Abstract
Background
The present study examined if increases in creatine kinase (CK) levels during high-dose atorvastatin treatment are associated with changes in skeletal muscle function and symptoms.
Methods
The Effect of Statins on Muscle Performance study (STOMP) investigated the effects of atorvastatin 80 mg daily for 6 months on muscle performance, exercise capacity, and the incidence of statin-associated muscle complaints in healthy adults.
Results
CK levels increased with atorvastatin (n = 202) from 132.3 ± 120.9 U/L (mean ± SD) at baseline to 159.7 ± 170.4 and 153.1 ± 139.4 U/L at 3 and 6 months, respectively (P≤0.002 for both). Changes in CK with atorvastatin treatment were not associated with changes in muscle function or the incidence of myalgia. More subjects on atorvastatin (n = 24) compared to placebo (n = 12 of 217) doubled their CK level at 6 months (P = 0.02). No differences in muscle function or physical activity were observed between atorvastatin-treated subjects who did or did not double their CK.
Conclusions
Results of the present investigation extend the findings of STOMP by demonstrating that greater increases in CK levels with high-dose atorvastatin treatment did not deleteriously impact skeletal muscle function or predict skeletal muscle complaints.
Keywords: statins, myalgia, exercise, muscle
INTRODUCTION
Statins inhibit hydroxy-methyl-glutaryl coenzyme A reductase, effectively decreasing low-density lipoprotein cholesterol (LDL-C) concentrations and markedly reducing cardiovascular events [1]. Mild myopathy, with symptoms of myalgia, muscle cramps, and weakness, can occur with statin use. The Effect of Statins on Muscle Performance study (STOMP; National Heart, Lung, and Blood Institute 5R01HL081893, NCT00609063) demonstrated that 6 months of treatment with atorvastatin 80 mg daily doubled the incidence of muscle complaints compared with placebo from 4.6% to 9.4% [2].
Most clinical trials used creatine kinase (CK) levels greater than 10 times the upper limit of normal (ULN) to define statin-associated myopathy [3–9]. In STOMP, no subject demonstrated a CK level persistently greater than 10 times the ULN [2]. CK increased slightly (27 and 21 U/L, P≤0.002 for both) after 3 and 6 months of atorvastatin treatment [2], suggesting low-level muscle injury. There was no change in multiple measures of skeletal muscle strength in STOMP [2], and it is not clear if larger increases in CK were associated with decreases in muscle performance. The present investigation examined if greater increases in CK with atorvastatin were associated with changes in muscle function and symptoms.
METHODS
STOMP was a double-blind, random-assignment trial investigating the effects of atorvastatin 80 mg daily for 6 months on muscle performance, exercise capacity, and the incidence of statin-associated muscle complaints in healthy adults [2]. Serological markers, aerobic exercise performance, knee extensor endurance, as well as handgrip, elbow and knee strength were measured at baseline and during the last week of treatment. Subjects were contacted twice monthly by study personnel and queried about muscle complaints using the Short-Form Brief Pain Inventory. The Institutional Review Boards at Hartford Hospital, University of Massachusetts, and University of Connecticut approved the study and the study was monitored by a Data Safety and Monitoring Board.
The study definition of myalgia required that: 1) Subjects reported new or increased muscle pain, cramps, or aching not associated with exercise; 2) these symptoms persisted for two weeks; 3) symptoms resolved within two weeks of drug cessation; and 4) symptoms reoccurred within four weeks of restarting the study drug. Subjects who met these criteria had measurements repeated as soon as possible and were withdrawn from the study. A total of 419 subjects completed the study of which 202 received atorvastatin.
Statistical analyses for the present report were performed with SPSS version 19.0 (SPSS, Inc., Chicago, IL). Data were assessed for normality and log transformed as necessary. The proportion of subjects who increased CK > twice their baseline level was compared between treatment groups, between myalgics and non-myalgics, and within the atorvastatin group using a Pearson χ2 test. Baseline values in atorvastatin-treated subjects who did or did not increase CK > twice their baseline level were evaluated using one-way analysis of variance (ANOVA) (or a Mann-Whitney U test for CK). An independent samples t-test was used to evaluate differences in the absolute change in muscle function in atorvastatin-treated subjects who did or did not increase CK > twice their baseline level. Linear regression was performed to evaluate if changes in CK predicted changes in muscle function. Further models were run controlling for sex and age. Significance was determined at P<0.05.
