Abstract
Background
Eye movements in essential tremor (ET) are poorly described and may present useful information on the underlying pathophysiology of the disorder.
Methods
Sixty patients with ET, including 15 de novo untreated patients, and 60 age-matched controls constitute the study population. A video-based eye tracker was used to assess binocular eye position. Oculomotor function was assessed while subjects followed random horizontally and vertically step-displaced targets.
Results
For all reflexive saccades, latencies were increased in ET subjects by a mean of 16.3% (p<0.01). Saccades showed reduced peak velocities with a lengthy, wavering velocity plateau, followed by slowed decelerations. For larger 30°+ saccades, peak velocities were decreased by a mean of 25.2% (p<0.01) and durations increased by 31.8% (p<0.01). The frequency of square wave jerks (SWJs) in patients was more than triple that of controls (p<0.0001). Despite frequent interruptions by SWJs, fixations were otherwise stable and indistinguishable from controls (root mean square [RMS] velocity, p = 0.324). The abnormal eye movement parameters were independent of disease duration, tremor severity, and medication therapy.
Discussion
In contrast to normally swift onset and efficient acceleration/deceleration movements, saccades in ET are characterized by abnormally prolonged latencies and slowed velocity profiles. Although ET subjects maintain highly stable fixations, they are interrupted by increased numbers of SWJs. This study reveals novel oculomotor deficits in ET, which are distinct from the eye movement dysfunction of other movement disorders, supporting a role for eye tracking to assist in the differential diagnoses of not only atypical, but also more common movement disorders.
Keywords: Essential tremor, ocular motility, saccades, square wave jerks, cerebellum, main sequence
Introduction
Essential Tremor (ET) affects approximately 4% of people over 40 years of age1 and is commonly characterized by bilateral postural arm tremor, with or without tremor with action.2 The tremor predominantly involves the hands and forearms, and less commonly affects the head or voice.3 Even though ET is considered to be the most prevalent movement disorder,4 the associated oculomotor deficits have not been well defined. In the only two published reports on eye movements in ET,5,6 abnormalities were found in initiation of smooth pursuit and in suppressing vestibular nystagmus, deficits likely attributable to cerebellar dysfunction.7–9 These groups also reported that saccades were unaffected; however, analysis of the collected data suggested the need to more thoroughly investigate this relevant issue. Using comparatively more in-depth and rigorous analyses of saccades, subjects in ET were found here to show distinct abnormalities in saccadic behavior. Moreover, the present study findings suggest novel oculomotor abnormalities in ET that are distinctly different from both controls and other movement disorders, such as Parkinson’s disease (PD). Finally, the eye movement abnormalities may lend support to the cerebellar pathophysiology of ET.
Methods
Sixty patients diagnosed with ET (age 63.4±12.9 years, [range 26–88 years]; sex 48 [80%] males) and 60 similarly aged control subjects (65.3±7.4 years, sex 40 [66.6%] male) completed oculomotor testing and constituted the study population. Patients were recruited consecutively from the Southeast/Richmond Veterans Affairs Medical Center Parkinson’s Disease Research, Education and Clinical Center (PADRECC). All patients were screened by a movement disorder specialist (M.S.B.) and considered to have ET based upon the diagnostic criteria set forth by the consensus statement of the Movement Disorder Society.2 Patients with deep brain stimulators or significant superimposed neurological or ophthalmic conditions were excluded. Control subjects were recruited among spouses, relatives, and friends and were screened and excluded if they had any significant neurological or ophthalmic conditions. The study was approved by the Institutional Review Board at the Hunter Holmes McGuire Veterans Affairs Hospital, and written informed consent was obtained from all subjects prior to testing.
Among the 60 study patients, the average duration of tremor was 11.3 years (SD±13.7). To maintain consistency across other studies in our center, tremor severity was scored (0–4) according to the Unified Parkinson’s Disease Rating Scale (UPDRS) Examination item 21, Action or Postural Tremor of Hands, and averaged 1.9 (±0.9). See Table 1 for a summary of subject enrollment.
Table 1. Subject Enrollment.
| Essential Tremor (n = 60) | Controls (n = 60) | |
|---|---|---|
| Age (years) | 63.4±12.9 | 65.3±7.4 |
| Duration of symptoms (years) | 11.3±13.7 | – |
| Gender: number of males (%) | n = 48 (80%) | n = 40 (66.6%) |
| Postural tremor score | 1.9±0.9 | – |
| Square wave jerks per minute | 26.9±20.01 | 8.4±8.3 |
| Saccadic latency (ms) | 255.3±80.91 | 220.8±46.4 |
| Q-ratio of saccades (peak/mean velocity) | 2.37±2.651 | 1.81±0.39 |
Statistically significant with a maximum p<0.01.
Mean±standard deviation.
