Abstract
A large proportion of the world population harbors herpes simplex virus type 1 (HSV-1) in a latent state in their trigeminal ganglia (TG). TG-resident CD8+ T cells appear important for preventing HSV-1 reactivation from latency and recurrent herpetic disease. In C57BL/6J mice half of these cells are specific for an immunodominant epitope on HSV-1 glycoprotein B while the other half are specific for 18 subdominant epitopes. Here, we show that the CD8+ T cell dominance hierarchy in the TG established during acute infection is maintained during latency. However, CD8+ T cells specific for subdominant epitopes lose functionality while those specific for the immunodominant epitope exhibit increased functionality in latently infected TG. Furthermore, we show that IL-10 produced by 16.4 ± 2.8% of TG-resident CD4+ T cells maintains the immunodominance hierarchy in part through selective inhibition of subdominant CD8+ T cell proliferation. Upon systemic anti-IL-10 receptor antibody treatment, we observed a significant expansion of functional subdominant CD8+ T cells, resulting in significantly improved protection from viral reactivation. In fact, systemic anti-IL-10 receptor antibody treatment prevented viral reactivation in up to 50% of treated mice. Our results not only demonstrate that HSV-1 reactivation from latency can be prevented by expanding the repertoire of functional TG-resident CD8+ T cells, but also that IL-10 receptor blockade might have therapeutic potential to reduce or eliminate recurrent herpetic disease.
Introduction
Herpes simplex virus type 1 (HSV-1) infects a large portion of the world population and can establish a quiescent (latent) infection in neurons of the trigeminal ganglion (TG). Periodic reactivation of HSV-1 from latency can cause recurrent lesions on the gums (stomatitis), lips (cold sores, fever blisters), and cornea (keratitis); and less commonly the brain (encephalitis). HSV-1 stromal keratitis (HSK) is a frequent cause of blindness, and HSV-1 encephalitis is often fatal(1). Mechanisms controlling HSV-1 latency in neurons remain somewhat enigmatic, but appear to involve a complex interaction involving viral micro RNAs (miRNA), host cell epigenetic repression of viral gene expression(2–5), and monitoring by TG-resident CD8+ T cells.
HSV-specific CD8+ T cells infiltrate the mouse TG following corneal infection and are maintained in direct apposition to neurons throughout life-long viral latency. A significant proportion of the TG-resident HSV-specific CD8+ T cells persistently exhibit an activation phenotype and form apparent immunological synapses with neurons, rendering untenable the concept that HSV-1 can hide from the host immune system during latency(6–8). Latently infected neurons in human TG are also surrounded by CD8+ T cells with a similar activation phenotype. The capacity of the TG resident CD8+ T cells to block HSV-1 reactivation from latency has been demonstrated, and an inverse relationship between the size of the TG-resident CD8+ T cell population and the frequency of HSV-1 reactivation from latency in ex vivo TG cultures was established(9, 10). Therapeutic vaccines that increase the frequency of circulating HSV-specific CD8+ T cells could theoretically reduce the rate of recurrent herpetic disease by increasing the size of the HSV-specific memory CD8+ T cell pool in latently infected TG(11, 12). However, TG-resident CD8+ T cells appear to be of the tissue resident memory (TRM) type(13) and exhibit little if any replenishment by circulating HSV-specific CD8+ T cells, potentially limiting vaccine efficacy(14).
All or the vast majority of the TG-resident CD8+ T cells in C57BL/6 mice are HSV-specific, with 50% recognizing a single immunodominant epitope on HSV glycoprotein B (gB498-505) and the remainder recognizing 18 subdominant epitopes(15–17). This 1:1 ratio of immunodominant to subdominant CD8+ T cells is established during acute infection and strictly maintained throughout latency(18). In TG harboring latent HSV-1 of the KOS or RE strain, gB498-505-specific CD8+ T cells maintain functionality (19), even when latency is interrupted by serial reactivation events (20). Moreover, the resident gB498-505-specific CD8+ T cells in HSV-1 latently infected TG can use IFN-γ and lytic granules containing granzyme B (GrB) to block HSV-1 reactivation from latency, while sparing the infected neuron (21–23).
Having identified the subdominant epitopes recognized by the 50% of TG-resident CD8+ T cells that are not specific for the immunodominant gB498-505 epitope (16), it was now possible to interrogate the retention and functional characteristics of these cells during viral latency, and their potential role in preventing viral reactivation from latency. Here we establish that subdominant CD8+ T cells are regulated by IL-10 produced by TG-resident CD4 T cells. The number of functional TG-resident subdominant CD8+ T cells can be dramatically increased by treating latently infected mice with anti-IL-10 receptor (αIL-10R) antibody, which reduces the frequency of TG that reactivate the virus from 100% to 50%.
Materials and Methods
Mice and virus
Wild-type HSV-1 strain RE was grown in Vero cells, and intact virions were isolated on Optiprep gradients according to the manufacturer’s instructions (Accurate Chemical and Scientific, Westbury, NY). Six- to eight-week-old female wild-type C57BL/6 mice and B6.129S6-Il10tm1Flv/J were anesthetized by i.p. injection of 3.0 mg ketamine hydrochloride and 0.04 mg xylazine (Phoenix Scientific, San Marcos, CA) in 0.2 ml HBSS (Bio Whittaker, Walkersville, MD). The abraded central corneas of anesthetized mice were infected by topical application of 3 μl RPMI 1640 (Bio Whittaker) containing 1 × 105 PFU HSV-1. All animal experiments were conducted in accordance with guidelines established by the University of Pittsburgh Institutional Animal Care and Use Committee.
Flow Cytometry Reagents
PE/APC-conjugated H-2Kb tetramers complexed with the gB498-505, RR1982-989, RR1822-829, ICP8171-178, or ICP8876-883 peptides (16) were provided by the National Institute of Allergy and Infectious Diseases Tetramer Core Facility (Emory University Vaccine Center, Atlanta, GA). Rat anti-mouse Pacific Blue-conjugated anti-CD8α (clone 53-6.7), PE-Cy7 conjugated anti-CD4 (clone RM4-5), APC-conjugated anti-IFN-gamma (XMG1.2), PerCP-conjugated anti-CD45 (30-F11), FITC-conjugated CD107a (H4A3), FITC-conjugated anti-BrdU (3D4), and BD Cytofix/Cytoperm Fixation/Permeablization Solution Kit were purchased from BD Pharmingen (San Diego, CA). The appropriate isotype control antibodies were purchased from BD Pharmingen (San Diego, CA). Mouse anti-human APC-conjugated anti-granzyme B (GB11) and the appropriate isotype control were purchased from Invitrogen. All flow cytometry samples were collected on a FACSAria cytometer and analyzed by FACSDiva software (BD Biosciences).
Tissue preparation
At specified times after infection, mice were anesthetized and euthanized by exsanguination. Trigeminal ganglia (TG) were harvested and digested in 100 ul per ganglion of DMEM (Bio Whittaker) containing 10% FBS (Atlanta Biologicals) and 400 U/ml collagenase type I (Sigma-Aldrich) for 1 h at 37°C. TG were dispersed into single-cell suspensions by trituration through a p-200 pipette tip. Spleens were dispersed mechanically and treated with RBC lysis buffer prior to cell counting. Cell counting was performed using the Becman Coulter Vi-Cell XR cell viability analyzer. Tissue harvest and preparation were performed under sterile conditions.
