene Assay
Following co-transfection with an NF-κB (NF-κB-Luc) or Notch-1 (pmirGlo-Notch-1) luciferase reporter plasmid and specific combination of plasmids as indicated, the medium was changed, and the cells were treated with drugs and/or irradiation. After 6 h, the cells were washed twice with cold PBS and lysed in reporter lysis buffer (Promega). The lysates were vortexed and centrifuged at 12,000 × g for 1 min at 4 °C. The lysate and luciferase assay reagent were then mixed at room temperature. Luciferase activity in the solution was measured with a luminometer (AutoLumat LB 953, EG & G Berthold, Bad Wildbad, Germany).
Apoptosis Assay
Apoptosis induction was assessed by analyzing cytoplasmic histone-associated DNA fragmentation. In brief, cells were plated in 96-well plates and allowed to attach overnight. The cells were then subjected to the desired treatment with transfection, irradiation, and/or drugs. Cytoplasmic histone-associated DNA fragmentation was monitored using a cell death detection kit (Roche Applied Science) according to the manufacturer's instructions.
Transwell Cell Migration Assay
A cell migration assay was performed using a 24-well Transwell chamber (Corning, Corning, NY). Cells cultured in the presence or absence of treatments (specific transfection, irradiation, and/or drugs) for 60 h (NCI-H1299) or 72 h (NCI-H460) were seeded in the upper chamber of an insert with 5-μm pores. The lower chamber was filled with 600 μl of RPMI 1640 medium containing 2% FBS. 6 h later, the upper membrane surface was wiped with a cotton swab to remove cells that had not migrated to the lower chamber. Cells that had migrated and attached to the lower membrane surface were fixed with 4% paraformaldehyde, stained with hematoxylin, and counted. The migration index was calculated and normalized to the number of untreated cells that had migrated.
Wound Healing Assay
Cells were cultured to 70% confluency in RPMI 1640 medium with 1% FBS. The cell monolayers were then scratched with a 200-μl pipette tip. The cells were further incubated with fresh medium with or without treatment for 15 h (NCI-H1299) or 48 h (NCI-H460). Photomicrographs were taken at ×100 magnification with an Olympus IX71 inverted microscope (Olympus Optical Co. Ltd.).
Tumor Xenografts in Nude Mice
BALB/c athymic nude mice (n = three per group) were injected with 2 × 106 NCI-H1299 cells in the flank, and palpable tumors were allowed to develop. When the tumor had acquired a minimal volume of 200 mm3, DMSO or drug (200 μg/kg body weight) was administered intraperitoneally every day for 25 days. The animals were also irradiated with 10 Gy once a week for 3 weeks. On day 25, the tumors were excised and subjected to further analyses. Tumor length (L) and width (W) were measured with a caliper, and tumor volumes were calculated with the formula (L × W2)/2.
Statistical Analysis
All numeric data are presented as the mean ± S.D. for at least three independent experiments. Experimental results were analyzed using a one-way analysis of variance for ranked data followed by a Tukey's honestly significant difference test, and a two-way analysis of variance for ranked data followed by a Bonferroni post test. Prism 4 software (GraphPad Software, San Diego) was used to conduct all statistical analyses. A p value < 0.05 was considered statistically significant.
RESULTS
Irradiation Increases Notch-1 Expression in the NSCLC Cell Lines
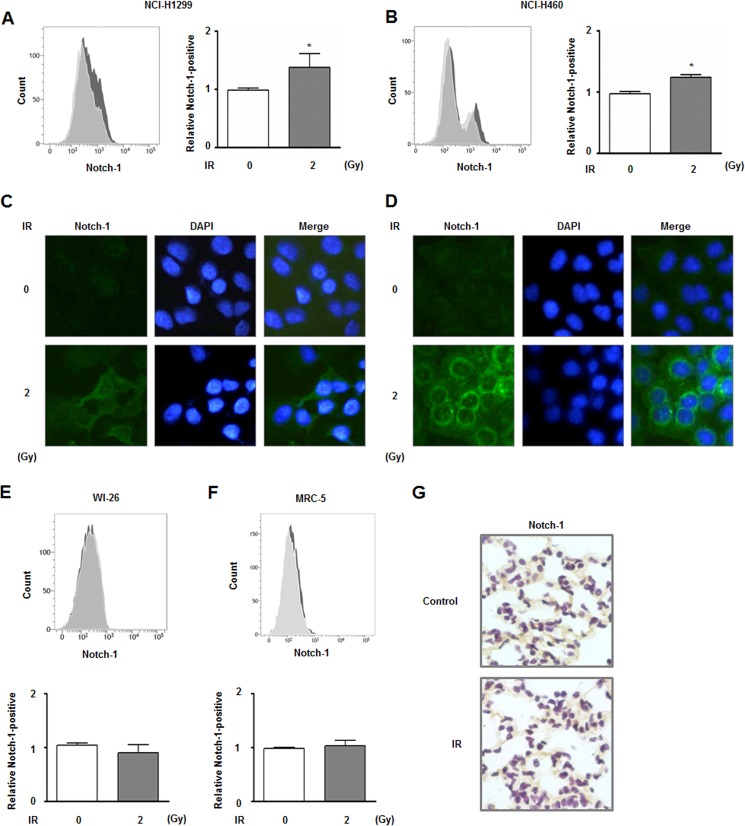
Notch-1 is highly overexpressed in NSCLC cells and could be involved in the survival of these cells (44, 45). Based on this information and our previous transcriptome analysis (46), we measured the expression of Notch-1 in irradiated NCI-H1299 and NCI-H460 cells in this study. After a single dose of irradiation (2 Gy), the expression of Notch-1 in NCI-H1299 and NCI-H460 cells was analyzed by flow cytometry. The populations of Notch-1-positive NCI-H1299 and NCI-H460 cells were increased ∼1.38- and 1.24-fold, respectively, after irradiation (Fig. 1, A and B). Radiation-induced overexpression of Notch-1 in both types of cells was also observed with an IF staining assay (Fig. 1, C and D). In addition, this experiment showed that Notch-1, possibly the active form of the protein, was detected in the cytoplasm and nucleus in response to irradiation. Next, we showed that no significant alteration of Notch-1 expression was observed in two normal lung cell lines, human lung epithelial cells (WI-26 VA4) and human fetal lung fibroblast (MRC-5), by flow cytometry (Fig. 1, E and F). IHC data concurred with the flow cytometry result in that no differences in Notch-1 expression patterns were observed between normal lung tissue from irradiated mice and the control mouse lung tissue (Fig. 1G).
FIGURE 1.
Irradiation increases Notch-1 expression in NCI-H1299 and NCI-H460 cells. A, altered Notch-1 expression in NCI-H1299 cells in response to 2 Gy of radiation was analyzed by flow cytometry. The number of Notch-1-positive cells that had been irradiated (gray) was compared with those of cells that had not been irradiated (white). The relative sizes of Notch-1-positive populations of NCI-H1299 cells are indicated in the graph based on the flow cytometry data. *, p < 0.05 for nonirradiated cells versus irradiated cells. B, same experiments shown in A were performed using NCI-H460 cells. *, p < 0.05 for nonirradiated cells versus irradiated cells. C, altered Notch-1 expression in NCI-H1299 cells after receiving 2 Gy of radiation was also assessed by IF. Notch-1 and cell nuclei are shown as green and blue (DAPI) signals, respectively. D, same experiments shown in C were performed using NCI-H460 cells. E and F, same experiments shown in A were performed using two normal lung cell lines, WI-26 VA4 and MRC-5. G, effect of Notch-1 expression on the lung tissue from mice exposed to 20 Gy of radiation was assessed by IHC.
Considering the diverse biological functions of Notch-1 in cell survival, we hypothesized that radiation-induced overexpression of Notch-1 could be a major factor for radioresistance, and Notch-1-specific inhibitors could induce radiosensitization in NSCLC cells. Because several flavonoids have been reported to regulate the Notch-1 pathway in various cancer cell lines, a large number of flavonoids were prepared for screening (37, 38). Based on their effect on Notch-1 expression, fewer than 10 candidates were selected and analyzed according to solubility and molecular weight along with Lipinski-like criteria (42). Ultimately, two compounds were selected for further investigation, rhamnetin and cirsiliol (Fig. 2A).
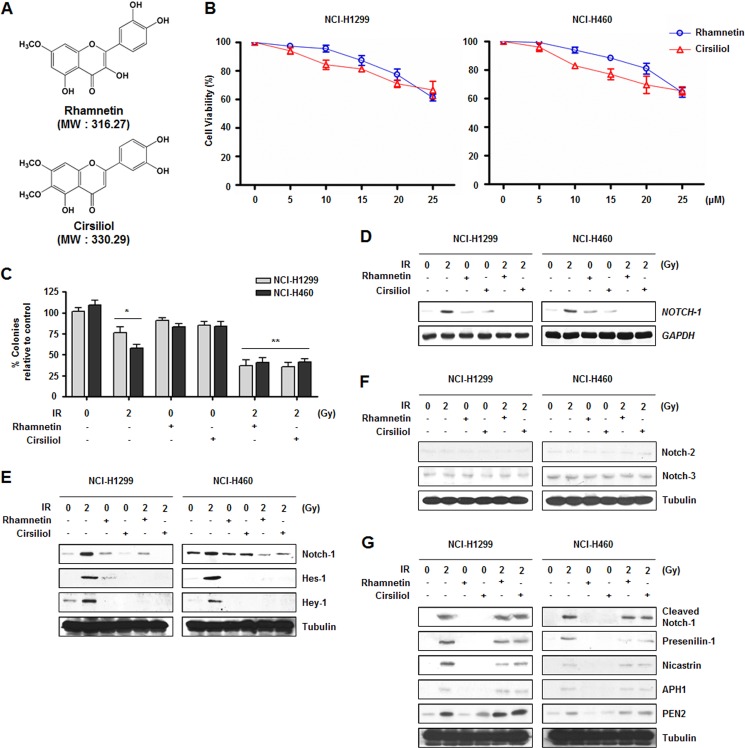
FIGURE 2.