At the time of testing, 45 patients (75%) were medicated for tremor and benefitted from single agents: 21 (35%) were receiving topiramate, 12 (20%) a β-blocker, and 12 (20%) primidone. Of the 15 untreated patients, eight subsequently initiated treatment and all exhibited improvement of clinical symptoms at follow-up examinations.
Binocular horizontal and vertical eye positions were recorded at 500 Hz using a video-based eye tracker (Eyelink II, SR Research Ltd) with a reported resolution of 0.01° root mean square (RMS). The system tracks the center of the dark pupil utilizing infrared light and two cameras placed just below each eye, beyond the field of vision. The eye tracker system rests comfortably on the subject’s head and is quick to set up and calibrate. To account for potential head movement, head position was recorded (at 125 Hz) with a six degree of freedom magnetic tracking system (trakSTAR, Ascension Technology Corp, Burlington VT) and synchronized with the eye position recordings.
Subjects were seated in a darkened room in front of a 26-inch diagonal LCD monitor placed 75 cm from their eyes and covering ±20° horizontally and ±13° vertically. The height of the monitor was adjusted so that the center of the screen corresponded to the center of the pupillary plane. Subjects were asked to fixate on a target stimulus and calibration and validation of the eye tracker was performed at three points along each of the cardinal axes, four times per subject, or until the reported gaze error was less that 0.5°. The target stimulus was a white dot sized to occupy 0.2° of visual angle on a black background. Eye and head position data were subsequently collected while subjects followed approximately 100 random and unpredictable discrete step changes in target position along the horizontal or vertical cardinal axes. Subjects were encouraged to close their eyes and rest between each recording to prevent fatigue. Patients were assessed while taking their prescribed medications.
Data were analyzed off line by a researcher blinded to the patient’s diagnosis using an interactive custom-written plotting program (P.A.W). Fixations were analyzed for duration and stability. Saccades were analyzed for duration, peak velocity, acceleration, amplitude, and accuracy. Saccadic beginning and end points were determined by a velocity threshold set at 20°/second, and saccadic velocity was calculated by way of a two-point central difference. Saccadic gain (accuracy) was calculated by dividing the amplitude of the initial reflexive saccade by the amplitude of the target displacement. The main sequence, a well-established method originally described by Bahill and colleagues,10 was used to examine the relationship between the amplitude of a saccade and its duration and peak velocity. Additionally, Q-ratios (peak/mean velocities) were calculated. Normal values11 are between 1.8 and 2.0, and values above 2.0 indicate single or multiple transient decelerations, with larger values signifying increasing amounts of deceleration.12
All statistical analysis was conducted using SPSS Statistics 17.0. For statistical analyses; α was set to 0.05. Data were assessed for normality using the Shapiro–Wilk test. Parameters that were not normally distributed (Q-ratio, saccadic duration at various amplitudes) were then log transformed and confirmed to be log-normal distributions, and analysis was run on these values. Independent sample, unpaired, two-tailed t-tests were conducted to assess for differences between ET and control population data. Levene’s test for the equality of variances was calculated, and if the significance was found to be less than 0.05 equal variances were not assumed. In the later instance, Welch’s t-test was used to compare the means, which has the ability to compensate for samples of unequal variance. Analyses of variance (ANOVA) were used to assess differences between different pharmaceutical therapies.
Results
Fixation stability
Subjects with ET showed highly stable ocular fixations, similar to controls. RMS velocity, an excellent measure of ocular stability during fixation,13 did not differ between subjects with ET (2.83±0.88) and controls (2.99±0.57, p = 0.331). Head tremor was detected with the magnetic tracking system in two ET subjects. Large amplitude head tremor was readily evident clinically in one subject. In the other subject, head tremor was detected first with the magnetic tracking system, and upon clinical re-evaluation a subtle head tremor was noted. Owing to potential activation of the vestibulo-ocular reflex, the data from those two subjects were excluded specifically from fixation stability analyses. Since head tremor would not affect other oculomotor parameters, data from these two subjects were included in subsequent analyses.