In vitro stimulation and intracellular staining
B6WT3 fibroblast targets were pulsed with individual or pooled peptides, RR1982-989, RR1822-829, RR1372-379, ICP8171-178, ICP8168-174 or ICP8876-883, at a concentration of 1.0 ug/mL for 45 min at 37°C/5% CO2. Dispersed TG cells were stimulated with peptide pulsed fibroblasts in RPMI 1640 containing 10% FBS, 50 uM 2-Mercaptoethanol (Fisher), anti-CD107a and Golgi-Plug (BD Biosciences) for 6 h at 37°C/5% CO2. After stimulation, cells were stained for surface expression of CD8α, followed by intracellular staining for IFN-γ after permeabilization and fixation via Cytofix/Cytoperm (BD Biosciences).
For granzyme B expression or BrdU incorporation, dispersed TGs were stained with anti-CD45, CD8alpha and gB498-505 tetramer for 1 h at room temperature. After incubation, cells were permeabilized and fixed with Cytofix/Cytoperm (BD Biosciences) and stained for intracellular granzyme B, or cells were fixed and stained for BrdU per manufacturer’s instructions using the BD Phamingen BrdU FITC Flow Kit (BD Phamigen).
In vivo IL-10R blockade and BrdU administration
Systemic IL-10 signaling was inhibited by intraperitoneal (i.p.) injections of 250 ug of anti-IL-10R mAb (1B1.3A) every 3 days for up to 21 days. The same clone, dose, and route of administration of this antibody was successfully employed to block IL-10 signaling in several other (24–26). Control mice were treated with i.p. injection of PBS because we demonstrated in a previous study that similar treatment with a rat antibody of irrelevant specificity did not affect the CD8+ T cell infiltrate in HSV-1 infected TG (27). After 17 days of treatment, mice were given a single i.p. injection of 1 mg of BrdU (Sigma-Aldrich), and 4 h later their TGs and spleens were harvested and stained for BrdU incorporation as stated above.
Detecting HSV-1 reactivation from ex vivo TG cultures
Following bilateral HSV-1 corneal infection, 4 TGs from 2 mice were removed and the TG cells dispersed as described above. Dissociated cells were suspended in DMEM containing 10% FBS, 10mM HEPES buffer (GIBCO), 10 U/ml recombinant murine IL-2 (R&D Systems), and 50 uM 2-Mercaptoethanol. Twenty-five percent (1 TG equivalent) of each suspensions was removed for phenotypic and functional analysis. The remaining cells (3 TG equivalents) were split into 2 aliquots that were supplemented with anti-CD8alpha mAb (100ug/ml, clone 2.43) to inhibit the fundtion of the endogenous CD8+ T cells, while the other aliquot of TG cells was supplemented with and the same concentration of control rat IgG. Pools of 1.5 TGs were then dispersed into 10 cultures (0.15 TG per culture well). Individual culture wells were monitored for reactivation by serially testing supernatant fluid for live virus using a standard plaque assay on Vero cell monolayers as previously described.
Statistical Analyses
Statistical comparisons of all data except the reactivation frequency data in Figure 6 were based on a Student’s t test as computed with GraphPad Prism software. A Mann-Whitney test was used for statistical comparisons of data in Figure 6.
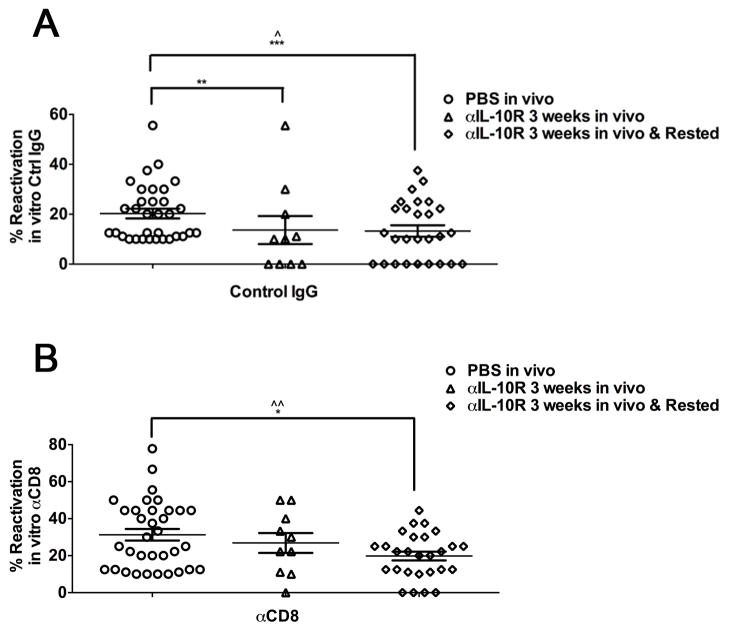
Figure 6. Treatment with anti-IL-10R mAb reduces viral reactivation from latency.
Latently infected mice were treated with PBS or anti-IL-10R mAb for 3 weeks. Immediately after or 3 weeks after termination of treatment, TG cells from groups of 2 mice were pooled and cultured (0.15 TG/well). Cultures were treated with control IgG (A) or anti-CD8α mAb(B). Culture supernatants were serially sampled every 2 days, and assayed for infectious virus on monolayers of Vero cells and reactivation frequency was calculated (For PBS treated: 5 experiments, anti-IL-10R Treated: 2 experiments, anti-IL-10R Treated and rested: 3 experiments). Comparison of the mean reactivation frequencies between groups was performed using a square root transformation of the data presented. Statistical significance between mean reactivation frequencies was determined using an ANOVA (^ p ≤ 0.05, ^^ p ≤ 0.005). Additionally, a Fisher’s Exact Test was used to compare the number of pools that had zero reactivation events between the treatment groups (* p ≤ 0.05, ** p ≤ 0.005, *** p ≤ 0.0005).
Results
CD8+ T cells specific for subdominant HSV-1 epitopes lose functionality in latently infected TG
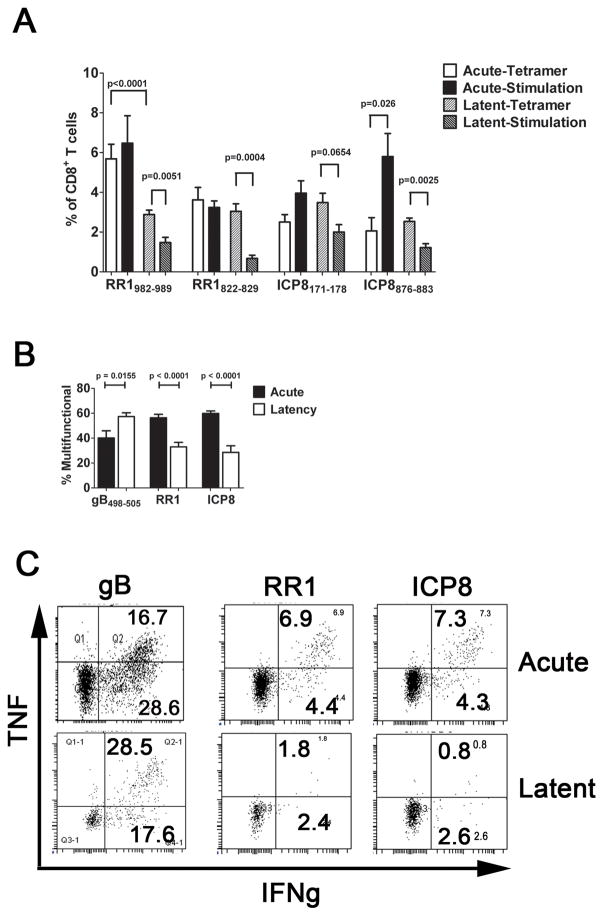
CD8+ effector T cells accumulate to peak levels in TG of C57BL/6 mice by 8 days post HSV-1 corneal infection (dpi), and contract to form a stable memory population by 30 dpi (27). Half of the CD8+ T cells in the effector population in acutely infected TG (8 dpi) and the memory population during latent infection (>30 dpi) are specific for a single immunodominant epitope on HSV-1 glycoprotein B (gB498-505) as assessed by tetramer staining, and all of these immunodominant cells maintain the ability to produce IFN-γ when stimulated with the gB498-505 epitope directly ex vivo (19). Two subdominant epitopes on HSV-1 ribonucleotide reductase 1 (RR1982-989 and RR1822-829) and two on HSV-1 infected cell protein 8 (ICP8171-178 and ICP8876-884) together represent 27.8% of the subdominant population at 8 dpi based on tetramer staining (Fig. 1A). Tetramer staining tended to underestimate the frequency of epitope reactive cells during acute infection since the frequency based on tetramer staining was lower than the frequency based on intracellular staining for IFN-γ following epitope stimulation, though the difference was statistically significant only for ICP8876. Although there was some individual variation, the frequency of the subdominant CD8+ T cell populations remained fairly constant during latency as assessed by tetramer staining, representing in aggregate 23.9% of the subdominant population at 30 dpi (Fig. 1A).