Rhamnetin and cirsiliol in combination with radiation reduce cell proliferation through the inhibition of Notch-1 expression in NSCLC cells. A, chemical structures of rhamnetin and cirsiliol. B, effects of rhamnetin and cirsiliol on the viability of NCI-H1299 and NCI-H460 cells were measured by a triazolyl blue tetrazolium bromide assay. Cells were treated for 4 h with the rhamnetin or cirsiliol at concentrations up to 25 μm. Absorbance was measured at 570 nm. C, effects of the rhamnetin and cirsiliol on proliferation of the NSCLC cells were measured with a colony forming assay. *, p < 0.05 for nonirradiated cells versus irradiated cells; **, p < 0.05 for irradiated cells versus irradiated cells treated with each drug. D, effects of rhamnetin and cirsiliol on radiation-induced NOTCH-1 mRNA expression in NSCLC cells were evaluated with a Northern blot analysis. Total RNA was analyzed using a α-32P-labeled NOTCH-1 cDNA probe. GAPDH was used for normalization. E, effects of rhamnetin and cirsiliol on the radiation-induced expression of Notch-1, and downstream target proteins were detected by Western blot analysis. F, effects of radiation, rhamnetin, and cirsiliol on the expression of Notch-2 and Notch-3 were detected by Western blot analysis. G, effects of rhamnetin and cirsiliol on radiation-induced increase of cleaved-Notch-1 and γ-secretase complex proteins were assessed by Western blot analysis.
Rhamnetin or Cirsiliol in Combination with Radiation Reduces NSCLC Cell Proliferation through Inhibition of Notch-1 Expression
To determine the concentration of rhamnetin and cirsiliol that could be used without affecting cell viability, NCI-H1299 and NCI-H460 cells were treated with different concentrations of rhamnetin and cirsiliol (Fig. 2B). Neither rhamnetin nor cirsiliol affected cell viability up to concentrations of 15 or 10 μm, respectively. The noncytotoxic concentrations of these compounds used for this study were identified.
To determine whether rhamnetin and cirsiliol can regulate radiosensitivity in NSCLC cells, a colony forming assay was performed (Fig. 2C). Treatment with rhamnetin or cirsiliol alone did not affect cell proliferation, whereas combinational treatment with radiation significantly suppressed proliferation of the NCI-H1299 and NCI-H460 cells compared with cells treated with radiation alone. Taken together, these results suggest that rhamnetin and cirsiliol could be a radiosensitizer in NSCLC cells.
Given the essential roles of Notch-1 in cell proliferation, we next evaluated the effect of rhamnetin- and cirsiliol-administered with radiation on Notch-1 signaling in the NSCLC cells. Both radiation-induced Notch-1 mRNA and protein expression were down-regulated by rhamnetin- and cirsiliol-radiation combinations (Fig. 2, D and E), whereas expressions of Notch-2 and Notch-3 were not significantly affected by radiation-alone exposure or combinational treatments of radiation and each compound (Fig. 2F). Additionally, reduced expression of Notch-1 targets, Hes-1 and Hey-1, was observed (Fig. 2E). We next determined whether initial activation of Notch-1 signaling is regulated by rhamnetin- and cirsiliol-radiation combinational treatment (Fig. 2G). The radiation-induced expression of cleaved Notch-1 and all components of the γ-secretase complex (presenilin-1, nicastrin, APH1, and PEN2) were not affected by either the rhamnetin- or cirsiliol-radiation combinations. These data suggest that the combined administration of rhamnetin or cirsiliol with radiation inhibits the expression but not the activation of Notch-1.
Rhamnetin or Cirsiliol Increases the Expression of miR-34a in a p53-dependent Manner in NSCLC Cells
The expression of miRNA is regulated by several cellular stress stimuli such as radiation or hypoxia and in turn influences tumor progression (17, 20, 47). Here, we determined whether certain miRNA molecules could regulate the gene expression of Notch-1 in the presence of rhamnetin or cirsiliol with radiation because several types of miRNA have been identified as key regulators of Notch-1 signaling (48, 49). We first examined changes in the expression of three oncogenic miRNAs (miR-92, miR-93, and miR-106b) and three tumor-suppressive miRNAs (miR-7–1, miR-34a, and miR-99a) in NSCLC cells after treatments with rhamnetin and cirsiliol (50). First, we performed qRT-PCR using miRNA-specific primers. As shown in Fig. 3A, the expression of miR-34a was increased by treatments with rhamnetin and cirsiliol, whereas expression of the other five miRNAs was not significantly changed in either cell line after the treatments. Our real time qRT-PCR results confirmed that the expression of miR-34a was up-regulated in both cell lines after treatments with rhamnetin and cirsiliol compared with the DMSO-treated control cells (Fig. 3B). In addition, the relative change in miR-34a expression was more significant when the cells were treated with combinations of rhamnetin or cirsiliol and radiation compared with the cells that were only irradiated. The most dramatic changes in the relative expression of miR-34a (8.5-fold) were observed in NCI-H1299 cells after treatment with 15 μm rhamnetin.
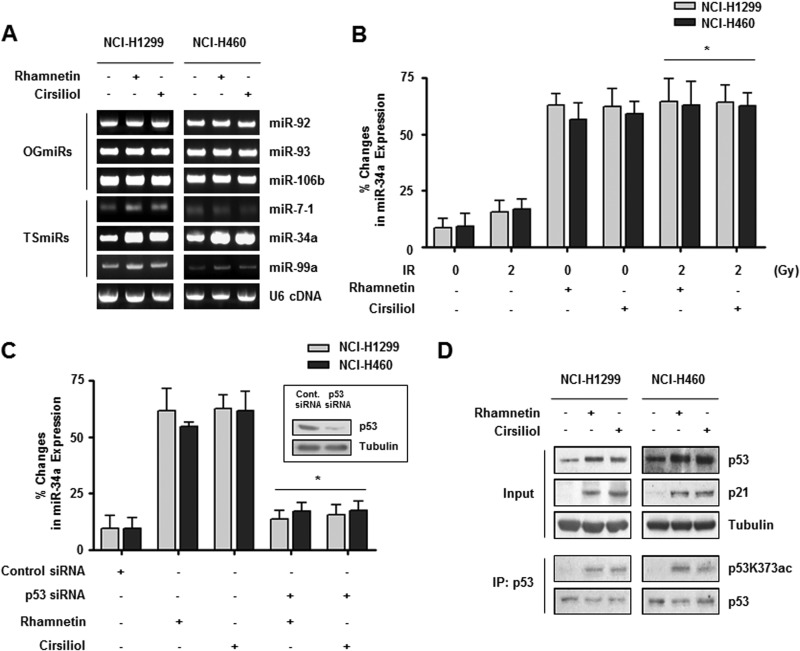
FIGURE 3.
Rhamnetin and cirsiliol increase the expression of miR-34a in a p53-dependent manner in NSCLC cells. A, effects of rhamnetin and cirsiliol on the expression of oncogenic miRNA and tumor-suppressive miRNA in NCI-H1299 and NCI-H460 cells were measured by qRT-PCR. Amplified PCR products were analyzed by 2.2% agarose gel electrophoresis. B, effects of rhamnetin and cirsiliol on miR-34a expression were measured by real time qRT-PCR. Data showing the percent change in miR-34a expression in NSCLC cells normalized to U6 RNA are presented. *, p < 0.05 for irradiated cells versus irradiated cells treated with each drug. C, direct involvement of p53 in rhamnetin- and cirsiliol-mediated overexpression of miR-34a was confirmed by p53 siRNA expression and real time qRT-PCR. The inset shows that siRNA oligonucleotides specific for p53 significantly reduced p53 expression. *, p < 0.05 for cells treated with each drug versus p53 siRNA-transfected cells treated with each drug. D, effects of rhamnetin and cirsiliol on the expression of p53 and p21 in NSCLC cells were evaluated by Western blot analysis. In addition, the effects of rhamnetin and cirsiliol on p53 acetylation at lysine 373 were assessed with an immunoprecipitation (IP) assay using p53K373ac antibody.
A previous report has shown that members of the miR-34 family are direct targets of p53, and their up-regulation induces several cellular responses such as G1 cell cycle arrest, apoptosis, senescence, and inhibition of migration (17). To determine whether the expression of miR-34a increased by rhamnetin and cirsiliol is mediated in a p53-dependent manner, siRNA specific for p53 (p53 siRNA) was prepared, and real time qRT-PCR was performed on the cells expressing p53 siRNA. Transient p53 knockdown significantly decreased miR-34a expression in the presence of rhamnetin and cirsiliol (Fig. 3C). Additionally, the levels of total p53 expression, p53 acetylated at residue 373, and p21 expression were increased by treatment with rhamnetin and cirsiliol in both types of NSCLC cells (Fig. 3D). These results suggest that rhamnetin and cirsiliol enhance p53 activity and stability by promoting acetylation at lysine 373 in the C terminus of p53. This in turn induced the specific expression of miR-34a, a representative tumor-suppressive miRNA, and the effect was synergistically enhanced by irradiation in NSCLC cells (51, 52).