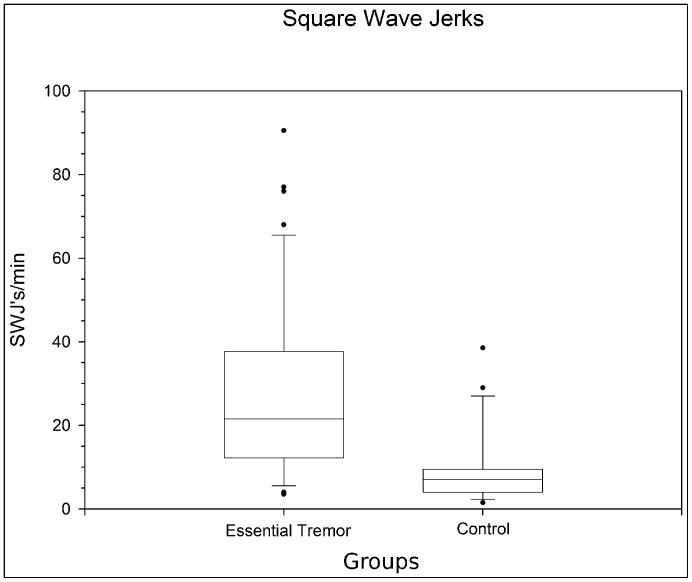
Although otherwise stable, the fixations of ET subjects were interrupted by an increased occurrence of square wave jerks (SWJs, p<0.0001). During fixation, subjects with ET exhibited on average 26.9±20 SWJ/minute compared with 8.4±8.3 in controls (Figure 1). Fundamental characteristics of the SWJs, including mean amplitudes (0.62±0.31° [ range: 0.13–2.16°] in ET subjects, 0.58±0.23°; range 0.14–1.56° for controls) and durations (257.8±89.1 ms [range 72–536 ms] for ET subjects; 265.6±91.3 ms [range 108–628 ms] for controls) did not differ between patients and controls (p = 0.19, and p = 0.22 respectively).
Figure 1. Frequency of Square Wave Jerks while Fixating.
Essential tremor patients exhibit appreciably more square wave jerks than age matched controls (p<0.0001). Middle bars inside boxes represent sample means, while the edges of boxes indicate first and third quartiles. Bottom and top bars represent minimum and maximum, respectively. Circles denote statistical outliers.
Saccadic dynamics
Latency
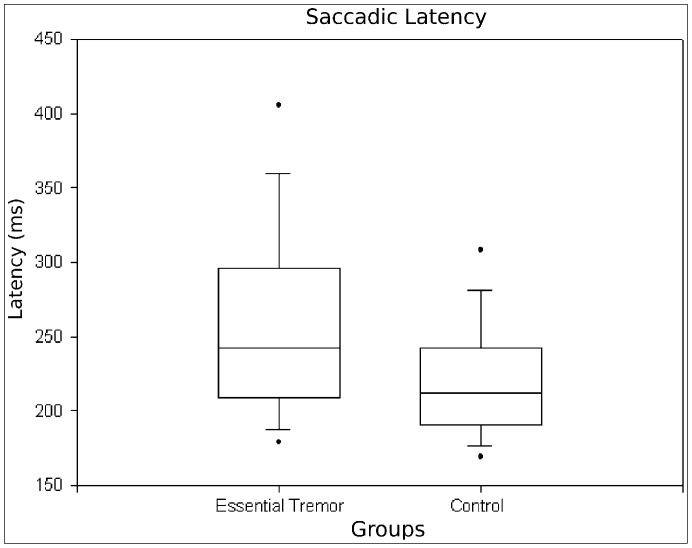
Latency to initiate reflexive saccades was increased by a mean of 16.3% (p<0.01) in ET patients (255.3±80.9 ms) compared with controls (220.8±46.4 ms). Figure 2 shows a boxplot of these differences.
Figure 2. Latency of Reflexive Saccades to Randomly Displaced Targets.
Patients with essential tremor have increased saccadic latencies compared with age-matched controls (p<0.01).
Velocity and duration
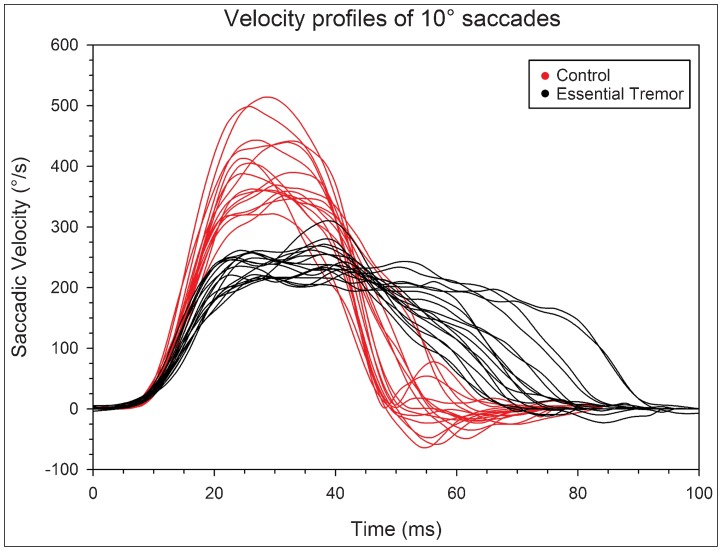
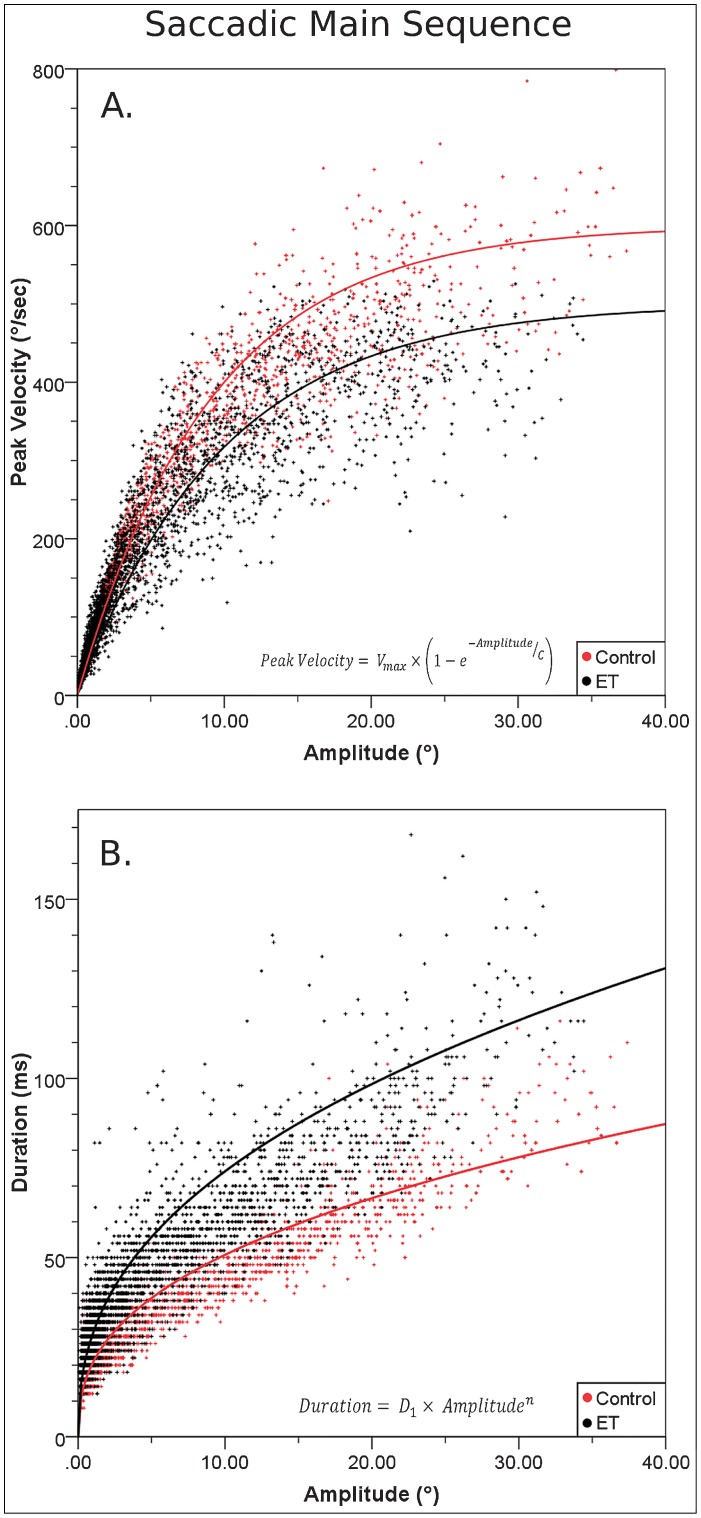
