Abstract
Mesenchymal stem cells (MSCs) secrete paracrine factors that could be cytoprotective and serve roles in immunoregulation during tissue injury. Although MSCs express HIV receptors, and co-receptors, and are susceptible to HIV infection, whether HIV-1 may affect biological properties of MSCs needs more study. We evaluated cellular proliferation, differentiation and paracrine functions of MSCs isolated from compact bones of healthy control mice and Tg26 HIV-1 transgenic mice. The ability of MSCs to protect against cisplatin toxicity was studied in cultured renal tubular cells as well as in intact mice. We successfully isolated MSCs from healthy mice and Tg26 HIV-1 transgenic mice and found the latter expressed viral Nef, Vpu, NL4-3 and Vif genes. The proliferation and differentiation of Tg26 HIV-1 MSCs was inferior to MSCs from healthy mice. Moreover, transplantation of Tg26 HIV-1 MSCs less effectively improved outcomes compared with healthy MSCs in mice with acute kidney injury. Also, Tg26 HIV-1 MSCs secreted multiple cytokines, but at significantly lower levels than healthy MSCs, which resulted in failure of conditioned medium from these MSCs to protect cultured renal tubular cells from cisplatin toxicity. Therefore, HIV-1 had adverse biological effects on MSCs extending to their proliferation, differentiation, function, and therapeutic potential. These findings will help in advancing mechanistical insight in renal injury and repair in the setting of HIV-1 infection.
Introduction
Bone marrow- or bone-derived mesenchymal stem cells (MSCs) are multipotent with the ability to differentiate along various lineages and to serve roles in tissue homeostasis, repair, and immunoregulation [1]. Such properties of MSCs are relevant for HIV-1 and related pathophysiological mechanisms, since MSCs express HIV receptors, CD4, and also coreceptors, CCR5 and CXCR4, and are thus susceptible to HIV infection or its persistence [2, 3, 4 and 5]. Previously, HIV was found to suppress clonogenicity and differentiation potential of MSCs [6], which was in agreement with reduced bone density in HIV-infected patients [7 and 8], because osteoblasts are needed for new bone formation may originate, in part, from bone marrow MSCs [9 and 10]. At the molecular level, HIV-1 proteins, such as p55, gag and REV, may perturb osteogenic signaling in differentiating MSCs [11]. However, whether HIV-1 could affect paracrine signals emanating from MSCs has not yet been defined. This will be important since MSCs may influence other cells via paracrine signals.
MSCs secrete multiple substances, including growth factors, cytokines, and chemokines [12] and [13], and respond to chemoattractants while migrating to sites of tissue inflammation or injury [14 and 15]. These properties of MSCs generated much interest for cell transplantation, including for immunomodulation and tissue engineering [16]. For instance, cell therapy with transplantation of MSCs has been studied in the model of cisplatin-induced acute kidney injury (AKI) [13, 15, and 17]. Recently, we found transplanted MSCs improved outcomes in cisplatin-induced AKI by paracrine signals and not by replacing lost cells [18]. These MSCs secreted several soluble factors, including GCSF, VEGF, HGF, IL-10, EGF, IGF, etc. [18], that are well-known to possess antiapoptotic, anti-inflammatory, mitogenic, angiogenic [13], and renoprotective properties [19, 20, 21, 22, 23, 24 and 25].
As acute kidney injury is a common problem in patients with HIV-1, we hypothesized that expression of HIV-1 genes in MSCs might compromise their biological properties, including lower efficacy of cell therapy with such MSCs for recovery of injured renal tubular cells, especially via paracrine signaling from transplanted MSCs. In the present study, we isolated MSCs from long bones of Tg26 HIV-1 transgenic mice characterized by the proviral transgene, pNL4-3:d1443, which encodes all HIV-1 genes, except for gag and pol [26], and express viral Nef, Vpu, NL4-3 and Vif mRNAs. Studies of these Tg26 HIV-1 MSCs in comparison with MSCs from healthy mice showed that HIV-1 deleteriously affected proliferation, differentiation, and paracrine functions of MSCs.
Materials and methods
Animals
The Ethics Review Committee for Animal Experimentation of Feinstein Institute for Medical Research-North Shore LIJ Health System and the Animal Care and Use Committee of Albert Einstein College of Medicine approved study protocols. C57BL/6J, FVB/N and C57BL/6-TgCAG-EGFP/1Osb/J mice (GFP mice) with ubiquitous expression of green fluorescent protein (GFP) were from Jackson Labs (Bar Harbor, ME). Tg26 HIV-1 transgenic mice in FVB/N background were from Dr. P.E. Klotman (Mount Sinai Medical Center, New York, NY). Tg26 mice typically develop proteinuria and glomerulosclerosis by 4 weeks with worsening of renal functions by 8-10 weeks of age [26]. We used 6-8 weeks old mice. All animals were housed under standard lighting conditions with unlimited access to pelleted food and water.
Isolation and characterization of MSCs
MSCs were isolated from long bones of C57BL/6J, C57BL/6-GFP, FVB/N and Tg26 HIV-1 transgenic mice, essentially as described previously [27]. Briefly, fragments of compact bones were suspended in Mesencult basal medium (StemCell Technologies Inc; Vancouver, CA) containing 10% FBS and 1 mg/ml of collagenase II (Sigma-Aldrich, USA), followed by digestion for 1-2 hours at 37°C. Cells and bone fragments were incubated in Mesencult basal medium with supplements (StemCell Technologies Inc, CA) in humidified 37°C incubator with 4% O2 and 5% CO2 for 3 days. Non-adherent cells and tissue debris were removed by changing medium after 3 days and twice weekly. MSCs were used for studies after three to five passages in culture. To Characterize cellular phenotype, MSCs were stained with PE-conjugated monoclonal antibodies specific for CD11b, CD14, CD34, CD86, CD90, and CD105 (BD Pharmingen) and analyzed by flow cytometry (FACSAriaII, Biosciences).
