Abstract
Objective
Acute lung injury (ALI) with subsequent pneumonia and sepsis represents a major cause of morbidity and mortality in thermally injured patients. Production of nitric oxide (NO) by the neuronal and inducible NO synthase (nNOS, iNOS) may be critically involved in the pathophysiology of the disease process at different time points and thus specific inhibition at different times may represent an effective treatment regimen.
Design
Prospective, controlled, randomized trial.
Setting
University research laboratory.
Subjects
Eighteen chronically instrumented, adult, female sheep.
Interventions
Following baseline measurements, the animals were allocated to either sham-injured, non-treated controls (sham), injured, non-treated controls (control), or injured animals treated with continuous infusion of 7-nitroindazole, a specific nNOS inhibitor, during the first 12 hrs post-injury and infusion of BBS-2, a specific iNOS inhibitor, during the next 12 hrs. Injury was induced by 48 breaths of cotton smoke and subsequent instillation of Ps. aeruginosa into the lungs. All sheep were mechanically ventilated and fluid resuscitated for the entire duration of the 24-hour experiment.
Measurements and Main Results
The injury induced severe pulmonary dysfunction which was associated with increases in lung edema formation, airway obstruction, and vascular endothelial growth factor (VEGF), 3-nitrotyrosine (3-NT), and poly(ADP ribose) (PAR) expression in lung tissue. The treatment reduced the degree of airway obstruction and improved pulmonary gas exchange, whereas the development of lung edema was not affected. The increases in lung tissue VEGF, 3-NT, and PAR expression were attenuated by the treatment.
Conclusions
The combination of early nNOS and delayed iNOS inhibition shows potential benefit in ovine ALI by reducing nitrosative stress in the lung and limiting the degree of airway obstruction.
Keywords: 3-nitrotyrosine, peroxynitrite, poly(ADP ribose), smoke inhalation, vascular endothelial growth factor
Introduction
Acute lung injury (ALI) by smoke inhalation trauma represents a major cause of morbidity and mortality among thermally injured patients (1). The pathophysiological changes following smoke inhalation injury are characterized by increases in airway blood flow, pulmonary microvascular permeability and inflammatory cell influx, together leading to a fluid shift from the intravascular to the interstitial space of the lung, ultimately resulting in pulmonary edema formation and severe impairment of respiratory gas exchange (2-8). These changes are associated with the occurrence of distinct airway obstruction and increases in ventilatory pressures (9). When ALI is complicated by subsequent bacterial infection, pneumonia and sepsis, mortality rates further increase by 40% (10); and the pathophysiological reactions of the lung are more pronounced than with smoke inhalation injury alone, causing more severe histological changes associated with early and fulminant perturbations of pulmonary gas exchange (11).
Nitric oxide (NO) is generated from L-arginine by a family of enzymes called NO synthases (NOS). Three different forms of NOS are presently known in mammals (12): the constitutively expressed endothelial NOS (eNOS) and neuronal NOS (nNOS), and the inducible NOS (iNOS). It has been generally believed that constitutively produced NO is involved in various regulatory, physiological processes including neurotransmission and the regulation of vascular tone and blood flow, whereas excessive NO production by iNOS is critically involved in the pathophysiology of several diseases such as ALI and sepsis (13, 14). However, recent evidence suggests that not only iNOS-, but also nNOS-derived NO may play a detrimental role in the pathogenesis of various diseases including ALI (15-17). Significantly, the results from our previous experiments and other studies in models of sepsis and ALI suggest that inhibition of different NOS isoforms may attenuate the disease process at different time points post-injury (15, 18, 19).
Our hypothesis for the present study was that NO production from nNOS is mainly involved in the early response to ALI and instillation of bacteria into the lungs in our model while iNOS-derived NO is responsible during the later phase of the disease process. To test this hypothesis, we investigated the effects of continuous infusion of a specific nNOS inhibitor during the initial phase post-injury and subsequent continuous infusion of a specific iNOS inhibitor on pulmonary morbidity of sheep subjected to smoke inhalation injury and bacterial challenge.
Materials and Methods
This study was approved by the Animal Care and Use Committee of the University of Texas Medical Branch and conducted in compliance with the guidelines of the National Institutes of Health and the American Physiological Society for the care and use of laboratory animals.