Figure 1. Subdominant CD8+ T cells become functionally compromised during latent HSV-1 infection.
TG were obtained from HSV-1 infected mice during acute (8 dpi) or latent (30–35 dpi) infection, and the dispersed cells (A) were stained with H2-Kb tetramers containing peptides corresponding to two subdominant epitopes on HSV-1 ribonucleotide reductase 1 (RR1) or infected cell protein 8 (ICP8); or were stimulated with B6WT3 fibroblasts pulsed with the same peptides, stained for intracellular IFN-γ, and analyzed by flow cytometry. The bars represent the cumulative mean ± SEM percent of CD8+ T cells that are tetramer positive during acute (2 experiments, total n = 8–10 TG per peptide) and latent (4 experiments, total n ≥ 20 TG/peptide) infection, or frequency of CD8+ T cells that are IFN-γ positive during acute or latent infection (2 experiments, n ≥ 8 TG/peptide). (B&C) TG cells were stimulated with B6WT3 fibroblasts pulsed with 3 subdominant epitopes on RR1 (RR1982-989, RR1822-829, and RR1372-380) or on ICP8 (ICP8171-178, ICP8168-176, and ICP8876-883) and monitored for surface CD107a (lytic granule release) and intracellular IFN-γ and TNFα by flow cytometry. Bars represent the cumulative mean ± SEM frequency of multifunctional (IFN-γ+, TNFα+ and CD107a+) cells (2 experiments, n ≥ 9 TG/peptide pool). (C) Representative dot plots displaying the loss of multi-functionality in RR1- and ICP-specific CD8+ T cells from acute infection to latent infection. The p values were determined by an unpaired Student’s T test.
The TG-resident subdominant CD8+ T cells did, however, exhibit a rather dramatic loss of functionality during latency as indicated by a significantly reduced frequency of IFN-γ producing cells relative to tetramer staining cells in latently infected TG (Fig. 1A&C). This comparison might underestimate the functional impairment of the subdominant CD8+ T cells, as some appear refractory to tetramer staining as noted above. Moreover, the frequency of multifunctional subdominant CD8+ T cells (capable of producing IFN-γ, TNFα, and releasing lytic granules when stimulated directly ex vivo) was also significantly reduced in latently infected compared to acutely infected TG (Fig. 1B&C). No functional compromise was observed in the corresponding subdominant CD8+ T cells in the spleen (data not shown). In our model HSV-1 latent infections are restricted to the TG and brainstem, suggesting that the functional compromise of TG-resident subdominant CD8+ T cells likely requires antigenic exposure. Functional impairment was restricted to the subdominant population, since the TG-resident immunodominant gB498-505-specific cells showed a slight increase in multifunctional cells during latency in agreement with previous findings(19).
TG-resident CD4+ T cells produce IL-10 during latent HSV-1 infection
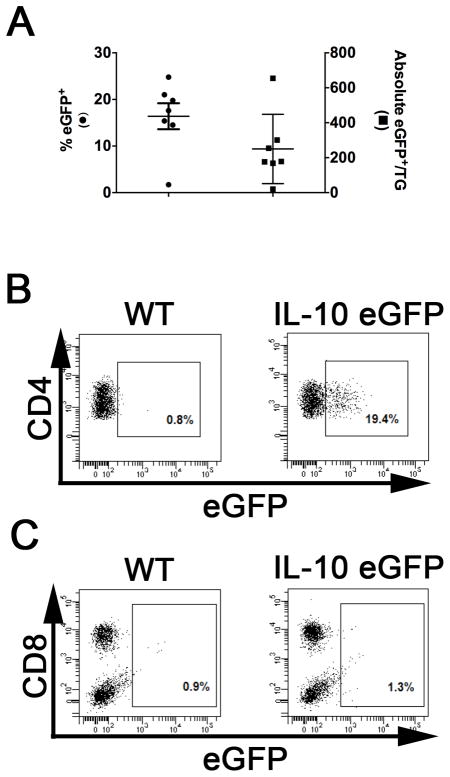
Functional compromise of CD8+ T cells during chronic viral infections is mediated in part by IL-10, though the main source of IL-10 in these models has not been definitively proven(24, 25, 28–30). Moreover, our HSV-1 latent infection model is unique in that loss of function during latency is restricted to the CD8+ T cells that recognize subdominant viral epitopes. To identify a role for IL-10 in our model, we first interrogated latently infected TG for IL-10 producing cells using reporter mice that express green fluorescent protein from the IL-10 promoter. Using flow cytometric analysis we demonstrate that 16.4 ± 2.8% of CD4+ T cells produce IL-10 in latently infected TG (Fig. 2A & B), and they appear to be the sole source of IL-10 (Fig. 2B&C).
Figure 2. TG-resident CD4+ T cells produce IL-10 during latent HSV-1 infection.
TG were obtained from latently HSV-1 infected WT C57BL/6 and B6.129S6-Il10tm1Flv/Jmice, dispersed into single-cell suspensions, and stained for expression of the surface markers CD45, CD8α, CD4, and CD11c. Detection of surface marker expression and intracellular eGFP expression (from the IL-10 promoter) was performed on live cells using flow cytometry. (A) Scatter plot illustrates mean ± SEM frequency(left axis)and absolute number (right axis)of eGFP expressing CD4+CD11c− cells in individual TG (2 experiments, total n = 7). (B & C) Representative dot plots comparing the eGFP populations gated on CD4+CD11c- cells (B) or CD45+CD4- cells(c) from WT and B6.129S6-Il10tm1Flv/J.