Proliferation and osteogenic differentiation of HIV-1 MSCs
To examine proliferation ability, MSCs were continuously sub-passaged 1:3 with trypsin-EDTA. Population doublings were measured by manual counting of cell numbers. To induce osteogenic differentiation, MSCs were cultured in Mesencult medium containing 10% FBS, 100 nM dexamethasone, 50 μM ascorbic acid-2-phosphate and 10 mM β-glycerophosphate (Sigma) for 3 weeks with fresh medium every 3-4 days. To examine osteogenic differentiation, cells were fixed in 10% formalin for 1 hour, washed with PBS, and incubated with 40 mM Alizarin Red (Sigma) for 10 minutes.
Viral gene expression in Tg26 HIV-1 MSCs
Total RNA was extracted from MSCs at 90% confluence by Trizol Reagent (Invitrogen Life Technologies, Carlsbad, CA). After incubation with Amplification Grade DNase I (Invitrogen), RNAs were reverse transcribed with Omniscript RT system (Qiagen Inc., Valencia, CA). Polymerase chain reactions (PCR) for HIV-1 genes were performed with following primers: Nef (forward: 5′-GGTGGGTTTTCCAGTCACAC-3′, reverse: 5′-GGGAGTGAATTAGCCCTTCC-3′); NL4-3 (forward: 5′-GGCGTTACTCGACAGAGGAG-3′, reverse: 5′-TGCTTTCATAGAGAAGCTTGATG-3′); Vif (forward: 5′-ACCTGGCAGACCAACTAATTCACCT-3′, reverse: 5′-GGCCCTTGGTCTTCTGGGGCTT-3′); and Vpu (forward: 5′-AGCACCTTGGAACGATACCTGGGA-3′, reverse: 5′-TGGTCCTTTGATGGGAGGGGCA-3′). Mouse β-actin (forward: 5′-GAGCTATGAGCTGCCTGAC-3′, reverse: 5′-CTGATCCACATCTGCTGGAA-3′) was used as control for integrity of input RNA. PCR used equal amounts of templates in Platinum PCR Supermix (Life Sciences, Carlsbad, CA) with following conditions: denaturation at 94°C × 5 min, 35 cycles at 94°C × 30 sec, annealing at 55°C × 30 sec, and extension at 72°C × 1 min. PCR products were resolved in 1.5% agarose gels containing ethidium bromide.
Protective effect of transplanted MSCs in cisplatin-induced AKI
HIV-1 MSCs and FVB/N MSCs were trypsinized and passed through 70-μM nylon mesh. Male mice (n=5 each) were transplanted with either 5 × 105 HIV-1 MSCs or FVB/N-MSCs through tail vein injection 24 hours before cisplatin (Sigma Chemical Co., St. Louis, MO), which was freshly dissolved in saline to 1.2 mg/ml. Mice were injected subcutaneously with 12 mg/kg cisplatin. The vehicle-treated controls received saline alone. To demonstrate differences in renal histology and renal tests, kidney and blood samples were collected on day 3 after cisplatin or vehicle alone.
Identification of transplanted MSCs in kidneys
Groups of C57BL/6J mice (n = 3–5 each) were given 5 × 105 MSCs-GFP cells via tail vein. Mice were sacrificed 1 hour, 1 day, 3 days and 7 days after MSCs-GFP transplantation. Kidney samples were incubated in graded sucrose solutions and then frozen in methylbutane cooled to −80°C. Transplanted cells were identified by immunostaining for GFP. Briefly, cryosections of 5 μm thickness were fixed in 4% paraformaldehyde in PBS for 10 minutes at 4°C and blocked in 3% goat serum for 1 hour at room temperature. Primary rabbit anti-GFP antibody (Molecular Probes, 1:300) was applied for 1 hour at room temperature followed by FITC-conjugated goat anti-rabbit IgG for 30 minutes. Sections were counterstained with DAPI-Antifade.
Histological evaluation of nephrotoxicity
Tissue samples were fixed in 10% buffered formalin for 24 hours and then transferred to 100% ethanol. Tissues were embedded in paraffin to cut 5 μm thick sections and stained with hematoxylin and eosin and Periodic Acid Schiff (PAS). The extent of injury in cortex and outer medulla was graded by identifying fractions of tubules with loss of brush borders, necrosis, cast formation, or dilatation, as follows: 0 = none, 1 = <10%, 2 = 10-25%, 3 = 26-45%, 4 = 46-75%, and 5 = >75%. At least 20 microscopic fields were reviewed per tissue section under high power with blinded observers.
BUN assay
Serum BUN was measured as previously described [28]. Briefly, 0.15 ml Urease Buffer Reagent (U-3383, Sigma) was added to 50 μL serum for 20 minutes at room temperature followed by 0.3 ml Phenol Nitroprusside Solution (P-6994, Sigma), 0.3 ml alkaline hypochlorite solution (A-1727, Sigma), and 1.5 ml deionized water, in that order. The mixture was incubated for 30 minutes at room temperature and absorbance at 540 nm was measured. Standards were prepared with 10 mg/ml urea (U-5128, Sigma).
