Abstract
Zn2+ is an essential ion that is stored in and co-released from glutamatergic synapses and it modulates neurotransmitter receptors involved in long-term potentiation (LTP). However, the mechanism(s) underlying Zn2+-induced modulation of LTP remain(s) unclear. As the purinergic P2X receptors are relevant targets for Zn2+ action, we have studied their role in LTP modulation by Zn2+ in the CA1 region of rat hippocampal slices. Induction of LTP in the presence of Zn2+ revealed a biphasic effect – 5–50 μm enhanced LTP induction, whereas 100–300 μm Zn2+ inhibited LTP. The involvement of a purinergic mechanism is supported by the fact that application of the P2X receptor antagonists 2′,3′-O-(2,4,6-trinitrophenyl) ATP (TNP-ATP) and periodate-oxidized ATP fully abolished the facilitatory effect of Zn2+. Notably, application of the P2X7 receptor-specific antagonist Brilliant Blue G did not modify the Zn2+-dependent facilitation of LTP. Exogenous ATP also produced a biphasic effect – 0.1–1 μm ATP facilitated LTP, whereas 5–10 μm inhibited LTP. The facilitatory effect of ATP was abolished by the application of TNP-ATP and was modified in the presence of 5 μm Zn2+, suggesting that P2X receptors are involved in LTP induction and that Zn2+ leads to an increase in the affinity of P2X receptors for ATP. The latter confirms our previous results from heterologous expression systems. Collectively, our results indicate that Zn2+ at low concentrations enhances LTP by modulating P2X receptors. Although it is not yet clear which purinergic receptor subtype(s) is responsible for these effects on LTP, the data presented here suggest that P2X4 but not P2X7 is involved.
Keywords: hippocampus, long-term potentiation, P2X, purinergic receptors, rat, zinc
Introduction
Long-term potentiation (LTP) in the CA1 region of the hippocampus is the most comprehensive model for studying activity-dependent synaptic modifications that underlie learning and memory (Malenka & Nicoll, 1999; Whitlock et al., 2006). Hippocampal glutamatergic synapses play a critical role in LTP. Notably, the vesicles within these synapses co-store neurotransmitter with Zn2+ (Assaf & Chung, 1984; Howell et al., 1984), a metal ion that inhibits both γ-aminobutyric acid (GABA) and N-methyl-d-aspartic acid (NMDA) receptor activity (Westbrook & Mayer, 1987), and potentiates α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor activity (Rassendren et al., 1990).
Although the effect of Zn2+ on synaptic plasticity has been widely investigated within the mossy fiber-CA3 synapses of the hippocampus, it has not been possible to draw clear conclusions as to its role in LTP induction. Studies using dietary depletion of zinc (Lu et al., 2000) and the Zn2+ chelators dithizone (Lu et al., 2000) or Ca-EDTA (Li et al., 2001) suggested that endogenous Zn2+ is required for the induction of LTP. However, another that analysed the effects of Ca-EDTA and the mouse mocha mutation (mutants lack vesicular Zn2+ within mossy fibers) on LTP suggested that Zn2+ is dispensible (Vogt et al., 2000), and in another study exogenous application of 100–300 μm Zn2+ blocked LTP (Xie & Smart, 1994). The role of Zn2+ in the Schaffer collateral-CA1 synapses of the hippocampus is even less clear. Two reports (Xie & Smart, 1994; Izumi et al., 2006) indicate that Zn2+ decreased NMDA receptor-mediated synaptic responses and reduced LTP, whereas others documented Zn2+-induced increases in NMDA receptor responses but no corresponding effect on LTP (Kim et al., 2002). Furthermore, a recent report showed that applying Zn2+ at low micromolar concentrations led to increased LTP induction (Takeda et al., 2009). These conflicting results led us to hypothesize that within the CA1 region, Zn2+ modulates LTP by acting on receptors other than NMDA receptors.
P2X purinergic receptors represent another possible target for Zn2+ in the regulation of LTP. P2X receptors constitute a family of proteins that are homo- and heteromeric channels; each channel is composed of three subunits of the seven cloned subtypes and is activated by ATP and/or structurally related nucleotide analogs (North, 2002). P2X receptors are widely expressed in the hippocampus (Kanjhan et al., 1999; Norenberg & Illes, 2000; Rubio & Soto, 2001) and contribute to ATP-mediated enhancement of LTP in the CA1 region (Wang et al., 2004). Zn2+ applied at concentrations of 1–100 μm potentiates the ATP-evoked cationic currents generated by both P2X2 and P2X4 receptor subtypes when expressed in a heterologous system (Xiong et al., 1999; Acuña-Castillo et al., 2000; Lorca et al., 2005; Huidobro-Toro et al., 2008), but inhibits the ATP-evoked currents of the P2X7 receptor expressed in the same context (Virginio et al., 1997; Acuña-Castillo et al., 2007).
Here we report that Zn2+ at low concentration enhances LTP, probably due to enhancement of P2X receptor function as two P2X receptor antagonists completely blocked this facilitatory effect. Higher Zn2+ concentrations reduced LTP, probably through inhibition of NMDA receptor activity. This dual role of Zn2+ may have important consequences for the regulation of plasticity, in the contexts of both normal physiologic and disease-associated changes in conditions of the brain circuitry.
Materials and methods
Ethical guidelines
All animal care and procedures described below are in accordance with the Chilean Council for Science and Technology Research (CONICYT) guidelines, and were reviewed and approved by the University of Santiago de Chile Animal Care and Experimental Use Committee.
Hippocampal slice preparations
Male Sprague-Dawley rats 3–4 weeks of age were decapitated under halothane anesthesia (4% inhaled until stimulation of the limb withdrawal reflex failed to elicit a response). Hippocampi were then dissected and transverse slices (350 μm thick) were cut with a vibratome (Campden Instruments, London, UK), in ice-cold dissection buffer containing (in mm): 212.7 sucrose, 5 KCl, 1.25 NaH2PO4, 3 MgSO4, 1 CaCl2, 26 NaHCO3 and 10 dextrose (pH 7.4, in 95% O2/5% CO2). Slices were transferred to a storage chamber and maintained at room temperature in artificial cerebrospinal fluid (ACSF) containing (in mm): 124 NaCl, 5 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, 26 NaHCO3 and 10 dextrose (pH 7.4, in 95% O2/5% CO2) for at least 1 h prior to recording.
Electrophysiology
In the recording chamber, slices were superfused with ACSF at a rate of 1–2 mL/min at 30 °C. Field excitatory postsynaptic potentials (fEPSPs) were evoked by square wave stimuli (0.2 ms) delivered with a concentric, bipolar stimulation electrode (200 μm diameter; FHC Inc., Bowdoinham, ME, USA) positioned in the Schaeffer collateral–commissural fibers, and recorded from the stratum radiatum in the CA1 region using a glass-pipette electrode (0.5–2 MΩ) filled with ACSF. A stable baseline was established for 15 min, with test pulses applied every 15 s, adjusted to evoke 50% of the maximal response. While the same stimulus intensity was applied, LTP was induced with theta burst stimulation (TBS: 10 trains, each with 10 bursts at 5Hz, each burst consisting of four pulses at 100 Hz). In most experiments, the recordings were continued for 60 min after TBS. The evoked responses were filtered at 10 kHz and digitized at 5 kHz using Igor Pro (WaveMetrics Inc., Lake Oswego, OR, USA). Each drug studied was diluted in ACSF and was applied by superfusion for 20 min in total (10 min before and 10 min after TBS). In one set of experiments, d(−)-2-amino-5-phosphonopentanoic acid (AP5) was co-applied with Zn2+ prior to and following TBS; subsequently, AP5 and Zn2+ were washed out over the course of 55 min. The same slice was then superfused with 10 μm Zn2+ for 20 min. An additional TBS was applied in the presence of Zn2+, and recordings were continued for a further 30 min. In the experiments using picrotoxin (PTX), a TBS was applied 20 min after PTX-superfused slices had produced stable basal responses; PTX was continuously applied throughout the course of the experiment. In these tests, the CA3 area of the hippocampus was dissected surgically under a microscope; the Schaffer collaterals were cut to avoid epileptiform activity induced by synaptic stimulation. In another set of experiments, NMDA receptor-mediated fEPSPs were isolated by blocking AMPA receptors with CNQX in Mg2+-free ACSF, as previously described (Andreasen et al., 1989). To differentiate between pre- and postsynaptic effects, we performed paired-pulse stimulation protocols: two pulses were given every 15 s, with an interstimulus interval of 20–2560 ms, and the time between pulses was doubled after each stimulation. This protocol, which assesses response facilitation by comparing the response to the second pulse with the response to the first pulse (paired pulse facilitation; PPF), was applied 20 min before and 30 min after TBS. PPF was also determined in the absence of TBS (baseline responses) and in the absence and presence of Zn2+ (10 μm) or ATP (1 or 5 μm). The results are presented as the ratio between the fEPSP slopes of the second and first responses.