Animal model
The ovine model of ALI induced by smoke inhalation and instillation of live Pseudomonas aeruginosa into the lungs has been previously described in detail (11, 20). In brief, 18 adult female sheep (body weight, 32±2 kg) were surgically prepared for chronic study with a femoral artery catheter, a thermodilution catheter, and a left atrial catheter. After a recovery period of 5-7 days, the animals received inhalation injury with 48 breaths of cotton smoke (<40°C) using a modified bee smoker. Afterwards, a stock solution of live Pseudomonas aeruginosa (2-5 × 1011 colony-forming units, from a burn patient at Brooke Army Medical Center; San Antonio, TX) suspended in 30 mL of 0.9 % NaCl solution was instilled into the right middle and lower lobe and left lower lobe of the lung (10 mL each). Anesthesia was then discontinued, and the sheep were allowed to awaken. The 18 animals were randomly allocated to the following three study groups:
Sham: Sham-injured, non-treated animals (n=6).
Control: Injured, non-treated animals (n=6).
Treatment: Injured animals (n=6) treated with 7-nitroindazole (1 mg·kg−1·h−1; Sigma-Aldrich, St. Louis, MO) (15) from 1-12 hours post-injury and with BBS-2 (100 μg·kg−1·h−1; Berlex, Richmond, CA) (18) from 12-24 hours post-injury.
All sheep were mechanically ventilated (Servo Ventilator 900C, Siemens; Elema, Sweden) with a tidal volume of 12-15 mL/kg and a positive end expiratory pressure of 5 mmHg during the study period of 24 hours. The respiratory rate was initially set at 20 breaths/min and adjusted according to blood gas analysis. All animals were fluid resuscitated, initially started with an infusion rate of 2 mL·kg−1·h−1 lactated Ringer's solution and adjusted to maintain hematocrit at baseline values ± 3. During the study period, all animals had free access to food, but not water. After completion of the experiment, the animals were deeply anesthetized with ketamine and xylazine and sacrificed by intravenous injection of saturated potassium choride.
Measurements
Mean pulmonary arterial pressure, pulmonary capillary wedge pressure, and left atrial pressure were measured using pressure transducers (model PX3X3, Baxter Edwards Critical Care Division) which were connected to a hemodynamic monitor (model 7830A, Hewlett Packard; Santa Clara, CA). Pulmonary capillary pressure was calculated by a standard equation. Blood gases were measured using a blood gas analyzer (Synthesis 15, Instrumentation Laboratories; Lexington, MA). Tracheal blood flow was measured by injection of colored microspheres (Interactive Medical Technologies, Los Angeles, CA) as described previously (20).
Lung histopathology
Immediately after death, the right lung was removed and a 1-cm thick section was taken from the middle of the lower lobe and inflated with 10% formalin for histological examination. Fixed samples were embedded in paraffin, sectioned into 4-µm pieces and stained with hematoxylin-eosin. A pathologist who was unaware of the group assignment analyzed the histological changes as previously described (9, 11). Twenty-four areas of lung parenchyma in each animal were observed at ×10 objective magnification and graded on a scale of 0-4 (0: absent, appears normal; 1: light; 2: moderate, 3: strong; 4: intense) for congestion, septal edema, alveolar edema, septal inflammation, and hemorrhage. Airway obstruction was evaluated at the same time when the injury score was assigned. For each cross-sectioned airway, a score of 0–100% was made as an estimate of the degree of luminal obstruction. Each airway was classified as a bronchus or a bronchiole (9). From the remaining half of the right lower lobe, bloodless lung wet/dry weight ratio was calculated as an index of lung water content
Immunoblotting
Lung tissue was rinsed twice with ice-cold PBS. The tissue was mechanically sheared, washed three times in ice cold PBS, then lysed in a buffer containing 50mM Tris (pH 7.4), 150 mM NaCl2, 1mM EDTA, 1mM EGTA, 1 mM NaF, 1 mM sodium orthovanadate, 1% Triton X-100, 1 mM PMSF, and supplemented with a protease inhibitor cocktail. Cell lysates were centrifuged at 14000×g (4°C) for 10 min. Protein concentrations were normalized using the Bio-Rad Protein Assay. Cell lysates containing 20 μg of protein were separated by a 4-20% linear gradient SDS-PAGE followed by electrophoretic transfer onto nitrocellulose membranes for 1.5 h at 120 mA. Membranes were pre-blocked with 5% milk in TTBS (150mM NaCl, 25mM KCl, 250mM Tris, pH 7.5, and 0.5% Tween-20) for 1 h at room temperature, and then incubated with primary antibodies overnight at 4°C. After incubation with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature, the bands were visualized by enhanced SuperSignal West Pico Chemiluminescent Substrate chemiluminescence system. Membranes were stripped and re-probed with anti-actin, which was used as a sample loading control. Blots were quantified by NIH IMAGE J scanning densitometry, and normalized to total actin expression.