Conditioned medium and cytoprotection assay with renal tubular cells
For harvesting conditioned medium (CM), MSCs were cultured to confluence and then shifted to serum-free medium (Mesencult basal medium, StemCell Technologies Inc) for 24 hours. Cytokine content of CM from Tg26 HIV-1 MSCs and FVB/N MSCs was analyzed by mouse cytokine antibody array to probe for 97 cytokines (RayBiotech, Norcross, GA). Briefly, samples from CM and basal media were incubated with membranes for 1 hour at room temperature. After washing, membranes were incubated with biotin-conjugated anti-cytokine primary antibodies followed by washes and development with HRP-conjugated streptavidin. Chemiluminescence was used for signal detection. Pixel densities of cytokine spots were analyzed using Image J software (NIH, Bethesda). The density of cytokine spots was normalized with respect to positive controls on each membrane and to corresponding cytokine spots on basal medium control membrane. Signal intensity was averaged (+/-SD) and analyzed by log2 transformation. Differences of 2-fold or greater (log2 = 1) were considered to be significant. The set of cytokines expressed in CM were assigned to protein families, molecular functions, biological processes, cellular component and pathways using Protein Analysis through Evolutionary Relationships (PANTHER) classification system (http://www.pantherdb.org). For cytotoxicity assays, mouse proximal tubular epithelial cells (MTC), which were well characterized and expressed tubular cell markers [29], were cultured at density of 5 × 105 cells/cm2 in 24-well culture dishes and incubated with 8, 10, 15, 25 and 50 μM of cisplatin with or without CM for 24 hours at 37°C in 5% CO2. Growth inhibition of cells was evaluated by thiazolyl blue (MTT) utilization assays. All experiments were in triplicate and repeated for reproducibility (n=3).
Statistical Analysis
Data were expressed as means ± standard deviation. Significances were determined by Student's t test or analysis of variance with SigmaStat 3.1 (Systat Software Inc., Point Richmond, CA). P < 0.05 was considered significant.
Results
Characterization of MSCs
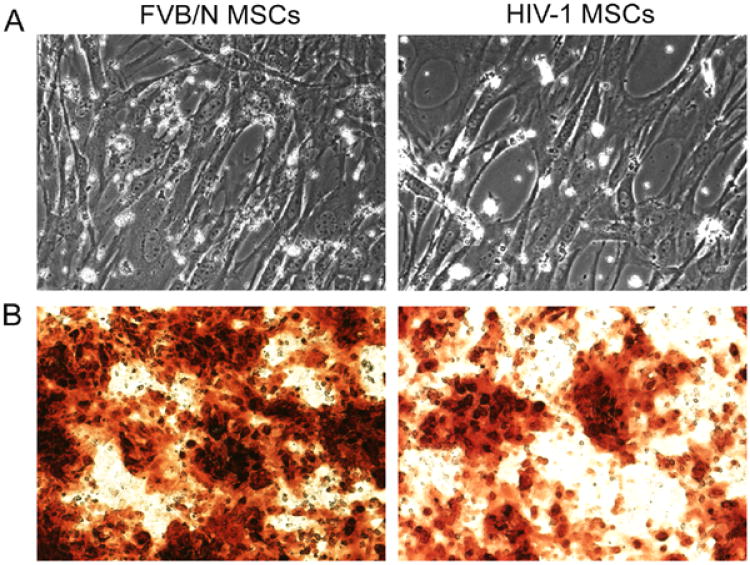
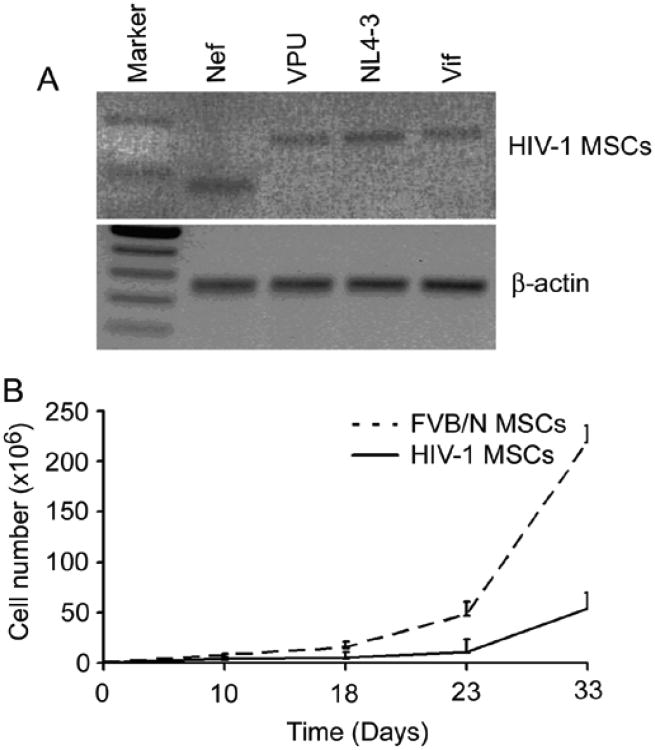
Morphologically, Tg26 HIV-1 MSCs resembled FVB/N MSCs during expansion (up to passages 8) (Fig. 1A). MSCs were homogenously positive for the mesenchymal markers, CD90 and CD105, and were negative for the hematopoietic markers, CD11b, CD14, and CD34, as well as the costimulating molecule, CD86 (not shown). Expression of viral genes in HIV-1 MSCs was confirmed since HIV-1 MSCs expressed Nef, Vpu, NL4-3 and Vif (Fig. 2A), which was expected.
Figure 1.
Osteogenic differentiation of HIV-1 MSCs. (A) Phase contrast microscopy showing morphology of HIV-1 MSCs and FVB/N MSCs cells was similar. (B) Staining with Alizarin Red showing less osteogenic differentiation in HIV-1 MSCs compared with FVB/N MSCs. The cells were cultured in differentiation conditions for 3 weeks before staining with Alizarin Red, ×100.
Figure 2.
HIV-1 MSCs expressed HIV-1 genes and exhibited less proliferation ability. (A) RT-PCR demonstrated Nef, Vpu, NL4-3 and Vif mRNA in HIV-1 MSCs. (B) Proliferation ability of HIV-1 MSCs was lower compared with FVB/N MSCs after 30 days expansion.
