Abstract
There is growing evidence of heterogeneity among responses to bitter stimuli at the peripheral, central and behavioral levels. For instance, the glossopharyngeal (GL) nerve and neurons receiving its projections are more responsive to bitter stimuli than the chorda tympani (CT) nerve, and this is particularly true for some bitter stimuli like PROP & cycloheximide that stimulate the GL to a far greater extent. Given this information, we hypothesized that cutting the GL would have a greater effect on behavioral avoidance of cycloheximide and PROP than quinine and denatonium, which also stimulate the CT, albeit to a lesser degree than salts and acids. Forty male SD rats were divided into 4 surgery groups: bilateral GL transection (GLX), chorda tympani transection (CTX), SHAM surgery, and combined transection (CTX + GLX). Post-surgical avoidance functions were generated for the 4 bitter stimuli using a brief-access test. GLX significantly compromised avoidance compared to both CTX and SHAM groups for all stimuli (p < .02), while CTX and SHAM groups did not differ. Contrary to our hypothesis, GLX had a greater effect on quinine than cycloheximide (mean shift of 1.02 vs. 0.27 log10 units). Moreover, combined CTX + GLX transection shifted the concentration-response function further than GLX alone for every stimulus except cycloheximide (p’s < .03), suggesting that the GSP nerve is capable of maintaining avoidance of this stimulus to a large degree. This hypothesis is supported by reports of cycloheximide-responsive cells with GSP-innervated receptive fields in the NST and PBN.
Keywords: bitter, glossopharyngeal, taste, GSP, brief-access test
INTRODUCTION
Recent evidence suggests that, as in humans (Behrens, Foerster, Staehler, Raguse, & Meyerhof, 2007; Delwiche, Buletic, & Breslin, 2001; McBurney, Smith, & Shick, 1972), responses to taste stimuli considered “bitter” to rodents may be of a heterogeneous nature. Differences across responses to bitter stimuli exist at the level of the taste receptor cell (Caicedo & Roper, 2001; Hacker, Laskowski, Feng, Restrepo, & Medler, 2008), gustatory nerves (Dahl, Erickson, & Simon, 1997; Damak et al., 2006; Danilova & Hellekant, 2003; Frank, Bouverat, MacKinnon, & Hettinger, 2004; Kuwabara, Shiraishi, & Tateda, 1970; Ninomiya, Kajiura, Ishibashi, & Imai, 1994), central nervous system (Geran & Travers, 2006, 2009) and behavior (Brasser, Mozhui, & Smith, 2005; Frank, Bouverat, MacKinnon, & Hettinger, 2004). This is not to say that significant overlap does not occur among bitter stimuli, and in fact some stimuli appear more similar than others. For instance, quinine and denatonium often activate the same units (Dahl, Erickson, & Simon, 1997; Damak et al., 2006; Geran & Travers, 2009; Lemon & Smith, 2005) and result in similar behavioral responses (Brasser, Mozhui, & Smith, 2005; Dotson, Roper, & Spector, 2005; Frank, Bouverat, MacKinnon, & Hettinger, 2004; Spector & Kopka, 2002), while quinine and cycloheximide, appear less similar both at the neural and behavioral levels (see also Danilova & Hellekant, 2003), although discrimination has not been explicitly tested. For instance, in a previous experiment recording taste responses from single gustatory neurons in the rat parabrachial nucleus (PBN), we found that broadly-tuned neurons responding optimally to acids and sodium salts (“AN” neurons) often had small sideband responses to quinine and denatonium but did not respond to cycloheximide and PROP. In contrast, neurons responding maximally to bitters (“B-best” neurons) not only had larger responses to quinine and denatonium than AN neurons, but were also activated by PROP and cycloheximide (Geran & Travers, 2009). In addition to these differences in chemospecificity, most B-best cells had receptive fields located in the foliate papillae, a region innervated by the glossopharyngeal (GL) nerve, whereas the receptive field for the majority of AN neurons were situated in the chorda tympani (CT)-innervated fungiform papillae (Geran & Travers, 2009; Travers & Geran, 2009).
From this finding, we hypothesized that the GL nerve, which innervates taste buds in the foliate and circumvallate papillae, would be more important for the avoidance of PROP and cycloheximide than for quinine and denatonium, which were capable of stimulating the CT nerve as well as the GL. Prior studies have shown that rats given pre-surgical experience with quinine were able to use either the GL or CT to maintain normal behavioral avoidance of this stimulus (St John, Garcea, & Spector, 1994). However, when naïve rats were used, GL transection (GLX) resulted in a significant increase in avoidance threshold while CTX was without effect, suggesting that the GL is important for unconditioned avoidance of bitter stimuli (Markison, St John, & Spector, 1999). We used a between-subjects design, similar to that used by Markison et al. (1999), where rats were only given access to bitter stimuli after surgery. This was done to reduce potential confounds due to experience and to better assess the effect of GLX. Forty rats were divided into four surgical groups (bilateral CT, GL, CT + GL, or SHAM transection) and tested for unconditioned avoidance to several concentrations of four bitter stimuli (quinine, denatonium, PROP and cycloheximide). Our findings show that the GL is necessary for normal unconditioned avoidance across an array of bitter stimuli and further indicate that input from the CT and greater superficial petrosal (GSP) nerves can help guide performance in the absence of the GL. However, whether the CT or GSP is more important for residual avoidance after GLX depends upon which bitter stimulus is used.
METHODS
Subjects
Forty adult male Sprague-Dawley (Harlan) rats were split into 4 groups; chorda tympani transected (CTX), glossopharyngeal transected (GLX), sham (SHAM) and combined transection (CTX + GLX). All rats weighed an average of 293 ± 3 g prior to testing and all procedures were performed in accordance with the Institutional Laboratory Care and Use Committee (ILACUC) at the Ohio State University. Animals were run in 4 phases of 10 rats each and access to water was restricted in order to encourage licking in the chamber. Throughout the experiment, subjects were weighed daily and closely monitored to assess hydration status. None of the subjects dropped below 90% of their weekly ad libitum body weight or showed other signs of illness or distress. Each of the 4 bitter stimuli tested required 1 week of testing. All stimuli were purchased from Sigma Chemical (St. Louis, MO).
