Abstract
Mucopolysaccharidosis IVA (MPS IVA) is caused by deficiency of N-acetylgalactosamine-6-sulfate sulfatase (GALNS), leading to systemic skeletal dysplasia because of excessive storage of keratan sulfate (KS) in chondrocytes. In an effort to determine a precise prognosis and personalized treatment, we aim to characterize clinical, biochemical, and molecular findings in MPS IVA patients, and to seek correlations between genotype, phenotype, and blood and urine KS levels. Mutation screening of GALNS gene was performed in 55 MPS IVA patients (severe: 36, attenuated: 13, undefined: 6) by genomic PCR followed by direct sequence analysis. Plasma and urine KS levels were measured by ELISA method. Genotype/phenotype/KS correlations were assessed when data were available. Fifty-three different mutations including 19 novel ones (41 missense, 2 nonsense, 4 small deletions, 1 insertion, and 5 splice-site) were identified in 55 patients and accounted for 93.6% of the analyzed mutant alleles. Thirty-nine mutations were associated with a severe phenotype and ten mutations with an attenuated one. Blood and urine KS concentrations in MPS IVA patients were age-dependent and markedly higher than those in age-matched normal controls. Plasma and urine KS levels in MPS IVA patients with the severe phenotype were higher than in those with an attenuated form.
This study provides evidence for extensive allelic heterogeneity of MPS IVA. Accumulation of mutations as well as clinical descriptions and KS levels allow us to predict clinical severity more precisely and should be used for evaluation of responses to potential treatment options.
Keywords: Mucopolysaccharidosis IVA, Genotype, Phenotype, Biomarker, Keratan Sulfate
INTRODUCTION
Mucopolysaccharidosis IVA (MPS IVA, Morquio A disease; OMIM# 253000) is an autosomal recessive lysosomal storage disorder (LSD) characterized by a loss of activity of the N-acetylgalactosamine 6-sulfate sulfatase (GALNS) enzyme. The estimated incidence of Morquio A disease varies widely between 1 in 75,000 to 500,000 births [1–8]. Deficiency of GALNS results in a build-up of the glycosaminoglycans (GAGs), keratan sulfate (KS), and chondroitin-6-sulfate (C6S) in lysosomes throughout the body, but specifically in the cartilage and cornea, where KS is synthesized. In MPS IVA, the degradation of KS is defective. KS is predominantly found in cartilage and cornea, the major organs affected in MPS IVA. The specific mechanism, by which excess storage of KS results in the skeletal dysplasia unique to MPS IVA, remains unknown.
The most widespread pathological findings are related to a systemic skeletal dysplasia including short trunk dwarfism, kyphoscoliosis, platyspondyly, odontoid hypoplasia, genu valgum, pectus carinatum, and dental anomalies. Other findings include characteristic ligamentous laxity, corneal clouding, coarse facies, hearing loss, and valvular heart disease. Unlike other MPS disorders, there is no central nervous system involvement and intelligence is preserved [9]. There is variable severity, but patients with severe phenotype usually do not survive past the second or third decade of life. Patients with the attenuated form of MPS IVA have been reported to survive into the seventh decade of life [10]. Based on a natural history study by the International Registry program [9], around 50% of the subjects underwent orthopedic surgical procedures. The patients with more severe short stature and those who underwent surgical procedures were reported to have more difficulties ambulating. The current clinical criteria establish that reduced growth and final height are associated with a more severe clinical phenotype. The GALNS gene, located on chromosome 16q24.3, contains 14 exons spanning 50 kb and encodes a 522-amino acid protein, including a signal peptide of 26 residues [11, 12]. GALNS has been purified from human placenta as an oligomer of 40 and 15 kDa polypeptides [13], with the oligomers inter-linked by disulphide bonding. Mature human GALNS enzyme is stabilized in a complex with two other lysosomal enzymes (β-galactosidase and α-neuraminidase) and the protective protein cathepsin A [14]. Until now, molecular analysis of different ethnic populations has revealed 185 different GALNS mutations including 19 novel mutations in this study [15]. This extensive allelic heterogeneity of GALNS gene mutations was consistent with the wide spectrum of clinical phenotypes observed in MPS IVA patients.
Genotype-phenotype correlation has been used to predict clinical severity in some groups of MPS IVA patients [9, 15–28]. The amount of KS in plasma and urine of MPS IVA patients, together with patient genotype, has been suggested as a useful biomarker of disease severity [15, 29, 30]. Previous studies on correlations among genotype, phenotype, and KS were carried out with small numbers of MPS IVA patients [30]. Clear understanding of correlation between phenotype, genotype, and biochemical markers based upon the study of a larger group of MPS IVA patients may help us make early diagnosis, early treatment, and better prognosis.
Here we present 53 mutations including 19 novel mutations in GALNS gene in a cohort of 55 patients. The report includes clinical and biochemical findings in addition to the patients’ genotypes. The conclusions pertain to improving the criteria for delineation of severe and attenuated forms. In this report, with cases described from a large multinational series of patients with MPS IVA, we detail the correlations between genotype, phenotype, and biomarker.
MATERIALS AND METHODS
A questionnaire was created by The International Morquio Organization (IMO) (www.morquio.com) and our research team collaboratively. The questionnaire was used for each patient, covering demographic information, family history, birth history, age of onset and diagnosis, signs and symptoms, clinical course, surgical interventions, growth chart, and physical activity. This study was approved by the institutional review board at Saint Louis University. All experimental procedures were conducted at Saint Louis University.
Patients
This study describes the clinical, biochemical, and molecular findings in the largest cohort reported in one study. Fifty-five MPS IVA patients (30 males and 25 females, ages 6 months – 46.8 years) were evaluated clinically and screened for mutations in GALNS gene and KS levels measured in blood and urine when the specimens were available. Four cases of siblings were included. Clinical diagnosis was confirmed when leukocytes or skin fibroblasts had low levels of GALNS activity (< 10% of the enzyme activity in normal control). The phenotypes were assigned based on growth evaluations compared with a standard growth chart of MPS IVA patients [10]. Among 55 patients, 36 patients had a severe phenotype (their height ≦ 75th percentile on the growth chart of MPS IVA patients), and 13 patients had an attenuated phenotype (their height > 75th percentile on the growth chart of MPS IVA patients) (Table 1). Clinical information in six patients was not obtained to define the phenotype.
Table 1.
Characterization of MPS IVA patients in this study
| MO | Sex | Phenotype | Age tested for height and weight (years) |
Present height (cm) | Percentile in Morquio patients |
Present weight (kg) | Percentile in Morquio patients |
Age onset | Age diagnosis | Growth arrest | Orthopedic Surgery | 1st allele: Nucleotide change |
2nd allele: Nucleotide change |
Ethnicity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MO 58 | M | undefined | 8.0 | NA | NA | NA | NA | NA | NA | c.1023C>G | undefined | SL | ||
| MO 59 | F | undefined | 6.0 | NA | NA | NA | NA | NA | NA | c.1023C>G | undefined | SL | ||
| MO 100 | M | severe | 9.9 | 98.4 | 25th | 15.9 | 10th | 0.5 | 0.6 | no | + | c.853_855delTTC | undefined | Am-Ca (Br/Ge/Pt) |
| MO 101 | M | attenuated | 12.0 | 127 | 75th | 38.6 | 90th | 5 | 5 | no | − | c.758G>A | c.922T>C | Am-Ca (Al) |
| MO 104 | M | attenuated | 24.0 | 127 | 75th | 52.5 | 75th | 1 | 2 | yes | − | c.121A>T | c.121A>T | Am-Ca (Fr) |
| MO 105 | F | severe | 13.0 | 102 | 50th | 21 | 50th | 0.5 | 6 | yes | + | c.898+1G>A | c.121-1G>C | Uk |
