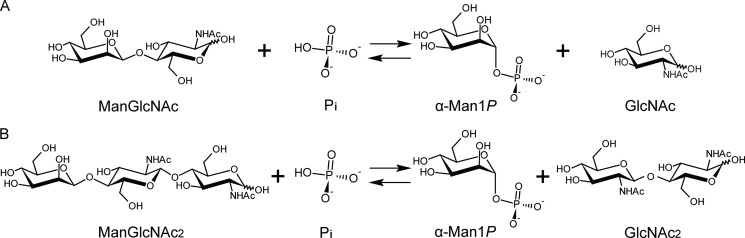
Background: N-Glycans are metabolized by sequential glycoside hydrolase-catalyzed reactions.
Results: A phosphorylase encoded in a gene cluster involved in N-glycan metabolism in the genome of Bacteroides thetaiotaomicron catalyzed reversible phosphorolysis of β-1,4-d-mannosyl-N-acetyl-d-glucosamine.
Conclusion: An N-glycan metabolic pathway containing a unique phosphorylase was discovered.
Significance: B. thetaiotaomicron efficiently utilizes the energy of ATP via a phosphorylase-dependent metabolic pathway.
Keywords: Carbohydrate Chemistry; Carbohydrate Metabolism; Enzyme Catalysis; Glycoside Hydrolases; Phosphorylase; Beta-1,4-D-Mannosyl-N-acetyl-D-glucosamine Phosphorylase; Bacteroides thetaiotaomicron; N-Glycans; Glycoside Hydrolase Family 130
Abstract
A gene cluster involved in N-glycan metabolism was identified in the genome of Bacteroides thetaiotaomicron VPI-5482. This gene cluster encodes a major facilitator superfamily transporter, a starch utilization system-like transporter consisting of a TonB-dependent oligosaccharide transporter and an outer membrane lipoprotein, four glycoside hydrolases (α-mannosidase, β-N-acetylhexosaminidase, exo-α-sialidase, and endo-β-N-acetylglucosaminidase), and a phosphorylase (BT1033) with unknown function. It was demonstrated that BT1033 catalyzed the reversible phosphorolysis of β-1,4-d-mannosyl-N-acetyl-d-glucosamine in a typical sequential Bi Bi mechanism. These results indicate that BT1033 plays a crucial role as a key enzyme in the N-glycan catabolism where β-1,4-d-mannosyl-N-acetyl-d-glucosamine is liberated from N-glycans by sequential glycoside hydrolase-catalyzed reactions, transported into the cell, and intracellularly converted into α-d-mannose 1-phosphate and N-acetyl-d-glucosamine. In addition, intestinal anaerobic bacteria such as Bacteroides fragilis, Bacteroides helcogenes, Bacteroides salanitronis, Bacteroides vulgatus, Prevotella denticola, Prevotella dentalis, Prevotella melaninogenica, Parabacteroides distasonis, and Alistipes finegoldii were also suggested to possess the similar metabolic pathway for N-glycans. A notable feature of the new metabolic pathway for N-glycans is the more efficient use of ATP-stored energy, in comparison with the conventional pathway where β-mannosidase and ATP-dependent hexokinase participate, because it is possible to directly phosphorylate the d-mannose residue of β-1,4-d-mannosyl-N-acetyl-d-glucosamine to enter glycolysis. This is the first report of a metabolic pathway for N-glycans that includes a phosphorylase. We propose 4-O-β-d-mannopyranosyl-N-acetyl-d-glucosamine:phosphate α-d-mannosyltransferase as the systematic name and β-1,4-d-mannosyl-N-acetyl-d-glucosamine phosphorylase as the short name for BT1033.
Introduction
Bacteroides thetaiotaomicron is a Gram-negative anaerobic inhabitant of the human distal gut, an environment deficient in mono- and oligosaccharides as carbon sources. Because most of these saccharides are absorbed by the host and various gut microbiota in the upper intestinal tract (1), B. thetaiotaomicron possesses superior capacities to degrade a variety of host-derived glycans elaborated on the surfaces of intestinal epithelial cells, in addition to the dietary polysaccharides that are indigestible by the host (1–4). Recent whole genome transcriptional analyses of B. thetaiotaomicron revealed that B. thetaiotaomicron dedicates ∼18% of its 6.26-Mb genome to 88 individual polysaccharide utilization loci (1, 3–6) for metabolism of various glycans such as N-glycans (7), mucin O-glycans (8), xyloglucan (9), and arabinogalactan (9). At least one of these loci is involved in the conventional metabolism for complex type N-glycans that contain N-acetyl-d-lactosamine (β-1,4-d-galactosyl-N-acetyl-d-glucosamine) units attached to the α-1,3- and/or α-1,6-mannosyl residues linked to the core saccharide β-1,4-d-mannosyl-N,N′-diacetylchitobiose (ManGlcNAc2) and commonly terminate with sialic acid residues (10). The complex type N-glycans are metabolized in this conventional pathway as follows (7). The oligosaccharide chain is cleaved from the glycoprotein by endo-β-N-acetylhexosaminidase (EC 3.2.1.96), belonging to glycoside hydrolase family (GH)2 18 (11). The oligosaccharide is transported into the periplasmic space by a starch utilization system (Sus)-like system (SusC and SusD) and is sequentially degraded by α-sialidase (EC 3.2.1.18), GH2 β-galactosidase (EC 3.2.1.23), GH20 β-N-acetylhexosaminidase (EC 3.2.1.52), and GH92 α-mannosidase (EC 3.2.1.24) (7). The remaining β-1,4-d-mannosyl-N-acetyl-d-glucosamine (ManGlcNAc) is converted by GH2 β-mannosidase (EC 3.2.1.25) into d-mannose and GlcNAc (7). The resultant monosaccharides are transported into the cytoplasm, are phosphorylated by ATP-dependent carbohydrate kinases, and enter glycolysis or amino sugar metabolism (12). The genes encoding the transporter system and glycoside hydrolases, except for endo-β-N-acetylhexosaminidase, constitute a gene cluster in the genome of B. thetaiotaomicron and are well conserved in the genus Bacteroides (see Fig. 1A) (7, 13–17).
FIGURE 1.
Gene loci in the gene cluster involved in the metabolism of complex type N-glycans. A, gene clusters involved in the conventional metabolism by sequential glycoside hydrolase-catalyzed reactions in the genomes of the genus Bacteroides. B, gene clusters involved in the metabolic pathway where a phosphorylase (gray rectangle) participates. Dotted rectangle, the gene cluster of B. thetaiotaomicron of focus in this article. amnA, B, and C, α-mannosidase; bgaA, β-galactosidase; bmnA, β-mannosidase; enhA, endo-β-N-acetylhexosaminidase; estA and S, arylesterase; mfsT, major facilitator superfamily transporter; nahA, B, and C, β-N-acetylhexosaminidase; nanH, α-sialidase; nanM, N-acetylneuramic acid mutarotase; phoA, GH130 phosphorylase; pufA, protein with unknown function; susC, TonB-dependent oligosaccharide transporter; susD, outer membrane lipoprotein.