Data analysis
Data are presented as mean ± SEM and normalized relative to baseline (averaged fEPSP slope obtained during at least 15 min of baseline response) prior to drug application or TBS induction. LTP magnitude (percentage baseline) was measured as the average of the final 10 min of recording (50–60 min after TBS, unless otherwise noted). Student’s t-test (for two groups) or anova (for three or more groups) were used for statistical comparison of mean fEPSP slopes, and were carried out using Graph Pad Prism software (San Diego, CA, USA); P < 0.05 was the cutoff for consideration as a significant result. Curve fitting and interpolation of the median effective concentration (EC50) were also obtained using Graph Pad Prism software. Amplitudes of responses during TBS were normalized to the maximal response of the initial burst and were fit with exponential functions of the form: 1 – Ae−B/τ, where B is the number of bursts in the train, τ is the number of bursts producing an e-fold decline in response amplitude, and 1 – A is the steady-state amplitude.
Drugs and chemicals
Zinc chloride (ZnCl2), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), AP5, PTX, adenosine 5′-triphosphate (ATP), adenosine 5′-(3-thiotriphosphate) tetralithium (ATPγS), 2-methylthio adenosine 5′-diphosphate (2-MeSADP), uridine-5′-triphosphate (UTP), adenosine, ivermectin (IVM), 2′,3′-O-(2,4,6-trinitrophenyl) adenosine 5′-triphosphate (TNP-ATP), periodate-oxidized adenosine 5′-triphosphate (oATP), Brilliant Blue G (BBG), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), 6-N,N-diethyl-β-γ-dibromomethylene-d-adenosine-5′-triphosphate (ARL67156), as well as all salts used in ACSF and dissection solutions were purchased from Sigma (St. Louis, MO, USA). 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM241385) was purchased from Tocris (Ellisville, MO, USA). Stock solutions of PTX, DPCPX and ZM241385 were prepared in dimethyl sulfoxide (DMSO); the final concentration of DMSO in the perfusion solution was < 0.01%.
Results
The effect of Zn2+ on LTP is biphasic
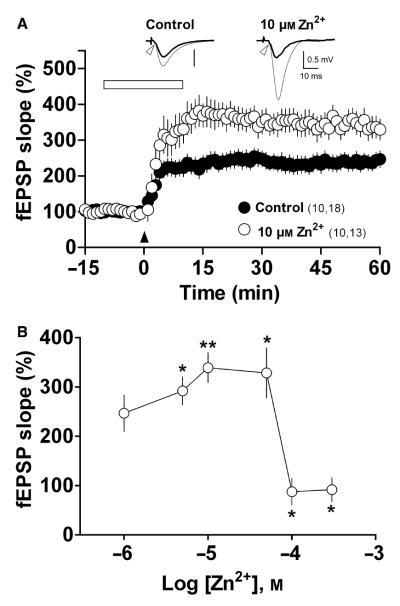
Application of 10 μm Zn2+ enhanced the LTP evoked by TSB in comparison with that evoked under control conditions (n = 13–18, respectively, P = 0.0024, Fig. 1A and B, Table 1). Similar increases were observed when 5 and 50 μm Zn2+ were applied (n = 10, P = 0.02 and n = 7, P = 0.04, respectively, Fig. 1B, Table 1). Notably, application of 1 μm Zn2+ caused no significant change in LTP (n = 11, P = 0.48, Fig. 1B, Table 1), and application of Zn2+ at higher concentrations (100 and 300 μm) abolished LTP (n = 4, P = 0.01, and n = 6, P = 0.03, respectively, Fig. 1B, Table 1). Together, these results indicate that Zn2+ has a biphasic effect on LTP: one that is clearly facilitatory across the range 5–50 μm, and one that is inhibitory across the range 100–300 μm.
Fig. 1.
Zn2+ modulates LTP in a biphasic manner. (A) LTP induced in the absence (control) or presence of Zn2+, with the metal applied to slices for 10 min before and 10 min after (open horizontal bar) the application of TBS (arrowhead). Symbols indicate the normalized fEPSP slope (means ± SEM). Numbers in parentheses: number of rats, number of slices. Inset – mean of five field responses obtained 12 min before (thick line) and 50 min after TBS (thin line), under both conditions. Open arrowheads indicate the presynaptic volley. (B) Zn2+concentration–response curve. Each symbol represents the fEPSP slope obtained 50–60 min after TBS (means ± SEM). *P < 0.05 and **P < 0.01 compared with LTP in control slices for each condition.
Table 1.
Effects of several concentrations of Zn2+ on LTP
| Percentage EPSP slope 50–60 min after TBS [mean ± SEM (n)] |
|||
|---|---|---|---|
| Zn2+ concentration (μm) |
Control | +Zn2+ | Significance* |
| 1 | 220.1 ± 26.7 (8) | 246.6 ± 37 (11) | NS |
| 5 | 218.4 ± 24 (10) | 292.1 ± 28.2 (10) | ↑ |
| 10 | 226.7 ± 19.1 (18) | 339.1 ± 30.2 (13) | ↑ ↑ |
| 50 | 209.6 ± 14.2 (7) | 328.5 ± 50.7 (7) | ↑ |
| 100 | 227.3 ± 27.4 (4) | 87.5 ± 27.1 (4) | ↓ |
| 300 | 203.7 ± 27.2 (3) | 91.5 ± 24.3 (6) | ↓ |
Key: ↑ and ↑↑, significant increase compared with control (P < 0.05 and P < 0.01, respectively); ↓, significant decrease compared with control (P < 0.05); NS, no significant difference compared with control.
The facilitatory effect of Zn2+ is a consequence of postsynaptic events
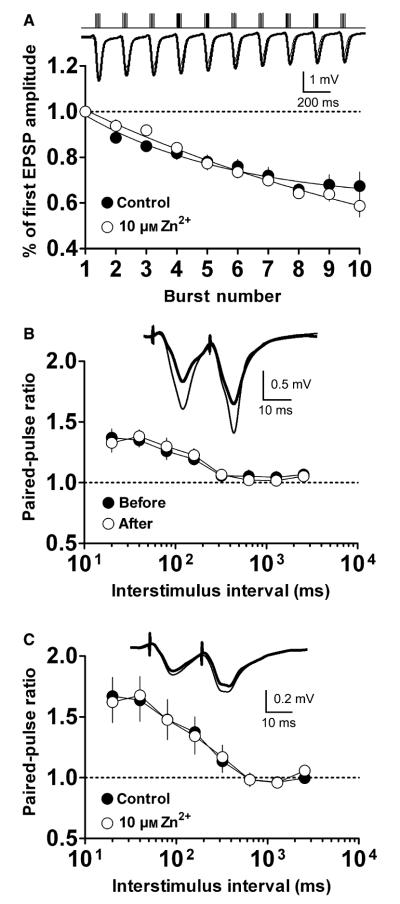
The enhancement of LTP by Zn2+ was not attributable to changes in the recruitment of presynaptic fibers, as 10 μm Zn2+ did not modify the presynaptic fiber volley response (Fig. 1A). Moreover, this concentration of Zn2+ did not affect synaptic responses, either to basal stimulation (as shown by an unaltered baseline fEPSPs during Zn2+ application; Fig. 1A) or to TBS (Fig. 2A). In PPF experiments, the paired-pulse ratio during any interstimulus interval remained unchanged by 10 μm Zn2+ facilitation of LTP (Fig. 2B); as in the case of the 20-ms interval, ratio values were not significantly modified (1.42 ± 0.04 before LTP vs. 1.48 ± 0.05 after Zn2+-facilitated LTP, n = 5, P = 0.21, Fig. 2B). Additionally, in control, unstimulated samples (basal stimulation only) there were no statistical differences between paired-pulse ratios at 20-ms intervals, regardless of whether 10 μm Zn2+ was present (1.67 ± 0.16 vs. 1.62 ± 0.17, respectively, n = 4, P = 0.44, Fig. 2C). In further control experiments in which no Zn2+ was applied, no significant difference in the paired-pulse ratio during the 20-ms interval was observed, either before or after LTP (1.37 ± 0.07 vs. 1.33 ± 0.08, n = 6, P = 0.35, data not shown). These results strongly suggest that the mechanism underlying the observed facilitation of LTP by Zn2+ is postsynaptic in nature.
Fig. 2.