| MO 106 | M | attenuated | 13.0 | 150 | 90th | 45 | 90th | 5.5 | 6.4 | no | − | c.320-1G>T | undefined | Chil |
| MO 107 | F | attenuated | 18.0 | 120 | 75th | 31.8 | 75th | 4 | 4.5 | yes | + | c.1354T>A | c.1485C>G | Ca-Ca (Ir/En) |
| MO 109 | M | severe | 13.0 | 116 | 75th | 24.5 | 50th | 1 | 3 | yes | + | c.415G>A | c.1219A>C | Am-Bl, Am-Ca |
| MO 110 | M | severe | 12.5 | 105 | 50th | 19.85 | 25th | NA | 3.8 | yes | − | c.715G>T | c.715G>T | Br |
| MO 112 | M | severe | 7.0 | 94.5 | 25th | 20 | 75th | NA | 5.7 | no | − | c.901G>T | c.901G>T | Br |
| MO 113 | M | severe | 7.4 | 100 | 50th | 17.6 | 50th | 1 | NA | no | + | c.1023C>G | c.1156C>T | Br |
| MO 114 | M | severe | 11.6 | 95 | 25th | 16 | 10th | NA | NA | yes | − | c.1023C>G | c.1023C>G | Br |
| MO 115 | F | severe | 11.5 | 90 | 10th | 13 | 10th | NA | 3.3 | yes | − | c.280C>T | c.608C>T | Br |
| MO 116 | M | severe | 12.4 | 109 | 75th | 21.3 | 25th | NA | 7.1 | yes | − | c.280C>T | c.608C>T | Br |
| MO 117 | F | severe | 8.3 | 91.4 | 25th | 13.6 | 10th | 1.3 | 2 | no | + | c.346G>A | c.1156C>T | Am-Ca |
| MO 117b | F | severe | 6.8 | 86.4 | 10th | 14.1 | 25th | NA | 3.5 | no | − | c.346G>A | c.1156C>T | Am-Ca (It) |
| MO 120 | F | undefined | 4.5 | NA | NA | NA | NA | NA | NA | c.612C>G | c.612C>G | Am-Ca | ||
| MO 121 | M | attenuated | 16.0 | 150 | 90th | 63.4 | 90th | 9 | 14 | yes | + | c.181C>T | c.498delC | Am-Ca |
| MO 121s | F | attenuated | 11.0 | 142.8 | 90th | 8 | 8 | yes | − | c.181C>T | c.498delC | Am-Ca | ||
| MO 122 | M | attenuated | 9.0 | 130 | 90th | 35 | 90th | NA | NA | no | − | c.975G>T | c.1156C>T | Ca-Ca |
| MO 125 | M | attenuated | 7.2 | 124 | 90th | 26.4 | 90th | 3 | 6 | no | + | c.29G>A | c.G1215>A | Au |
| MO 126 | M | severe | 9.0 | 104.1 | 50th | 17.7 | 25th | at birth | 1.4 | yes | − | c.278T> A | undefined | Am-Ca |
| MO 127 | M | severe | 14.7 | 102 | 50th | 20 | 25th | 0.5 | 9 | yes | + | c.3G > A | c.257T>C | Fi |
| MO 129 | F | undefined | 0.8 | NA | NA | NA | NA | NA | − | c.634-1 G>T | c.860C>T | Gr | ||
| MO 133 | M | severe | 24.0 | 97 | 25th | 28 | 50th | 0.65 | 3 | yes | + | c.139G>A | c.139G>A | Po |
| MO 134 | M | severe | 2.7 | 85 | 25th | 10 | 10th | 0.6 | 1.7 | yes | − | c.697G>A | undefined | Po |
| MO 138 | F | severe | 12.7 | NA | NA | NA | NA | NA | − | c.477G>A | c.477G>A | Am-Ca | ||
| MO 139 | F | severe | 11.0 | 94 | 25th | 17 | 25th | 1.5 | 1.5 | yes | + | c.230C>G | c.230C>G | SA |
| MO 141 | M | undefined | 33.0 | NA | NA | NA | NA | NA | NA | c.1171A>G | undefined | Ca-Ca | ||
| MO 143 | M | severe | 4.0 | 93.6 | 50th | 16 | 50th | 2 | 4.5 | no | − | c.860C>T | c.860C>T | Mac |
| MO 144 | F | severe | 23.3 | 101.6 | 50th | 22.7 | 25th | 4 | 4 | yes | + | c.953T>G | c.1567T>G | Ch |
| MO 145 | M | severe | 12.0 | 113 | 75th | 20 | 25th | NA | 3 | yes | + | c.1156C>T | c.1219A>C | Fr |
| MO 146 | F | severe | 11.0 | 111.8 | 75th | 20.86 | 50th | 2 | 6.5 | yes | − | c.1171A>G | c.1171A>G | Am-Ca (En/Ge) |
| MO 147 | F | severe | 4.1 | 91.4 | 75th | 13.2 | 50th | at birth | 0.5 | no | + | c.901G>T | c.901G>T | Am-Ca |
| MO 148 | M | severe | 8.0 | 107 | 50th | 22 | 75th | 2.2 | 4.3 | yes | − | c.422+2_+3insT | c.1195delA | Ira |
| MO 148s | F | severe | 5.3 | 103 | 50th | 18 | 90th | 1.5 | 1.7 | yes | − | c.422+2_+3insT | c.1195delA | Ira |
| MO 150 | F | undefined | 6.9 | NA | NA | NA | NA | NA | NA | c.485C>T | c.485C>T | Co | ||
| MO 151 | M | severe | 4.0 | 91.44 | 50th | 13.6 | 50th | 1.5 | 2.5 | no | + | c.121-1G>A | c.337A>T | Am-Ca |
| MO 154 | F | severe | 1.8 | 81 | 50th | 11.5 | 25th | 0.5 | 1.5 | no | + | c.1156C>T | c.1156C>T | Am-Ca (His/Gr) |
| MO 155 | M | severe | 14.4 | 94.3 | 25th | 18.6 | 25th | 2.5 | 2.9 | yes | − | c.452C>T | c.452C>T | Pa |
| MO 157 | M | severe | 5.5 | 89.2 | 25th | NA | NA | NA | NA | NA | c.938C>T | c.938C>T | Ca-Ca | |
| MO 158 | F | severe | 3.8 | 88.9 | 75th | 12.08 | 50th | 2.3 | 2.6 | no | + | c.740G>A | c.901G>T | Am-Ca (Ge/Sw) |
| MO 159 | F | severe | 3.9 | 90 | 75th | 13.2 | 50th | 1 | 1.5 | no | + | c.346G>A | c.860C>T | Tu |
| MO 160 | F | severe | 4.0 | 91.4 | 75th | 12.7 | 50th | 0.7 | 1.3 | no | + | c.346G>A | c.1485C>G | Am-Ca |
| MO 161 | M | severe | 18.0 | 117 | 75th | 33 | 50th | 2 | 1.6 | yes | + | c.125G>A | c.374C>T | It |
| MO 162 | M | severe | 8.2 | 101 | 50th | 16 | 25th | 1.5 | 2 | yes | − | c.415G>A | c.901G>T | Sp |
| MO 163 | M | attenuated | 9.3 | 139.7 | 90th | 34.1 | 90th | 2.5 | 3.8 | no | + | c.1171A>G | c.1354T>A | Ca-Ca |
| MO 163s | F | attenuated | 5.0 | 116.8 | 90th | 25 | 90th | 2.3 | 0.8 | no | − | c.1171A>G | c.1354T>A | Ca-Ca |
| MO 165 | M | severe | 7.0 | 81.3 | 10th | 10.9 | 10th | 0.8 | 1.7 | no | − | c.405_422+1del19 | c.1480A>G | Ca-Ca |
| MO 166 | F | attenuated | 10.2 | 126.6 | 90th | NA | NA | NA | no | NA | c.244T>C | c.244T>C | Ca | |
| MO 167 | F | attenuated | 46.9 | 139.7 | 75th | 47.2 | 75th | 7 | 5 | yes | − | c.740G>A | c.761A>G | Am-Ca |
| MO 168 | F | attenuated | 10.1 | 114 | 75th | 22.2 | 75th | 2 | 6.5 | no | − | c.850T>G | c.850T>G | Tu |
| MO 170 | F | severe | 27.0 | 91.4 | 25th | 22.7 | 25th | 2 | 3 | yes | + | c.122T>A | c.122T>A | Am-Ca |
| MO 172 | M | severe | 18.8 | 91.4 | 10th | 19.07 | 10th | 2 | 3.2 | yes | + | c.245C>T | c.498delC | Am-Ca |
The DNA mutation numbering is based on cDNA sequence. Nucleotides numbered from the ATG initiator codons as suggested in den Dunnen and Antonarakis (2000).
Al: Albanian, Am-Ca: American Caucasian, Am-Bl: American-Black, Ar: Argentine, Au: Austrian, Br: Brazilian, Bt: British, Ca-Ca: Canadian Caucasian, Ch: Chinese, Chil: Chilean, Co: Colombian, Fi: Finnish, Fr: French, Ge: German, Gr: Greek, Hi: Hispanic, It: Italian, Ira: Iraq, Ir: Irish, Jp: Japanese, Mac: Macedonian, Pk: Pakistani, Po: Polish, Pt: Portuguese, SA: Saudi Arabian, Sw: Swedish, SL: Sri Lanka, Sp: Spanish, Tu: Turkish, un: unknown, Uk: Ukrainian
Highlighted in gray: novel mutations
Mutation analysis of GALNS gene
Genomic DNAs of MPS IVA patients were isolated from peripheral blood leukocytes or cultured skin fibroblasts. To investigate the GALNS gene, genomic PCR was performed as described previously with slight modification [26]. Briefly, we split up the GALNS gene into 5 fragments to enable rapid and efficient DNA amplification. Five sets of primers covering all 14 exons and the exon-intron boundaries of the GALNS gene were assigned. Fragment 1 consists of exon 1 (300 bp), fragment 2 is made up of exons 2 through 4 (2 kb), fragment 3 consists of exons 5 through 9 (5.8 kb), fragment 4 has exons 10 through 12 (5 kb) and fragment 5 contains exons 13 and 14 (3.7 kb). After heating the reaction mixtures, PCR amplification was carried out with these cycling conditions: 94°C for 3 min, followed by 35 cycles of 45 sec denaturation at 94°C, 45 sec annealing at the temperature for a particular primer pair (range 60–65°C), and 20 sec – 6 min at 68–72°C, with a final extension at 68–72°C for 7 min. Purified PCR-amplified fragments including 14 exons and their exon-intron boundaries were sequenced directly using fluorescent-labelled dideoxynucleotides (ABI, Foster City, WA). The sets of primers for genomic PCR were described previously [26].
KS determination
Plasma and urine KS concentrations of these patients and age-matched normal controls were measured by using an ELISA KS kit (Seikagaku Co, Tokyo Japan) [29]. The duplicate values for each KS standard and sample were averaged. The KS concentrations for each sample were determined using the generated KS standard curve.
Creatinine (Cr) was measured by mixing 10 μl of a 10-fold diluted urine sample with 50 μL saturated picric acid (Sigma, St. Louis, MO) and 50 μl 0.2 M NaOH. Absorbance at 490 nm was read after 15 min. All urine KS levels were normalized with urine Cr levels.
Urine GAG determination
Urine total GAGs were measured using dimethylmethylene blue (DMB) method. The method is based on spectrophotometric detection of metachromatic changes to the dye 1,9-dimethylmethylene blue resulting from GAG binding. Total GAG concentrations were subsequently normalized to urine Cr concentrations (milligrams of total GAGs per gram of urine Cr).