IL-10R blockade alters the immunodominance hierarchy in latently infected TG by selectively augmenting expansion of subdominant CD8+ T cells
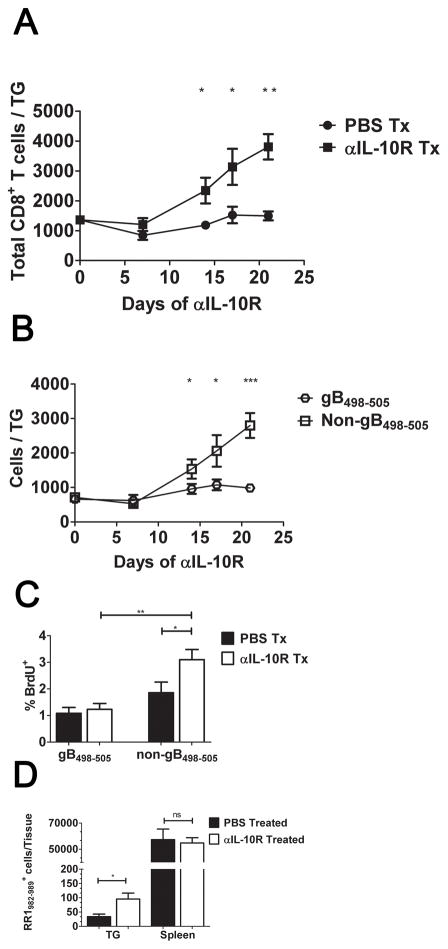
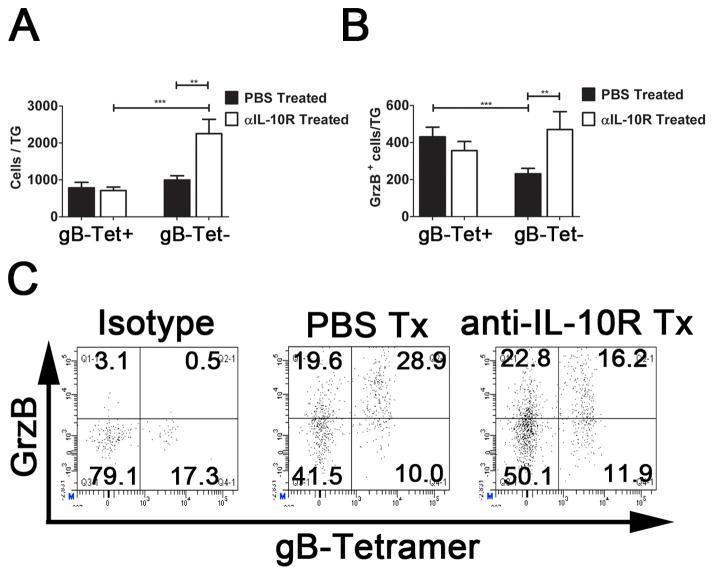
We next attempted to restore function in the subdominant CD8+ T cell population by treating mice with monoclonal antibodies (mAb) capable of blocking binding of IL-10 to the IL-10 receptor (IL-10R). Mice that had established a latent HSV-1 infection (35 dpi) were systemically treated every three days with anti-IL-10R mAb or PBS for a period of 3 weeks. After treatment, the TG were excised and the dispersed cells stained with anti-CD8 and with H-2Kb tetramers containing the immunodominant gB498-505 epitope. Flow cytometry revealed a dramatic increase in total CD8+ T cells in anti-IL-10R treated mice (Fig. 3A), that was almost entirely accounted for within the tetramer negative subdominant CD8+ T cell population (Fig. 3B). As expected, the control mice did not show an increase in TG-resident CD8+ T cells during treatment, and maintained an approximate 1:1 ratio of gB498-505-tetramer positive to tetramer negative cells. The increased number of subdominant CD8+ T cells was associated with a significant increase in their rate of proliferation as indicated by BrdU incorporation (Fig. 3C). The increased frequency of subdominant CD8+ T cells in anti-IL-10R treated mice appears to be restricted to the TG, since the anti-IL-10R treatment increased the number of RR1982-specific CD8+ T cells in the TG approximately 2-fold, while not affecting their frequency in the spleen (Fig. 3D). Since HSV-1 is confined to the TG during latency, these data are consistent with the notion that IL-10 regulates proliferation resulting from antigenic exposure rather than homeostatic proliferation of the subdominant CD8+ T cells in latently infected mice.
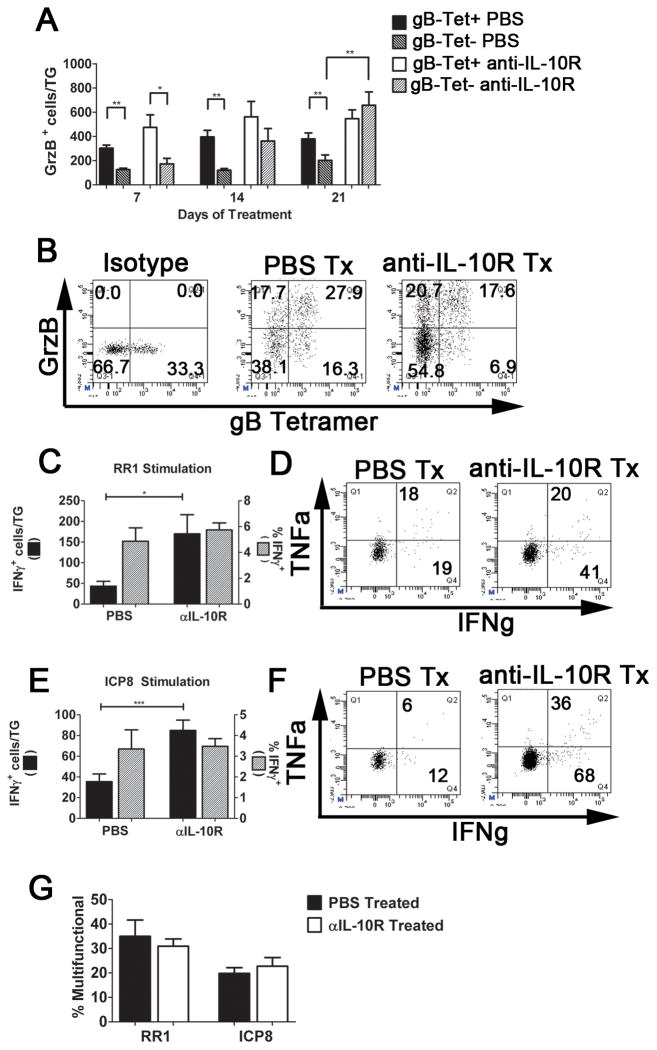
Figure 3. Anti-IL-10R treatment preferentially increases proliferation of subdominant CD8+ T cells in the TG.
HSV-1 latently infected (35 dpi) mice received intraperitoneal (i.p.) injections of PBS or anti-IL-10R every 3 days for up to 21 days (A&B). TGs were harvested and dispersed cells stained for CD8α and with tetramers containing the immunodominant gB498-505 epitope (gB-Tet) and analyzed by flow cytometry. Symbols represent the cumulative mean ± SEM number of total CD8+ T cells (A) or gB-Tet positive and negative CD8+ T cells (B) per TG at 7, 14, 17, and 21 days of treatment (at least 2 independent experiments, total n = 6–15 mice/time). (C&D) Latently infected mice were treated with PBS or anti-IL-10R mAb for 17 days, and then given an intraperitoneal (i.p.) injection of 2mg of BrdU. (C) After 4 hours, TGs were dispersed into single-cell suspensions, stained for CD8α, gB-Tet, and BrdU, and analyzed by flow cytometry. Bars represent the cumulative mean ± SEM percentage of BrdU positive cells (2 experiments, total n = 10 mice). (D) TGs and spleens were dispersed into single-cell suspensions, stained for CD8α and tetramers containing the subdominant RR1982-989 epitope (RR1-Tet) and analyzed by flow cytometry. Bars represent the cumulative mean ± SEM number of RR1-Tet positive cells (2 experiments, total n = 10 mice). Statistical significance was determined using an unpaired Student’s T test. *p ≤ 0.05, ** p ≤ 0.005, *** p ≤ 0.0005.
