Abstract
Protein haptenation by polyurethane industrial intermediate methylene diphenyl diisocyanate (MDI) is thought to be an important step in the development of diisocyanate (dNCO)-specific allergic sensitization; however, MDI haptenated albumins used to screen specific antibody are often poorly characterized. Recently, the need to develop standardized immunoassays using a consistent, well characterized dNCO-haptenated protein to screen for the presence of MDI-specific IgE and IgG from workers’ sera has been emphasized and recognized. This has been challenging to achieve due to the bivalent, electrophilic nature of dNCO leading to the capability to produce multiple cross-linked protein species and polymeric additions to proteins. In the present study, MDI was reacted with human serum albumin (HSA) and hemoglobin (Hb) at molar ratios ranging from 1:1 to 40:1 MDI: protein. Adducts were characterized by (1) loss of available trinitrobenzene sulfonic acid (TNBS) binding to primary amines, (2) electrophoretic migration in polyacrylamide gels, (3) quantification of methylene diphenyl diamine following acid hydrolysis and (4) immunoassay. Concentration dependent changes in all the above noted parameters were observed demonstrating increase in both number and complexity of conjugates formed with increasing MDI concentration. In conclusion, a series of bio-analytical assays should be performed to standardize MDI-antigen preparations across lots and laboratories for measurement of specific antibody in exposed workers which in total indicate degree of intra- and inter-molecular cross-linking, number of dNCO bound, number of different specific binding sites on the protein and degree of immuno-reactivity.
Keywords: Occupational Asthma, Immunoassay standardization, Human serum albumin, Hemoglobin, Methylene diphenyl diisocyanate
Introduction
Diisocyanates (dNCO), such as 4, 4′-methylenediphenyl diisocyanate (MDI), are high volume production chemicals used in the production of polyurethane foams, elastomers, paints and other related products (1;2). Exposure to dNCO has most commonly been reported in the occupational setting (3;4); however, concern of potential exposure through leaching of dNCOs from cured, semi-cured or non-cured products has recently been raised in domestic settings(5).
dNCOs are low molecular weight (LMW) compounds that must first react with (haptenated) autologous proteins to produce a functional antigen (6;7). Apart from directly reacting with proteins at the site of exposure, diisocyanates can react with glutathione or thiol containing proteins, forming labile thiocarbamate adducts that may possibly be transported to sites distal to the site of exposure. It is not known if the antigenicity of proteins adducted by isocyanates regenerated from thiocarbamates is different than via direct haptenation. Synthetic methods have been reported for amino acid conjugation using thiocarbamates (8) which may possibly find utility in haptenation of whole proteins for specific-antibody detection. The fate of the dNCO in the body and the ultimate protein adduct responsible for immunologic sensitization currently remains unknown (9).
Diisocyanate asthma has been one of the most commonly reported causes of occupational asthma (OA); however, a downward trend in the number of reported cases has been noted in recent years.(10–12). This may be attributed to implementation of exposure limits and increased surveillance. Diagnosis of dNCO-induced OA remains confounded by methodological limitations(13). Although dNCO asthma displays the pathological hallmarks of allergic asthma, including eosinophilic inflammation and increased airway reactivity, testing for dNCO-specific IgE for diagnosis of dNCO asthma is specific (96–98%), but not sensitive (18–27%)(14). The low prevalence of detectable dNCO-specific IgE has been attributed to both assay limitations and to a potential IgE- independent dNCO-asthma mechanism(s)(15). Ott et al. (14) reviewed issues related to dNCO antibody testing for use in diagnosis of dNCO asthma. Immunoassay standardization is critical for comparison of results across studies (16). A number of factors that may confound results from these immunoassays include the dNCO used, the carrier protein employed, dNCO-protein reaction conditions, and post-reaction processing of the haptenated protein. The variability of results obtained in these immunoassays may also be in part due to a lack of standardization in conjugate preparation and characterization.
dNCOs are reactive, bi-functional electrophilic compounds. When reacted to protein under aqueous conditions, dNCO can covalently bind to protein amines and reversibly, to thiols (17). Such binding is in competition with hydrolysis of the dNCO to a diamine. Multiple species are formed with reaction of dNCO to protein including [a] monomeric dNCO binding (1 N=C=O moiety reacts to the protein and the other hydrolyses to an amine), [b] polymeric binding onto a protein site (a second dNCO reacts to the amine formed from NCO hydrolysis), [c] intra-molecular crosslinking by the dNCO through two nucleophilic sites on a protein, or [d] intermolecular crosslinking by the dNCO through nucleophilic sites on two different protein molecules. A single protein molecule such as human serum albumin (HSA) may also be haptenated at multiple nucleophilic sites by one or more of the species noted above(18). HSA is the most common carrier protein used for dNCO antibody immunoassays (19) due to its prevalence in plasma (20) to form MDI adducts. Other molecules such as keratin 18(20), tubulin (21) and the peptide, glutathione (22) have been found to be modified by dNCO exposure. Hb-MDI haptenation occurs in vivo following MDI exposure. Sabbioni et al reported MDI bound to the N-terminal valine of hemoglobin in MDI exposed rats and proposed Hb-MDI as a biological marker of MDI exposure (23). The same authors also found the N-terminal adduct with valine in globin of a TDI-exposed worker and in two women with polyurethane covered breast implants(24). The immunogenicity of adducted proteins that have been identified other than albumin has not been tested; however, as haptenated keratin and tubulin were identified immunochemically suggest that multiple haptenated-protein species formed following exposure may be antigens.
Due to the lack of characterization of protein dNCO adducts, antibody reactivity toward other endogenous haptenated proteins may be overlooked using conventional detection approaches, however other proteins assessed as carrier proteins for dNCOs have not been as effective at detecting dNCO-specific antibody in the blood of exposed workers. It is not known if this is due to varying degrees of dNCO adduction to different proteins or antibody recognition. Characterization of different dNCO-protein conjugates is therefore important to further our understanding of dNCO haptenation and adduction.
Our previous research has been directed toward delineating the concentration-dependent increase in specific dNCO such as toluene diisocyanate (TDI) binding sites on HSA (18). In these studies, the predominant TDI binding sites on HSA were lysine residues, although binding to the N-terminal arginine and to glutamine were also observed. In another study performed in our lab (25), MDI bound to the same sites as TDI, but overall the reactivity was reduced. The extent of conjugation was also influenced by buffers as conjugation was greater in phosphate buffer compared to ammonia carbonate buffer as a result of faster kinetics of the competing hydrolysis of the dNCO in ammonium carbonate buffer compared to phosphate buffered saline (PBS).
