Abstract
Environmental transmission of extremely resistant Toxoplasma gondii oocysts has resulted in infection of diverse species around the world, leading to severe disease and deaths in human and animal populations. This review explores T. gondii oocyst shedding, survival, and transmission, emphasizing the importance of linking laboratory and landscape from molecular characterization of oocysts to watershed-level models of oocyst loading and transport in terrestrial and aquatic systems. Building on discipline-specific studies, a One Health approach incorporating tools and perspectives from diverse fields and stakeholders has contributed to an advanced understanding of T. gondii and is addressing transmission at the rapidly changing human–animal–environment interface.
Keywords: Toxoplasma gondii, One Health, Oocyst, Modeling, Transport, Molecular characterization, Climate change, Land use change, Zoonotic disease, Environmental resistance
1. Introduction
Understanding the ecology and epidemiology of disease in the context of ecosystems is critical for preserving human and animal population health. Over 7 billion people now depend upon the earth's resources [1], and global environmental change has made disease migration to new hosts and landscapes a reality rather than a potential threat [2,3]. Anthropogenic activities, in particular, reshape landscapes, climate, and species distributions and interactions across the globe, with significant potential to alter patterns of pathogen emergence and spread [4–7]. Habitat conversion, introduction of non-native species, and increased contact between human, domestic animal, and wildlife populations have been linked to emerging viral, bacterial, and parasitic diseases [8]. Climate change also has the potential to alter cycles of disease transmission by influencing vector and host ranges, pathogen survival, and dynamics of water-borne transmission [8,9]. Of the 1415 organisms documented as human pathogens, over 60% are believed to have come from domestic or wild animal reservoirs [10]. Examining animal, human, or environmental health alone ignores the vital links between these components.
Historical biological and geographic boundaries of disease transmission will likely continue to shift significantly with changing environmental conditions, making a more holistic, One Health approach to pathogen research and management essential. By linking diverse non-academic stakeholder communities and researchers from different disciplines, the One Health approach builds a synergistic base from which to study and address the unique health challenges emerging at the human–animal–environment interface in a changing global environment [11,12].
Toxoplasma gondii, a globally distributed, zoonotic, protozoan parasite capable of infecting a wide range of warm-blooded animals [13], provides a broadly applicable example of the complexity of pathogen transmission among diverse hosts and environments and illustrates the need for a One Health approach to better understand disease ecology and epidemiology. Although long-studied in terrestrial landscapes, T. gondii has also emerged as a significant aquatic pathogen linked to marine mammal infection and water-borne outbreaks of disease in humans around the world [14]. Oocysts, the exceptionally hardy free-living environmental stage of the parasite, play a key role in transmission of T. gondii to newly recognized hosts and ecosystems. As wild and domestic felids are the only known hosts capable of shedding T. gondii oocysts in their feces [15–17], infection of people and animals through contaminated terrestrial and aquatic sources emphasizes the need to jointly examine human, domestic animal, and wildlife populations. While parasitologists, physicians, veterinarians, ecologists, and molecular biologists have studied T. gondii independently, understanding how a traditionally terrestrial pathogen is emerging in new environments requires more integrated knowledge. For T. gondii and other pathogens, creating a more collaborative approach to research and management from molecular to landscape levels has enhanced our understanding of health at the human–animal–environment interface.
2. Importance of oocysts in transmission of T. gondii infections
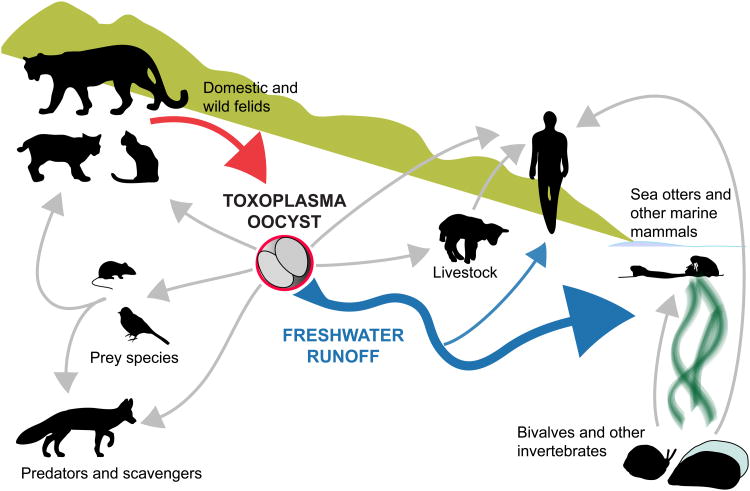
Warm-blooded animals, including humans, are typically infected with T. gondii through one of three pathways: ingesting oocysts from the environment (through contaminated water, soil, or food), eating an infected intermediate host with T. gondii cysts in its tissues, or congenital transmission from infected mothers to offspring [13,18]. Additional potential routes of transmission, including T. gondii tachyzoite-contaminated sperm and unpasteurized milk, have been demonstrated, but are thought to be rare sources of infection [19–21]. Uncommon cases of human infection following blood transfusion or organ transplantation from T. gondii-infected donors have also been reported [22,23]. However, oocyst-induced infections are increasingly recognized as a significant route of T. gondii transmission (Fig. 1) [14,24].
Fig. 1.
Hardy, free-living Toxoplasma gondii oocysts, which can be transported in freshwater runoff (blue arrow), likely play a significant role in environmental transmission of T. gondii in terrestrial and aquatic systems. Domestic and wild felids are the only known source (red arrow) of T. gondii oocysts. Light gray arrows indicate possible routes of T. gondii transmission by exposure directly to oocysts or indirectly through food sources.
A key feature of oocyst-borne infections is that the majority of human cases with clinical symptoms were reported in immunocompetent individuals, which contrasts with the traditional dogma that 90% of acquired cases of toxoplasmosis in healthy hosts are asymptomatic [25]. Clinical disease from acquired toxoplasmosis has been linked to strain virulence (as reviewed by [26]), and may be more severe when intermediate hosts acquire the infection through the oocyst versus tissue stages of T. gondii [27-29]. As reviewed by Grigg and Sundar [30], a number of studies link oocyst infections to symptomatic disease in immunocompetent individuals, with signs ranging from mild flu-like illness to more disseminated and severe outcomes, such as chorioretinitis, neurologic deficits, aborted fetuses, and even death. While outbreaks associated with consumption of bradyzoite tissue cysts in undercooked meat have been reported, the numbers of individuals affected are minimal as compared with documented oocyst outbreaks [30].
2.1. Terrestrial oocyst transmission
Widespread infection in chickens, grazing livestock, and herbivorous wildlife species from diverse geographic locations provides evidence of environmental exposure to T. gondii oocysts [31,32]. Omnivorous animals may acquire T. gondii infection through ingestion of tissue cysts, but infection from oocyst-contaminated food or water has also been reported [33]. Additionally, human infection has been repeatedly linked to terrestrial environmental sources including oocysts in domestic cat (Felis catus) litter boxes and soil as well as ingestion of contaminated unwashed fruits and vegetables [34–36]. Oocysts have been recovered from soil under natural conditions [37,38], and epidemiological studies indicate that there is an increased risk of acquired T. gondii associated with soil exposure (Tables 1 and 2[39]). While differentiating routes of T. gondii acquisition has been historically difficult, a recently recognized oocyst-specific antigen [27,40,41] applied in a study of mothers of congenitally infected infants in the United States demonstrated that 78% of these women (59 of 76), had oocyst-acquired infections [41].
Table 1. Reported human clinical toxoplasmosis associated with oocysts in the environment.
| Reporting period | Location | Predominant habitat type | Implicated source | Cases | Reference |
|---|---|---|---|---|---|
| 1972 | Panama | Rain forest | River | 32 | [176] |
| 1977 | Georgia, USA | Horse stable | Soil | 37 | [177] |
| 1980 | Alabama, USA | Urban | Soil | 10 | [178] |
| 1995 | Victoria, Canada | Urban | Municipal water supply | 100 (3000–7000 suspected) | [179] |
| 1995–2002 | French Guiana | Rain forest | Unknown | 16 | [169] |
| 2001–2002 | Santa Isabel do Ivai, Brazil | Urban | Municipal water supply | 426 | [180] |
| 2004–2005 | Lublin, Poland | Farm | Well water | 1 clinical (24 exposed) | [181] |
| 2004–2005 | Coimbatore City, India | Urban | Municipal drinking supply | 248 | [182,183] |
| 1998–2006 | French Guiana | Rain forest | Surface water | 44 | [169, 170, 184, 185] |
Table 2. Epidemiological investigations linking Toxoplasma gondii infection with environmental exposure to oocysts.
| Reporting period | Location | Implicated source | No. infected (percent of tested) | Reference |
|---|---|---|---|---|
| 1985 | Ciego de Avila, Cuba | Non potable water | 284(55.9) | [186] |
| 1995 | Grenada, West Indies | Soil or water | 305(57) | [187] |
| 1997–1999 | Campos dos Goytacazes, Brazil | Unfiltered water | 823(57.3) | [188] |
| 2001 | Iauareté, Brazil | Unfiltered water | 191 (73.5) | [189] |
| 2002 | Rondonia, Brazil | Well or river water | 195(73.3) | [189] |
| 2003 | Cascavel, Brazil | Homemade ice | 161 (69.7) | [190] |
| 2003 | Guatamala | Well water | 215(43) | [191] |
| 2003–2004 | Democratic Republic of São Tomé and Príncipe | Unboiled water | 375(75.2) | [192] |
| 2004 | Aydin, Turkey | Non bottled water | 185(30.1) | [193] |
| 2004 | Nunavik, Canada | Municipal and environmental waters | 548(59.8) | [194] |
| 2005 | Fortaleza, Brazil | Homemade ice | 666(69.1) | [195] |
| 2005 | Salvador, Brazil | Non-treated piped water | 213(17.5) | [24] |
| 2009–2010 | Thailand | Pipe, tap, or rain water | 181 (28.3) | [196] |
2.2. Water-borne oocyst transmission
Furthering the understanding of T. gondii epidemiology at a level that integrates environmental, human, and animal factors necessitates novel insight on the ecology of this parasite in water as well as terrestrial landscapes. Contamination of water with disease-causing microorganisms is a global health issue, with implications for human and animal health. Increasing reports of epidemic and endemic waterborne T. gondii infection in humans have emphasized the zoonotic potential for oocyst-based transmission [14,42]. Outbreaks of clinical toxoplasmosis in humans exposed to oocyst-contaminated water have been described in both developing and developed countries [43], with numerous additional reports identifying water as a risk factor for endemic T. gondii infection (Tables 1 and 2).
Some of the strongest support for the importance of oocyst-based transmission comes from T. gondii morbidity and mortality in aquatic mammals. Infection with T. gondii has been described for numerous marine mammal species living in near-shore and open ocean waters around the world, with clinical disease observed in seals, dolphins, whales, sea otters, and manatees [44–49]. Animals living in freshwater systems are also at risk; over 85% (82 of 95) of free-ranging Amazon River dolphins had antibodies to T. gondii [50]. Widespread T. gondii infection in aquatic mammals suggests that contamination of terrestrial watersheds with T. gondii is prevalent, and that sufficient numbers of oocysts are distributed in freshwater and marine ecosystems to infect and cause disease in both near-shore and pelagic mammals. T. gondii in marine mammal tissues also presents a public health concern, as widespread consumption of these animals, especially in indigenous communities, can provide an additional route of zoonotic transmission [51].
In California, T. gondii is a significant cause of mortality in threatened Southern sea otters (Enhydra lutris nereis) [52]. Because the only known definitive hosts of T. gondii are felids, otter infection supports land-to-sea transmission of this parasite through contaminated freshwater runoff [53]. Although a case of vertical transmission in sea otters has been reported [54], infection is more likely to occur through eating oocyst-contaminated invertebrate prey [55] or by direct exposure to oocysts in seawater. Southern sea otters, though they eat numerous marine prey species, have only rarely been observed to consume warm-blooded intermediate hosts of T. gondii [56,57]. Filter-feeding marine invertebrates, such as mussels and oysters, do not appear to become infected, but can concentrate viable T. gondii oocysts [58,59]. These invertebrates can then serve as transport hosts of the parasite and a potential source of infection for otters and other marine predators as well as humans, underscoring the public health risks of T. gondii in the near-shore marine environment [60].