IL-10R blockade expands both functional and non-functional subdominant CD8+ T cells in latently infected TG
To determine if the function of the subdominant CD8+ T cells in latently infected TG was also rescued by IL-10R blockade, latently infected mice were treated for up to three weeks with anti-IL-10R mAb as described above. TG cells of treated and control mice were dispersed and CD8+ T cells were either stained directly ex vivo for GrzB, or stimulated with B6WT3 cells pulsed with pools of peptides corresponding to the three subdominant epitopes on RR1 or on ICP8 in the presence of anti-CD107a mAb (to detect lytic granule release) and then stained for intracellular IFN-γ and TNFα. Consistent with previous findings, the immunodominant gB498-505-specific CD8+ T cells expressed more GrzB than the non-gB498-505-specific cells prior to treatment (Fig. 4A&B). However after a 1 week lag period, GrzB expression increased dramatically in the non-gB498-505-specific cells, reaching comparable levels to the gB498-505-specific cells by the end of the 3-week anti-IL-10R treatment. A slight increase in the mean number of GrzB+ gB498-505-specific cells during the first two weeks of treatment did not achieve statistical significance(Fig. 4A&B). There was a significant increase in the absolute number, but not the frequency, of CD8+ T cells capable of producing IFN-γ in response to the RR1 or ICP8 subdominant epitopes (Figs 4C–F). Moreover, anti-IL-10R mAb treatment increased the number (not shown) but not the frequency (Fig. 4G) of multifunctional cells capable of producing IFN-γ, TNFα, and releasing lytic granules when stimulated with subdominant epitopes. Together our findings suggest that anti-IL-10R mAb treatment does not rescue the function of impaired subdominant CD8+ T cells, but rather increases the rate of proliferation of both functional and non-functional cells.
Figure 4. Blocking IL-10 signaling during latency increases the number of functional subdominant CD8+ T cells in TG.
Latently infected mice were treated with PBS or anti-IL-10R mAb for 7–21 days (A&B), or 14 days (C-G). (A&B)After treatment, TG cells were stained for CD8α, granzyme B (GrzB), and with gB498-505 tetramers (gB-Tet), and analyzed by flow cytometry. Bars represent the cumulative mean ± SEM number of GrzB+ cells (PBS: 2 experiments, total n = 6 mice/time; anti-IL-10R: at least 2 experiments, total n = 5, 12, and 17 mice at 7, 14, and 17 days, respectively. (B) Representative dot plots gated on CD8+ T cells showing GrzB expression in gB-Tet+ and gB-Tet- cells in TG after 21 days of PBS or anti-IL-10R treatment. (C-G) After treatment, TG cells were incubated for 6 hours with B6WT3 fibroblasts pulsed with three RRI epitopes (C, D, & G) or three ICP8 epitopes (E-G). Cells were analyzed for surface CD8α and intracellular IFNγ (C&E), or surface CD8α and CD107a and intracellular IFN-γ and TNFα (D, F, & G) and analyzed by flow cytometry. (C&E)Bars represent cumulative mean ± SEM number (black bar) or frequency (gray bar) IFN-γ+ cells/TG (3 experiments, n = 11 mice). (D&F) Representative dot plots displaying IFN-γ and TNFα production after stimulation with RR1 peptides (D) or ICP8 peptides (F). Numbers indicate the cells numbers for the denoted quadrants. (G) Bars represent cumulative mean ± SEM percentage multifunctional (IFN γ+, TNFα+, and CD107a+) CD8+ T cells (3 experiments, total n = 7–12 mice). Statistical significance was determined using unpaired Student’s T test. * p ≤ 0.05, ** p ≤ 0.005, *** p ≤ 0.0005.
The effect of anti-IL-10R treatment persists for at least 3 weeks
It was of interest to determine if the augmented number of functional subdominant CD8+ T cells persist after anti-IL-10R treatment is terminated. TG were excised 3 weeks after PBS or anti-IL-10R treatment was discontinued and the number of total and GrzB+ immunodominant and subdominant CD8+ T cells was determined by flow cytometry. Although the total number of TG-resident subdominant CD8+ T cells and the number of GrzB+ subdominant CD8+ T cells declined somewhat during the 3 weeks after termination of anti-IL-10R treatment (compare Fig. 3B with Fig. 5A, and Fig. 4A with Fig 5B), both populations remained significantly elevated compared to the controls. Again, the gB498-505-specific CD8+ T cells were not affected by treatment.
Figure 5. The effects of anti-IL-10R mAb are maintained for at least 3 weeks after terminating treatment.
Latently infected mice (35 dpi) were treated with PBS or anti-IL-10R mAb for 3 weeks and were then rested for an additional 3 weeks. After rest, TG cells were stained for CD8α, granzyme B (GrzB), and with gB498-505 tetramers (gB-Tet), and analyzed by flow cytometry. (A) Bars represent the cumulative mean ± SEM number of gB-Tet+ and gB-Tet-CD8+ T cells per TG (2 experiments, n = 9 – 16 mice). (B) Bars represent the cumulative mean ± SEM number of gB-Tet+ and gB-Tet-cells that express GrzB(3 experiments, n=16–20 mice). (C) Representative dot plots gated on CD8 showing GrzB. Statistical significance was determined by an unpaired Student’s T tests. *p ≤ 0.05, ** p ≤ 0.005, *** p ≤ 0.0005.
In vivo anti-IL-10R antibody treatment during latency reduces viral reactivation
HSV reactivation frequency in ex vivo TG cultures is directly proportional to the viral genome copy number, and inversely proportional to the number of CD8+ T cells in the TG (10). The anti-IL-10R antibody treatment did not significantly alter the HSV-1 genome copy number in latently infected TG (data not shown), or the number of immunodominant CD8+ T cells (Fig. 3). Therefore, we predicted that the increased number of functional subdominant CD8+ T cells in anti-IL-10R treated mice would result in a decreased frequency of HSV-1 reactivation from latency in ex vivo TG cultures only if these cells are capable of blocking reactivation. TG were excised from anti-IL-10R treated and control mice at the end of treatment or 3 weeks after treatment was terminated, the cells from each TG were dispersed into cultures (the equivalent of 0.15 TG/culture), and supernatants of each culture were sampled every other day for 10 days and assayed for infectious virus (reactivation) as previously described(21–23). The data are presented as the frequency of TG cultures from each mouse that reactivated. Control mice showed a low frequency of reactivated cultures that was significantly increased when the function of the CD8+ T cells was compromised by addition of anti-CD8 antibody to the cultures (Fig. 6A&B). This is consistent with our previous findings that CD8+ T cells in latently infected TG are never able to completely block HSV-1 reactivation in ex vivo cultures of latently infected TG(21). In contrast, TG obtained at the end of the anti-IL-10R treatment, or 3 weeks after treatment was terminated exhibited a significant reduction in reactivation frequency, with 4/10 (40%) and 9/27 (33%), respectively exhibiting no detectable reactivation in cultures with intact CD8+ T cell function (Fig. 6A). Compromising CD8+ T cell function increased the reactivation frequency in cultures of TG from anti-IL-10R treated mice, although TG from a few treated mice showed no evidence of reactivation even when CD8+ T cell function was compromised in TG cultures (Fig. 6B).