Malondialdehyde (MDA) formation
Lung tissue MDA, an index of lipid peroxidation, was quantified with a commercially available assay (Northwest Life Science Specialties; Vancouver, WA) and expressed as MDA/ mg protein, measured by a commercially available assay (Fluka BioChemika; Buchs, Switzerland).
Myeloperoxidase (MPO) activity
The activity of MPO, an index of neutrophil accumulation, was evaluated in homogenized lung tissue using a commercially available assay (CytoStore; Calgary, AB, Canada). MPO activity was normalized to lung wet/dry weight ratio and reported as U/ g dry tissue.
Statistical analysis
All values are expressed as means±SEM. After confirming normal distribution (Kolmogorov-Smirnov test), a two-way analysis of variance for repeated measurements with appropriate Student-Newman-Keuls post hoc comparisons to compare differences within and between groups. A value of p<.05 was regarded as statistically significant.
Results
Respiratory gas exchange and ventilatory pressures
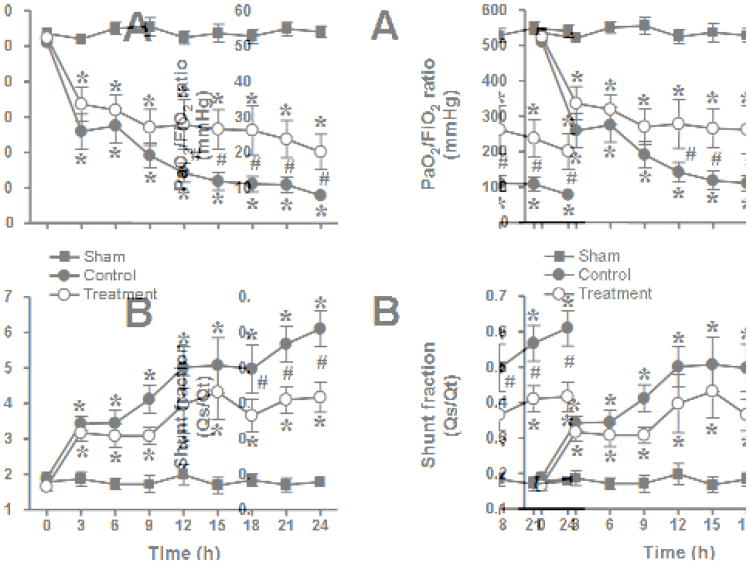
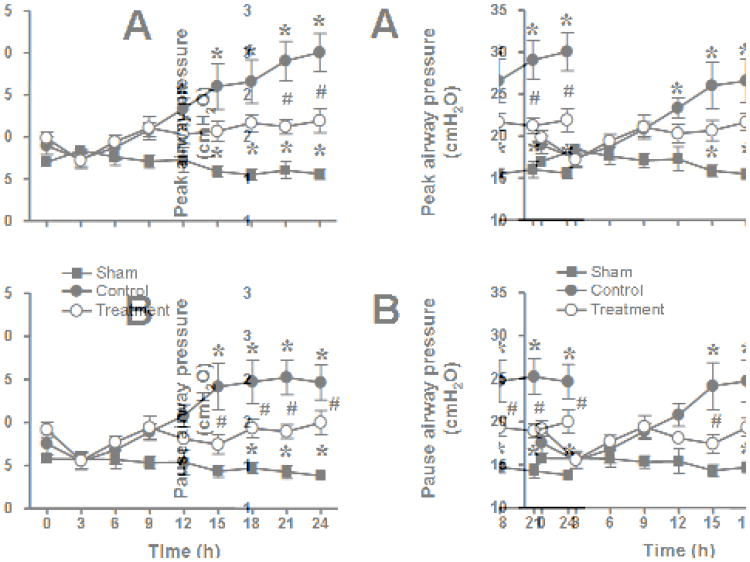
The injury was associated with a severe impairment in respiratory gas exchange as indicated by a decline in PaO2/FiO2 ratio below 200 mmHg and a concurrent increase in pulmonary shunt fraction in untreated control animals. In the treatment group, the PaO2/FiO2 ratio remained above 200 mmHg during the entire study period and was significantly higher than in the control group from 12-24 hours. Pulmonary shunt fraction was significantly lower in treated than in control animals from 18-24 hours. Pulmonary gas exchange remained stable in sham animals (Fig. 1). The injury was further associated with marked increases in ventilatory pressures which were significantly attenuated by the treatment (Fig. 2). There were no significant differences in pulmonary hemodynamics, calculated pulmonary vascular resistance, cardiac filling pressures, and cardiac output between groups (Table 1).