Several intestinal anaerobes such as Bifidobacterium longum (18) and Clostridium perfringens (19) possess a unique metabolic pathway that efficiently uses the energy of ATP, without an ATP-dependent carbohydrate kinase, to process host-derived O-glycans. In the metabolic pathway, a GH112 β-1,3-d-galactosyl-N-acetyl-d-hexosamine phosphorylase (EC 2.4.1.211) directly produces α-d-galactose 1-phosphate from galacto-N-biose (β-1,3-d-galactosyl-N-acetyl-d-galactosamine) and lacto-N-biose I (β-1,3-d-galactosyl-N-acetyl-d-glucosamine) as degradation products from mucin O-glycans and human milk oligosaccharides, respectively (18, 19). In addition, Bacteroides fragilis and Ruminococcus albus, a member of the human colon microbiota and a ruminal bacterium, respectively, possess metabolic pathways for plant polysaccharide β-1,4-mannan in which GH130 β-1,4-mannosyl-d-glucose phosphorylase (EC 2.4.1.281) and β-1,4-mannooligosaccharide phosphorylase (EC 2.4.1.-) participate (20, 21). These pathways including the phosphorolysis that enables the anaerobes to produce phosphorylated sugars directly without consuming ATP are energetically efficient, in comparison with the conventional metabolic pathway containing sequential glycoside hydrolase-catalyzed reactions, because only three molecules of ATP are available via the glycolytic pathway from glucose 6-phosphate. However, there has been no report of energy-efficient N-glycan metabolism via phosphorylase.
In this study, we identified a gene cluster including a gene encoding a novel GH130 phosphorylase, BT1033, for the energy-efficient metabolism of complex type N-glycans in the genome of the human gut bacterium B. thetaiotaomicron VPI-5482. BT1033 phosphorylase is a key enzyme in a new metabolic pathway of N-glycans, allowing an alternative to the β-mannosidase-containing conventional metabolic pathway. BT1033 plays a crucial role to convert the ManGlcNAc—transported from the periplasmic space into the cytoplasm by the major facilitator superfamily transporter—into α-d-mannose 1-phosphate (α-Man1P) and N-acetyl-d-glucosamine.
EXPERIMENTAL PROCEDURES
Sequence Analysis
Similarity searches were performed at the Swiss Institute of Bioinformatics using the basic local alignment search tool (BLAST) network service. The National Center for Biotechnology Information (NCBI) BLASTP tool was used to search the Swiss-Prot/TrEMBL database (22). Prediction of protein localization and signal peptide was conducted using PSORTb version 3.0.2 (23) and SignalP 4.1 server (24), respectively.
Cloning, Expression, and Purification
The gene encoding BT1033 (GenBankTM accession number AAO76140.1) was amplified by PCR from genomic DNA of B. thetaiotaomicron VPI-5482, using KOD-plus DNA polymerase (Toyobo, Osaka, Japan) with the following oligonucleotides based on the genome sequence (GenBankTM accession number AE015928) (2): 5′-ggaattccatatgaataagattcaaattc-3′ as the forward primer containing an NdeI site (underlined) and 5′-tttctcgaggataatgctcgttcgttttg-3′ as the reverse primer containing an XhoI site (underlined). The amplified gene was purified using a FastGene Gel/PCR extraction kit (Nippon Genetics Co., Tokyo, Japan), digested by NdeI and XhoI (New England Biolabs, Beverly, MA), and inserted into pET24a (+) (Novagen, Madison, WI) to encode a His6 tag fusion at the C terminus of the recombinant protein. The expression plasmid was propagated in Escherichia coli DH5α (Toyobo), purified by a FastGene Plasmid Mini Kit (Nippon Genetics Co.), and verified by sequencing (Operon Biotechnologies, Tokyo, Japan). An E. coli BL21 (DE3) (Novagen) transformant harboring the expression plasmid was grown at 37 °C in 200 ml of Luria-Bertani medium (1% tryptone, 0.5% yeast extract, and 0.5% NaCl) containing 50 μg/ml kanamycin, until the absorbance reached 0.6 at 600 nm. The expression was induced by 0.1 mm isopropyl β-d-thiogalactopyranoside and continued at 18 °C for 24 h. The cells were harvested by centrifugation at 20,000 × g for 20 min and suspended in 50 mm HEPES-NaOH buffer (pH 7.0) containing 500 mm NaCl (buffer A). The suspended cells were disrupted by sonication (Branson sonifier 250A; Branson Ultrasonics, Emerson Japan, Kanagawa, Japan). The supernatant collected by centrifugation at 20,000 × g for 20 min was applied to a HisTrap HP column (GE Healthcare), equilibrated with buffer A containing 10 mm imidazole, using ÄKTA Prime (GE Healthcare). After washing with buffer A containing 22 mm imidazole and subsequent elution using a 22–400 mm imidazole linear gradient in buffer A, fractions containing recombinant protein (BT1033) were pooled, dialyzed against 10 mm HEPES-NaOH buffer (pH 7.0), and concentrated (AMICON Ultra-15 filter; Millipore, Billerica, MA). The protein concentration was determined spectrophotometrically at 280 nm using a theoretical extinction coefficient of ϵ = 37,823 m−1 cm−1, based on the amino acid sequence (25). The molecular mass of purified BT1033 was estimated by SDS-PAGE (Mini-PROTEAN Tetra electrophoresis system; Bio-Rad) and by gel filtration (HiLoad 26/600 Superdex, 200 pg; GE Healthcare) equilibrated with 10 mm HEPES-NaOH buffer (pH 7.0) containing 150 mm NaCl at a flow rate of 0.5 ml/min, using Marker Proteins for molecular Weight Determination on High Pressure Liquid Chromatography (Oriental Yeast Co., Tokyo, Japan) as standards.
Measurement of Synthetic Activity
The synthetic activity was routinely determined by measuring the increase in inorganic phosphate (Pi) using a reaction mixture containing 10 mm α-Man1P (α-Man1P bis(cyclohexylammonium) salt; Sigma-Aldrich) and 10 mm GlcNAc (Wako Pure Chemicals, Osaka, Japan) in 40 mm sodium acetate buffer (pH 5.5) at 30 °C by following the method of Lowry and Lopez (26) as described previously (27). One unit of the synthetic activity was defined as the amount of enzyme that catalyzed the release of 1 μmol of Pi per min under the above conditions.
Acceptor Specificity Analysis
To investigate the acceptor specificity of BT1033 (85 μm), the synthetic reaction was performed under the standard conditions described above, by substituting GlcNAc with putative carbohydrate acceptors (d-altrose, d-fructose, d-glucosamine, d-glucose, isomaltose, kojibiose, lactose, lactulose, maltose, d-mannose, melibiose, methyl-α-d-glucoside, methyl-β-d-glucoside, nigerose, l-rhamnose, d-ribose, sophorose, sucrose, d-talose, trehalose, xylobiose, d-xylose (Wako Pure Chemicals), N-acetyl-d-galactosamine, N-acetyl-d-mannosamine, d-allose, 1,5-anhydro-d-glucitol, 2-deoxy-d-glucose, d-galactosamine, d-galactose, d-galacturonic acid, gentiobiose, α-d-glucose 1-phosphate, d-glucose 6-phosphate, d-lyxose, 3-O-methyl-d-glucose (Sigma-Aldrich), d-arabinose, l-arabinose, cellobiose, β-d-glucose 1-phosphate, d-glucuronic acid (Tokyo Chemical Industry, Tokyo, Japan), β-1,4-mannobiose (Megazyme, Bray, Ireland), N,N′-diacetylchitobiose (β-1,4-N-acetyl-d-glucosaminyl-N-acetyl-d-glucosamine (GlcNAc2)), and laminaribiose (Seikagaku Biobusiness, Tokyo, Japan)) for 2 h. The reaction mixture was spotted on a TLC plate (Kieselgel 60 F254; Merck), and the plate was developed with a mobile phase of 80% acetonitrile in water. The TLC plates were soaked in 5% sulfuric acid-methanol solution and heated in an oven until the bands were visible.