Genotype, phenotype, and KS correlation analyses
Determination of mutations, clinical severity, and plasma and urine KS measurements were performed independently and blinded before cross-referencing to clinical data. Of an original cohort of 55 patients, 46 patients who completely filled out the clinical questionnaire were studied for the genotype-phenotype correlation analysis. MPS IVA patients in this study originated from 20 countries. Among the 55 patients, 36.4% were from the USA, 14.5% from Canada, 18.2% from European countries, 14.5% from Latin America, and 16.4% from Asian countries.
The genotype/phenotype relation for each mutation was determined, based on the following four factors: i) the homozygosity of the mutation, ii) the level of residual activity determined by in vitro expression study with reported mutations, iii) prediction of the likely change in the protein structure, and iv) patients with an attenuated phenotype with an allele permitting residual enzyme activity [15, 28].
Variability of plasma and urine KS levels in both MPS IVA patients and normal controls were plotted according to age. Student’s t-test was applied for the comparison of patient vs normal control samples and patients with a severe phenotype vs patients with an attenuated phenotype. All data analyses were performed using SPSS Student Version 15.0 statistical software.
RESULTS
Clinical characteristics (Table 1)
Based on their growth charts, 36 patients were defined as severe and the other 13 were defined as attenuated.
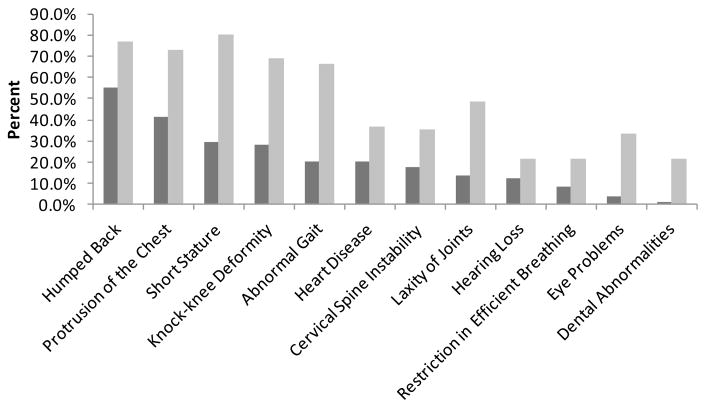
The mean of birth length for boys and girls was 51.8 ± 2.9 cm (n = 25) and 51.8 ± 3.7 cm (n = 19), respectively, which corresponded to +0.67 SD for boys (50 ± 2.7 cm) and +0.8 SD for girls (49.3 ± 2.2 cm) on the CDC growth charts. The final heights for male and female patients with severe phenotypes were 102 ± 9.74 cm and 99.1 ± 7.74 cm, respectively. The final heights for male and female patients with attenuated phenotypes were 136 ± 12.37 cm and 134 ± 12.37 cm, respectively. Initial and current symptoms exhibited in MPS IVA patients are shown in Figure 1, indicating the progression of skeletal dysplasia. Orthopedic complications are the most critical issues for MPS IVA patients. In this study, 47.9% (23 out of 48) of the patients underwent orthopedic interventions. Cervical fusion was performed starting at 2 years old prophylactically, without any clinical sign and/or symptom. Thereafter, the femoral realignment osteotomies and hip reconstructive surgery were performed.
Figure 1. Comparison of initial and current clinical symptoms in MPS IVA patients.
Distribution of clinical symptoms among the 55 patients who responded to the questionnaire. Dark gray bars show initial symptoms while light gray bars show current symptoms.
Spectrum of mutations
In total, 53 different disease-causing mutations were identified including 19 novel mutations. One hundred-three of 110 mutant alleles (93.6%) were defined, and seven alleles remain unidentified. These undefined mutations could be located in the control region or an intron. Alternatively, the mutation could be large deletion or large rearrangement in which we may not be able to define the site by the current mutation detection strategy. The identified mutations are summarized in Table 2. Among 53 mutations, most were missense mutations (77%). The frequency of each type of mutation in a total of 103 mutant alleles showed 86 alleles with missense mutations, 3 with nonsense mutations, 7 with deletions, 5 with splice-site mutations, and 2 with insertions. Thirty-three mutations were previously reported [15, 26–28, 30]. Nineteen of the mutations are described here for the first time (Table 2). No mutation in exon 14 of the GALNS gene has previously been reported; however, we have identified two novel mutations in exon 14. The mutation c.1567T>G (p.X523EextX93) creating a stop codon was identified in a Chinese patient. The mutation c.1485C>G (p.N495K) was identified in two unrelated Caucasian patients. All the novel mutations above were not found in any of over 200 normal control alleles.
Table 2.
Mutations identified in this study
| Nucleotide change | Effect on amino acide | Exon | Degree of conservation of aa | Conservativeness of aa change | Phenotype | Times Identified | Ethnicity | References |
|---|---|---|---|---|---|---|---|---|
| c.3G > A | p.M1I | 1 | Conserved | Conservative | severe | 1 | Fi | |
| c.29G>A | p.W10X | 1 | Non-conservative | severe | 1 | Au, It | Bunge et al, [1997], Tomatsu et al. [2004d] | |
| c.121-1G>A | p.M41RfsX46 | intron 1 | severe | 1 | Am-Ca | |||
| c.121-1G>C | p.M41RfsX46 | intron 1 | severe | 1 | Uk | Tomatsu et al. [2005] | ||
| c.121A>T | p.M41L | 2 | Conserved | Conservative | attenuated | 2 | Am-Ca (Fr), Ac, Sp | Tomatsu et al. [2004c] |
| c.122T>A | p.M41K | 2 | Conserved | Non-conservative | severe | 2 | Am-Ca | |
| c.125G>A | p.G42E | 2 | Conserved | Non-conservative | severe | 1 | It | Tomatsu et al. [2005] |
| c.139G>A | p.G47R | 2 | Conserved | Non-conservative | severe | 2 | Po, Ho | Bunge et al. [1997], Tomatsu et al. [2004c] |
| c.181C>T | p.R61W | 2 | Mammal-specific | Non-conservative | attenuated | 1 | Am-Ca | |
| c.230C>G | p.P77R | 2 | Conserved | Non-conservative | severe | 2 | SA, In, Am-Ca | Tomatsu et al. [1995b], Tomatsu et al. [2004c] |
| c.244T>C | p.S82P | 2 | Conserved | Semi-conservative | attenuated | 2 | Ca | |
| c.245C>T | p.S82L | 3 | Conserved | Non-conservative | severe | 1 | Am-Ca | |
| c.257T>C | p.L86P | 3 | Conserved | Non-conservative | severe | 1 | Fi | |
| c.278T> A | p.I93N | 3 | Conserved | Non-conservative | severe | 1 | Am-Ca | |
| c.280C>T | p.R94C | 3 | Conserved | Non-conservative | severe | 2 | Br, Am-Ca, Ca, Cr | Cole et al., [1996] |
| c.320-1G>T | p.A107GfsX54 | intron 3 | undefined | 1 | Chil | |||
| c.337A>T | p.I113F | 4 | Conserved | Conservative | severe | 1 | Am-Ca, Ir, Bt, Ge, Da | Tomatsu et al. [1995], Yamada et al. [1998], Tomatsu et al. [2004a] |
| c.346G>A | p.G116S | 4 | Conserved | Conservative | severe | 4 | Am-Ca, Br, Tu | Tomatsu et al. [2004c], Tomatsu [2005] |
| c.374C>T | p.P125L | 4 | Conserved | Non-conservative | severe | 1 | It, Jp | Tomatsu et al. [1997], It [2004b] |
| c.405_422+1del19 | p.S135fsX94 | exon & intron 4 | severe | 1 | Ca -Ca | |||
| c.415G>A | p.G139S | 4 | Conserved | Conservative | severe | 2 | Sp, Am-Bl, Ir, Ar, Am-Ca, Br | Tomatsu et al. [1997], Tomatsu et al. [2004a], Tomatsu et al. [2004c], Tomatsu [2005] |
| c.422+2_+3insT | p.W141fsX41 | intron 4 | severe | 2 | Iraqi | |||
| c.452C>T | p.P151L | 5 | Conserved | Non-conservative | severe | 2 | Pa, Pk, Jp | Tomatsu et al. [1995b] |
| c.477G>A | p.W159X | 5 | Non-conservative | severe | 2 | Am-Ca | ||
| c.485C>T | p.S162F | 5 | Mammal-specific | Non-conservative | severe | 2 | Co | Kato et al. [1997] |
| c.498delC | p.H166fsX32 | 5 | severe | 2 | Br, Am-Ca | unpublished | ||
| c.608C>T | p.A203V | 6 | Non-conserved | Semi-conservative | severe | 2 | Br | unpublished |
| c.612C>G | p.N204K | 6 | Conserved | Non-conservative | attenuated | 2 | Am-Ca, Jp | Fukuda et al. [1992], Ogawa et al., [1995], Tomatsu et al., [2004c] |
| c.634-1 G>T | p.E212VfsX9 | intron 6 | undefined | 1 | Gr | |||
| c.697G>A | p.D233N | 7 | Conserved | Conservative | severe | 1 | Ge, Po | Tomatsu et al. [2004c] |
| c.715G>T | p.V239F | 7 | Conserved | Conservative | severe | 2 | Br | Tomatsu et al. [2005] |
| c.740G>A | p.G247D | 7 | Conserved | Non-conservative | severe | 2 | Am-Ca, Ge, Nw | Bunge et al. [1997] |
| c.758G>A | p.R253Q | 7 | Mammal-specific | Conservative | attenuated | 1 | Al | |
| c.761A>G | p.Y254C | 8 | Conserved | Non-conservative | undefined | 1 | Am-Ca | |
| c.776G>A | p.R259Q | 8 | Non-conserved | Conservative | attenuated | 1 | Au, Po | Bunge et al. [1997], Tomatsu et al. [2004c] |
| c.850T>G | p.F284V | 8 | Vertebrate-specific | Conservative | attenuated | 2 | Tu | Yamada et al. [1998] |
| c.853_855delTTC | p.F285del | 8 | severe | 1 | Am-Ca (Br/Ge/Port), It | Tomatsu et al. [2004d] | ||