In the present study, we further characterize MDI-HSA conjugation/adduction with the aim of developing a standardized approach for screening IgE and IgG-specific dNCO-haptenated HSA. MDI reactivity toward hemoglobin was additionally examined and compared to HSA, since dNCO adducted hemoglobin has been measured from the blood of exposed workers and used as a biological marker of dNCO exposure (26). Although diisocyanate adducted hemoglobin in vivo immunogenicity or antigenicity has not yet been reported in the literature, Wong J.L et al(27) reported that acrylonitrile adducted hemoglobin was antigenic. In another study, Pien et al(28) found that rats exposed to the respiratory allergen and inducer of late respiratory systemic syndrome, trimellitic anhydride (TMA) by inhalation produced IgG that recognized both TMA haptenated-albumin and -hemoglobin. They demonstrated through cross-inhibition studies that TMA-albumin and TMA-hemoglobin share antigenic determinants. Collectively, these studies suggest that haptenated hemoglobin can be antigenic.
Materials and Methods
Chemicals
Unless otherwise specified, all reagents were acquired from Sigma-Aldrich (St. Louis, MO) and used without further purification. Ethyl acetate (reagent grade) was purchased from J.T Baker/Avantor Performance Materials, Inc. (Center Valley, PA). Sodium tetra borate, sodium hydroxide, hydrochloric acid, dialysis membranes (MWCO 12,000–14,000), 98% sulfuric acid, N-acetyl glycine were purchased from Fischer Scientific (Fair Lawn, NJ).
Preparation of MDI-Protein adducts
MDI-protein adducts were prepared as previously described (25). Briefly, protein solutions were prepared in 0.1M phosphate buffered saline (PBS, pH 7.4) at 0.5 mg/mL. MDI was dissolved in dry acetone at 1.8, 9, 18, and 72 µg/mL for HSA conjugations and 1.84, 9.2, 18.4, 73.6 μg/mL for Hb conjugation, immediately before use. Each MDI solution was added at 34.5 μL to 5 mL of 0.5 mg/mL protein with mixing, resulting in MDI: protein molar ratios of 1:1, 5:1, 10:1 and 40:1. Samples were then incubated at room temperature for 1 hour. Following incubation, the samples were dialyzed for 18 hours against 4 L of distilled deionized water using 12,000–14,000 MWCO dialysis tubing (Sigma Aldrich). The samples were stored at −20°C until analysis.
Analysis of number of moles of MDI bound per mole protein
MDI conjugated proteins (2 mL of 0.5 mg/mL of HSA-MDI or Hb-MDI) were hydrolyzed by incubating with 1 ml of 3 M H2SO4 at 100°C for 16 h. MDA (1–16000 ng/mL) spiked protein standards were run in parallel. Following hydrolysis, samples and standards were cooled to room temperature. Five mL of saturated sodium hydroxide was added, vortexed, and put in an ice bath to cool for 10 min. The resulting MDA from samples and standards was extracted into 6 mL ethyl acetate and subsequently evaporated at 40°C under N2 to 1 mL. The ethyl acetate extracts were then back extracted into 500 μL of 0.5% H2SO4. Two hundred fifty μL saturated borate buffer (pH 8.5) and 450 μL acetonitrile were added to 250 μL of each H2SO4 extract, vortexed for 1 minute, and then 50 μL of 14.4 mg/mL fluorescamine in acetonitrile was added. This was vortexed for 1 minute and 100 μL injected onto a Supelco (Bellefonte, PA) LC-SI C18 column (25 cm × 4.6 mm, 5 μm). Samples and standards were analyzed on a Shimadzu (Columbia, MD) Prominence HPLC system consisting of an online vacuum degasser (model DGU-20A5), a quaternary pump (model LC-20AT), an auto sampler (model SIL-10AD-VP) and a fluorescence detector (model RF-10AXL). The HPLC system was controlled by EZStart software version 7.3. Samples and standards were eluted from the column at 1 mL/min over 20 minutes using a linear gradient of 10% to 50% acetonitrile/water over 13 min, and held at 50% for 5 min. The resulting MDA-fluorescamine complex was excited at 410 nm and emission was measured at 510 nm.
Assessment of cross-linking (loss of primary amines)
The 2, 4, 6-trinitrobenzenesulfonic acid (TNBS) assay was performed on HSA and MDI-HSA conjugates(29). HSA (600, 500, 400, 200, 100, 50 μg/mL) were prepared in 0.1M sodium tetra borate (pH 9.3). TNBS (5% w/v) was diluted 1: 5.48 with 0.1M borate buffer. To 500 μL of samples, 12.5 μL of TNBS was added, mixed well, and left to react for 30 min. Absorbance at 420 nm was measured on a Beckman Coulter spectrophotometer Model DU 800 (Somerset, NJ). Hb has a strong absorbance at 420 nm that prevented measurement of primary amine content by TNBS. Semi-quantitative evaluation of intra- and inter-molecular cross linking in MDI -Hb and MDI -HSA was also performed using gel electrophoresis.
Assessment of cross-linking (gel electrophoresis)
For denaturing gels, HSA, Hb, and MDI-protein conjugates were mixed with 950μl of Laemmli sample buffer and 50 μl of 2-mercaptoethanol. Sodium dodecyl sulfate (SDS) acrylamide precast gels (4–20%) gradient gels were obtained from Bio-Rad (Hercules, CA). Samples were run on 8% (MDI-HSA) and 4–20% gradient (MDI-Hb) polyacrylamide gels. Following electrophoretic separation of proteins, the gels were stained with imperial protein stain (Pierce, Rockford, IL) and de-stained with water. Unmodified HSA and Bio-Rad pre-stained molecular weight markers were used for relative molecular weight determination. Intermolecular cross-linked proteins will migrate at a slower rate than the native protein, while extensive intra-molecular cross-linking prevents complete protein denaturation causing an apparent migration of a molecule smaller that the native protein.
For native gels, HSA and MDI-HSA samples were mixed with native sample buffer from BIO-RAD and run on an 8% native gel in parallel with unstained protein markers from (Life Technologies, Carlsbad, CA). Gels were stained and de-stained as described previously.
Trypsin digestion of albumin samples
In preparation for MS/MS analysis of MDI conjugation sites of Hb by ultra-performance liquid chromatography quadrupole time of flight mass spectrometer (UPLC-qTOF MS), 200 μL aliquots of MDI-Hb samples were treated with tributylphosphine for 30 min at room temperature to reduce the disulfide bonds, followed by alkylation with iodoacetamide for 1 h at room temperature. Alkylation was quenched by further addition of tributylphosphine for 15 min at room temperature. Porcine trypsin in 25 mM NH4HCO3 was then added at a 40:1 (protein: trypsin) ratio. Samples were incubated overnight at 37°C.
Ultra-performance liquid chromatography
Tryptic peptides of Hb and MDI-Hb were separated on a Waters (Milford, MA) nanoACQUITY ultra-performance liquid chromatography system. Aliquots (1 μL) of the digest mixture were injected and trapped/desalted on a 5 μm SymmetryC18 (180 μm × 20 mm) trapping column with 99.5/0.5 A/B (A: 0.1% formic acid; B: 0.1% formic acid in acetonitrile) at a flow rate of 15 μL/min for 1 minute. Separation was performed on a 1.7 μm BEH 130 C18 (100 μm × 100 mm) analytical column utilizing gradient elution at a flow rate of 400 nL/min and a gradient of 99/1 to 60/40 A/B over 90 min.
