Abstract
Ceruloplasmin is a protective ferroxidase. Although some studies suggest that plasma ceruloplasmin levels are raised by exercise, the impact of exercise on brain ceruloplasmin is unknown. The present study examined whether striatal ceruloplasmin is raised with treadmill exercise and/or is correlated with spontaneous physical activity in rhesus monkeys. Parkinson’s disease is characterized by a loss in ceruloplasmin and, similarly, Parkinson’s models lead to a loss in antioxidant defenses. Exercise may protect against Parkinson’s disease and is known to prevent antioxidant loss in experimental models. We therefore examined whether treadmill exercise prevents ceruloplasmin loss in monkeys treated unilaterally with the dopaminergic neurotoxin MPTP. We found that exercise raised ceruloplasmin expression in the caudate and accumbens, but not the putamen of intact monkeys. However, putamen ceruloplasmin was correlated with spontaneous activity in a home pen. MPTP alone did not cause unilateral loss of ceruloplasmin but blocked the impact of exercise on ceruloplasmin. Similarly, the correlation between putamen ceruloplasmin and activity was also lost with MPTP. MPTP elicited loss of tyrosine hydroxylase in the treated hemisphere and the remaining tyrosine hydroxylase was correlated with overall daily activity (spontaneous activity plus that induced by the treadmill). These data reveal that treadmill activity can raise ceruloplasmin, but that this impact and the link with spontaneous activity are both diminished in parkinsonian primates. Furthermore, low overall physical activity predicts greater loss of dopaminergic phenotype in MPTP-treated primates. These data have implications for the maintenance of active lifestyles in both healthy and neurodegenerative conditions.
Keywords: Parkinson’s disease, neurodegeneration, monkey, antioxidant, exercise, MPTP
Introduction
Peripheral ceruloplasmin is a copper chaperone and ferroxidase and typically functions as an antioxidant. Recent studies also reveal a protective role for brain ceruloplasmin (Kaneko et al. 2008, Hineno et al. 2011, Texel et al. 2011). Humans lacking functional ceruloplasmin exhibit iron toxicity, basal ganglia neurodegeneration, and parkinsonism (Berg et al. 2001, Texel et al. 2008, Torsdottir et al. 2010). Conversely, Parkinson’s patients exhibit deficits in serum and cerebrospinal fluid ceruloplasmin and lower serum ceruloplasmin levels correlate with earlier disease onset (Bharucha et al. 2008, Boll et al. 2008, Torsdottir et al. 2010). Physical activity is thought to be protective against Parkinson’s motor deficits (Xu et al. 2010) and has been shown to stimulate antioxidant defenses as a compensatory adaptation to the mild stress of exercise (Radak et al. 2008). We previously observed that mild cellular stressors elicit compensatory adaptations against the 6-hydroxydopamine model of Parkinson’s disease (Leak et al. 2006, Leak et al. 2008) and raise ceruloplasmin mRNA in vitro (Leak, Garbett, and Mirnics, unpublished observations). Therefore, one might expect the mild stress of exercise to raise ceruloplasmin in the brain and/or protect against loss of ceruloplasmin in Parkinson’s models. For example, long-distance running raises serum ceruloplasmin in dogs and humans (Liesen et al. 1977, Kenyon et al. 2011). However, playing soccer results in a loss in serum ceruloplasmin (Resina et al. 1991), suggesting that the form of exercise also influences peripheral antioxidant status. In contrast, the effect of exercise on brain ceruloplasmin is unknown.
The basal ganglia play an indispensable role in movement. Removal of projections from the nigra into the dorsal striatum (caudate and putamen) leads to the motor deficits associated with Parkinsonism. The ventral striatum (accumbens) mediates aspects of motivation and is a critical interface between limbic and motor systems. The accumbens also modulates motor responses via projections to the substantia nigra (Groenewegen et al. 1996, Hauber 1998). The caudate, putamen, and accumbens are thus uniquely poised to impact and respond to physical activity. Therefore, we investigated the effect of treadmill exercise on ceruloplasmin in the caudate, putamen, and accumbens of healthy rhesus macaques (Macaca mulatta). Our regimen of 1 h/day, 5 days/week of treadmill running reflects guidelines by the American Heart Association and the College of Sports Medicine for middle-aged humans to improve cardiovascular health and regulate body weight (Haskell et al. 2007). Furthermore, treadmill running at 80% of maximal heart rate, as undertaken in the present study, increases vascular volume and improves cognitive function in primates (Rhyu et al. 2010).
The dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induces a well-accepted model of Parkinsonism in primates and mice. MPTP causes a loss in CuZn superoxide dismutase and Mn superoxide dismutase, whereas exercise can protect against these changes (Lau et al. 2011). In a second experiment, sedentary or exercising monkeys were therefore infused unilaterally with MPTP. If MPTP causes a similar loss in ceruloplasmin, one might expect exercise to also reverse this deficit. The impact of MPTP on ceruloplasmin has not been measured before in any species.
In addition to measurements of brain ceruloplasmin, daily mean physical activity levels were monitored with accelerometers to identify potential correlations between motor activity and ceruloplasmin. Spontaneous activity in a home pen was measured at baseline before the treadmill study was begun and overall activity was measured during the treadmill exercise study (spontaneous activity plus that induced by treadmill running). In order to confirm the effectiveness of MPTP, levels of tyrosine hydroxylase (TH), the rate-limiting enzyme for dopamine biosynthesis, were also measured. We tested four hypotheses: 1) treadmill exercise raises striatal ceruloplasmin, 2) physical activity levels correlate with ceruloplasmin, 3) MPTP leads to loss of ceruloplasmin and TH, and 4) exercise protects against MPTP-induced loss of ceruloplasmin and TH.
Methods
All efforts were made to minimize animal suffering. Experiments were approved by the University of Pittsburgh IACUC and carried out in strict accordance with the principles outlined in the NIH Guide for the Care and Use of Laboratory Animals. Female rhesus monkeys (15-20 yr, 5-8 kg) were housed in social living pens (4m × 4m × 4.7m) with perches and toys in a 12 h LD cycle at 24±2°C. All monkeys had access to primate biscuits, fresh fruits, vegetables, seeds, nuts, and water. Monkeys were outfitted with a collar housing an accelerometer (Respironics, Phoenix AZ), measuring minute-by-minute activity counts, as previously described (Sullivan and Cameron 2010). Healthy monkeys were matched for spontaneous activity levels and divided into sedentary controls and runners (n=6 each). Runners were placed on treadmills (model 910e, Precor Inc., Bothell WA) and first adapted to walking until they were running 1 h/day, 5 days/week, at a target speed determined from a maximal exercise test, as previously described (Sullivan and Cameron 2010). Target speed was then adjusted so that monkeys ran on the treadmill at 80% maximal heart rate. Sedentary animals sat on a silent treadmill for the same duration.
For the second set of monkeys, MPTP was administered after 12 weeks of running at 60% maximal heart rate (n=3), 80% maximal heart rate (n=3), or after remaining sedentary (n=3). At 80% maximal heart rate, the monkeys were jogging on the treadmill. At 60% maximal heart rate, monkeys were only walking briskly. We added the 60% maximal heart rate group to the MPTP study because jogging on the treadmill at 80% maximal heart rate may have been too stressful on parkinsonian animals. However, we subsequently found that these hemiparkinsonsian animals were able to jog for an hour without much difficulty and retained this group. A dose of 0.8 mg MPTP-HCl (0.2mg/ml, Sigma-Aldrich, St. Louis MO) was infused into the right carotid of all 9 monkeys, as previously described (Ding et al. 2008, Grondin et al. 2008). After one week in quarantine, MPTP monkeys ran for an additional six weeks before sacrifice.
Animals were anesthetized with sodium pentobarbital (30mg/kg, Abbott Laboratories, North Chicago IL) and exsanguinated after 12 weeks of running in the first experiment on intact monkeys or 7 weeks after MPTP in the second experiment on parkinsonian monkeys. Striatal tissue was sonicated in a Triton-based lysis buffer. Equal amounts of protein were separated by standard gel electrophoresis and infrared immunoblotting (Leak et al. 2010). Membranes were incubated in anti-ceruloplasmin (1:1000; BD Biosciences, San Diego CA) or anti-TH (1:20,000, Millipore, Billerica MA) in conjunction with anti-β-actin (1:20,000; Sigma-Aldrich). Blots were quantified on an Odyssey Imager (Li-COR Biosciences, Lincoln NE). The two-tailed t-test was used to analyze the intact runners versus sedentary controls (GraphPad Prism 5.0d, San Diego CA). For the MPTP monkeys, data were analyzed by two-way ANOVA followed by the Bonferroni post hoc test. Linear regression analyses were used to determine whether activity levels predicted ceruloplasmin or TH levels. Data were deemed significant at p ≤ 0.05.