| c.860C>T | p.S287L | 8 | Conserved | Non-conservative | severe | 4 | Am-Ca, Au, Mac, Tu, Gr, Po | Bunge et al. [1997], Tomatsu et al. [2004c] |
| c.898+1G>A | p.G300DfsX34 | intron 8 | severe | 1 | Bt, Le, Uk | Bunge et al. [1997], Tomatsu [2005] | ||
| c.901G>T | p.G301C | 9 | Vertebrate-specific | Non-conservative | severe | 6 | Am-Ca, Bt, Br, Fr, Sp, Co, Mo, Pt | Kato et al. [1997], Bunge et al. [1997], Tomatsu et al. [2004c], Tomatsu [2005] |
| c.922T>C | p.C308R | 9 | Conserved | Non-conservative | severe | 1 | Al | |
| c.938C>T | p.T313M | 9 | Conserved | Non-conservative | severe | 2 | Ca -Ca | |
| c.953T>G | p.M318R | 9 | Conserved | Non-conservative | severe | 1 | Ch, Jp | Ogawa et al. [1995] |
| c.975G>T | p.W325C | 9 | Conserved | Non-conservative | attenuated | 1 | Ca -Ca | unpublished |
| c.1023C>G | p.S341R | 10 | Mammal-specific | Non-conservative | severe | 5 | Br, SL | Tomatsu et al. [2004e], Tomatsu [2005] |
| c.1156C>T | p.R386C | 11 | Conserved | Non-conservative | severe | 7 | Bt, Ca -Ca, Jp, It, Mx, Po, Tu, Ge, Ar, Ch, Br, Am-Ca, Co | Fukuda et al. [1996a], Tomatsu et al. [1997], Bunge et al. [1997], Tomatsu et al. [2004c], Tomatsu et al. [2004e],Tomatsu et al. [2004d], Tomatsu [2005] |
| c.1171A>G | p.M391V | 11 | Conserved | Conservative | attenuated | 5 | Am-Ca, Br, Ca -Ca, Ir | Tomatsu et al. [1995b], Tomatsu et al. [1997], Tomatsu et al. [2004c] |
| c.1195delA | p.K399fsX41 | 11 | severe | 1 | Ira | |||
| c.1219A>C | p.N407H | 11 | Conserved | Semi-conservative | severe | 2 | Am-Ca, Du, Fr, Ge, It | Bunge et al. [1997], Tomatsu [2005] |
| c.1354T>A | p.F452I | 12 | Mammal-specific | Conservative | attenuated | 3 | Ca -Ca, Am-Ca (Ir/En) | Tomatsu [2005] |
| c.1480A>G | p.M494V | 13 | Vertebrate-specific | Conservative | severe | 1 | Ca -Ca, Tu | Bunge et al. [1997] |
| c.1485C>G | p.N495K | 14 | Conserved | Non-conservative | undefined | 2 | Am-Ca, Ir/En | |
| c.1567T>G | p.X523EextX93 | 14 | severe | 1 | Ch |
Al: Albanian, Am-Ca: American Caucasian, Am-Bl: American-Black, Ar: Argentine, Au: Austrian, Br: Brazilian, Bt: British, Ca-Ca: Canadian Caucasian, Ch: Chinese, Chil: Chilean, Co: Colombian,
Da: Danish, Du: Dutch, Fi: Finnish, Fr: French, Ge: German, Gr: Greek, Hi: Hispanic, In: Indian, It: Italian, Ira: Iraq, Ir: Irish, Jp: Japanese, Mac: Macedonian, Mx: Mexican, Nw: Norwegian, Pk: Pakistani, Po: Polish, Pt: Portuguese, SA: Saudi Arabian, Sw: Swedish, SL: Sri Lanka, Sp: Spanish, Tu: Turkish, un: unknown, Uk: Ukrainian
Highlighted in gray: novel mutations
Genotype-phenotype correlations (Table 1)
Missense Mutation
Nine novel missense mutations were identified. Three of these mutations (p.M41K, pS82P, p.T313M) were detected in a homozygous form. A Caucasian American female patient (MO 170) with a severe clinical phenotype had p.M41K in a homozygous state. By 24 years of age, she had severe bone dysplasia and four surgeries of hip, knee, leg, and neck.
A 5.5-year-old Caucasian Canadian patient (MO 157) was homozygous for p.T313M mutation; his height suggested a severe clinical form with rapid progression.
A 10.2-year-old Caucasian Canadian patient (MO 166) had a c.244T>C (p.S82P) mutation in a homozygous state. The c.244T>C mutation may change the exonic consensus sequence of the splice donor site at the exon 2-intron 2 junction (ATgt>ACgt), leading to aberrant splicing.
In addition, 13 patients were found to be homozygous for previously reported missense mutations (p.M41L, p.G47R, p.P77R, p.P151L, p.S162F, p.N204K, p.V239F, p.F284V, p.S287L, p.G301C, p.S341R, p.R386C, p.M391V). A Caucasian American female patient (MO 154) homozygous for p.R386C mutation had a classical form of MPS IVA. Kyphoscoliosis was noticed at the age of 6 months. She developed progressive bone dysplasia (knock-knee, laxity of joints, protrusion of the chest, and abnormal gait) within one year, and fell frequently. The patient underwent cervical fusion at 2.2 years old.
A 4.1-year-old female patient (MO 147) had a p.G301C mutation in a homozygous state. Hump back was noticed at birth, and she was diagnosed at 6 months old. She underwent cervical fusion at the age of 2 years and both hip reconstruction and bilateral knee plates at the age of 4 years.
Three mutations (p.M41L, p.N204K, p.F284V) were defined as an attenuated type, while other mutations were defined as a severe type.
In total, 16 out of the 55 (29.1%) patients were homozygous for missense mutations. The missense mutations (p.G301C, p.R386C, p.M391V) are three of the more frequent mutations, accounting for 7.8%, 9.8%, and 9.8% of mutant alleles, respectively, in pan-ethnic populations [15].
Eight novel missense mutations were identified in a heterozygous state (p.M1I, p.S82L, p.L86P, p.I93N, p.Y254C, p.C308R, p.N495K, p.X523EextX93, p.R253Q). Two missense mutations of p.M1I and p.L86P were detected in a Filipino patient with a severe phenotype (MO 127), who had systemic bone deformity and underwent three surgeries on his neck. He has been wheelchair-bound from 8 years of age.
The mutation p.I93N was found in a Caucasian American boy (MO 126), who had severe growth retardation. He was presented with a kyphotic back detected at birth.
The p.Y254C and p.G247D mutations were identified in a 47-year-old Caucasian American female case (MO 167). The p.G247D mutation was previously detected in Caucasian German, Norwegian, and American patients with severe phenotypes [23, 26].
The p.N495K novel mutation was found in two unrelated Caucasian patients. The patient with an attenuated form was heterozygous for p.N495K and p.F452I (MO 107). The p.F452I mutation was also identified in two Caucasian Canadian siblings with an attenuated phenotype (MO 163, MO 163s). The other 4-year-old patient (MO 160) was a compound heterozygote for p.N495K and p.G116S. She was presented with kyphosis and had a prophylactic cervical fusion at 2 years of age. The p.G116S mutation was previously reported in Brazilian and Caucasian American patients defining a severe phenotype [15, 26] (MO 117, MO 117b, MO 159, MO 160 in this study).
The p.X523EextX93 mutation in exon 14 was identified in a Chinese patient with a severe phenotype (MO 144). The second mutant allele was p.M318R reported in a Japanese patient with a severe form [17].
A Caucasian American patient with a severe phenotype (MO 172) possessed the mutation c.245C>T (p.S82L), which could alter the acceptor splice site at the intron 2-exon 3 junction (agCG>agTG).An Albanian patient with an attenuated form (MO 101) was a compound heterozygote for novel missense mutations, c.758G>A (p.R253Q) and c.922T>C (p.C308R). The c.758G>A mutation is located in the exonic consensus sequence of the splice donor site at the exon 7-intron 7 junction (CGgt>CAgt) and may cause aberrant splicing.
Nonsense mutation
Two different nonsense mutations [c.29G>A (p.W10X), c.477G>A (p.W159X)] were seen in 2 of the 55 patients. The p.W10X mutation was found in an Austrian patient with an attenuated form (MO 125). The p.W10X mutation defining a severe phenotype accounted for 10 mutant alleles reported in the GALNS gene to date. The second mutation was p.R259Q defining an attenuated form [23].
The patient with a severe phenotype possessed a novel nonsense mutation, p.W159X, in a homozygous form, and became wheel-chair bound at 11 years old (MO 138).
Small deletion
Four deletion mutations were identified and two of them (c.405_422+1del19, c.1195_1196delA) were novel. These novel deletions were private and confined to the individual. The c.405_422+1del19 mutation was found in a Caucasian Canadian boy with a severe phenotype (MO 165). At 7.0 years old, he presented short stature, cervical spine instability, and hearing loss. The mutation was a 19 base-pair deletion [c.405_422+1del19 (p.S135fsX94)], spanning the exon 4-intron 4 junction and resulting in a 94 amino acid frame-shift. Thesecond mutation of this patient was a missense mutation, p.M494V. This mutation was previously described in two mutant alleles from Turkish patients with a severe phenotype [23]. Both mutations are consistent with the severe phenotype of this patient.