RESULTS
Subjects in the atorvastatin and placebo (n = 217) groups were similar at baseline and had similar medication compliance as assessed by pill count [2]. CK levels increased with atorvastatin from 132.3 ± 120.9 U/L (mean ± SD) at baseline to 159.7 ± 170.4 (n = 186) and 153.1 ± 139.4 U/L (n = 202) at 3 and 6 months, respectively (P≤0.002 for both). There was no relationship between final CK and changes in measures of muscle function or physical activity levels (all P≥0.23) in atorvastatin-treated subjects by linear regression analysis. There was also no relationship between absolute (P≥0.17) or relative change (P≥0.21) in CK and most parameters of muscle function. Interestingly, change in handgrip strength was directly related to both absolute (R2 = 0.017, P = 0.068) and relative change in CK (R2 = 0.041, P = 0.063), but neither reached statistical significance.
The absolute change in CK levels ranged from −532 to1039 U/L at 3 months and from −485 to 1049 U/L at 6 months. In contrast, CK levels in the placebo subjects were 135.1 ± 136.0, 129.2 ± 135.7, and 136.6 ± 163.0 U/L at baseline, 3, and 6 months, respectively. Their absolute change ranged from −987 to 978 U/L at 3 months and from −938 to 1306 U/L at 6 months. The STOMP protocol required that subjects with CK levels >10 times ULN refrain from exercise and have a repeat CK value obtained as soon as possible. Two subjects on atorvastatin, a 31 year old man and a 20 year old woman, had CK values >10 times ULN at 3 months of 4557 and 2120 U/L, which decreased to 63 and 356 U/L, 8 and 3 days later, respectively. Elevated CK levels were attributed to recent exercise in the man and to unknown factors in the woman; the repeat values were used in analysis. No subject had CK values >10 times ULN at 6 months.
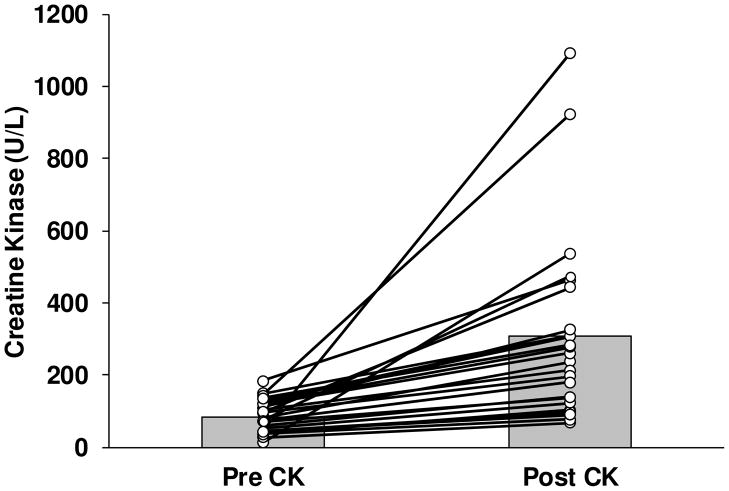
Thirty six subjects doubled their CK value at 6 months compared to baseline, 24 in the atorvastatin and 12 in the placebo groups (χ2 = 5.37, P = 0.02). The mean CK change in the 24 atorvastatin-treated subjects was 221.9 ± 248.7 U/L and increased from 85.0 ± 44.6 to 306.9 ± 255.5 U/L (P<0.0001) (Figure 1). Subjects on atorvastatin who did not double their CK (n = 178) had a mean change of −6.3 ± 91.5 U/L (138.7 ± 126.5 to 132.4 ± 99.7 U/L, P = 0.64). Subjects who did or did not double their CK were generally similar, although baseline CK levels were lower (P = 0.01) in those subjects who doubled their baseline value (Table 1). STOMP subjects were equally recruited by age into three groups. Doubling of the CK was more common in the middle-aged group (7 of 75 (9.3%) adults <40 y, 14 of 66 (21.2%) adults 40–54 y, and 3 of 61 (4.9%) adults >55 y) (χ2 = 8.78, P = 0.01). Ten subjects, 5 on atorvastatin, at least tripled their CK levels from baseline.
Figure 1.