Fig. 1.
Impact of combined neuronal and inducible nitric oxide synthase inhibitor treatment on PaO2/FiO2 ratio (A) and pulmonary shunt fraction (Qs/Qt, B) in sheep with acute lung injury (n=6 per group). *p<.05 vs. sham; #p<.05 control vs. treatment.
Fig. 2.
Impact of combined neuronal and inducible nitric oxide synthase inhibitor treatment on ventilatory pressures (A, peak airway pressure and B, pause airway pressure) in sheep with acute lung injury (n=6 per group). *p<.05 vs. sham; #p<.05 control vs. treatment.
Table 1.
Pulmonary hemodynamics, filling pressures, cardiac output, and tracheal blood flow.
| Time after injury | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| 0 hrs | 3 hrs | 6 hrs | 12 hrs | 18 hrs | 24 hrs | |
| MPAP, mmHg | ||||||
| Sham | 20 ± 1 | 25 ± 2 | 27 ± 1 | 28 ± 2 | 27 ± 2 | 24 ± 2 |
| Control | 20 ± 0 | 23 ± 1 | 24 ± 1 | 28 ± 0 | 29 ± 2 | 27 ± 2 |
| Treatment | 20 ± 0 | 24 ± 1 | 27 ± 1 | 28 ± 2 | 27 ± 1 | 28 ± 1 |
| PVR, mmHg | ||||||
| Sham | 154 ± 8 | 195 ± 29 | 197 ± 20 | 252 ± 42 | 248 ± 31 | 223 ± 31 |
| Control | 166 ± 7 | 176 ± 19 | 137 ± 23 | 168 ± 18a | 163 ± 29a | 118 ± 11a |
| Treatment | 174 ± 15 | 146 ± 11 | 165 ± 11 | 170 ± 29a | 157 ± 25a | 159 ± 23a |
| PCWP, mmHg | ||||||
| Sham | 11 ± 1 | 14 ± 1 | 16 ± 1 | 16 ± 1 | 15 ± 2 | 14 ± 2 |
| Control | 10 ± 0 | 13 ± 1 | 14 ± 1 | 16 ± 1 | 16 ± 1 | 17 ± 2 |
| Treatment | 10 ± 1 | 14 ± 1 | 15 ± 1 | 16 ± 1 | 14 ± 1 | 16 ± 1 |
| CVP, mmHg | ||||||
| Sham | 7 ± 1 | 11 ±2 | 10 ± 2 | 12 ± 2 | 11 ± 1 | 10 ± 1 |
| Control | 5 ± 0 | 7 ± 1 | 7 ± 1 | 11 ± 1 | 13 ± 1 | 14 ± 2 |