Rhamnetin- and Cirsiliol-activated miR-34a Directly Targets NOTCH-1 through Interaction with the 3′-UTR
Because we found that combinations of rhamnetin or cirsiliol with radiation down-regulated Notch-1 expression and increased p53-dependent miR-34a expression, we further examined the direct relationship between these two effects. Given the well known mode of miRNA action, we hypothesized that miR-34a might increase cell proliferation by regulating NOTCH-1 genes in NSCLC cells treated with rhamnetin- or cirsiliol-radiation combinations. As shown in Fig. 4A, NOTCH-1 was predicted to have a putative miR-34a-binding site within its 3′-UTR according to the PicTar-Vert and TargetScan miRNA Release 5.1 target prediction programs. Overexpression of miR-34a mimics decreased Notch-1 protein levels, indicating that miR-34a negatively regulated Notch-1 expression in NSCLC cells (Fig. 4B). Next, we performed a luciferase reporter assay to confirm the direct regulation of NOTCH-1 by miR-34a with rhamnetin or cirsiliol treatment in the two NSCLC cell lines. Our findings demonstrated that miR-34a could directly bind to the 3′-UTR of NOTCH-1 and suppressed luciferase expression in the presence of rhamnetin or cirsiliol. The binding of miR-34a to the NOTCH-1 3′-UTR was specific because the luciferase construct with a mutated NOTCH-1 3′-UTR was not affected by miR-34a expression (Fig. 4C). These data indicated that NOTCH-1 is a direct target gene of miR-34a in NSCLC cells treated with rhamnetin- or cirsiliol-radiation combinations.
FIGURE 4.
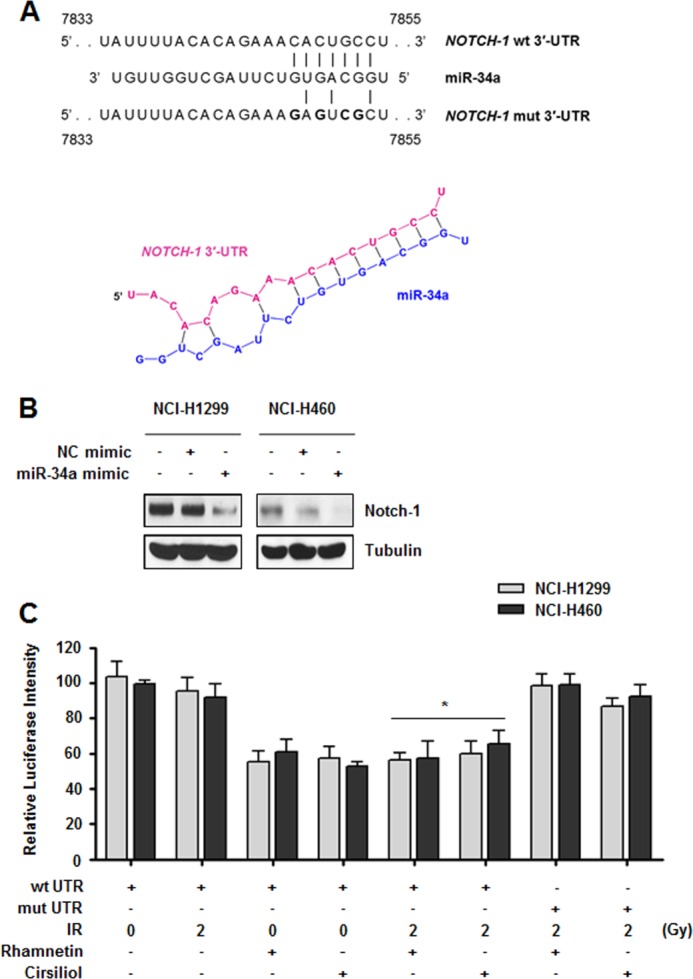
Rhamnetin- and cirsiliol-activated miR-34a directly targets NOTCH-1 through interaction with the 3′-UTR. A, 3′-UTR of wild-type NOTCH-1 mRNA contains an miR-34a-binding site. The mutated NOTCH-1 3′-UTR is also shown (upper panel). The predicted secondary structure of the NOTCH-1 3′-UTR with miR-34a is shown in red and blue, respectively (lower panel). B, effect of miR-34a overexpression on Notch-1 expression was measured by Western blot analysis. Before the assay, NCI-H1299 and NCI-H460 cells were transfected with either the miR-34a mimic or negative control miRNA mimic for 48 h. C, direct regulation of Notch-1 by miR-34a with rhamnetin and cirsiliol treatment was measured with a luciferase assay. NSCLC cells were transfected with luciferase reporter plasmids expressing the wild-type (wt UTR) or mutated (mut UTR) 3′-UTR. The cells were then treated with rhamnetin or cirsiliol and radiation. After 6 h, luciferase activity was measured. *, p < 0.05 for irradiated cells versus irradiated cells treated with each drug.
Inhibition of Notch-1 Expression Increases Apoptosis and Radiosensitivity of NSCLC Cells
Because the function of miR-34a (whose expression is induced by rhamnetin and cirsiliol) may be associated with Notch-1 in NSCLC cells, the function and effect of rhamnetin and cirsiliol on radioresistance was assessed. In the two types of NSCLC cells, we evaluated treatment with rhamnetin- and cirsiliol-enhanced radiation-induced apoptosis. These effects were similar to ones observed with miR-34a overexpression and Notch-1 knockdown (inset; Fig. 5A). In addition, overexpression of Notch-1 by cDNA transfection rescued the cells from rhamnetin- and cirsiliol-induced apoptosis to a certain degree (Fig. 5, B and C).
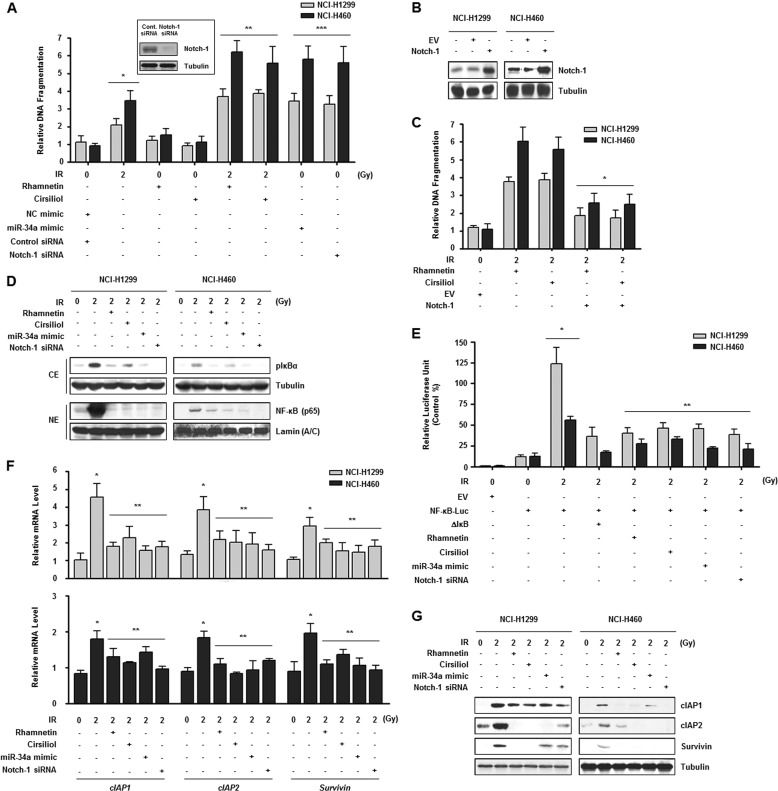
FIGURE 5.
Inhibition of Notch-1 expression increases apoptosis and radiosensitivity of NSCLC cells. A, involvement of rhamnetin- and cirsiliol-induced miR-34a overexpression and Notch-1 down-regulation on radiosensitization was evaluated by monitoring the apoptosis of NCI-H1299 and NCI-H460 cells. The inset shows that siRNA oligonucleotides specific for Notch-1 significantly reduced Notch-1 expression. *, p < 0.05 for nonirradiated cells versus irradiated cells; **, p < 0.05 for irradiated cells versus irradiated cells treated with each drug; ***, p < 0.05 for irradiated cells versus nonirradiated cells treated with the miR-34a mimic or Notch-1 siRNA. B, Notch-1 overexpression after transfection with a Notch-1 expression vector was measured by Western blot analysis. C, rescue from rhamnetin- and cirsiliol-induced apoptosis by Notch-1 overexpression in the NSCLC cells was examined by an apoptosis assay. *, p < 0.05 for irradiated cells treated with each drug versus irradiated cells treated with each drug and Notch-1 siRNA. D, effects of rhamnetin and cirsiliol on radiation-induced IκBα phosphorylation and NF-κB (p65 subunit) nuclear translocation in NSCLC cells were detected by Western blot analysis. CE, cytoplasmic extract; NE, nuclear extract. E, effect of rhamnetin and cirsiliol on irradiation-induced NF-κB transcriptional activity was assessed with a luciferase assay. *, p < 0.05 for nonirradiated cells versus irradiated cells; **, p < 0.05 for irradiated cells versus irradiated cells treated with each drug, the miR-34a mimic, or Notch-1 siRNA. F, effects of rhamnetin and cirsiliol on the expression of pro-survival genes in irradiated NSCLC cells were analyzed by real time qRT-PCR. *, p < 0.05 for nonirradiated cells versus irradiated cells; **, p < 0.05 for irradiated cells versus irradiated cells treated with each drug, the miR-34a mimic or Notch-1 siRNA. G, effects of rhamnetin and cirsiliol on the expression of pro-survival proteins in irradiated NSCLC cells were analyzed by Western blot analysis.