The facilitatory effect of Zn2+ on LTP is postsynaptic. (A) fEPSPs evoked during TBS application were normalized to the first fEPSP and are plotted as the normalized response. Open circles – 10 μm Zn2+ (n = 6), closed circles – control (n = 6); means ± SEM. Curves were fitted to a single exponential (see Materials and methods). Inset – representative recordings of TBS responses for control (thick line) and 10 μm Zn2+- (thin line) treated slices; upper trace represents stimulus bursts. (B, C) Paired pulses separated by 20- to 2560-ms intervals recorded (B) before (closed circles, n = 6) or after Zn2+-facilitated (open circles, n = 6) LTP induction; and (C) during baseline stimulation before (control, closed circles, n = 4) and during 10 μm Zn2+ application (open circles, n = 4). Symbols represent ratios of the fEPSP slope of the second vs. the first pulse ± SEM, for each interval. Insets – representative traces of three mean field responses at 20-ms intervals.
Zn 2+-dependent LTP facilitation does not depend on NMDA and GABAA receptors
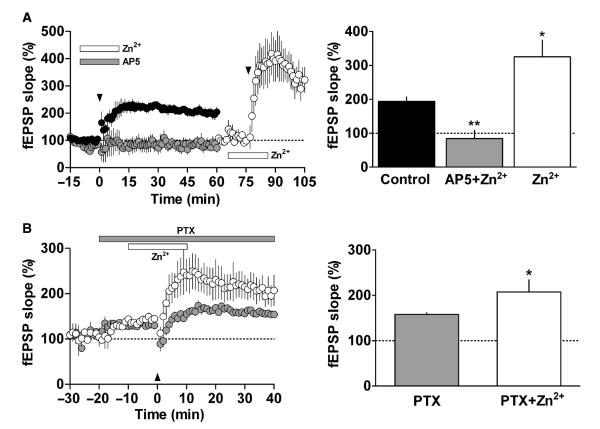
To examine a possible role for the NMDA receptor in LTP facilitation by Zn2+, we isolated the NMDA receptor-mediated component of the EPSP. Specifically, we blocked AMPA receptors with 1 μm CNQX and removed Mg2+ from the ACSF. Although basal NMDA-isolated fEPSPs were unaffected by application of 10 μm Zn2+ (a concentration that facilitates LTP), application of 100 μm Zn2+ noticeably inhibited these receptors (n = 9, F2,24 = 39.02, P < 0.01 compared with baseline, Fig. 3). In addition, we tested the effect of 10 μm Zn2+ on NMDA-isolated fEPSPs at several stimulation intensities, and discovered that Zn2+ did not change the input–output curve or the presynaptic volley–fEPSP correlation (Supporting Information Fig. S1). These observations suggest that the facilitation of LTP seen at low Zn2+ concentrations does not directly involve NMDA receptors. To assess whether NMDA receptors are necessary for the Zn2+-induced facilitation of LTP, we applied the NMDA receptor-specific antagonist AP5. This drug reversibly blocked induction of LTP in the presence of 10 μm Zn2+ (from 196.3 ± 18% under control conditions to 84.2 ± 24.1% with 10 μm Zn2+plus 50 μm AP5, n = 6, F2,15 = 21.89, P < 0.01; Fig. 4A). Following AP5 washout, however, LTP could again be generated and 10 μm Zn2+ clearly enhanced this effect (325.4 ± 48.9%, n = 6, F2,15 = 21.89, P < 0.05 compared with control LTP; Fig. 4A). Thus, the data derived from our experimental paradigm provide no evidence for a direct involvement of NMDA receptors in Zn2+-induced LTP facilitation, even though NMDA receptors are necessary for LTP as such.
Fig. 3.
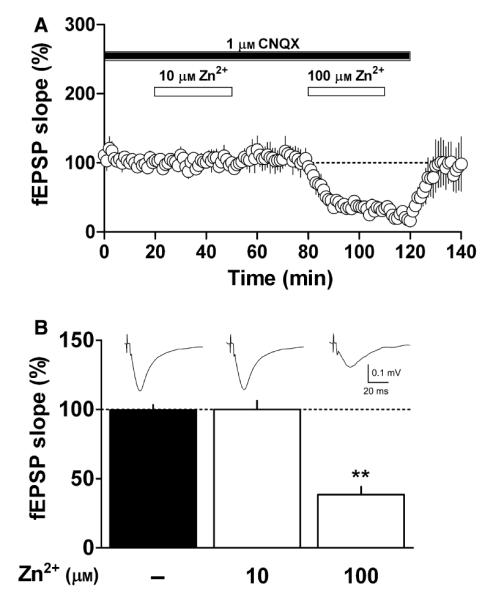
Effect of Zn2+ on isolated NMDA receptor-mediated responses. (A) Isolation of the NMDA receptor-mediated responses during superfusion of 1 μm CNQX in Mg2+-free ACSF (closed bar). After NMDA responses had been monitored for 20 min, slices were superfused with 10 μm Zn2+ for 30 min (open bar). Following 30 min of metal washout, 100 μm Zn2+ was again applied for 30 min (open bar): mean fEPSP ± SEM. (B) Bar graph of the experimental data shown in A. Columns correspond to the average fEPSP slope (± SEM) calculated 10–20 min after CNQX application, 10–20 min after 10 μm Zn2+ application and 10–20 min after 100 μm Zn2+ application (n = 9, **P < 0.01 compared with fEPSP before Zn2+ superfusion). Inset – mean of five field responses obtained under each condition.
Fig. 4.
Influence of NMDA and GABAA receptors on the facilitatory effect of Zn2+ on LTP. (A) Left – LTP induced under control conditions (closed circles) or in the presence of 10 μm AP5 plus 10 μm Zn2+ (gray circles). AP5 and Zn2+ (gray and open horizontal bar, respectively) were applied by superfusion for 10 min before and 10 min after TBS (arrowhead). Sixty-five minutes after TBS slices were superfused with 10 μm Zn2+ for 20 min, a second TBS was applied (arrowhead): means ± SEM. Right – summary and statistical analysis of the experimental data shown in the left panel. Columns correspond to the normalized average fEPSP slope obtained 50–60 min after the first TBS (for control and AP5 + Zn2+) or 20–30 min after the second TBS (for Zn2+ alone). Error bars, SEM; n = 6, *P < 0.05, **P < 0.01 compared with control. (B) Left – LTP induced in the presence of 10 μm PTX (gray circles) or 10 μm PTX plus 10 μm Zn2+ (open circles). Slices were superfused with PTX for 20 min before TBS was applied (arrowhead) and until the end of the experiment (gray horizontal bar). Zn2+ was applied 10 min before and 10 min after TBS (open horizontal bar). Right – summary and statistics of the experimental data shown in the left-hand panel. Columns correspond to normalized means of the fEPSP slope ± SEM of values 30–40 min after TBS (n = 5, *P < 0.05 compared with 10 μm PTX).
To assess whether GABAergic transmission influences the Zn2+- induced increase in LTP, we superfused hippocampal slices with 10 μm PTX, a non-competitive GABAA-receptor antagonist. This treatment increased basal responses by 32.9 ± 2.3% (n = 5, P = 0.004 compared with basal responses before PTX, Fig. 4B) but did not reduce the facilitatory effect of 10 μm Zn2+on LTP (157.6 ± 3.4% with PTX vs. 207.3 ± 26.8% with PTX plus Zn2+, n = 5, P = 0.02, Fig. 4B). Thus, it is unlikely that GABAergic interneurons play a relevant role in the facilitatory effect of Zn2+.
The effect of exogenous ATP on LTP is biphasic
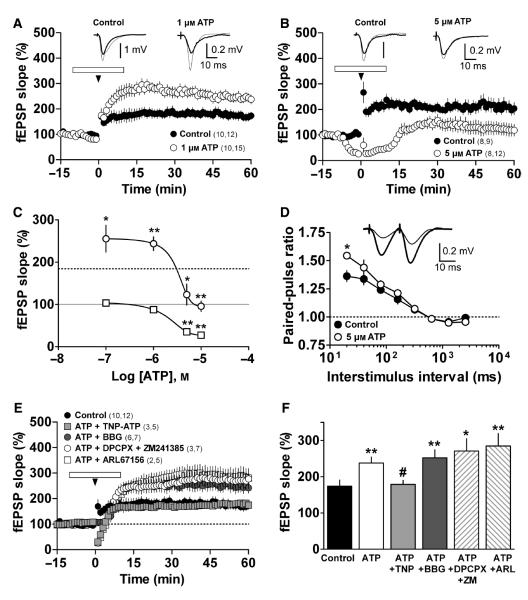
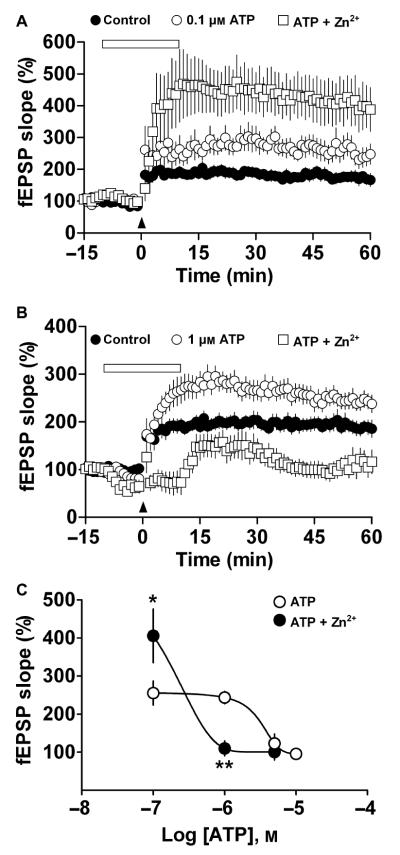
We next examined whether exogenous ATP or structurally related analogs could modify LTP induction. In slices superfused with 0.1 and 1 μm ATP, LTP was increased relative to that in controls (n = 9, P = 0.047 or n = 15, P = 0.003, respectively, Table 2, Fig. 5A and C). In slices superfused with 5 or 10 μm ATP, however, LTP was significantly reduced (n = 12, P = 0.0086 and n = 11, P = 0.0015, respectively; Table 2, Fig. 5B and C) as was the amplitude of the baseline response (64.6 ± 6.4% of inhibition, n = 12, P < 0.0001, and 72.5 ± 7.3% of inhibition, n = 11, P < 0.0001, respectively; Fig. 5B and C). The effect of ATP on baseline activity was concentration-dependent; neither 0.1 nor 1 μm ATP elicited a significant reduction in baseline activity (−3.4 ± 4.3% of inhibition, n = 8, P = 0.4796 and 12 ± 4.7% of inhibition, n = 15, P = 0.0835, respectively; Fig. 5A and C).