Spout adaptation
All training and testing sessions took place in the Davis rig (MS-160 lickometer, DiLog Instruments, Tallahassee FL). Water bottles were removed from home cages in the afternoon and training (spout adaptation) began in the Davis rig approximately 18 h later. On Day 1 of adaptation, animals were given free access to distilled water from a single stationary spout for a period of 30 min. On Day 2, the session was extended to 40 min and water trials alternated in duration between 2 and 5s to mimic the testing session. Intertrial interval was set at 10s. Two rats failed to adequately learn the task on Day 2 (i.e. initiated half as many trials as the other subjects) and were given an additional training session approximately 2 hours later. Water bottles were then replaced and the animals were allowed ad libitum access for 2–3 days prior to surgery. Subjects were counterbalanced for surgical group based on ad libitum body weight and total number of trials initiated on Day 2 of spout training.
Surgery
Animals were deeply anesthetized with a ketamine/xylazine mixture (85:13 mg/kg) injected intraperitoneally. A heating pad and telethermometer were used to maintain body temperature at approximately 37 °C. For rats receiving bilateral CTX, the pinna was retracted, the tympanic membrane punctured and the ossicle and underlying CT nerve cauterized at the points where the nerve entered and exited the bulla and along the rim of the ear canal to stimulate cerumen production (St John, Markison, Guagliardo, Hackenberg, & Spector, 1997). The ossicle was then removed with forceps. For rats with bilateral GLX, an incision was made in the ventral neck, the salivary glands and musculature retracted and approximately 10 mm of the GL nerve excised using microscissors and forceps (King, Garcea, & Spector, 2000; Spector & Grill, 1992). The wound was then closed with sterile clips. Subjects in the SHAM surgery group had the pinna retracted, tympanic membrane punctured, ventral incision in the neck, and exposure of the GL on either side. Rats in the double cut (CTX+GLX) group received both CTX and GLX as described above. All rats, regardless of surgery group, were given 1 dose of carprofen (5 mg/kg, s.c.) for pain control immediately after surgery and ampicillin (100 mg/kg s.c.) for 3 days following surgery. Rats were given 1 week to recover from surgery prior to testing. During this time rats were given free access to food and water.
Post-surgical testing
For each of the 4 weeks of post-surgical testing, water bottles were removed from home cages on Sunday afternoon approximately 18 hours prior to the start of testing on Monday morning. Each Monday, the test session mimicked Day 2 of pre-surgical spout adaptation, with distilled water as the only stimulus. This was done to remind the rats of the test parameters. Stimulus testing occurred Tuesday through Friday with a different bitter stimulus tested each week. The subjects were tested in randomized blocks of eight with 7 concentrations of a bitter stimulus and 1 distilled water stimulus. Each test trial lasted 5s and was preceded by a 2s distilled water rinse to reduce potential adaptation from one test stimulus to the next and to encourage sampling. Subjects typically initiated between 120 and 130 trials per session, with 7 to 8 trials for each stimulus concentration. One bitter stimulus was tested each week. Stimulus order was counterbalanced across rats to avoid order effects, with the exception that cycloheximide was the last of the 4 stimuli tested for each group due to potential post-ingestive cues related to its toxicity (see Hettinger, Formaker, & Frank, 2007). The concentration ranges for each stimulus were picked such that the highest concentration produced an equal number of gapes in prior studies (see Chan, Yoo, & Travers, 2004; Geran & Travers, 2006). Concentrations tested include 0.003, 0.03, 0.01, 0.1, 0.3, 1 and 3 mM quinine-HCl, 0.01, 0.03, 0.1, 0.3, 1, 3 and 7 mM PROP, 0.01, 0.03, 0.1, 0.3, 1, 3 and 10 mM denatonium benzoate, and 0.01, 0.03, 0.1, 0.3, 1, 3 and 10 uM cycloheximide. A fan was positioned directly over the stimulus access slot facing downward in an attempt to lessen potential odor cues related to the stimuli. Tube placement was also changed daily so that rats could not learn to associate sound cues with a particular stimulus concentration. Rats received ad libitum home cage access to distilled water every Friday afternoon through Sunday afternoon.
Histology
Following testing, rats were deeply anesthetized and perfused transcardially with buffered saline and formalin. Tongues were kept in formalin until the tissue could be processed and the taste pores, taste buds and fungiform papillae counted to confirm transection. Taste pores in fungiform papillae were stained with methylene blue and counted under a light microscope to confirm CTX (see Parks & Whitehead, 1998; Spector, Schwartz, & Grill, 1990) while circumvallate tissue was embedded in paraffin, cut into 5 −10 um sections, mounted and stained using a hemoxylin/eosin procedure to confirm GLX (Guth, 1957; Spector & Grill, 1992). A taste bud was counted in the circumvallate papilla if there was a visible taste pore or a group of well-organized elongated cells very close to the trench, suggesting the apical taste pore was just out of the plane of section (Geran, Garcea, & Spector, 2004).
Data Analysis
The number of licks taken by each subject was averaged together across the 4-day test period (Tuesday through Friday) for each of the 7 stimulus concentrations and for the 5-s water stimulus. Trials without licks were removed from analysis. The mean number of licks for each stimulus concentration was then divided by the mean number of licks to water for that animal (see St John, Garcea, & Spector, 1994). Thus, the Avoidance Ratio Score was adjusted for the lick rate of each subject.
Avoidance ratio scores were then plotted and a 3-parameter logistic function (see below) fitted to the data for each subject and bitter stimulus such that the inflection points (or c-values) could be analyzed statistically.
Where a = maximum asymptote, b = slope, c = stimulus concentration corresponding to a lick ratio score of 0.5, x = stimulus concentration. Performance for some subjects became so compromised by surgery that in some cases the data could not be fit to a logistic function (see Geran, Garcea, & Spector, 2004). These cases included 1 subject in the GLX group and 4 subjects in the CTX+GLX group for quinine performance and 1 subject in the CTX+GLX group for denatonium performance. These 6 cases were not used in the analyses of curve parameters. It was also often necessary to extrapolate c-values in order to fit curves to the flattened quinine and denatonium performance of subjects in the CTX+GLX group, making them less accurate than parameters for the other surgical and stimulus conditions.