Results
Impact of treadmill exercise and MPTP on striatal ceruloplasmin
Ceruloplasmin was significantly raised by treadmill exercise in the caudate (p=0.0231, t=2.896, df=7) and the accumbens (p=0.026, t=2.724, df=8) of intact monkeys (Fig.1a, b). There was no effect of exercise on putamen ceruloplasmin in these animals (data not shown; p=0.9961, t=0.005063, df=10). We did not end up with an of 6 each group for the caudate assay because β-actin bands were smeared along the entire upper half of the blot for 2 animals and extremely high fluorescence on the ceruloplasmin band from one animal made it a significant outlier by the Grubb’s test. Furthermore, the accumbens was not harvested from the first pair of exercising and sedentary monkeys (n=5/group).
Figure 1.
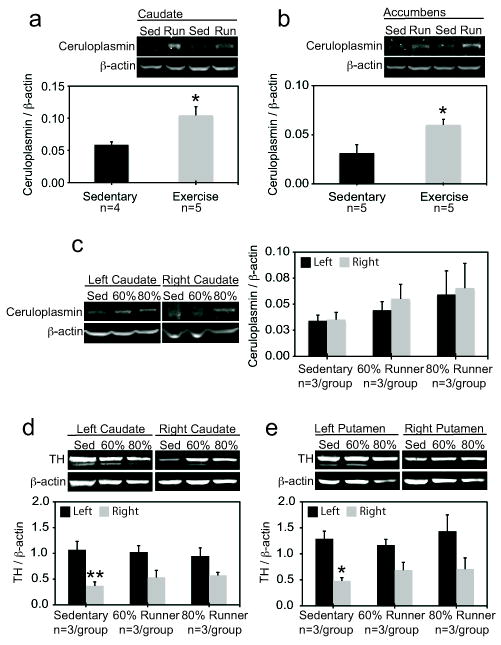
Ceruloplasmin in the (a) caudate and (b) accumbens of sedentary monkeys and monkeys that ran on a treadmill at 80% maximal heart rate. *p<0.05 (two tailed t-test). c: Ceruloplasmin in the caudate of monkeys infused in the right hemisphere with MPTP. MPTP monkeys were sedentary or ran at 60% or 80% maximal heart rates. d and e: Tyrosine hydroxylase (TH) levels in the (d) caudate and (e) putamen of the same MPTP-treated monkeys as in Figure 1c. *p<0.05 **p<0.01 compared to left, control hemisphere (Bonferroni post hoc test following two way ANOVA).
MPTP did not cause unilateral ceruloplasmin loss in the caudate (Fig.1c) or putamen (not shown). The accumbens of MPTP monkeys was not assayed in the present study. However, exercise failed to significantly raise ceruloplasmin in the caudate (Fig.1c) or putamen (not shown) of MPTP animals. MPTP did cause unilateral loss of TH in the caudate of sedentary animals (Fig.1d; for the Bonferroni comparison of left versus right hemisphere in sedentary controls: p<0.01, t=3.891; for the ANOVA on hemisphere p<0.001, F(1,7)=25.18) and the putamen of sedentary animals (Fig.1e; for the Bonferroni comparison of left versus right hemisphere in sedentary controls, p<0.05, t=3.074; for the ANOVA on hemisphere, p<0.001, F(1,7)=19.56). Although we cannot be certain without isoform-specific antibodies that the fluorescent band under the larger TH band is a smaller isoform of TH (Le Bourdelles et al. 1991), it is lost with MPTP and is therefore likely to be TH (R.G. Perez, personal communication). The impact of MPTP on loss of caudate or putamen TH was not significant in the 60% and 80% runners. However, the Bonferroni comparison of the right hemispheres of sedentary controls versus either of the runners was not significant, suggesting that treadmill exercise, when considered alone, did not protect the lesioned hemisphere. However, this comparison does not take into account the influence of overall daily activity levels on loss of TH (see below).