The mean numbers were similar of HIV-1 MSCs or FVB/N MSCs harvested per mouse after 5 days in culture, 7.3 × 105 HIV-1- MSCs vs. 7.2 × 105 FVB/N MSCs, p= N.S. However, after 30 days in culture (passage 4), FVB/N-MSCs expanded by mean of 300-fold, whereas HIV-1 MSCs expanded by 75-fold, which was 4-fold lower, p<0.001 (Fig. 2B), indicating HIV-1 inhibited proliferation of MSCs. Similarly, evaluation of lineage differentiation of MSCs revealed significantly less Alizarin Red staining on repeated occasions in HIV-1 MSCs versus FVB/N MSCs (Fig. 1B), indicating HIV-1 adversely affected osteogenic potential of MSCs.
Distribution and engraftment of transplanted MSCs
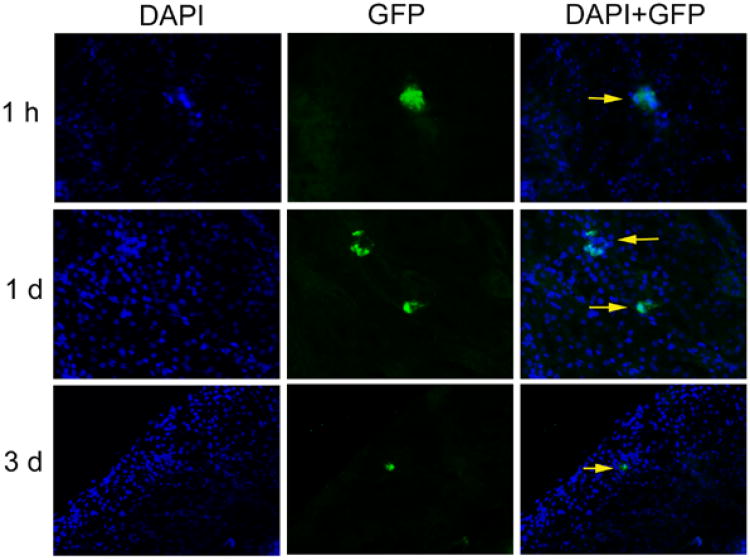
Transplanted MSCs were readily observed in pulmonary capillaries after 1 hour (not shown). Presence of transplanted GFP+ MSCs in kidneys was verified by GFP immunostaining after 1 hour and 1 day. After 3 days, transplanted cells could be identified only rarely (Fig. 3). After 7 days, transplanted GFP+ MSCs were not found in kidneys. This indicated that MSCs transplanted intravenously were largely entrapped in pulmonary capillaries, reached kidneys infrequently, and were rapidly cleared without long-term engraftment in kidneys.
Figure 3.
Distribution and engraftment of transplanted MSCs. GFP immunostaining verified transplanted GFP+ MSCs in kidneys 1 hour and 1 day after transplantation (arrows). Transplanted cells were found only rarely after 3 days, ×200.
Effects of intravenous transplantation of HIV-1 MSCs on cisplatin-induced AKI
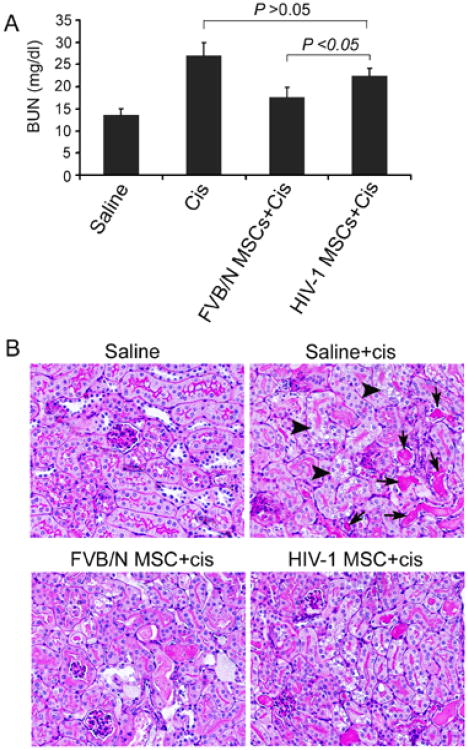
In mice receiving FVB/N MSCs followed by cisplatin, BUN was significantly lower than vehicle-treated control mice, i.e., 17.6 ± 1.7 mg/dl vs. 29.6 ± mg/dl, p<0.05 (Fig. 4A). By contrast, BUN in mice receiving HIV-1 MSCs followed by cisplatin was higher, 22.5 ± 1.2 mg/dl, which was greater than in animals treated with FVB/N MSCs and cisplatin (Fig. 4A). These blood test results were in agreement with renal histology, which showed attenuation of cisplatin-induced tubular injury and cast formation in mice receiving FVB/N MSCs, and less so after transplantation of Tg26 HIV-1 MSCs (Fig. 4B). The renal histology score in vehicle-treated mice receiving cisplatin was 3.0 ± 0.4. By contrast, renal histology score in mice receiving FVB/N MSCs was 0.9 ± 0.3, which was lower than mice receiving HIV-1 MSCs, 2.7 ± 0.6, p<0.05.
Figure 4.
Effects of intravenous transplantation of HIV-1 MSCs on cisplatin-induced AKI. (A) BUN in mice receiving FVB/N MSC was significantly lower than in vehicle-treated controls. Mice receiving HIV-1 MSCs showed higher BUN levels. (B) Renal histology showed less tubular injury (arrowhead) and cast formation (black arrow) in mice receiving FVB/N MSCs compared with vehicle alone. Mice receiving HIV-1 MSCs did not show improvement in renal injury. PAS stain, ×200.