One- and two-way analyses of variance (ANOVAs), Tukey’s post-hoc analyses, two-group and paired t-tests and Pearson’s correlations were performed where appropriate. Statistical significance was set at p <.05.
RESULTS
Behavior
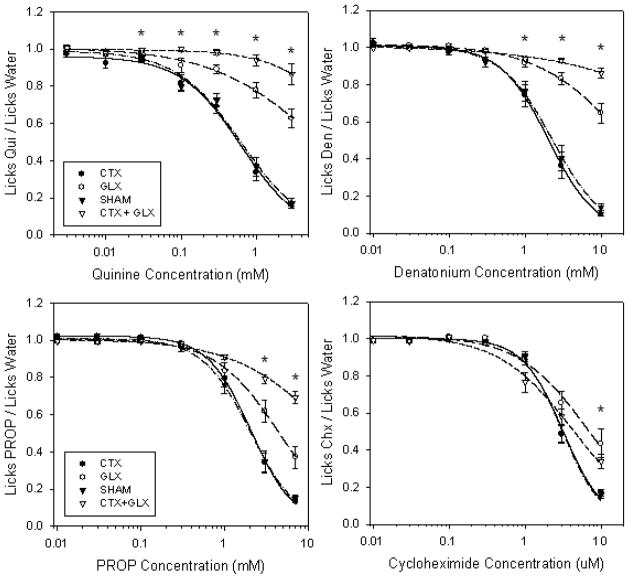
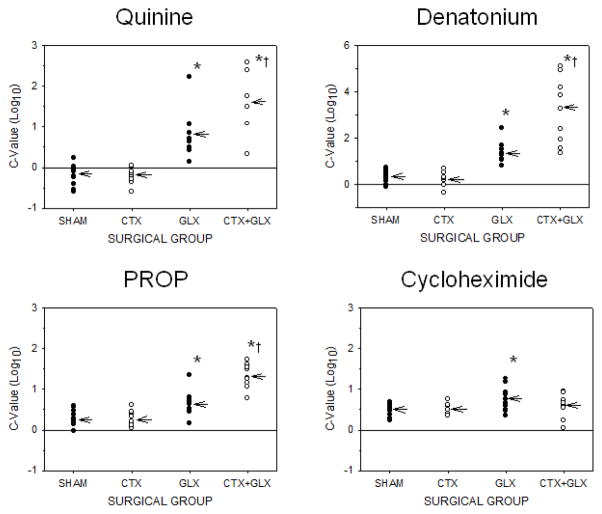
Two-way ANOVAs (concentration x surgical group) performed on individual lick ratios from all subjects indicated that performance for all 4 bitter stimuli was significant for each main term and for the interaction (p’s < .04). Bonferroni adjusted post-hoc tests showed a group effect at the 5 highest quinine concentrations (0.03, 0.1, 0.3, 1 & 3 mM; p’s < .0071), the 3 highest denatonium concentrations (1, 3 & 10mM; p’s < .003), the 2 highest PROP concentrations (3 & 7 mM; p < .001), and the highest concentration of cycloheximide (10 uM; p < .001). Logistic curves were fit to the lick ratio data with a mean R2 value of 0.97 ± .004. One-way ANOVAs for the 4 surgical groups indicated that the c-value differed significantly for 3 of the 4 bitter stimuli tested (F(3,30–35) > 24.0, p < .001 for each). See Figure 1. The ANOVA for cycloheximide performance was not significant when all 4 surgical groups were included, but did reach significance when the CTX+GLX group was dropped from analysis (F(2,26)=5.05, p< .02). A post-hoc Tukey’s test revealed the GLX group had significantly higher cycloheximide avoidance thresholds than either CTX or SHAM rats (p’s < .04). Post-hoc Tukey’s HSD tests for the remaining 3 bitter stimuli (quinine, denatonium and PROP) across all 4 surgical groups also indicated that CTX and SHAM groups were similar for each, but the GLX group had a higher avoidance threshold (p < .03 for each) than either CTX or SHAM (Table 1). The double cut group (CTX + GLX) showed an even greater effect on avoidance than GLX alone for the same 3 stimuli (p < .03 for each, Figure 2). When comparing performance to the SHAM condition for each stimulus and surgical group, we found that GLX and CTX+GLX produced the greatest mean increases in threshold for quinine (1.02 & 1.80 log10 units, respectively) and denatonium (1.01 & 2.84 log10 units), with the effect on PROP (0.36 & 1.02 log10 units) and cycloheximide (0.27 & 0.14 log10 units) commonly less than half these amounts (see Figure 2).
Figure 1.
Mean concentration-response functions for all surgical groups and stimuli. Note that GLX (open circles) had a greater impact on performance on the quinine and denatonium tests (top) than PROP and cycloheximide (bottom). Combined CTX + GLX transection (open triangles) had an even greater effect on avoidance than GLX alone for all stimuli except cycloheximide. CTX was not different from SHAM for any stimulus tested. Asterisks represent significance in a 2-way (surgical group x concentration) ANOVA.
Table 1.
Mean avoidance threshold (c-values) ± standard error by surgery group
| Surgical groups | ||||
|---|---|---|---|---|
| Stimuli | SHAM | CTX | GLX | CTX+GLX |
| Quinine HCl | −0.19 ±.08 log10 | −0.22±.07 log10 | 0.83±.20 log10 | 1.61±.34 log10 |
| Denatonium | 0.34 ±.09 log10 | 0.25±.10 log10 | 1.34±.15 log10 | 3.18±.48 log10 |
| PROP | 0.30 ±.07 log10 | 0.29±.06 log10 | 0.66±.10 log10 | 1.32±.09 log10 |
| Cycloheximide | 0.49 ±.05 log10 | 0.50±.04 log10 | 0.76±.10 log10 | 0.63±.09 log10 |
Figure 2.