Individual changes in creatine kinase (CK) from baseline (Pre) in atorvastatin-treated subjects (Post) who doubled their CK value from baseline (n = 24). Mean CK levels (grey bars) increased significantly (P<0.0001).
Table 1.
Baseline Characteristics of Atorvastatin-Treated Subjects Who Did or Did Not Double Their Baseline Creatine Kinase Value
| CK < 2x baseline (n = 178) | CK > 2x baseline (n = 24) | P | |
|---|---|---|---|
| Male, n (%) | 88 (49.4) | 12 (50.0) | 0.96 |
| Age, yrs | 43.4 ± 16.4 | 43.3 ± 12.1 | 0.97 |
| Height, cm | 170.9 ± 9.5 | 171.8 ± 8.9 | 0.67 |
| Weight, kg | 77.4 ± 17.1 | 79.1 ± 17.8 | 0.65 |
| BMI, kg/m2 | 26.2 ± 4.7 | 26.8 ± 5.3 | 0.61 |
| Waist, cm | 85.1 ± 13.7 | 87.8 ± 14.6 | 0.40 |
| Resting HR, bpm | 68 ± 11 | 71 ± 13 | 0.24 |
| Resting SBP, mmHg | 119 ± 13 | 122 ± 13 | 0.30 |
| Resting DBP, mmHg | 76 ± 10 | 74 ± 8 | 0.48 |
| Total-C, mg/dL | 198.8 ± 38.3 | 196.8 ± 47.6 | 0.82 |
| HDL-C, mg/dL | 57.7 ± 17.2 | 57.1 ± 17.0 | 0.88 |
| LDL-C, mg/dL | 119.3 ± 34.4 | 116.5 ± 44.1 | 0.72 |
| TG, mg/dL | 109.4 ± 54.3 | 118.3 ± 64.7 | 0.46 |
| CK, U/L | 138.7 ± 126.5 | 85.0 ± 44.6 | 0.01 |
Data are means ± SD. BMI = body mass index; C = cholesterol; CK = creatine kinase; DBP = diastolic blood pressure; HDL-C = high-density lipoprotein; HR = heart rate; LDL = low-density lipoprotein; SBP = systolic blood pressure; TG = triglycerides.
There were also no differences in baseline muscle function or physical activity or change in these parameters (Table 2) between atorvastatin-treated subjects who did or did not double their CK. Among the 24 atorvastatin-treated subjects who exhibited a two-fold or greater increase in CK, final CK values were not related to changes in measures of muscle function or physical activity levels after adjustment for sex and age (P≥0.11). However, both the absolute (Figure 2A) and relative increase in CK were directly related to an increase in handgrip strength (R2 = 0.25 and 0.17 for absolute and relative increase in CK, respectively; P≤0.047), but this relationship was no longer significant with adjustment for sex and age (P≥0.063). Change scores for all other parameters of muscle function, including isometric elbow extension (Figure 2B) and isometric knee extension (Figure 2C), were not related to increases in CK (P≥0.096).
Table 2.
Change in Exercise Performance and Strength Values in Atorvastatin-Treated Subjects Who Did or Did Not Double Their Baseline Creatine Kinase Value
| CK < 2x baseline (n = 178) | CK > 2x baseline (n = 24) | P | |
|---|---|---|---|
| Resting RER | 0.0 ± 0.1 | 0.0 ± 0.1 | 0.42 |
| VO2max, ml/kg/min | −0.9 ± 3.8 | −0.1 ± 2.0 | 0.36 |
| VT, ml/kg/min | −0.8 ± 5.1 | −0.6 ± 4.0 | 0.82 |
| Handgrip, kg | 0.2 ± 4.5 | 0.4 ± 6.3 | 0.83 |
| Arm Strength (APT), N-m | |||
| Isom Ext | 1.1 ± 9.0 | 1.7 ± 6.8 | 0.78 |
| Isom Flex | 0.3 ± 1.0 | 1.0 ± 4.6 | 0.67 |
| Isok Ext at 60°/s | 0.2 ± 6.7 | 1.3 ± 4.0 | 0.47 |
| Isok Flex at 60°/s | 0.0 ± 5.6 | 1.2 ± 2.9 | 0.30 |
| Isok Ext at 180°/s | 0.2 ± 5.9 | 1.6 ± 3.9 | 0.26 |
| Isok Flex at 180°/s | 0.4 ± 5.5 | 1.3 ± 2.9 | 0.41 |
| Leg Strength (APT), N-m | |||
| Isom Ext | −1.0 ± 23.5 | −6.9 ± 20.0 | 0.24 |
| Isom Flex | −1.8 ± 10.1 | −1.3 ± 9.2 | 0.80 |
| Isok Ext at 60°/s | 2.6 ± 15.7 | −3.8 ± 19.1 | 0.07 |
| Isok Flex at 60°/s | 1.1 ± 10.6 | 2.5 ± 10.2 | 0.54 |
| Isok Ext at 180°/s | 4.3 ± 13.8 | 5.9 ± 23.1 | 0.63 |
| Isok Flex at 180°/s | 2.8 ± 9.0 | 4.1 ± 12.5 | 0.52 |
| Knee Endurance Fatigue Index | 1.2 ± 8.3 | 1.0 ± 6.9 | 0.92 |
Data are means ± SD. APT = average peak torque; CK = creatine kinase; Ext = Extension; Flex = Flexion; Isom = Isometric; Isok = Isokinetic; RER = respiratory exchange ratio; VO2max = maximal oxygen uptake; VT = ventilator threshold.