Structural Determination
Reaction products for structural determination were generated in 500 μl of reaction mixture (pH 5.5) containing BT1033 (1.5 and 3.7 μm GlcNAc and GlcNAc2, respectively), 50 mm α-Man1P, and 50 mm GlcNAc or GlcNAc2. The reaction mixtures were incubated at 30 °C for 24 h, followed by desalting using Amberlite MB-3 (Organo, Tokyo, Japan). The reaction products were purified using an HPLC system (Prominence; Shimadzu, Kyoto, Japan) equipped with a Shodex Asahipak NH2P-50 4E column (4.6-mm internal diameter × 25 cm; Showa Denko KK, Tokyo, Japan) at 30 °C under a constant flow (1.0 ml/min) of 75% acetonitrile in water as the mobile phase. Fractions containing the reaction products were collected, followed by lyophilization. The amounts of products obtained were 4 and 5 mg from GlcNAc and GlcNAc2 as the acceptors, respectively. One-dimensional (1H and 13C) and two-dimensional (double-quantum filtered correlation spectroscopy, heteronuclear single-quantum coherence, and heteronuclear multiple-bond correlation) NMR spectra of the product were acquired in D2O with 2-methyl-2-propanol as an internal standard using a Bruker Avance 800 spectrometer (Bruker Biospin, Rheinstetten, Germany). Proton signals were assigned based on the double-quantum filtered correlation spectra. 13C signals were assigned using the heteronuclear single-quantum coherence spectra, based on the assignment of the proton signals. The linkage position of each disaccharide was determined by detecting the inter-ring cross-peaks in each heteronuclear multiple-bond correlation spectrum.
Measurement of Phosphorolytic Activity
The substrates for phosphorolysis of BT1033 were generated in 5 ml of reaction mixture (pH 5.5) containing 15 μm BT1033, 500 mm α-Man1P, and 500 mm GlcNAc or 500 mm GlcNAc2. After incubation at 30 °C for 24 h, the reaction mixtures were desalted using Amberlite MB-3 and loaded onto a Toyopearl HW-40S column (50-mm internal diameter × 950 mm; Tosoh) equilibrated with distilled water at a flow rate of 1.0 ml/min. Fractions containing the reaction products were collected, followed by lyophilization. The amounts of products obtained were 123 and 40 mg from GlcNAc and GlcNAc2 as the acceptors, respectively. The phosphorolytic activity was routinely determined by quantifying the α-Man1P released during a phosphorolytic reaction in 40 mm sodium acetate buffer (pH 5.5) containing 10 mm substrate and 10 mm Pi at 30 °C by the colorimetric method as described previously (28). One unit of the phosphorolytic activity was defined as the amount of enzyme that catalyzed the liberation of 1 μmol of α-Man1P from the substrates per min under the above conditions.
Temperature and pH Profile
The effects of pH on the phosphorolytic and synthetic activities using 212 nm BT1033 were measured under the standard conditions described above, by substituting 40 mm sodium acetate buffer (pH 5.5) with the following 40 mm buffers: sodium citrate (pH 3.0–5.5), bis(2-hydroxyethyl)aminotris(hydroxymethyl)methane-HCl (pH 5.5–7.0), HEPES-NaOH (pH 7.0–8.5), and glycine-NaOH (pH 8.5–10.5). The thermal and pH stabilities were evaluated by measuring the residual synthetic activity under the standard conditions after incubation of BT1033 (7 and 11 μm to study thermal and pH stabilities, respectively) at a temperature range of 30–90 °C for 15 min in 67 mm sodium acetate buffer (pH 5.5) and in the various pH values at 4 °C for 24 h, respectively.
Kinetic Analysis
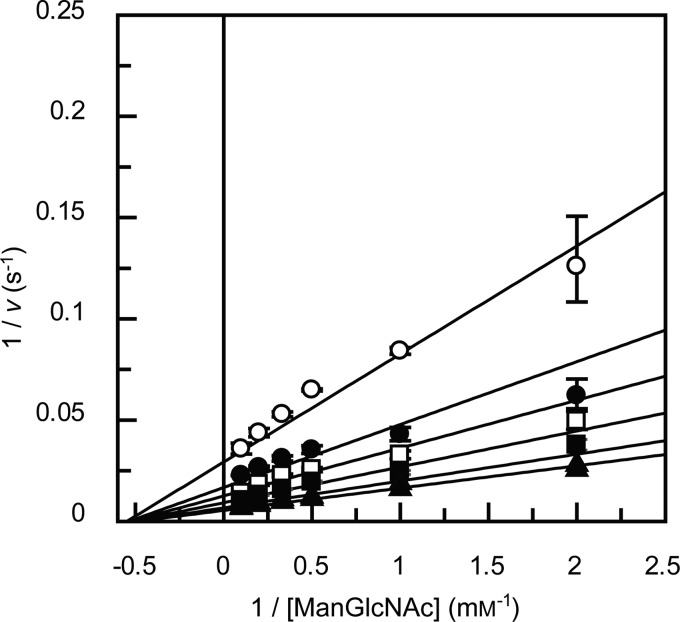
The initial velocities of the phosphorolytic reaction with ManGlcNAc were determined under the standard conditions with 42 nm BT1033 and a combination of initial concentrations of ManGlcNAc (0.5, 1.0, 2.0, 3.0, 5.0, and 10 mm) and Pi (0.1, 0.2, 0.3, 0.5, 1.0, and 2.0 mm). The kinetic parameters were calculated by curve-fitting the experimental data to theoretical Equation 1 for a sequential Bi Bi mechanism using GraFit version 7.0.2 (Erithacus Software Ltd., London, UK).
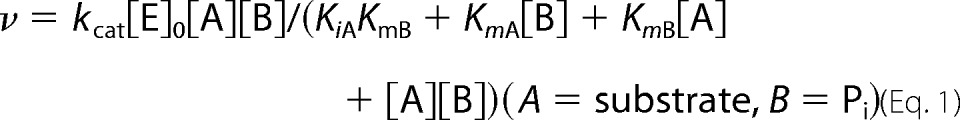 |
The initial velocities of the phosphorolytic reaction with ManGlcNAc2 were determined under the standard conditions with 210 nm BT1033 by substituting 10 mm substrate with various concentrations of ManGlcNAc2 (0.5, 1.0, 2.0, 3.0, 5.0, and 10 mm). The kcat/Km values were obtained from the slope of a linear fit of the initial velocities versus the initial concentrations of ManGlcNAc2.
Kinetic analysis of the synthetic reaction with GlcNAc as the acceptor was performed under the standard conditions (42 and 210 nm BT1033 for GlcNAc and α-Man1P, respectively) and various concentrations of GlcNAc (1.0, 2.0, 3.0, 5.0, 10, 20, and 60 mm) or α-Man1P (0.5, 1.0, 2.0, 3.0, 5.0, and 10 mm) as the donor with 10 mm each opposite substrate. In addition, kinetic analyses of the synthetic reaction with other acceptors were conducted under the standard conditions (42, 210, and 1100 nm BT1033 for GlcNAc2, d-glucose, and d-mannose, respectively) and various concentrations of the acceptors (1.0, 2.0, 3.0, 5.0, 10, 20, and 60 mm). The kinetic parameters for GlcNAc2 were calculated by curve fitting the experimental data to the Michaelis-Menten equation {v = kcat [E]0[S]/(Km + [S])} using GraFit version 7.0.2, whereas the values of kcat/Km for d-glucose and d-mannose were obtained from the slope of a linear fit of the initial velocities versus the initial concentrations of the acceptors.