Analysis of CM from MSCs
FVB/N MSCs expressed multiple soluble factors, including IL-6, KC, CXCL16, decorin, Galectin-1, Gas 6, MCP1, MIP1α, MIP2, osteopontin, osteoporotegerin, RANTES, sTNFR1, VCAM1, etc., which was similar to previous findings [18]. However, CM of HIV-1 MSCs contained significantly lower levels of many factors (Table 1). Noteworthy decreases were as follows: Lungkine (10-fold), 6Ckine (8-fold), IGFBP-5 (7-fold), MIP1α (5-fold), HGF (4-fold), GSCF (4-fold), I-TAC (4-fold), IL12p70 (4-fold), FcgRIIB (4-fold), IL-17 (4-fold) and E-cadherin (4-fold).
Table 1.
Analysis of conditioned medium from HIV-1 MSCs and FVB/N MSCs.
| Cytokines | Log2 (HIV/FVBN) | Cytokines | Log2 (HIV/FVBN) | Cytokines | Log2 (HIV/FVBN) |
|---|---|---|---|---|---|
|
| |||||
| IL-15 | 3 | IL-1 rα | - 3 | L-Selectin | - 2 |
| IGFBP6 | 2 | IL-17B R | - 3 | Axl | - 2 |
| MMP-2 | 1 | EGF | - 3 | HAI-1 | - 2 |
| Lungkine | -10 | Flt-3 Ligand | - 3 | MadVAM-1 | - 2 |
| 6Ckine | - 8 | GITR | - 3 | IL-2 Rα | - 2 |
| IGFBP-5 | - 7 | SCF | - 3 | TROY | - 2 |
| MIP-1α | - 5 | TPO | - 3 | E-Selectin | - 2 |
| HGF | - 4 | Decorin I | - 3 | IL13 | - 2 |
| GCSF | - 4 | TAC | - 3 | Leptin R | - 2 |
| I-TAC | - 4 | MDC | - 2 | IL-10 | - 2 |
| IL-12 p70 | - 4 | IL-21 | - 2 | Fas Ligand | - 2 |
| Fcg RIIB | - 4 | Osteoponin | - 2 | sTNF RII | - 2 |
| IL17 | - 4 | IL-2 | - 2 | IL-1 β | - 2 |
| E-cadherin | - 4 | TARC | - 2 | TRANCE | - 2 |
| IL17F | - 4 | IL-1 α | - 2 | CD36/SR-B3 | - 1 |
| Prolactin | - 3 | Amphiregulin | - 2 | MIP-1γ | - 1 |
| GITR Ligand | - 3 | Dkk-1 | - 2 | Leptin | - 1 |
| IL17E | - 3 | IFNγ | - 2 | IGF-II | - 1 |
| ALK1 | - 3 | CD30L | - 2 | MFG-E8 | - 1 |
| Osteoporotegerin | - 3 | Gas6 | - 2 | VEGF-D | - 1 |
| Granzyme B | - 3 | Fractalkine | - 2 | BLC | - 1 |
| TNF-α | - 3 | VCAM-1 | - 2 | IL-3 | - 1 |
| JAM-A | - 3 | VEGF R3 | - 2 | VEGF R1 | - 1 |
| IL-20 | - 3 | Pro-MMP-9 | - 2 | IL-5 | - 1 |
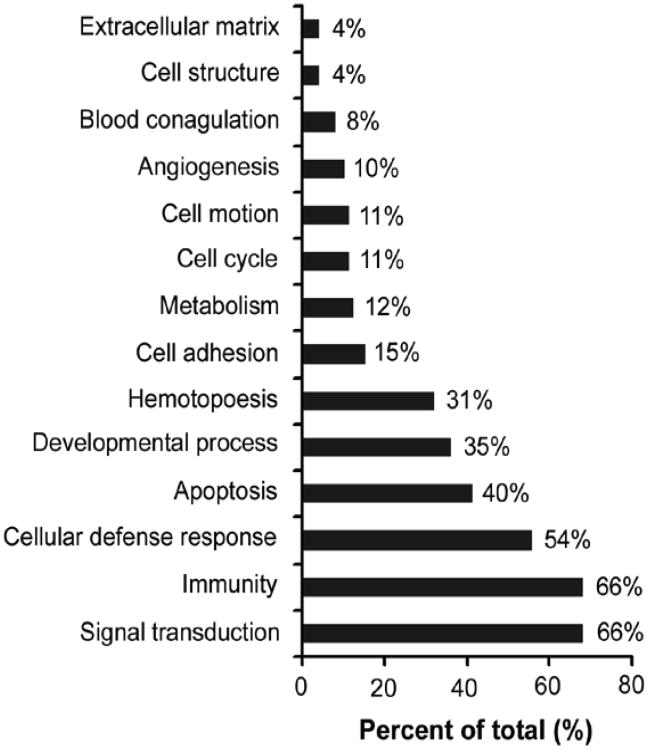
The distribution of various substances according to ontology based on biological processes and molecular function using PANTHER classification system was informative. Keeping in mind that an individual factor could be involved in multiple biological processes and several factors could share similar functions, we found these factors were grouped along the following major classes: signal transduction (66%), immunity (66%), cellular defense response (54%), apoptosis (40%), developmental process (35%), hematopoiesis (31%), cell adhesion (15%), and metabolism (12%) (Fig. 5).
Figure 5.
Biological classification of soluble factors in CM by PANTHER system. The distribution of each biological category is presented as percentage of total calculated pathways.