Tandem mass spectrometry of Hb peptides
The eluent from the ultra-performance liquid chromatography system was directed to the nanoelectrospray source of a Waters SYNAPT MS quadrupole time-of-flight (qTOF) mass spectrometer. Positive ion nano-electrospray was performed utilizing 10 μm Pico-Tip (Waters) emitters held at a potential of +3.5 kV. The cone voltage was held constant at +40 V for all experiments. Dry nitrogen desolvation gas was supplied to the instrument via a nitrogen generator (NitroFlowLab, Parker Hannifin Corp., Haverhill, MA). [Glu]1-Fibrinopeptide B (100 fmol/μL in 75/25 A/B) was supplied to an orthogonal reference probe and the [M+2H]2+ ion (m/z = 785.84265u) measured as an external calibrant at 30 sec intervals. Ultra-high purity (UHP) argon was used as collision gas. Spectra were acquired in an “MSe” fashion [34[. Alternating one-second mass spectra were acquired. The collision energy was set to 6 eV (1 sec low energy scan) and a 15–30 eV ramp (1 sec high energy scan).
Immunoassay for percent of number of HSA conjugated
Binding of a murine IgM 15D4 monoclonal antibody (mAB) to 4,4′-MDI conjugated HSA was analyzed using a sandwich enzyme-linked immuno-sorbent assay (ELISA). Ninety six well plates (Corning, Corning, NY) were coated with 4 μg/mL Affini-Pure goat anti-mouse IgM, μ chain specific IgG (Jackson Immuno-Research Laboratories Inc., West Grove, PA) overnight at 4°C. After washing three times with PBST, wells were incubated on a shaker for 1 hour with 2 μg/ml 15D4 mAb at room temperature (RT). The plates were then blocked with 3% skim milk/PBS-Tween 20 (SMPBST) for 1 hour at 37°C. Duplicate 4,4′-MDI-HSA conjugates were then added to the blocked plates at a concentration of 25 μg/mL and incubated for 1 hour at 37°C. Plates were then washed three times with PBST and incubated for 1 hour at 37°C with biotin-conjugated affinity purified rabbit anti HSA (Rockland, Gilbertsville, PA) diluted 1:5000 (v/v) in SMPBST. The plates were then washed three times with PBST and incubated for 1 hour at 37°C with alkaline-phosphatase-conjugated streptavidin (Jackson Immuno-Research Laboratories Inc, West Grove, PA) diluted 1:5000 (v/v) in SMPBST. Following incubation, the plates were washed with PBST and binding of the 15D4 mAb to the conjugates was visualized using 0.5 mg/ml p-nitrophenyl phosphate (Sigma Aldrich, St. Louis, MO) in alkaline phosphatase substrate. The optical density was determined at 405 nm after 30 minutes.
Data analysis for MDI binding sites on Hb
Data were analyzed with BioPharmaLynx v. 1.2 (Waters), a software program for analysis of peptide mass maps and identification of sites of modification on known protein sequences. Default peptide mass map analysis criteria of 30 ppm mass error in both low and high collision energy mode were specified. Trypsin was specified as the digestion enzyme and 2 missed cleavages were allowed. Identification of an isocyanate binding site proceeded via a rigorous procedure that involved the following steps: 1) observation of a potential peptide-dNCO conjugation product with less than 30 ppm m/Δm mass error in the analyte peptide mass map, 2) comparison of analyte and control peptide mass map from unmodified Hb shows that observed m/z and chromatographic retention time are unique to analyte, and 3) MS/MS data contains bn-and yn-type ions consistent with the assigned sequence and modifier.
Results
Mapping the binding sites of MDI on Hb
Hb was reacted in vitro (PBS, pH7.4) to 4, 4-MDI at MDI: Hb ratios of 1:1 to 40:1. The conjugates produced were digested with trypsin and resultant peptides analyzed by UPLC-MS/MS to determine MDI binding sites. Examination of the tandem mass spectra of the tryptic peptides allowed assignment of conjugation sites on Hb (30). Figure 1 is a representative tandem mass spectrum of the β subunit of Hb tryptic fragment 1–17 conjugated to MDI on the N-terminal valine. The conjugated peptide has a mass of 2098.07 which is in agreement with the theoretical mass of the fragment. The b2* and b7* ions were conjugated to MDI, appearing 250.07 u higher in mass than the theoretical b2 and b7 ions, indicating that MDI is covalently bound to the N-terminal valine at position one. On the other hand, yn-type ions, y2–y14, all appear at the correct theoretical masses; again further confirming MDI is bound to the N-terminal valine. In addition, when MDI reacts with proteins in aqueous solution, a variety of products are formed as previously described. Polymerization was observed on lysine 66 at 10:1 MDI: Hb molar conjugation ratio and above.
Figure 1.
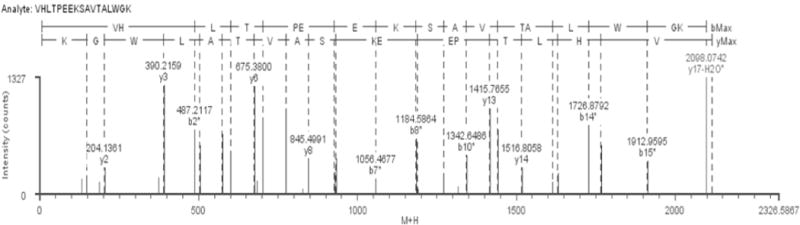
Determination of MDI binding sites on hemoglobin. Tandem mass spectrum of MDI conjugated hemoglobin β-fragment 1–17 conjugated on the N-terminal valine residue.
Table 1 shows the concentration-dependent specific binding sites identified for MDI on Hb when MDI was added at molar ratios from 1:1 to 40:1 MDI: Hb. A total of 8 binding sites were identified at the highest concentration of MDI used, including the N-terminal amine of valine on both the alpha and beta chains. MDI bound to two lysines on the alpha chain and four additional lysines on the beta chain. The two N-terminal valines of the alpha and beta chains as well as lysine 66 on the beta chain were observed to bind at the lowest conjugation ratio of 1:1 MDI: Hb. Increasing MDI concentration increased the number of sites bound to a maximum of eight. All eight binding sites were conjugated at 10:1 mole ratio. Increasing the concentration to 40:1 did not increase the number of different amino acids bound.
Table 1.