The novel small deletion in exon 11 (c.1195_1196delA) was found in a boy with a severe form from Iraq (MO 148). At 4.4 years of age, he had systemic bone dysplasia, abnormal gait, hearing loss, and laxity of joints, and fell frequently. The second mutation of this patient is a novel insertion mutation (c.422+2_+3insT) as described below. His sister was 2 years old when presented with the same clinical manifestations (MO 148s).
The deletion, c.498delC, results in a 198-amino acid protein product; hence, it is likely to be nonfunctional and degraded. This mutation was identified in 3 patients. Two siblings with a unique clinical course had compound heterozygous mutations for c.498delC and p.R61W (MO 121, MO 121s) (Fig. 4-2). The mutation p.R61W, a non-conservative amino acid change at a non-conserved residue, was classified as attenuated [15]. By 2 years of age, the older brother was not talking and seemed to have a hearing problem. Chronic and recurrent ear infections prompted tonsillectomy and adenoidectomy, and a set of middle ear tubes was placed by 3 years of age. Behavior problems were noted at 6 years of age with complex partial seizure, and later multiple behavioral/psychiatry diagnoses were suggested. By the same age the patient’s appetite picked up and he began to gain significant weight. He was evaluated for Prader Willi syndrome and Fragile X syndrome; both tests were negative. Genu valgum was noted at 8 years of age, and ankle/foot orthoses were provided. He was 21 kg overweight for his height. Additional features included: coarse curly brown scalp hair, up and outward slanting palpebral fissures, broad nasal bridge at the root, and dental crowding. Skeletal habitus revealed a short trunk, significant genu valgum, and bilateral pronated pes planus. Thoraco-lumbar platyspondyly was noted as was odontoid hypoplasia without instability. Epiphyseal changes were present throughout. He was clinically diagnosed with an unclassified form of spondyloepiphyseal dysplasia. Distal femoral realignment osteotomies were performed for the genu valgum at ages 10 and 14 years. He underwent posterior spinal fusion for thoracic kyphoscoliosis at 16 years of age. There was no significant joint laxity noted. Ophthalmologic evaluation revealed corneal clouding. Initial urine screening studies showed elevated KS, and further biochemical and molecular studies confirmed the abnormality in GALNS. Following this discovery, the younger sister was studied and found to have the same MPS IVA variant. Mild corneal clouding was seen. Radiographic features of spondyloepiphyseal dysplasia were present. Growth in both children ceased before puberty.
A 19-year-old Caucasian American patient with a severe phenotype (MO 172) had compound heterozygosity for c.498delC and a novel splice-site mutation c.245C>T. He needed a wheelchair from 18 years of age.
The mutation c.853_855delTTC was found in a Caucasian American boy with a severe form (MO 100). This deletion c.853_855delTTC that produces a 285-amino acid product was reported previously in an Italian patient with a severe phenotype [26].
Insertion
Only one novel insertion (c.422+2_+3insT) was identified in this study. This insertion mutation at exon/intron boundary changes the consensus splicing sequence, presumably producing a 182-amino acid protein product and resulting in absence of intron 4 splicing. The Iraqi patient with a severe phenotype (MO 148, MO 148s) was a compound heterozygote for c.422+2_+3insT and c.1195delA as mentioned above.
Splicing site mutation
Five splice-site mutations (c.121-1G>A, c.121-1G>C, c.320-1G>T, c.634-1G>T, c.898+1G>A) were identified in 4 out of the 55 MPS IVA patients studied and accounted for 5% of mutant alleles. Four of these splice-site mutations were novel (all except c.898+1G>A).
The c.121-1G>A novel intronic mutation is a private mutation that results in a 124-base deletion, corresponding to the 5′end of exon 2. This skipping results in a 46 amino acid frame-shift and a premature stop codon, giving a truncated protein (p.M41RfsX46) (MO 151). The second disease causing alteration is a reported missense mutation (p.I113F). The p.I113F is one of the most prevalent recurrent mutations in the GALNS gene. This mutation is specific to British/Irish populations; accounting for 18% of all British/Irish mutations and correlating with a severe clinical form [19, 25]. The patient with these two mutations displayed severe bone dysplasia: short stature, cervical spine instability, teeth problems, hearing loss, hepatomegaly, and laxity of joints. He had undergone three surgeries by 3.8 years of age.
A Chilean patient (MO 106) possessed a novel c.320-1G>T (p.A107GfsX54) mutation and had an attenuated phenotype. The mutation, c.320-1G>T, occurred at the acceptor site of intron 3 and causes exon 3 skipping to continue into intron 3, resulting in a frame-shift and premature stop codon. The novel splice-site mutation, c.634-1 G>T (IVS6-1g>t), was identified in a Greek infant (0.8 year old) (MO 129). This mutation occurs at the acceptor site of intron 6 and results in a 9 amino acid frame-shift and a premature stop codon (p.E212VfsX9). The second mutation of this patient was p.S287L. This missense mutation was found in Polish, Austrian, and Caucasian American patients with a severe phenotype [23, 26]. The p.S287L (c.860C>T) mutation was identified in 3 patients in this study (a homozygous form in a Macedonian patient with a severe phenotype; a heterozygous form in a Turkish and Greek patient). The c.898+1G>A mutation (p.G300DfsX34) was seen in a Caucasian Ukrainian with a severe phenotype (MO 105). This mutation occurs in intron 8 at the donor site. The patient possessed another splicing mutation, c.121-1G>C (p.M41RfsX46). Both splicing mutations led to premature termination. She presented severe bone dysplasia: severe short stature, corneal clouding, hearing loss, hepatomegaly, valvular heart disease, and laxity of joints.
Overall, we have identified 39 mutations as severe and 10 mutations as attenuated, while the other 4 mutations were undefined.
Plasma and urine KS concentrations
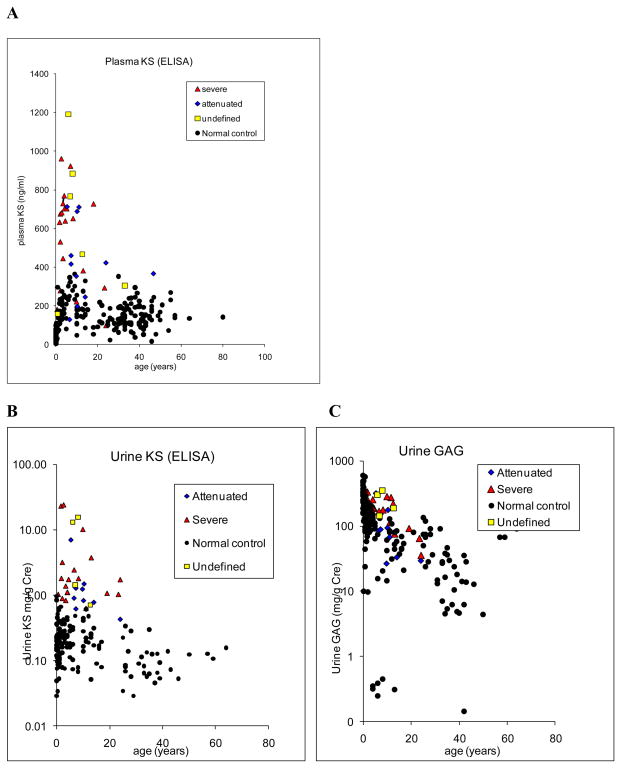
Blood KS concentrations were found to vary markedly with age (Fig. 2A). For the normal control individuals, during the first 5 years of life, blood KS concentrations rose progressively reaching a peak between 5 and 15 years of age. After reaching teen ages, blood KS concentrations decreased until 20 years old and stabilized thereafter. In MPS IVA patients the trend was the same. KS levels in patients between 0.5 and 5 years were 3.8 times higher than those in age-matched controls (Table 3). The values in patients between 5 and 15 years were 2.7 times higher than those in age-matched controls (Table 3). When MPS IVA patients over 15 years of age were compared with the age-matched controls, the blood concentrations of KS were different between the groups. The values in patients over 15 years were 2.2 times higher (Table 3). Six patients under 20 years had the values within the normal range. Blood KS in severe MPS IVA patients was 1.3 times higher than that in attenuated patients (550 ± 245 ng/ml vs 427 ± 203 ng/ml; p = 0.073). Urine KS concentrations varied with age (Fig. 2B). During the first 6 months of life, urine KS concentrations for the control group were ranged between 0.01 and 0.97 mg/g Cr. For the normal control individuals between 0.5 and 15 years of life, urine KS concentrations were not changed (Table 3). After 15 years, urine KS concentrations decreased until age 20 years and stabilized thereafter (Table 3). In MPS IVA patients, urine KS concentration was the highest between 0.5 and 5 years. Values in patients between 0.5 and 5 years were 22.7 times higher than those in age-matched controls (Table 3). The values in patients between 5 and 15 years were 14.6 times higher than those in age-matched controls (Table 3). When compared with the age-matched controls over 15 years of age, urine KS concentrations were different between the groups (Table 3). After age 5 years, urine KS levels in MPS IVA began to decrease gradually. After 15 years of age, the KS excretion continued to decline and reached a plateau after 20 years of age. Urine KS in severe MPS IVA patients (n = 19) was three times higher than that in attenuated patients (n = 10) (4.4 mg/g vs 1.5 mg/g Cr, p = 0.111). Three patients under 20 years had the values within the upper limit of the normal range.
Figure 2. KS concentration in blood and urine.
A. Concentrations of blood KS in MPS IVA patients and normal individuals. Blood samples taken from 38 MPS IVA subjects (mean age, 9.8 years; range, 0.5–46.9 years) and 234 control subjects (mean age, 18.6 years; 0–50 years). KS levels ranged between 97 ng/ml and 1,192 ng/ml (mean, 526 ± 263 ng/ml) for MPS IVA patients and 3 and 323 ng/ml (122 ± 73 ng/ml) for control subjects.