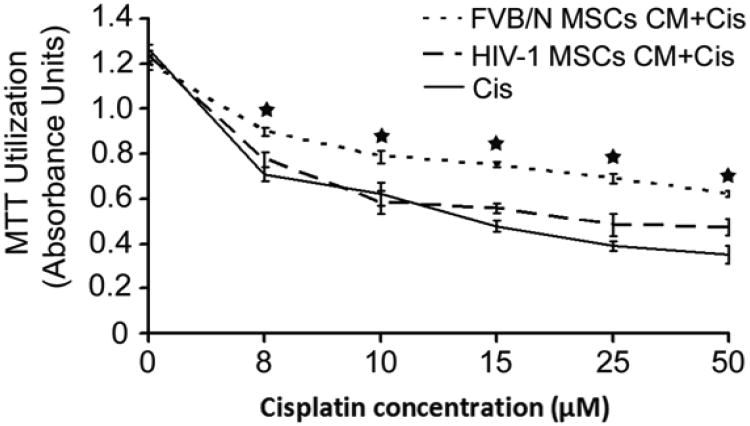
Effects of CM from MSCs on renal tubular cytotoxicity in vitro
Treatment of MTC cells with cisplatin in basal medium led to dose-dependent cytotoxicity within 24 hours. FVB/N-MSCs CM decreased cisplatin-induced cytotoxicity, as indicated by MTT assays (P<0.05, Fig. 6). By contrast, in response to CM from HIV-1 MSCs, viability of MTC cells was not significantly different from that noted for basal medium (P>0.05), indicating that HIV-1 MSCs did not protect from cisplatin-induced cytotoxicity.
Figure 6.
Effects of CM from MSCs on cisplatin-induced toxicity in MTC. Cells were incubated with cisplatin for 24 hours. CM from HIV-1 MSCs did not protect MTC from cisplatin toxicity compared with CM from FVB/N MSCs.
Discussion
These studies provided significant insights into the effects of HIV-1 on bone-derived MSCs. First, use of Tg26 HIV-1 transgenic mice was effective for addressing differences in the biology of MSCs since cells isolated from these mice expressed HIV-1 genes. Second, HIV-1 had significant effects upon MSCs, including less proliferation and osteogenic differentiation ability, along with impaired function of HIV-1 MSCs, as demonstrated by inferior expression of multiple cytokines and chemokines. Consequently, HIV-1 MSCs were less capable of cytoprotection in the setting of cisplatin-induced AKI. The underlying mechanisms responsible for inferior cytoprotection from HIV-1 MSCs involved differences in secretion of paracrine factors.
The Tg26 line was established in transgenic mice containing a replication-defective HIV-1 construct that lacked gag/pol region of pNL4-3 provirus, but included 5' and 3' long terminal repeats, env, tat, nef, rev, vif, vpr, and vpu genes [26]. These mice expressed HIV-1 genes abundantly in skin, muscle, tail, and variably in kidneys, intestine, and thymus [30]. The Tg26 mouse line has been widely used as a model of HIV-1-associated nephropathy [31]. However, whether bone marrow cells in Tg26 mice expressed HIV-1 had not previously been investigated. Similarly, to our knowledge, the effects of HIV-1 on MSCs in this transgenic line had not been established. While previous studies suggested possible connections between HIV-1 gene products and regulation of gene expression in human MSCs [11], these were of unclear significance. For instance, HIV-1 rev increased the level or activity of genes governing development of MSCs, e.g., CTGF, RUNX-2 and BMP-2, whereas HIV-1 p55 or gag, had opposite effects on CTGF, RUNX-2 and BMP-2. Our results showed that HIV-1 MSCs secreted less osteopoinin, osteopontin, decorin, Dkk-1, and pro-MMP-9, which contribute in osteogenesis and matrix maturation [32]. Therefore, it should seem reasonable that these paracrine factors accounted for lower osteogenic capacity of HIV-1 MSCs, although additional mechanisms may be involved. Similarly, paracrine factors, and perhaps additional mechanisms, likely accounted for lower proliferation of HIV-1 MSCs. These findings of impaired proliferation and paracrine functions of MSCs should be noteworthy for helping explain the observation of reduced bone density in HIV-1-infected individuals [7] and [8].
We consider that HIV-1-induced alterations in MSCs carry significance for their therapeutic applications. For instance, among the list of factors produced least well by HIV-1 MSCs were GSCF, SCF, HGF, VEGF and VEGF receptor (Table 1). This could be especially significant because these factors were previously identified to contribute in renal tubular repair and regeneration in cisplatin-induced AKI [1], 20, 21, 22, 23, 24 and 25]. Recently, we reported that CM from healthy mouse MSCs improved cisplatin-induced AKI [18]. We found renoprotection by MSCs was due to paracrine signaling from transplanted MSCs and not replacement of damaged renal cells [18]. Similarly, in the present study, we only infrequently or rarely detected transplanted MSCs in kidneys after 1 h and 1 day or 3 days, which reiterated that outcomes in cisplatin-induced AKI after cell therapy reflected beneficial paracrine signaling from transplanted cells and that HIV-1 MSCs were inferior for this purpose.
Another mechanism in tissue repair and/or regeneration may concern release of inflammatory cytokines or chemokines. In this context, MSCs are well recognized to exert immunomodulatory effects by inhibiting T, B, dendritic and NK cell proliferation through paracrine effectors [33 and 34]. Moreover, numerous cytokines modulate HIV-1 infection and replication in CD4 cells and macrophages, including HIV-1-suppressor, e.g., IFN-α, IL-10, MIP-1α, MIP-1β and RANTES, and HIV-1 inducer, e.g., TNF-α, IL-1β, IL-6, IL-15 and MCP-1 [35 and 36]. Although we did not measure blood levels of cytokines in Tg26 mice, recent studies in HIV-1- infected individuals established differences in cytokine expression, e.g., IL-6, TNF-α, M-CSF, IL-10, and IL-1 [36 and 37]. Whether these differences in cytokine or chemokine levels reflected impaired expression from MSCs perturbed by HIV-1 is one possibility. Similarly, we did not examine differences in cytokine or chemokine levels in this study, although whether transplanted HIV-1 MSCs were less effective in modulating cytokine or chemokine release from phagocytes, macrophages or other inflammatory cells in cisplatin-treated animals is another possibility.