Several studies have demonstrated that cancer cells may acquire resistance to radiation-induced apoptosis through the dynamic interplay and regulation of multiple pro-survival factors (40, 42). Therefore, we next investigated the relationship of apoptosis induced by rhamnetin- or cirsiliol-radiation treatment with activation of the nuclear factor-κ B (NF-κB) pathway in NSCLC cells. As shown in Fig. 5, D and E, IκBα phosphorylation, nuclear translocation of p65, and NF-κB transcriptional activation increased by irradiation in NSCLC cells was suppressed by treatment of rhamnetin and cirsiliol. Similar results were produced by miR-34a overexpressed and Notch-1 knockdown in the NSCLC cells. Our findings indicated that the transcriptional activity of NF-κB could be affected by rhamnetin- and cirsiliol-dependent overexpression of miR-34a as well as inhibition of Notch-1 expression. Ultimately, these events could impede NF-κB-mediated pro-survival gene expression. We subsequently examined changes in the transcription of cIAP1 (cellular inhibitor of apoptosis 1), cIAP2, and Survivin, three well known NF-κB-regulated pro-survival genes, induced by rhamnetin and cirsiliol (53). Significant reduction of cIAP1, cIAP2, and Survivin mRNA levels with concomitant reduction of protein expression was observed in rhamnetin- and cirsiliol-treated NSCLC cells following irradiation. In addition, expression of these genes was reduced by Notch-1 down-regulation (Fig. 5, F and G). These results suggest that rhamnetin- and cirsiliol-induced down-regulation of pro-survival signaling such as NF-κB signaling decreases pro-survival gene expression, regulates apoptosis, and ultimately confers radiosensitization in NSCLC cells.
miR-34a-mediated Suppression of Notch-1 Expression by Rhamnetin and Cirsiliol Reduces EMT in NSCLC Cells
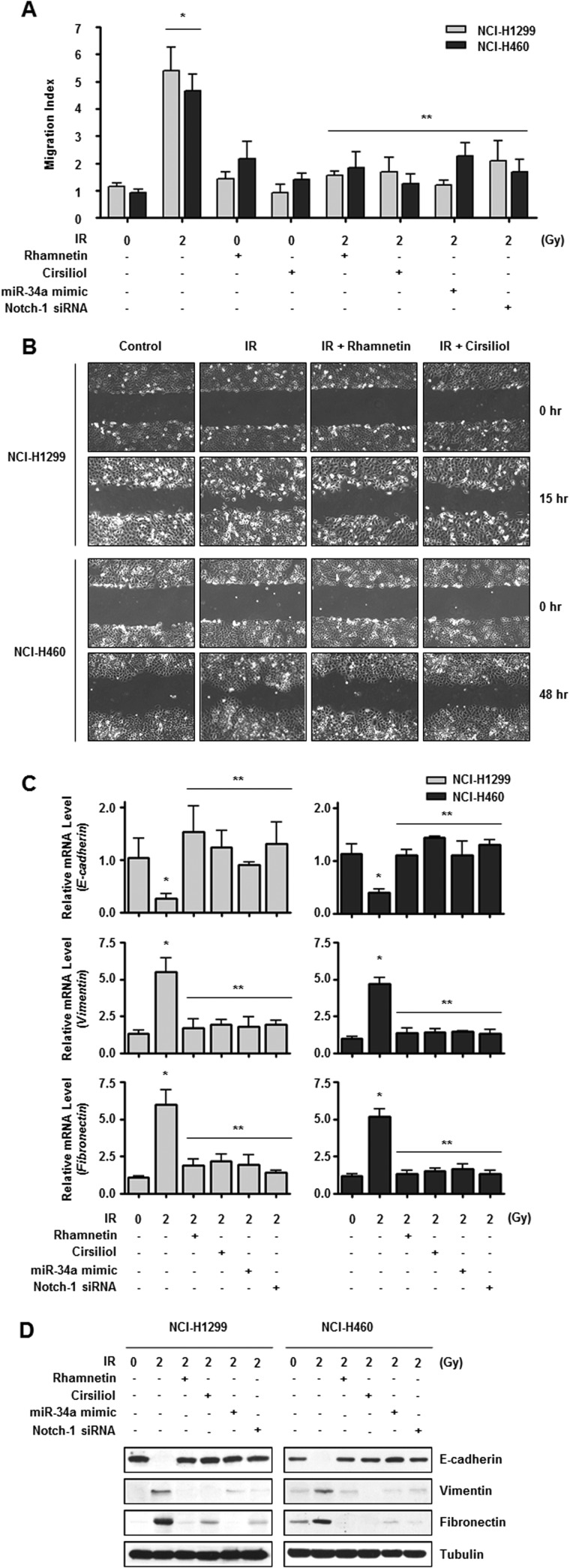
Several lines of evidence have demonstrated the critical role of Notch-1 signaling in EMT (23, 29). To examine the impact of rhamnetin and cirsiliol on Notch-1-mediated EMT, we measured the migration capacity and EMT marker expression of NSCLC cells treated with rhamnetin and cirsiliol. NSCLC cells treated with either compound exhibited reduced motility as determined by both Transwell cell migration and wound healing assays (Fig. 6, A and B). Rhamnetin and cirsiliol treatment alleviated radiation-induced EMT by increasing expression of the epithelial phenotype marker E-cadherin and decreasing the expression of mesenchymal markers vimentin and fibronectin at both the mRNA and protein levels (Fig. 6, C and D). Reduced EMT was also observed in the NSCLC cells with miR-34a overexpression and Notch-1 knockdown. Taken together, these results suggest that miR-34a-mediated inhibition of Notch-1 expression by rhamnetin and cirsiliol reduces EMT in NSCLC cells.
FIGURE 6.
miR-34a-mediated inhibition of Notch-1 by rhamnetin and cirsiliol prevents EMT in NSCLC cells. A, inhibitory effects of rhamnetin and cirsiliol on irradiation-induced migration of NCI-H1299 and NCI-H460 cells were measured with a Transwell migration assay. The results are expressed as fold-increase of migration compared with the control group and based on the relative number of cells in a randomly selected field from three representative experiments. *, p < 0.05 for nonirradiated cells versus irradiated cells; **, p < 0.05 for irradiated cells versus irradiated cells treated with each drug, the miR-34a mimic, or Notch-1 siRNA. B, inhibitory effects of rhamnetin and cirsiliol on irradiation-induced migration of NSCLC cells were measured with a wound healing assay. C, effects of rhamnetin and cirsiliol on mRNA expression of E-cadherin, Vimentin, and Fibronectin in irradiated NSCLC cells were analyzed by real time qRT-PCR. *, p < 0.05 for nonirradiated cells versus irradiated cells; **, p < 0.05 for irradiated cells versus irradiated cells treated with each drug, the miR-34a mimic, or Notch-1 siRNA. D, effects of rhamnetin and cirsiliol on the protein expression of E-cadherin, vimentin, and fibronectin in irradiated NSCLC cells were analyzed by Western blot analysis.
Rhamnetin and Cirsiliol Increase in Vivo Radiosensitization and Decrease in Vivo EMT in a Xenograft Mouse Model
To evaluate the combined effects of rhamnetin or cirsiliol with radiation on tumor growth in vivo, a xenograft mouse model was established (Fig. 7A). In vivo data from nude mice bearing tumors formed by NCI-H1299 cells, a relatively radioresistant NSCLC cell line, indicated that rhamnetin and cirsiliol had an in vivo radiosensitization effect (Fig. 7B). Tumor volumes of the mice treated with radiation and rhamnetin or cirsiliol on day 25 were significantly reduced by ∼57 and 62%, respectively, compared with mice receiving radiation alone. In addition, irradiation-induced expressions of Notch-1, pro-survival proteins, and EMT-related proteins were significantly reduced in the extracted tumor tissue lysates when rhamnetin and cirsiliol were directly administered at the tumor site in the mice (Fig. 7C). Moreover, the levels of miR-34a in the tumor tissue lysates were dramatically increased by treatment with rhamnetin and cirsiliol (Fig. 7D). Thus, we suggest that rhamnetin and cirsiliol significantly increase in vivo radiosensitization while inhibiting EMT.
FIGURE 7.
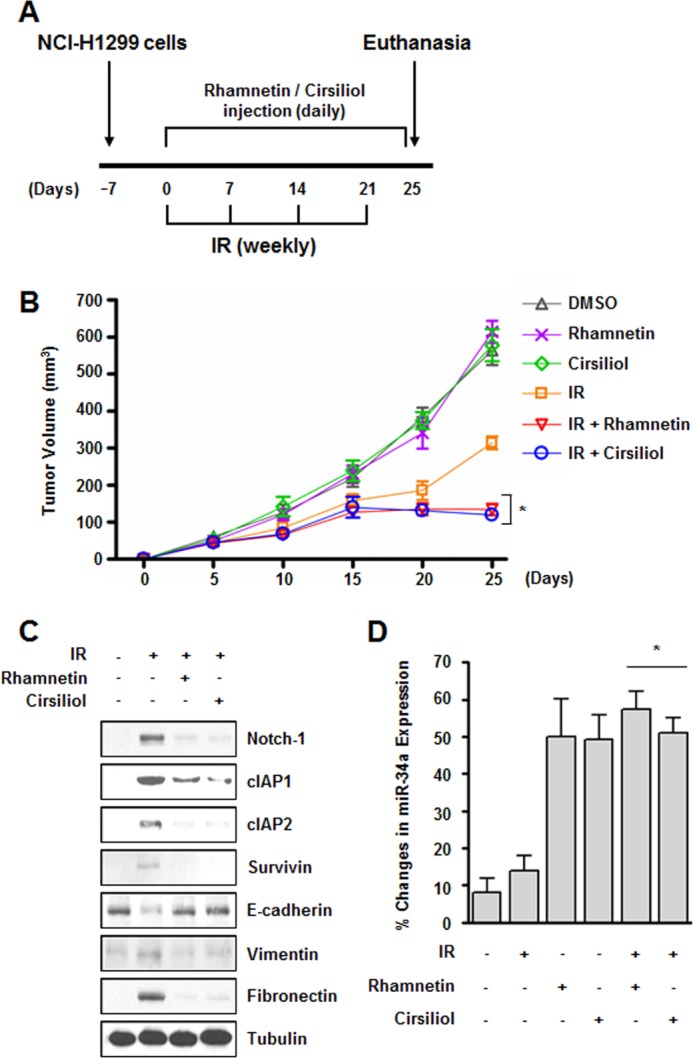
Rhamnetin and cirsiliol increase in vivo radiosensitization and decrease in vivo EMT in a xenograft mouse model. A, experimental protocol. B, effect of rhamnetin and cirsiliol on in vivo radiosensitization was measured in a xenograft mouse model. *, p < 0.05 for tumor volume on day 25 in mice treated with radiation versus animals treated with radiation and each drug. C, in vivo effects of rhamnetin and cirsiliol on the expression of Notch-1, pro-survival proteins, and EMT-related proteins were evaluated by Western blot analysis. D, effects of rhamnetin and cirsiliol on the expression of miR-34a were measured in tumor tissue lysates by real time qRT-PCR. *, p < 0.05 for tumor tissues from irradiated mice versus tumor tissues from irradiated animals treated with each drug.