Discussion
The successful design of a therapeutic or prophylactic treatment for HSV infection remains elusive. Recent evidence from several laboratories suggests that strategies aimed at augmenting the TG-resident CD8+ T cell population could potentially reduce the frequency of recurrent disease(8, 14, 21, 23, 31). HSV-1 therapeutic vaccines could be designed to increase the number and function of circulating HSV-specific CD8+ T cells, but clinical trials of HSV vaccines to date have been restricted to genital infections and have established limited efficacy in preventing viral transmission or the frequency of recurrent disease (12, 32, 33). Failure of therapeutic HSV vaccines might be related in part to their focus on increasing antibody titer rather than T cell responses, but a recent study also demonstrated limited if any access of circulating HSV-specific CD8+ T cells to latently infected TG(14). Therefore, alternative approaches to augmenting the TG-resident CD8+ T cell population may be required.
The unusually strong immunodominance of the gB498-505 epitope in C57BL/6 mice is well documented(15, 27, 34). These cells represent 50% of the HSV-1 specific CD8+ T cell repertoire in spleens and infected TG. The remaining 50% of HSV-specific CD8+ T cells recognize 18 different subdominant epitopes on 11 HSV-1 proteins (16, 17). Using tetramers containing four of the subdominant epitopes that together represent approximately 30% of the subdominant population, we demonstrate little if any change in the CD8+ T cell repertoire within the memory population that is retained in the TG during viral latency. How the CD8+ T cell dominance hierarchy is maintained during latency is not clear. The apparent isolation of these cells combined with their nearly uniform expression of CD69 suggests that they might represent a tissue resident memory (TRM) population that is maintained by proliferation rather than infiltration (13). Retention of CD8+ T cells in the latently infected TG appears to require antigenic exposure (27), and is less dependent on homeostatic proliferation signals from IL-15 than their counterparts in the spleen (18). Together these findings suggest that the TG-resident CD8+ T cell population is established through a relatively uniform contraction of the effector population present in acutely infected TG (maintaining the dominance hierarchy) and is maintained by proliferation that is antigen driven.
Maintenance of the dominance hierarchy during latency would suggest relatively uniform proliferation of the immunodominant gB498-505-specific and subdominant CD8+ T cells, and by extension uniform exposure to the viral proteins containing the epitopes. However, the subdominant CD8+ T cells in latently infected TG actually divide at a significantly higher rate than the immunodominant cells (Fig. 3). Moreover, IL-10 has been detected in latently infected TG(35), and our current study demonstrates that blocking IL-10 binding to its receptor dramatically increases proliferation of CD8+ T cells specific for subdominant HSV-1 epitopes in latently infected TG, while having minimal if any effect on the immunodominant cells. Therefore, the TG-resident subdominant CD8+ T cells would proliferate at an even higher rate were it not for selective inhibition of their proliferation by IL-10. IL-10 selectively inhibits proliferation of subdominant HSV-specific CD8+ T cells in TG that harbors latent virus and not in the spleen. This finding could indicate selective expression of IL-10R on subdominant CD8+ T cells in the TG, and/or selective regulation of antigen driven, but not homeostatic proliferation of subdominant CD8+ T cells by IL-10.
The elevated rate of proliferation of subdominant CD8+ T cells in latently infected TG would be consistent with increased antigenic exposure. The fact that they divide at a higher rate while maintaining a 1:1 ratio with immunodominant cells suggests that the subdominant cells are being pushed to a state of exhaustion and deletion by persistent over exposure to antigen. Indeed, the subdominant CD8+ T cells express a higher level of programed death 1 (PD-1), a molecule that is expressed on exhausted CD8+ T cells in chronic viral infection models. Moreover, PD-1 interaction with its ligand PD-L1 in latently infected TG results in a significantly enhanced level of apoptosis in subdominant CD8+ T cells, maintaining their constant frequency in spite of more rapid cell division(36).
We now show that subdominant CD8+ T cells exhibit reduced functionality during latency whereas their immunodominant counterparts retain full functionality. This observation is also consistent with subdominant CD8+ T cells selectively becoming functionally exhausted during latency. The functional exhaustion of subdominant CD8+ T cells is characterized by reduced expression of GrzB, reduced lytic granule release, reduced cytokine production, and reduced frequency of multifunctional cells capable of producing IFN-γ, TNF-α, and releasing lytic granules. Blocking binding of IL-10 to its receptor during latency appeared to reverse exhaustion as indicated by a significant increase in the number of Grzb positive and multifunctional cells. Similar observations have been made in mice with chronic viral infections, where it was proposed that anti-IL-10R treatment rescued the function of exhausted virus specific CD8+ T cells. However, here we show that anti-IL-10 treatment increases the number, but not the frequency of functional subdominant cells. The implication of our finding is that IL-10 is not causing functional compromise, but rather inhibiting proliferation of both functionally exhausted and functional subdominant CD8+ T cells in latently infected TG. Thus, subdominant CD8+ T cells in latently infected TG, presumably due to overexposure to antigen undergo sequential changes characterized by initial up-regulation of IL-10R, followed by functional impairment, and finally deletion.
During chronic lymphocytic choriomeningitis virus (LCMV) infections, CD8+ T cells specific for immunodominant epitopes become preferentially exhausted, presumably due in part to their higher TCR affinity. In our HSV-1 model, epitope affinity for MHC and TCR do not seem to significantly influence immunodominance, since similar MHC I and TCR affinities are observed for the strongly immunodominant gB498-505 epitope and the subdominant RR1982 epitope (16). Moreover, in HSV-1 latently infected TG gB498-505-specific CD8+ T cells remain fully functional, while the subdominant CD8+ T cells exhibit partially functional exhaustion. While several factors might influence the susceptibility of subdominant CD8+ T cells to exhaustion, perhaps the simplest would be that HSV-1 gB is expressed at a level that maintains a low rate of proliferation and optimal function of the immunodominant cells, whereas the viral proteins containing subdominant epitopes are expressed at a higher level, driving subdominant CD8+ T cells to enhanced proliferation and ultimately exhaustion. This would be consistent with our observation that functional exhaustion is limited to HSV-specific subdominant CD8+ T cells in the TG that harbors latent HSV-1, and not their counterparts in the spleen where there is no antigenic exposure.
While a role for CD8+ T cells in maintaining HSV-1 latency is suggested by several recent studies, the involvement of CD4+ T cells remains largely unexplored despite the fact that they represent at least half of the T cells in latently infected TG (Fig. 2). One could argue that the exclusive retention of latent HSV-1 in neurons that are not known to express MHC class II (required for antigen recognition by CD4+ T cells) would preclude CD4+ T cells from responding to latent virus. However, our current data demonstrate production of IL-10 by 16.4% of CD4+ T cells in latently infected mice. This observation demonstrates that CD4+ T cells are persistently stimulated in latently infected TG, and contribute to the maintenance of the dominance hierarchy of TG-resident CD8+ T cells. The phenotype of IL-10 producing CD4+ T cells remains to be determined. A likely candidate would be FoxP3+ Tregs, but the harsh fixation and permeabilization procedures necessary to detect FoxP3 via flow cytometry, precluded concurrent assessment of IL-10eGFP and FoxP3. However, our previous study (18)demonstrated that only 4% TG-resident CD4+ T cells express FoxP3, suggesting that a majority of the IL-10 producing CD4+ T cells do not express FoxP3. Moreover, IL-10-TRegs that do not express FoxP3 have been shown to prevent the proliferation of activated T cells similar to the observations seen in our study (37). Our findings encourage the exploration of a possible direct role for TG-resident CD4+ T cells in controlling HSV-1 latency. In addition to defining the phenotype of these cells, it will also be of interest to determine if these CD4+ T cells are stimulated by HSV-1 antigens, and if antigen presentation is directly on neurons or on other antigen presenting cells within the latently infected TG.