| Treatment | 6 ± 1 | 8 ± 0 | 10 ± 1 | 11 ± 1 | 13 ± 2 | 13 ± 2 |
| LAP, mmHg | ||||||
| Sham | 8 ± 1 | 11 ± 1 | 12 ± 1 | 11 ± 2 | 12 ± 1 | 11 ± 1 |
| Control | 7 ± 1 | 9 ± 1 | 11 ± 0 | 15 ± 1a | 16 ± 1 | 17 ± 2a |
| Treatment | 7 ± 1 | 10 ± 1 | 11 ± 1 | 13 ± 2 | 14 ± 2 | 15 ± 2a |
| CO, mmHg | ||||||
| Sham | 4.6 ± 0.2 | 4.8 ± 0.4 | 4.4 ± 0.3 | 4.3 ± 0.3 | 4.0 ± 0.2 | 4.0 ± 0.2 |
| Control | 4.6 ± 0.2 | 4.8 ± 0.5 | 5.6 ± 0.4 | 5.6 ± 0.5 | 6.9 ± 0.7a | 6.8 ± 0.5a |
| Treatment | 4.6 ± 0.5 | 5.7 ± 0.5 | 6.0 ± 0.8 | 6.6 ± 0.8a | 7.3 ± 0.7a | 6.7 ± 1.0a |
| TBF, mL·min−1·g−1 | ||||||
| Sham | 0.27 ± 0.04 | 0.32 ± 0.07 | 0.20 ± 0.01 | 0.23 ± 0.02 | 0.17 ± 0.02 | 0.25 ± 0.02 |
| Control | 0.19 ± 0.04 | 1.42 ± 0.37a | 1.66 ± 0.48a | 1.38 ± 0.30a | 0.98 ± 0.24a | 0.85 ± 0.25a |
| Treatment | 0.18 ± 0.02 | 1.72 ± 0.19a | 2.08 ± 0.17a | 1.68 ± 0.23a | 1.14 ± 0.23a | 1.24 ± 0.22a |
CO, cardiac output; CVP, central venous pressure; LAP, left atrial pressure; MPAP, mean pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; TBF, tracheal blood flow.
p<.05 vs. sham;
p<.05 control vs. treatment.
Tracheal blood flow and lung water content
The time changes in tracheal blood flow are shown in Table 1. In the control group, tracheal blood flow dramatically increased after the injury. However, this increase was not affected by the combination treatment with 7-NI and BBS-2. The injury-related increase in lung wet/dry weight ratio, a marker of lung water content, was not significantly attenuated by the treatment (sham 3.5±0.2, control 6.2±1.0, treatment 5.3±0.5; p<.05 sham vs. control and treatment).
Lung histopathology and airway obstruction