We conclude that HIV-1 expression in MSCs not only modulates their proliferation and differentiation properties but also alters their therapeutic potential. These findings provide mechanistical insight in renal injury and repair in the setting of HIV-1 infection.
Highlights.
MSCs isolated from HIV mice displayed HIV genes.
MSCs isolated from HIV mice exhibited attenuated growth and paracrine functions.
AKI mice with transplanted HIV-MSC displayed poor outcome.
HIV-1 MSC secreted multiple cytokines but at a lower level.
Acknowledgments
This work was supported by grants RO1DK084910, 1R01DK098074, and RO1 DK083931 (PCS) and R01 DK088561, R01 DK071111 and P30-DK41296 (SG). from National Institutes of Health, Bethesda, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chamberlain G, Fox J, Ashton B, Middleton J. Mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 2.Cotter EJ, Chew N, Powderly WG, Doran PP. HIV type 1 alters mesenchymal stem cell differentiation potential and cell phenotype ex vivo. AIDS Res Hum Retroviruses. 2011;27:187–199. doi: 10.1089/aid.2010.0114. [DOI] [PubMed] [Google Scholar]
- 3.Gibellini D, Alviano F, Miserocchi A, Tazzari PL, Ricci F, Clò A, Morini S, Borderi M, Viale P, Pasquinelli G, Pagliaro P, Bagnara GP, Re MC. HIV-1 and recombinant gp120 affect the survival and differentiation of human vessel wall-derived mesenchymal stem cells. Retrovirology. 2011;8:40. doi: 10.1186/1742-4690-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canque B, Marandin A, Rosenzwajg M, Louache F, Vainchenker W, Gluckman JC. Susceptibility of human bone marrow stromal cells to human immunodeficiency virus (HIV) Virology. 1995;208:779–783. doi: 10.1006/viro.1995.1211. [DOI] [PubMed] [Google Scholar]
- 5.Gibellini D, Miserocchi A, Tazzari PL, Ricci F, Clò A, Morini S, Ponti C, Pasquinelli G, Bon I, Pagliaro P, Borderi M, Re MC. Analysis of the effects of HIV-1 Tat on the survival and differentiation of vessel wall-derived mesenchymal stem cells. J Cell Biochem. 2012;113:1132–1141. doi: 10.1002/jcb.23446. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Mondal D, La Russa VF, Agrawal KC. Suppression of Clonogenic Potential of Human Bone Marrow Mesenchymal Stem Cells by HIV Type 1: Putative Role of HIV Type 1 Tat Protein and Inflamatory Cytokines. AIDS Res Hum Retroviruses. 2002;18:917–931. doi: 10.1089/088922202760265597. [DOI] [PubMed] [Google Scholar]
- 7.Bongiovanni M, Tincati C. Bone diseases associated with human immunodeficiency virus infection: pathogenesis, risk factors and clinical management. Curr Mol Med. 2006;6:395–400. doi: 10.2174/156652406777435435. [DOI] [PubMed] [Google Scholar]
- 8.McComsey GA, Tebas P, Shane E, Yin MT, Overton ET, Huang JS, Aldrovandi GM, Cardoso SW, Santana JL, Brown TT. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51:937–946. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borderi M, Gibellini D, Vescini F, De Crignis E, Cimatti L, Biagetti C, Tampellini L, Re MC. Metabolic bone disease in HIV infection. AIDS. 2009;23:1297–1310. doi: 10.1097/QAD.0b013e32832ce85a. [DOI] [PubMed] [Google Scholar]
- 10.Cotter EJ, Malizia AP, Chew N, Powderly WG, Doran PP. HIV proteins regulate bone marker secretion and transcription factor activity in cultured human osteoblasts with consequent potential implications for osteoblast function and development. AIDS Res Hum Retroviruses. 2007;23:1521–1530. doi: 10.1089/aid.2007.0112. [DOI] [PubMed] [Google Scholar]
- 11.Cotter EJ, Ip HS, Powderly WG, Doran PP. Mechanism of HIV protein induced modulation of mesenchymal stem cell osteogenic differentiation. BMC Musculoskelet Disord. 2008;9:33–45. doi: 10.1186/1471-2474-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eliopoulos N, Zhao J, Bouchentouf M, Forner K, Birman E, Yuan S, Boivin MN, Martineau D. Human marrow-derived mesenchymal stromal cells decrease cisplatin renotoxicity in vitro and in vivo and enhance survival of mice post-intraperitoneal injection. Am J Physiol Renal Physiol. 2010;299:F1288–298. doi: 10.1152/ajprenal.00671.2009. [DOI] [PubMed] [Google Scholar]
- 14.Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, Alison M, Remuzzi G. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–1804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 15.Herrera MB, Bussolati B, Bruno S, Morando L, Mauriello-Romanazzi G, Sanavio F, Stamenkovic I, Biancone L, Camussi G. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 2007;72:430–441. doi: 10.1038/sj.ki.5002334. [DOI] [PubMed] [Google Scholar]
- 16.Ozawa K, Sato K, Oh I, Ozaki K, Uchibori R, Obara Y, Kikuchi Y, Ito T, Okada T, Urabe M, Mizukami H, Kume A. Cell and gene therapy using mesenchymal stem cells (MSC) J Autoimmun. 2008;30:121–127. doi: 10.1016/j.jaut.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, Oh DJ, Lu L, Klein CL, Dinarello CA, Edelstein CL. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther. 2007;322:8–15. doi: 10.1124/jpet.107.119792. [DOI] [PubMed] [Google Scholar]