Peak saccadic velocities were appreciably reduced and saccadic durations increased across ET subjects compared with controls. Figure 3 shows examples of saccadic velocity profiles of study subjects, illustrating the normal, bell shaped, slightly positively skewed curves, with a single velocity peak in control subjects.12 In contrast, ET subjects characteristically showed reduced peak velocities, and a lengthy fluctuating plateau of velocity, followed by slowed final decelerations. Using main sequence comparisons, a well-established means to define saccadic differences,10 Figure 4A illustrates the prominent reductions in peak saccadic velocities relative to saccadic amplitude in ET subjects compared with controls. While affecting all amplitudes, dynamic differences of saccades progressively increase as saccades become larger, with peak velocities for amplitudes of 30° or larger saccades reduced by 25.2% in ET subjects compared with controls (p<0.01). The divergent main sequence regression lines in Figure 4B similarly demonstrate progressively greater differences between ET subjects and controls in saccadic duration with increasing movement amplitude (31.8% for 30° or larger saccades, p<0.01). The Q-ratio of saccades (peak velocity/mean velocity), used to quantify and detect abnormalities of the velocity waveform of a saccade,11 was on average 30.9% larger in ET subjects (2.37±2.65) than controls (1.81±0.39, p<0.001).
Figure 3. Velocity profiles of 10° saccades. Single velocity traces are shown for 18 representative essential tremor (ET) subjects and 18 control subjects.