Individual c-values for each surgical condition grouped by stimulus. Arrows represent means for each. Change in avoidance threshold was more pronounced with GLX and CTX+GLX for quinine and denatonium (top) than for PROP and cycloheximide (bottom). Note that the y-axis scale for denatonium is different from that of the other stimuli. Asterisks represent a significant difference from SHAM in a one-way ANOVA, while crosses represent a significant increase compared to GLX (p <.05 for each). Cycloheximide significance is based on a one-way ANOVA across 3 surgical groups instead of 4. See Results section for description.
One-way ANOVAs were also performed across groups on the slope parameter (b-value) for each of the 4 stimuli (F(3,28) > 2.6, p < .05 for all). Post hoc analyses revealed the slope was significantly lower for CTX+GLX than for either CTX or SHAM for the PROP, cycloheximide and denatonium tasks (p’s < .04). Furthermore, in the cycloheximide and denatonium conditions GLX was also significantly lower than either CTX or SHAM (p < .02 for each), indicating a flatter curve. Post hoc tests did not reveal any significant differences between surgery groups for quinine slope, even though the ANOVA was significant.
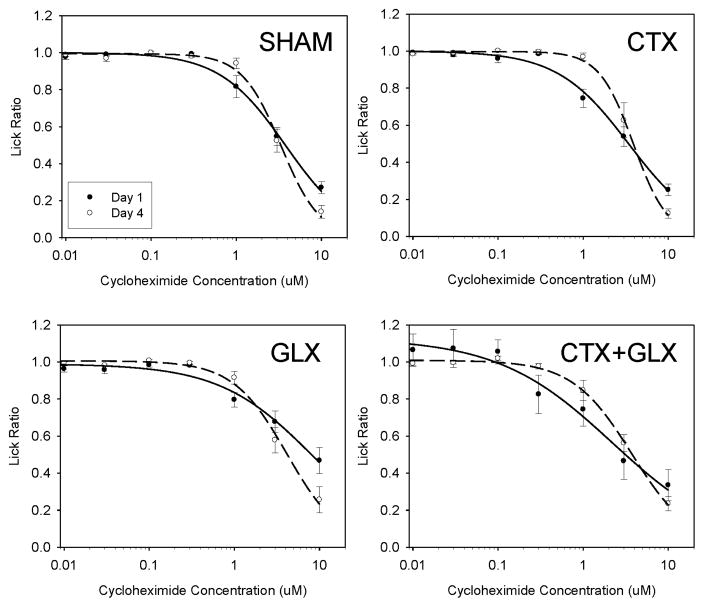
Because nerve transection had less of an effect on cycloheximide than the other bitters, we also wanted to determine whether postingestive or other non-gustatory factors might have influenced licking to this stimulus. To this end, we fitted curves to data from the first day of cycloheximide testing for each animal and compared the c-values for each surgical group to that generated from the mean lick ratio scores for the last day of cycloheximide testing using paired t-tests. Avoidance threshold did not decrease significantly over the course of testing for any surgical group (p > .11. See Figure 3).
Figure 3.
Cycloheximide Day 1 (closed symbols) means vs. Day 4 means (open) for each surgery group. Avoidance thresholds did not significantly improve over the course of testing for any of the four surgical groups as assessed by paired t-tests, suggesting that cycloheximide performance was not affected by postingestive conditioning.
Histology
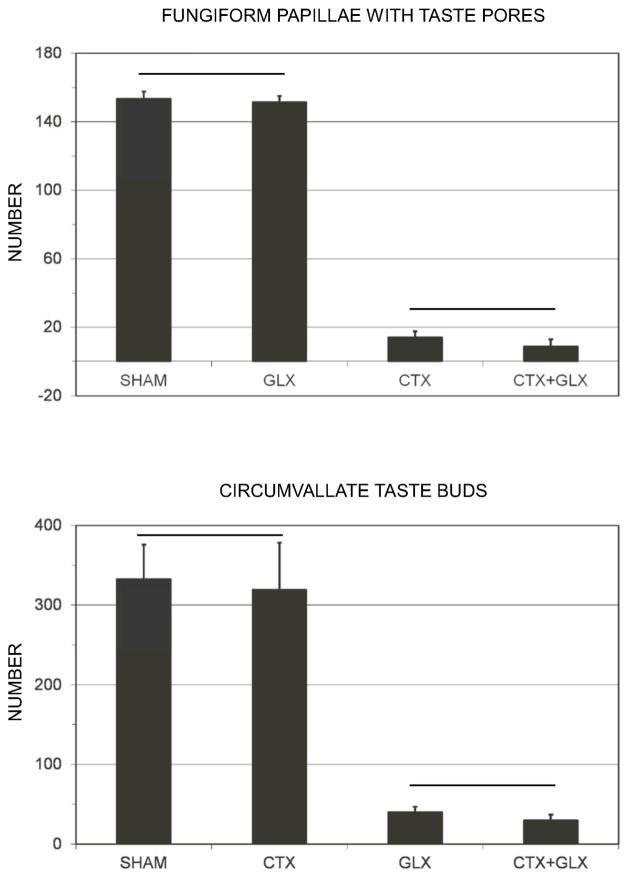
One of the 20 rats with chorda tympani transection (10 CTX and 10 CTX+GLX) had round, well-stained pores in over 90% of fungiform papillae indicating incomplete CT transection (Spector, Schwartz, & Grill, 1990). This animal was dropped from analysis leaving an n=9 for the CTX group. An average of 36.9 ± 0.2 days passed between surgery and perfusion for the 39 rats analyzed. Similar methods were used to achieve transection without significant regeneration for over 74 days previously (Geran, Garcea, & Spector, 2004). One-way ANOVAs for number of fungiform papillae and percentage of intact pores were significant across the 4 surgery groups (F(3,35) > 16, p < .001 for both). Post-hoc testing with Tukey’s HSD test indicated that the CTX and CTX+GLX groups had both significantly fewer fungiform papillae and a smaller percentage of intact fungiform pores than either the GLX or SHAM conditions (p < .007 for all). See Figure 4. The circumvallate papilla, innervated by the GL nerve, also showed significant differences in number of taste buds across groups (F(3,35) = 22.8, p < .001). A post-hoc Tukey’s comparison revealed significantly fewer circumvallate taste buds in the GLX and CTX+GLX groups compared to either the CTX or SHAM conditions (p < .001 for all). Taste bud loss was bilateral for all subjects.