DISCUSSION
Tumor radioresistance remains a critical obstacle for the effective treatment of NSCLC with radiotherapy. Thus, many efforts were made to develop radiosensitizers for combination with radiotherapy to enhance therapeutic efficacy. Traditional radiosensitizers, including classic chemotherapeutic agents that affect tumor microenvironments and characteristics (such as high levels of DNA replication, mitotic division, and hypoxia), have shown poor specificity for cancer cells. Consequently, many research groups made great efforts to establish target-based radiosensitizing strategies focused on tumor selectivity and the availability of molecular markers (54–56). In the same context, we found that Notch-1 served as a potential pharmacological target for the radiosensitization of NSCLC cells because overexpression of Notch-1 has been shown to inhibit apoptosis in many human cancers (11, 12).
In our study, NSCLC cells underwent apoptosis after a combinational treatment with irradiation and rhamnetin or cirsiliol. Because the studies of rhamnetin and cirsiliol have been rarely conducted, we focused on whether these compounds show their own anti-cancer activities like other flavonoids (33, 34). The viability of NSCLC cells was decreased at treatment with a nonpharmacological dose of two compounds (Fig. 2). These results implicated that rhamnetin and cirsiliol have possible tumor cell killing properties. In addition, expression of miR-34a was increased in the presence of two flavonoids regardless of radiation exposure (Fig. 3). From these data, we could not exclude the possibility that many genes might be regulated by miR-34a overexpression. It has been reported that miR-34a down-regulated various cancer-related genes, including c-Met, E2F3, MYC, and AXL (57). Because we wanted to develop promising radiosensitizers based on targeting specific molecules or signaling pathways responsible for radiation response, not on showing anti-cancer activities of drugs themselves, the noncytotoxic doses of both compounds were determined. Even though we still observed the tendency that the level of colony formation was slightly reduced in NSCLC cells treated with noncytotoxic doses of rhamnetin or cirsiliol compared with nontreated cells under nonirradiation conditions, these phenomena did not have statistical significance (Fig. 2). Moreover, apoptosis was not induced under drug-only-treated cells (Fig. 5). This means that basal level regulation of several genes, including NOTCH-1 affected by overexpression of miR-34a, might negligibly exert influence on cell fate decision. Therefore, we believe that these two compounds could act as novel candidates for radiosensitizers to regulate radiation-induced specific events, such as suppression of overexpression of Notch-1, not to show their own cytotoxic activity. Additionally, sensitization of NSCLC cells to radiation by rhamnetin and cirsiliol through p53- and miR-34a-mediated signaling could represent a useful strategy for treating other radioresistant cancers, including colon, lung, head, neck, and pancreatic cancers, in which Notch-1 is overexpressed (58–60). Our findings demonstrate that understanding tumor-specific responses to radiation and investigating molecular mechanism of radioresistance in tumors can serve as a basis for identifying pharmacological targets for increasing susceptibility to radiation and subsequent development of target-based radiosensitizers.
According to several investigations, inhibition of Notch-1 activation by effectively using GSIs, which inhibit cleavage of the Notch-1 intracellular domain, leads to anti-cancer effects (61–63). Although these studies have presented that GSIs could act as potent chemotherapeutic agents and radiosensitizers for several types of cancer, some of these drugs could have potential risks as well (64–66). Treatment of GSIs might result in suppression of the basal level of Notch-1 signaling in not only tumor cells but also nearby normal cells, which could lead to normal cell damage as one of adverse effects for chemotherapy. These inevitable side effects could subsequently induce normal cell dysfunction and disruption of cell-cell communication, because it has been well studied that Notch-1 signaling is in charge of cell fate decision, control of interactions between cellular neighbors, and developmental processes (67). In this study, rhamnetin and cirsiliol affect only Notch-1 overexpression, not activation, in response to irradiation (Fig. 2). Thus, rhamnetin and cirsiliol as Notch-1-regulating compounds could have the merit of not suppressing the basal level of Notch-1 signaling, which might allow Notch-1 to maintain its fundamental cellular functions. This possibility could be supported by the cases of hypoxia-inducible factor-1 (HIF-1) inhibitor developments. Because HIF-1 plays a critical role in survival of cells under hypoxia, which ultimately renders adaptation properties of cancer cells against hypoxic stress, there are many investigations for the development of HIF-1 inhibitors for better cancer therapy. The accumulated data have shown that drugs targeting the HIF-1 expression levels, such as EZN-2208, EZN-2968, and aminoflavone, are currently in clinical trials of phase I or II (68–70). However, some clinical drugs that affect HIF-1 activities such as DNA binding and transcriptional activities of HIF-1, including echinomycin and chetomin, have been halted due to no significant activity or high toxicity in clinical trials (71, 72). These results implicated that drugs regulating HIF-1 expression could be preferred as more potent HIF-1 inhibitors for clinical usage. Therefore, we expect that rhamnetin and cirsiliol could be promising alternatives to GSIs as radiosensitizers with minimal side effects when it comes to modulation of radiation-induced Notch-1 overexpression in NSCLC.
As discussed above, we demonstrated that rhamnetin and cirsiliol reduce NSCLC cell proliferation by inhibiting the expression (but not the activation) of Notch-1 (Fig. 2). A recent report has shown that Notch-1 expression regulates cell death by modulating both apoptosis and cell cycle pathways by affecting the expression of survival genes such as JNK1, Bcl-xL, p21CIP1, p27KIP1, RB1, and NFKB1 (73). We observed that down-regulation of Notch-1 by rhamnetin and cirsiliol reduced NF-κB activity and expression of its target pro-survival genes, including cIAP1, cIAP2, and Survivin (Fig. 5). Because NF-κB pathways are key regulators of numerous cellular processes such as proliferation, differentiation, and inflammation, our results clearly provide molecular evidence for potential cross-talk between Notch-1 and NF-κB pathways and suggest that cell growth inhibition and apoptosis promoted by the down-regulation of Notch-1 after treatment of rhamnetin and cirsiliol may be partly mediated by NF-κB (73–75). Because an approach using Notch-1 siRNA is not yet practical for therapeutic purposes, we recommend the use of rhamnetin and cirsiliol found to be potent agents capable of regulating NF-κB pathway by Notch-1 inhibition. We found that rhamnetin and cirsiliol down-regulated the transcription and translation of Notch-1 and its downstream targets Hes-1, Hey-1, and NF-κB. In addition, these two compounds had a dramatic effect on growth inhibition and apoptosis induction in NSCLC cells. Overexpression of Notch-1 by transfection with NOTCH-1 cDNA abrogated rhamnetin- and cirsiliol-induced apoptosis to a certain degree. Therefore, we strongly believe that down-regulation of Notch-1 expression by rhamnetin and cirsiliol is mechanistically linked to cell proliferation and pro-apoptotic processes. The molecular mechanism(s) by which rhamnetin and cirsiliol exert their inhibitory effects on NSCLC cells as revealed in the present study has opened up exciting avenues for devising novel therapeutic strategies. Therefore, the down-regulation of Notch-1 and NF-κB by rhamnetin and cirsiliol could be useful for developing novel therapeutic approaches to treat lung cancer.
In this study, we investigated the use of flavonoid compounds as novel therapeutic drugs to enhance radiosensitivity via inhibiting Notch-1 expression in the NSCLC cell lines, NCI-H1299 and NCI-H460, with different levels of radiosensitivity. Previously, our group and others measured the radiosensitivity of a subset of NSCLC cells after exposure to various doses of radiation (40, 42, 76). In this study, NCI-H1299 and NCI-H460 cells were used as representative radioresistant and radiosensitive NSCLC cells, respectively, which is supported by a previous investigation (77). We selected these two cell lines because we wanted to develop general radiosensitizers that would be effective in all NSCLC cells regardless of intratumor heterogeneity. Recent multiregion sequencing data revealed that the majority of somatic genetic mutations are not ubiquitously expressed by all cells within a tumor, and this heterogeneity presents a considerable therapeutic challenge because choosing the treatments based on a biomarker present in a single biopsy specimen or cell line may not be generally relevant (78). More focus on general biomarkers and regulators should therefore be given to promote the widespread development of radiosensitizers in translational radiation biological research for treating NSCLC.
Previous studies have found that EMT could be induced by Notch-1 activation via mediating expression of various EMT-related genes, which is associated with cancer cell resistance to therapy and metastasis (79, 80). Inhibition of Notch-1 activation by N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (one of the GSIs) during EMT attenuates the expression of E-cadherin and Snail, and it increases the expression of α-SMA, MMP-2, and MMP-9 (81). Consistent with these findings, results from this study showed that inhibition of Notch-1 expression by rhamnetin and cirsiliol resulted in increased expression of E-cadherin and decreased expression of vimentin and fibronectin, which consequently alleviated radiation-induced EMT both in in vitro and in vivo models (Figs. 6 and 7). Taken together, these results further elucidate the effect of Notch-1 on EMT and suggest that inactivation of Notch-1 signaling leads to the reversal of EMT and a return to a mesenchymal-epithelial transition phenotype associated with less invasive characteristics. Thus, down-regulation of Notch-1 might represent a novel mechanism for enhancing the effect of irradiation and delaying resistance to radiotherapy in patients with NSCLC because irradiation has been correlated with development of an EMT phenotype in cases of NSCLC (26, 27).