MDI Hemoglobin conjugation sites
| MDI molar Conjugation Ratio | ||||
|---|---|---|---|---|
| → | 1:1 | 5:1 | 10:1 | 40:1 |
| Alpha subunit | ||||
| Val 1 | x | X | x | x |
| Lys 7 | X | x | x | |
| Lys 40 | X | x | x | |
| Beta subunit | ||||
| Val 1 | x | X | x | x |
| Lys 8 | x | x | ||
| Lys 61 | x | x | ||
| Lys 65 | x | x | ||
| Lys 66 | x | X | x | x |
In contrast, we have previously reported that MDI conjugates 5 different HSA lysines at 1:1 MDI: HSA. Conjugation to the N-terminal Asp in HSA was not observed until the MDI concentration was raised to 5:1 MDI: HSA and 2 glutamine sites were bound at the higher conjugation ratios(25). In addition, the number of different amino acid binding sites increased through the entire MDI concentration range with 20 different binding sites observed at 40:1 MDI: HSA. Table 2 compares the number of observed binding sites for Hb and HSA. HSA has more lysine binding sites accessible for MDI binding compared to Hb through the entire MDI concentration range. However, relative to the number of potential lysine binding sites in the two proteins, both proteins showed relatively equivalent percentage binding, where the percentage binding was calculated as the number of observed binding sites divided by the total number of potential binding sites (lysines + N-terminal amines), expressed as a percentage.
Table 2.
Comparison of MDI binding sites in Hb and HSA
| Molar Conjugation Ratio | Binding sites on Hb | Binding sites on HSA | % Binding Hb | % Binding HSA |
|---|---|---|---|---|
| 1:1 MDI: Protein | 3 | 5 | 13 | 9 |
| 5:1 MDI: Protein | 5 | 11 | 21 | 19 |
| 10:1 MDI: Protein | 8 | 13 | 33 | 22 |
| 40:1 MDI: Protein | 8 | 20 | 33 | 34 |
| Total number of potential binding sites | 24 | 59 |
Quantification of MDI binding in Hb and HSA
MDI conjugated HSA and Hb were hydrolyzed under strong acid conditions to obtain free methylene dianiline (MDA) and derivatized with fluorescamine as described above. The MDA-fluorescamine complex was then quantified using HPLC with fluorescence detection. Quantification of the number of moles of MDI bound to Hb and HSA measured following acid hydrolysis are reported in Table 3. On a per mole basis, HSA bound a greater amount of MDI than Hb. In addition, while the number of different amino acid sites did not increase between 10:1 and 40:1 MDI:Hb, the absolute amount of MDI bound increased 3-fold. This may be indicative of increasing number of specific lysines bound as well as increased MDI polymerization onto a single protein site.
Table 3.
Moles of MDI bound to Hb and HSA
| MDI:protein Molar Conjugation Ratio | * Moles MDI per mole Hb | Number of binding sites on Hb | * Moles MDI per mole HSA | Moles MDA(HSA)/Moles MDA(Hb) |
|---|---|---|---|---|
| 1:1 | 0.03 ± 0.01 | 3 | 0.59 ± 0.11 | 17.96 |
| 5:1 | 0.85 ± 0.04 | 5 | 2.73 ± 0.29 | 3.20 |
| 10:1 | 1.24 ± 0.03 | 8 | 3.93 ± 0.33 | 3.17 |
| 40:1 | 3.87 ± 0.12 | 8 | 8.15 ± 0.95 | 2.10 |
Average±RSD
Cross linking in MDI-HSA
Table 4 shows a concentration-dependent loss of available primary amines with increasing MDI concentration, and thus an increase in the amount of dNCO cross-linking of protein residues. Approximately 60% of primary amines in HSA are cross-linked at the highest MDI concentration. MDI-Hb could not be assessed by TNBS due to spectral interference. Preliminary results using fluorescamine to assess free amines on Hb could not be used to assess loss of primary amines in Hb-MDI conjugates due to fluorescent quenching by Hb at 510 nm (data not shown).
Table 4.
Evaluation of cross linking in HSA-MDI by loss of amine reactivity with TNBS
| Molar Conjugation Ratio | TNBS Abs(as a % of HSA control) | *% cross linking in MDI: HSA |
|---|---|---|
| No MDI | 100.00 | 0.00 ± 0.00 |
| 1:1 MDI: Protein | 83.42 | 16.58 ± 5.21 |
| 5:1 MDI: Protein | 75.04 | 24.96 ± 2.14 |
| 10:1 MDI: Protein | 60.29 | 39.71± 4.51 |
| 40:1 MDI: Protein | 42.12 | 57.88 ± 7.07 |
Average±RSD
Gel Electrophoresis: Qualitative assessment of Conjugation and Cross linking in MDI-HSA and MDI-Hb
SDS-PAGE was employed to qualitatively examine the extent of binding and cross-linking. Under denaturing conditions, inter-molecular cross-linked proteins migrated at a slower rate compared to native protein, and additionally prevented complete protein denaturation causing an apparent migration like that of a smaller protein. Figure 2 shows an 8% SDS-PAGE gel of 0.5 mg/mL HSA reacted to MDI at different molar ratios MDI: HSA. dNCO conjugation of HSA at a 1:1 MDI: HSA conjugation ratio (Figure 2, lane 3) resulted in 68 kD and 71 kD equivalent bands. At 5:1 MDI: HSA (Figure 2, lane 4), the 68 kD HSA band and the 71kD band are still observable with reduced resolution At the higher 10:1 and 40:1 MDI:HSA conjugation ratios (lanes 5 and 6), broad bands that migrated like smaller molecules with leading edges of the bands at apparent masses of 63 and 53 kD were observed. In addition, a faint band between 100 and 150 kD was also observed in lane 6, presumably due to intermolecular MDI-HSA cross-linking. Native gel electrophoresis of MDI-HSA conjugates was also assessed (Figure 3); however, higher molecular weight bands 2- to 3-fold the mass of HSA were observed in control samples (Lane 2) and identified by digestion and UPLC-MS-MS to be HSA. These results demonstrated the aggregation of HSA under the assay conditions, and prevented the observation of potential intra-molecular cross-linking. An MDI concentration-dependent increase in migration of the conjugate band was also observed in the MDI-HSA conjugates similar to that observed under denaturing conditions suggestive of intramolecular cross-linking. The faster migration (apparent smaller size) may be possible attributable to exclusion of intramolecular water(s) effectively decreasing the size.
Figure 2.
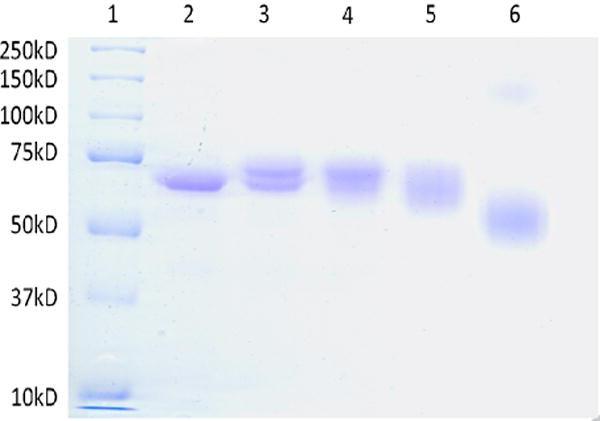
Determination of cross linking in MDI-HSA by gel electrophoresis. An 8% denaturing gel of MDI: HSA. Lane 1 is the molecular weight marker, lane 2 is HSA, lanes 3, 4, 5 and 6 are 1:1, 5:1, 10:1 and 40:1 MDI:HSA conjugates respectively.