Table 2.
Effects of purinergic agonists, antagonists and a modulator on LTP
| Percentage EPSP slope 50–60 min after TBS [mean ± SEM (n)] |
||||
|---|---|---|---|---|
| Concentration (μm) |
Control | + Agonist/antagonist/ modulator |
Significance* | |
| Agonist | ||||
| ATP | 0.1 | 182.4 ± 24.0 (9) | 255.4 ± 31.7 (9) | ↑ |
| 1 | 173.9 ± 17.1 (12) | 243.6 ± 16.4 (15) | ↑ ↑ | |
| 5 | 209.1 ± 22.5 (9) | 123.3 ± 24.7 (12) | ↓ ↓ | |
| 10 | 180.8 ± 12.3 (9) | 95.8 ± 12.3 (11) | ↓ ↓ | |
| ATPγS | 1 | 190.6 ± 16.0 (3) | 327.7 ± 47.5 (3) | ↑ |
| 2-MeSADP | 0.1 | 199.4 ± 33.7 (4) | 162.4 ± 25.0 (5) | NS |
| UTP | 1 | 170.5 ± 24.7 (6) | 203.1 ± 23.8 (12) | NS |
| Adenosine | 1 | 208.4 ± 14.3 (8) | 213.7 ± 28.6 (12) | NS |
| Antagonist | ||||
| TNP-ATP | 10 | 194.0 ± 6.1 (8) | 178.0 ± 7.9 (4) | NS |
| 30 | 164.7 ± 14.2 (6) | 119.6 ± 8.0 (3) | ↓ | |
| oATP | 10 | 208.2 ± 15.5 (10) | 191.2 ± 19.8 (6) | NS |
| 30 | 200.2 ± 10.9 (7) | 135.1 ± 13.8 (9) | ↓ | |
| DPCPX+ZM241385 | 0.05 + 0.1 | 159.3 ± 18.4 (5) | 157.7 ± 7.1 (4) | NS |
| ARL67156 | 50 | 190.5 ± 11.7 (6) | 185.0 ± 27.3 (6) | NS |
| Modulator | ||||
| IVM | 3 | 208.5 ± 30.3 (6) | 335.1 ± 46.3 (13) | ↑ |
Key: ↑ and ↑↑, significant increase compared with control (P < 0.05 and P < 0.01, respectively); ↓ and ↓↓, significant decrease compared with control (P < 0.05 and P < 0.01, respectively); NS, no significant difference compared with control.
Fig. 5.
Biphasic effect of ATP on LTP induction. (A, B) LTP induced under control conditions (closed circles) and in the presence of 1 or 5 μm ATP (open circles), respectively. ATP was applied (open horizontal bar) for 10 min before and 10 min after TBS (arrowheads). Symbols indicate mean normalized fEPSP slope ± SEM. Numbers in parentheses: number of rats, number of slices. Insets – mean of five fEPSP responses obtained 12 min before (thick line) or 50 min after TBS (thin line, 1 or 5 μm ATP). (C) ATP concentration–response curves. Open circles represent the average fEPSP slope obtained 50–60 min after TBS (means ± SEM): *P < 0.05 and **P < 0.01 compared with control LTP for each experiment. Dotted line shows the average LTP value of all control experiments. Open squares represent the average fEPSP slope obtained 5–10 min after ATP superfusion and in the absence of TBS (means ± SEM): **P < 0.01 compared with fEPSP before superfusion of ATP (gray line). (D) Paired-pulse ratio interstimulus curve shows a significant difference in basal activity at 20-ms intervals in the presence of 5 μm ATP (open circles, n = 5, *P < 0.05), vs. no ATP superfusion (control, closed circles). Symbols represent ratios of the fEPSP slope of the second vs. the first pulse ± SEM for each interval. Inset – representative traces of three averaged field responses at 20-ms intervals recorded in the absence (thick line) or presence of 5 μm ATP (thin line). (E) LTP modulation in the presence of 1 μm ATP plus 10 μm TNP-ATP (light gray squares), 100 nm BBG (dark gray circles), 50 μm ARL67156 (open squares) or 50 nm DPCPX and 100 nm ZM241385 (open circles). (F) Summary and statistical results of the experimental data shown in E. Columns correspond to the average fEPSP slope obtained 50–60 min after TBS. Bars indicate SEM. *P < 0.05 and **P < 0.01 compared with control, #P < 0.05 compared with 1 μm ATP.
Investigation of the pre- or postsynaptic nature of the ATP effect on synaptic plasticity revealed that paired-pulse ratios obtained at all intervals after TBS in the context of 1 μm ATP were not significantly different from those observed before TBS. At a 20-ms interval, the ratio was 1.30 ± 0.06 before and 1.37 ± 0.1 after 1 μm ATP-facilitated LTP (n = 12, P = 0.23, data not shown). Furthermore, the paired-pulse ratio at a 20-ms interval obtained during baseline activity did not differ significantly from that obtained in the presence of 1 μm ATP (1.25 ± 0.04 vs. 1.30 ± 0.04, respectively, n = 4, P = 0.1, data not shown). However, 5 μm ATP induced a significant increase in the 20-ms interval paired-pulse ratio compared with baseline responses (1.39 ± 0.04 vs. 1.50 ± 0.03, respectively, n = 5, P = 0.03; Fig. 5D), but did not significantly change the 20-ms interval paired-pulse ratio before and after 5 μm ATP-induced inhibition of LTP (1.42 ± 0.11 vs. 1.44 ± 0.09, n = 8, P = 0.34, data not shown). Together, these results indicate that ATP-induced inhibition of basal activity might be due, at least in part, to a presynaptic component.
The facilitatory effect observed with 1 μm ATP was mimicked by application of the non-hydrolysable analog ATPγS at the same concentration (n = 5, P = 0.03; Table 2, Supporting Information Fig. S2). Given that ATPγS might undergo minute localized hydrolysis, which would lead to activation of adenosine receptors (Cunha et al., 1998), we also tested the possibility that ATP might be hydrolysed by ecto-NTPDases, and found that the ATP-dependent facilitation of LTP was not affected by the application of 50 μm ARL67156, an NTPDase inhibitor (n = 5, P = 0.13; Fig. 5E and F); ARL67156 itself did not modify LTP induction (n = 6, P = 0.2; Table 2), suggesting that the facilitatory effect of exogenous ATP is due to ATP itself and not to any of its metabolites. In a further series of experiments, we assessed the latter possibility by applying structurally-related ATP agonists that selectively activate several different purinergic receptors: 2-MeSADP (0.1 μm, n = 5, P = 0.28; Table 2), an agonist of the P2Y1, P2Y12 and P2Y13 receptors; UTP (1 μm, n = 12, P = 0.30; Table 2), an agonist of P2Y2, P2Y4 and P2Y6 receptors; and adenosine (1 μm, n = 12, P = 0.49, Table 2). Notably, none of these agonists modified LTP. Furthermore, co-application of 1 μm ATP with both 50 nm DPCPX and 100 nm ZM241385 (selective antagonists of the A1 and A2A receptors, respectively) did not affect ATP-induced facilitation of LTP (n = 7, P = 0.22; Fig. 5E and F). Moreover, application of 50 nm DPCPX and 100 nm ZM241385 in the absence of exogenous ATP did not affect LTP (Table 2). Collectively, these results rule out the possibility that P2Y or adenosine receptors play roles in the facilitatory effect of ATP on LTP under the conditions used in our study. Interestingly, the P2X receptor antagonist TNP-ATP (North, 2002) fully abolished the ATP-induced facilitation of LTP (n = 5, P = 0.04; Fig. 5E and F), indicating that the facilitatory effect of ATP depends on the activation of P2X receptors. However, the P2 antagonist BBG, which is specific for the P2X7 receptor subtype at 100 nm (Jiang et al., 2000), failed to reduce ATP-dependent facilitation of LTP when applied (n = 7, P = 0.31; Fig. 5E and F), suggesting that P2X7 receptors do not participate in LTP facilitation by ATP.