In summary, our results indicate that administration of rhamnetin or cirsiliol with radiation has significant potential as an effective anti-lung cancer therapeutic strategy. However, it is still unclear whether rhamnetin and cirsiliol have a single major target or several targets. In Fig. 3, both compounds induced p53 acetylation, which is critical for expression of miR-34a, leading to radiosensitization effects. Because Sirt1 is one of the well known deacetylases for p53, we hypothesized that Sirt1 might be a potential target of rhamnetin and cirsiliol (82). Rhamnetin and cirsiliol have very similar structures of fisetin and quercetin, which are known of Sirt1 activators (83). Although it has been identified that fisetin and quercetin induced Sirt1 deacetylase activity, we assumed that rhamnetin and cirsiliol might inhibit Sirt1 activity due to a few different functional groups. The most common difference of structure between rhamnetin/cirsiliol and fisetin/quercetin is the C7 group of each flavonoid, in which rhamnetin and cirsiliol have –OCH3 (methoxy group), whereas fisetin and quercetin have –OH (hydroxyl group). The methoxy group of rhamnetin and cirsiliol might render the property to the compounds for maintaining hydrophobic interactions with nonpolar amino acids. One study has been recently reported that the EX527 analog (a Sirt1 inhibitor) can bind to the Sirt1 catalytic domain via formation of hydrophobic networks with Ile-347, Ile-270, Ile-279, Phe-273, Phe-297, Ile-347, Ile-411, and Phe-413 (84). Thus, we expect that rhamnetin and cirsiliol might be stabilized via interactions with several nonpolar amino acids of the Sirt1 catalytic domain, like the EX527 analog does. For better understanding, further investigations for interaction between Sirt1 and rhamnetin or cirsiliol should be performed. This will allow us to understand the missing link between these compounds and p53 acetylation responsible for consequent radiosensitization effects. Furthermore, it will serve the opportunity for optimizing the capacity of target inhibition to improve the efficiency of these drugs and to minimize potential side effects based on structure-activity relationship analysis.
Until now, the exact molecular mechanism governing the development of radioresistance in NSCLC cells has been unclear. In this study, we demonstrate that overexpression of Notch-1 is a potential mediator of radioresistance in NSCLC cells. We have therefore proposed a novel regulatory mechanism of radioresistance involving the functional orchestration of Notch-1-targeted inhibitors, p53 and miR-34a, in response to irradiation in NSCLC cells (Fig. 8). More importantly, we have provided the first evidence showing that rhamnetin and cirsiliol may be effective candidate radiosensitizers. Although we did not evaluate the potential effect of the tumor microenvironment on radioresistance, the results of our study demonstrate that targeting of Notch-1 with pharmacological agents in combination with radiotherapy could overcome radioresistance and eventually increase the efficacy of radiation therapy for treating NSCLC.
FIGURE 8.
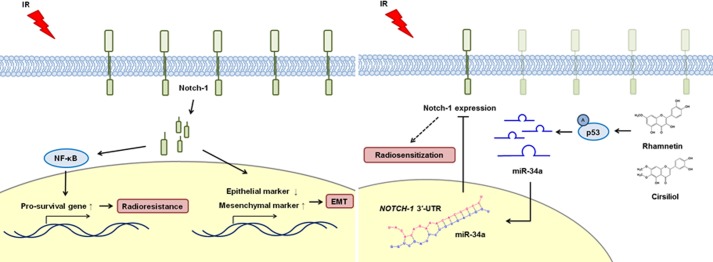
Schematic diagrams illustrating Notch-1 signaling in response to irradiation and the radiosensitizing effects of rhamnetin and cirsiliol in NSCLC cells. Irradiation induced Notch-1 overexpression specifically in NSCLC cells and not in normal lung cells or tissues. Treatment with rhamnetin or cirsiliol also reduced the proliferation of NSCLC cells through the suppression of radiation-induced Notch-1 expression. Additionally, rhamnetin and cirsiliol increased the expression of miR-34a (tumor-suppressive miRNA) in a p53-dependent manner in the cells. Reduced Notch-1 expression increased NSCLC cell apoptosis through significant down-regulation of NF-κB signaling. Finally, EMT induced by radiation was notably attenuated in the presence of rhamnetin and cirsiliol in the NSCLC cells. These results demonstrated that two flavonoids, rhamnetin and cirsiliol, can act as novel radiosensitizers to enhance the efficacy of radiotherapy by inhibiting irradiation-induced Notch-1 expression and its signaling pathways associated with radioresistance.
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012-0003201), a grant from the National R&D Program for Cancer Control, Ministry for Health and Welfare, Republic of Korea (1320100), and the Radiation Technology R&D Program through the National Research Foundation of Korea funded by the Ministry of Science, ITC & Future Planning (2013M2A2A7042502).
- NSCLC
- non-small cell lung cancer
- EMT
- epithelial-mesenchymal transition
- GSI
- γ-secretase inhibitor
- IF
- immunofluorescence
- IHC
- immunohistochemistry
- IP
- immunoprecipitation
- qRT-PCR
- quantitative RT-PCR
- NF-κB
- nuclear factor-κB
- cIAP
- cellular inhibitor of apoptosis
- HIF-1
- hypoxia-inducible factor-1
- miRNA
- microRNA
- Gy
- gray.
REFERENCES
- 1. Baumann M., Krause M., Zips D., Petersen C., Dittmann K., Dörr W., Rodemann H. P. (2004) Molecular targeting in radiotherapy of lung cancer. Lung Cancer 45, S187–S197 [DOI] [PubMed] [Google Scholar]
- 2. Koh P. K., Faivre-Finn C., Blackhall F. H., De Ruysscher D. (2012) Targeted agents in non-small cell lung cancer (NSCLC): clinical developments and rationale for the combination with thoracic radiotherapy. Cancer Treat. Rev. 38, 626–640 [DOI] [PubMed] [Google Scholar]
- 3. Bussink J., van der Kogel A. J., Kaanders J. H. (2008) Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol. 9, 288–296 [DOI] [PubMed] [Google Scholar]
- 4. Nishimura Y., Nakagawa K., Takeda K., Tanaka M., Segawa Y., Tsujino K., Negoro S., Fuwa N., Hida T., Kawahara M., Katakami N., Hirokawa K., Yamamoto N., Fukuoka M., Ariyoshi Y. (2007) Phase I/II trial of sequential chemoradiotherapy using a novel hypoxic cell radiosensitizer, doranidazole (PR-350), in patients with locally advanced non-small cell lung cancer (WJTOG-0002). Int. J. Radiat. Oncol. Biol. Phys. 69, 786–792 [DOI] [PubMed] [Google Scholar]
- 5. Hillman G. G., Singh-Gupta V., Runyan L., Yunker C. K., Rakowski J. T., Sarkar F. H., Miller S., Gadgeel S. M., Sethi S., Joiner M. C., Konski A. A. (2011) Soy isoflavones radiosensitize lung cancer while mitigating normal tissue injury. Radiother. Oncol. 101, 329–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toschi L., Cappuzzo F. (2010) Impact of biomarkers on non-small cell lung cancer treatment. Target. Oncol. 5, 5–17 [DOI] [PubMed] [Google Scholar]
- 7. Westhoff B., Colaluca I. N., D'Ario G., Donzelli M., Tosoni D., Volorio S., Pelosi G., Spaggiari L., Mazzarol G., Viale G., Pece S., Di Fiore P. P. (2009) Alterations of the Notch pathway in lung cancer. Proc. Natl. Acad. Sci. U.S.A. 106, 22293–22298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oswald F., Täuber B., Dobner T., Bourteele S., Kostezka U., Adler G., Liptay S., Schmid R. M. (2001) p300 acts as a transcriptional coactivator for mammalian Notch-1. Mol. Cell. Biol. 21, 7761–7774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Z., Zhang Y., Li Y., Banerjee S., Liao J., Sarkar F. H. (2006) Down-regulation of Notch-1 contributes to cell growth inhibition and apoptosis in pancreatic cancer cells. Mol. Cancer Ther. 5, 483–493 [DOI] [PubMed] [Google Scholar]
- 10. Wang Z., Banerjee S., Li Y., Rahman K. M., Zhang Y., Sarkar F. H. (2006) Down-regulation of notch-1 inhibits invasion by inactivation of nuclear factor-κB, vascular endothelial growth factor, and matrix metalloproteinase-9 in pancreatic cancer cells. Cancer Res. 66, 2778–2784 [DOI] [PubMed] [Google Scholar]
- 11. Jundt F., Anagnostopoulos I., Förster R., Mathas S., Stein H., Dörken B. (2002) Activated Notch1 signaling promotes tumor cell proliferation and survival in Hodgkin and anaplastic large cell lymphoma. Blood 99, 3398–3403 [DOI] [PubMed] [Google Scholar]
- 12. Miele L., Osborne B. (1999) Arbiter of differentiation and death: Notch signaling meets apoptosis. J. Cell. Physiol. 181, 393–409 [DOI] [PubMed] [Google Scholar]
- 13. Eliasz S., Liang S., Chen Y., De Marco M. A., Machek O., Skucha S., Miele L., Bocchetta M. (2010) Notch-1 stimulates survival of lung adenocarcinoma cells during hypoxia by activating the IGF-1R pathway. Oncogene 29, 2488–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keum J. S., Kong G., Yang S. C., Shin D. H., Park S. S., Lee J. H., Lee J. D. (1999) Cyclin D1 overexpression is an indicator of poor prognosis in resectable non-small cell lung cancer. Br. J. Cancer 81, 127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calin G. A., Croce C. M. (2006) MicroRNA signatures in human cancers. Nat. Rev. Cancer 6, 857–866 [DOI] [PubMed] [Google Scholar]