One-pot Enzymatic Synthesis of ManGlcNAc
One-pot synthesis of ManGlcNAc was performed in 1 ml of reaction mixture (pH 7.0) containing 250 mm sucrose, 250 mm GlcNAc, 25 mm Pi, and 20 μg/ml d-glucose 1,6-bisphosphate (Sigma-Aldrich), 33 μg/ml sucrose phosphorylase, 0.34 mg/ml α-phosphoglucomutase (Sigma-Aldrich), 0.37 mg/ml d-glucose 6-phosphate isomerase, 0.23 mg/ml d-mannose-6-phosphate isomerase, 2.4 mg/ml α-phosphomannomutase, and 83 μg/ml BT1033 at 30 °C for 276 h. Sucrose phosphorylase, d-glucose 6-phosphate isomerase, d-mannose-6-phosphate isomerase, and α-phosphomannomutase were prepared as described previously (28, 29). The concentration of ManGlcNAc was monitored by a HPLC system (Prominence; Shimadzu) equipped with a Shodex Asahipak NH2P-50 4E column at 30 °C under a constant flow (1.0 ml/min) of 75% acetonitrile in water as the mobile phase. The reaction mixture was treated with 0.3 mg/ml invertase (Sigma-Aldrich) at 30 °C for 20 h to eliminate remaining sucrose, followed by desalting using Amberlite MB-3 (Organo). The synthesized ManGlcNAc was purified by a Toyopearl HW-40S column (50-mm internal diameter × 950 mm; Tosoh) equilibrated with distilled water at a flow rate of 1.0 ml/min. Fractions containing the reaction product were collected, followed by lyophilization. The amount of ManGlcNAc obtained was 22 mg.
RESULTS
Prediction of the Enzymatic Function of BT1033
A gene cluster involved in complex type N-glycan metabolism was identified in the genome of B. thetaiotaomicron VPI-5482 (Fig. 1B). Based on assignments using BLASTP (22) (supplemental Table S1), the gene cluster contains nine unidirectionally transcribed ORFs (Fig. 1B) that encode four glycoside hydrolases (GH92 α-mannosidase (BT1032), GH20 β-N-acetylhexosaminidase (BT1035), exo-α-sialidase (BT1036), and GH18 endo-β-N-acetylglucosaminidase (BT1038)), a major facilitator superfamily transporter (BT1034), a hypothetical protein with unknown function (BT1037), an outer membrane lipoprotein (BT1039) and a TonB-dependent oligosaccharide transporter (BT1040) constituting a Sus-like protein, and a GH130 phosphorylase (BT1033). The sequence analysis including prediction of protein localization based on PSORTb version 3.0.2 (23) and signal peptide identification using version SignalP 4.1 (24) suggests that BT1033 plays a role in the intracellular phosphorolysis of ManGlcNAc liberated from complex type N-glycans by sequential glycoside hydrolase-catalyzed reactions in the periplasmic space and transported into the cytoplasm. However, there have been no reports on the N-glycans metabolism that a phosphorylase participates. In this study, BT1033 was recombinantly expressed in E. coli BL21 (DE3) to investigate the detail enzymatic properties, as described below.
Preparation of Recombinant BT1033
Recombinant BT1033 was purified by nickel chelate affinity chromatography with a yield of 24 mg from the cell lysate of a 200-ml culture. Purified BT1033 migrated in SDS-PAGE as a single protein band with an estimated size of 35 kDa in agreement with the theoretical molecular mass of 37,823. However, the molecular mass was estimated by gel filtration to be 147 kDa, indicating that BT1033 is a homotetramer in solution, whereas R. albus GH130 β-1,4-d-mannosyl-d-glucose phosphorylase (EC 2.4.1.281) and β-1,4-d-mannooligosaccharide phosphorylase (EC 2.4.1.-) showing 39 and 70% sequence similarities with BT1033, respectively, have been reported to be homodimeric and homohexameric, respectively (21).
Synthetic Reaction Catalyzed by BT1033
The acceptor specificity in the synthetic reaction was examined using various carbohydrate acceptor candidates (see “Experimental Procedures”) together with α-Man1P as the donor. BT1033 utilized GlcNAc and GlcNAc2 as the suitable acceptors, with specific activities of 41 and 8 units/mg, respectively. Each synthetic reaction gave a single product from GlcNAc and GlcNAc2. The products from GlcNAc and GlcNAc2 were identified by 1H and 13C NMR spectroscopic analysis to be the corresponding β-1,4-d-mannopyranosyl-β-1,4-N-acetyl-d-glucosamine(ManGlcNAc) (supplemental Fig. S1) and β-1,4-d-mannopyranosyl-β-1,4-N-acetyl-d-glucosaminyl-β-1,4-N-acetyl-d-glucosamine (ManGlcNAc2) (supplemental Fig. S2), respectively. In addition BT1033 showed weak synthetic activities with d-glucose and d-mannose. Therefore, the kinetic parameters for the four acceptors (GlcNAc, GlcNAc2, d-glucose, and d-mannose) were determined to investigate the accepter preference of BT1033 in the presence of α-Man1P as the donor (Table 1). The Km value for GlcNAc was in the millimolar range and was 12 times lower than that for GlcNAc2. However, the kcat value for GlcNAc was in the same range with that for GlcNAc2. These results indicate that the catalytic efficiency (kcat/Km) values for GlcNAc and GlcNAc2 mainly depend on the Km values. In addition, the fact that the kcat/Km value for GlcNAc was 140–200 times greater than those for the other acceptors indicates that GlcNAc is the most effective acceptor for BT1033. Based on the acceptor specificity and considering that the kinetic parameters for α-Man1P are in the same ranges as those of the other inverting phosphorylases for their specific donors (20, 21, 27, 30–36), we here propose 4-O-β-d-mannopyranosyl-N-acetyl-d-glucosamine:phosphate α-d-mannosyltransferase as the systematic name and β-1,4-d-mannosyl-N-acetyl-d-glucosamine phosphorylase as the short name for BT1033 (Fig. 2).
TABLE 1.
Kinetic parameters for the synthetic reactions catalyzed by BT1033
| Km | kcat | kcat/m | |
|---|---|---|---|
| mm | s−1 | s−1mm−1 | |
| Acceptora | |||
| GlcNAc | 3.8 ± 0.19 | 37 ± 0.57 | 9.6 |
| GlcNAc2 | 27 ± 1.9 | 22 ± 0.77 | 0.8 |
| Glucose | NDc | NDc | 0.067d |
| Mannose | NDc | NDc | 0.047d |
| Donorb | |||
| α-Man1P | 2.6 ± 0.6 | 25 ± 3.4 | 9.6 |
a Kinetic parameters were calculated by fitting the initial velocities toward various concentrations of acceptor substrates in the presence of 10 mm α-Man1P to the Michaelis-Menten equation. The data are the means ± S.D. for three independent experiments.
b Kinetic parameters were calculated by fitting the initial velocities toward various concentrations of donor substrates in the presence of 10 mm GlcNAc to the Michaelis-Menten equation. The data are the means ± S.D. for three independent experiments.
c ND, not determined because of the high Km value.
d Kinetic parameters were calculated from the slope of the linear [A]-v plots, where A represents the concentration of acceptor substrate in the presence of 10 mm α-Man1P.
FIGURE 2.
Schematic representation of the reversible phosphorolysis of ManGlcNAc (A) and ManGlcNAc2 (B) catalyzed by BT1033.
Phosphorolytic Reaction Catalyzed by BT1033
BT1033 catalyzed the phosphorolysis of ManGlcNAc and ManGlcNAc2 with inversion of the anomeric configuration to release α-Man1P and GlcNAc or GlcNAc2, respectively. The specific activity values on ManGlcNAc and ManGlcNAc2 were 258 and 30 units/mg of protein, respectively. In addition, BT1033 did not cleave the substrates in the absence of Pi. Double reciprocal plots of the initial velocities against various initial concentrations of ManGlcNAc and Pi gave a series of lines intersecting at a single point (Fig. 3). These results indicate that the phosphorolytic reactions on ManGlcNAc follow a sequential Bi Bi mechanism, similar to inverting phosphorylases (21, 27, 30–35). In addition, BT1033 did not phosphorolyze β-1,4-d-mannosyl-d-glucose and β-1,4-mannooligosaccharides (degree of polymerization of 2–6), known substrates of the GH130 β-1,4-d-mannosyl-d-glucose phosphorylase (20, 21), and β-1,4-mannooligosaccharide phosphorylase (21), respectively. The kinetic parameters for ManGlcNAc and ManGlcNAc2 are summarized in Table 2. The kcat/Km value for ManGlcNAc was 58 times greater than that for ManGlcNAc2. In addition, the parameters for ManGlcNAc are in the same ranges with those of the other inverting phosphorylases (21, 27, 30–35), indicating that ManGlcNAc is the true substrate of BT1033.