Figure 2.
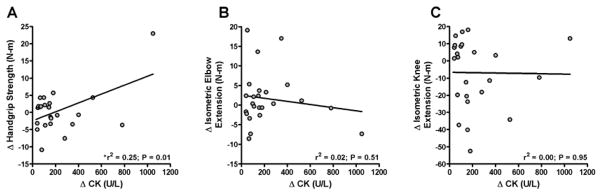
Relationship between changes (Post - Pre) in creatine kinase (CK) and handgrip strength (A), isometric elbow extension (B), and isometric leg extension (C) in atorvastatin-treated subjects who exhibited a two-fold or greater increase in CK (n = 24). Linear regression was performed to evaluate relationships between variables. Data were log transformed for analyses but raw data are depicted in the figure. *The relationship between changes in CK and handgrip strength was no longer significant when sex and age were controlled (r2 = 0.30, P = 0.063).
More subjects on atorvastatin (n = 19) met the study definition of myalgia than on placebo (n = 10) (P = 0.05) [2], but baseline, change, and final CK levels did not differ between these groups (all P≥0.35). Three atorvastatin-treated myalgics, but no placebo-treated myalgics doubled their CK (χ2 = 1.87, P = 0.17). Baseline, change, and final CK levels did not differ between atorvastatin-treated myalgics (n = 3) and non-myalgics (n = 21) who doubled their CK from baseline (P≥0.40).
DISCUSSION
STOMP demonstrated that high-dose statin treatment for 3 and 6 months increased CK levels in healthy adults by 27 and 21 U/L, respectively, but did not impair skeletal muscle function or exercise performance [2]. We presently report that more atorvastatin-treated than placebo subjects at least doubled their baseline CK. Despite ostensibly more muscle injury, these atorvastatin treated subjects did not demonstrate deleterious effects on skeletal muscle function. Thus, the original STOMP report [2] and the present data demonstrate that high-dose statin treatment does not detrimentally impact muscular performance, even in those individuals displaying exaggerated CK responses.
Most prior clinical trials defined statin-associated myopathy as CK levels > than 10 times the ULN [3–9]. No subject in STOMP persistently demonstrated such a large CK response. Two subjects did temporarily demonstrate CK values of 4557 and 2120 U/L, respectively, which were not present on repeat analysis. At least one of these remarkable CK increases occurred after recent self-reported exercise confirming prior observations that statins augment the increase in CK that occurs with strenuous exercise [10, 11]. Few placebo controlled statin studies have reported average CK values at baseline and follow-up, but a systematic review [12] of muscle symptoms in statin trials identified 4 of 42 studies reporting baseline and change in CK values [3, 13–15]. CK increased 5.7, 6.8, and 13.3 U/L in three [3, 14, 15] of these four studies after 0.5, 5.4, and 3.4 years of treatment.