The gB498-505-specific CD8+ T cells in latently infected TG can block HSV-1 reactivation from latency through IFN-γ production and release of lytic granules containing GrzB(21, 22, 38). The capacity of TG-resident CD8+ T cells specific for subdominant epitopes to similarly prevent HSV-1 reactivation has not been established. The in vivo anti-IL-10R treatment increased the number of subdominant CD8+ T cells in the TG that loaded GrzB into their lytic granules in vivo and produce IFN-γ when stimulated directly ex vivo without significantly affecting the number or function of the gB498-505-specific cells. The increased number of functional subdominant CD8+ T cells persisted for at least 3 weeks after anti-IL-10R treatment was terminated.
We predicted that the expanded number of TG resident subdominant CD8+ T cells in anti-IL-10R treated mice would enhance T cell-mediated protection from HSV-1 reactivation in ex vivo TG cultures. Indeed, there was a significant reduction of reactivation in cultures of TG from anti-IL-10R treated mice. All of the TG from control mice reactivated the virus as expected since in our hands latently infected TG always reactivate the virus to some degree. In contrast, nearly half of TG from anti-IL-10R treated mice failed to reactivate the virus when CD8+ T cell function was intact in ex vivo TG cultures. The increased protection from reactivation was mediated by CD8+ T cells, as it was eliminated when anti-CD8α mAb was added to cultures. Since the functionality of the TG-resident immunodominant gB498-505-specific CD8+ T cells was unchanged by anti-IL-10R treatment and their numbers slightly declined, it is highly unlikely that the increased protection was mediated by augmentation of the immunodominant CD8+ T cell response in the TG. In contrast, anti-IL-10R treatment significantly increased the number of functional TG-resident subdominant CD8+ T cells in the TG. These findings suggest that the reduced reactivation frequency observed in TG from anti-IL-10-treated mice reflected a normal contribution of immunodominant CD8+ T cells combined with an augmented response of subdominant CD8+ T cells. Interestingly, some TG from anti IL-10R treated mice failed to reactivate the virus even when anti-CD8 mAb was added to the ex vivo cultures. It is possible that in vivo anti-IL-10R treatment led to emergence of cells other than CD8+ T cells that can block HSV-1 reactivation in ex vivo TG cultures. Alternatively, in vivo interaction with increased numbers of subdominant CD8+ T cells might have resulted in a more repressed viral genome or elimination of neurons that would normally reactivate the virus in ex vivo TG cultures. These exciting possibilities are being explored.
Our studies suggest that transiently blocking IL-10 signaling might be a useful adjunct to antiviral therapy in controlling recurrent herpetic disease. However, herpes stromal keratitis is a potentially blinding immunopathological process that is reportedly exacerbated by IL-10 neutralization(39–42). Additionally, IL-10 neutralization can exacerbate pathology in mouse models of inflammatory bowel disorder(43). Nonetheless, 3 weeks of anti-IL-10R treatment did not result in any observable corneal pathology or signs of inflammatory bowel disease in mice. Moreover, our treatment regimen resulted in complete protection from viral reactivation in up to 50% of TGs, even under conditions of extreme stress resulting from neuronal axotomy, transient separation from CD8+ T cells and culturing in vitro. Therefore, short-term interference with IL-10 signaling could effectively inhibit HSV-1 reactivation and shedding in vivo, thus obviating concern about the exacerbation of subsequent pathology induced by the virus.
Acknowledgments
We would like to thank Nancy Zurowski for flow cytometry acquisition and the National Institue of Allergy and Infectious Diseases Tetramer Core Facility (Emory University Vaccine Center, Atlanta, GA) for supplying tetramers.
Footnotes
This work was supported by National Institutes of Health grants P30-EY08098 (R.L.H.), R01-EY005945 (R.L.H.), T32-EY017271 (A.J.S.), an unrestricted grant from Research to Prevent Blindness (New York, NY), the Eye and Ear Foundation of Pittsburgh
References
- 1.Liesegang TJMD. Herpes Simplex Virus Epidemiology and Ocular Importance. Cornea. 2001;20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Kubat NJ, Tran RK, McAnany P, Bloom DC. Specific histone tail modification and not DNA methylation is a determinant of herpes simplex virus type 1 latent gene expression. J Virol. 2004;78:1139–1149. doi: 10.1128/JVI.78.3.1139-1149.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kubat NJ, Amelio AL, Giordani NV, Bloom DC. The herpes simplex virus type 1 latency-associated transcript (LAT) enhancer/rcr is hyperacetylated during latency independently of LAT transcription. J Virol. 2004;78:12508–12518. doi: 10.1128/JVI.78.22.12508-12518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloom DC, Giordani NV, Kwiatkowski DL. Epigenetic regulation of latent HSV-1 gene expression. Biochimica et biophysica acta. 2010;1799:246–256. doi: 10.1016/j.bbagrm.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amelio AL, Giordani NV, Kubat NJ, O’Neil JE, Bloom DC. Deacetylation of the Herpes Simplex Virus Type 1 Latency-Associated Transcript (LAT) Enhancer and a Decrease in LAT Abundance Precede an Increase in ICP0 Transcriptional Permissiveness at Early Times Postexplant. Journal of Virology. 2006;80:2063–2068. doi: 10.1128/JVI.80.4.2063-2068.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verjans GMGM, Hintzen RQ, van Dun JM, Poot A, Milikan JC, Laman JD, Langerak AW, Kinchington PR, Osterhaus ADME. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proceedings of the National Academy of Sciences. 2007;104:3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theil D, Derfuss T, Paripovic I, Herberger S, Meinl E, Schueler O, Strupp M, Arbusow V, Brandt T. Latent Herpesvirus Infection in Human Trigeminal Ganglia Causes Chronic Immune Response. Am J Pathol. 2003;163:2179–2184. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes Simplex Virus-Specific Memory CD8+ T Cells Are Selectively Activated and Retained in Latently Infected Sensory Ganglia. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lekstrom-Himes JA, Pesnicak L, Straus SE. The Quantity of Latent Viral DNA Correlates with the Relative Rates at Which Herpes Simplex Virus Types 1 and 2 Cause Recurrent Genital Herpes Outbreaks. J Virol. 1998;72:2760–2764. doi: 10.1128/jvi.72.4.2760-2764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoshino Y, Pesnicak L, Cohen JI, Straus SE. Rates of Reactivation of Latent Herpes Simplex Virus from Mouse Trigeminal Ganglia Ex Vivo Correlate Directly with Viral Load and Inversely with Number of Infiltrating CD8+ T Cells. J Virol. 2007;81:8157–8164. doi: 10.1128/JVI.00474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koelle DM, Corey L. Herpes Simplex: Insights on Pathogenesis and Possible Vaccines. Annual Review of Medicine. 2008;59:381–395. doi: 10.1146/annurev.med.59.061606.095540. [DOI] [PubMed] [Google Scholar]