- 16. Zhao L., Bode A. M., Cao Y., Dong Z. (2012) Regulatory mechanisms and clinical perspectives of miRNA in tumor radiosensitivity. Carcinogenesis 33, 2220–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hermeking H. (2010) The miR-34 family in cancer and apoptosis. Cell Death Differ. 17, 193–199 [DOI] [PubMed] [Google Scholar]
- 18. Chang T. C., Wentzel E. A., Kent O. A., Ramachandran K., Mullendore M., Lee K. H., Feldmann G., Yamakuchi M., Ferlito M., Lowenstein C. J., Arking D. E., Beer M. A., Maitra A., Mendell J. T. (2007) Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol. Cell 26, 745–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He L., He X., Lim L. P., de Stanchina E., Xuan Z., Liang Y., Xue W., Zender L., Magnus J., Ridzon D., Jackson A. L., Linsley P. S., Chen C., Lowe S. W., Cleary M. A., Hannon G. J. (2007) A microRNA component of the p53 tumour suppressor network. Nature 447, 1130–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zenz T., Mohr J., Eldering E., Kater A. P., Bühler A., Kienle D., Winkler D., Dürig J., van Oers M. H., Mertens D., Döhner H., Stilgenbauer S. (2009) miR-34a as part of the resistance network in chronic lymphocytic leukemia. Blood 113, 3801–3808 [DOI] [PubMed] [Google Scholar]
- 21. Thiery J. P., Sleeman J. P. (2006) Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 7, 131–142 [DOI] [PubMed] [Google Scholar]
- 22. Kalluri R., Weinberg R. A. (2009) The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sahlgren C., Gustafsson M. V., Jin S., Poellinger L., Lendahl U. (2008) Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc. Natl. Acad. Sci. U.S.A. 105, 6392–6397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rhyu D. Y., Yang Y., Ha H., Lee G. T., Song J. S., Uh S. T., Lee H. B. (2005) Role of reactive oxygen species in TGF-β1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J. Am. Soc. Nephrol. 16, 667–675 [DOI] [PubMed] [Google Scholar]
- 25. Kawamoto A., Yokoe T., Tanaka K., Saigusa S., Toiyama Y., Yasuda H., Inoue Y., Miki C., Kusunoki M. (2012) Radiation induces epithelial-mesenchymal transition in colorectal cancer cells. Oncol. Rep. 27, 51–57 [DOI] [PubMed] [Google Scholar]
- 26. Park C. C., Henshall-Powell R. L., Erickson A. C., Talhouk R., Parvin B., Bissell M. J., Barcellos-Hoff M. H. (2003) Ionizing radiation induces heritable disruption of epithelial cell interactions. Proc. Natl. Acad. Sci. U.S.A. 100, 10728–10733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andarawewa K. L., Erickson A. C., Chou W. S., Costes S. V., Gascard P., Mott J. D., Bissell M. J., Barcellos-Hoff M. H. (2007) Ionizing radiation predisposes nonmalignant human mammary epithelial cells to undergo transforming growth factor β induced epithelial to mesenchymal transition. Cancer Res. 67, 8662–8670 [DOI] [PubMed] [Google Scholar]
- 28. Niessen K., Fu Y., Chang L., Hoodless P. A., McFadden D., Karsan A. (2008) Slug is a direct Notch target required for initiation of cardiac cushion cellularization. J. Cell Biol. 182, 315–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zavadil J., Cermak L., Soto-Nieves N., Böttinger E. P. (2004) Integration of TGF-β/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 23, 1155–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bauer R. A., Wurst J. M., Tan D. S. (2010) Expanding the range of “druggable” targets with natural product-based libraries: an academic perspective. Curr. Opin. Chem. Biol. 14, 308–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang H. J., Youn H., Seong K. M., Yun Y. J., Kim W., Kim Y. H., Lee J. Y., Kim C. S., Jin Y. W., Youn B. (2011) Psoralidin, a dual inhibitor of COX-2 and 5-LOX, regulates ionizing radiation (IR)-induced pulmonary inflammation. Biochem. Pharmacol. 82, 524–534 [DOI] [PubMed] [Google Scholar]
- 32. Kim W., Yang H. J., Youn H., Yun Y. J., Seong K. M., Youn B. (2010) Myricetin inhibits Akt survival signaling and induces Bad-mediated apoptosis in a low dose ultraviolet (UV)-B-irradiated HaCaT human immortalized keratinocytes. J. Radiat. Res. 51, 285–296 [DOI] [PubMed] [Google Scholar]
- 33. Romagnolo D. F., Selmin O. I. (2012) Flavonoids and cancer prevention: a review of the evidence. J. Nutr. Gerontol. Geriatr. 31, 206–238 [DOI] [PubMed] [Google Scholar]
- 34. Seelinger G., Merfort I., Wölfle U., Schempp C. M. (2008) Anti-carcinogenic effects of the flavonoid luteolin. Molecules 13, 2628–2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang G., Cao X., Zhang X., Chang H., Yang Y., Du W., Wilson J. X. (2009) Effects of soybean isoflavone on the notch signal pathway of the brain in rats with cerebral ischemia. J. Nutr. Sci. Vitaminol. 55, 326–331 [DOI] [PubMed] [Google Scholar]
- 36. Okuhashi Y., Itoh M., Nara N., Tohda S. (2011) Effects of combination of notch inhibitor plus hedgehog inhibitor or Wnt inhibitor on growth of leukemia cells. Anticancer Res. 31, 893–896 [PubMed] [Google Scholar]
- 37. Pan H., Zhou W., He W., Liu X., Ding Q., Ling L., Zha X., Wang S. (2012) Genistein inhibits MDA-MB-231 triple-negative breast cancer cell growth by inhibiting NF-κB activity via the Notch-1 pathway. Int. J. Mol. Med. 30, 337–343 [DOI] [PubMed] [Google Scholar]
- 38. Wang A. M., Ku H. H., Liang Y. C., Chen Y. C., Hwu Y. M., Yeh T. S. (2009) The autonomous notch signal pathway is activated by baicalin and baicalein but is suppressed by niclosamide in K562 cells. J. Cell. Biochem. 106, 682–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Niyazi M., Niyazi I., Belka C. (2007) Counting colonies of clonogenic assays by using densitometric software. Radiat. Oncol. 2, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim W., Youn H., Seong K. M., Yang H. J., Yun Y. J., Kwon T., Kim Y. H., Lee J. Y., Jin Y. W., Youn B. (2011) PIM1-activated PRAS40 regulates radioresistance in non-small cell lung cancer cells through interplay with FOXO3a, 14-3-3, and protein phosphatases. Radiat. Res. 176, 539–552 [DOI] [PubMed] [Google Scholar]
- 41. Griffiths-Jones S. (2004) The microRNA registry. Nucleic Acids Res. 32, D109–D111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim W., Youn H., Kwon T., Kang J., Kim E., Son B., Yang H. J., Jung Y., Youn B. (2013) PIM1 kinase inhibitors induce radiosensitization in non-small cell lung cancer cells. Pharmacol. Res. 70, 90–101 [DOI] [PubMed] [Google Scholar]
- 43. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2(−ΔΔCT) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 44. Li Y., Burns J. A., Cheney C. A., Zhang N., Vitelli S., Wang F., Bett A., Chastain M., Audoly L. P., Zhang Z. Q. (2010) Distinct expression profiles of Notch-1 protein in human solid tumors: Implications for development of targeted therapeutic monoclonal antibodies. Biologics 4, 163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang S., Long H., Yang Y. L., Wang Y., Hsieh D., Li W., Au A., Stoppler H. J., Xu Z., Jablons D. M., You L. (2013) Inhibition of CK2α down-regulates Notch1 signalling in lung cancer cells. J. Cell. Mol. Med. 17, 854–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang H. J., Kim N., Seong K. M., Youn H., Youn B. (2013) Investigation of radiation-induced transcriptome profile of radioresistant non-small cell lung cancer A549 cells using RNA-seq. PLoS One 8, e59319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Simone N. L., Soule B. P., Ly D., Saleh A. D., Savage J. E., Degraff W., Cook J., Harris C. C., Gius D., Mitchell J. B. (2009) Ionizing radiation-induced oxidative stress alters miRNA expression. PLoS One 4, e6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ji X., Wang Z., Geamanu A., Goja A., Sarkar F. H., Gupta S. V. (2012) δ-Tocotrienol suppresses Notch-1 pathway by up-regulating miR-34a in non-small cell lung cancer cells. Int. J. Cancer 131, 2668–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kashat M., Azzouz L., Sarkar S. H., Kong D., Li Y., Sarkar F. H. (2012) Inactivation of AR and Notch-1 signaling by miR-34a attenuates prostate cancer aggressiveness. Am. J. Transl. Res. 4, 432–442 [PMC free article] [PubMed] [Google Scholar]
- 50. Shohet J. M., Ghosh R., Coarfa C., Ludwig A., Benham A. L., Chen Z., Patterson D. M., Barbieri E., Mestdagh P., Sikorski D. N., Milosavljevic A., Kim E. S., Gunaratne P. H. (2011) A genome-wide search for promoters that respond to increased MYCN reveals both new oncogenic and tumor suppressor microRNAs associated with aggressive neuroblastoma. Cancer Res. 71, 3841–3851 [DOI] [PubMed] [Google Scholar]
- 51. Kim W. J., Rivera M. N., Coffman E. J., Haber D. A. (2012) The WTX tumor suppressor enhances p53 acetylation by CBP/p300. Mol. Cell 45, 587–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhu Z., Luo Z., Li Y., Ni C., Li H., Zhu M. (2009) Human inhibitor of growth 1 inhibits hepatoma cell growth and influences p53 stability in a variant-dependent manner. Hepatology 49, 504–512 [DOI] [PubMed] [Google Scholar]
- 53. Li F., Sethi G. (2010) Targeting transcription factor NF-κB to overcome chemoresistance and radioresistance in cancer therapy. Biochim. Biophys. Acta 1805, 167–180 [DOI] [PubMed] [Google Scholar]