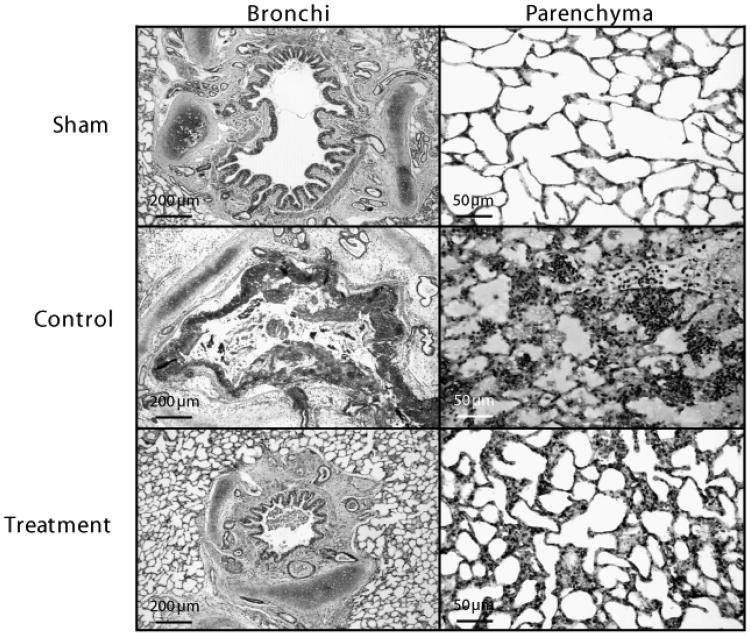
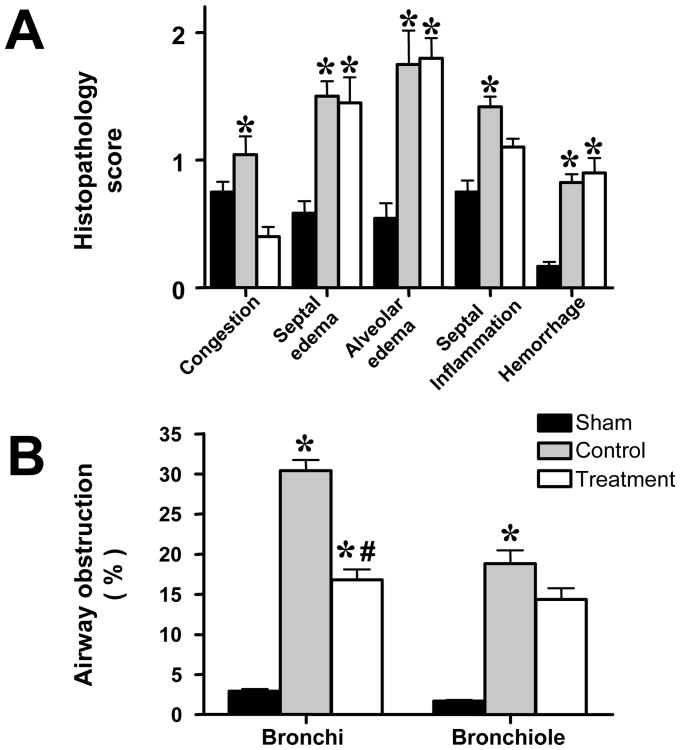
All injured animals showed severe epithelial damage and formation of obstructive material within the airways consisting of neutrophils, cellular debris, fibrin, and mucus (Fig. 3). The histopathology and airway obstruction scores are summarized in Fig. 4. Histology and obstruction scores were significantly increased by the injury. The combination treatment attenuated the increases in congestion and inflammation as well as bronchi and bronchiole obstruction scores.
Fig. 3.
Representative light micrographs of hematoxylin-eosin-stained tissue showing bronchi (×50) and lung parenchyma (×200) of sheep in the sham, control, and treatment group.
Fig. 4.
Impact of combined neuronal and inducible nitric oxide synthase inhibitor treatment on histopathology scores (A) and airway obstruction scores (B) in sheep with acute lung injury (n=6 per group). *p<.05 vs. sham; #p<.05 control vs. treatment.
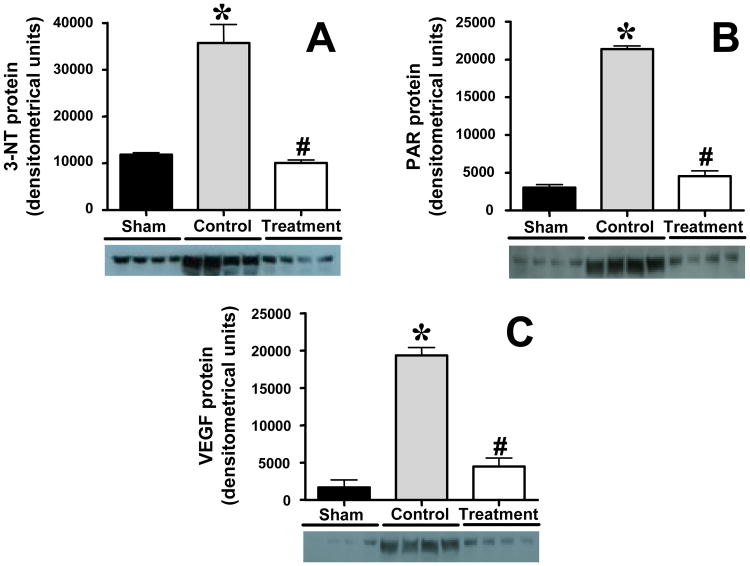
Markers of oxidative/nitrosative stress and VEGF in lung tissue
Immunoblotting in lung homogenates revealed significant increases in 3-nitrotyrosine (3-NT), poly(ADP ribose) (PAR), and vascular endothelial growth factor (VEGF) protein expression in untreated control animals. The expression of 3-NT, PAR, and VEGF was significantly decreased by combined nNOS and iNOS inhibition (Fig. 5). MDA formation was equally increased in control and treated animals after the injury (sham 3.3±0.2, control 4.5±0.2, treatment 4.6±0.2 nmol/mg protein; p<.05 sham vs. control and treatment).