Figure 4.
Mean ± SE number of taste pores located in the fungiform papillae (above) and number of intact circumvallate taste buds (below) for each surgical group. Fungiform papillae are located on the anterior tongue and innervated by the chorda tympani (CT) branch of cranial nerve VII. The circumvallate papilla is located on the posterior tongue and innervated by the glossopharyngeal nerve (GL), cranial nerve IX. Horizontal lines indicate that groups under the line were not significantly different from one another. All other comparisons were significant (p<.05).
DISCUSSION
Glossopharyngeal nerve transection significantly compromised unconditioned avoidance of all 4 bitter stimuli tested, while CT transection was without effect. This result is consistent with the difference in response magnitude to bitter stimuli observed in these nerves (Dahl, Erickson, & Simon, 1997; Damak et al., 2006; Danilova & Hellekant, 2003; Frank, Bouverat, MacKinnon, & Hettinger, 2004; Yamada, 1966) and in bitter-responsive brainstem neurons with foliate vs. fungiform receptive fields (Geran & Travers, 2006, 2009). The current results support those of a previous study where GLX compromised unconditioned quinine avoidance (Markison, St John, & Spector, 1999) and extend this finding to other bitters. Quinine avoidance thresholds for SHAM rats were similar to those previously reported (Geran, Garcea, & Spector, 2004; Markison, St John, & Spector, 1999), however GLX compromised quinine performance to a larger degree than that observed in the Markison study (mean shift = 1.01 log10 units compared to 0.44 from Markison et al., 1999). This is likely due to differences in parameters such as trial duration (5s in this task vs. 10s previously). When both the GL and CT were cut simultaneously, avoidance threshold increased still further (by 1.8 log10 units). This is somewhat larger than the within-subject shifts of 1.18 and 1.35 log10 units reported for CTX + GLX rats with pre-surgical quinine experience (Geran, Garcea, & Spector, 2004; St John, Garcea, & Spector, 1994), suggesting that the remaining quinine avoidance with combined CT+GL transection, presumably due to GSP input, may be improved with presurgical conditioning. However, given that these are separate studies with differing parameters we cannot say for sure.
We found that the effects of transection on denatonium mirrored those observed for quinine. This is not surprising given that the 2 stimuli have been reported to taste quite similar to rodents (Frank, Bouverat, MacKinnon, & Hettinger, 2004; Spector & Kopka, 2002) and also to stimulate similar groups of nerve fibers and neurons (see Dahl, Erickson, & Simon, 1997; Geran & Travers, 2009; Lemon & Smith, 2005) - although differences have also been reported (Geran & Travers, 2006). Although the effectiveness of GLX varied across bitter stimuli, the results were contrary to our hypothesis, as this manipulation had less of an effect on PROP and cycloheximide than on quinine and denatonium (see Figures 1 & 2). The effects of CT transection were more consistent with our initial hypothesis, in that CTX alone had a negligible impact on unconditioned avoidance regardless of stimulus, but in combination with GLX magnified these deficits for bitter compounds shown to stimulate the CT (i.e. quinine and denatonium). We were somewhat surprised, however, that CTX+GLX also compromised PROP performance compared to GLX alone given the small proportion of T2Rs in the fungiform papillae compared to other gustatory fields (Adler et al., 2000) and the lack of PROP responsiveness generally reported for this nerve and neurons receiving its projections in rodents (Damak et al., 2006; Danilova & Hellekant, 2003; Geran & Travers, 2009). Perhaps in the absence of the GL, this response however slight becomes more important for taste-guided behavior. Nevertheless, denervating taste receptor cells on the anterior tongue did not affect cycloheximide performance, consistent with previous reports from the CT nerve and associated neurons (Damak et al., 2006; Danilova & Hellekant, 2003; Frank, Bouverat, MacKinnon, & Hettinger, 2004; Travers & Geran, 2009).
The comparatively small effects of GLX and CTX+GLX on cycloheximide and to a lesser extent PROP made us wonder whether non-gustatory factors such as aversive olfactory or somatosensory cues could have contributed to behavioral avoidance. Hettinger and colleagues (2007) reported that hamsters with prior cycloheximide experience showed no intake suppression when given the pungent breakdown products of 500 uM cycloheximide in a 1 bottle, 1-hour test but showed strong suppression to cycloheximide itself. Pilot studies in our laboratory using the same alkalizing procedure described by Hettinger (2007), likewise found that the breakdown products of cycloheximide failed to suppress licking in rats either before or after cycloheximide experience (see Figure 5). This suggests that it was not the olfactory component of this stimulus, or even the association between olfactory and postingestive factors that produced cycloheximide avoidance in the brief-access test. We were also concerned that because T2Rs are located in the gastrointestinal tract as well as the oral cavity (Wu et al., 2002), over time they could contribute to avoidance via post-ingestive conditioning. Glendinning and colleagues (2008) found that pairing intragastric administration of the T2R ligand denatonium with an oral stimulus made that stimulus less preferred. In addition, injection of a highly toxic bitter substance has been shown to work as an unconditioned stimulus; reducing intake of associated gustatory stimuli even without stimulating T2Rs in the oral cavity or gut (St John, Pour, & Boughter, 2005). Given that cycloheximide is both a T2R ligand and toxic to rats at higher concentrations (Chandrashekar et al., 2000; Hettinger, Formaker, & Frank, 2007), we wanted to determine whether such conditioning could have affected performance to this stimulus. To accomplish this, we compared Day 1 of cycloheximide testing to Day 4 for each surgical group using paired t-tests. We found that mean avoidance thresholds (c-values) were similar for each group (p > .11 for each). Thus, performance did not improve over the course of testing; strongly suggesting that cycloheximide avoidance was not due to postingestive factors.