Correlation of striatal ceruloplasmin and TH with activity levels
Although we assayed all 12 putamen tissue punches from intact animals for ceruloplasmin, activity data was only available for 9 animals. Putamen ceruloplasmin in intact monkeys was found to be significantly correlated with spontaneous activity levels, that is, mean daily activity measured at baseline before the 12-week treadmill session (Fig.2a). Putamen ceruloplasmin was not significantly correlated with overall mean daily activity levels (spontaneous plus induced by jogging) during the treadmill study (Fig.2b). In other words, adding treadmill exercise to the monkey’s daily physical activity masks this underlying (still valid) correlation so that the p value rose to 0.1295. Ceruloplasmin in the caudate and accumbens was not correlated with either activity measure (not shown).
Figure 2.
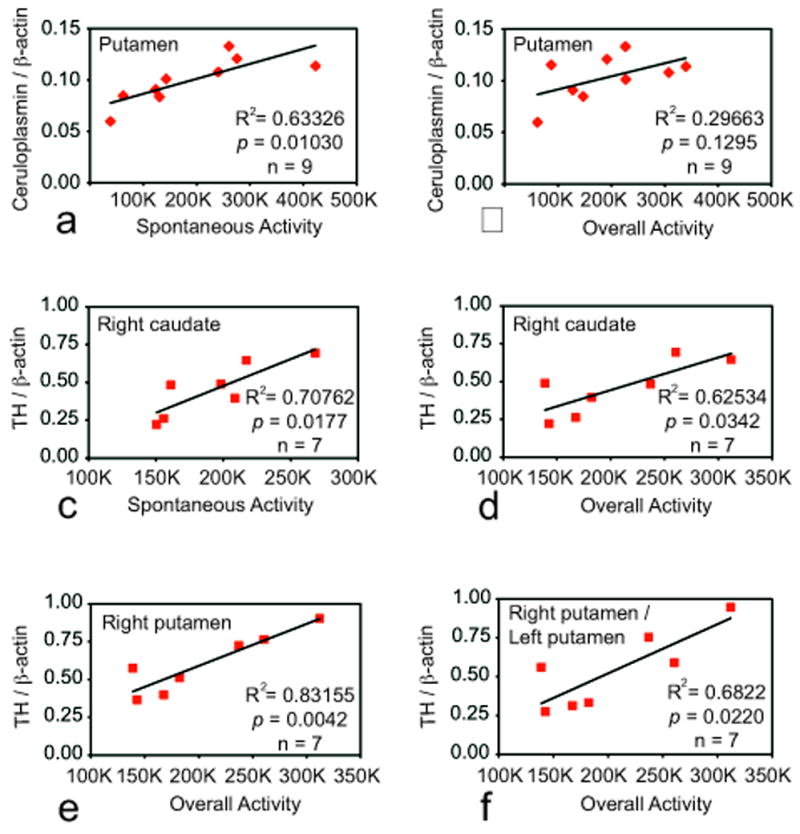
Putamen ceruloplasmin as a function of (a) spontaneous baseline activity (before initiation of the treadmill running study) or (b) overall activity during the study (includes spontaneous daily activity and treadmill running activity) in intact, healthy monkeys. c, d, e, and f: Correlations between spontaneous or overall activity and tyrosine hydroxylase (TH) in indicated brain regions of MPTP-treated monkeys. The right hemisphere was infused with MPTP.
Activity data was not available for one MPTP monkey. Activity levels of a second monkey were found to be 2.6 standard deviations greater than the group mean (Grubb’s outlier), significantly higher than any other monkey we have ever recorded activity for. Therefore, both were excluded from analyses. Putamen and caudate ceruloplasmin in MPTP monkeys failed to correlate with activity (not shown). For example, for the right (MPTP-treated) putamen, R2=0.18560 (p=0.39370) for ceruloplasmin versus spontaneous activity. For the left putamen, R2=0.13490 (p=0.37080) for ceruloplasmin versus spontaneous activity.
TH levels in the MPTP-treated right caudate were correlated with both spontaneous activity and overall activity (Fig.2c, d). TH levels in the MPTP-treated right putamen were correlated with overall activity (Fig.2e) but not spontaneous activity (not shown). Importantly, right putamen TH expressed as a fraction of TH on the untreated side was also positively correlated with overall activity (Fig.2f). Thus, overall daily activity predicted protection against MPTP-induced loss of TH. TH in the untreated left hemisphere was not correlated with any measure (not shown).