Fig. 5.
Impact of combined neuronal and inducible nitric oxide synthase inhibitor treatment on 3-nitrotyrosine (3-NT), poly(ADP ribose) (PAR), and vascular endothelial growth factor (VEGF) protein expression measured by immunoblotting in sheep with acute lung injury (n=4 per group). *p<.05 vs. sham; #p<.05 control vs. treatment.
MPO in lung tissue
The activity of MPO in lung tissue, an index of neutrophil accumulation, was likewise increased in control and treated animals (sham 8.5±1.8, control 21.3±4.4, treatment 18.3±1.5 U/g dry tissue; p<0.05 sham vs. control and treatment).
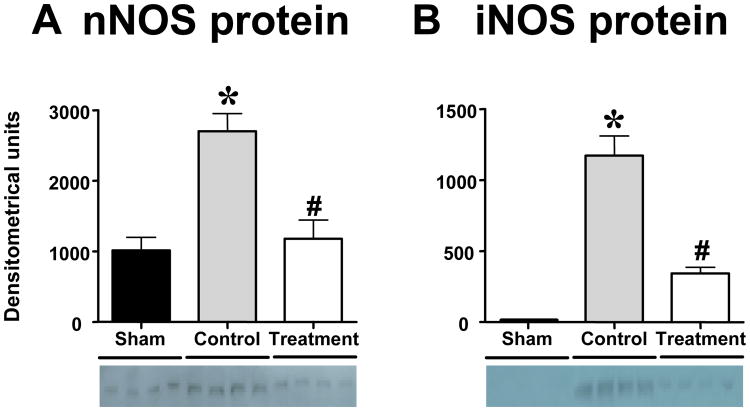
Neuronal and inducible NOS expression in lung tissue
nNOS protein was expressed in lung homogenates of healthy sham animals whereas iNOS protein was not detected. Both nNOS and iNOS protein were significantly increased in control animals. In the treatment group, both nNOS and iNOS expression was significantly lower than in controls (Fig. 6).
Fig. 6.
Impact of the combination treatment on neuronal nitric oxide synthase (nNOS) and inducible nitric oxide synthase (iNOS) protein expression measured by immunoblotting in sheep with acute lung injury (n=4 per group). p<.05 vs. sham; #p<.05 control vs. treatment.
Discussion
The present study demonstrates that a combination therapy consisting of early nNOS and delayed iNOS inhibition reduces markers of nitrosative stress and poly(ADP ribolysation) in lung tissue and limits histological damage and airway obstruction, resulting in partial attenuation of pulmonary dysfunction in sheep with ALI and bacterial challenge.
The role of NOS inhibition in ALI has been previously investigated in the same animal model that is used in the present study. However, sole treatment with different nNOS or iNOS inhibitors was not (21) or only partially (15, 18) effective in preventing pulmonary dysfunction. Non-selective NOS inhibition in humans with septic shock mainly originating from the respiratory tract has even been associated with an increase in 28-mortality in a recent phase III trial (22). On the other hand, several other experimental and clinical studies reported beneficial effects of NOS inhibition on cardiovascular and pulmonary function during sepsis (23-26). These on the first view contradicting results raise the important question whether NOS inhibition during sepsis is beneficial or rather harmful. Notably, it has been suggested that the overproduction of NO in septic and hemorrhagic shock may be due to both the early activation of constitutive NOS and the delayed activation of iNOS (13, 27, 28). It can be speculated that, depending on the type of injury, different NOS isoforms are increasingly expressed and/or activated at different time points during the disease process. While non-selective NOS inhibition or selective NOS inhibition at the wrong time points may be ineffective or even harmful, selective inhibition of different NOS isoforms when they are actually responsible for NO overproduction may effectively reduce the progression of pulmonary dysfunction.