The only difference between subjects who did and did not double their CK was lower baseline CK values in the subjects with the more robust response. CK levels are higher in subjects with greater lean body mass [16] so it is possible that subjects with lower baseline CK had less muscle mass and would therefore receive a higher dose of statin per kg of muscle thereby producing more muscle injury. There was no difference in body weight or BMI between the two groups making this possibility unlikely although we do not have a better estimate of skeletal muscle mass. It is also possible that regression to the mean contributed to doubling CK since this was observed in those with the lower pretreatment CK values.
We assume that the increase in CK in STOMP [2] is indicative of muscle injury, but the absence of muscle dysfunction in those subjects doubling their CK values challenges this assumption. Statins could also increase CK levels by reducing inflammation. CK levels are lower in patients with inflammatory conditions such as rheumatoid arthritis and systemic lupus [17]. Statins inhibit activation of the pro-inflammatory transcription factor NF-κB in cultured human endothelial and vascular smooth muscle cells [18] and reduce systemic inflammation in adults [1]. It is not clear why the CK increase would double in some STOMP subjects and not others, but this could be due to genetic difference in CK related genes [19, 20] or to differences in genes regulating statin metabolism [21]. Furthermore, it is noteworthy that only 3 of the 24 subjects in STOMP who doubled their CK levels satisfied the study definition of myalgia and conversely that only 3 of the 19 myalgics were in the group that doubled their CK. This confirms prior clinical reports, not obtained from a double blind protocol, that CK levels are not predictive of skeletal muscle symptoms [22, 23].
Results of the present investigation extend the findings of STOMP [2] by demonstrating that greater increases in CK levels with high-dose atorvastatin treatment, presumed to indicate greater statin-induced muscle damage, did not translate into measurable changes in muscle function. Although we cannot exclude the possibility of low-level skeletal muscle injury with high-dose atorvastatin use in STOMP, any potential deleterious effect of statins on skeletal muscle [24] did not translate into greater reductions in muscle strength [2]. Interestingly, 33% and 50% of subjects who exhibited CK increases greater than two-times and three-times their baseline level, respectively, were on placebo. Why some placebo-treated subjects doubled their CK is unknown and highlights that increases in CK during statin therapy are not necessarily caused by the statin. We cannot exclude the contribution of recent physical activity as an alternate explanation of our observation of a two-fold increase in CK in ~12% and ~6% of atorvastatin- and placebo-treated subjects, respectively. However, even if exercise contributed, the putative exercise CK increase can be exacerbated by statins [10, 11]. We consider prior exercise as an unlikely explanation as no differences in self-reported and directly assessed physical activity levels were observed between treatment groups in STOMP [2], suggesting that differences in recent physical activity would not be likely to contribute to differential CK increases. Future studies are required to examine the impact of long-term statin use on muscular performance and their effect on muscle injury in asymptomatic individuals.
Conclusion
The present data demonstrate that exaggerated CK responses occurring with high-dose atorvastatin treatment in healthy adults do not deleteriously affect skeletal muscle function. Furthermore, exaggerated CK levels do not predict pre-defined skeletal muscle complaints [2] confirming that increased CK is not necessarily indicative of statin-associated myopathy.
Highlights.
CK increased slightly with atorvastatin, suggesting low-level muscle injury.
More subjects on atorvastatin doubled their CK level at 6 months.
CK increases with atorvastatin were not associated with changes in muscle function.
CK increases with atorvastatin did not predict the incidence of myalgia.
Increased CK is not necessarily indicative of statin-associated myopathy.
Acknowledgments
The following individuals are acknowledged for their participation on the Data Safety Monitoring Board: JoAnne Foody, M.D., Pamela Hartigan, Ph.D., and Ira Ockene, M.D. (chair).
FUNDING SOURCE
Funding provided by NHLBI/NIH grant RO1 HL081893 (P. Thompson).
Footnotes
This study was registered at ClinicalTrials.gov (NCT00609063)
DISCLOSURES
Paul D. Thompson reports receiving research grants from the National Institutes of Health, GlaxoSmithKline, Anthera, B. Braun, Genomas, Roche, Aventis, and Novartis; serving as a consultant for Astra Zenica, Regeneron, Merck, Roche, Genomas, Abbott, Runners World, Genzyme, Sanolfi, Pfizer, and GlaxoSmithKline; receiving speaker honoraria from Merck, Pfizer, Abbott, Astra Zenica, GlaxoSmithKline, and Kowa: owning stock in General Electric, JA Wiley Publishing, J&J, Sanolfi-Aventis and Abbott; and serving as a medical legal consultant on cardiac complications of exercise, statin myopathy, tobacco, ezetimibe and non-steroidals. The other authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kevin D. Ballard, Email: kballard@harthosp.org.