FIGURE 3.
Double reciprocal plot for the phosphorolysis of ManGlcNAc by BT1033. The initial velocities for phosphorolysis of ManGlcNAc at various concentrations of ManGlcNAc and Pi were measured. The concentrations of Pi were 0.1 mm (open circles), 0.2 mm (filled circles), 0.3 mm (open squares), 0.5 mm (filled squares), 1 mm (open triangles), and 2 mm (filled triangles). The data are the means ± S.D. (error bars) for three independent experiments.
TABLE 2.
Kinetic parameters for the phosphorolytic reactions catalyzed by BT1033
| Substrate | kcat | KmA | KmB | KiA | kcat/KmA |
|---|---|---|---|---|---|
| s−1 | mm | mm | mm | s−1 mm−1 | |
| ManGlcNAca | 240 | 2.1 | 0.6 | 1.7 | 110 |
| ManGlcNAc2b | NDc | NDc | NDc | NDc | 1.9 |
a Kinetic parameters were calculated by fitting the initial velocities toward 0.5−10 mm ManGlcNAc in the presence of 0.1−2.0 mm Pi to the following theoretical equation for sequential bi-bi mechanism using Grafit version 7.0.2: v = kcat[E]0[A][B]/(KiAKmB + KmA [B] + KmB [A] + [A][B]), where A is ManGlcNAc, and B is Pi.
b Kinetic parameters were calculated from the slope of the linear [A]-v plots, where A is 0.5−10 mm ManGlcNAc2 in the presence of 10 mm Pi.
c ND, not determined.
Basic Properties of BT1033
BT1033 was stable up to 55 °C during 15 min of incubation (Fig. 4A) and in the range of pH 4.5–10.5 at 4 °C for 24 h (Fig. 4B). The optimum pH for both the phosphorolytic and synthetic reactions was pH 5.5 (Fig. 4C).
FIGURE 4.
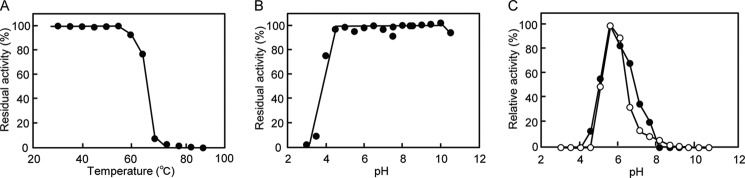
Effect of pH and temperature on the activity and stability of BT1033. A, stability of 7 μm BT1033 at the temperature range of 30–90 °C for 15 min. B, pH stability of 11 μm BT1033 at 4 °C for 24 h. C, pH activity dependence of the phosphorolysis and synthesis of ManGlcNAc by 212 nm BT1033 in 40 mm sodium citrate (pH 3.0–5.5), bis(2-hydroxyethyl)aminotris(hydroxymethyl)methane-HCl (pH 5.5–7.0), HEPES-NaOH (pH 7.0–8.5), and glycine-NaOH (pH 8.5–10.5). The closed and open symbols represent synthetic and phosphorolytic activities, respectively.
Synthesis of ManGlcNAc from Sucrose and GlcNAc
A one-pot enzymatic reaction by concomitant actions of sucrose phosphorylase (EC 2.4.1.7), α-phosphoglucomutase (EC 5.4.2.2), d-glucose 6-phosphate isomerase (EC 5.3.1.9), d-mannose-6-phosphate isomerase (EC 5.3.1.8), α-phosphomannomutase (EC 5.4.2.8), and BT1033 was demonstrated to synthesize ManGlcNAc from sucrose and GlcNAc as inexpensive starting materials (Fig. 5A). As shown in Fig. 5B, synthesis of ManGlcNAc was observed along with a decrease in GlcNAc at the early stage of the reaction. After 276 h of reaction, the product formation reached a plateau, and 58 mm ManGlcNAc was produced in the reaction mixture.
FIGURE 5.
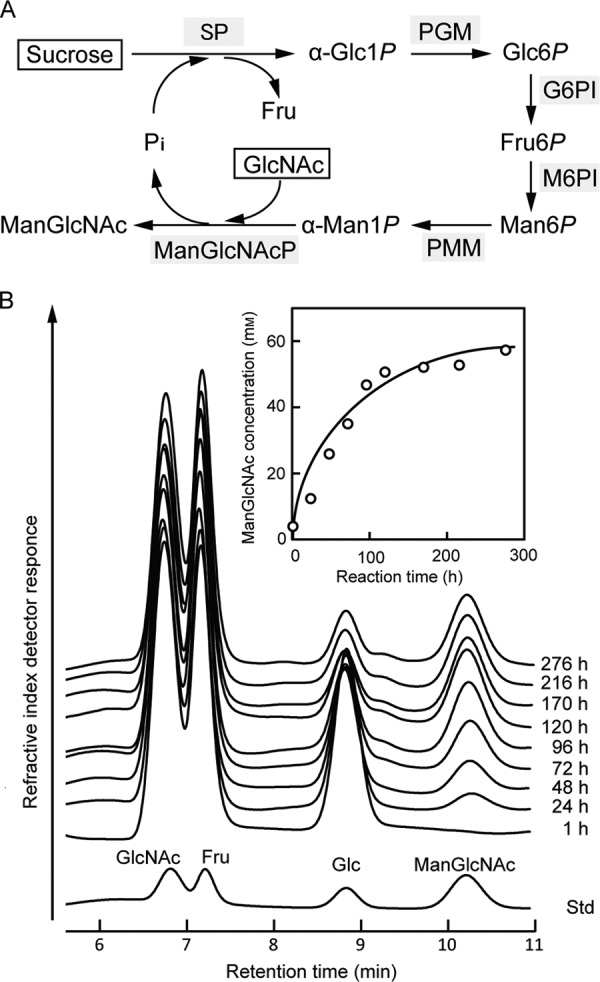
One-pot enzymatic synthesis of ManGlcNAc from sucrose and GlcNAc. A, reaction scheme for the synthesis of ManGlcNAc. Sugars used as the starting materials are boxed. Enzymes used are represented using gray shading. SP, sucrose phosphorylase; PGM, α-phosphoglucomutase; G6PI, d-glucose 6-phosphate isomerase; M6PI, d-mannose-6-phosphate isomerase; PMM, α-phosphomannomutase; ManGlcNAcP, β-1,4-d-mannosyl-N-acetyl-d-glucosamine phosphorylase. B, HPLC monitoring of the one-pot enzymatic synthesis of ManGlcNAc. The remaining sucrose in the reaction mixture was removed by incubating with invertase, allowing the quantification of ManGlcNAc. Inset, time course of the ManGlcNAc concentration calculated from the peak areas.