B. Concentrations of urine KS in MPS IVA patients and normal individuals. Urine KS results taken from 33 MPS IVA subjects (mean age, 8.6 years; 0.5– 24 years) and 178 control subjects (mean, 9.7 years; range, 0–50 years).
C. Concentrations of urine GAGs in MPS IVA patients and normal individuals. Urine GAG results taken from 36 MPS IVA subjects (mean, 8.8 years; 0.5–24 years) and 165 control subjects (mean, 9.9 years; range, 0–50 years).
Table 3.
KS and GAG levels in MPS IVA patients and control subjects
| Control | Blood KS (ng/ml) | Urine KS (mg/gCr) | Urine GAGs (mg/gCr) | MPS IVA | Blood KS (ng/ml) | Urine KS (mg/gCr) | Urine GAGs (mg/g Cr) | |
|---|---|---|---|---|---|---|---|---|
| Under 0.5 years# | Mean | 46.5 | 0.22 | 287 | Mean | |||
| SD | 24.9 | 0.18 | 125 | SD | ||||
| n | 51 | 44 | 44 | n | ||||
|
| ||||||||
| 0.5 years to 5 years* | Mean | 149 | 0.24 | 144 | Mean | 567 | 5.5 | 217 |
| SD | 60 | 0.14 | 88 | SD | 240 | 9.0 | 85.1 | |
| n | 45 | 53 | 54 | n | 13 | 11 | 11 | |
|
| ||||||||
| 5 to 15 years** | Mean | 203.5 | 0.24 | 53.6 | Mean | 548 | 3.6 | 161 |
| SD | 82.7 | 0.14 | 42.6 | SD | 286 | 4.7 | 100 | |
| n | 32 | 44 | 29 | n | 19 | 18 | 21 | |
|
| ||||||||
| Over 15 years*** | Mean | 145.1 | 0.12 | 44.2 | Mean | 323 | 1.07 | 81.6 |
| SD | 59.4 | 0.07 | 32.9 | SD | 207 | 0.53 | 28.7 | |
| n | 106 | 37 | 38 | n | 6 | 4 | 4 | |
Data is not available in MPS IVA patient
Blood KS, P < 0.0001; urine KS, P = 0.082; urine GAGs, P = 0.021
Blood KS, P < 0.0001; urine KS, P = 0.008; urine GAGs, P < 0.0001
Blood KS, P = 0.089; urine KS, P = 0.037; urine GAGs, P = 0.073
In contrast to urine KS concentration, urine GAG concentration did not clearly distinguish the MPS IVA patients from normal individuals. Over 30% of MPS IVA patients (12 out of 35 patients) overlapped with the normal range. The results of urine GAG from 36 MPS IVA patients, and 165 control subjects are grouped by age and clinical severity (Fig. 2C). Urine GAGs concentrations varied markedly with age. During the first six months of life, the mean concentration of GAGs in urine for the control group was the highest between 9.9 and 607 mg/g Cr (Table 3). For the normal control individuals, urine GAGs concentrations declined gradually with age (Table 3). After 15 years, urine GAGs concentrations decreased further and stabilized thereafter. In MPS IVA patients, urine GAGs concentration was the highest between 0.5 and 5 years. Values in patients between 0.5 and 5 years were 1.5 times higher than those in age-matched controls (Table 3). The values in patients between 5 and 15 years were 3 times higher than those in age-matched controls (Table 3). When compared with the age-matched controls over 15 years of age, urine GAGs concentrations were not significantly different between the groups (Table 3). There was a significant relationship between clinical severity and urine GAGs excretion. Patients with a severe form (n = 21) demonstrated 185 mg/g Cr on average, whereas the patients with a milder form had 108 mg/g Cr (n = 10) (p < 0.05).
There was no significant correlation coefficient (r = 0.31) between urine KS and GAGs in MPS IVA patients. The same thing was true for the correlation coefficients between urine KS and blood KS and between blood KS and urine GAGs (r = 0.4 or r = 0.32).
DISCUSSION
We have characterized 55 MPS IVA patients and have attempted to predict which type of mutation could result in which phenotype as well as how KS levels correlated with the clinical phenotype. Results of this study indicate that (i) MPS IVA patients have extensive clinical and genetic heterogeneity, (ii) that genotype/phenotype correlation was defined in 88.5% of identified mutations, (iii) that clinical severity correlates with plasma and urine KS levels, demonstrating a potential biomarker for monitoring status and prognosticating MPS IVA, and (iv) that there was no strong correlation between blood KS and urine KS, indicating that the origin of blood and urine KS is different.
Clinical spectrum of MPS IVA
The age of presentation of MPS IVA is variable, as are the presenting signs and disease complications. It is important to note that individuals, who are diagnosed with an attenuated form of the disease, may still have symptoms and complications with significant morbidity and disability. Although the clinical course for the more severely affected patients is relatively predictable, there is considerable variability in the clinical phenotype and progression of the more attenuated form of the disease. The patients at the severe end of the spectrum are found to have skeletal manifestations of MPS IVA (gibbus and/or protrusion of the chest) by 6 months of age. At that time, it should be common to observe initial bone abnormalities detected by radiological methods, particularly ovoid vertebrae with a beaking sign, platyspondyly, and widening of the ribs. It is likely that most parents have noticed a hump back at birth or within a few months of age. Eventually, skeletal dysplasia involving the bones is observed in all phenotypes of MPS IVA to some degree.
The patients with an attenuated form like MO 121s were not diagnosed until 10 years old since the clinical phenotype was unlike Morquio-like change at an early stage. It is likely that the patients with an attenuated phenotype could have been misdiagnosed or undiagnosed until the spine X-ray pictures were taken.
Spectrum of mutations
Over 300 MPS IVA patients including the 55 current cases has been analyzed to date. A total of 185 mutations have been found to cause MPS IVA [15, 32–34]. Most mutations change single DNA building blocks (nucleotides) in the gene. We identified two exonic mutations in the last exon (exon 14) where no mutation was found in the previous studies. Thus, we proved that the mutations are distributed along the entire gene and exons.
Missense mutations are the most prevalent among GALNS mutations. The 10 most frequent mutations accounting for over seven mutant alleles are represented by single nucleotide changes except for one small deletion c.334delG. They make up around 35% of all described mutations. Among the most prevalent mutations, c.1156C>T (p.R386C), c.901G>T (p.G301C), c.337A>T (p.I113F), and c.1171A>G (p.M391V) were found also in this study. The remaining mutations occur fewer than six times in the total populations. The analysis of GALNS mutations in MPS IVA reveals considerable molecular heterogeneity, reflecting the diversity of clinical phenotypes.
Genotype/phenotype correlations
The genotype/phenotype relation for each of 185 mutations was determined as described in Materials and Methods [35]. Thus, 35 mutations were associated with an attenuated phenotype, while 127 mutations were associated with a severe phenotype. The other 23 mutations were not defined by the current information. Seventy-nine and 34 of 128 missense mutations were associated with severe and attenuated phenotypes, respectively. All 11 nonsense mutations and 2 large deletions were associated with severe phenotypes. Eighteen small deletions were associated with severe phenotypes. Five insertions were associated with severe phenotypes, and one with an attenuated one. Fourteen splice site mutations were associated with severe phenotypes, and one with an attenuated one.
The novel p.R253Q missense mutation was not located at an evolutionarily conserved position among sulfatase genes and the amino acid change was conservative, leading to an attenuated phenotype. The current and previous results showed that the likelihood of a missense variant causing MPS IVA was directly correlated with the level of evolutionary conservation and inversely correlated with conservativeness and that the combination of two factors, evolutionary conservation and conservativeness, provides a better association between missense variants and clinical severity with higher sensitivity (80–85%) and specificity (70–90%), than that obtained by either factor alone [28]. These findings suggest that the combination of evolutionary conservation and conservativeness is a useful tool to predict the effect of each mutation on the clinical phenotype in MPS IVA.
Phenotype and KS correlation
Identifying an accurate biomarker for assessing clinical severity, prognosis, and therapeutic efficacy of the patient is indispensable. A specific biomarker associated with improvement of physical activity or clinical severity is preferable. KS, which contributes more than 25% of the cartilage GAG in adults, is one of the most important components in bone. The degraded products of KS diffuse rapidly out of the chondrocytes into the blood stream and extracellular matrix (ECM). When KS is not degraded properly, it is stored mainly in chondrocytes and their ECM. Although pathological examinations of the bone and cartilage cells are useful for diagnosing and staging MPS IVA patients, it is not realistic to obtain biopsy samples from each individual patient. Measurements of KS concentrations in blood and urine should provide critical information of the clinical status. Age-dependent changes in KS turnover showed that the blood KS level rose progressively during the first 5 years of life, remained elevated until 12 years of age, and declined after the teenage years until it stabilized at age 20. Elongation of the long bones during growth occurs through a process of endochondral ossification where new cartilage is continuously laid down before it is degraded and replaced by bone. The decreased KS level after the teenage years is consistent with the fact that the growth rate in normal children begins to decline as children reach skeletal maturity. A lower rate of catabolism of proteoglycans in adult cartilage and/or decrease of mass of cartilage per body weight causes primary reduction of blood KS concentration. These maturation-related changes are supported by the present study on MPS IVA. Young MPS IVA patients have progressive destruction of cartilage tissues, releasing large quantities of KS. When the growth plate is closed or torn, synthesis of KS in cartilage will decline markedly. Patients with high levels of KS in blood likely represent severe cases of MPS IVA whose cartilage is overloaded with undegraded KS. The blood KS concentration in MPS IVA patients was the highest between 5 and 15 years of age. These findings indicate that the blood KS concentration in MPS IVA patients directly reflect the amount of stored KS in cartilage tissues. After 20 years of age, most MPS IVA patients are found to have blood KS levels within the normal range, suggesting that elevation of KS in MPS IVA is related to the rate of cartilage catabolism.