Figure 3.
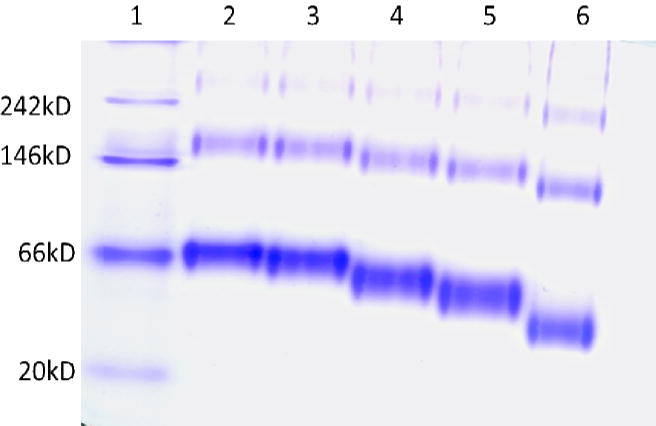
Determination of cross linking in MDI-HSA by gel electrophoresis. An 8% native gel of MDI: HSA. Lane 1 is the molecular weight marker, lane 2 is HSA and lanes 3, 4, 5 and 6 are 1:1, 5:1, 10:1 and 40:1 MDI:HSA respectively.
Figure 4 demonstrates a 4–20 % gradient gel of 0.5 mg/mL Hb reacted to MDI at 4 different mole ratios of MDI:Hb. Denaturation of Hb resulted in the dissociation of the alpha and beta subunits that migrated at 15 kD and 30 kD, respectively. A dark unresolved smear of high molecular weight compounds above 45 kD suggests extensive inter-subunit cross linking and the intensity of this band increased with increasing MDI concentration. A downward shift of the 15 kD and 35 kD subunits with increased MDI concentration was also observed, similar to that found with the MDI-HSA conjugates.
Figure 4.
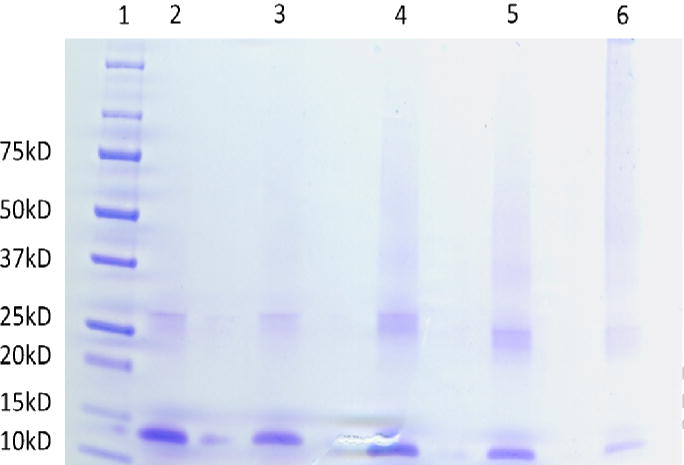
Determination of cross linking in MDI-Hb by gel electrophoresis. A denaturing gradient gel of MDI-Hb. Lane 1 is the molecular weight marker, lane 2 is Hb, and lanes 3, 4, 5 and 6 are 1:1, 5:1, 10:1 and 40:1 MDI:Hb respectively.
ELISA assessment of percent of albumin molecules conjugated
An immunoassay was developed using a mAb IgM developed in our laboratory that detects MDI-HSA. The MDI mAb was used as the capture antibody in solid phase and MDI-HSA conjugates were detected using an alkaline phosphatase conjugated anti-human albumin antibody. In this assay, the amount of HSA with at least one nucleophilic site conjugated by MDI (vs. number of MDI bound per albumin molecule) was quantified. As shown in Fig 5, the ELISA absorbance becomes saturated at a binding ratio of 20:1 with no further increase at 40:1. These results demonstrate that all HSA molecules are conjugated to at least 1 MDI at the 20:1 MDI:HSA conjugation ratio.
Figure 5.
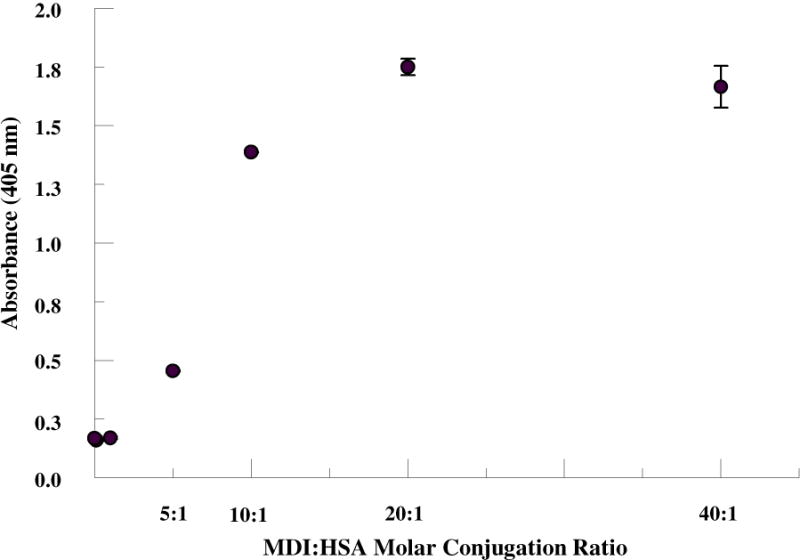
Immunoassay of MDI haptenated HSA. MDI was reacted to HSA at 1:1 up to 40:1 MDI:HSA molar ratios. Resultant haptenated protein was captured using an anti-MDI-protein IgM and detected using an alkaline phosphatase anti-HSA IgG. At ≥ 20:1 MDI:HSA all HSA have at least 1 MDI bound.
Discussion
dNCO specific antibodies (IgG and IgE) are not consistently observed in workers with dNCO asthma and this is thought to be, in part, due to limitations associated with antibody-based immunoassays. Need for standardization of immunoassays for the detection of specific dNCO antibody in workers has been noted in several publications (14;31). Wisnewski (13) has reported limitations associated with immunoassay standardization that have prevented researchers in the field to compare results between occupational cohorts. The development of monoclonal antibodies (mAb) to detect particular dNCO-protein conjugates has been previously reported (32;33) and such tools may be used along with the proteomic methods noted above to provide dNCO haptenated albumin with both chemical and immune reactivity consistency.