The velocity profiles were aligned at t = 10 ms at the defined saccadic onset threshold (20°/second). Note the lower peak velocity, velocity plateau, and prolonged duration of saccades in ET subjects. The integral was taken of each individual velocity curve, and no difference was found between each group (p = 0.59). This further supports that despite abnormal velocity behavior during saccadic flight, that subjects with ET maintain accurate saccadic amplitudes, and reach the intended target position.
Figure 4. Saccadic Dynamics along the Main Sequence.
(A) Peak velocity vs. amplitude of saccades. For all movement amplitudes, subjects with essential tremor generally exhibit lower peak velocities than controls (Vmax = 400°/second for essential tremor [ET] subjects vs. 500°/second for controls, C = 19.9 for ET subjects vs. 9.1 for controls). (B) Saccadic duration vs. amplitude. Saccades are generally slower for subjects with ET compared to controls, with the group differences becoming progressively greater with increasing saccadic amplitude (average duration of a one degree saccade (D1) = 28.8 ms for ET subjects vs. 20.6 ms for controls, exponential value (n) for ET subjects = 0.41 vs. 0.39 for controls).
Accuracy
Saccadic gain (amplitude of primary saccade/amplitude of target displacement) did not differ between ET subjects (0.95±0.08) and control subjects (0.95±0.09, p = 0.297).
Fifteen patients (25%) had SWJs that were 2 SD above the control mean. Eleven patients (18.3%) had latencies that were 2 SD above the control mean. Seven patients (11.6%) had a Q-ratio that was 2 SD above the control mean. Of the above-mentioned subjects with values more than 2 SD from the control mean, none of those had both Q-ratios and latencies 2 SD away. However, four subjects had Q-ratio and SWJs that were both 2 SD from the control mean, and four other subjects had latencies and SWJs that were 2 SD from the control mean. None of the subjects had all three categories 2 SD above the control mean. Of these patients with values 2 SD above the control mean, there was a very weak negative correlation between SWJs and postural tremor scores (R2 –0.14), with a slightly stronger negative correlation between SWJs and action tremor (–0.21). There was no correlation between tremor scores and Q-ratios or latencies.
All of the oculomotor measures were independent of disease duration, severity of tremor, and gender, both individually and as a group. Further, individual ANOVA, comparing de novo untreated, topiramate, primidone, and β-blocker-treated subjects, show that differences in saccadic latencies (F = 0.521, p = 0.671), SWJs (F = 0.310, p = 0.817), and Q-ratios (F = 0.471, p = 0.704) were not influenced by ET-targeted therapies (post hoc power analyses revealed power [1 – β err prob] >0.999).
Discussion
The major finding in this study was that ET is associated with abnormalities in saccadic dynamics, including slowed peak velocities and prolonged durations. Additionally, ET subjects were observed to maintain stable fixations, which however are interrupted by more than triple the normal number of SWJs. These abnormal oculomotor features were independent of disease duration and tremor severity. Furthermore, there were no differences in eye movement parameters between de novo untreated subjects and those taking tremor-targeted medications, grouped together or by specific medications, suggesting that these abnormalities are inherent to the disease. To our knowledge, this is the first study to reveal appreciable saccadic abnormalities and increased SWJs in ET.
In distinction from the highly efficient acceleration–deceleration movements observed in control subjects, saccades in ET subjects are characterized by lengthy, reduced, fluctuating peak velocities and slowed final decelerations, resulting in prolonged total movement times. Although present at all amplitudes, these saccadic abnormalities became progressively more evident as the amplitude of the saccade increased. Additionally, despite having abnormally altered movement dynamics, ET subjects, importantly, are nonetheless able to accurately capture step-displaced visual targets, as reflected by saccadic gains equal to that of normal controls. Finally, saccadic latencies were modestly prolonged in ET subjects by an average of 16.3% relative to control subjects.
In conflict with our findings, Helmchen and colleagues5 found no abnormalities in saccadic latencies and velocities in ET subjects. The investigators, however, specifically assessed only 10° and 20° saccades rather than a continuum of amplitudes, analyzed only 20 saccades from 17 patients and 11 controls, and the main sequence and Q-ratios were not calculated. Conceivably, the comparatively greater statistical power in our study (∼12,000 vs. ∼560 saccades) largely accounts for why we found differences in saccades whereas Helmchen and colleagues did not. Although actual statistical values were unreported for the saccadic portion of that manuscript, their table possibly suggests that Helmchen et al5 had similar findings to this study, but the differences were subtle enough to not reach significance. According to the values in their table, subjects with ET appeared to show lower peak saccadic velocities than controls and slightly increased saccadic latencies in a subset of ET subjects with intention tremor. Additionally, ET subjects in their study appear to have normal saccadic gain, also consistent with our findings. Since all of the findings we report here are subtle changes from normal, we suggest that the discrepancies between the two reports are likely to be accounted for by differences in statistical power, despite similar results. Trillenberg et al6 reported that saccadic gain was normal in subjects with ET, which our findings confirm. Yet, in discrepancy with our study findings, the authors did not observe abnormalities in saccadic latency. Notably, Trillenberg and colleagues,6 however, only investigated a single saccade from each of just 12 subjects with ET.