P2X4 receptors play a role in LTP induction
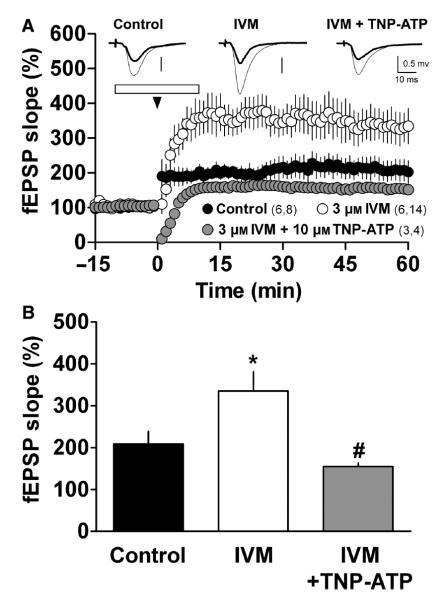
Among the P2X receptor subtypes expressed in the hippocampus (Norenberg & Illes, 2000; Rubio & Soto, 2001), only the P2X4 subtype has been directly reported to modulate LTP (Sim et al., 2006). To evaluate the participation of P2X4 receptors on LTP, we tested the effect of applying ivermectin (IVM), a P2X4-specific positive allosteric modulator (Khakh et al., 1999), to our assay system. As previously shown in mouse hippocampal slices (Sim et al., 2006), we found that 3 μm IVM enhanced LTP induced in the CA1 region of the rat hippocampus (n = 13, P = 0.04; Table 2, Fig. 6). The facilitatory effect of IVM was fully prevented by the application of 10 μm TNP-ATP (n = 4, P = 0.002; Fig. 6). These data support the notion that P2X4 receptors actively participate in LTP modulation, and highlight the role of endogenous extracellular ATP in synaptic plasticity.
Fig. 6.
Ivermectin enhances LTP. (A) LTP induced under control conditions (closed circles), with superfusion of 3 μm IVM (open circles) or 3 μm IVM plus 10 μm TNP-ATP (gray circles) superfused 10 min before and 10 min after (open horizontal bar) TBS (arrowhead). Symbols indicate the normalized fEPSP slope (mean ± SEM). Numbers in parentheses: number of rats, number of slices. Inset – mean of five field responses obtained 12 min before (thick line) and 50 min after TBS (thin line), for each condition. (B) Summary of the experimental data shown in A. Columns correspond to the average fEPSP slope obtained 50–60 min after TBS. Bars indicate SEM. *P < 0.05 compared with control, #P < 0.01 compared with 3 μm IVM.
The effect of ATP on LTP is modulated by Zn 2+
Based on the observation that Zn2+ is a positive modulator of the receptor function of P2X2 and P2X4 (Xiong et al., 1999; Acuña-Castillo et al., 2000), we evaluated whether superfusing hippocampal slices with Zn2+plus ATP would further modulate LTP. We applied 5 μm Zn2+, a concentration that enhanced LTP slightly (Table 1, Fig. 1B), with ATP at various concentrations. Indeed, the facilitation of LTP by 0.1 μm ATP plus 5 μm Zn2+ was greater than that observed in the presence of ATP alone (405.8 ± 70.5 vs. 255.4 ± 31.7%, n = 6 and 9, respectively, P = 0.03; Fig. 7A and C). Interestingly, increasing the ATP concentration to 1 μm or more resulted in a significant reduction of the ATP-induced facilitation of LTP in the presence of Zn2+ (236.1 ± 26.3 vs. 110.1 ± 19.5%, n = 12 and 10, respectively, P = 0.001; Fig. 7B and C). Plotting these results as an ATP concentration–response curve revealed a leftward shift in the curve, and an eight-fold reduction in the EC50, from 3.18 ± 2.26 to 0.41 ± 0.45 μm (n = 6-12, P < 0.01; Fig. 7C), suggesting that Zn2+ increases the affinity of the P2X receptor for ATP, a notion consistent with data from in vitro assays (Acuña-Castillo et al., 2000; Lorca et al., 2005).
Fig. 7.
Zn2+ modifies ATP facilitation of LTP. (A,B) LTP modulation in the presence of 0.1 or 1 μm ATP (open circles) or ATP plus 5 μm Zn2+ (open squares). Slices were superfused with ATP or ATP plus Zn2+ (open horizontal bar) 10 min before and 10 min after TBS (arrowheads). (C) ATP concentration–response curves. Open circles represent the effect of ATP on LTP (as seen in Fig. 5C). Closed circles represent the effect of ATP plus 5 μm Zn2+ on LTP (n = 6–12): *P < 0.05 and **P < 0.01 compared with ATP alone.
Role of the P2X receptors in Zn 2+-dependent facilitation of LTP
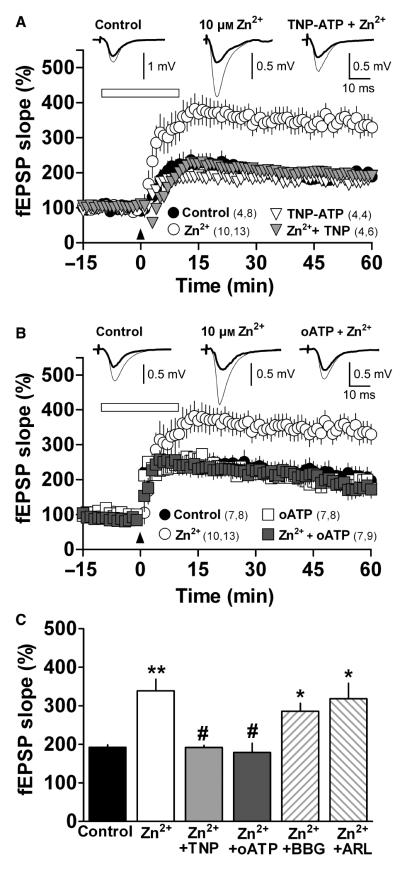
To assess whether nucleotide receptors play an active role in the Zn2+-mediated facilitation of LTP, we applied TNP-ATP or oATP (Murgia et al., 1993; North, 2002; Solini et al., 2007), two antagonists of P2X receptors that are not subtype-selective. As shown in Fig. 8 and Table 2, neither 10 μm TNP-ATP nor 10 μm oATP significantly affected LTP induction (n = 4, P = 0.1, and n = 6, P = 0.3962, respectively). However, at 30 μm concentration each antagonist significantly inhibited LTP (n = 3, P = 0.02, and n = 9, P = 0.002, respectively, Table 2). Furthermore, when hippocampal slices were superfused with either 10 μm TNP-ATP or 10 μm oATP plus 10 μm Zn2+, the Zn2+-mediated facilitation of LTP was abolished (n = 6, P = 0.006 and n = 9, P = 0.002, respectively; Fig. 8). Therefore, a P2X purinergic receptor is probably involved in the facilitatory effect of Zn2+ on LTP. The joint application of 100 nm BBG and 10 μm Zn2+ did not reduce the facilitatory effect of the metal significantly (n = 4, P = 0.28; Fig. 8C and Supporting Information Fig. S3), indicating that the P2X7 receptors do not play a major role in Zn2+-dependent facilitation of LTP. To test whether Zn2+ modifies the activity of ecto-NTPDases, thus changing the levels of extracellular ATP rather than directly modulating P2X receptors, we superfused hippocampal slices with 10 μm Zn2+ in the presence of the NTPDase inhibitor 50 μm ARL67156. However, this NTPDase inhibitor did not affect the Zn2+-dependent facilitation of LTP (n = 4, P = 0.32; Fig. 8C and Supporting Information Fig. S3). Together, these results support the idea that LTP facilitation by Zn2+ occurs through P2X receptors, probably of the P2X4 subtype, and not by other purinergic mechanisms.
Fig. 8.