Beth A. Parker, Email: bparker03@harthosp.org.
Jeffrey A. Capizzi, Email: jac00012@gmail.com.
Adam S. Grimaldi, Email: adam.grimaldi@yahoo.com.
Priscilla M. Clarkson, Email: clarkson@kin.umass.edu.
Stephanie M. Cole, Email: smcole@kin.umass.edu.
Justin Keadle, Email: jkeadle@gmail.com.
Stuart Chipkin, Email: schipkin@kin.umass.edu.
Linda S. Pescatello, Email: linda.pescatello@uconn.edu.
Kathleen Simpson, Email: katie.simpson1@us.army.mil.
C. Michael White, Email: charles.white@uconn.edu.
Paul D. Thompson, Email: pthomps@harthosp.org.
References
- 1.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 2.Parker BA, Capizzi JA, Grimaldi AS, Clarkson PM, Cole SM, Keadle J, Chipkin S, Pescatello LS, Simpson K, White CM, Thompson PD. Effect of statins on skeletal muscle function. Circulation. 2013;127:96–103. doi: 10.1161/CIRCULATIONAHA.112.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Effects of pravastatin in patients with serum total cholesterol levels from 5.2 to 7.8 mmol/liter (200 to 300 mg/dl) plus two additional atherosclerotic risk factors. The Pravastatin Multinational Study Group for Cardiac Risk Patients. Am J Cardiol. 1993;72:1031–7. doi: 10.1016/0002-9149(93)90858-a. [DOI] [PubMed] [Google Scholar]
- 4.Bradford RH, Shear CL, Chremos AN, Dujovne CA, Franklin FA, Grillo RB, Higgins J, Langendorfer A, Nash DT, Pool JL, et al. Expanded Clinical Evaluation of Lovastatin (EXCEL) study results: two-year efficacy and safety follow-up. Am J Cardiol. 1994;74:667–73. doi: 10.1016/0002-9149(94)90307-7. [DOI] [PubMed] [Google Scholar]
- 5.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM., Jr Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–22. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 6.Maningat P, Breslow JL. Needed: pragmatic clinical trials for statin-intolerant patients. N Engl J Med. 2011;365:2250–1. doi: 10.1056/NEJMp1112023. [DOI] [PubMed] [Google Scholar]
- 7.Pitt B, Mancini GB, Ellis SG, Rosman HS, Park JS, McGovern ME. Pravastatin limitation of atherosclerosis in the coronary arteries (PLAC I): reduction in atherosclerosis progression and clinical events. PLAC I investigation. J Am Coll Cardiol. 1995;26:1133–9. doi: 10.1016/0735-1097(95)00301-0. [DOI] [PubMed] [Google Scholar]
- 8.Serruys PW, de Feyter P, Macaya C, Kokott N, Puel J, Vrolix M, Branzi A, Bertolami MC, Jackson G, Strauss B, Meier B. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;287:3215–22. doi: 10.1001/jama.287.24.3215. [DOI] [PubMed] [Google Scholar]
- 9.Serruys PW, Foley DP, Jackson G, Bonnier H, Macaya C, Vrolix M, Branzi A, Shepherd J, Suryapranata H, de Feyter PJ, Melkert R, van Es GA, Pfister PJ. A randomized placebo-controlled trial of fluvastatin for prevention of restenosis after successful coronary balloon angioplasty; final results of the fluvastatin angiographic restenosis (FLARE) trial. Eur Heart J. 1999;20:58–69. doi: 10.1053/euhj.1998.1150. [DOI] [PubMed] [Google Scholar]
- 10.Parker BA, Augeri AL, Capizzi JA, Ballard KD, Troyanos C, Baggish AL, D’Hemecourt PA, Thompson PD. Effect of statins on creatine kinase levels before and after a marathon run. Am J Cardiol. 2012;109:282–7. doi: 10.1016/j.amjcard.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 11.Thompson PD, Zmuda JM, Domalik LJ, Zimet RJ, Staggers J, Guyton JR. Lovastatin increases exercise-induced skeletal muscle injury. Metabolism. 1997;46:1206–10. doi: 10.1016/s0026-0495(97)90218-3. [DOI] [PubMed] [Google Scholar]