Discussion
The present study is the first examination of ceruloplasmin in the brains of animals as a function of spontaneous physical activity or the physical activity of treadmill exercise. Ceruloplasmin was indeed linked with activity in the striatum of intact, healthy primates, a link that was lost with MPTP. First, we found that treadmill exercise raised ceruloplasmin in the intact caudate and accumbens. Although we did not gather support for an MPTP-induced loss in ceruloplasmin, we observed that there was no significant rise in ceruloplasmin with exercise in MPTP-treated primates in either hemisphere. The negative impact of MPTP on this response suggests that endogenous adaptations to exercise are more difficult to elicit in parkinsonian animals and require an intact dopaminergic system. As the MPTP-induced loss of the dopaminergic marker TH was not accompanied by a parallel loss in ceruloplasmin, striatal ceruloplasmin is not likely to be contained within dopaminergic terminals. This is entirely consistent with the heavily astrocytic expression of this ferroxidase (Patel and David 1997).
Second, putamen ceruloplasmin levels were not raised by exercise but were correlated with spontaneous activity levels measured before the treadmill running session was begun. These topographic differences in the ceruloplasmin responses of striatal subregions may reflect the underappreciated heterogeneity of astrocytes within different brain regions (Zhang and Barres 2010). Once daily physical activity included that induced by treadmill running (overall activity), the correlation with putamen ceruloplasmin was lost. These subtle differences suggest that spontaneous and treadmillinduced activities do not impact ceruloplasmin in precisely the same way. This interpretation assumes that the link between activity and ceruloplasmin is causal. An alternative explanation, however, is that it is not motor activity per se that determines putamen ceruloplasmin, but that the correlation between spontaneous activity and ceruloplasmin reflects the independent impact of some other process on both these factors. On the other hand, if the link were causal, one might conclude that spontaneous activity initiated intrinsically by the monkey to explore its home pen exerts different effects on the brain than the motor activity initiated externally by a moving treadmill. This would not be surprising given that spontaneous physical activity is an exceedingly complex behavior influenced by limbic functions such as internal motivation and eagerness to explore.
Third, the correlation between putamen ceruloplasmin and spontaneous physical activity was lost with MPTP. Again, these data suggest that the link between physical activity and ceruloplasmin is broken with loss of the dopaminergic phenotype. In this respect, it is noteworthy that treadmill exercise did not robustly protect against MPTP-induced loss of TH in this primate model. It might therefore be speculated that exercise would have elicited protection against dopaminergic loss had the ceruloplasmin response remained intact in parkinsonian monkeys. As mentioned earlier, the effect of the treadmill session per se does not take into consideration physical activity levels over the course of the day. Notably, TH levels in the MPTP-treated caudate and putamen were both correlated with overall activity. In addition, overall daily activity correlated negatively with loss of the dopaminergic phenotype relative to the untreated hemisphere. In other words, more TH remained behind after MPTP relative to the control hemisphere in the active animals. These findings support the hypothesis that daily spontaneous and induced physical activities as a whole predict the degree of protection against Parkinson’s models and have potentially critical implications for the maintenance of an active lifestyle in humans.
Finally, regarding the lack of effect on striatal ceruloplasmin with MPTP, one human study employing ELISAs suggests that ceruloplasmin levels in the caudate and putamen are not affected significantly by Parkinson’s disease (Loeffler et al. 1996), although there are losses in cerebrospinal fluid and serum ceruloplasmin in Parkinson’s patients (Bharucha et al. 2008, Boll et al. 2008, Torsdottir et al. 2010). These findings indicate that the impact of the Parkinson’s phenotype on ceruloplasmin is not uniform and depends on cell type. Added to this complexity is the topographical heterogeneity within the brain, even in three structures all defined as striatal, as is evident from our study. Striatal heterogeneity is also strongly supported by the pattern of neurodegeneration in Parkinson’s disease. The putamen usually suffers the greatest loss of dopaminergic terminals with Parkinsonism, whereas the caudate and accumbens are less affected (Jan et al. 2003). In this respect, it is noteworthy that treadmill exercise only raises ceruloplasmin in the primate caudate and accumbens, the two structures less affected in Parkinson’s disease.
In sum, we interpret our findings to suggest that treadmill exercise increases ceruloplasmin expression in the caudate and accumbens, but only in healthy monkeys with an intact dopaminergic system. Putamen ceruloplasmin is not responsive to treadmill exercise, consistent with the topographic heterogeneity of the primate striatum. Instead, there is an association between spontaneous activity and putamen ceruloplasmin that may or may not be a causal link. This association also disappears in MPTP-treated animals, suggesting again that the Parkinson’s phenotype adversely affects antioxidant defenses. When measurements of activity include that induced by daily treadmill running, the association with putamen ceruloplasmin is lost, suggesting that spontaneous and induced locomotion are not interchangeable forms of physical activity. Nonetheless, animals that were overall more active throughout the day suffered less loss of TH in response to MPTP. Taken as a whole, these data all support the notion that humans with sedentary lifestyles have fewer endogenous defenses and are more vulnerable to disease states.