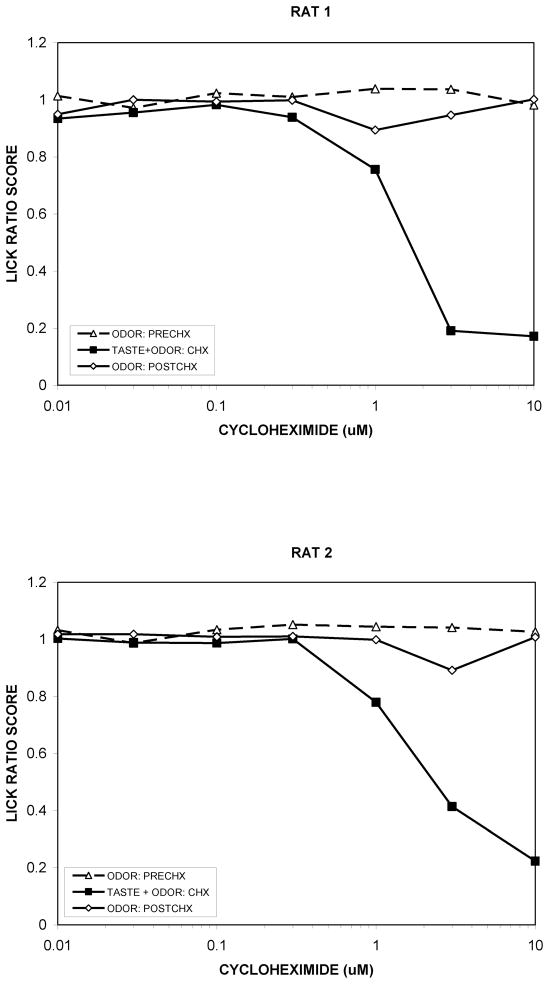
Figure 5.
Pilot data from 2 intact rats trained on quinine for 10 weeks then exposed to the tasteless but pungent breakdown products of cycloheximide for 4 days (open triangles with dotted line), cycloheximide (CHX) itself (filled squares), and the breakdown products again (open circles). This was done to determine whether rats find the odor associated with cycloheximide aversive either with or without exposure to its taste. Cycloheximide was alkalized to produce the stimulus on “ODOR” days as described in (Hettinger, Formaker, & Frank, 2007).
The fact that cycloheximide was tested last (all other stimuli were counterbalanced for order of presentation) and least affected by the surgical manipulations, suggested that perhaps nerve regeneration in the affected fields was responsible for avoidance, even though similar numbers of taste buds were unable to maintain quinine performance in previous tasks (Geran, Garcea, & Spector, 2004; King, Garcea, & Spector, 2000). To test this hypothesis, we performed Pearson’s correlations on the number of taste buds in each papillary field and cycloheximide performance for each surgical group. No significant inverse correlations (i.e. low c-value: high number of taste buds) were found. Consequently, taste bud regeneration does not appear to be a factor in cycloheximide performance, although it is possible that other mechanisms may have occurred over the course of testing which compensated for the loss of taste buds. These could include an upregulation of T2Rs or other receptor elements in innervated tissue or changes to central mechanisms (see Blonde, Jiang, Garcea, & Spector, 2010; Green & George, 2004; Hill & Phillips, 1994).
Although the possibility remains that somatosensory cues could play a role, it appears that the most likely explanation for the residual avoidance of cycloheximide and PROP following CTX+GLX is that the intact greater superficial petrosal (GSP) nerve was able to maintain this behavior to a large degree. The GSP, a branch of CN VII, innervates taste buds in the nasoincisor ducts and soft palate and is most responsive to sweeteners, although activity to quinine has also been reported (Harada, Yamamoto, Yamaguchi, & Kasahara, 1997; Sollars & Hill, 1998, 2005). To our knowledge, electrophysiology has not been performed on this nerve with other bitter stimuli. However, as in the GL-innervated circumvallate and foliate papillae, immunohistochemistry revealed T2R bitter receptors and gustducin (Miura et al., 2007) in every taste bud of the GSP-innervated geschmackstreiffen region of the soft palate (Adler et al., 2000). Moreover, our recordings from the PBN identified neurons with GSP-innervated nasoincisor duct receptive fields that responded optimally to bitter stimuli, including cycloheximide and PROP (Geran & Travers, 2009; Travers & Geran, 2009). Thus, perhaps it is more surprising that CTX+GLX had such a profound effect on quinine and denatonium performance since the central neurophysiology suggests these compounds activate the same GSP-innervated PBN neurons that respond to PROP and cycloheximide (Geran & Travers, 2009). Perhaps the answer to this discrepancy lies in the fact that these bitter-responsive neurons also frequently receive convergent input from the GL. Given the GL’s putative role in unconditioned negative hedonic function, (see Spector, 2003; St John & Spector, 1998) it is possible that in the absence of GL input, ionic bitters like quinine become less discriminable from the salts and acids stimulating many of the same neurons and hence are less avoided until the subjects have enough experience with the altered quinine signal to respond appropriately to it. In a study using rats with presurgical bitter experience, CTX+GSPX significantly compromised quinine performance compared to CTX alone (St John, Garcea, & Spector, 1994) suggesting that GSP input can help guide quinine avoidance when the GL is intact. Furthermore, a recent study showed that CTX+GLX rats initially unable to perform a salt discrimination task were able to re-learn the task over several weeks using input from the GSP (Blonde, Jiang, Garcea, & Spector, 2010). Perhaps taste-guided behavior to quinine and/or denatonium would also improve in CTX+GLX subjects given sufficient time and experience.
As in previous experiments, the current results suggest bitter stimuli consist of a heterogeneous group, with the distinction between responses to quinine/denatonium and those to cycloheximide particularly apparent. We know that T2Rs often have overlapping molecular ranges and bind to more than one ligand (see Behrens & Meyerhof, 2009; Meyerhof et al., 2010), that bitter responses have been observed in the absence of functioning T2Rs (Damak et al., 2006; Dotson, Roper, & Spector, 2005; Sawano, Seto, Mori, & Hayashi, 2005), and that variation in anatomical features like receptor cell density have been shown to affect behavioral sensitivity to bitter compounds (Hayes, Bartoshuk, Kidd, & Duffy, 2008). Given this information, it is likely that differential expression of bitter receptors across gustatory fields leads to the heterogeneity observed in this and other papers.