- 12.Ganga HV, Slim J, Thompson PD. A systematic review of statin-induced muscle problems in clinical trials. Unpublished work. [DOI] [PubMed] [Google Scholar]
- 13.Comparative efficacy and safety of pravastatin and cholestyramine alone and combined in patients with hypercholesterolemia. Pravastatin Multicenter Study Group II. Arch Intern Med. 1993;153:1321–9. [PubMed] [Google Scholar]
- 14.Keech A, Collins R, MacMahon S, Armitage J, Lawson A, Wallendszus K, Fatemian M, Kearney E, Lyon V, Mindell J, et al. Three-year follow-up of the Oxford Cholesterol Study: assessment of the efficacy and safety of simvastatin in preparation for a large mortality study. Eur Heart J. 1994;15:255–69. doi: 10.1093/oxfordjournals.eurheartj.a060485. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen TR, Berg K, Cook TJ, Faergeman O, Haghfelt T, Kjekshus J, Miettinen T, Musliner TA, Olsson AG, Pyorala K, Thorgeirsson G, Tobert JA, Wedel H, Wilhelmsen L. Safety and tolerability of cholesterol lowering with simvastatin during 5 years in the Scandinavian Simvastatin Survival Study. Arch Intern Med. 1996;156:2085–92. [PubMed] [Google Scholar]
- 16.Swaminathan R, Ho CS, Donnan SP. Body composition and plasma creatine kinase activity. Ann Clin Biochem. 1988;25 (Pt 4):389–91. doi: 10.1177/000456328802500411. [DOI] [PubMed] [Google Scholar]
- 17.Lee YH, Choi SJ, Ji JD, Song GG. Serum creatine kinase in patients with rheumatic diseases. Clin Rheumatol. 2000;19:296–300. doi: 10.1007/s100670070049. [DOI] [PubMed] [Google Scholar]
- 18.Dichtl W, Dulak J, Frick M, Alber HF, Schwarzacher SP, Ares MP, Nilsson J, Pachinger O, Weidinger F. HMG-CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:58–63. doi: 10.1161/01.atv.0000043456.48735.20. [DOI] [PubMed] [Google Scholar]
- 19.Clarkson PM, Hoffman EP, Zambraski E, Gordish-Dressman H, Kearns A, Hubal M, Harmon B, Devaney JM. ACTN3 and MLCK genotype associations with exertional muscle damage. J Appl Physiol. 2005;99:564–9. doi: 10.1152/japplphysiol.00130.2005. [DOI] [PubMed] [Google Scholar]
- 20.Hubal MJ, Devaney JM, Hoffman EP, Zambraski EJ, Gordish-Dressman H, Kearns AK, Larkin JS, Adham K, Patel RR, Clarkson PM. CCL2 and CCR2 polymorphisms are associated with markers of exercise-induced skeletal muscle damage. J Appl Physiol. 2010;108:1651–8. doi: 10.1152/japplphysiol.00361.2009. [DOI] [PubMed] [Google Scholar]
- 21.Ghatak A, Faheem O, Thompson PD. The genetics of statin-induced myopathy. Atherosclerosis. 2010;210:337–43. doi: 10.1016/j.atherosclerosis.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 22.Phillips PS, Haas RH, Bannykh S, Hathaway S, Gray NL, Kimura BJ, Vladutiu GD, England JD. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137:581–5. doi: 10.7326/0003-4819-137-7-200210010-00009. [DOI] [PubMed] [Google Scholar]
- 23.Soininen K, Niemi M, Kilkki E, Strandberg T, Kivisto KT. Muscle symptoms associated with statins: a series of twenty patients. Basic Clin Pharmacol Toxicol. 2006;98:51–4. doi: 10.1111/j.1742-7843.2006.pto_193.x. [DOI] [PubMed] [Google Scholar]
- 24.Mohaupt MG, Karas RH, Babiychuk EB, Sanchez-Freire V, Monastyrskaya K, Iyer L, Hoppeler H, Breil F, Draeger A. Association between statin-associated myopathy and skeletal muscle damage. CMAJ. 2009;181:E11–8. doi: 10.1503/cmaj.081785. [DOI] [PMC free article] [PubMed] [Google Scholar]