- 18.Cheng K, Rai P, Plagov A, Lan X, Kumar D, Salhan D, Rehman S, Malhotra A, Bhargava K, Palestro CJ, Gupta S, Singhal PC. Transplantation of Bone Marrow-derived MSCs Improves Cisplatinum-induced Renal Injury through Paracrine Mechanisms. Exp Mol Pathol. 2013;94:466–73. doi: 10.1016/j.yexmp.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bengatta S, Arnould C, Letavernier E, Monge M, de Préneuf HM, Werb Z, Ronco P, Lelongt B. MMP9 and SCF protect from apoptosis in acute kidney injury. J Am Soc Nephrol. 2009;20:787–797. doi: 10.1681/ASN.2008050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001;60:2118–2128. doi: 10.1046/j.1523-1755.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 21.Deuse T, Peter C, Fedak PM, Doyle T, Reichenspurner H, Zimmermann WH, Eschenhagen T, Stein W, Wu JC. Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell–based myocardial salvage after acute myocardial infarction. Circulation. 2009;120:S247–S254. doi: 10.1161/CIRCULATIONAHA.108.843680. [DOI] [PubMed] [Google Scholar]
- 22.Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L, Rottoli D, Valsecchi F, Benigni A, Wang J, Abbate M, Zoja C, Remuzzi G. Insulin-like growth factor-1 sustains stem cell-mediated renal repair. J Am Soc Nephrol. 2007;18:2921–2928. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki M, Adachi Y, Minamino K, Suzuki Y, Zhang Y, Okigaki M, Nakano K, Koike Y, Wang J, Mukaide H, Taketani S, Mori Y, Takahashi H, Iwasaka T, Ikehara S. Mobilization of bone marrow cells by G-CSF rescues mice from cisplatin-induced renal failure, and M-CSF enhances the effects of G-CSF. J Am Soc Nephrol. 2005;16:658–666. doi: 10.1681/ASN.2004010067. [DOI] [PubMed] [Google Scholar]
- 24.Tögel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772–1784. doi: 10.1111/j.1523-1755.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 25.Yuan L, Wu MJ, Sun HY, Xiong J, Zhang Y, Liu CY, Fu LL, Liu DM, Liu HQ, Mei CL. VEGF-modified human embryonic mesenchymal stem cell implantation enhances protection against cisplatin-induced acute kidney injury. Am J Physiol Renal Physiol. 2011;300:207–218. doi: 10.1152/ajprenal.00073.2010. [DOI] [PubMed] [Google Scholar]
- 26.Kopp JB, Klotman ME, Adler SH, Bruggeman LA, Dickie P, Marinos NJ, Eckhaus M, Bryant JL, Notkins AL, Klotman PE. Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc Natl Acad Sci USA. 1992;89:1577–1581. doi: 10.1073/pnas.89.5.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu H, Guo ZK, Jiang XX, Li H, Wang XY, Yao HY, Zhang Y, Mao N. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc. 2010;5:550–560. doi: 10.1038/nprot.2009.238. [DOI] [PubMed] [Google Scholar]
- 28.Cheng K, Benten D, Bhargava K, Inada M, Joseph B, Palestro C, Gupta S. Hepatic targeting and biodistribution of human fetal liver stem/progenitor cells and adult hepatocytes in mice. Hepatology. 2009;50:1194–1203. doi: 10.1002/hep.23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf G, Neilson EG. Isoproterenol induces mitogenesis in MCT and LLC-PK1 tubular cells. J Am Soc Nephrol. 1994;4:1995–2002. doi: 10.1681/ASN.V4121995. [DOI] [PubMed] [Google Scholar]
- 30.Bruggeman LA, Thomson MM, Nelson PJ, Kopp JB, Rappaport J, Klotman PE, Klotman ME. Patterns of HIV-1 mRNA expression in transgenic mice are tissue-dependent. Virology. 1994;202:940–948. doi: 10.1006/viro.1994.1416. [DOI] [PubMed] [Google Scholar]
- 31.Cheng K, Rai P, Plagov A, Lan X, Subrati A, Husain M, Malhotra A, Singhal PC. MicroRNAs in HIV-associated nephropathy (HIVAN) Exp Mol Pathol. 2013;94:65–72. doi: 10.1016/j.yexmp.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulterer B, Friedl G, Jandrositz A, Sanchez-Cabo F, Prokesch A, Paar C, Scheideler M, Windhager R, Preisegger KH, Trajanoski Z. Gene expression profiling of human mesenchymal stem cells derived from bone marrow during expansion and osteoblast differentiation. BMC Genomics. 2007;8:70. doi: 10.1186/1471-2164-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Armstrong MA, Li G. Mesenchymal stem cells in immunoregulation. Immunol Cell Biol. 2006;84:413–421. doi: 10.1111/j.1440-1711.2006.01458.x. [DOI] [PubMed] [Google Scholar]
- 34.Yang SH, Park MJ, Yoon IH, Kim SY, Hong SH, Shin JY, Nam HY, Kim YH, Kim B, Park CG. Soluble mediators from mesenchymal stem cells suppress T cell proliferation by inducing IL-10. Exp Mol Med. 2009;41:315–324. doi: 10.3858/emm.2009.41.5.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kedzierska K, Crowe SM. Cytokines and HIV-1: interactions and clinical implications. Antivir Chem Chemother. 2001;12:133–150. doi: 10.1177/095632020101200301. [DOI] [PubMed] [Google Scholar]
- 36.Barqasho B, Nowak P, Tjernlund A, Kinloch S, Goh LE, Lampe F, Fisher M, Andersson J, Sönnerborg A. Kinetics of plasma cytokines and chemokines during primary HIV-1 infection and after analytical treatment interruption. HIV Med. 2009;10:94–102. doi: 10.1111/j.1468-1293.2008.00657.x. [DOI] [PubMed] [Google Scholar]
- 37.Isgrò A, Aiuti A, Leti W, Gramiccioni C, Esposito A, Mezzaroma I, Aiuti F. Immunodysregulation of HIV disease at bone marrow level. Autoimmun Rev. 2005;4:486–490. doi: 10.1016/j.autrev.2005.04.014. [DOI] [PubMed] [Google Scholar]