Unlike the GL, the CT nerve appears to be important for fine taste discriminations and learned behaviors (Spector, 2003; St John & Spector, 1998). Perhaps this nerve responds to less toxic, and in some cases even adaptive bitter stimuli such as quinine (see Vitazkova, Long, Paul, & Glendinning, 2001), so that when food is scarce these can be discriminated from more toxic stimuli like cycloheximide that do not appreciably stimulate this nerve. Our findings indicate that the GL is necessary for unconditioned avoidance of bitter stimuli, regardless of which other nerves might also be activated, and suggest that the GSP plays a greater role in bitter taste function and/or unconditioned avoidance than previously considered.
Acknowledgments
The authors wish to thank Pumla Pamla-Gutter, Tamara Kolli, Scott Herness and Mary Lloyd for help with histology and Nicole Kinzeler for advice on the manuscript. This research was funded by the National Institute on Deafness and Other Communication Disorders (NIDCD) grants R03-DC008678 (LCG) and R01-000416 (SPT).
Literature Cited
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100(6):693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Behrens M, Foerster S, Staehler F, Raguse JD, Meyerhof W. Gustatory expression pattern of the human TAS2R bitter receptor gene family reveals a heterogenous population of bitter responsive taste receptor cells. J Neurosci. 2007;27(46):12630–12640. doi: 10.1523/JNEUROSCI.1168-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens M, Meyerhof W. Mammalian bitter taste perception. Results Probl Cell Differ. 2009;47:203–220. doi: 10.1007/400_2008_5. [DOI] [PubMed] [Google Scholar]
- Blonde G, Jiang E, Garcea M, Spector AC. Learning-based recovery from perceptual impairment in salt discrimination after permanently altered peripheral gustatory input. Am J Physiol Regul Integr Comp Physiol. 2010;299(4):R1027–1036. doi: 10.1152/ajpregu.00843.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasser SM, Mozhui K, Smith DV. Differential covariation in taste responsiveness to bitter stimuli in rats. Chem Senses. 2005;30(9):793–799. doi: 10.1093/chemse/bji071. [DOI] [PubMed] [Google Scholar]
- Caicedo A, Roper SD. Taste receptor cells that discriminate between bitter stimuli. Science. 2001;291(5508):1557–1560. doi: 10.1126/science.291.5508.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CY, Yoo JE, Travers SP. Diverse bitter stimuli elicit highly similar patterns of Fos-like immunoreactivity in the nucleus of the solitary tract. Chem Senses. 2004;29(7):573–581. doi: 10.1093/chemse/bjh062. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, et al. T2Rs function as bitter taste receptors. Cell. 2000;100(6):703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Dahl M, Erickson RP, Simon SA. Neural responses to bitter compounds in rats. Brain Res. 1997;756(1–2):22–34. doi: 10.1016/s0006-8993(97)00131-5. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Perez CA, Shigemura N, et al. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31(3):253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- Danilova V, Hellekant G. Comparison of the responses of the chorda tympani and glossopharyngeal nerves to taste stimuli in C57BL/6J mice. BMC Neurosci. 2003;4:5. doi: 10.1186/1471-2202-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche JF, Buletic Z, Breslin PA. Covariation in individuals’ sensitivities to bitter compounds: evidence supporting multiple receptor/transduction mechanisms. Percept Psychophys. 2001;63(5):761–776. doi: 10.3758/bf03194436. [DOI] [PubMed] [Google Scholar]
- Dotson CD, Roper SD, Spector AC. PLCbeta2-independent behavioral avoidance of prototypical bitter-tasting ligands. Chem Senses. 2005;30(7):593–600. doi: 10.1093/chemse/bji053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank ME, Bouverat BP, MacKinnon BI, Hettinger TP. The distinctiveness of ionic and nonionic bitter stimuli. Physiol Behav. 2004;80(4):421–431. doi: 10.1016/j.physbeh.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Geran LC, Garcea M, Spector AC. Nerve regeneration-induced recovery of quinine avoidance after complete gustatory deafferentation of the tongue. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1235–1243. doi: 10.1152/ajpregu.00137.2004. [DOI] [PubMed] [Google Scholar]
- Geran LC, Travers SP. Single neurons in the nucleus of the solitary tract respond selectively to bitter taste stimuli. J Neurophysiol. 2006;96(5):2513–2527. doi: 10.1152/jn.00607.2006. [DOI] [PubMed] [Google Scholar]
- Geran LC, Travers SP. Bitter-responsive gustatory neurons in the rat parabrachial nucleus. J Neurophysiol. 2009;101(3):1598–1612. doi: 10.1152/jn.91168.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning JI, Yiin YM, Ackroff K, Sclafani A. Intragastric infusion of denatonium conditions flavor aversions and delays gastric emptying in rodents. Physiol Behav. 2008;93(4–5):757–765. doi: 10.1016/j.physbeh.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, George P. ‘Thermal taste’ predicts higher responsiveness to chemical taste and flavor. Chem Senses. 2004;29(7):617–628. doi: 10.1093/chemse/bjh065. [DOI] [PubMed] [Google Scholar]
- Guth L. The effects of glossopharyngeal nerve transection on the circumvallate papilla of the rat. Anat Rec. 1957;128(4):715–731. doi: 10.1002/ar.1091280406. [DOI] [PubMed] [Google Scholar]
- Hacker K, Laskowski A, Feng L, Restrepo D, Medler K. Evidence for two populations of bitter responsive taste cells in mice. J Neurophysiol. 2008;99(3):1503–1514. doi: 10.1152/jn.00892.2007. [DOI] [PubMed] [Google Scholar]
- Harada S, Yamamoto T, Yamaguchi K, Kasahara Y. Different characteristics of gustatory responses between the greater superficial petrosal and chorda tympani nerves in the rat. Chem Senses. 1997;22(2):133–140. doi: 10.1093/chemse/22.2.133. [DOI] [PubMed] [Google Scholar]
- Hayes JE, Bartoshuk LM, Kidd JR, Duffy VB. Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chem Senses. 2008;33(3):255–265. doi: 10.1093/chemse/bjm084. [DOI] [PubMed] [Google Scholar]