Marine filter-feeding fish, which are prey species for many pelagic marine mammals, may also be a source of T. gondii infection. Using PCR and mouse bioassay detection methods, anchovies and sardines were experimentally shown to filter viable, sporulated oocysts from T. gondii spiked seawater into their alimentary canals [61]. Whether this process occurs in nature is unknown, but if migratory filter-feeding fish serve as transport hosts of Toxoplasma, they could carry oocysts from coastal runoff to diverse marine environments.
3. Keys to oocyst success
Oocysts pose a serious threat to susceptible hosts because of their robust environmental resistance, low infectious dose for some species, and the lack of methods to reliably detect oocysts in water and other environmental substrates to identify sources of exposure. Multiple experiments evaluating the survival of oocysts in soils showed that they may remain viable for at least 1 year when covered and in cool temperatures (4°C) [38,62–64]. Under warm climate conditions in dry soils from Kansas, USA, oocysts remained viable for 18 months [63]. In fresh or marine waters, oocysts were shown to be viable for at least 4.5 and 2 years, respectively [65] (reviewed by [66]). Although T. gondii oocysts have been reported to be inactivated by exposure to >60 °C for 1 min [67], higher temperature and longer duration may be necessary for complete and reliable inactivation. Two chemicals commonly used to treat water, sodium hypochlorite (chlorine) and ozone, failed to inactivate all infective oocysts at concentrations well in excess of those used to treat both sewage and drinking water [68,69]. Furthermore, physical inactivation by ultraviolet (UV) irradiation at doses ≥500 mJ/cm2, far exceeding doses commonly used to treat water, did not fully inactivate T. gondii oocysts [70].
Although nearly all warm-blooded animals are susceptible to oocyst-borne infections, the infectious dose for most species is unknown. Some intermediate hosts, including mice and pigs, are highly susceptible and become infected at doses as low as 1–10 T. gondii oocysts [71,72]. Even rats and horses, which appear resistant to clinical disease following infection with T. gondii oocysts, develop tissue cysts, indicating their potential to serve as sources of infection for other hosts [73,74].
4. How molecular characteristics contribute to oocyst success
Molecular description of the oocyst has been extremely limited for several reasons: (1) no method exists for maintaining or expanding oocysts in vitro, therefore, infection of the definitive felid host is required to produce oocysts; (2) oocysts are refractory to routine protocols used to isolate nucleic acids and proteins, thus requiring special equipment and procedures; and (3) live oocysts pose a biohazard risk as they are not readily killed by laboratory disinfectants [75–77]. However, recent molecular characterization of T. gondii oocysts provides preliminary clues to the critical proteins that comprise the oocyst wall and likely confer oocyst resistance to destruction [75,77]. Comprehensive mining of the recently published oocyst transcriptome and proteome revealed many genes/proteins that were found to be uniquely expressed in the oocyst stage and appear to be located in the oocyst wall or otherwise contribute to oocyst resistance. Among these genes/proteins were tyrosine-rich proteins, T. gondii oocyst wall proteins (OWPs), PAN-domain-containing proteins, and late embryogenesis abundant domain-containing proteins (LEAs) [75,77].
4.1. How the oocyst wall confers resistance
Evidence suggests that the oocyst wall is comprised of groups of highly cross-linked proteins that form a sturdy outer matrix contributing to the oocyst's resistance to destruction. The T. gondii oocyst wall is a bilayered structure with an outer electron dense layer and an inner electron lucent layer, both roughly 40 nm thick [78]. Oocyst wall formation takes place within feline intestinal epithelial cells as the macrogamete develops. The outer layer of the oocyst wall is reportedly composed primarily of proteins and carbohydrates, providing structural strength, and the inner wall is primarily lipid, providing protection from chemical insult [79,80]. Under UV excitation (330–385 nm) the oocyst wall is autofluorescent, and the wall retains its autofluorescence following bleach treatment and boiling [80]. Because molecular data on T. gondii oocysts were largely unavailable, predictions for oocyst wall formation and composition in T. gondii have been made based on what has been described in two closely related, extensively studied, and environmentally resistant protozoan genera: Eimeria and Cryptosporidium.
Similarities in oocyst characteristics, including: environmental stability; mechanism of wall formation; and autofluorescence of oocyst walls under UV excitation, indicate that the T. gondii oocyst wall is likely very similar to Eimeria spp. A unique feature of the identified proteins in the Eimeria oocyst wall is tyrosine-richness [81,82] with tyrosine-protein cross-linkages reported to be responsible for structural robustness and autofluorescence [82]. BLAST searches of the tyrosine-rich proteins identified in Eimeria failed to turn up any homologs in the predicted genome of T. gondii [75]. However, six tyrosine-rich proteins (>5% tyrosine) were found to be abundantly expressed in the proteome and transcriptome of T. gondii oocysts [75,77], suggesting that like Eimeria, T. gondii oocysts contain tyrosine-rich proteins. Cross-linking via dityrosine bonds has been described in a number of organisms and is associated with the formation of insoluble and physicochemical resistant matrices; examples include bacterial spores, fungal cell walls, sea urchin eggs, and coccidian oocysts [83–87]. The putative location of tyrosine-rich proteins in the walls of the oocysts and their ability to form bridges across tyrosine residues has yet to be experimentally investigated.
Oocyst wall proteins (OWPs) in Cryptosporidium spp. are cysteine-rich and predicted to be stabilized via disulfide bridges [88,89]. Seven homologs to the cysteine-rich family of OWPs identified in Cryptosporidium spp. are present in the T. gondii genome (designated TgOWPs 1–7) [89]. Three were recently confirmed in the T. gondii oocyst wall (TgOWPs 1–3), and all were identified in oocysts by mass spectrometry and/or microarray [75,77]. Also abundantly detected in T. gondii oocysts by mass spectrometry were PAN domain-containing proteins [77]. The structural conformation of PAN-domain containing proteins is also achieved through disulfide bridges that result in a pattern of folding that creates recognition and binding sites [90]. The role of these PAN domain-containing proteins has not yet been investigated in T. gondii oocysts, but they may be of structural significance in the oocyst wall given their large size and predicted extensive disulfide bridging.
4.2. Other molecules that confer resistance
While it has been presumed that the T. gondii oocyst achieves its resistance to environmental destruction through structures present in the wall, a group of proteins designated ‘late embryogenesis abundant domain-containing proteins’ (LEAs) may comprise another critical dimension to oocyst resistance. Four LEAs were discovered in oocysts by microarray and mass spectrometry [75,77]. While the function of these proteins in T. gondii is unknown, LEA proteins have been described in a number of other organisms including plants, invertebrates, and microorganisms [91]. There is significant diversity in the LEA families and their respective functions are still under investigation. However, a commonly ascribed role is resistance to environmental stresses including drought, high salinity, and freezing [92].
Molecular characterizations of the oocyst will aid in developing targets for oocyst inactivation and development of reagents to concentrate and detect oocysts in water and other environmental substrates. Several methods have been evaluated for detection of T. gondii in water, soil, and felid feces, including molecular approaches, microscopy, and mouse bioassays as shown in Table 3 [66]. However, detection of oocysts in terrestrial and aquatic habitats remains challenging as sensitive, standardized techniques for large-scale environmental testing are not currently commercially available. The difficulty of detecting T. gondii oocysts in environmental sources has likely led to underestimation of the importance of this route of transmission and limited the ability to identify and address high-risk areas of oocyst exposure.
Table 3. Published methods for detection of Toxoplasma gondii oocysts in water.
| Water tested | Laboratory or field study | Water concentration method | T. gondii detection assay(s) used | Quantitative, yes/no | Reference |
|---|---|---|---|---|---|
| Tap, fresh, marine | Laboratory | Ultrafiltration and capsule filtration | Microscopy, real time PCR, conventional PCR | Yes | [197] |
| Tap, fresh | Laboratory | Continuous separation channel centrifugation | Microscopy | Yes | [198] |
| Fresh | Laboratory | Capsule filtration | Real time PCR | Yes | [199] |
| Fresh | Laboratory and field | Capsule filtration | PCR, mouse bioassay | No | [200] |
| Fresh | Laboratory and field | Flocculation (Al2(SO4)3) | Loop-mediated isothermal amplification | No | [201] |
| Tap, fresh | Laboratorya and field | Capsule filtration | Immunomagnetic separation | Yes | [202,203] |
| Tap, fresh, marine | Laboratory and field | Flocculation (Al2(SO4)3) | Conventional PCR | No | [204] |
| Deionized, fresh | Laboratory and field | Capsule filtration | PCR, mouse bioassay | No | [205] |
| Fresh | Laboratory | Flocculation (Al2(SO4)3 and Fe2(SO4)3) | PCR | No | [206] |
| Deionized, tap | Laboratory | Centrifugation and Flocculation (Al2(SO4)3 and Fe2(SO4)3) | Microscopy | Yes | [207] |
| Tap | Laboratorya and field | Capsule filtration | Bioassay | No | [208] |
T. gondii successfully detected only in spiked laboratory samples.
5. Oocysts at the animal–human–environment interface
Evaluating the role of oocysts in T. gondii transmission cycles requires a broad understanding that encompasses: the ecology of felids, aquatic mammals, and terrestrial and marine prey species; chemical and physical properties that determine oocyst resistance; human influences on domestic animal and wildlife populations; and the impact of environmental factors, such as land use, climate, and freshwater runoff. Considering the diverse factors that contribute to T. gondii oocyst loading, survival, and transport facilitates interdisciplinary research and management approaches to reduce environmental exposure.
5.1. Domestic and wild sources of oocysts
Experimentally, domestic cats typically shed millions to hundreds of millions of oocysts over a single 1- to 3-week period following initial infection with T. gondii bradyzoite cysts [76,93]. The route of infection, strain of T. gondii, and cat age may impact the time from infection to shedding, percentage of infected cats that shed T. gondii, and number of oocysts shed. Lower prevalence and delayed onset of shedding were reported in cats fed T. gondii oocysts compared to those infected with tachyzoites or bradyzoites [15]. Although experimental infection with certain strains of T. gondii yielded higher shedding prevalence and quantities of oocysts, the limited number of strains tested does not reflect the diverse T. gondii genotypes circulating in free-ranging populations [16,94–96]. Young cats may shed higher numbers of oocysts following primary infection, and higher prevalence of shedding was observed in outdoor pet and feral kittens compared to adults [18,97]. However, older cats may still play an important role in environmental loading, as oocyst shedding was detected in naturally infected adult domestic cats (1–18 years old) in diverse geographical locations [98–100]. Reported shedding prevalences of molecularly confirmed T. gondii oocysts in pet and feral domestic cat populations ranged from zero to nine percent, with higher shedding levels observed in feral cats than pet cats [14,99,100].
Even in experimental settings, few reports of prevalence, duration, and frequency of oocyst shedding in wild felids exist. The prevalence of oocyst shedding by bobcats (Lynx rufus) was lower than that in domestic cats experimentally infected with the same strain of T. gondii [15,16]. However, the strain used for infection was isolated from sheep, and the experimental response of bobcats may not have reflected natural exposure to strains circulating in wild populations. Asian leopard cats (Prionailurus bengalensis) exposed to T. gondii strains isolated from domestic and wild animal sources only shed oocysts following infection with the wild strain [16]. Although the number of oocysts shed by experimentally infected wild felids has not been quantified, a free-ranging mountain lion (Puma concolor) in Canada shed 1.25 × 106 oocysts per gram of feces, which is similar to quantities of oocysts shed by naturally infected domestic cats [14,101]. In the limited reports of T. gondii-like oocyst shedding in naturally infected, free-ranging wild felids, shedding prevalences ranged from 0% to 33%, suggesting that shedding can vary widely among wild felid populations (Table 4).