DISCUSSION
Enzymatic Function of BT1033
BT1033 has unique substrate specificity in the phosphorolytic reaction; it recognizes ManGlcNAc and ManGlcNAc2 as the substructures of the N-glycans. The amino acid sequence of BT1033 shows 42, 39, and 79% similarities with those of B. fragilis and R. albus β-1,4-mannosyl-d-glucose phosphorylases and R. albus β-1,4-mannooligosaccharide phosphorylase belonging to GH130 (supplemental Table S1), respectively, which are involved in the catabolism for plant polysaccharide β-1,4-mannan (20, 21). To date, no three-dimensional structures of reported GH130 phosphorylases have been solved. In addition, there has been no report of enzymatic activities of four putative GH130 glycosidases from Bacteroides ovatus, B. thetaiotaomicron, Parabacteroides distasonis, and Thermotoga maritima, of which the three-dimensional structures are available (Protein Data Bank codes 3QC2, 3R67, 3TAW, and 1VKD, respectively). Furthermore, information regarding the amino acid residues involved in the substrate recognition or catalysis is not available because the substrate-enzyme complex structure has not been solved for GH130. However, together with the fact that BT1033 showed no phosphorolytic activity toward β-1,4-mannosyl-d-glucose, the substrate for the reported GH130 β-1,4-mannosyl-d-glucose phosphorylase (20, 21), the much lower kcat/Km values of d-glucose and d-mannose substituted at C2 position of GlcNAc as an suitable acceptor in the synthetic reaction with strict β-1,4-regioselectivity clearly suggest that BT1033 recognizes the C2 N-acetyl group at subsite +1. Regarding the recognition of the C2 N-acetyl group of the acceptor molecule at subsite +1, a GH94 N,N′-diacetylchitobiose phosphorylase (EC 2.4.1.280) from Vibrio proteolyticus has been reported to recognize the C2 N-acetyl group of GlcNAc at subsite +1 by interaction with the methyl group in a small hydrophobic pocket formed by Cys-493 and Val-631. In contrast, the space is occupied by a well conserved bulky Tyr residue in case of GH 94 cellobiose phosphorylase (EC 2.4.1.20) (37, 38). In addition, BT1033 did not utilize N-acetyl-d-galactosamine with an axial hydroxyl group at the C4 position as the acceptor in the synthetic reaction, clearly indicating that the equatorial C4 hydroxyl group of GlcNAc is essential for acceptor binding at subsite +1. Notably, GlcNAc2 acted as the acceptor in the synthetic reaction, suggesting that BT1033 possesses a moderately large binding space to accommodate the oligosaccharide acceptor. Therefore, addition of an excess of the enzyme was confirmed to cause elongation of successive d-mannose residue at the nonreducing end of the acceptor molecule, although d-mannose was not a suitable acceptor at the subsite +1.
It should be noted that the enzymatic properties of BT1033, such as strict regioselectivity and acceptor specificity, enable the enzymatic syntheses of ManGlcNAc and ManGlcNAc2, which are common core structures shared by all N-glycans such as high mannose, complex, and hybrid types (10). Stereoselective formation of the β-mannosidic linkages is a considerable challenge in synthetic glycochemistry because the vicinal C2 hydroxyl group blocks access to the β-face because of its steric and polar effects (39). Therefore, the new synthetic method for core structure of N-glycans employing the synthetic reaction of this unique mannoside phosphorylase would be a strong tool for the efficient preparation of various N-glycans and glycoconjugates used in the research field of glycobiology and glycotechnology. In addition, ManGlcNAc was synthesized by a one-pot enzymatic reaction from sucrose and GlcNAc as the starting materials, which are industrially prepared and are available at reasonable cost (Fig. 5A). The details of ManGlcNAc synthesis are as follows: (i) sucrose is phosphorolyzed into α-d-glucose 1-phosphate and fructose by sucrose phosphorylase; (ii) α-d-glucose 1-phosphate is converted into α-Man1P via d-glucose 6-phosphate, d-fructose 6-phosphate, and d-mannose 6-phosphate by the sequential reactions of α-phosphoglucomutase, d-glucose 6-phosphate isomerase, d-mannose-6-phosphate isomerase, and α-phosphomannomutase; and (iii) ManGlcNAc is generated from α-Man1P and GlcNAc by BT1033 together with the release of Pi. Because Pi is recycled in the reaction, the overall reaction can be described as the transformation of sucrose and GlcNAc to ManGlcNAc and fructose by the concomitant action of the six enzymes in the presence of catalytic amounts of Pi. Notably, this strategy circumvents the addition of costly α-Man1P and is compatible with large scale production. Moreover, starch and cellobiose/cellodextrin biomass are available as the starting materials by substituting sucrose phosphorylase with starch phosphorylase (EC 2.4.1.1) and cellobiose/cellodextrin phosphorylase (EC 2.4.1.20/EC 2.4.1.49), respectively, which catalyze the release of α-d-glucose 1-phosphate by the phosphorolysis. This one pot enzymatic approach using the inexpensive starting materials can be extended to include the production of a variety of valuable β-mannosides using β-1,4-d-mannosyl-d-glucose and β-1,4-mannooligosaccharide phosphorylases belonging to GH130 with known acceptor specificities in the synthetic reaction (21).
Physiological Role of BT1033
The complex type N-glycans had been considered to be metabolized in a conventional pathway where β-mannosidase and ATP-dependent hexokinase participate. In this study, a gene cluster including a gene encoding a β-1,4-d-mannosyl-N-acetyl-d-glucosamine phosphorylase (BT1033) for the energy-efficient metabolism of complex type N-glycans was identified in the genome of B. thetaiotaomicron VPI-5482. This is the first report of a metabolic pathway for complex type N-glycans in which a unique phosphorylase participates. A possible metabolic pathway for complex type N-glycans in B. thetaiotaomicron is illustrated in Fig. 6. In the pathway, an oligosaccharide chain is cleaved from glycoprotein by endo-β-N-acetylhexosaminidase (BT1038) and transported into periplasmic space by a Sus-like system that comprises one pair of outer membrane proteins homologous to SusC (BT1040) and SusD (BT1039) (40). The transported oligosaccharide chain is sequentially degraded by α-sialidases (BT1036), β-galactosidase, β-N-acetylhexosaminidase (BT1035), and α-mannosidase (BT1032). The resultant ManGlcNAc is transported into the cytoplasm by an major facilitator superfamily transporter (BT1034), followed by phosphorolysis by BT1033 to produce α-Man1P and N-acetyl-d-glucosamine. The α-Man1P released is converted into d-fructose 6-phosphate via d-mannose 6-phosphate by the sequential reaction of phosphomannomutases (BT1548 or BT3950) and d-mannose-6-phosphate isomerase (BT0373) to enter glycolysis. The GlcNAc released is also converted into d-fructose 6-phosphate via GlcNAc 6-phosphate and d-glucosamine 6-phosphate by sequential reaction of hexokinase (BT2430) and d-glucosamine 6-phosphate deaminase (BT0258, BT3587, or BT4127) to enter the glycolysis. Thus, BT1033 plays a necessary role to phosphorolyze the intracellular ManGlcNAc, as an alternative to β-mannosidase in the conventional metabolic pathway for complex type N-glycans. Notably, intestinal anaerobes such as B. fragilis (16, 17), Bacteroides helcogenes (13), Bacteroides salanitronis (14), Bacteroides vulgatus (15), Prevotella denticola, Prevotella dentalis, Prevotella melaninogenica, P. distasonis (15), and Alistipes finegoldii were also identified to possess the similar metabolic pathway for complex type N-glycans (Fig. 1B), according to sequence analyses using BLAST (22) and PSORTb (23). One notable feature of the new N-glycan metabolic pathway is that these intestinal anaerobes efficiently use the energy of ATP, because the d-mannose residue of ManGlcNAc can be directly phosphorylated without consuming ATP by ATP-dependent carbohydrate kinase. Hence, the energy-efficient strategy for N-glycan metabolism in which a unique β-1,4-d-mannosyl-N-acetyl-d-glucosamine phosphorylase participates would have provided intestinal anaerobic bacteria with evolutionary advantages because anaerobic respiration is in general less energy-efficient than aerobic respiration.
FIGURE 6.