P2X antagonists abolish the facilitator effect of Zn2+. (A) LTP induced under control conditions (closed circles), or in the presence of 10 μm Zn2+ (open circles), 10 μm TNP-ATP (open triangles) or 10 μm TNP-ATP plus 10 μm Zn2+ (gray triangles), with superfusion carried out for 10 min before and 10 min after (open horizontal bar) the TBS (arrowhead). (B) Control LTP (closed circles), LTP induced in the presence of 10 μm Zn2+ (open circles), 10 μm oATP (open squares) or 10 μm Zn2+ plus 10 μm oATP (gray squares), with superfusion carried out as described in A. Numbers in parentheses: number of rats, number of slices. Insets – mean of five field responses obtained 12 min before (thick line) and 50 min after TBS (thin line), under each condition. (C) Average fEPSP slope obtained 50–60 min after TBS for control slices (n = 11), and slices superfused with 10 μm Zn2+ (n = 13) and 10 μm Zn2+ plus either 10 μm TNP-ATP (n = 6), 10 μm oATP (n = 9), 100 nM BBG (n = 5) or 50 μm ARL67156 (n = 4). *P < 0.05 and **P < 0.01 compared with control, #P < 0.01 compared with 10 μm Zn2+.
Discussion
Zn2+-induced facilitation of LTP
The present study confirms that Zn2+ plays a role in LTP induction, and highlights a mechanism of action whereby this metal ion modulates P2X receptors in the Schaffer collateral–CA1 synapses of the rat hippocampus. Low micromolar concentrations of Zn2+, applied before and during TBS, increased LTP, the largest increase being observed at 10 μm Zn2+. This facilitation of LTP by Zn2+ does not have a presynaptic component, as the concentration of 10 μm did not modify the PPF curve during LTP or the TBS-evoked response amplitude.
Zn2+ modulates a wide array of receptors, including glutamate, GABA, glycine and purinergic receptors (Westbrook & Mayer, 1987; Bloomenthal et al., 1994; Virginio et al., 1997; Xiong et al., 1999; Acuña-Castillo et al., 2000; Mathie et al., 2006; Huidobro-Toro et al., 2008). Previously, we showed that micromolar concentrations of Zn2+ potentiate ATP-evoked currents produced by heterologously expressed P2X2 and P2X4 receptors (Acuña-Castillo et al., 2000; Coddou et al., 2002; Lorca et al., 2005), but that they inhibit ATP-elicited currents produced by P2X7 receptors (Acuña-Castillo et al., 2007). In view of this evidence, and considering that purinergic receptors are widely distributed throughout the rat hippocampus (Ralevic & Burnstock, 1998; Kanjhan et al., 1999; Norenberg & Illes, 2000; Rubio & Soto, 2001; Rodrigues et al., 2005), we propose that P2X receptors are targets for the modulatory action of Zn2+ on LTP induction.
LTP facilitation by Zn 2+ requires activation of P2X receptors
Our demonstration here that the Zn2+ -dependent increase in LTP can be fully reversed by the P2X-receptor agonists TNP-ATP and oATP (Murgia et al., 1993; North, 2002; Solini et al., 2007) indicates that endogenous ATP, through P2X receptor activation, is involved in the Zn2+ facilitatory effect on LTP. Given that neither oATP nor TNP-ATP is selective for particular P2X receptor subtypes, our data do not pinpoint which P2X receptor subtypes, homomers or heteromers, are involved. Nevertheless, our finding that TNP-ATP blocks LTP facilitiation by the P2X4 receptor-specific positive modulator IVM suggests that TNT-ATP may act by inhibiting at least the activity of P2X4 receptors. In addition, as the Zn2+-mediated LTP increase was not significantly modified by BBG, in spite of its application at a concentration that selectively inhibits the P2X7 receptor (Jiang et al., 2000), we can rule out a primary role for P2X7 in this process.
Purinergic modulation of LTP
The endogenous activation of P2X receptors may be relevant to LTP modulation, consistent with the reduction in LTP by both 30 μm TNP-ATP or oATP and the increase by IVM. Thus, the endogenous activation of P2X receptors may be increased by the presence of Zn2+, which enhances the ATP-evoked P2X currents several fold (Xiong et al., 1999; Acuña-Castillo et al., 2000; Lorca et al., 2005). Furthermore, superfusion of hippocampal slices with exogenous ATP mimics the effects of activating nucleotide receptors that modulate LTP. The application of exogenous ATP revealed biphasic effects, suggesting that this nucleotide modulates LTP through more than one mechanism. These effects are postsynaptic, given that we did not observe differences in the paired-pulse ratio before or after LTP modulation in response to the application of 1 or 5 μm ATP. In addition, we consistently observed that 5 μm ATP reduced the magnitude of basal synaptic activity; the increase in the paired-pulse ratio experiments suggests that this effect might be explained, at least in part, by a presynaptic component. As extracellular ATP is rapidly metabolized to adenosine in the hippocampus, the newly formed adenosine may act through presynaptic A1 receptors to reduce synaptic transmission, thereby decreasing neurotransmitter release in the CA1 area (Cunha et al., 1998). Alternatively, ATP itself could act on P2 receptors to enhance the release of adenosine and facilitate LTP induction (Almeida et al., 2003). However, in our experiments this latter possibility seems unlikely. First, the lack of an effect of exogenous adenosine or P2Y-selective agonists on LTP suggests that they have only a minor role or do not participate under the experimental conditions used here. Secondly, exogenous application of the nonhydrolysable analog ATPγS, like that of ATP, facilitates LTP. Thirdly, the facilitatory effect of ATP on LTP did not change in the presence of the A1 and A2A receptor antagonists DPCPX and ZM241385, respectively.
The dual effect of ATP may account for the complex purinergic modulation of LTP and basal synaptic activity. In a recent review, Pankratov et al. (2009) proposed that both the facilitatory (Wang et al., 2004; Sim et al., 2006) and inhibitory (Pankratov et al., 2002) roles of P2X receptors in LTP induction are consistent with the hypothesis of a bi-directional synaptic plasticity. Our results support this notion that P2X receptors may modulate synaptic plasticity in one direction or another, as reflected by the biphasic effect of exogenous ATP application. We have considered that another potential target for ATP action might be glial cells, as P2X receptors are expressed in hippocampal astrocytes (Kukley et al., 2001), and ATP can elicit currents in these cells through P2X receptor activation (Walz et al., 1994) and also induce the release of glutamate from cultured astrocytes (Fellin et al., 2006). However, a recent study reports that ATP does not evoke currents from astrocytes in acute hippocampal slices or freshly dissociated cell suspension (Jabs et al., 2007). Thus, the contribution of P2X receptor activation in astrocytes during LTP induction is not yet clear and needs further elucidation.
The facilitatory effect of exogenous ATP on LTP is modulated by Zn 2+
Zn2+ reduces the EC50 derived from the ATP concentration–response curve on LTP. We propose that Zn2+ might exert this effect by increasing the affinity of certain P2X receptor subtypes for ATP. We previously reported that cultured cells expressing either P2X2 or P2X4 receptors show a similar modulation of the ATP concentration–response curve in the presence of Zn2+ (Acuña-Castillo et al., 2000; Lorca et al., 2005). The P2X receptor family contains seven subtypes (P2X1–7), all of which are expressed in the hippocampus (Kanjhan et al., 1999; Norenberg & Illes, 2000; Rodrigues et al., 2005). Among these members, the P2X2, P2X3 and P2X4 subtypes are putative targets for Zn2+ because the ATP-evoked cationic currents they evoke are facilitated by this metal (Wildman et al., 1999; Huidobro-Toro et al., 2008). However, it is unlikely that the facilitatory effect of Zn2+ on LTP induction is mediated by P2X3 modulation, as the metal concentrations capable of facilitating ATP-evoked currents generated by this receptor are higher than those used in our study (Wildman et al., 1999). Of the P2X2 and P2X4 receptor populations, the latter seems to be the better candidate for Zn2+ modulation, as in heterologous expression systems 10 μm Zn2+ has a 10-fold greater effect on ATP-evoked currents generated by P2X4 than on those generated by the P2X2 receptor (Huidobro-Toro et al., 2008). Moreover, only the P2X4 receptor has two Zn2+ binding sites – a high-affinity site that potentiates the ATP-induced current, and a low-affinity site that inhibits it (Coddou et al., 2007). This may account for the biphasic effect observed in the modulation of LTP by Zn2+. In addition, in experiments using IVM, a selective positive modulator of P2X4 receptors (Khakh et al., 1999), we observed a significant increase in LTP that was fully reversed by the application of TNP-ATP. These results, together with the findings of Sim et al. (2006) describing reduced LTP in mice lacking P2X4 receptors, constitute strong evidence that P2X4 receptors play a role in LTP facilitation, and in the mechanism of Zn2+-mediated modulation of synaptic plasticity in the hippocampus. Furthermore, we hypothesize that a P2X-dependent increase of intracellular Ca2+ may be necessary to increase AMPA receptor insertion into postsynaptic membranes, as was demonstrated in other brain nuclei (Gordon et al., 2005).