Table 4. Published reports of natural Toxoplasma gondii oocyst shedding in free-ranging wild felids. Reports of T. gondii-like oocysts (diagnosed by microscopy) and confirmed T. gondii oocysts (diagnosed by bioassay) are included.
| Region and felid | Sampling location | Diagnostic method | No. sampled (no. positive) | Shedding prevalence | Reference |
|---|---|---|---|---|---|
| North America | |||||
| Bobcat (Lynx rufus)a | New Mexico, Montana, USA | Microscopy and mouse bioassay | 9 (3) | 33.3% | [209] |
| Mountain lion (Puma concolor) | Montana, USA | Microscopy and mouse bioassay | 5 (0) | 0% | [209] |
| Mountain lion (Puma concolor ssp. vancouverensis) | Vancouver Island, British Columbia, Canada | Microscopy and mouse bioassay | 23 (2)b | 8.7% | [101,210] |
| Central/South America | |||||
| Ocelot (Leopardus pardalis) | Cockscomb Basin, Belize | Microscopy | 8 (2) | 25% | [211] |
| Jaguar (Panthera onca) | Cockscomb Basin, Belize | Microscopy | 25 (1) | 4% | [211] |
| Geoffrey's cat (Leopardus geoffroyi) Pampas cat (Leopardus colocolo) Jaguarundi (Puma yagouaroundi) | Cordoba Province, Argentina | Pig/mouse bioassy | 73 (27)c | 37% | [212] |
| Asia | |||||
| Leopard (Panthera pardus) | Huai Kha Wildlife Sanctuary (HKWS), Thailand | Microscopy | 54(1) | 1.9% | [213] |
| Tiger (Panthera tigris) | HKWS, Thailand | Microscopy | 19 (0) | 0% | [213] |
| Leopard cat (Prionailurus bengalensis) | HKWS, Thailand | Microscopy | 3 (0) | 0% | [213] |
| Iriomote cat (Prionailurus iriomotensis) | Iriomote-jima Island, Japan | Microscopy and mouse bioassay | 45 (2)d | 4.4% | [214] |
Felid scientific names listed according to International Union for Conservation of Nature (IUCN) classifications [215]. Mountain lions (also commonly called pumas and cougars) identified as Felis concolor and Felis concolar in previous publications [14,39,101,209] all belong to a single species, Puma concolor.
16 fecal samples (1 positive) collected from dead mountain lions, 7 fecal samples (1 positive) collected in the environment from an unknown number of mountain lions. Overlap may exist between the sampled mountain lions and environmentally collected feces.
The number and species of felids with confirmed shedding of T. gondii is unknown as T. gondii-like oocysts were pooled for bioassay.
45 fecal samples collected in the environment from an unknown number of Iriomote cats.
Although discussions of oocyst shedding usually focus on fecal excretion following initial infection of felids, repeat or intermittent oocyst shedding may increase their contribution to parasite burden in the terrestrial environment. No evidence of persistent oocyst shedding exists for domestic or wild felids, but repeat shedding may occur under certain conditions. Chronically infected domestic cats have been experimentally shown to reshed oocysts after being exposed to novel strains of T. gondii, as well as when receiving immunosuppressive doses of glucocorticoids or when co-infected with Isospora felis, a common feline parasite [14,94,102–104]. Interestingly, co-infection with immunosuppressive pathogens, such as feline immunodeficiency virus (FIV) has not been associated with repeat shedding [105]. Malnutrition was linked to repeat shedding in domestic cats, but similar results were obtained with well-nourished cats [97]. The repeat shedding observed in these cats may be due to novel strains of T. gondii used for reinfection rather than nourishment status. Natural repeat shedding of T. gondii oocysts has been observed in diverse species of captive wild felids kept in zoos and has been attributed to repeated exposures to the parasite in raw meat [106]. By impacting nutritional and immune status as well as exposure to I. felis and new strains of T. gondii, diet also has the potential to influence repeat shedding in free-ranging felids.
At this time, there is no commercially available vaccine or treatment that can be administered to felids to prevent or reduce oocyst shedding. Two candidate vaccines were successful in preventing oocyst shedding in domestic cats that were subsequently challenged with infective strains [107–110]. However, these vaccines are not feasible for widespread application because they utilized live tissue cysts maintained in chronically infected mice.
5.2. Environmental transmission – oocyst loading and transport from terrestrial to aquatic systems
Both anthropogenic and natural conditions strongly influence T. gondii oocyst loading in the terrestrial environment as well as transmission to freshwater, estuarine, and marine systems (Table 5). As T. gondii oocysts cannot replicate outside of felid hosts, spatial variation in the burden of oocysts on land reflects the distribution of T. gondii shedding by these populations. Native wild felids as well as introduced pet domestic cats and un-owned or feral domestic cats, have the potential to contribute massive quantities of oocysts to terrestrial and aquatic habitats [111,112]. The likelihood of oocyst shedding in a given location is influenced by felid distribution, availability of prey or other food resources, and human management of domestic and wild felid populations.
Table 5. Factors influencing Toxoplasma gondii oocyst-based transmission in terrestrial and aquatic environments.
| Oocyst loading | Oocyst survival | Transport of oocysts overland and in waterways |
|---|---|---|
Distribution of wild and domestic felids
|
Terrestrial conditions:
|
Felid defecation behavior:
|
Felid defecation behavior:
|
Chemical and physical surface properties of oocysts | |
Prevalence and frequency of oocyst shedding in wild and domestic cats
|
Aquatic conditions:
|
Terrestrial conditions:
|
Precipitation patterns:
|
||
| Duration and quantity of oocyst shedding by wild and domestic felids | Aquatic conditions:
|
The distribution of pet cats and feral domestic cat colonies is closely linked to human households that supply food and shelter [113]. Even unmanaged, more solitary feral domestic cats not fed or cared for by humans may have home ranges linked to developed landscapes, inhabiting urban and agricultural lands in addition to less developed areas [113]. Human activities may also influence the distribution of wild felids, as many species were reported to be sensitive to habitat fragmentation and avoided areas of high human population density [114–117]. In protected natural areas with public access, abundance of bobcats declined with increasing human and domestic dog visitors [118], illustrating that even temporary human land use activities may influence felid distributions. However, some wild felids frequently used edge environments or riparian corridors of developed urban and agricultural areas [119]. Although many wild felids and unmanaged feral cats use a wide variety of habitats throughout their ranges, habitat choice may be influenced by prey availability, intra or inter-specific territorial interactions, competition wit other predators for access to prey, environmental stress, as well as vegetation and topography [113,120,121]. Research on domestic and wild felid ecology builds the understanding of and ability to predict loading of T. gondii oocysts in natural environments.
Even within a shared habitat, exposure of wild and domestic felids to T. gondii may increase or decrease based upon access to prey and dietary preferences. Pet and feral domestic cats showed different prey consumption patterns, with higher predation levels observed in feral cat populations [122,123]. Reports of specific prey preferences for both wild and domestic felids vary drastically both among felid species and populations of a single species living in different environments [124,125]. In regions where humans, livestock, and wildlife live in close contact, wild felid prey frequently included domestic livestock such as sheep, goats, and cattle as well as wildlife [126]. Prey availability and preferences of wild and domestic felids not only contribute to the distribution of these hosts in the terrestrial landscape, but may also influence their risk of infection and oocyst shedding, as T. gondii infection levels varied dramatically in sampled intermediate host species [32,127]. In a meta-analysis of studies on T. gondii infection in small mammals, reported seroprevalence of infection was significantly higher in large rodents and rabbits than smaller rodent species [127]. Additional information on local prey populations and dietary preferences of domestic and wild felids will help to determine the risk of T. gondii infection and oocyst shedding for diverse felids in a given region. Indirect measures of the abundance of intermediate hosts of T. gondii, including habitat distribution and precipitation patterns, may facilitate estimates of prey populations at landscape scales that would be impractical for field investigations [128–131].
Historic and current human land use and animal management strategies can directly and indirectly impact wild and domestic felid distribution, and T. gondii oocyst shedding and transport. Extensive and rapid land use change around the world has converted many natural habitats to developed urban and agricultural lands [132]. These environments are frequently used by both pet and feral domestic cats, whose populations will likely expand with increased development [113,133]. Changing land use may also alter unmanaged feral cat and wild felid distributions by shifting habitat and the numbers or types of prey in a given location. Individual pet owner decisions determine the amount of time pet cats spend outside the home, and therefore the likelihood that they will be exposed to infected intermediate hosts and defecate outdoors, contributing oocysts to the terrestrial environment. People feeding stray or feral cats influence the diet and behavior of these animals, and government-level management decisions also affect loading and transport of oocysts. City and regional pet and feral cat management policies, including trap-neuter-return programs and mandatory spay-neuter laws shape numbers and distribution of these felids [134]. National and regional policies on wildlife hunting and protection have also influenced population sizes of wild felids [135,136]. Additionally, the California legislature Bill AB2485 directly targeted oocyst loading and transport by requiring cat litter labels to direct owners to put used litter in landfills rather than depositing it outside or flushing it through the sewage system where it might eventually reach the ocean and sensitive marine wildlife [137]. Considering T. gondii environmental transmission in the context of felid distribution and oocyst shedding, as well as human landscape and animal management decisions, will facilitate identification and management of high-risk areas for parasite exposure.
Transport and potential accumulation of T. gondii oocysts in environmental matrices, such as soil or water, is determined by felid behavior, environmental attributes, and oocyst surface properties. In addition to affecting sporulation and survival, felid defecation behaviors can alter the number of oocysts that can be entrained and transported in freshwater flow. Fewer T. gondii oocysts may be mobilized from feces that are buried under the soil, and fecal burying behavior can differ greatly among different species and populations of felids. Many free-ranging pet domestic cats bury feces, but kittens in Costa Rica, dominant individuals in feral cat colonies, and unmanaged feral cats left feces exposed [97,138]. In a zoo setting, mountain lions buried feces and occasionally defecated in water, whereas bobcats and tigers (Panthera tigris) frequently defecated in water (personal communication, Lynn Dowling, keeper Folsom Zoo Sanctuary). Semi-captive bobcats have also been reported to frequently defecate in running streams and/or shallow ponds, and free-ranging bobcats commonly leave their feces exposed at sites along roads or trails [138,139]. For some terrestrial carnivores, including mountain lions, the tendency to bury feces may also change with season or reproductive status [140,141]. Location of defecation can limit or enhance oocyst transport, with oocysts deposited near waterways more likely to be transported in freshwater runoff into aquatic environments. In some regions, mountain lions and bobcats commonly use riparian habitats [119,142], which could increase their contribution to the number of oocysts reaching aquatic systems. Understanding defecation behavior for wild and domestic felids in a given environment will help to predict oocyst deposition, survival, and transport.
Substrate, vegetation, and climate at the site where oocysts are deposited all affect transport. Permeable soils may allow oocysts and other pathogens to percolate vertically, reducing the number of oocysts mobilized and carried in freshwater flow to aquatic systems. In contrast, impervious surfaces, like asphalt and concrete in developed environments can lead to increased mobilization of contaminants into runoff [143]. Natural fresh and saltwater wetlands play a vital role in reducing the levels of these contaminants in runoff [144,145], and vegetation in constructed wetlands and terrestrial vegetation buffers was also effective in decreasing pathogens in effluent waters [146,147]. Physical attributes of watersheds and local climate characteristics also impact T. gondii environmental transmission. The slope of the watershed as well as its size can influence the amount of freshwater runoff and transported pathogens reaching aquatic systems where humans and animals can be exposed [148,149]. Freshwater runoff and particle transport are also directly influenced by storm patterns and the quantity, duration, intensity, and frequency of precipitation in the watershed [150,151]. Infections and deaths in marine mammals due to terrestrially derived fecal protozoa and bacteria have been temporally and spatially linked to increased precipitation or land-based runoff [152,153].
Surface properties of the environmental stages of protozoan pathogens are particularly relevant for driving their environmental transmission patterns [154]. The surface electrophoretic mobility (surface charge) and hydrophobicity (affinity to water) are two properties that influence the behavior of suspended particles in water. T. gondii oocysts were determined to have a negative charge in fresh water solutions, but their charge was neutralized in estuarine and seawater [155]. Oocysts were also determined to have a very low contact angle, indicating that they have a strong affinity to water molecules. The strongly hydrophilic nature and negative charge of T. gondii oocysts in freshwater could facilitate their widespread contamination in waterways. The loss of charge observed in saline waters suggests that enhanced flocculation and subsequent accumulation of T. gondii oocysts may occur in locations where fresh and marine waters mix, increasing the risk of exposure to humans and wildlife.