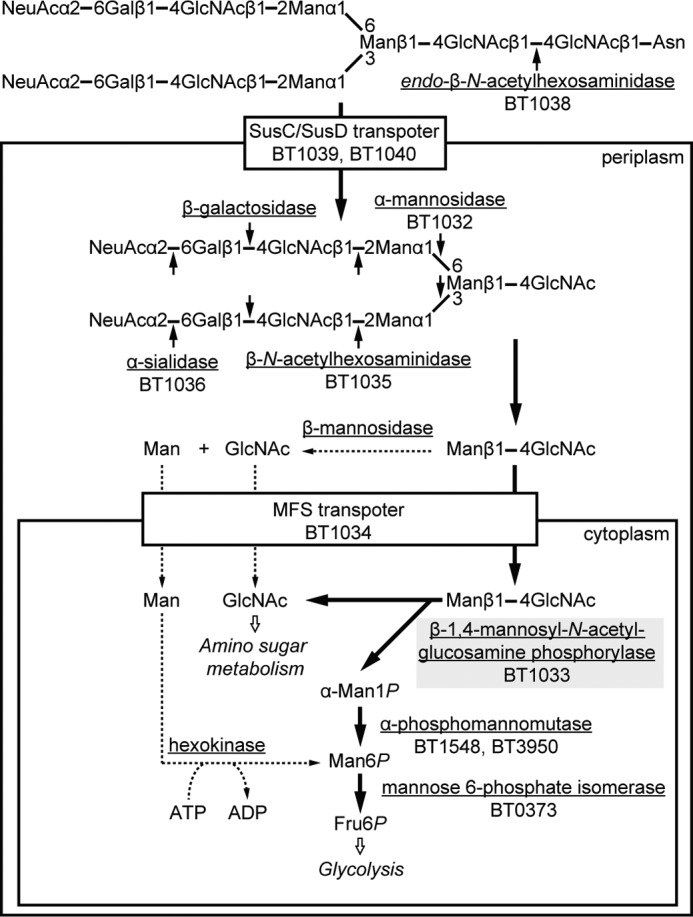
Predicted metabolic pathway for complex type N-glycans in B. thetaiotaomicron. Underlined, enzymes involved in the metabolism of complex type N-glycans; bold arrows, predicted new metabolic pathway; dotted arrows, conventional metabolic pathway; solid arrows, cleavage sites by the N-glycan catabolic enzymes; gray shading, key enzyme involved in the new metabolic pathway for the N-glycans. Fru6P, d-fructose 6-phosphate; Gal, d-galactose; Man, d-mannose; α-Man1P, α-d-mannose 1-phosphate; Man6P, d-mannose 6-phosphate; MFS, major facilitator superfamily; NeuAc, N-acetyl-d-neuraminic acid; SusC, TonB-dependent oligosaccharide transporter; SusD, outer membrane lipoprotein.
Conclusions
A gene cluster involved in complex type N-glycan metabolism was identified in the genome of B. thetaiotaomicron VPI-5482. It was demonstrated that BT1033 encoded in the gene cluster catalyzed the reversible phosphorolysis of ManGlcNAc in a typical sequential Bi Bi mechanism. This is the first report of a metabolic pathway for complex type N-glycans in which a unique phosphorylase participates. In addition, several intestinal anaerobes were also identified to possess the similar metabolic pathway for the N-glycans. One notable feature of the new metabolic pathway for N-glycans is that these intestinal anaerobes efficiently use the energy of ATP, in comparison with a conventional pathway in which β-mannosidase and ATP-dependent hexokinase participate, because it is possible to directly phosphorylate the d-mannose residue of ManGlcNAc to enter glycolysis.
Supplementary Material
Acknowledgments
We thank the staff of the Instrumental Analysis Center for Food Chemistry of the National Food Research Institute for recording NMR spectra.
This work was supported in part by the MEXT Promotion of Environmental Improvement for Independence of Young Researchers Program under the Special Coordination Funds for Promoting Science and Technology and Sapporo Bioscience Foundation Research Grant.

This article contains supplemental Table S1 and Figs. S1 and S2.
- GH
- glycoside hydrolase family
- GlcNAc2
- N,N′-diacetylchitobiose
- α-Man1P
- α-d-mannose 1-phosphate
- ManGlcNAc
- β-1,4-d-mannosyl-N-acetyl-d-glucosamine
- ManGlcNAc2
- β-1,4-d-mannosyl-N,N′-diacetylchitobiose
- Sus
- starch utilization system.
REFERENCES
- 1. Koropatkin N. M., Cameron E. A., Martens E. C. (2012) How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 10, 323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu J., Gordon J. I. (2003) Honor thy symbionts. Proc. Natl. Acad. Sci. U.S.A. 100, 10452–10459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zocco M. A., Ainora M. E., Gasbarrini G., Gasbarrini A. (2007) Bacteroides thetaiotaomicron in the gut. Molecular aspects of their interaction. Dig. Liver Dis. 39, 707–712 [DOI] [PubMed] [Google Scholar]
- 4. Martens E. C., Chiang H. C., Gordon J. I. (2008) Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe 4, 447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bjursell M. K., Martens E. C., Gordon J. I. (2006) Functional genomic and metabolic studies of the adaptations of a prominent adult human gut symbiont, Bacteroides thetaiotaomicron, to the suckling period. J. Biol. Chem. 281, 36269–36279 [DOI] [PubMed] [Google Scholar]
- 6. Martens E. C., Roth R., Heuser J. E., Gordon J. I. (2009) Coordinate regulation of glycan degradation and polysaccharide capsule biosynthesis by a prominent human gut symbiont. J. Biol. Chem. 284, 18445–18457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nakayama-Imaohji H., Ichimura M., Iwasa T., Okada N., Ohnishi Y., Kuwahara T. (2012) Characterization of a gene cluster for sialoglycoconjugate utilization in Bacteroides fragilis. J. Med. Invest. 59, 79–94 [DOI] [PubMed] [Google Scholar]
- 8. Tap J., Mondot S., Levenez F., Pelletier E., Caron C., Furet J. P., Ugarte E., Muñoz-Tamayo R., Paslier D. L., Nalin R., Dore J., Leclerc M. (2009) Towards the human intestinal microbiota phylogenetic core. Environ. Microbiol. 11, 2574–2584 [DOI] [PubMed] [Google Scholar]
- 9. Mohnen D. (2008) Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 11, 266–277 [DOI] [PubMed] [Google Scholar]
- 10. Stanley P., Schachter H., Taniguchi N. (2009) N-Glycans, in Essentials of Glycobiology (Varki A., Cummings R. D., Esko J. D., Freeze H. H., Stanley P., Bertozzi C. R., Hart G. W., Etzler M. E., eds) 2nd Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- 11. Henrissat B. (1991) A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 280, 309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. White R. J. (1968) Control of amino sugar metabolism in Escherichia coli and isolation of mutants unable to degrade amino sugars. Biochem. J. 106, 847–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pati A., Gronow S., Zeytun A., Lapidus A., Nolan M., Hammon N., Deshpande S., Cheng J. F., Tapia R., Han C., Goodwin L., Pitluck S., Liolios K., Pagani I., Ivanova N., Mavromatis K., Chen A., Palaniappan K., Land M., Hauser L., Chang Y. J., Jeffries C. D., Detter J. C., Brambilla E., Rohde M., Göker M., Woyke T., Bristow J., Eisen J. A., Markowitz V., Hugenholtz P., Kyrpides N. C., Klenk H. P., Lucas S. (2011) Complete genome sequence of Bacteroides helcogenes type strain (P 36–108). Stand. Genomic Sci. 4, 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gronow S., Held B., Lucas S., Lapidus A., Del Rio T. G., Nolan M., Tice H., Deshpande S., Cheng J. F., Pitluck S., Liolios K., Pagani I., Ivanova N., Mavromatis K., Pati A., Tapia R., Han C., Goodwin L., Chen A., Palaniappan K., Land M., Hauser L., Chang Y. J., Jeffries C. D., Brambilla E. M., Rohde M., Göker M., Detter J. C., Woyke T., Bristow J., Markowitz V., Hugenholtz P., Kyrpides N. C., Klenk H. P., Eisen J. A. (2011) Complete genome sequence of Bacteroides salanitronis type strain (BL78). Stand. Genomic Sci. 4, 191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu J., Mahowald M. A., Ley R. E., Lozupone C. A., Hamady M., Martens E. C., Henrissat B., Coutinho P. M., Minx P., Latreille P., Cordum H., Van Brunt A., Kim K., Fulton R. S., Fulton L. A., Clifton S. W., Wilson R. K., Knight R. D., Gordon J. I. (2007) Evolution of symbiotic bacteria in the distal human intestine. PLoS Biol. 5, e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuwahara T., Yamashita A., Hirakawa H., Nakayama H., Toh H., Okada N., Kuhara S., Hattori M., Hayashi T., Ohnishi Y. (2004) Genomic analysis of Bacteroides fragilis reveals extensive DNA inversions regulating cell surface adaptation. Proc. Natl. Acad. Sci. U.S.A. 101, 14919–14924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cerdeño-Tárraga A. M., Patrick S., Crossman L. C., Blakely G., Abratt V., Lennard N., Poxton I., Duerden B., Harris B., Quail M. A., Barron A., Clark L., Corton C., Doggett J., Holden M. T., Larke N., Line A., Lord A., Norbertczak H., Ormond D., Price C., Rabbinowitsch E., Woodward J., Barrell B., Parkhill J. (2005) Extensive DNA inversions in the B. fragilis genome control variable gene expression. Science 307, 1463–1465 [DOI] [PubMed] [Google Scholar]