The frequency of SWJs has been previously reported to be increased in many neurological disorders and is generally attributed to cerebellar involvement.14,15 Although ET subjects were observed presently to show more frequent SWJs, their fixations were otherwise normally stable and, therefore, would not necessarily be expected to be clinically relevant.16 Additionally, the characteristics of individual SWJs were not altered in ET subjects compared with controls.16,17 Further, features of the SWJs, apart from their frequency, are consistent with what has been reported in controls17,18 and other disorders.19
In addition to the increased SWJs, findings of transient slowing of saccades may support a principal role of cerebellar purkinje cells (PCs) in the pathogenesis of ET. Although not uniformly found,20,21 the prevailing pathological finding in ET brains has been reduced numbers of PCs, along with increased PC axonal swellings (“torpedoes”).22–24 Furthermore, in late onset Tay–Sachs (LOTS), in which PCs in the vermis appear to be largely destroyed,25 saccades are similarly characterized by transient slowing of saccadic velocities,26,27 albeit more profoundly than ET cases. Moreover, findings of transient slowing of saccades in ET and LOTS are consistent with current theories suggesting that normally precisely timed inhibitory signals from PCs in the vermis “choke off” saccadic drive signals originating from the superior colliculus.28,29
The presently defined abnormal oculomotor dynamics in ET are distinctly different from those previously seen in PD using the same protocols.13 In contrast to the eye movement abnormalities in ET, subjects with PD exhibit normal latencies and movement dynamics during reflexive saccades. Further, PD subjects show normal frequencies of SWJs while exhibiting characteristic highly unstable, oscillatory fixations. Although unconfirmed,30–33 this distinctive ocular tremor was suggested to be highly sensitive for distinguishing PD patients from control subjects.13 Though the presently defined oculomotor abnormalities similarly largely distinguished subjects with ET from controls, there was nevertheless appreciable overlap between these two groups. One potential explanation for this overlap is that the present distinguishing ocular findings represent relative differences rather than being unique to those with ET. Another possibility is that since ET is likely to represent a heterogeneous disorder,34 those with the presently defined abnormal eye findings may represent a specific pathological subset of the disorder. Since SWJs are a normal phenomenon, with appreciable overlap in frequencies among ET patients and healthy controls, we suggest that slowed saccades are a primary feature of ET, while an increased frequency of SWJs is a secondary or supportive feature. As saccade velocity by itself cannot fully separate all controls from ET subjects, numbers of SWJs, and saccadic latencies, and can be helpful distinguishing features of ET. Particularly early in the course of the disease, differentiating between tremor due to ET vs. PD can be difficult even for experienced movement disorder specialists.35–38 However, since the oculomotor abnormalities in ET are distinctly different from those in PD, sensitive oculomotor testing could prove to be a valuable means to differentiate these conditions. Further studies will be required to determine the accuracy of eye movements for differential diagnoses.
The present study has a number of limitations. Although the eye movement abnormalities did not differ between treated and untreated patients, nor were there any differences between various medications; testing of individual subjects with and without medication therapy however was not performed to more directly address specific effects of individual medications. Secondly, objective visual acuity was not formally assessed and, therefore, the clinical impact of the observed oculomotor abnormalities could not be determined. Finally, as brain scans were only infrequently performed, we cannot fully exclude the potential contribution of additional processes such as strokes in a percentage of patients.
In summary, eye movements in ET are characterized by an increased frequency of SWJs, which interrupt otherwise stable fixations, and by delayed initiation of saccades, with reduced, wavering peak velocities and prolonged, though accurate, movements. While none of these features are of sufficient magnitude to be detectable on routine ophthalmological examinations,39,40 the extent to which these abnormalities could potentially negatively impact normal visual function remains to be defined. The present study findings suggest that oculomotor testing could serve a valuable means to assist in the clinical differential diagnosis of ET. Future studies are particularly needed to assess the value of eye movement tracking for differentiating between ET and PD.
Acknowledgements
We thank Abu Qutubuddin M.D. (Physiatrist, Southeast PADRECC, McGuire Veterans Affairs Medical Center) and Peggy Roberge R.N. (Clinical Nurse Coordinator, Southeast PADRECC, McGuire Veterans Affairs Medical Center) for their assistance in recruiting subjects.
Footnotes
Funding: This study was supported by the Department of Veterans Affairs.
Conflict of Interests: The authors report no conflicts of interest.
Financial disclosures: None.