Aggregation studies further support the hypothesis that T. gondii oocysts are likely to concentrate in estuarine and marine ecosystems [156]. In laboratory studies, attachment of oocysts with aquatic macroaggregates (i.e. “marine snow”) was observed in fresh, estuarine, and marine water samples, but was greatest in waters with increased salinity. Concentration of T. gondii in estuarine or marine aggregates was enriched 3–4 orders of magnitude as compared with oocyst concentration in surrounding water. Aggregation of oocysts is significant because attached oocysts are likely to move through an aquatic habitat differently and may have an altered ecological role as compared with unattached oocysts. Oocysts associated with aggregates have increased settling velocities and larger effective surface area that can facilitate their removal from the water column by emergent vegetation. Distinct high-risk sites of infection may thus form at locations where contaminated freshwater mixes with saline waters as aggregates deposit in the benthos or are trapped by vegetation. The high seroprevalence of T. gondii in California sea otters may be partially accounted for by the fact that otters spend the majority of their lives within 500 m of the coastline, exactly where oocyst concentration may be greatest. Furthermore, invertebrates are known to retain particles that are ingested within aggregates more readily than particles that are freely suspended in the water column [157]. Thus, the association of T. gondii with aquatic aggregates may facilitate their uptake into the marine food chain, with eventual ingestion by a susceptible warm-blooded host, such as a sea otter or human. For decades, oceanographers have recognized that aquatic aggregates are crucial vehicles for vertical and horizontal movement of materials in the ocean [158,159]. However, the role of aggregates in the transport of terrestrially derived zoonotic pathogens is only now starting to emerge, and future efforts are still needed to characterize when and where T. gondii and other zoonotic pathogens become a threat to human and animal health in aquatic habitats.
6. Land use and climate change – anthropogenic influences on oocyst transmission
Oocyst-borne infections with T. gondii may increase in animals and humans as climate and habitat changes reshape environments worldwide. As most of the human population and their domesticated animals are distributed along waterways, there has been an associated increase in the amount of fecal deposition within watersheds that drain into collecting freshwater bodies, estuaries, and coastlines. The physical forces that drive overland runoff events and mobilize transport of fecal matter are likely to increase with climatic factors that are forecasted to change in the coming decades. Across most latitudes, weather simulation models have projected reduced predictably of storms coupled with increased intensity of rainfall events, leading to precipitation patterns that are likely to result in a net increase of land-based runoff [160]. High amounts of rainfall that occur within a shorter duration of time would provide enhanced force for mobilizing overland runoff, which acts as a conduit of storm-driven pollutants, including fecal pathogens. In addition, increased runoff can indirectly exacerbate pollution by overcoming the ability of sewage treatment facilities to cope with large volumes, leading to treatment failures and discharge of untreated waste to receiving water bodies.
The potential for permeable soils and terrestrial and aquatic vegetation to reduce overland transport of fecal pathogens is increasingly threatened by conversion of natural habitats to developed environments. As one example, degradation of coastal wetlands has resulted in a net loss of nearly 67% of saltwater marshes in the United States, eliminating water-cleansing services in coastal regions where human development, and the associated production of fecal matter, is greatest [161]. Recent work that examined the effect of estuarine wetland degradation on transport of T. gondii revealed that erosion of wetlands to mudflats could result in 6 orders of magnitude greater flux of oocysts to coastal waters [144]. Coupled with wetland destruction, modification to historic flow of waterways through channelization and increased urban runoff through storm drains will likely increase pathogen transport in surface waters. Just as landscape change can exacerbate impacts of climate change on pollution, climate can also facilitate the speed of landscape change. Regions that are susceptible to sea level rise are predicted to suffer further loss of wetlands in areas where accretion cannot compensate submergence due the speed of rising sea levels, reduced delivery of sediment, or because higher grounds have already been converted to urbanized or agricultural lands [162]. The combination of landscape change coupled with climate variability may therefore increase fecal pollution of waterways [163].
The presence of T. gondii oocysts in water is only a health concern if the parasite remains infectious to susceptible hosts. Persistence of T. gondii in terrestrial and aquatic environments is closely governed by climatic factors [66]. Humid environments and cooler temperatures are generally more favorable for oocyst survival [63]. Conversely, extremes in weather including freezing temperatures or hot and arid conditions are less likely to support prolonged oocyst viability [64,164]. In regions where long-term data are available, including the United States, a trend of increasing surface soil moisture was detected [165], a climate change that could prolong viability of T. gondii oocysts. In middle and higher latitudes, the duration of time the earth is covered by ice or snow is expected to decline, rendering those environments more favorable to oocyst survival. Higher latitude and mountainous regions that to date have reported comparatively lower prevalence of T. gondii infections in humans [166,167] may experience a rising infection rate if a warmer climate facilitates oocyst infectivity [168].
In addition to impacting the spatial distribution and potential viability of oocysts, landscape change may lead to alterations in T. gondii strain pathogenicity, or the likelihood that humans are exposed to more virulent strains. In French Guiana, virulent strains of T. gondii were associated with waterborne outbreaks of infection and even mortality in some immunocompetent adults [169–171]. Unique genotypes of T. gondii in French Guiana were identified in anthropogenically altered environments, which differed from T. gondii strains isolated from surrounding natural habitats [172]. Exposure of seronegative persons to a particularly virulent strain of T. gondii that emerged from strain hybridization may have led to fatal outbreaks of toxoplasmosis in local communities. The desperate need for livelihood improvement in many regions of the world will likely lead to increasing encroachment of people into undeveloped lands, resulting in greater exposure of humans to T. gondii strains that typically circulate within wild felid populations.
7. Why a One Health modeling approach enhances understanding of T. gondii oocyst transmission
Currently, there is a notable lack of literature on the relationship between T. gondii oocyst properties, felid shedding patterns, oocyst transport from terrestrial to aquatic systems, and climate and habitat change. This knowledge gap illustrates the vital need for a more integrative method to examine the impacts of environmental change on oocyst-based infection in human and animal populations. Uniting ecologists, veterinarians, physicians, epidemiologists, molecular biologists, and physical scientists including hydrologists, oceanographers, and engineers, as well as local stakeholders and policymakers is critical to developing models of T. gondii oocyst transmission and management in terrestrial and aquatic environments.
Previous efforts to model T. gondii transmission have focused predominantly on the interactions of felids and their prey in maintaining infection. Given the complexity of multiple predator-prey interactions in natural environments, these models were designed to simulate transmission in several small habitat patches or on a single farm, and they focused on a single or few prey species [173,174]. In reality, overlapping wild and domestic felid populations sharing an environment likely consume a wide diversity of prey species that would be difficult to incorporate into traditional transmission models. In a unique model including a domestic cat population, multiple prey species, and direction of hydrologic flow, water-borne transmission was identified as a critical component of T. gondii infection [175]. While these models provide important insight on parasite transmission dynamics, a broader scale approach is still needed to investigate environmental transmission in areas with multiple felid populations. Watershed-level models integrating human, domestic animal, and wildlife ecology with oocyst properties, landscape structure, and hydrology offer a new direction for examining the impacts of environmental change on T. gondii oocyst loading and transport from terrestrial to aquatic systems. For T. gondii and many other pathogens, incorporating the tools and perspectives of diverse disciplines through a One Health approach will provide essential new insights on health from the molecular to landscape level.
Acknowledgments
Funding sources supporting the authors included NSF-Ecology of Infectious Disease Grants (0525765, 1065990), a National Institutes of Health K01 award (5K01RR031487) to H. Fritz, and a fellowship from the National Oceanic and Atmospheric Administration Oceans and Human Health Initiative (S08-67884) to K. Shapiro. The authors thank Alison Kent, UC Davis Wildlife Health Center, for assistance in developing the transmission graphic.
Role of funding sources: Funders did not have any involvement in the writing of or decision to submit this manuscript.
Footnotes
Conflict of interest: None.
References
- 1.UNFPA. The state of world population 2011: people and possibilities in a world of 7 billion. 2011:1–7. [Google Scholar]
- 2.Plowright RK, Sokolow SH, Gorman ME, Daszak P, Foley JE. Causal inference in disease ecology: investigating ecological drivers of disease emergence. Frontiers in Ecology and the Environment. 2008;6:420–9. [Google Scholar]
- 3.Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife-threats to biodiversity and human health. Science. 2000;287:443–9. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- 4.Daszak P, Cunningham A, Hyatt A. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Tropica. 2001;78:103–16. doi: 10.1016/s0001-706x(00)00179-0. [DOI] [PubMed] [Google Scholar]