There are several methods that have been used to characterize dNCO-protein conjugates (19;34) in association with dNCO-specific antibody detection immunoassays and also for bio-monitoring assays of dNCO exposure. Measurement of total conjugated and free dNCO hydrolysis products from urine and plasma, such as MDA analyzed following derivatization, by GC-MS has been extensively employed as a biomonitoring tool (35;36). These methods are based on determination of MDA (a hydrolysis product of MDI) after strong acid or base hydrolysis of MDI-protein adducts similar to that employed in the present study. These methods are quantitative, but do not provide qualitative information about the nature of the conjugates. While measurement of the diamine based hydrolysis product has been utilized for bio-monitoring, only one study has reportedly applied this approach to characterize in vitro prepared HSA conjugates (37). Several solvents are commercially available that have been used for the extraction of diamines derived from dNCO hydrolysis; these include toluene, dichloromethane, and ethyl acetate (38;39). Toluene is the most common extraction solvent reported in the literature; however, MDA has reduced solubility in toluene. The extraction efficiencies of the dichloromethane(DCM) and ethyl acetate(EA) were tested and determined to be 36.2% ± 17.0 (s.d.) and 96.2% ± 4.3 (s.d.), respectively. EA was chosen as the extraction solvent; although loss of MDA was noted upon complete solvent evaporation under nitrogen. Partial evaporation of EA with subsequent back extraction into 0.5% H2SO4 provided higher recoveries and was used to transfer the MDA into an aqueous solvent for subsequent derivatization.
Several approaches were evaluated for the characterization of MDI-HSA and MDI-Hb conjugates. The objective of evaluating MDI-Hb in comparison to MDI-HSA was to evaluate if our approach to dNCO-albumin characterization can be applied to non-albumin protein carriers and to evaluate differences in reactivity to MDI that may have potential utility in understanding disease mechanisms. An HPLC method based on fluorescence detection was developed for absolute quantification of MDA following conjugate hydrolysis. This method is inexpensive, simple and sensitive (LOD = 1ng/ml). The derivatization employed here is a modification of the one used by S.E Toker et al (40). Gas or liquid-chromatographic-mass spectral (MS) methods for MDA measurement are an alternative to the HPLC-fluorescence method developed for antigen characterization. The MS methods incorporate an isotopic internal standard which may increase accuracy especially where matrix effects may alter extraction efficiency. However, we believe that the external standard methodology used for quantification of laboratory haptenated proteins where there is a uniform matrix provides sufficient accuracy for quantification of mass of MDI/protein conjugated. The method used in the present study is reproducible for both Hb and HSA. Using this method, HSA was 2-fold more reactive to MDI than Hb at 40:1 MDI: protein. This could be because Hb has 4 subunits, 2 alpha and 2 beta. This arrangement may mask potential binding sites from conjugating to MDI. Moreover, Hb contains the porphyrin ring that also contains iron, potentially affecting its reactivity with MDI. This contrasts sharply with HSA, a single polypeptide with 585 residues containing 17 pairs of disulphide bridges and one free cysteine.
Previous studies in our lab have delineated the specific dNCO binding sites on HSA using UPLC-qTOF MS/MS on tryptic dNCO-HSA digests. These studies have provided further insight into dNCO polymerization and cross linking of HSA under varying conjugation conditions (25). In the present study, tandem protein mass spectrometry was used to delineate specific MDI binding sites on Hb. An MDI concentration dependent increase in the number of MDI binding sites was observed. Except for the N-terminal valines on both the alpha and beta subunits, lysine 66 was the only non-terminal binding site that was observed at the lowest MDI concentration studied (1:1 MDI:Hb). All 8 observed binding sites on Hb were bound at 10:1 MDI:Hb and no new sites were identified by increasing the MDI:Hb reaction ratio of 40:1. Three of the 8 binding sites identified were on the alpha subunit and 5 were on the beta subunit. Although additional MDI binding sites were not observed, the number of moles of MDI bound per mole Hb increased 3-fold when the binding ratio was increased from 10:1 to 40:1.
Trinitrobenzene sulfonic acid (TNBS), a primary amine specific spectrophotometric probe, has also been used for the characterization of dNCO-protein conjugates. However, studies have often misinterpreted experimental results and interpreted the absolute loss of amine reactivity to TNBS as a quantitative marker of dNCO binding (34). Although this may be an approximate measure of mono-isocyanates conjugation, with dNCO there is a high likelihood of conjugation of one isocyanate moiety with subsequent hydrolysis of the other isocyanate to regenerate a primary amine. In this case, there would be no net loss of amine causing an underestimation of moles dNCO bound to protein by the TNBS assay. Loss of TNBS reactivity in dNCO conjugated proteins only occurs when the dNCO cross-links two amine sites. Based on these findings, we propose that the TNBS assay should be used in conjunction with other analytical methods to better indicate cross-linking (intra- and inter-molecular) of total dNCO. There is a concentration-dependent loss of available primary amines with increasing MDI:HSA molar reaction ratios, and thus an increase in the amount of diisocyanate cross-linking of protein residues with loss of 58% of primary amine reactivity at 40:1 MDI:HSA. The estimate of the number of MDI involved in cross-linking assumes an insignificant number of protein residues are cross-liked by MDI polymer. Due to spectral interference from Hb, cross linking in MDI-Hb conjugates could not be assessed quantitatively.
Qualitative assessment by polyacrylamide gel electrophoresis is a commonly employed useful method for assessing dNCO haptenated proteins; however, resultant migration patterns are complex and subject to interpretation. Inter-molecular cross-linking was observed in both MDI haptenated HSA and Hb at the higher conjugation ratios by appearance of bands approximately 2-fold the monomeric protein masses progressing with increased conjugation ratios to unresolved staining suggesting further polymerization. The lack of higher molecular size band resolution is most likely also due to the great diversity of species with intra- and intermolecular crosslinking and MDI self-polymerization occurring on the same protein molecules. Intra-molecular cross-linking was additionally indicated by the MDI concentration dependent increase in conjugate mobility presumably due to inhibition of complete denaturation under denaturing conditions and exclusion of intra-molecular water under native conditions.
Immunoassays using a recently developed mAb specific for dNCO conjugated protein can be formatted in a variety of configurations to provide further information to develop a standardized antigen for diisocyanate-specific antibody screening. The ELISA format employed in the present study uses an anti-MDI-protein IgM as the capture antibody and a labeled anti-HSA as the detection antibody and thus measures the number of albumin with at least one MDI molecule covalently bound. No increase in immuno-reactivity was observed at reaction ratios ≥ 20:1 MDI:HSA, suggesting that any increase in bound MDI was due to polymerization and reaction to additional HSA nucleophilic sites vs. reaction to unbound HSA. Direct binding of the haptenated HSA to an ELISA plate with measurement of binding by a mAb (data not shown) is also an approach that can be used to standardize the antigenicity of the haptenated protein. Wisnewski et al. (41) reported maximal MDI-specific IgG reactivity of exposed worker’s sera against MDI-HSA in their immunoassay was observed at a conjugation ratio of 200 μg MDI/mg HSA (approx. 50:1). It must be noted that the HSA concentration reacted to MDI was 10X greater than that used in the present study which very possibly altered the final product as we would expect greater intermolecular cross-linking and polymerization associated with these higher protein and MDI concentrations.