References
- 1.Zesiewicz TA, Chari A, Jahan I, Miller AM, Sullivan KL. Overview of essential tremor. Neuropsychiatric Dis Treat. 2010;6:401–408. doi: 10.2147/NDT.S4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deuschl G, Bain P, Brin M, Ad Hoc Scientific Committee Consensus statement of the Movement Disorder Society on tremor. Mov Disord. 1998;13(Suppl. 3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 3.Whaley NR, Putzke JD, Baba Y, et al. Essential Tremor: phenotypic expression in a clinical cohort. Parkinsonism Relat Disord. 2007;13:333–339. doi: 10.1016/j.parkreldis.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Louis ED, Ottoman R, Hauser WA. How common is the most common adult movement disorder? Estimates of the prevalence of essential tremor throughout the world. Mov Disord. 1998;13:5–10. doi: 10.1002/mds.870130105. [DOI] [PubMed] [Google Scholar]
- 5.Helmchen C, Hagenow J, Miesner A, et al. Eye movement abnormalities in essential tremor may indicate cerebellar dysfunction. Brain. 2003;126:1319–1332. doi: 10.1093/brain/awg132. [DOI] [PubMed] [Google Scholar]
- 6.Trillenberg P, Führer J, Sprenger A, et al. Eye-hand coordination in essential tremor. Mov Disord. 2006;21:373–379. doi: 10.1002/mds.20729. [DOI] [PubMed] [Google Scholar]
- 7.Straube A, Scheurer W, Eggert T. Unilateral cerebellar lesions affect initiation of ipsilateral smooth pursuit eye movements in humans. Ann Neurol. 1997;42:891–898. doi: 10.1002/ana.410420611. [DOI] [PubMed] [Google Scholar]
- 8.Moschner C, Crawford TJ, Heide W, Trillenburg P, Kömpf D, Kennard C. Deficits of smooth pursuit initiaion in patinets with degenerative cerebellar lesions. Brain. 1999;122:2147–2158. doi: 10.1093/brain/122.11.2147. [DOI] [PubMed] [Google Scholar]
- 9.Hain TC, Zee DS, Maria BL. Tilt suppression of vestibulo-ocular reflex in patients with cerebellar lesions. Acta Otolaryngol. 1988;105:13–20. doi: 10.3109/00016488809119440. [DOI] [PubMed] [Google Scholar]
- 10.Bahill AT, Clark MR, Stark L. The main sequence, a tool for studying human eye movements. Math Biosci. 1975;24:194. doi: 10.1016/0025-5564(75)90075-9. [DOI] [Google Scholar]
- 11.Harwood MR, Mezey LE, Harris CM. The spectral main sequence of human saccades. J Neurosci. 1999;19:9098–9106. doi: 10.1523/JNEUROSCI.19-20-09098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baloh RW, Konrad HR, Sills AW, Honrubia V. The Saccade Velocity Test. Neurology. 1975;25:1071–1076. doi: 10.1212/WNL.25.11.1071. [DOI] [PubMed] [Google Scholar]
- 13.Gitchel GT, Wetzel PA, Baron MS. Pervasive ocular tremor in Parkinson’s disease. Arch Neurol. 2012;69:1011–1017. doi: 10.1001/archneurol.2012.70. [DOI] [PubMed] [Google Scholar]
- 14.Rabiah PK, Bateman JB, Demer JL, et al. Ophthalmologic findings in patients with cerebellar ataxia. Am J Ophthalmol. 1997;123:108–117. doi: 10.1016/s0002-9394(14)71000-1. [DOI] [PubMed] [Google Scholar]
- 15.Garbutt S, Riley DE, Kumar AN, et al. Abnormalities of optokinetic nystagmus in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2004;75:1386–1394. doi: 10.1136/jnnp.2003.027367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herishanu Y, Sharpe JA. Normal Square Wave Jerks. Invest Ophthalmol Vis Sci. 1981;20:268–272. [PubMed] [Google Scholar]
- 17.Shallo-Hoffmann J, Sendler B, Muhlendyck H. Normal square wave jerks in differing age groups. Invest Ophthalmol Vis Sci. 1990;31:1649–1652. [PubMed] [Google Scholar]