- 5.Patz JA, Daszak P, Tabor GM, Aguirre AA, Pearl M, Epstein J, et al. Unhealthy landscapes: policy recommendations on land use change and infectious disease emergence. EHP. 2004;112:1092–8. doi: 10.1289/ehp.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley CA, Altizer S. Urbanization and the ecology of wildlife diseases. Trends in Ecology and Evolution. 2006;22:95–102. doi: 10.1016/j.tree.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–3. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patz JA, Olson SH, Uejio CK, Gibbs HK. Disease emergence from global climate and land use change. Medical Clinics of North America. 2008;92:1473–91. doi: 10.1016/j.mcna.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Gould E, Higgs S. Impact of climate change and other factors on emerging arbovirus diseases. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009;103:109–21. doi: 10.1016/j.trstmh.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor L, Latham S, Woolhouse M. Risk factors for human disease emergence. Philosophical Transactions of the Royal Society B. 2001;356:983–9. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conrad P, Mazet J, Clifford D, Scott C, Wilkes M. Evolution of a transdisciplinary “One Medicine-One Health” approach to global health education at the University of California, Davis. Preventive Veterinary Medicine. 2009;92:268–74. doi: 10.1016/j.prevetmed.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazet J, Clifford D, Coppolillo P, Deolalikar A, Erickson J, Kazwala R. A “One Health” approach to address emerging zoonoses: the HALI Project in Tanzania. PLoS Medicine. 2009;6:e1000190. doi: 10.1371/journal.pmed.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. International Journal for Parasitology. 2000;30:1217–58. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones J, Dubey J. Waterborne toxoplasmosis -recent developments. Experimental Parasitology. 2010;124:10–25. doi: 10.1016/j.exppara.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Dubey JP, Miller NL, Frenkel JK. The Toxoplasma gondii oocyst from cat feces. Journal of Experimental Medicine. 1970;132:636–62. doi: 10.1084/jem.132.4.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller NL, Frenkel JK, Dubey JP. Oral infections with Toxoplasma cysts and oocysts in felines, other mammals, and in birds. Journal of Parasitology. 1972;58:928–37. [PubMed] [Google Scholar]
- 17.Hutchison WM, Dunachie JF, Siim JC, Work K. Life cycle of Toxoplasma gondii. British Medical Journal. 1969;4:806. doi: 10.1136/bmj.4.5686.806-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubey JP, Beattie CP. Toxoplasmosis of animals and man. Boca Raton, FL: CRC Press; 1988. [Google Scholar]
- 19.Sacks JJ, Roberto RR, Brooks NF. Toxoplasmosis infection associated with raw goat's milk. Journal of the American Medical Association. 1982;248:1728–32. doi: 10.1001/jama.1982.03330140038029. [DOI] [PubMed] [Google Scholar]
- 20.Walsh C, Hammond S, Zajac A, Lindsay D. Survival of Toxoplasma gondii tachyzoites in goat milk: potential source of human toxoplasmosis. Journal of Eukaryotic Microbiology. 1999;46:73S–4S. [PubMed] [Google Scholar]
- 21.Arantes TP, Lopes WD, Ferreira RM, Pieroni JS, Pinto VM, Sakamoto CA, et al. Toxoplasma gondii: evidence for the transmission by semen in dogs. Experimental Parasitology. 2009;123:190–4. doi: 10.1016/j.exppara.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Rosalinde D. Infectious hazards associated with blood-transfusion. Adverse Drug Reactions and Toxicological Reviews. 1995;14:157–74. [PubMed] [Google Scholar]
- 23.Derouin F, Pelloux H. Prevention of toxoplasmosis in transplant patients. Clinical Microbiology and Infection. 2008;14:1089–101. doi: 10.1111/j.1469-0691.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- 24.Dattoli VC, Veiga RV, Cunha SS, Pontes-de-Carvalho L, Barreto ML, Alcantara-Neves NM. Oocyst ingestion as an important transmission route of Toxoplasma gondii in Brazilian urban children. Journal of Parasitology. 2011;97:1080–4. doi: 10.1645/GE-2836.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–76. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 26.Robert-Gangneux F, Darde ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clinical Microbiology Reviews. 2012;25:264–96. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munoz-Zanzi CA, Fry P, Lesina B, Hill D. Toxoplasma gondii oocyst-specific antibodies and source of infection. Emerging Infectious Diseases. 2010;16:1591–3. doi: 10.3201/eid1610.091674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubey JP. A review of toxoplasmosis in pigs. Veterinary Parasitology. 1986;19:181–223. doi: 10.1016/0304-4017(86)90070-1. [DOI] [PubMed] [Google Scholar]
- 29.Hill D, Dubey JP. Toxoplasma gondii: transmission, diagnosis and prevention. Clinical Microbiology and Infection. 2002;8:634–40. doi: 10.1046/j.1469-0691.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- 30.Grigg ME, Sundar N. Sexual recombination punctuated by outbreaks and clonal expansions predicts Toxoplasma gondii population genetics. International Journal for Parasitology. 2009;39:925–33. doi: 10.1016/j.ijpara.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubey JP. Toxoplasma gondii infections in chickens (Gallus domesticus): prevalence, clinical disease, diagnosis and public health significance. Zoonoses and Public Health. 2010;57:60–73. doi: 10.1111/j.1863-2378.2009.01274.x. [DOI] [PubMed] [Google Scholar]
- 32.Dubey JP, Jones JL. Toxoplasma gondii infection in humans and animals in the United States. International Journal for Parasitology. 2008;38:1257–78. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Dubey JP. Toxoplasmosis in pigs – the last 20 years. Veterinary Parasitology. 2009;164:89–103. doi: 10.1016/j.vetpar.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 34.Baril L, Ancelle T, Goulet V, Thulliez P, Tirard-Fleury V, Carme B. Risk factors for Toxoplasma infection in pregnancy: a case-control study in France. Scandinavian Journal of Infectious Diseases. 1999;31:305–9. doi: 10.1080/00365549950163626. [DOI] [PubMed] [Google Scholar]
- 35.Kapperud G, Jenum PA, Stray-Pedersen B, Maelby KK, Eskiid A, Eng J. Risk factors for Toxoplasma gondii infection in pregnancy. American Journal of Epidemiology. 1996;144:405–12. doi: 10.1093/oxfordjournals.aje.a008942. [DOI] [PubMed] [Google Scholar]
- 36.Jones JL, Kruszon-Moran D, Wilson M, McQuillan G, Navin T, McAuley JB. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. American Journal of Epidemiology. 2001;154:357–65. doi: 10.1093/aje/154.4.357. [DOI] [PubMed] [Google Scholar]
- 37.Lass A, Pietkiewicz H, Modzelewska E, Dumetre A, Szostakowska B, Myjak P. Detection of Toxoplasma gondii oocysts in environmental soil samples using molecular methods. European Journal of Clinical Microbiology and Infectious Diseases. 2009;28:599–605. doi: 10.1007/s10096-008-0681-5. [DOI] [PubMed] [Google Scholar]
- 38.Ruiz A, Frenkel JK, Cerdas L. Isolation of toxoplasma from soil. Journal of Parasitology. 1973;59:204–6. [PubMed] [Google Scholar]
- 39.Elmore SA, Jones JL, Conrad PA, Patton S, Lindsay DS, Dubey JP. Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends in Parasitology. 2010;26:190–6. doi: 10.1016/j.pt.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Hill D, Coss C, Dubey JP, Wroblewski K, Sautter M, Hosten T, et al. Identification of a sporozoite-specific antigen from Toxoplasma gondii. Journal of Parasitology. 2011;97:328–37. doi: 10.1645/GE-2782.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boyer K, Hill D, Mui E, Wroblewski K, Karrison T, Dubey JP, et al. Unrecognized ingestion of Toxoplasma gondii oocysts leads to congenital toxoplasmosis and causes epidemics in North America. Clinical Infectious Diseases. 2011;53:1081–9. doi: 10.1093/cid/cir667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubey JP. Toxoplasmosis – a waterborne zoonosis. Veterinary Parasitology. 2004;126:57–72. doi: 10.1016/j.vetpar.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Baldursson S, Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks – an update 2004–2010. Water Research. 2011;45:6603–14. doi: 10.1016/j.watres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Holshuh HJ, Sherrod AE, Taylor CR, Andrews BF, Howard EB. Toxoplasmosis in a feral northern fur seal. Journal of the American Veterinary Medical Association. 1985;187:1229–30. [PubMed] [Google Scholar]
- 45.Honnold SP, Braun R, Scott DP, Sreekumar C, Dubey JP. Toxoplasmosis in a Hawaiian monk seal (Monachus schauinslandi) Journal of Parasitology. 2005;91:695–7. doi: 10.1645/GE-469R. [DOI] [PubMed] [Google Scholar]
- 46.Lapointe JM, Duignan PJ, Barr BC, Petrich AK, MacPherson DW, Gulland FM, et al. Meningoencephalitis associated with an unidentified apicomplexan protozoan in a Pacific harbor seal. Journal of Parasitology. 2003;89:859–62. doi: 10.1645/GE-62R1. [DOI] [PubMed] [Google Scholar]
- 47.Migaki G, Sawa TR, Dubey JP. Fatal disseminated toxoplasmosis in a spinner dolphin (Stenella longirostris) Veterinary Pathology. 1990;27:463–4. doi: 10.1177/030098589902700615. [DOI] [PubMed] [Google Scholar]
- 48.Mikaelian I, Boisclair J, Dubey JP, Kennedy S, Martineau D. Toxoplasmosis in beluga whales (Delphinapterus leucas) from the St Lawrence estuary: two case reports and a serological survey. Journal of Comparative Pathology. 2000;122:73–6. doi: 10.1053/jcpa.1999.0341. [DOI] [PubMed] [Google Scholar]
- 49.Buergelt CD, Bonde RK. Toxoplasmic meningoencephalitis in a West Indian manatee. Journal of the American Veterinary Medical Association. 1983;183:1294–6. [PubMed] [Google Scholar]
- 50.Santos PS, Albuquerque GR, da Silva VM, Martin AR, Marvulo MF, Souza SL, et al. Seroprevalence of Toxoplasma gondii in free-living Amazon River dolphins (Inia geoffrensis) from central Amazon, Brazil. Veterinary Parasitology. 2011;183:171–3. doi: 10.1016/j.vetpar.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 51.Robards MD, Reeves RR. The global extent and character of marine mammal consumption by humans: 1970–2009. Biological Conservation. 2011;144:2770–86. [Google Scholar]
- 52.Kreuder C, Miller MA, Jessup DA, Lowenstine LJ, Harris MD, Ames JA, et al. Patterns of mortality in southern sea otters (Enhydra lutris nereis) from 1998–2001. Journal of Wildlife Diseases. 2003;39:495–509. doi: 10.7589/0090-3558-39.3.495. [DOI] [PubMed] [Google Scholar]
- 53.Miller MA, Gardner IA, Kreuder C, Paradies DM, Worcester KR, Jessup DA, et al. Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis) International Journal for Parasitology. 2002;32:997–1006. doi: 10.1016/s0020-7519(02)00069-3. [DOI] [PubMed] [Google Scholar]
- 54.Miller M, Conrad P, James ER, Packham A, Toy-Choutka S, Murray MJ, et al. Transplacental toxoplasmosis in a wild southern sea otter (Enhydra lutris nereis) Veterinary Parasitology. 2008;153:12–8. doi: 10.1016/j.vetpar.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 55.Johnson CK, Tinker MT, Estes JA, Conrad PA, Staedler M, Miller MA, et al. Prey choice and habitat use drive sea otter pathogen exposure in a resource-limited coastal system. Proceedings of the National Academy of Sciences of the United States of America; 2009; pp. 2242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riedman ML, Estes JA. Predation on seabirds by sea otters. Canadian Journal of Zoology. 1988;66:1396–402. [Google Scholar]
- 57.Estes JA, Riedman ML, Staedler MM, Tinker MT, Lyon BE. Individual variation in prey selection by sea otters: patterns, causes and implications. Journal of Animal Ecology. 2003;72:144–55. [Google Scholar]
- 58.Arkush KD, Miller MA, Leutenegger CM, Gardner IA, Packham AE, Heckeroth AR, et al. Molecular and bioassay-based detection of Toxoplasma gondii oocyst uptake by mussels (Mytilus galloprovincialis) International Journal for Parasitology. 2003;22:1087–97. doi: 10.1016/s0020-7519(03)00181-4. [DOI] [PubMed] [Google Scholar]
- 59.Lindsay DS, Collins MV, Mitchell SM, Wetch CN, Rosypal AC, Flick GJ, et al. Survival of Toxoplasma gondii oocysts in eastern oysters (Crassostrea virginica) Journal of Parasitology. 2004;90:1054–7. doi: 10.1645/GE-296R. [DOI] [PubMed] [Google Scholar]
- 60.Jones JL, Dargelas V, Roberts J, Press C, Remington JS, Montoya JG. Risk factors for Toxoplasma gondii infection in the United States. Clinical Infectious Diseases. 2009;49:878–84. doi: 10.1086/605433. [DOI] [PubMed] [Google Scholar]
- 61.Massie GN, Ware MW, Villegas EN, Black MW. Uptake and transmission of Toxoplasma gondii oocysts by migratory, filter-feeding fish. Veterinary Parasitology. 2010;169:296–303. doi: 10.1016/j.vetpar.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 62.Lindsay DS, Blagburn BL, Dubey JP. Survival of nonsporulated Toxoplasma gondii oocysts under refrigerator conditions. Veterinary Parasitology. 2002;103:309–13. doi: 10.1016/s0304-4017(01)00554-4. [DOI] [PubMed] [Google Scholar]
- 63.Frenkel JK, Ruiz A, Chinchilla M. Soil survival of Toxoplasma oocysts in Kansas and Costa Rica. American Journal of Tropical Medicine and Hygiene. 1975;24:439–43. doi: 10.4269/ajtmh.1975.24.439. [DOI] [PubMed] [Google Scholar]