In conclusion, we suggest that multiple methods be utilized to characterize dNCO-HSA conjugates for use in standardized immunoassays for screening dNCO specific IgE and IgG from workers’ sera. These analyses should include (1) measurement of amount of diamine/mole HSA following acid hydrolysis, (2) TNBS measurement of cross-linking, (3) denature gel electrophoresis for indication of intermolecular and intra-molecular cross-linking, and (4) use of mAb for antigenicity standardization. Both qualitative and quantitative differences in MDI binding were observed between Hb and HSA conjugates suggesting normalization to conjugation ratio is not sufficient to compare antigenicity of different diisocyante haptenated proteins. Comparative studies of antigen preparations normalized to each of these parameters are needed additionally to provide information as to which of the proposed above measure(s) would be the best to standardize the dNCO haptenated antigens for specific antibody and possibly cell stimulation based diagnostic assays.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institute for Occupational Safety and Health. This work was supported by an Interagency Agreement with the NIEHS (IAG#Y1-ES-0001-12) and Grant Number CHE 1056311 from the National Science Foundation. We also acknowledge Dr. Ajay Nayak for his guidance in optimization of SDS-PAGE assessment of the MDI-proteins.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hettick JM, Siegel PD, Green BJ, Liu J, Wisnewski AV. Vapor conjugation of toluene diisocyanate to specific lysines of human albumin. Anal Biochem. 2012;421(2):706–711. doi: 10.1016/j.ab.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munn SJ, Hansen BG. EU risk assessment: science and policy. Toxicology. 2002;181–182:281–285. doi: 10.1016/s0300-483x(02)00456-0. [DOI] [PubMed] [Google Scholar]
- 3.Thomasen JM, Fent KW, Nylander-French LA. Development of a sampling patch to measure dermal exposures to monomeric and polymeric 1,6-hexamethylene diisocyanate: a pilot study. J Occup Environ Hyg. 2011;8(12):709–717. doi: 10.1080/15459624.2011.626744. [DOI] [PubMed] [Google Scholar]
- 4.Jarand CW, Akapo SO, Swenson LJ, Kelman BJ. Diisocyanate emission from a paint product: a preliminary analysis. Appl Occup Environ Hyg. 2002;17(7):491–494. doi: 10.1080/10473220290035705. [DOI] [PubMed] [Google Scholar]
- 5.Krone CA. Diisocyanates and nonoccupational disease: a review. Arch Environ Health. 2004;59(6):306–316. [PubMed] [Google Scholar]
- 6.Baur X, Czuppon A. Diagnostic validation of specific IgE antibody concentrations, skin prick testing, and challenge tests in chemical workers with symptoms of sensitivity to different anhydrides. J Allergy Clin Immunol. 1995;96(4):489–494. doi: 10.1016/s0091-6749(95)70292-x. [DOI] [PubMed] [Google Scholar]
- 7.Biagini RE, Bernstein IL, Gallagher JS, Moorman WJ, Brooks S, Gann PH. The diversity of reaginic immune responses to platinum and palladium metallic salts. J Allergy Clin Immunol. 1985;76(6):794–802. doi: 10.1016/0091-6749(85)90750-x. [DOI] [PubMed] [Google Scholar]
- 8.Sabbioni G, Dongari N, Schneider S, Kumar A. Synthetic approaches to obtain amino acid adducts of 4,4′-methylenediphenyl diisocyanate. Chem Res Toxicol. 2012;25(12):2704–2714. doi: 10.1021/tx300347e. [DOI] [PubMed] [Google Scholar]
- 9.Mapp CE. Agents, old and new, causing occupational asthma. Occup Environ Med. 2001;58(5):354–60. 290. doi: 10.1136/oem.58.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diller WF. Frequency and trends of occupational asthma due to toluene diisocyanate: a critical review. Appl Occup Environ Hyg. 2002;17(12):872–877. doi: 10.1080/10473220290107075. [DOI] [PubMed] [Google Scholar]
- 11.Tarlo SM, Liss GM. Diisocyanate-induced asthma: diagnosis, prognosis, and effects of medical surveillance measures. Appl Occup Environ Hyg. 2002;17(12):902–908. doi: 10.1080/10473220290107101. [DOI] [PubMed] [Google Scholar]
- 12.Buyantseva LV, Liss GM, Ribeiro M, Manno M, Luce CE, Tarlo SM. Reduction in diisocyanate and non-diisocyanate sensitizer-induced occupational asthma in Ontario. J Occup Environ Med. 2011;53(4):420–426. doi: 10.1097/JOM.0b013e3182122376. [DOI] [PubMed] [Google Scholar]
- 13.Wisnewski AV. Developments in laboratory diagnostics for isocyanate asthma. Curr Opin Allergy Clin Immunol. 2007;7(2):138–145. doi: 10.1097/ACI.0b013e3280895d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ott MG, Jolly AT, Burkert AL, Brown WE. Issues in diisocyanate antibody testing. Critical Reviews in Toxicology. 2007;37(7):567–585. doi: 10.1080/10408440701419553. [DOI] [PubMed] [Google Scholar]
- 15.Budnik LT, Preisser AM, Permentier H, Baur X. Is specific IgE antibody analysis feasible for the diagnosis of methylenediphenyl diisocyanate-induced occupational asthma? Int Arch Occup Environ Health. 2012 doi: 10.1007/s00420-012-0772-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wisnewski AV, Stowe MH, Cartier A, Liu Q, Liu J, Chen L, Redlich CA. Isocyanate vapor-induced antigenicity of human albumin. J Allergy Clin Immunol. 2004;113(6):1178–1184. doi: 10.1016/j.jaci.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Chipinda I, Zhang XD, Simoyi RH, Siegel PD. Mercaptobenzothiazole allergenicity-role of the thiol group. Cutan Ocul Toxicol. 2008;27(2):103–116. doi: 10.1080/15569520701713008. [DOI] [PubMed] [Google Scholar]