- 12.Koelle DM, Corey L. Recent Progress in Herpes Simplex Virus Immunobiology and Vaccine Research. Clin Microbiol Rev. 2003;16:96–113. doi: 10.1128/CMR.16.1.96-113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proceedings of the National Academy of Sciences. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Himmelein S, St Leger A, Knickelbein J, Rowe A, Freeman M, Hendricks R. Circulating herpes simplex type 1 (HSV-1)-specific CD8+ T cells do not access HSV-1 latently infected trigeminal ganglia. Herpesviridae. 2011;2:5. doi: 10.1186/2042-4280-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace ME, Keating R, Heath WR, Carbone FR. The Cytotoxic T-Cell Response to Herpes Simplex Virus Type 1 Infection of C57BL/6 Mice Is Almost Entirely Directed against a Single Immunodominant Determinant. J Virol. 1999;73:7619–7626. doi: 10.1128/jvi.73.9.7619-7626.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.StLeger AJ, Peters B, Sidney J, Sette A, Hendricks RL. Defining the Herpes Simplex Virus-Specific CD8+ T Cell Repertoire in C57BL/6 Mice. The Journal of Immunology. 2011;186:3927–3933. doi: 10.4049/jimmunol.1003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvucci L, Bonneau R, Tevethia S. Polymorphism within the herpes simplex virus (HSV) ribonucleotide reductase large subunit (ICP6) confers type specificity for recognition by HSV type 1-specific cytotoxic T lymphocytes. J Virol. 1995;69:1122–1131. doi: 10.1128/jvi.69.2.1122-1131.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheridan BS, Khanna KM, Frank GM, Hendricks RL. Latent Virus Influences the Generation and Maintenance of CD8+ T Cell Memory. The Journal of Immunology. 2006;177:8356–8364. doi: 10.4049/jimmunol.177.12.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frank GM, Lepisto AJ, Freeman ML, Sheridan BS, Cherpes TL, Hendricks RL. Early CD4+ T Cell Help Prevents Partial CD8+ T Cell Exhaustion and Promotes Maintenance of Herpes Simplex Virus 1 Latency. J Immunol. 2010;184:277–286. doi: 10.4049/jimmunol.0902373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackay LK, Wakim L, van Vliet CJ, Jones CM, Mueller SN, Bannard O, Fearon DT, Heath WR, Carbone FR. Maintenance of T Cell Function in the Face of Chronic Antigen Stimulation and Repeated Reactivation for a Latent Virus Infection. The Journal of Immunology. 2012;188:2173–2178. doi: 10.4049/jimmunol.1102719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. Cd8+ T Cells Can Block Herpes Simplex Virus Type 1 (HSV-1) Reactivation from Latency in Sensory Neurons. The Journal of Experimental Medicine. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Decman V, Kinchington PR, Harvey SAK, Hendricks RL. Gamma Interferon Can Block Herpes Simplex Virus Type 1 Reactivation from Latency, Even in the Presence of Late Gene Expression. J Virol. 2005;79:10339–10347. doi: 10.1128/JVI.79.16.10339-10347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic Lytic Granule-Mediated CD8+ T Cell Inhibition of HSV-1 Reactivation from Neuronal Latency. Science. 2008;322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MBA. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brooks DG, Walsh KB, Elsaesser H, Oldstone MBA. IL-10 directly suppresses CD4 but not CD8 T cell effector and memory responses following acute viral infection. Proceedings of the National Academy of Sciences. 2010;107:3018–3023. doi: 10.1073/pnas.0914500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MB. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. The Journal of experimental medicine. 2008;205:533–541. doi: 10.1084/jem.20071948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheridan BS, Cherpes TL, Urban J, Kalinski P, Hendricks RL. Reevaluating the CD8 T-Cell Response to Herpes Simplex Virus Type 1: Involvement of CD8 T Cells Reactive to Subdominant Epitopes. J Virol. 2009;83:2237–2245. doi: 10.1128/JVI.01699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brooks DG, Ha SJ, Elsaesser H, Sharpe AH, Freeman GJ, Oldstone MBA. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proceedings of the National Academy of Sciences. 2008;105:20428–20433. doi: 10.1073/pnas.0811139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones M, Ladell K, Wynn KK, Stacey MA, Quigley MF, Gostick E, Price DA, Humphreys IR. IL-10 Restricts Memory T Cell Inflation during Cytomegalovirus Infection. The Journal of Immunology. 2010;185:3583–3592. doi: 10.4049/jimmunol.1001535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng CT, Oldstone MBA. Infected CD8α dendritic cells are the predominant source of IL-10 during establishment of persistent viral infection. Proceedings of the National Academy of Sciences. 2012;109:14116–14121. doi: 10.1073/pnas.1211910109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. Painful Failure of Promising Genital Herpes Vaccine. Science. 2010;330:304. doi: 10.1126/science.330.6002.304. [DOI] [PubMed] [Google Scholar]
- 33.Nesburn A, Ghiasi H, Wechsler S. Ocular safety and efficacy of an HSV-1 gD vaccine during primary and latent infection. Invest Ophthalmol Vis Sci. 1990;31:1497–1502. [PubMed] [Google Scholar]
- 34.Stock AT, Jones CM, Heath WR, Carbone FR. CTL response compensation for the loss of an immunodominant class I-restricted HSV-1 determinant. Immunol Cell Biol. 2006;84:543–550. doi: 10.1111/j.1440-1711.2006.01469.x. [DOI] [PubMed] [Google Scholar]
- 35.Halford WP, Gebhardt BM, Carr DJ. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J Immunol. 1996;157:3542–3549. [PubMed] [Google Scholar]
- 36.Jeon S, St Leger TJ, Cherpes TL, Sheridan BS, Hendricks RL. PD-L1/B7-H1 regulates the survival, but not the function of CD8+ T cells in HSV-1 latently infected Trigeminal Ganglia. J Immunol. 2013 doi: 10.4049/jimmunol.1300582. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vieira PL, Christensen JR, Minaee S, O’Neill EJ, Barrat FJ, Boonstra A, Barthlott T, Stockinger B, Wraith DC, O’Garra A. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. Journal of immunology. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 38.Knickelbein J, Khanna K, Yee M, Baty C, Kinchington P, Hendricks R. Noncytotoxic lytic granule-mediated CD8+ Tcell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daheshia M, Kuklin N, Kanangat S, Manickan E, Rouse BT. Suppression of ongoing ocular inflammatory disease by topical administration of plasmid DNA encoding IL-10. Journal of immunology. 1997;159:1945–1952. [PubMed] [Google Scholar]
- 40.Tumpey TM, V, Elner M, Chen SH, Oakes JE, Lausch RN. Interleukin-10 treatment can suppress stromal keratitis induced by herpes simplex virus type 1. Journal of immunology. 1994;153:2258–2265. [PubMed] [Google Scholar]
- 41.Keadle TL, Stuart PM. Interleukin-10 (IL-10) ameliorates corneal disease in a mouse model of recurrent herpetic keratitis. Microbial pathogenesis. 2005;38:13–21. doi: 10.1016/j.micpath.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Rajasagi NK, Reddy PB, Suryawanshi A, Mulik S, Gjorstrup P, Rouse BT. Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1. J Immunol. 2011;186:1735–1746. doi: 10.4049/jimmunol.1003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, Powrie F, Cheever AW, Maloy KJ, Sher A. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]