- 64.Yilmaz SM, Hopkins SH. Effects of different conditions on duration of infectivity of Toxoplasma gondii oocysts. Journal of Parasitology. 1972;58:938–9. [PubMed] [Google Scholar]
- 65.Lindsay DS, Dubey JP. Long-term survival of Toxoplasma gondii sporulated oocysts in seawater. Journal of Parasitology. 2009;95:1019–20. doi: 10.1645/GE-1919.1. [DOI] [PubMed] [Google Scholar]
- 66.Dumetre A, Darde ML. How to detect Toxoplasma gondii oocysts in environmental samples? FEMS Microbiology Review. 2003;27:651–61. doi: 10.1016/S0168-6445(03)00071-8. [DOI] [PubMed] [Google Scholar]
- 67.Dubey JP. Toxoplasma gondii oocyst survival under defined temperatures. Journal of Parasitology. 1998;84:862–5. [PubMed] [Google Scholar]
- 68.Wainwright KE, Miller MA, Barr BC, Gardner IA, Melli AC, Essert T, et al. Chemical inactivation of Toxoplasma gondii oocysts in water. Journal of Parasitology. 2007;93:925–31. doi: 10.1645/GE-1063R.1. [DOI] [PubMed] [Google Scholar]
- 69.Dumetre A, Le Bras C, Baffet M, Meneceur P, Dubey JP, Derouin F, et al. Effects of ozone and ultraviolet radiation treatments on the infectivity of Toxoplasma gondii oocysts. Veterinary Parasitology. 2008;153:209–13. doi: 10.1016/j.vetpar.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 70.Wainwright KE, Lagunas-Solar M, Miller MA, Barr BC, Gardner IA, Pina C, et al. Physical inactivation of Toxoplasma gondii oocysts in water. Applied and Environment Microbiology. 2007;73:5663–6. doi: 10.1128/AEM.00504-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dubey JP, Lunney JK, Shen SK, Kwok OC, Ashford DA, Thulliez P. Infectivity of low numbers of Toxoplasma gondii oocysts to pigs. Journal of Parasitology. 1996;82:438–43. [PubMed] [Google Scholar]
- 72.Rejmanek D, VanWormer E, Mazet JA, Packham AE, Aguilar B, Conrad PA. Congenital transmission of Toxoplasma gondii in deer mice (Peromyscus maniculatus) after oral oocyst infection. Journal of Parasitology. 2010;96:516–20. doi: 10.1645/GE-2372.1. [DOI] [PubMed] [Google Scholar]
- 73.Dubey JP. Persistence of encysted Toxoplasma gondii in tissues of equids fed oocysts. American Journal of Veterinary Research. 1985;46:1753–4. [PubMed] [Google Scholar]
- 74.Dubey JP. Pathogenicity and infectivity of Toxoplasma gondii oocysts for rats. Journal of Parasitology. 1996;82:951–6. [PubMed] [Google Scholar]
- 75.Fritz HM, Buchholz KR, Chen X, Durbin-Johnson B, Rocke DM, Conrad PA, et al. Transcriptomic analysis of Toxoplasma development reveals many novel functions and structures specific to sporozoites and oocysts. PLoS One. 2011;7:e29998. doi: 10.1371/journal.pone.0029998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fritz H, Barr B, Packham A, Melli A, Conrad PA. Methods to produce and safely work with large numbers of Toxoplasma gondii oocysts and bradyzoite cysts. Journal of Microbiological Methods. 2012;88:47–52. doi: 10.1016/j.mimet.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fritz HM, Bowyer PW, Bogyo M, Conrad PA, Boothroyd JC. Proteomic analysis of fractionated Toxoplasma oocysts reveals clues to their environmental resistance. PLoS One. 2012;7:e29955. doi: 10.1371/journal.pone.0029955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferguson DJ, Hutchison WM, Siim JC. The ultrastructural development of the macrogamete and formation of the oocyst wall of Toxoplasma gondii. Acta Pathologica et Microbiologica Scandinavica B. 1975;83:491–505. doi: 10.1111/j.1699-0463.1975.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 79.Ferguson DJP, Dubremetz JF. The ultrastructure of Toxoplasma gondii. In: Weiss LM, Kim K, editors. Toxoplasma gondii, the model apicomplexan: perspectives and methods. London: Elsevier; 2007. pp. 19–48. [Google Scholar]
- 80.Belli SI, Smith NC, Ferguson DJ. The coccidian oocyst: a tough nut to crack! Trends in Parasitology. 2006;22:416–23. doi: 10.1016/j.pt.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 81.Belli SI, Wallach MG, Luxford C, Davies MJ, Smith NC. Roles of tyrosine-rich precursor glycoproteins and dityrosine- and 3,4-dihydroxyphenylalanine-mediated protein cross-linking in development of the oocyst wall in the coccidian parasite Eimeria maxima. Eukaryotic Cell. 2003;2:456–64. doi: 10.1128/EC.2.3.456-464.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mai K, Smith NC, Feng ZP, Katrib M, Slapeta J, Slapetova I, et al. Peroxidase catalysed cross-linking of an intrinsically unstructured protein via dityrosine bonds in the oocyst wall of the apicomplexan parasite, Eimeria maxima. International Journal for Parasitology. 2011;41:1157–64. doi: 10.1016/j.ijpara.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 83.Mai K, Sharman PA, Walker RA, Katrib M, De Souza D, McConville MJ, et al. Oocyst wall formation and composition in coccidian parasites. Memorias do Instituto Oswaldo Cruz. 2009;104:281–9. doi: 10.1590/s0074-02762009000200022. [DOI] [PubMed] [Google Scholar]
- 84.Kay ES, Shapiro BM. Ovoperoxidase assembly into the sea urchin fertilization envelope and dityrosine crosslinking. Developmental Biology. 1987;121:325–34. doi: 10.1016/0012-1606(87)90168-0. [DOI] [PubMed] [Google Scholar]
- 85.Suda Y, Rodriguez RK, Coluccio AE, Neiman AM. A screen for spore wall permeability mutants identifies a secreted protease required for proper spore wall assembly. PLoS One. 2009;4:e7184. doi: 10.1371/journal.pone.0007184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smail EH, Briza P, Panagos A, Berenfeld L. Candida albicans cell walls contain the fluorescent cross-linking amino acid dityrosine. Infection and Immunity. 1995;63:4078–83. doi: 10.1128/iai.63.10.4078-4083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.DiMarco T, Giulivi C. Current analytical methods for the detection of dityrosine, a biomarker of oxidative stress, in biological samples. Mass Spectrometry Reviews. 2007;26:108–20. doi: 10.1002/mas.20109. [DOI] [PubMed] [Google Scholar]
- 88.Templeton TJ, Lancto CA, Vigdorovich V, Liu C, London NR, Hadsall KZ, et al. The Cryptosporidium oocyst wall protein is a member of a multigene family and has a homolog in Toxoplasma. Infection and Immunity. 2004;72:980–7. doi: 10.1128/IAI.72.2.980-987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Possenti A, Cherchi S, Bertuccini L, Pozio E, Dubey JP, Spano F. Molecular characterisation of a novel family of cysteine-rich proteins of Toxoplasma gondii and ultrastructural evidence of oocyst wall localisation. International Journal for Parasitology. 2010;40:1639–49. doi: 10.1016/j.ijpara.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 90.Brown PJ, Gill AC, Nugent PG, McVey JH, Tomley FM. Domains of invasion organelle proteins from apicomplexan parasites are homologous with the Apple domains of blood coagulation factor XI and plasma pre-kallikrein and are members of the PAN module superfamily. FEBS Letters. 2001;497:31–8. doi: 10.1016/s0014-5793(01)02424-3. [DOI] [PubMed] [Google Scholar]
- 91.Tunnacliffe A, Wise MJ. The continuing conundrum of the LEA proteins. Naturwissenschaften. 2007;94:791–812. doi: 10.1007/s00114-007-0254-y. [DOI] [PubMed] [Google Scholar]
- 92.Hundertmark M, Hincha DK. LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genomics. 2008;9:118. doi: 10.1186/1471-2164-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dubey JP. Oocyst shedding by cats fed isolated bradyzoites and comparison of infectivity of bradyzoites of the VEG strain Toxoplasma gondii to cats and mice. Journal of Parasitology. 2001;87:215–9. doi: 10.1645/0022-3395(2001)087[0215:OSBCFI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 94.Dubey J. Duration of immunity to shedding of Toxoplasma gondii oocysts by cats. Journal of Parasitology. 1995;81:410–5. [PubMed] [Google Scholar]
- 95.Khan A, Dubey JP, Su C, Ajioka JW, Rosenthal BM, Sibley LD. Genetic analyses of atypical Toxoplasma gondii strains reveal a fourth clonal lineage in North America. International Journal for Parasitology. 2011;41:645–55. doi: 10.1016/j.ijpara.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wendte JM, Gibson AK, Grigg ME. Population genetics of Toxoplasma gondii: new perspectives from parasite genotypes in wildlife. Veterinary Parasitology. 2011;182:96–111. doi: 10.1016/j.vetpar.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ruiz A, Frenkel J. Toxoplasma gondii in Costa Rican cats. American Journal of Tropical Medicine and Hygiene. 1980;29:1150–60. doi: 10.4269/ajtmh.1980.29.1150. [DOI] [PubMed] [Google Scholar]
- 98.Schares G, Vrhovec MG, Pantchev N, Hermann DC, Conraths FJ. Occurrence of Toxoplasma gondii and Hammondia hammondii oocysts in the faeces of cats from Germany and other European countries. Veterinary Parasitology. 2008;152:34–45. doi: 10.1016/j.vetpar.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 99.Hermann DC, Pantchev N, Vrhovec MG, Varutzki D, Wilking H, Frolich A, et al. Atypical Toxoplasma gondii genotypes identified in oocysts shed by cats in Germany. International Journal for Parasitology. 2010;40:285–92. doi: 10.1016/j.ijpara.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 100.Berger-Schoch AE, Hermann DC, Schares G, Muller N, Bernet D, Gottstein B, et al. Prevalence and genotypes of Toxoplasma gondii in feline faeces (oocysts) and meat from sheep, cattle and pigs in Switzerland. Veterinary Parasitology. 2011;177:290–7. doi: 10.1016/j.vetpar.2010.11.046. [DOI] [PubMed] [Google Scholar]
- 101.Aramini JJ, Stephen C, Dubey JP. Toxoplasma gondii in Vancouver Island cougard (Felis concolor vancouverensis): serology and oocyst shedding. Journal of Parasitology. 1998;84:438–40. [PubMed] [Google Scholar]
- 102.Chessum BS. Reactivation of Toxoplasma oocyst production in the cat by infection with Isospora felis. British Veterinary Journal. 1972;128:33–6. [PubMed] [Google Scholar]
- 103.Dubey J, Frenkel J. Immunity to feline toxoplasmosis: modification by administration of corticosteroids. Veterinary Pathology. 1974;11:350–79. doi: 10.1177/030098587401100407. [DOI] [PubMed] [Google Scholar]
- 104.Dubey J. Reshedding of Toxoplasma oocysts by chronically infected cats. Nature. 1976;262 doi: 10.1038/262213a0. [DOI] [PubMed] [Google Scholar]
- 105.Lappin MR, George JW, Pedersen NC, Barlough JE, Murphy CJ, Morse LS. Primary and secondary Toxoplasma gondii infection in normal and feline immunodeficiency virus-infected cats. Journal of Parasitology. 1996;82:733–42. [PubMed] [Google Scholar]
- 106.Lukesova D, Literak I. Shedding of Toxoplasma gondii oocysts by Felidae in zoos in the Czech Republic. Veterinary Parasitology. 1998;74:1–7. doi: 10.1016/s0304-4017(97)00155-6. [DOI] [PubMed] [Google Scholar]
- 107.Frenkel JK, Pfefferkorn ER, Smith DD, Fishback JL. Prospective vaccine prepared from a new mutant of Toxoplasma gondii for use in cats. American Journal of Veterinary Research. 1991;52:759–63. [PubMed] [Google Scholar]
- 108.Freyre A, Choromanski L, Fishback JL, Popiel I. Immunization of cats with tissue cysts, bradyzoites, and tachyzoites of the T-263 strain of Toxoplasma gondii. Journal of Parasitology. 1993;79:716–9. [PubMed] [Google Scholar]
- 109.Choromanski L, Freyre A, Popiel R, Brown K, Grieve R, Shibley G. Safety and efficacy of modified live feline Toxoplasma gondii vaccine. Developments in Biological Standardization. 1995;84:269–81. [PubMed] [Google Scholar]
- 110.Innes EA, Bartley PM, Maley S, Katzer F, Buxton D. Veterinary vaccines against Toxoplasma gondii. Memorias do Instituto Oswaldo Cruz. 2009;104:246–51. doi: 10.1590/s0074-02762009000200018. [DOI] [PubMed] [Google Scholar]
- 111.Dabritz H, Miller M, Atwill E, Gardner I, Leutenegger C, Melli A, et al. Detection of Toxoplasma gondii-like oocysts in cat feces and estimates of the environmental oocyst burden. Journal of the American Veterinary Medical Association. 2007;231:1676–84. doi: 10.2460/javma.231.11.1676. [DOI] [PubMed] [Google Scholar]
- 112.Afonso E, Thulliez P, Gilot-Fromont E. Local meteorological conditions, dynamics of seroconversion to Toxoplasma gondii in cats (Felis catus)and oocyst burden in a rural environment. Epidemiology and Infection. 2010;138:1105–13. doi: 10.1017/S0950268809991270. [DOI] [PubMed] [Google Scholar]