We could not observe a significant correlation between clinical severity and KS levels in blood and urine because of the small size of study. However, if we add the previous data [29, 30], urine and blood KS levels were significantly different between the patients (over 5 years) with attenuated and severe phenotypes (p < 0.003 and p < 0.05, respectively) (Table 4). There was also a significant difference in blood and urine KS between 5 and 15 years; however, the difference disappeared over 15 years old, suggesting that clinical severity is distinguished by KS levels only at a progressive stage.
Table 4.
KS levels in MPS IVA patients with attenuated and severe phenotypes
| Attenuated | Urine KS (mg/gCre) | Blood KS (ng/ml) | Severe | Urine KS (mg/gCre) | Blood KS (ng/ml) | |
|---|---|---|---|---|---|---|
| Over 5 years* | Mean | 2.4 | 410.4 | Mean | 6.7 | 564.4 |
| SD | 4.3 | 196.9 | SD | 6.4 | 359.4 | |
| n | 21 | 24 | n | 43 | 43 | |
|
| ||||||
| Under 5 years# | Mean | Mean | 11.0 | 573.9 | ||
| SD | SD | 12.7 | 224.1 | |||
| n | n | 20 | 23 | |||
|
| ||||||
| 5 to 15 years** | Mean | 3.5 | 486.4 | Mean | 8.6 | 689.5 |
| SD | 5.5 | 204.7 | SD | 7.1 | 349.1 | |
| n | 12 | 14 | n | 28 | 29 | |
|
| ||||||
| Over 15 years*** | Mean | 1.0 | 392.4 | Mean | 3.0 | 305.3 |
| SD | 1.0 | 361.7 | SD | 2.1 | 217.9 | |
| n | 9 | 10 | n | 15 | 14 | |
Urine KS, P = 0.0029; blood KS, P = 0.0385
Urine KS, P = 0.0199; blood KS, P = 0.0196
Urine KS, P = 0.6647; P = 0.7614
Data for urine KS is not available in the patients with an attenuated phenotype.
Although elevation of blood and urine KS is observed in MPS IVA patients, there is no explanation why correlation coefficient between blood and urine is weak. One of the hypotheses is that the origin of blood and urine KS is different. While blood KS directly derives from the chondrocytes, urine KS is filtered in kidneys or originated from stored KS in kidneys, resulting in a smaller molecule compared with blood KS.
To understand age dependency of blood and urine KS concentrations, normalization of KS in adult MPS IVA, and property difference between blood and urine KS, it is required to measure KS levels sequentially at different ages in the same individuals. It is also noteworthy to investigate blood and urine KS from a group of the patients with the same genotype. Further studies including a larger number of cases lead to clarification of the correlation between a certain genotype and the extent of elevation of KS.
The diagnosis of MPS IVA is established in the metabolic laboratory for patients with characteristic physical and radiographic features. At the initial stage, urine screening supports the diagnosis. However, urine GAGs in 10–20% of patients are within normal limits, leading to misdiagnosis of the patient [31]. This fact is explained by the findings that KS accounts for only a few percents of urine GAGs and that an increase of urine KS does not always reflect the level of urine GAGs. Therefore, once the patient is clinically suspected as MPS IVA, the assay for KS in blood and urine is preferable, followed by GALNS enzyme assay in peripheral leucocytes or fibroblasts. GALNS mutation screening provides solid confirmation of the diagnosis of MPS IVA and valuable information of the patient’s prognosis.
Some proportion of MPS IVA patients should be recognizable clinically at birth with gibbus deformity and successive spine X-ray pictures. Given an unknown mutation at the early stage of MPS IVA, it is important to predict the clinical course and to understand therapeutic efficacy (such as hematopoietic stem cell therapy and ERT) before its application. Drawing up ground-rules for assessing the nature of each mutation and predicting genotype/phenotype/biomarker correlations should contribute to significant improvements in both the design and efficacy of treatment strategies. In addition, early knowledge of the genotype should favor gathering clinical information more prospectively and may influence the development of successful treatments. Cataloguing of GALNS mutations and cross sectional and longitudinal KS levels will provide more accurate information.
In conclusion, this study provides a new approach to determining the prognosis of the disease and clear correlations between genotype, phenotype, and KS levels.
Highlights.
Characterize clinical, biochemical, and molecular findings in MPS IVA patients.
Seek correlations between genotype, phenotype, and blood and urine KS levels.
The data here allow us to predict clinical severity more precisely.
Blood and urine KS concentrations in MPS IVA patients were age-dependent.
Provides evidence for extensive allelic heterogeneity of MPS IVA
Acknowledgments
This work was supported by grants from Ariana’s Cure Fund for Morquio, Austrian MPS Society, Bennett Foundation, International Morquio Organization (Carol Ann Foundation), and Jacob Randall Foundation. The content of the paper has not been influenced by the sponsors.
Abbreviations
- C6S
Chonroitin-6-sulfate
- CDC
Centers for Disease Control and Prevention
- Cr
Creatinine
- DMB
dimethylmethylene blue
- DMSO
dimethylsulfoxide
- GAGs
Glycosaminoglycans
- GALNS
N-acetylgalactosamine-6-sulfate sufatase
- IMO
International Morquio Organization
- KS
Keratan sulfate
- LSD
Lysosomal storage disorder
- MPS IVA
Mucopolysaccharidosis IVA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lowry RB, Applegarth DA, Toone JR, MacDonald E, Thunem NY. An update on the frequency of mucopolysaccharide syndromes in British Columbia. Hum Genet. 1990;85:389–390. doi: 10.1007/BF00206770. [DOI] [PubMed] [Google Scholar]
- 2.Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 3.Nelson J. Incidence of the mucopolysaccharidoses in Northern Ireland. Hum Genet. 1997;101:355–358. doi: 10.1007/s004390050641. [DOI] [PubMed] [Google Scholar]
- 4.Nelson J, Crowhurst J, Carey B, Greed L. Incidence of the mucopolysaccharidoses in Western Australia. Am J Med Genet A. 2003;123:310–313. doi: 10.1002/ajmg.a.20314. [DOI] [PubMed] [Google Scholar]
- 5.Pinto R, Caseiro C, Lemos M, Lopes L, Fontes A, Ribeiro H, Pinto E, Silva E, Rocha S, Marcao A, Ribeiro I, Lacerda L, Ribeiro G, Amaral O, Sa Miranda MC. Prevalence of lysosomal storage diseases in Portugal. Eur J Hum Genet. 2004;12:87–92. doi: 10.1038/sj.ejhg.5201044. [DOI] [PubMed] [Google Scholar]
- 6.Poorthuis BJ, Wevers RA, Kleijer WJ, Groener JE, de Jong JG, van Weely S, Niezen-Koning KE, van Diggelen OP. The frequency of lysosomal storage diseases in the Netherlands. Hum Genet. 1999;105:151–156. doi: 10.1007/s004399900075. [DOI] [PubMed] [Google Scholar]
- 7.Baehner F, Schmiedeskamp C, Krummenauer F, Miebach E, Bajbouj M, Whybra C, Kohlschutter A, Kampmann C, Beck M. Cumulative incidence rates of the mucopolysaccharidoses in Germany. J Inherit Metab Dis. 2005;28:1011–1017. doi: 10.1007/s10545-005-0112-z. [DOI] [PubMed] [Google Scholar]
- 8.Applegarth DA, Toone JR, Lowry RB. Incidence of inborn errors of metabolism in British Columbia, 1969–1996. Pediatrics. 2000;105:e10. doi: 10.1542/peds.105.1.e10. [DOI] [PubMed] [Google Scholar]
- 9.Montaño AM, Tomatsu S, Gottesman GS, Smith M, Orii T. International Morquio A registry: clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis. 2007b;30:165–74. doi: 10.1007/s10545-007-0529-7. [DOI] [PubMed] [Google Scholar]
- 10.Montaño AM, Tomatsu S, Brusius A, Smith M, Orii T. Growth charts for patients affected with Morquio A Disease. Am J Med Genet A 15. 2008;146A:1286–95. doi: 10.1002/ajmg.a.32281. [DOI] [PubMed] [Google Scholar]
- 11.Tomatsu S, Fukuda S, Masue M, Sukegawa K, Fukao T, Yamagishi A, Hori T, Iwata H, Ogawa T, Nakashima Y, Hanyu Y, Hashimoto T, Titani K, Oyama R, Suzuki M, Yagi K, Hayashi Y, Orii T. Morquio disease: isolation, characterization and expression of full-length cDNA for human N-Acetylgalactosamine-6-sulfate sulfatase. Biochem Biophys Res Commun. 1991;181:677–682. doi: 10.1016/0006-291x(91)91244-7. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima Y, Tomatsu S, Hori T, Fukuda S, Sukegawa K, Kondo N, Suzuki Y, Shimozawa Y, Orii T. Mucopolysaccharidosis IVA: molecular cloning of the human N-acetylgalactosamine-6-sulfatase (GALNS) gene and analysis of the 5N-flanking region. Genomics. 1994;20:99–104. doi: 10.1006/geno.1994.1132. [DOI] [PubMed] [Google Scholar]