Acknowledgments
We are deeply grateful to Deb Wilson, Mary Caruso, and Jackie Farrer for superb administrative support. This work was supported by a startup award to RKL and an R21 NS053471 award to JLC.
Footnotes
We declare no conflicts of interest.
References
- Berg D, Gerlach M, Youdim MB, Double KL, Zecca L, Riederer P, Becker G. Brain iron pathways and their relevance to Parkinson’s disease. Journal of neurochemistry. 2001;79:225–236. doi: 10.1046/j.1471-4159.2001.00608.x. [DOI] [PubMed] [Google Scholar]
- Bharucha KJ, Friedman JK, Vincent AS, Ross ED. Lower serum ceruloplasmin levels correlate with younger age of onset in Parkinson’s disease. J Neurol. 2008;255:1957–1962. doi: 10.1007/s00415-009-0063-7. [DOI] [PubMed] [Google Scholar]
- Boll MC, Alcaraz-Zubeldia M, Montes S, Rios C. Free copper, ferroxidase and SOD1 activities, lipid peroxidation and NO(x) content in the CSF. A different marker profile in four neurodegenerative diseases. Neurochem Res. 2008;33:1717–1723. doi: 10.1007/s11064-008-9610-3. [DOI] [PubMed] [Google Scholar]
- Ding F, Luan L, Ai Y, Walton A, Gerhardt GA, Gash DM, Grondin R, Zhang Z. Development of a stable, early stage unilateral model of Parkinson’s disease in middle-aged rhesus monkeys. Experimental neurology. 2008;212:431–439. doi: 10.1016/j.expneurol.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AVJ. The nucleus accumbens: gateway for limbic structures to reach the motor system? Amsterdam: Elsevier; 1996. [DOI] [PubMed] [Google Scholar]
- Grondin R, Zhang Z, Ai Y, Ding F, Walton AA, Surgener SP, Gerhardt GA, Gash DM. Intraputamenal infusion of exogenous neurturin protein restores motor and dopaminergic function in the globus pallidus of MPTP-lesioned rhesus monkeys. Cell transplantation. 2008;17:373–381. [PMC free article] [PubMed] [Google Scholar]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- Hauber W. Involvement of basal ganglia transmitter systems in movement initiation. Progress in neurobiology. 1998;56:507–540. doi: 10.1016/s0301-0082(98)00041-0. [DOI] [PubMed] [Google Scholar]
- Hineno A, Kaneko K, Yoshida K, Ikeda S. Ceruloplasmin protects against rotenone-induced oxidative stress and neurotoxicity. Neurochemical research. 2011;36:2127–2135. doi: 10.1007/s11064-011-0537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan C, Pessiglione M, Tremblay L, Tande D, Hirsch EC, Francois C. Quantitative analysis of dopaminergic loss in relation to functional territories in MPTP-treated monkeys. The European journal of neuroscience. 2003;18:2082–2086. doi: 10.1046/j.1460-9568.2003.02946.x. [DOI] [PubMed] [Google Scholar]
- Kaneko K, Hineno A, Yoshida K, Ikeda S. Increased vulnerability to rotenone-induced neurotoxicity in ceruloplasmin-deficient mice. Neurosci Lett. 2008;446:56–58. doi: 10.1016/j.neulet.2008.08.089. [DOI] [PubMed] [Google Scholar]
- Kenyon CL, Basaraba RJ, Bohn AA. Influence of endurance exercise on serum concentrations of iron and acute phase proteins in racing sled dogs. J Am Vet Med Assoc. 2011;239:1201–1210. doi: 10.2460/javma.239.9.1201. [DOI] [PubMed] [Google Scholar]
- Lau YS, Patki G, Das-Panja K, Le WD, Ahmad SO. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson’s disease with moderate neurodegeneration. The European journal of neuroscience. 2011;33:1264–1274. doi: 10.1111/j.1460-9568.2011.07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourdelles B, Horellou P, Le Caer JP, Denefle P, Latta M, Haavik J, Guibert B, Mayaux JF, Mallet J. Phosphorylation of human recombinant tyrosine hydroxylase isoforms 1 and 2: an additional phosphorylated residue in isoform 2, generated through alternative splicing. The Journal of biological chemistry. 1991;266:17124–17130. [PubMed] [Google Scholar]