- 113.Horn JA, Mateus-Pinilla N, Warner RE, Heske EJ. Home range, habitat use, and activity patterns of free-roaming domestic cats. Journal of Wildlife Management. 2011;75:1177–85. [Google Scholar]
- 114.Beier P. Dispersal of juvenile cougars in fragmented habitat. Journal of Wildlife Management. 1995;59:228–37. [Google Scholar]
- 115.Crooks KR. Relative sensitivities of mammalian carnivores to habitat fragmentation. Conservation Biology. 2002;16:488–502. [Google Scholar]
- 116.Hunter RD, Fisher RF, Crooks KR. Landscape-level connectivity in coastal southern California, USA, as assessed through carnivore habitat suitability. Natural Areas Journal. 2003;23:302–14. [Google Scholar]
- 117.Ordenana MA, Crooks KR, Boydston EE, Fisher RN, Lyren LM, Siudyla S, et al. Effects of urbanization on carnivore species distribution and richness. Journal of Mammalogy. 2010;91:1322–31. [Google Scholar]
- 118.Reed SE, Merenlender AM. Effects of management of domestic dogs and recreation on carnivores in protected areas in northern California. Conservation Biology. 2011;25:504–13. doi: 10.1111/j.1523-1739.2010.01641.x. [DOI] [PubMed] [Google Scholar]
- 119.Hilty JA, Merenlender AM. Use of riparian corridors and vineyards by mammalian predators in northern California. Conservation Biology. 2004;18:126–35. [Google Scholar]
- 120.Karanth KU, Sunquist ME. Behavioral correlates of predation by tiger (Panthera tigris), leopard (Panthera pardus) and dhole (Cuon alpinus) in Nagarahole, India. Journal of Zoology. 2000;250:255–65. [Google Scholar]
- 121.Pierce BM, Bleich VC, Bowyer RT. Social organization of mountain lions: does a land-tenure system regulate population size? Ecology. 2000;81:1533–43. [Google Scholar]
- 122.Winter L. Trap-neuter-release programs: the reality and the impact. Journal of the American Veterinary Medical Association. 2004;225:1369–76. doi: 10.2460/javma.2004.225.1369. [DOI] [PubMed] [Google Scholar]
- 123.Fitzgerald BM, Turner DC. Hunting behavior of domestic cats and their impact on prey populations. In: Turner DC, Bateson P, editors. The domestic cat: the biology of its behavior. 2nd. Cambridge: Cambridge University Press; 2000. pp. 152–75. [Google Scholar]
- 124.Platinga EA, Bosch G, Hendriks WH. Estimation of the dietary nutrient profile of free-roaming feral cats: possible implications for nutrition of domestic cats. British Journal of Nutrition. 2011;106:S35–48. doi: 10.1017/S0007114511002285. [DOI] [PubMed] [Google Scholar]
- 125.Hass CC. Competition and coexistence in sympatric bobcats and pumas. Journal of Zoology. 2009;278:174–80. [Google Scholar]
- 126.Treves A, Karanth KU. Human-carnivore conflict and perspectives on carnivore management worldwide. Conservation Biology. 2003;17:1491–9. [Google Scholar]
- 127.Afonso E, Thulliez P, Pontier D, Gilot-Fromont E. Toxoplasmosis in prey species and consequences for prevalence in feral cats: not all prey species are equal. Parasitology. 2007;134:1963–71. doi: 10.1017/S0031182007003320. [DOI] [PubMed] [Google Scholar]
- 128.Guisan A, Thuiller W. Predicting species distribution: offering more than simple habitat models. Ecology Letters. 2005;8:993–1009. doi: 10.1111/j.1461-0248.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 129.Porcasi X, Calderón G, Lamfri M, Gardenal N, Polop J, Sabattini M, et al. The use of satellite data in modeling population dynamics and prevalence of infection in the rodent reservoir of Junin virus. Ecological Modelling. 2005;185:437–49. [Google Scholar]
- 130.Orrock JL, Pagels JF, Mcshea WJ, Harper EK. Predicting presence and abundance of small mammal species: the effect of scale and resolution. Ecological Applications. 2000;10:1356–66. [Google Scholar]
- 131.Dickman CR, Mahon PS, Masters P, Gibson DF. Long-term dynamics of rodent populations in arid Australia: the influence of rainfall. Wildlife Research. 1999;26:389–403. [Google Scholar]
- 132.Foley JA, Defries R, Asner GP, Barford C, Bonan G, Carpenter SR, et al. Global consequences of land use. Science. 2005;309:570–4. doi: 10.1126/science.1111772. [DOI] [PubMed] [Google Scholar]
- 133.Sims V, Evans KL, Newson SE, Tratalos JA, Gaston KJ. Avian assemblage structure and domestic cat densities in urban environments. Diversity and Distributions. 2007;14:387–99. [Google Scholar]
- 134.Jessup DA. The welfare of feral cats and wildlife. Journal of the American Veterinary Medical Association. 2004;225:1377–83. doi: 10.2460/javma.2004.225.1377. [DOI] [PubMed] [Google Scholar]
- 135.Weaver RA. Changing status of mountain lion in California and livestock depredation problems. Proceeding of 8th Vertebrate Pest Conference; 1978; pp. 214–9. [Google Scholar]
- 136.Roberts NM, Crimmins SM. Bobcat population status and management in North America: evidence of large-scale population increase. Journal of Fish and Wildlife Management. 2010;1:169–74. [Google Scholar]
- 137.Defenders of Wildlife. [accessed 01.05.12];Govenor Schwarzenegger signs California sea otter bill. 2006 http://www.defenders.org/press-release/governor-schwarzenegger-signs-california-sea-otter-bill.
- 138.Macdonald DW. Patterns of scent marking with urine and faeces amongst carnivore communities. Symposia of the Zoological Society of London. 1980;45:107–39. [Google Scholar]
- 139.Yoakum J. Observations on bobcat-water relationships. Journal of Mammalogy. 1964;45:477–9. [Google Scholar]
- 140.Seidensticker JCI, Hornocker MG, Wildes WV, Messick JP. Mountain lion social organization in the Idaho primitive area. Wildlife Monographs. 1973;35:3–60. [Google Scholar]
- 141.Ralls K, Sharma S, Smith DA, Bremner-Harrison S, Cypher BL, Maldonado JE. Changes in kit fox defecation patterns during the reproductive season: implications for noninvasive surveys. Journal of Wildlife Management. 2010;74:1457–62. [Google Scholar]
- 142.Dickson BG, Beier P. Home-range and habitat selection by adult cougars in southern California. Journal of Wildlife Management. 2002;66:1235–45. [Google Scholar]
- 143.Mallin M, Ensign S, McIver M, Shank G, Fowler P. Demographic, landscape, and meteorological factors controlling the pollution of coastal waters. Hydrobiologia. 2001;460:185–293. [Google Scholar]
- 144.Shapiro K, Conrad PA, Mazet JAK, Wallender WW, Miller WA, Largier JL. Effect of estuarine wetland degradation on transport of Toxoplasma gondii surrogates from land to sea. Applied and Environment Microbiology. 2010;76:6821–8. doi: 10.1128/AEM.01435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Knox A, Dahgren R. Efficacy of natural wetlands to retain nutrient, sediment and microbial pollutants. Journal of Environment Quality. 2008;37:1837–46. doi: 10.2134/jeq2007.0067. [DOI] [PubMed] [Google Scholar]
- 146.Falabi JA, Gerba CP, Karpiscak MM. Giardia and Cryptosporidium removal from waste-water by a duckweed (Lemna gibba L.) covered pond. Letters in Applied Microbiology. 2002;34:384–7. doi: 10.1046/j.1472-765x.2002.01104.x. [DOI] [PubMed] [Google Scholar]
- 147.Miller WA, Lewis DJL, Lennox M, Periera MDG, Conrad PA, Tate KW, et al. Farm factors associated with reducing Cryptosporidium loading in storm runoff from dairy high use areas. Journal of Environment Quality. 2008;37:1875–82. doi: 10.2134/jeq2007.0413. [DOI] [PubMed] [Google Scholar]
- 148.Winter TC. The concept of hydrologic landscapes. Journal of the American Water Resources Association. 2001;37:335–49. [Google Scholar]
- 149.Trask JR, Kalita PK, Kuhlenschmidt MS, Smith RD, Funk TL. Overland and near-surface transport of Cryptosporidium parvum from vegetated and nonvegetated surfaces. Journal of Environment Quality. 2004;33:984–93. doi: 10.2134/jeq2004.0984. [DOI] [PubMed] [Google Scholar]
- 150.de Lima JLMP, Singh VP. The influence of the pattern of moving rainstorms on overland flow. Advances in Water Resources. 2002;25:817–28. [Google Scholar]
- 151.Nearing MA, Jetten V, Baffaut C, Cerdan O, Couturier A, Hernandez M, et al. Modeling response of soil erosion and runoff to changes in precipitation and cover. Catena. 2005;61:131–54. [Google Scholar]
- 152.Shapiro K, Miller MA, Mazet JAK. Temporal association between land-based runoff events and California sea otter (Enhydra lutris nereis) protozoal mortalities. Journal of Wildlife Diseases. 2012;48:394–404. doi: 10.7589/0090-3558-48.2.394. [DOI] [PubMed] [Google Scholar]
- 153.Stoddard RA, Atwill ER, Gulland FMD, Miller MA, Dabritz HA, Paradies DM, et al. Risk factors for infection with pathogenic and antimicrobial-resistant fecal bacteria in northern elephant seals in California. Public Health Reports. 2008;123:360–70. doi: 10.1177/003335490812300316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dumetre A, Aubert D, Puech PH, Hohweyer J, Azas N, Villena I. Interaction forces drive the environmental transmission of pathogenic protozoa. Applied and Environment Microbiology. 2012;78:905–12. doi: 10.1128/AEM.06488-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shapiro K, Largier J, Mazet JA, Bernt W, Ell JR, Melli AC, et al. Surface properties of Toxoplasma gondii oocysts and surrogate microspheres. Applied and Environment Microbiology. 2009;75:1185–91. doi: 10.1128/AEM.02109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shapiro K, Silver MW, Largier J, Conrad PA, Mazet JAK. Association of Toxoplasma gondii oocysts with fresh, estuarine, and marine macroaggregates. Limnology and Oceanography. 2012;57:449–56. [Google Scholar]
- 157.Ward JE, Kach DJ. Marine aggregates facilitate ingestion of nanoparticles by suspension-feeding bivalves. Marine Environment Research. 2009;68:137–42. doi: 10.1016/j.marenvres.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 158.Alldredge AL, Silver MW. Characteristics, dynamics and significance of marine snow. Progress in Oceanography. 1988;20:41–82. [Google Scholar]
- 159.Burd AB, Jackson GA. Particle aggregation. Annual Review of Materials Science. 2009;1:65–90. doi: 10.1146/annurev.marine.010908.163904. [DOI] [PubMed] [Google Scholar]
- 160.Bates BC, Kundzewicz ZW, Wu S, Palutikof JP. In: Climate change and water: technical paper of the Intergovernmental Panel on Climate Change. Bates BC, Kundzewicz ZW, Wu S, Palutikof JP, editors. Geneva: Intergovernmental Panel on Climate Change; p. 2008.p. 210. [Google Scholar]
- 161.Jackson J. Ecological extinction and evolution in the brave new ocean. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11458–65. doi: 10.1073/pnas.0802812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Scavia D, Field J, Boesch D, Buddemeier R, Burkett V, Cayan D, et al. Climate change impacts on US coastal and marine ecosystems. Estuaries. 2002;25:149–64. [Google Scholar]
- 163.Shapiro K. Climate and coastal habitat change: a recipe for a dirtier ocean. Marine Pollution Bulletin. 2012;64:1079–80. doi: 10.1016/j.marpolbul.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 164.Frenkel JK, Dubey JP. Effects of freezing on the viability of Toxoplasma oocysts. Journal of Parasitology. 1973;59:587–8. [PubMed] [Google Scholar]
- 165.Robock A, Vinnikov KY, Srinivasan G, Entin JK, Hollinger SE, Speranskaya NA, et al. The global soil moisture data bank. Bulletin of the American Meteorological Society. 2000;81:1281–99. [Google Scholar]
- 166.Jenum PA, Kapperud G, Stray-Pedersen B, Melby KK, Eskild A, Eng J. Prevalence of Toxoplasma gondii specific immunoglobulin G antibodies among pregnant women in Norway. Epidemiology and Infection. 1998;120:87–92. doi: 10.1017/s0950268897008480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hershey DW, McGregor JA. Low prevalence of Toxoplasma infection in a rocky-mountain prenatal population. Obstetrics and Gynecology. 1987;70:900–2. [PubMed] [Google Scholar]