Other possible Zn 2+ targets
Our data from hippocampal slices do not support the notion that the effects of Zn2+ on LTP involve the modulation of GABA receptors, a mechanism that has been proposed based on experiments carried out on cultured hippocampal neurons (Legendre & Westbrook, 1991). Specifically, neither the afferent volley nor synaptic transmission was modulated by the application of 10 μm Zn2+ and, more importantly, Zn2+-induced LTP facilitation was not inhibited by the application of PTX. On the other hand, we cannot completely discard the possibility that Zn2+ inhibits the NMDA receptor at low concentrations, as such an effect could be masked by residual AMPA receptor activity. Nevertheless, such an inhibitory effect would not account for the facilitatory modulation of LTP observed in the presence of Zn2+. Another reasonable target for Zn2+ modulation might be the AMPA/kainate receptors, which are potentiated by this metal at micromolar concentrations, but inhibited by millimolar concentrations (Rassendren et al., 1990). Although we cannot exclude the possibility that several neurotransmitter receptors may be integrated in Zn2+-based modulation of LTP, our findings show a full reversion of Zn2+-induced facilitation of LTP with P2X receptor antagonists, suggesting that these receptors are likely key targets for LTP facilitation. Patch-clamp recordings may provide further evidence of the specific participation of several postsynaptic neurotransmitter receptors, as well as a better understanding of the intracellular mechanisms involved in the Zn2+ and purinergic modulation of LTP.
The results from several studies, including those presented here, indicate that Zn2+ is an active modulator of synaptic strength and plasticity (Lu et al., 2000; Li et al., 2001; Izumi et al., 2006; Lorca et al., 2007; Morales et al., 2008; Takeda et al., 2008, 2009), yet the molecular mechanism(s) responsible for the Zn2+-mediated modulation of LTP remain(s) unknown. Our findings on the role of Zn2+ as a facilitator of LTP induction in the CA1 area of the hippocampus confirm recent observations (Takeda et al., 2009) and add mechanistic evidence for the role of this metal as a modulator of LTP. Our findings are not fully compatible with previously proposed mechanisms concerning the Zn2+-dependent facilitatory effect (Takeda et al., 2009). In particular, whereas Zn2+ has been proposed to act intracellularly by direct action on NMDA receptors, and thereby overcoming the negative modulator effect on NMDA receptors in the extracellular compartment, we hypothesize that Zn2+ facilitates LTP through the modulation of P2X receptors.
Conclusions
Based on the results presented here, we suggest that the Zn2+-evoked enhancement of LTP is mainly mediated through the modulation of P2X receptors. At least two mechanisms seem to be involved in the biphasic effect of Zn2+: (i) at low micromolar concentrations, Zn2+ facilitates P2X4 receptor activity to increase LTP, possibly through binding to high-affinity sites in the extracellular domain of this receptor (Coddou et al., 2007; Huidobro-Toro et al., 2008); (ii) at higher micromolar concentrations, Zn2+ inhibits NMDA receptors (Westbrook & Mayer, 1987), resulting in decreased LTP – at these concentrations the facilitation of P2X4 receptor activity by Zn2+ is significantly reduced, as reflected by the bell-shaped curve of the response to the metal of P2X4 receptors expressed in heterologous systems (Coddou et al., 2003, 2007). In conclusion, low concentrations of Zn2+ facilitate LTP induction in the CA1 region of the hippocampus at least partly by interacting with P2X receptors. This finding highlights the physiological role of Zn2+ as a synaptic plasticity modulator with certain implications for learning and memory.
Supplementary Material
Acknowledgements
We would like to thank Dr S. K. England, Dr A. J. Shepherd, Dr R. Cabeza and C. Blaumueller for their critical reading of this manuscript. This work was supported by FONDAP grant 13980001, Millennium Institute for Fundamental and Applied Biology, FFB 12/2007, FONDECYT grant 1080652, and DICYT-USACH. R.A.L. was supported by a CONICYT graduate student fellowship and a CONICYT AT 23070142 training fellowship. C.R. is supported by the Graduate Program in Biotechnology USACH.
Abbreviations
- 2-MeSADP
2-methylthio adenosine 5′-diphosphate
- ACSF
artificial cerebrospinal fluid
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AP5
d(−)-2-amino-5-phosphonopentanoic acid
- ARL67156
6-N,N-diethyl-b-γ-dibromomethylene-d-adenosine-5′-triphosphate
- ATP
adenosine 5′-triphosphate
- ATPγS
adenosine 5′-(3-thiotriphosphate)
- BBG
Brilliant Blue G
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- DMSO
dimethyl sulfoxide
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- EC50
median effective concentration
- fEPSP
field excitatory postsynaptic potential
- GABA
γ-aminobutyric acid
- IVM
ivermectin
- LTP
long-term potentiation
- NMDA
N-methyl-d-aspartic acid
- oATP
periodate-oxidized adenosine 5′-triphosphate
- PPF
paired-pulse facilitation
- PTX
picrotoxin
- TBS
theta-burst stimulation
- TNP-ATP
2′,3′-O-(2,4,6-trinitrophenyl) adenosine 5′-triphosphate
- UTP
uridine-5′-triphosphate
- ZM241385
4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol
Footnotes
Supporting Information Additional supporting information can be found in the online version of this article:
Fig. S1. Effect of Zn2+ on presynaptic volley–fEPSP interaction.
Fig. S2. ATPcS enhances LTP.
Fig. S3. Zn2+-induced facilitation of LTP is independent of P2X7 activation and NTPDase activity.
Please note: As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed, and may be re-organized for online delivery, but are not copy-edited or typeset by Wiley-Blackwell. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Acuña-Castillo C, Morales B, Huidobro-Toro JP. Zinc and copper modulate differentially the P2X4 receptor. J. Neurochem. 2000;74:1529–1537. doi: 10.1046/j.1471-4159.2000.0741529.x. [DOI] [PubMed] [Google Scholar]
- Acuña-Castillo C, Coddou C, Bull P, Brito J, Huidobro-Toro JP. Differential role of extracellular histidines in copper, zinc, magnesium and proton modulation of the P2X7 purinergic receptor. J. Neurochem. 2007;101:17–26. doi: 10.1111/j.1471-4159.2006.04343.x. [DOI] [PubMed] [Google Scholar]
- Almeida T, Rodrigues RJ, de Mendonca A, Ribeiro JA, Cunha RA. Purinergic P2 receptors trigger adenosine release leading to adenosine A2A receptor activation and facilitation of long-term potentiation in rat hippocampal slices. Neuroscience. 2003;122:111–121. doi: 10.1016/s0306-4522(03)00523-2. [DOI] [PubMed] [Google Scholar]
- Andreasen M, Lambert JD, Jensen MS. Effects of new non-N-methyl-D-aspartate antagonists on synaptic transmission in the in vitro rat hippocampus. J. Physiol. 1989;414:317–336. doi: 10.1113/jphysiol.1989.sp017690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf SY, Chung SH. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- Bloomenthal AB, Goldwater E, Pritchett DB, Harrison NL. Biphasic modulation of the strychnine-sensitive glycine receptor by Zn2+ Mol. Pharmacol. 1994;46:1156–1159. [PubMed] [Google Scholar]
- Coddou C, Villalobos C, Gonzalez J, Acuna-Castillo C, Loeb B, Huidobro-Toro JP. Formation of carnosine-Cu(II) complexes prevents and reverts the inhibitory action of copper in P2X4 and P2X7 receptors. J. Neurochem. 2002;80:626–633. doi: 10.1046/j.0022-3042.2001.00732.x. [DOI] [PubMed] [Google Scholar]
- Coddou C, Morales B, Gonzalez J, Grauso M, Gordillo F, Bull P, Rassendren F, Huidobro-Toro JP. Histidine 140 plays a key role in the inhibitory modulation of the P2X4 nucleotide receptor by copper but not zinc. J. Biol. Chem. 2003;278:36777–36785. doi: 10.1074/jbc.M305177200. [DOI] [PubMed] [Google Scholar]
- Coddou C, Acuna-Castillo C, Bull P, Huidobro-Toro JP. Dissecting the facilitator and inhibitor allosteric metal sites of the P2X4 receptor channel: critical roles of CYS132 for zinc potentiation and ASP138 for copper inhibition. J. Biol. Chem. 2007;282:36879–36886. doi: 10.1074/jbc.M706925200. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Sebastiao AM, Ribeiro JA. Inhibition by ATP of hippocampal synaptic transmission requires localized extracellular catabolism by ectonucleotidases into adenosine and channeling to adenosine A1 receptors. J. Neurosci. 1998;18:1987–1995. doi: 10.1523/JNEUROSCI.18-06-01987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Pozzan T, Carmignoto G. Purinergic receptors mediate two distinct glutamate release pathways in hippocampal astrocytes. J. Biol. Chem. 2006;281:4274–4284. doi: 10.1074/jbc.M510679200. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat. Neurosci. 2005;8:1078–1086. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- Howell GA, Welch MG, Frederickson CJ. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature. 1984;308:736–738. doi: 10.1038/308736a0. [DOI] [PubMed] [Google Scholar]