- Leak RK, Castro SL, Jaumotte JD, Smith AD, Zigmond MJ. Assaying multiple biochemical variables from the same tissue sample. J Neurosci Methods. 2010;191:234–238. doi: 10.1016/j.jneumeth.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak RK, Liou AK, Zigmond MJ. Effect of sublethal 6-hydroxydopamine on the response to subsequent oxidative stress in dopaminergic cells: evidence for preconditioning. J Neurochem. 2006;99:1151–1163. doi: 10.1111/j.1471-4159.2006.04149.x. [DOI] [PubMed] [Google Scholar]
- Leak RK, Zigmond MJ, Liou AK. Adaptation to chronic MG132 reduces oxidative toxicity by a CuZnSOD-dependent mechanism. J Neurochem. 2008;106:860–874. doi: 10.1111/j.1471-4159.2008.05459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesen H, Dufaux B, Hollmann W. Modifications of serum glycoproteins the days following a prolonged physical exercise and the influence of physical training. Eur J Appl Physiol Occup Physiol. 1977;37:243–254. doi: 10.1007/BF00430954. [DOI] [PubMed] [Google Scholar]
- Loeffler DA, LeWitt PA, Juneau PL, Sima AA, Nguyen HU, DeMaggio AJ, Brickman CM, Brewer GJ, Dick RD, Troyer MD, Kanaley L. Increased regional brain concentrations of ceruloplasmin in neurodegenerative disorders. Brain research. 1996;738:265–274. doi: 10.1016/s0006-8993(96)00782-2. [DOI] [PubMed] [Google Scholar]
- Patel BN, David S. A novel glycosylphosphatidylinositol-anchored form of ceruloplasmin is expressed by mammalian astrocytes. The Journal of biological chemistry. 1997;272:20185–20190. doi: 10.1074/jbc.272.32.20185. [DOI] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev. 2008;7:34–42. doi: 10.1016/j.arr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Resina A, Gatteschi L, Rubenni MG, Giamberardino MA, Imreh F. Comparison of some serum copper parameters in trained professional soccer players and control subjects. J Sports Med Phys Fitness. 1991;31:413–416. [PubMed] [Google Scholar]
- Rhyu IJ, Bytheway JA, Kohler SJ, Lange H, Lee KJ, Boklewski J, McCormick K, Williams NI, Stanton GB, Greenough WT, Cameron JL. Effects of aerobic exercise training on cognitive function and cortical vascularity in monkeys. Neuroscience. 2010;167:1239–1248. doi: 10.1016/j.neuroscience.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Cameron JL. A rapidly occurring compensatory decrease in physical activity counteracts diet-induced weight loss in female monkeys. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1068–1074. doi: 10.1152/ajpregu.00617.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Texel SJ, Xu X, Harris ZL. Ceruloplasmin in neurodegenerative diseases. Biochemical Society transactions. 2008;36:1277–1281. doi: 10.1042/BST0361277. [DOI] [PubMed] [Google Scholar]
- Texel SJ, Zhang J, Camandola S, Unger EL, Taub DD, Koehler RC, Harris ZL, Mattson MP. Ceruloplasmin deficiency reduces levels of iron and BDNF in the cortex and striatum of young mice and increases their vulnerability to stroke. PLoS One. 2011;6:e25077. doi: 10.1371/journal.pone.0025077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsdottir G, Kristinsson J, Snaedal J, Sveinbjornsdottir S, Gudmundsson G, Hreidarsson S, Johannesson T. Case-control studies on ceruloplasmin and superoxide dismutase (SOD1) in neurodegenerative diseases: a short review. J Neurol Sci. 2010;299:51–54. doi: 10.1016/j.jns.2010.08.047. [DOI] [PubMed] [Google Scholar]
- Xu Q, Park Y, Huang X, Hollenbeck A, Blair A, Schatzkin A, Chen H. Physical activities and future risk of Parkinson disease. Neurology. 2010;75:341–348. doi: 10.1212/WNL.0b013e3181ea1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Current opinion in neurobiology. 2010;20:588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]