- 18. Kitaoka M., Tian J., Nishimoto M. (2005) Novel putative galactose operon involving lacto-N-biose phosphorylase in Bifidobacterium longum. Appl. Environ. Microbiol. 71, 3158–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakajima M., Nihira T., Nishimoto M., Kitaoka M. (2008) Identification of galacto-N-biose phosphorylase from Clostridium perfringens ATCC13124. Appl. Microbiol. Biotechnol. 78, 465–471 [DOI] [PubMed] [Google Scholar]
- 20. Senoura T., Ito S., Taguchi H., Higa M., Hamada S., Matsui H., Ozawa T., Jin S., Watanabe J., Wasaki J., Ito S. (2011) New microbial mannan catabolic pathway that involves a novel mannosylglucose phosphorylase. Biochem. Biophys. Res. Commun. 408, 701–706 [DOI] [PubMed] [Google Scholar]
- 21. Kawahara R., Saburi W., Odaka R., Taguchi H., Ito S., Mori H., Matsui H. (2012) Metabolic mechanism of mannan in a ruminal bacterium, Ruminococcus albus, involving two mannoside phosphorylases and cellobiose 2-epimerase. Discovery of a new carbohydrate phosphorylase, β-1,4-mannooligosaccharide phosphorylase. J. Biol. Chem. 287, 42389–42399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. (1990) Basic local alignment search tool. J. Mol. Biol. 215, 403–410 [DOI] [PubMed] [Google Scholar]
- 23. Gardy J. L., Spencer C., Wang K., Ester M., Tusnády G. E., Simon I., Hua S., deFays K., Lambert C., Nakai K., Brinkman F. S. (2003) PSORT-B. Improving protein subcellular localization prediction for Gram-negative bacteria, Nucleic Acids Res. 31, 3613–3617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nielsen H., Engelbrecht J., Brunak S., von Heijne G. (1997) Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10, 1–6 [DOI] [PubMed] [Google Scholar]
- 25. Pace C. N., Vajdos F., Fee L., Grimsley G., Gray T. (1995) How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 4, 2411–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lowry O. H., Lopez J. A. (1946) The determination of inorganic phosphate in the presence of labile phosphate esters. J. Biol. Chem. 162, 421–428 [PubMed] [Google Scholar]
- 27. Nihira T., Nakai H., Chiku K., Kitaoka M. (2012) Discovery of nigerose phosphorylase from Clostridium phytofermentans. Appl. Microbiol. Biotechnol. 93, 1513–1522 [DOI] [PubMed] [Google Scholar]
- 28. Nihira T., Suzuki E., Kitaoka M., Nishimoto M., Ohtsubo K., Nakai H. (2013) Colorimetric quantification of α-d-mannose 1-phosphate. J. Appl. Glycosci. 60, 137–139 [Google Scholar]
- 29. Nishimoto M., Kitaoka M. (2007) Practical preparation of lacto-N-biose I, a candidate for the bifidus factor in human milk. Biosci. Biotechnol. Biochem. 71, 2101–2104 [DOI] [PubMed] [Google Scholar]
- 30. Nakajima M., Nishimoto M., Kitaoka M. (2009) Characterization of three β-galactoside phosphorylases from Clostridium phytofermentans. Discovery of d-galactosyl-β1→4-l-rhamnose phosphorylase. J. Biol. Chem. 284, 19220–19227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nihira T., Saito Y., Kitaoka M., Otsubo K., Nakai H. (2012) Identification of Bacillus selenitireducens MLS10 maltose phosphorylase possessing synthetic ability for branched α-d-glucosyl trisaccharides. Carbohydr. Res. 360, 25–30 [DOI] [PubMed] [Google Scholar]
- 32. Kitaoka M., Sasaki T., Taniguchi H. (1993) Purification and properties of laminaribiose phosphorylase (EC 2.4.1.31) from Euglena gracilis Z. Arch. Biochem. Biophys. 304, 508–514 [DOI] [PubMed] [Google Scholar]
- 33. Kitaoka M., Matsuoka Y., Mori K., Nishimoto M., Hayashi K. (2012) Characterization of a bacterial laminaribiose phosphorylase. Biosci. Biotechnol. Biochem. 76, 343–348 [DOI] [PubMed] [Google Scholar]
- 34. Nihira T., Saito Y., Kitaoka M., Nishimoto M., Otsubo K., Nakai H. (2012) Characterization of a laminaribiose phosphorylase from Acholeplasma laidlawii PG-8A and production of 1,3-β-d-glucosyl disaccharides. Carbohydr. Res. 361, 49–54 [DOI] [PubMed] [Google Scholar]
- 35. Nakai H., Kitaoka M., Svensson B., Ohtsubo K. (2013) Recent development of phosphorylases possessing large potential for oligosaccharide synthesis. Curr. Opin. Chem. Biol. 17, 301–309 [DOI] [PubMed] [Google Scholar]
- 36. Nihira T., Nakai H., Kitaoka M. (2012) 3-O-α-d-Glucopyranosyl-l-rhamnose phosphorylase from Clostridium phytofermentans. Carbohydr. Res. 350, 94–97 [DOI] [PubMed] [Google Scholar]
- 37. Hidaka M., Honda Y., Kitaoka M., Nirasawa S., Hayashi K., Wakagi T., Shoun H., Fushinobu S. (2004) Chitobiose phosphorylase from Vibrio proteolyticus, a member of glycosyl transferase family 36, has a clan GH-L-like (α/α)6 barrel fold. Structure 12, 937–947 [DOI] [PubMed] [Google Scholar]
- 38. Hidaka M., Kitaoka M., Hayashi K., Wakagi T., Shoun H., Fushinobu S. (2006) Structural dissection of the reaction mechanism of cellobiose phosphorylase. Biochem. J. 398, 37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ito Y., Ohnishi Y. (2001) Stereoselective synthesis of β-manno glycosides, in Glycoscience: Chemistry and Chemical Biology (Fraser-Reid B. O., Tatsuta K., Thiem J., eds) pp. 1589–1619, Springer-Verlag, Berlin [Google Scholar]
- 40. Martens E. C., Koropatkin N. M., Smith T. J., Gordon J. I. (2009) Complex glycan catabolism by the human gut microbiota. The Bacteroidetes Sus-like paradigm. J. Biol. Chem. 284, 24673–24677 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.