- Huidobro-Toro JP, Lorca RA, Coddou C. Trace metals in the brain: allosteric modulators of ligand-gated receptor channels, the case of ATP-gated P2X receptors. Eur. Biophys. J. 2008;37:301–314. doi: 10.1007/s00249-007-0230-7. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Auberson YP, Zorumski CF. Zinc modulates bidirectional hippocampal plasticity by effects on NMDA receptors. J. Neurosci. 2006;26:7181–7188. doi: 10.1523/JNEUROSCI.1258-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs R, Matthias K, Grote A, Grauer M, Seifert G, Steinhauser C. Lack of P2X receptor mediated currents in astrocytes and GluR type glial cells of the hippocampal CA1 region. Glia. 2007;55:1648–1655. doi: 10.1002/glia.20580. [DOI] [PubMed] [Google Scholar]
- Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Mol. Pharmacol. 2000;58:82–88. [PubMed] [Google Scholar]
- Kanjhan R, Housley GD, Burton LD, Christie DL, Kippenberger A, Thorne PR, Luo L, Ryan AF. Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. J. Comp. Neurol. 1999;407:11–32. [PubMed] [Google Scholar]
- Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA. Allosteric control of gating and kinetics at P2X(4) receptor channels. J. Neurosci. 1999;19:7289–7299. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TY, Hwang JJ, Yun SH, Jung MW, Koh JY. Augmentation by zinc of NMDA receptor-mediated synaptic responses in CA1 of rat hippocampal slices: mediation by Src family tyrosine kinases. Synapse. 2002;46:49–56. doi: 10.1002/syn.10118. [DOI] [PubMed] [Google Scholar]
- Kukley M, Barden JA, Steinhauser C, Jabs R. Distribution of P2X receptors on astrocytes in juvenile rat hippocampus. Glia. 2001;36:11–21. doi: 10.1002/glia.1091. [DOI] [PubMed] [Google Scholar]
- Legendre P, Westbrook GL. Noncompetitive inhibition of gamma-aminobutyric acidA channels by Zn. Mol. Pharmacol. 1991;39:267–274. [PubMed] [Google Scholar]
- Li Y, Hough CJ, Frederickson CJ, Sarvey JM. Induction of mossy fiber → Ca3 long-term potentiation requires translocation of synaptically released Zn2+ J. Neurosci. 2001;21:8015–8025. doi: 10.1523/JNEUROSCI.21-20-08015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca RA, Coddou C, Gazitua MC, Bull P, Arredondo C, Huidobro-Toro JP. Extracellular histidine residues identify common structural determinants in the copper/zinc P2X2 receptor modulation. J. Neurochem. 2005;95:499–512. doi: 10.1111/j.1471-4159.2005.03387.x. [DOI] [PubMed] [Google Scholar]
- Lorca RA, Moreira-Ramos S, Morales B, Huidobro-Toro JP. Neuroscience Meeting Planner. Society for Neuroscience; San Diego, CA: 2007. ATP and trace metals modulate long-term potentiation in CA1 of rat hippocampal slices. Program No. 584.9. 2007. Online. [Google Scholar]
- Lu YM, Taverna FA, Tu R, Ackerley CA, Wang YT, Roder J. Endogenous Zn(2+) is required for the induction of long-term potentiation at rat hippocampal mossy fiber-CA3 synapses. Synapse. 2000;38:187–197. doi: 10.1002/1098-2396(200011)38:2<187::AID-SYN10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation – a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Mathie A, Sutton GL, Clarke CE, Veale EL. Zinc and copper: pharmacological probes and endogenous modulators of neuronal excitability. Pharmacol. Ther. 2006;111:567–583. doi: 10.1016/j.pharmthera.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Morales B, Lorca RA, Huidobro-Toro JP. Neuroscience Meeting Planner. Society for Neuroscience; Washington, DC: 2008. Zinc increases long-term potentiation through P2X receptors in CA1 area of rat hippocampus. Program No. 434.3. 2008. Online. [Google Scholar]
- Murgia M, Hanau S, Pizzo P, Rippa M, Di Virgilio F. Oxidized ATP. An irreversible inhibitor of the macrophage purinergic P2Z receptor. J. Biol. Chem. 1993;268:8199–8203. [PubMed] [Google Scholar]
- Norenberg W, Illes P. Neuronal P2X receptors: localisation and functional properties. Naunyn Schmiedebergs Arch. Pharmacol. 2000;362:324–339. doi: 10.1007/s002100000311. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Pankratov YV, Lalo UV, Krishtal OA. Role for P2X receptors in long-term potentiation. J. Neurosci. 2002;22:8363–8369. doi: 10.1523/JNEUROSCI.22-19-08363.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Krishtal OA, Verkhratsky A. P2X receptors and synaptic plasticity. Neuroscience. 2009;158:137–148. doi: 10.1016/j.neuroscience.2008.03.076. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Rassendren FA, Lory P, Pin JP, Nargeot J. Zinc has opposite effects on NMDA and non-NMDA receptors expressed in Xenopus oocytes. Neuron. 1990;4:733–740. doi: 10.1016/0896-6273(90)90199-p. [DOI] [PubMed] [Google Scholar]
- Rodrigues RJ, Almeida T, Richardson PJ, Oliveira CR, Cunha RA. Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in the rat hippocampus. J. Neurosci. 2005;25:6286–6295. doi: 10.1523/JNEUROSCI.0628-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio ME, Soto F. Distinct localization of P2X receptors at excitatory postsynaptic specializations. J. Neurosci. 2001;21:641–653. doi: 10.1523/JNEUROSCI.21-02-00641.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim JA, Chaumont S, Jo J, Ulmann L, Young MT, Cho K, Buell G, North RA, Rassendren F. Altered hippocampal synaptic potentiation in P2X4 knock-out mice. J. Neurosci. 2006;26:9006–9009. doi: 10.1523/JNEUROSCI.2370-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solini A, Santini E, Chimenti D, Chiozzi P, Pratesi F, Cuccato S, Falzoni S, Lupi R, Ferrannini E, Pugliese G, Di Virgilio F. Multiple P2X receptors are involved in the modulation of apoptosis in human mesangial cells: evidence for a role of P2X4. Am. J. Physiol. Renal Physiol. 2007;292:F1537–F1547. doi: 10.1152/ajprenal.00440.2006. [DOI] [PubMed] [Google Scholar]
- Takeda A, Kanno S, Sakurada N, Ando M, Oku N. Attenuation of hippocampal mossy fiber long-term potentiation by low micromolar concentrations of zinc. J. Neurosci. Res. 2008;86:2906–2911. doi: 10.1002/jnr.21732. [DOI] [PubMed] [Google Scholar]
- Takeda A, Fuke S, Ando M, Oku N. Positive modulation of long-term potentiation at hippocampal CA1 synapses by low micromolar concentrations of zinc. Neuroscience. 2009;158:585–591. doi: 10.1016/j.neuroscience.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Virginio C, Church D, North RA, Surprenant A. Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology. 1997;36:1285–1294. doi: 10.1016/s0028-3908(97)00141-x. [DOI] [PubMed] [Google Scholar]
- Vogt K, Mellor J, Tong G, Nicoll R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron. 2000;26:187–196. doi: 10.1016/s0896-6273(00)81149-6. [DOI] [PubMed] [Google Scholar]
- Walz W, Gimpl G, Ohlemeyer C, Kettenmann H. Extracellular ATP-induced currents in astrocytes: involvement of a cation channel. J. Neurosci. Res. 1994;38:12–18. doi: 10.1002/jnr.490380104. [DOI] [PubMed] [Google Scholar]
- Wang Y, Haughey NJ, Mattson MP, Furukawa K. Dual effects of ATP on rat hippocampal synaptic plasticity. Neuroreport. 2004;15:633–636. doi: 10.1097/00001756-200403220-00012. [DOI] [PubMed] [Google Scholar]
- Westbrook GL, Mayer ML. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987;328:640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wildman SS, King BF, Burnstock G. Modulatory activity of extracellular H+ and Zn2+ on ATP-responses at rP2X1 and rP2X3 receptors. Br. J. Pharmacol. 1999;128:486–492. doi: 10.1038/sj.bjp.0702802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Smart TG. Modulation of long-term potentiation in rat hippocampal pyramidal neurons by zinc. Pflugers Arch. 1994;427:481–486. doi: 10.1007/BF00374264. [DOI] [PubMed] [Google Scholar]
- Xiong K, Peoples RW, Montgomery JP, Chiang Y, Stewart RR, Weight FF, Li C. Differential modulation by copper and zinc of P2X2 and P2X4 receptor function. J. Neurophysiol. 1999;81:2088–2094. doi: 10.1152/jn.1999.81.5.2088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.