- 18.Hettick JM, Siegel PD. Determination of the toluene diisocyanate binding sites on human serum albumin by tandem mass spectrometry. Anal Biochem. 2011;414(2):232–238. doi: 10.1016/j.ab.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 19.Budnik LT, Preisser AM, Permentier H, Baur X. Is specific IgE antibody analysis feasible for the diagnosis of methylenediphenyl diisocyanate-induced occupational asthma? Int Arch Occup Environ Health. 2012 doi: 10.1007/s00420-012-0772-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wisnewski AV, Srivastava R, Herick C, Xu L, Lemus R, Cain H, Magoski NM, Karol MH, Bottomly K, Redlich CA. Identification of human lung and skin proteins conjugated with hexamethylene diisocyanate in vitro and in vivo. Am J Respir Crit Care Med. 2000;162(6):2330–2336. doi: 10.1164/ajrccm.162.6.2002086. [DOI] [PubMed] [Google Scholar]
- 21.Lange RW, Lantz RC, Stolz DB, Watkins SC, Sundareshan P, Lemus R, Karol MH. Toluene diisocyanate colocalizes with tubulin on cilia of differentiated human airway epithelial cells. Toxicol Sci. 1999;50(1):64–71. doi: 10.1093/toxsci/50.1.64. [DOI] [PubMed] [Google Scholar]
- 22.Lantz RC, Lemus R, Lange RW, Karol MH. Rapid reduction of intracellular glutathione in human bronchial epithelial cells exposed to occupational levels of toluene diisocyanate. Toxicol Sci. 2001;60(2):348–355. doi: 10.1093/toxsci/60.2.348. [DOI] [PubMed] [Google Scholar]
- 23.Sabbioni G, Hartley R, Henschler D, Hollrigl-Rosta A, Koeber R, Schneider S. Isocyanate-specific hemoglobin adduct in rats exposed to 4, 4′-methylenediphenyl diisocyanate. Chem Res Toxicol. 2000;13(2):82–89. doi: 10.1021/tx990096e. [DOI] [PubMed] [Google Scholar]
- 24.Sabbioni G, Hartley R, Schneider S. Synthesis of adducts with amino acids as potential dosimeters for the biomonitoring of humans exposed to toluenediisocyanate. Chem Res Toxicol. 2001;14(12):1573–1583. doi: 10.1021/tx010053+. [DOI] [PubMed] [Google Scholar]
- 25.Hettick JM, Siegel PD. Comparative analysis of aromatic diisocyanate conjugation to human albumin utilizing multiplexed tandem mass spectrometry. International Journal of Mass Spectrometry. 2012;309:168–175. [Google Scholar]
- 26.Sabbioni G, Wesp H, Lewalter J, Rumler R. Determination of isocyanate biomarkers in construction site workers. Biomarkers. 2007;12(5):468–483. doi: 10.1080/13547500701395636. [DOI] [PubMed] [Google Scholar]
- 27.Wong JL, Liu DZ, Zheng YT. Lysine conjugate of acrylonitrile as antigenic sites in hemoglobin adducts. 2004;63(2):171–174. doi: 10.1111/j.1399-3011.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 28.Pien LC, Zeiss CR, Leach CL, Hatoum NS, Levitz D, Garvin PJ, Patterson R. Antibody response to trimellityl hemoglobin in trimellitic anhydride-induced lung injury. Allergy Clin Immunol. 1988;82(6):1098–1103. doi: 10.1016/0091-6749(88)90149-2. [DOI] [PubMed] [Google Scholar]
- 29.Snyder SL, Sobocinski PZ. An improved 2,4,6-trinitrobenzenesulfonic acid method for the determination of amines. Anal Biochem. 1975;64(1):284–288. doi: 10.1016/0003-2697(75)90431-5. [DOI] [PubMed] [Google Scholar]
- 30.Hettick JM, Ruwona TB, Siegel PD. Structural elucidation of isocyanate-peptide adducts using tandem mass spectrometry. J Am Soc Mass Spectrom. 2009;20(8):1567–1575. doi: 10.1016/j.jasms.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 31.Redlich CA, Karol MH. Diisocyanate asthma: clinical aspects and immunopathogenesis. Int Immunopharmacol. 2002;2(2–3):213–224. doi: 10.1016/s1567-5769(01)00174-6. [DOI] [PubMed] [Google Scholar]
- 32.Ruwona TB, Johnson VJ, Hettick JM, Schmechel D, Beezhold D, Wang W, Simoyi RH, Siegel PD. Production, characterization and utility of a panel of monoclonal antibodies for the detection of toluene diisocyanate haptenated proteins. J Immunol Methods. 2011;373(1–2):127–135. doi: 10.1016/j.jim.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Ruwona TB, Johnson VJ, Schmechel D, Simoyi RH, Beezhold D, Siegel PD. Monoclonal antibodies against toluene diisocyanate haptenated proteins from vapor-exposed mice. Hybridoma (Larchmt) 2010;29(3):221–229. doi: 10.1089/hyb.2009.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemus R, Lukinskeine L, Bier ME, Wisnewski AV, Redlich CA, Karol MH. Development of immunoassays for biomonitoring of hexamethylene diisocyanate exposure. Environ Health Perspect. 2001;109(11):1103–1108. doi: 10.1289/ehp.011091103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skarping G, Dalene M, Littorin M. 4,4′-Methylenedianiline in hydrolysed serum and urine from a worker exposed to thermal degradation products of methylene diphenyl diisocyanate elastomers. Int Arch Occup Environ Health. 1995;67:73–7. doi: 10.1007/BF00572229. [DOI] [PubMed] [Google Scholar]
- 36.Skarping G, Dalene M, Littorin M. 4,4′-Methylenedianiline in hydrolysed serum and urine from a worker exposed to thermal degradation products of methylene diphenyl diisocyanate elastomers. Int Arch Occup Environ Health. 1995;67:73–7. doi: 10.1007/BF00572229. [DOI] [PubMed] [Google Scholar]
- 37.Tse CS, Pesce AJ. Chemical characterization of isocyanate-protein conjugates. Toxicol Appl Pharmacol. 1979;51(1):39–46. doi: 10.1016/0041-008x(79)90006-1. [DOI] [PubMed] [Google Scholar]
- 38.Sakai T, Morita Y, Kim Y, Tao YX. LC-MS determination of urinary toluenediamine in workers exposed to toluenediisocyanate. Toxicol Lett. 2002;134(1–3):259–264. doi: 10.1016/s0378-4274(02)00174-1. [DOI] [PubMed] [Google Scholar]
- 39.Flack SL, Fent KW, Gaines LG, Thomasen JM, Whittaker SG, Ball LM, Nylander-French LA. Hemoglobin adducts in workers exposed to 1,6-hexamethylene diisocyanate. Biomarkers. 2011;16(3):261–270. doi: 10.3109/1354750X.2010.549242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toker SE, Sagirli O, Cetin SM, Onal A. A new HPLC method with fluorescence detection for the determination of memantine in human plasma. J Sep Sci. 2011;34(19):2645–2649. doi: 10.1002/jssc.201100489. [DOI] [PubMed] [Google Scholar]
- 41.Wisnewski AV, Liu J, Redlich CA. Antigenic changes in human albumin caused by reactivity with the occupational allergen diphenylmethane diisocyanate. Anal Biochem. 2010;400(2):251–258. doi: 10.1016/j.ab.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]