- 54. Bianco C., Tortora G., Bianco R., Caputo R., Veneziani B. M., Caputo R., Damiano V., Troiani T., Fontanini G., Raben D., Pepe S., Bianco A. R., Ciardiello F. (2002) Enhancement of antitumor activity of ionizing radiation by combined treatment with the selective epidermal growth factor receptor-tyrosine kinase inhibitor ZD1839 (Iressa). Clin. Cancer Res. 8, 3250–3258 [PubMed] [Google Scholar]
- 55. Chinnaiyan P., Huang S., Vallabhaneni G., Armstrong E., Varambally S., Tomlins S. A., Chinnaiyan A. M., Harari P. M. (2005) Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res. 65, 3328–3335 [DOI] [PubMed] [Google Scholar]
- 56. Wang M., Morsbach F., Sander D., Gheorghiu L., Nanda A., Benes C., Kriegs M., Krause M., Dikomey E., Baumann M., Dahm-Daphi J., Settleman J., Willers H. (2011) EGF receptor inhibition radiosensitizes NSCLC cells by inducing senescence in cells sustaining DNA double-strand breaks. Cancer Res. 71, 6261–6269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bader A. G. (2012) miR-34–a microRNA replacement therapy is headed to the clinic. Front. Genet. 3, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reedijk M., Odorcic S., Zhang H., Chetty R., Tennert C., Dickson B. C., Lockwood G., Gallinger S., Egan S. E. (2008) Activation of Notch signaling in human colon adenocarcinoma. Int. J. Oncol. 33, 1223–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lin J. T., Chen M. K., Yeh K. T., Chang C. S., Chang T. H., Lin C. Y., Wu Y. C., Su B. W., Lee K. D., Chang P. J. (2010) Association of high levels of Jagged-1 and Notch-1 expression with poor prognosis in head and neck cancer. Ann. Surg. Oncol. 17, 2976–2983 [DOI] [PubMed] [Google Scholar]
- 60. Büchler P., Gazdhar A., Schubert M., Giese N., Reber H. A., Hines O. J., Giese T., Ceyhan G. O., Müller M., Büchler M. W., Friess H. (2005) The Notch signaling pathway is related to neurovascular progression of pancreatic cancer. Ann. Surg. 242, 791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen S. M., Liu J. P., Zhou J. X., Chen C., Deng Y. Q., Wang Y., Tao Z. Z. (2011) Suppression of the notch signaling pathway by γ-secretase inhibitor GSI inhibits human nasopharyngeal carcinoma cell proliferation. Cancer Lett. 306, 76–84 [DOI] [PubMed] [Google Scholar]
- 62. Yu S., Zhang R., Liu F., Hu H., Yu S., Wang H. (2011) Down-regulation of Notch signaling by a γ-secretase inhibitor enhances the radiosensitivity of nasopharyngeal carcinoma cells. Oncol. Rep. 26, 1323–1328 [DOI] [PubMed] [Google Scholar]
- 63. Lin J., Zhang X. M., Yang J. C., Ye Y. B., Luo S. Q. (2010) γ-Secretase inhibitor-I enhances radiosensitivity of glioblastoma cell lines by depleting CD133+ tumor cells. Arch. Med. Res. 41, 519–529 [DOI] [PubMed] [Google Scholar]
- 64. Milano J., McKay J., Dagenais C., Foster-Brown L., Pognan F., Gadient R., Jacobs R. T., Zacco A., Greenberg B., Ciaccio P. J. (2004) Modulation of notch processing by γ-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol. Sci. 82, 341–358 [DOI] [PubMed] [Google Scholar]
- 65. Wong G. T., Manfra D., Poulet F. M., Zhang Q., Josien H., Bara T., Engstrom L., Pinzon-Ortiz M., Fine J. S., Lee H. J., Zhang L., Higgins G. A., Parker E. M. (2004) Chronic treatment with the γ-secretase inhibitor LY-411,575 inhibits β-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J. Biol. Chem. 279, 12876–12882 [DOI] [PubMed] [Google Scholar]
- 66. Searfoss G. H., Jordan W. H., Calligaro D. O., Galbreath E. J., Schirtzinger L. M., Berridge B. R., Gao H., Higgins M. A., May P. C., Ryan T. P. (2003) Adipsin, a biomarker of gastrointestinal toxicity mediated by a functional γ-secretase inhibitor. J. Biol. Chem. 278, 46107–46116 [DOI] [PubMed] [Google Scholar]
- 67. Artavanis-Tsakonas S., Rand M. D., Lake R. J. (1999) Notch signaling: cell fate control and signal integration in development. Science 284, 770–776 [DOI] [PubMed] [Google Scholar]
- 68. Sapra P., Zhao H., Mehlig M., Malaby J., Kraft P., Longley C., Greenberger L. M., Horak I. D. (2008) Novel delivery of SN38 markedly inhibits tumor growth in xenografts, including a camptothecin-11-refractory model. Clin. Cancer Res. 14, 1888–1896 [DOI] [PubMed] [Google Scholar]
- 69. Greenberger L. M., Horak I. D., Filpula D., Sapra P., Westergaard M., Frydenlund H. F., Albaek C., Schrøder H., Ørum H. (2008) A RNA antagonist of hypoxia-inducible factor-1α, EZN-2968, inhibits tumor cell growth. Mol. Cancer Ther. 7, 3598–3608 [DOI] [PubMed] [Google Scholar]
- 70. Terzuoli E., Puppo M., Rapisarda A., Uranchimeg B., Cao L., Burger A. M., Ziche M., Melillo G. (2010) Aminoflavone, a ligand of the aryl hydrocarbon receptor, inhibits HIF-1α expression in an AhR-independent fashion. Cancer Res. 70, 6837–6848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kong D., Park E. J., Stephen A. G., Calvani M., Cardellina J. H., Monks A., Fisher R. J., Shoemaker R. H., Melillo G. (2005) Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 65, 9047–9055 [DOI] [PubMed] [Google Scholar]
- 72. Kung A. L., Zabludoff S. D., France D. S., Freedman S. J., Tanner E. A., Vieira A., Cornell-Kennon S., Lee J., Wang B., Wang J., Memmert K., Naegeli H. U., Petersen F., Eck M. J., Bair K. W., Wood A. W., Livingston D. M. (2004) Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer Cell 6, 33–43 [DOI] [PubMed] [Google Scholar]
- 73. Jang M. S., Miao H., Carlesso N., Shelly L., Zlobin A., Darack N., Qin J. Z., Nickoloff B. J., Miele L. (2004) Notch-1 regulates cell death independently of differentiation in murine erythroleukemia cells through multiple apoptosis and cell cycle pathways. J. Cell. Physiol. 199, 418–433 [DOI] [PubMed] [Google Scholar]
- 74. Nickoloff B. J., Qin J. Z., Chaturvedi V., Denning M. F., Bonish B., Miele L. (2002) Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-κB and PPARγ. Cell Death Differ. 9, 842–855 [DOI] [PubMed] [Google Scholar]
- 75. Wang Y., Chan S. L., Miele L., Yao P. J., Mackes J., Ingram D. K., Mattson M. P., Furukawa K. (2004) Involvement of Notch signaling in hippocampal synaptic plasticity. Proc. Natl. Acad. Sci. U.S.A. 101, 9458–9462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kim W. Y., Oh S. H., Woo J. K., Hong W. K., Lee H. Y. (2009) Targeting heat shock protein 90 overrides the resistance of lung cancer cells by blocking radiation-induced stabilization of hypoxia-inducible factor-1α. Cancer Res. 69, 1624–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Das A. K., Sato M., Story M. D., Peyton M., Graves R., Redpath S., Girard L., Gazdar A. F., Shay J. W., Minna J. D., Nirodi C. S. (2006) Non-small-cell lung cancers with kinase domain mutations in the epidermal growth factor receptor are sensitive to ionizing radiation. Cancer Res. 66, 9601–9608 [DOI] [PubMed] [Google Scholar]
- 78. Gerlinger M., Rowan A. J., Horswell S., Larkin J., Endesfelder D., Gronroos E., Martinez P., Matthews N., Stewart A., Tarpey P., Varela I., Phillimore B., Begum S., McDonald N. Q., Butler A., Jones D., Raine K., Latimer C., Santos C. R., Nohadani M., Eklund A. C., Spencer-Dene B., Clark G., Pickering L., Stamp G., Gore M., Szallasi Z., Downward J., Futreal P. A., Swanton C. (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Matsuno Y., Coelho A. L., Jarai G., Westwick J., Hogaboam C. M. (2012) Notch signaling mediates TGF-β1-induced epithelial-mesenchymal transition through the induction of Snai1. Int. J. Biochem. Cell Biol. 44, 776–789 [DOI] [PubMed] [Google Scholar]
- 80. Saad S., Stanners S. R., Yong R., Tang O., Pollock C. A. (2010) Notch mediated epithelial to mesenchymal transformation is associated with increased expression of the Snail transcription factor. Int. J. Biochem. Cell Biol. 42, 1115–1122 [DOI] [PubMed] [Google Scholar]
- 81. Pannuti A., Foreman K., Rizzo P., Osipo C., Golde T., Osborne B., Miele L. (2010) Targeting Notch to target cancer stem cells. Clin. Cancer Res. 16, 3141–3152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ota H., Akishita M., Eto M., Iijima K., Kaneki M., Ouchi Y. (2007) Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J. Mol. Cell. Cardiol. 43, 571–579 [DOI] [PubMed] [Google Scholar]
- 83. Howitz K. T., Bitterman K. J., Cohen H. Y., Lamming D. W., Lavu S., Wood J. G., Zipkin R. E., Chung P., Kisielewski A., Zhang L. L., Scherer B., Sinclair D. A. (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae life span. Nature 425, 191–196 [DOI] [PubMed] [Google Scholar]
- 84. Zhao X., Allison D., Condon B., Zhang F., Gheyi T., Zhang A., Ashok S., Russell M., MacEwan I., Qian Y., Jamison J. A., Luz J. G. (2013) The 2.5 Å crystal structure of the SIRT1 catalytic domain bound to nicotinamide adenine dinucleotide (NAD+) and an indole (EX527 analogue) reveals a novel mechanism of histone deacetylase inhibition. J. Med. Chem. 56, 963–969 [DOI] [PubMed] [Google Scholar]