- 13.Masue M, Sukegawa K, Orii T, Hashimoto T. N-acetylgalactosamine-6-sulfate sulfatase in human placenta: purification and characteristics. J Biochem. 1991;110:965–970. doi: 10.1093/oxfordjournals.jbchem.a123697. [DOI] [PubMed] [Google Scholar]
- 14.Pshezhetsky AV, Potier M. Association of N-acetylgalactosamine-6-sulfate sulfatase with the multienzyme lysosomal complex of beta-galactosidase, cathepsin A, and neuraminidase. Possible implication for intralysosomal catabolism of keratan sulfate. J Biol Chem. 1996;271:28359–28365. doi: 10.1074/jbc.271.45.28359. [DOI] [PubMed] [Google Scholar]
- 15.Tomatsu S, Montaño AM, Nishioka T, Gutierrez MA, Pena OM, Tranda Firescu GG, Lopez P, Yamaguchi S, Noguchi A, Orii T. Mutation and polymorphism spectrum of the GALNS gene in mucopolysaccharidosis IVA (Morquio A) Hum Mutat. 2005;26:500–512. doi: 10.1002/humu.20257. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda S, Tomatsu S, Masue M, Sukegawa K, Iwata H, Ogawa T, Nakashima Y, Hori T, Yamagishi A, Hanyu Y, Morooka K, Kiman T, Hashimoto T, Orii T. Mucopolysaccharidosis type IVA N-Acetylgalactosamine-6-sulfate sulfatase exonic point mutation in classical Morquio and mild cases. J Clin Invest. 1992;90:1049–1053. doi: 10.1172/JCI115919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogawa T, Tomatsu S, Fukuda S, Yamagishi A, Rezvi GM, Sukegawa K, Kondo N, Suzuki Y, Shimozawa N, Orii T. Mucopolysaccharidosis IVA: screening and identification of mutations of the N-acetyl galactosamine-6-sulfate sulfatase gene. Hum Mol Genet. 1995;4:341–349. doi: 10.1093/hmg/4.3.341. [DOI] [PubMed] [Google Scholar]
- 18.Tomatsu S, Fukuda S, Cooper A, Wraith JE, Rezvi MG, Yamagishi A, Yamada N, Kato Z, Isogai K, Sukegawa K, Kondo K, Suzuki Y, Shimozawa N, Orii T. Mucopolysaccharidosis type IVA: identification of 6 novel mutations among non-Japanese patients. Hum Mol Genet. 1995a;4:741–743. doi: 10.1093/hmg/4.4.741. [DOI] [PubMed] [Google Scholar]
- 19.Tomatsu S, Fukuda S, Cooper A, Wraith JE, Rezvi GMM, Yamagishi A, Yamada N, Isogai K, Kato Z, Sukegawa K, Kondo N, Suzuki Y, Shimozawa N, Orii T. Mucopolysaccharidosis IVA: Identification of a common missense mutation I113F in the N-acetylgalactosamine-6-sulfate sulfatase gene. Am J Hum Genet. 1995b;57:556–563. [PMC free article] [PubMed] [Google Scholar]
- 20.Hori T, Tomatsu S, Nakashima Y, Uchiyama A, Fukuda S, Sukegawa K, Shimozawa N, Suzuki Y, Kondo N, Horiuchi T, Ogura S, Orii T. Mucopolysaccharidosis type IVA: common double deletion at the N-acetylgalactosamine-6-sulfate sulfatase gene. Genomics. 1995;26:535–542. doi: 10.1016/0888-7543(95)80172-i. [DOI] [PubMed] [Google Scholar]
- 21.Tomatsu S, Fukuda S, Yamagishi A, Cooper A, Wraith JE, Hori T, Kato Z, Yamada N, Isogai K, Sukegawa K, Kondo N, Suzuki Y, Shimozawa N, Orii T. Mucopolysaccharidosis IVA: four new exonic mutations in patients with N-acetylgalactosamine-6-sulfate sulfatase deficiency. Am J Hum Genet. 1996;58:950–962. [PMC free article] [PubMed] [Google Scholar]
- 22.Tomatsu S, Fukuda S, Cooper A, Wraith JE, Ferreira P, Di Natale P, Tortora P, Atsuko F, Kato Z, Yamada N, Isogai K, Yamagishi A, Sukegawa K, Suzuki Y, Shimozawa N, Kondo N, Sly WS, Orii T. Fourteen novel mucopolysaccharidosis IVA producing mutations in GALNS gene. Hum Mutat. 1997;10:368–375. doi: 10.1002/(SICI)1098-1004(1997)10:5<368::AID-HUMU6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 23.Bunge S, Kleijer WJ, Tylki-Szymanska A, Steglich C, Beck M, Tomatsu S, Fukuda S, Poorthuis BJH, Czartoryska B, Orii T, Gal A. Identification of 31 novel mutations in the N-acetylgalactosamine-6-sulfatase gene reveals excessive allelic heterogeneity among patients with Morquio A syndrome. Hum Mutat. 1997;10:223–232. doi: 10.1002/(SICI)1098-1004(1997)10:3<223::AID-HUMU8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 24.Kato Z, Fukuda S, Tomatsu S, Vega H, Yasunaga T, Yamagishi A, Yamada N, Valencia A, Barrera LA, Sukegawa K, Orii T, Kondo N. A novel common missense mutation G301C in the N-acetylgalactosamine-6-sulfate sulfatase gene in mucopolysaccharidosis IVA gene. Hum Genet. 1997;101:97–101. doi: 10.1007/s004390050594. [DOI] [PubMed] [Google Scholar]
- 25.Yamada N, Fukuda S, Tomatsu S, Muller V, Hopwood JJ, Nelson J, Kato Z, Yamagishi A, Sukegawa K, Suzuki Y, Shimozawa N, Kondo N, Orii T. Molecular heterogeneity in mucopolysaccharidosis IVA in Australia and Northern Ireland; nine novel mutations including T312S, common allele that confers a mild phenotype. Hum Mutat. 1998;11:202–208. doi: 10.1002/(SICI)1098-1004(1998)11:3<202::AID-HUMU4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 26.Tomatsu S, Nishioka T, Montaño AM, Gutierrez MA, Pena OS, Orii KO, Sly WS, Yamaguchi S, Orii T, Paschke E, Kircher SG, Noguchi A. Mucopolysaccharidosis IVA: identification of mutations and methylation study in GALNS gene. J Med Genet. 2004a;41:e98. doi: 10.1136/jmg.2003.018010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomatsu S, Filocamo M, Orii KO, Sly WS, Gutierrez MA, Nishioka T, Serrato OP, Di Natale P, Montaño AM, Yamaguchi S, Kondo N, Orii T, Noguchi A. Mucopolysaccharidosis IVA (Morquio A): identification of novel common mutations in the N-acetylgalactosamine-6-sulfate sulfatase (GALNS) gene in Italian patients. Hum Mutat. 2004b;24:187–188. doi: 10.1002/humu.9265. [DOI] [PubMed] [Google Scholar]
- 28.Tomatsu S, Montaño AM, Lopez P, Trandafirescu GG, Gutierrez MA, Oikawa H, Nishioka T, Vieira MB, Orii T, Noguchi A. Determinant factors of spectrum of missense variants in mucopolysaccharidosis IVA gene. Mol Genet Metab. 2006;89:139–149. doi: 10.1016/j.ymgme.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Tomatsu S, Okamura K, Taketani T, Orii KO, Nishioka T, Gutierrez MA, Velez-Castrillon S, Fachel AA, Grubb JH, Cooper A, Thornley M, Wraith E, Barrera LA, Giugliani R, Schwartz IV, Frenking GS, Beck M, Kircher SG, Paschke E, Yamaguchi S, Ullrich K, Isogai K, Suzuki Y, Orii T, Kondo N, Creer M, Noguchi A. Development and testing of new screening method for keratan sulfate in mucopolysaccharidosis IVA. Pediatr Res. 2004c;55:592–597. doi: 10.1203/01.PDR.0000113767.60140.E9. [DOI] [PubMed] [Google Scholar]
- 30.Tomatsu S, Dieter T, Schwartz IV, Sarmient P, Giugliani R, Barrera LA, Guelbert N, Kremer R, Repetto GM, Gutierrez MA, Nishioka T, Serrato OP, Montaño AM, Yamaguchi S, Noguchi A. Identification of a common mutation in mucopolysaccharidosis IVA: correlation among genotype, phenotype, and keratan sulfate. J Hum Genet. 2004d;49:490–494. doi: 10.1007/s10038-004-0178-8. [DOI] [PubMed] [Google Scholar]
- 31.Ohashi A, Montaño AM, Colón JE, Oguma T, Luisiri A, Tomatsu S. Sacral dimple: Incidental findings from newborn evaluation. Acta Paediatrica. 2009;98:768–769. 910–920. doi: 10.1111/j.1651-2227.2009.01134.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Zhang W, Wang Y, Meng Y, Su L, Shi H, Huang S. Mucopolysaccharidosis IVA mutations in Chinese patients: 16 novel mutations. J Hum Genet. 2010;55:534–540. doi: 10.1038/jhg.2010.65. [DOI] [PubMed] [Google Scholar]
- 33.Montaño AM, Sukegawa K, Kato K, Carrozzo R, Di Natale P, Christensen E, Orii KO, Orii T, Kondo N, Tomatsu S. Effect of ‘attenuated’ mutations in mucopolysaccharidosis IVA on molecular phenotypes of N-acetylgalactosamine-6-sulfate sulfatase. J Inherit Metab Dis. 2007a;5:758–67. doi: 10.1007/s10545-007-0702-z. [DOI] [PubMed] [Google Scholar]
- 34.Laradi S, Tukel T, Khediri S, Shabbeer J, Erazo M, Chkioua L, Chaabouni M, Ferchichi S, Miled M, Desnick RJ. Mucopolysaccharidosis type IV: N-acetylgalactosamine-6-sulfatase mutations in Tunisian patients. Mol Genet Metab. 2006;87:213–218. doi: 10.1016/j.ymgme.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Tomatsu S, Montaño AM, Dung VC, Grubb JH, Sly WS. Mutations and Polymorphisms in GUS Gene in Mucopolysaccharidosis VII (Sly Syndrome) Hum Mutat. 2009;30:511–519. doi: 10.1002/humu.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]