- Hettinger TP, Formaker BK, Frank ME. Cycloheximide: no ordinary bitter stimulus. Behav Brain Res. 2007;180(1):4–17. doi: 10.1016/j.bbr.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DL, Phillips LM. Functional plasticity of regenerated and intact taste receptors in adult rats unmasked by dietary sodium restriction. J Neurosci. 1994;14(5 Pt 1):2904–2910. doi: 10.1523/JNEUROSCI.14-05-02904.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CT, Garcea M, Spector AC. Glossopharyngeal nerve regeneration is essential for the complete recovery of quinine-stimulated oromotor rejection behaviors and central patterns of neuronal activity in the nucleus of the solitary tract in the rat. J Neurosci. 2000;20(22):8426–8434. doi: 10.1523/JNEUROSCI.20-22-08426.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara M, Shiraishi A, Tateda H. New Method for Testing Rat Repellents. Appl Ent Zool. 1970;5(2):84–94. [Google Scholar]
- Lemon CH, Smith DV. Neural representation of bitter taste in the nucleus of the solitary tract. J Neurophysiol. 2005;94(6):3719–3729. doi: 10.1152/jn.00700.2005. [DOI] [PubMed] [Google Scholar]
- Markison S, St John SJ, Spector AC. Glossopharyngeal nerve transection reduces quinine avoidance in rats not given presurgical stimulus exposure. Physiol Behav. 1999;65(4–5):773–778. doi: 10.1016/s0031-9384(98)00219-4. [DOI] [PubMed] [Google Scholar]
- McBurney DH, Smith DV, Shick TR. Gustatory cross adaptation: Sourness and bitterness. Percept Psychophys. 1972;11(3):228–232. [Google Scholar]
- Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, et al. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 2010;35(2):157–170. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- Miura H, Nakayama A, Shindo Y, Kusakabe Y, Tomonari H, Harada S. Expression of gustducin overlaps with that of type III IP3 receptor in taste buds of the rat soft palate. Chem Senses. 2007;32(7):689–696. doi: 10.1093/chemse/bjm036. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Kajiura H, Ishibashi T, Imai Y. Different responsiveness of the chorda tympani and glossopharyngeal nerves to L-lysine in mice. Chem Senses. 1994;19(6):617–626. doi: 10.1093/chemse/19.6.617. [DOI] [PubMed] [Google Scholar]
- Parks JD, Whitehead MC. Scanning electron microscopy of denervated taste buds in hamster: morphology of fungiform taste pores. Anat Rec. 1998;251(2):230–239. doi: 10.1002/(SICI)1097-0185(199806)251:2<230::AID-AR12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Sawano S, Seto E, Mori T, Hayashi Y. G-protein-dependent and -independent pathways in denatonium signal transduction. Biosci Biotechnol Biochem. 2005;69(9):1643–1651. doi: 10.1271/bbb.69.1643. [DOI] [PubMed] [Google Scholar]
- Sollars SI, Hill DL. Taste responses in the greater superficial petrosal nerve: substantial sodium salt and amiloride sensitivities demonstrated in two rat strains. Behav Neurosci. 1998;112(4):991–1000. doi: 10.1037//0735-7044.112.4.991. [DOI] [PubMed] [Google Scholar]
- Sollars SI, Hill DL. In vivo recordings from rat geniculate ganglia: taste response properties of individual greater superficial petrosal and chorda tympani neurones. J Physiol. 2005;564(Pt 3):877–893. doi: 10.1113/jphysiol.2005.083741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC. The Functional Organization of the Peripheral Gustatory System: Lessons From Behavior. Progress in Psychobiology and Physiological Psychology. 2003;18:101–161. [Google Scholar]
- Spector AC, Grill HJ. Salt taste discrimination after bilateral section of the chorda tympani or glossopharyngeal nerves. Am J Physiol. 1992;263(1 Pt 2):R169–176. doi: 10.1152/ajpregu.1992.263.1.R169. [DOI] [PubMed] [Google Scholar]
- Spector AC, Kopka SL. Rats fail to discriminate quinine from denatonium: implications for the neural coding of bitter-tasting compounds. J Neurosci. 2002;22(5):1937–1941. doi: 10.1523/JNEUROSCI.22-05-01937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC, Schwartz GJ, Grill HJ. Chemospecific deficits in taste detection after selective gustatory deafferentation in rats. Am J Physiol. 1990;258(3 Pt 2):R820–826. doi: 10.1152/ajpregu.1990.258.3.R820. [DOI] [PubMed] [Google Scholar]
- St John SJ, Garcea M, Spector AC. Combined, but not single, gustatory nerve transection substantially alters taste-guided licking behavior to quinine in rats. Behav Neurosci. 1994;108(1):131–140. doi: 10.1037//0735-7044.108.1.131. [DOI] [PubMed] [Google Scholar]
- St John SJ, Markison S, Guagliardo NA, Hackenberg TD, Spector AC. Chorda tympani transection and selective desalivation differentially disrupt two-lever salt discrimination performance in rats. Behav Neurosci. 1997;111(2):450–459. [PubMed] [Google Scholar]
- St John SJ, Pour L, Boughter JD., Jr Phenylthiocarbamide produces conditioned taste aversions in mice. Chem Senses. 2005;30(5):377–382. doi: 10.1093/chemse/bji032. [DOI] [PubMed] [Google Scholar]
- St John SJ, Spector AC. Behavioral discrimination between quinine and KCl is dependent on input from the seventh cranial nerve: implications for the functional roles of the gustatory nerves in rats. J Neurosci. 1998;18(11):4353–4362. doi: 10.1523/JNEUROSCI.18-11-04353.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers SP, Geran LC. Bitter-responsive brainstem neurons: characteristics and functions. Physiol Behav. 2009;97(5):592–603. doi: 10.1016/j.physbeh.2009.02.042. [DOI] [PubMed] [Google Scholar]
- Vitazkova SK, Long E, Paul A, Glendinning JI. Mice suppress malaria infection by sampling a “bitter” chemotherapy agent. Anim Behav. 2001;61:887–894. [Google Scholar]
- Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci U S A. 2002;99(4):2392–2397. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. Gustatory and thermal responses in the glossopharyngeal nerve of the rat. Jpn J Physiol. 1966;16(6):599–611. doi: 10.2170/jjphysiol.16.599. [DOI] [PubMed] [Google Scholar]