- 168.Meerburg BG, Kijlstra A. Changing climate-changing pathogen: Toxoplasma gondii in north-western Europe. Parasitology Research. 2009;105:17–24. doi: 10.1007/s00436-009-1447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Carme B, Bissuel F, Ajzenberg D, Bouyne R, Aznar C, Demar M, et al. Severe acquired toxoplasmosis in immunocompetent adult patients in French Guiana. Journal of Clinical Microbiology. 2002;40:4037–44. doi: 10.1128/JCM.40.11.4037-4044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Carme B, Demar M, Ajzenberg D, Darde ML. Severe acquired toxoplasmosis caused by wild cycle of Toxoplasma gondii, French Guiana. Emerging Infectious Diseases. 2009;15:656–8. doi: 10.3201/eid1504.081306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Demar M, Ajzenberg D, Maubon D, Djossou F, Panchoe D, Punwasi W, et al. Fatal outbreak of human toxoplasmosis along the Maroni River: epidemiological, clinical, and parasitological aspects. Clinical Infectious Diseases. 2007;45:E88–95. doi: 10.1086/521246. [DOI] [PubMed] [Google Scholar]
- 172.Mercier A, Ajzenberg D, Devillard S, Demar MP, de Thoisy B, Bonnabau H, et al. Human impact on genetic diversity of Toxoplasma gondii: example of the anthropized environment from French Guiana. Infection, Genetics and Evolution. 2011;11:1378–87. doi: 10.1016/j.meegid.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 173.Langlais M, Lélu M, Avenet C, Gilot-Fromont E. A simplified model system for Toxoplasma gondii spread within a heterogeneous environment. Nonlinear Dynamics. 2012;68:381–99. [Google Scholar]
- 174.Jiang W, Sullivan AM, Su C, Zhao X. An agent-based model for the transmission dynamics of Toxoplasma gondii. Journal of Theoretical Biology. 2012;293:15–26. doi: 10.1016/j.jtbi.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 175.Trejos DYA, Duarte IG. Contribution of waterborne transport in the spread of infection with Toxoplasma gondii. Biomat 2009 – Int Symp Math Comp Biol. 2009:366–76. [Google Scholar]
- 176.Benenson MW, Takafuji ET, Lemon SM, Greenup RL, Sulzer AJ. Oocyst-transmitted toxoplasmosis associated with ingestion of contaminated water. New England Journal of Medicine. 1982;307:666–9. doi: 10.1056/NEJM198209093071107. [DOI] [PubMed] [Google Scholar]
- 177.Teutsch SM, Juranek DD, Sulzer A, Dubey JP, Sikes RK. Epidemic toxoplasmosis associated with infected cats. New England Journal of Medicine. 1979;300:695–9. doi: 10.1056/NEJM197903293001302. [DOI] [PubMed] [Google Scholar]
- 178.Stagno S, Dykes AC, Amos CS, Head RA, Juranek DD, Walls K. An outbreak of toxoplasmosis linked to cats. Pediatrics. 1980;65:706–12. [PubMed] [Google Scholar]
- 179.Bowie WR, King AS, Werker DH, Isaac-Renton JL, Bell A, Eng SB, et al. Outbreak of toxoplasmosis associated with municipal drinking water. The BC Toxoplasma Investigation Team. Lancet. 1997;350:173–7. doi: 10.1016/s0140-6736(96)11105-3. [DOI] [PubMed] [Google Scholar]
- 180.de Moura L, Bahia-Oliveira LM, Wada MY, Jones JL, Tuboi SH, Carmo EH, et al. Waterborne toxoplasmosis, Brazil, from field to gene. Emerging Infectious Diseases. 2006;12:326–9. doi: 10.3201/eid1202.041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sroka J, Wojcik-Fatla A, Dutkiewicz J. Occurrence of Toxoplasma gondii in water from wells located on farms. Annals of Agricultural and Environmental Medicine. 2006;13:169–75. [PubMed] [Google Scholar]
- 182.Balasundaram MB, Andavar R, Palaniswamy M, Venkatapathy N. Outbreak of acquired ocular toxoplasmosis involving 248 patients. Archives of Ophthalmology. 2010;128:28–32. doi: 10.1001/archophthalmol.2009.354. [DOI] [PubMed] [Google Scholar]
- 183.Palanisamy M, Madhavan B, Balasundaram MB, Andavar R, Venkatapathy N. Outbreak of ocular toxoplasmosis in Coimbatore, India. Indian Journal of Ophthalmology. 2006;54:129–31. doi: 10.4103/0301-4738.25839. [DOI] [PubMed] [Google Scholar]
- 184.Demar M, Hommel D, Djossou F, Peneau C, Boukhari R, Louvel D, et al. Acute toxoplasmosis in immunocompetent patients hospitalized in an intensive care unit in French Guiana. Clinical Microbiology and Infection. 2012;18:E221–31. doi: 10.1111/j.1469-0691.2011.03648.x. [DOI] [PubMed] [Google Scholar]
- 185.Carme B, Darde ML. Severe aspects of toxoplasmic primary infection in French Guiana: are they Guianese or neotropical forest or more largely (or overall) tropical specificities? Proc Med Health Trop Congress. 2005:210. [Google Scholar]
- 186.Martinez Sanchez R, Machin Sanchez R, Fachado Carvajales A, Pividal Grana J, Cruz de la Paz R, Suarez Hernandez M. Several results of a Toxoplasma survey. Investigacion Clinica. 1991;32:13–26. [PubMed] [Google Scholar]
- 187.Asthana SP, Macpherson CNL, Weiss SH, Stephens R, Denny TN, Sharma RN, et al. Seroprevalence of Toxoplasma gondii in pregnant women and cats in Grenada, West Indies. Journal of Parasitology. 2006;92:644–5. doi: 10.1645/GE-762R.1. [DOI] [PubMed] [Google Scholar]
- 188.Bahia-Oliveira LM, Jones JL, Azevedo-Silva J, Alves CC, Orefice F, Addiss DG. Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro state, Brazil. Emerging Infectious Diseases. 2003;9:55–62. doi: 10.3201/eid0901.020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cavalcante GT, Aguiar DM, Camargo LMA, Labruna MB, de Andrade HF, Meireles LR, et al. Seroprevalence of Toxoplasma gondii antibodies in humans from rural Western Amazon, Brazil. Journal of Parasitology. 2006;92:647–9. doi: 10.1645/GE-774R.1. [DOI] [PubMed] [Google Scholar]
- 190.Heukelbach J, Meyer-Cirkel V, Moura RCS, Gomide M, Queiroz JAN, Saweljew P, et al. Waterborne toxoplasmosis, northeastern Brazil. Emerging Infectious Diseases. 2007;13:287–9. doi: 10.3201/eid1302.060686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jones JL, Lopez B, Mury MA, Wilson M, Klein R, Luby S, et al. Toxoplasma gondii infection in rural Guatemalan children. American Journal of Tropical Medicine and Hygiene. 2005;72:295–300. [PubMed] [Google Scholar]
- 192.Hung CC, Fan CK, Su KE, Sung FC, Chiou HY, Gil V, et al. Serological screening and toxoplasmosis exposure factors among pregnant women in the Democratic Republic of Sao Tome and Principe. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2007;101:134–9. doi: 10.1016/j.trstmh.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 193.Ertug S, Okyay P, Turkmen M, Yuksel H. Seroprevalence and risk factors for Toxoplasma infection among pregnant women in Aydin province, Turkey. BMC Public Health. 2005;5:66. doi: 10.1186/1471-2458-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Messier V, Levesque B, Proulx JF, Rochette L, Libman MD, Ward BJ, et al. Seroprevalence of Toxoplasma gondii among Nunavik Inuit (Canada) Zoonoses and Public Health. 2009;56:188–97. doi: 10.1111/j.1863-2378.2008.01177.x. [DOI] [PubMed] [Google Scholar]
- 195.Sroka S, Bartelheimer N, Winter A, Heukelbach J, Ariza L, Ribeiro H, et al. Prevalence and risk factors of toxoplasmosis among pregnant women in Fortaleza, Northeastern Brazil. American Journal of Tropical Medicine and Hygiene. 2010;83:528–33. doi: 10.4269/ajtmh.2010.10-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nissapatorn V, Suwanrath C, Sawangjaroen N, Ling LY, Chandeying V. Toxoplasmosis-serological evidence and associated risk factors among pregnant women in Southern Thailand. American Journal of Tropical Medicine and Hygiene. 2011;85:243–7. doi: 10.4269/ajtmh.2011.10-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shapiro K, Mazet JA, Schriewer A, Wuertz S, Fritz H, Miller WA, et al. Detection of Toxoplasma gondii oocysts and surrogate microspheres in water using ultrafiltration and capsule filtration. Water Research. 2010;44:893–903. doi: 10.1016/j.watres.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 198.Borchardt MA, Spencer SK, Bertz PD, Ware MW, Dubey JP, Alan Lindquist HD. Concentrating Toxoplasma gondii and Cyclospora cayetanensis from surface water and drinking water by continuous separation channel centrifugation. Journal of Applied Microbiology. 2009;107:1089–97. doi: 10.1111/j.1365-2672.2009.04316.x. [DOI] [PubMed] [Google Scholar]
- 199.Yang WL, Lindquist HDA, Cama V, Schaefer FW, Villegas E, Fayer R, et al. Detection of Toxoplasma gondii oocysts in water sample concentrates by real-time PCR. Applied and Environment Microbiology. 2009;75:3477–83. doi: 10.1128/AEM.00285-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Aubert D, Villena I. Detection of Toxoplasma gondii oocysts in water: proposition of a strategy and evaluation in Champagne-Ardenne Region, France. Memorias do Instituto Oswaldo Cruz. 2009;104:290–5. doi: 10.1590/s0074-02762009000200023. [DOI] [PubMed] [Google Scholar]
- 201.Sotiriadou I, Karanis P. Evaluation of loop-mediated isothermal amplification for detection of Toxoplasma gondii in water samples and comparative findings by polymerase chain reaction and immunofluorescence test (IFT) Diagnostic Microbiology and Infectious Disease. 2008;62:357–65. doi: 10.1016/j.diagmicrobio.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 202.Dumetre A, Darde ML. Immunomagnetic separation of Toxoplasma gondii oocysts using a monoclonal antibody directed against the oocyst wall. Journal of Microbiological Methods. 2005;61:209–17. doi: 10.1016/j.mimet.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 203.Dumetre A, Darde ML. Detection of Toxoplasma gondii in water by an immunomagnetic separation method targeting the sporocysts. Parasitology Research. 2007;101:989–96. doi: 10.1007/s00436-007-0573-0. [DOI] [PubMed] [Google Scholar]
- 204.Kourenti C, Karanis P. Evaluation and applicability of a purification method coupled with nested PCR for the detection of Toxoplasma oocysts in water. Letters in Applied Microbiology. 2006;43:475–81. doi: 10.1111/j.1472-765X.2006.02008.x. [DOI] [PubMed] [Google Scholar]
- 205.Villena I, Aubert D, Gomis P, Ferte H, Inglard JC, Denis-Bisiaux H, et al. Evaluation of a strategy for Toxoplasma gondii oocyst detection in water. Applied and Environment Microbiology. 2004;70:4035–9. doi: 10.1128/AEM.70.7.4035-4039.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kourenti C, Karanis P. Development of a sensitive polymerase chain reaction method for the detection of Toxoplasma gondii in water. Water Science and Technology. 2004;50:287–91. [PubMed] [Google Scholar]
- 207.Kourenti C, Heckeroth A, Tenter A, Karanis P. Development and application of different methods for the detection of Toxoplasma gondii in water. Applied and Environment Microbiology. 2003;69:102–6. doi: 10.1128/AEM.69.1.102-106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Isaac-Renton J, Bowie WR, King A, Irwin GS, Ong CS, Fung CP, et al. Detection of Toxoplasma gondii oocysts in drinking water. Applied and Environment Microbiology. 1998;64:2278–80. doi: 10.1128/aem.64.6.2278-2280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Marchiondo AA, Duszynski DW, Maupin GO. Prevalence of antibodies to Toxoplasma gondii in wild and domestic animals of New Mexico, Arizona, and Colorado. Journal of Wildlife Diseases. 1976;12:226–31. doi: 10.7589/0090-3558-12.2.226. [DOI] [PubMed] [Google Scholar]
- 210.Aramini JJ, Stephen C, Dubey JP, Engelstoft C, Schwantje H, Ribble CS. Potential contamination of drinking water with Toxoplasma gondii oocysts. Epidemiology and Infection. 1999;122:305–15. doi: 10.1017/s0950268899002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Patton S, Rabinowitz A, Randolph S, Johnson SS. A coprological survey of parasites of wild neotropical felidae. Journal of Parasitology. 1986;72:517–20. [PubMed] [Google Scholar]
- 212.Pizzi HL, Rico CM, Pessat OAN. Hallazgo del ciclo ontogenico selvatico del Toxoplasma gondii en felidos salvages (Oncifelis geofroyi Felis colocolo y Felis eira) de la Provincia de Cordoba. Revista Militar de Veterinaria. 1978;25:293–300. [Google Scholar]
- 213.Patton S, Rabinowitz AR. Parasites of wild felidae in Thailand: a coprological study. Journal of Wildlife Diseases. 1994;30:472–5. doi: 10.7589/0090-3558-30.3.472. [DOI] [PubMed] [Google Scholar]
- 214.Akuzawa M. Hematological and parasitological study of the Iriomote cat (Prionailurus iriomotensisi) Canadian Journal of Zoology. 1987;65:946–8. [Google Scholar]
- 215.IUCN. The IUCN red list of threatened species. [accessed 01.06.12];Version 2011. 2012 2 http://www.iucnredlist.org. [Google Scholar]