- 18.Abadi RV, Gowen E. Characteristics of saccadic intrusions. Vision Research. 2004;44:2675–90. doi: 10.1016/j.visres.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Rascol O, Sabatini U, Simonetta-Moreau M, et al. Square wave jerks in parkinsonian syndromes. J Neurol Neurosurg Psychiatry. 1991;54:599–602. doi: 10.1136/jnnp.54.7.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajput AH, Robinson CA, Rajput ML, Rajput A. Cerebellar purkinje cell loss is not pathognomonic of essential tremor. Parkinsonism Relat Disord. 2011;17:16–21. doi: 10.1016/j.parkreldis.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Rajput AH, Robinson CA, Rajput ML, Robinson SL, Rajput A. Essential tremor is not dependent upon cerebellar purkinje cell loss. Parkinsonism Relat Disord. 2012;18:626–628. doi: 10.1016/j.parkreldis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130(Pt 12):3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 23.Axelrad JE, Louis ED, Honig LS, et al. Reduced Purkinje cell number in essential tremor: a postmortem study. Arch Neurol. 2008;65:101–107. doi: 10.1001/archneurol.2007.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louis ED, Faust PL, Ma KJ, Yu M, Cortes E, Vonsattel JP. Torpedoes in the cerebellar vermis in essential tremor cases vs. controls. Cerebellum. 2011;10:812–819. doi: 10.1007/s12311-011-0291-0. [DOI] [PubMed] [Google Scholar]
- 25.Rucker JC, Leigh RJ, Optican LM, Keller EL, Büttner-Ennever JA. Ocular motor anatomy in a case of interrupted saccades. In: Kennard C, Leigh RJ, . Amsterdam: Elsevier B.V.; editors. Progress in brain research. (Vol 171. Chapter 6.11). [Google Scholar]
- 26.Rucker JC, Shapiro BE, Han YH, et al. Neuro-ophthalmology of late-onset Tay-Sachs disease (LOTS) Neurology. 2004;63:1918–1926. doi: 10.1212/01.WNL.0000144275.76658.F4. [DOI] [PubMed] [Google Scholar]
- 27.Rucker JC, Ying SH, Moore W, et al. Do brainstem omnipause neurons terminate saccades? Ann N Y Acad Sci. 2011;1233:48–57. doi: 10.1111/j.1749-6632.2011.06170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quaia C, Lefevre P, Optican LM. Model of the control of saccades by Superior colliculus and cerebellum. J Neurophysiol. 1999;82:999–1018. doi: 10.1152/jn.1999.82.2.999. [DOI] [PubMed] [Google Scholar]
- 29.Optican LM, Quaia C. Distributed model of collicular and cerebellar functions during saccades. Ann NY Acad Sci. 2002;956:164–177. doi: 10.1111/j.1749-6632.2002.tb02817.x. [DOI] [PubMed] [Google Scholar]
- 30.Kaski D, Saifee TA, Buckwell D, Bronstein AM. Ocular tremor in Parkinson’s disease is due to head oscillation. Mov Disord. 2013;28:534–537. doi: 10.1002/mds.25342. [DOI] [PubMed] [Google Scholar]
- 31.Baron MS, Gitchel GT, Wetzel PA. Ocular tremor in Parkinson’s disease is due to eye, not head oscillation. Mov Disord. 2013;28:844. doi: 10.1002/mds.25461. [DOI] [PubMed] [Google Scholar]
- 32.Leigh RJ, Martinez-Conde S. Tremor of the eyes, or of the head, in Parkinson’s disease? Mov Disord. 2013;28:691–693. doi: 10.1002/mds.25478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duval C. Ocular tremor in Parkinon’s disease: the debate is not over. Mov Disord. 2013;28:713–714. doi: 10.1002/mds.25514. [DOI] [PubMed] [Google Scholar]
- 34.Benito-León J, Louis ED. Update on essential tremor. Minerva Med. 2011;102:417–440. [PubMed] [Google Scholar]
- 35.Baumann CR. Epidemiology, diagnosis, and differential diagnosis in Parkinson’s disease tremor. Parkinsonism and Related Disorders. 2012;18(Suppl. 1):S90–S92. doi: 10.1016/S1353-8020(11)70029-3. [DOI] [PubMed] [Google Scholar]
- 36.Shahed J, Jankovic J. Exploring the relationship between essential tremor and Parkinson’s disease. Parkinsonism Relat Disord. 2007;13:67–76. doi: 10.1016/j.parkreldis.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 37.Jain S, Lo SE, Louis ED. Common misdiagnosis of a common neurological disorder: how are we misdiagnosing essential tremor? Arch Neurol. 2006;63:1100–1104. doi: 10.1001/archneur.63.8.1100. [DOI] [PubMed] [Google Scholar]
- 38.Pahwa R, Lyons KE. Early diagnosis of Parkinson’s disease: recommendations from diagnostic clinical guidelines. Am J Manag Care. 2010;16:S94–S99. [PubMed] [Google Scholar]
- 39.Kumar AN, Han YH, Liao K, et al. Evaluating large saccades in patients with brain-stem or cerebellar disorders. Ann NY Acad Sci. 2005;1039:404–16. doi: 10.1196/annals.1325.038. [DOI] [PubMed] [Google Scholar]
- 40.Leigh RJ, Kennard C. Using saccades as a research tool in the clinical neurosciences. Brain. 2004;127:460–477. doi: 10.1093/brain/awh035. [DOI] [PubMed] [Google Scholar]