When considering possible treatment strategies, however, it is crucial to know the respective time points when the different NOS isoforms are involved. Unfortunately, only little information on this topic can be found in the literature. As mentioned before, our own research group has previously tested the effects of both continuous nNOS and iNOS inhibition in the same animal model that is used in the present study (15, 18). While sole nNOS inhibition reduced plasma nitrite/nitrate levels and attenuated the decrease in PaO2/FiO2 ratio and the increase in pulmonary shunt fraction more effectively during the first 12 hours post-injury, sole iNOS inhibition proved more effective during the second 12 hours (15, 18). In addition, Okamoto et al. (19) evaluated the role of specific iNOS inhibition at different time points in a rat model of septic ALI following peritonitis. Inhibition of iNOS in this model improved survival only when given 12 hours post-injury, but even increased mortality when administered earlier. Okamoto et al. (19) speculated that the up-regulation of iNOS-derived NO may be a crucial defense mechanism against bacterial challenge in the early phase of sepsis-induced ALI; and early iNOS inhibition may therefore be detrimental. From these studies (15, 18, 19) we concluded that combined NOS inhibition may be most effective when a specific nNOS inhibitor is given during the first 12 hours after ALI followed by specific iNOS inhibition during the remainder of the experiment.
The pathophysiological changes of the lung in response to ALI have been well described in previous publications (3-7). It is believed that excessively produced NO impairs hypoxic pulmonary vasoconstriction and increases the pulmonary arterio-venous shunt (29). Furthermore, excessive NO may exert pro-inflammatory, cytotoxic effects by reacting with superoxide radicals from activated neutrophils, yielding reactive oxygen and nitrogen species such as peroxynitrite. Peroxynitrite may induce lung tissue damage and pulmonary microvascular hyperpermeability by oxidizing and nitrating/nitrosating proteins and lipids (30, 31) and inducing the expression of VEGF, a potent permeability factor (32). In addition, peroxynitrite can induce excessive activation of the nuclear repair enzyme poly(ADP ribose) polymerase (PARP) (33, 34) which in turn may cause ATP depletion and cell damage (34, 35). PARP can likewise activate nuclear factor-kappaB leading to further up-regulation of iNOS as well as chemokines such as interleukin-8 (36, 37). These changes can cause endothelial damage and consequently contribute to cast formation in the airways and pulmonary edema, ultimately resulting in impairments in pulmonary gas exchange. In fact, the present study demonstrates that ALI and instillation of bacteria into the lungs significantly increased indicators of neutrophil accumulation (MPO activity), markers of protein nitration (3-NT) and lipid peroxidation (MDA formation), as well as VEGF and PAR protein expression in lung tissue of untreated control animals. These changes were associated with signs of histological lung damage, increased airway obstruction, pulmonary edema formation, and impaired pulmonary gas exchange.
In sheep treated with combined nNOS and iNOS inhibition, pulmonary shunting was attenuated and the expression of both 3-NT and PAR was significantly reduced towards control animals, suggesting that the peroxynitrite-PARP pathway was effectively blocked by the treatment. As a result, the grade of histological tissue injury, and airway obstruction was reduced and the impairment of pulmonary gas exchange was limited. However, we noticed a discrepancy between the effectively blocked peroxynitrite-PARP pathway and only slightly attenuated histopathology scores, suggesting that other mechanisms may be involved in histological lung damage following ALI in our model such as direct effects of superoxide radicals.
Furthermore, it needs to be pointed out that the effects of combined NOS inhibition on pulmonary function in the current study were not superior to the effects of sole nNOS or iNOS inhibition in previous experiments with the same ovine model (15, 18). This failure may be explained by the fact that the combination treatment in the present study did not reduce the injury-related increase in airway (tracheal) blood flow and the degree of lung water content. In general, the lung water content can be increased by two means: endothelial damage and increases in hydrostatic pressure. While combined NOS inhibition apparently reduced the peroxynitrite, PARP, and VEGF-induced endothelial damage, it may even have increased the hydrostatic pressure in the pulmonary vasculature via vasoconstriction. Consequently, pulmonary edema formation was not significantly attenuated by the treatment. It is possible that the combination of two NOS inhibitors in the current study may have exponentiated the vasoconstrictive effects of both drugs. Unfortunately, we were not able to directly measure hydrostatic pressure into the lung capillaries and vascular resistance. However, mean pulmonary artery pressure and calculated pulmonary vascular resistance were not significantly different between groups. Future studies should be conducted to test if lower doses of combined NOS inhibitors exert beneficial effects with reduced vasoconstriction-related side effects.
Interestingly, we found that both nNOS and iNOS protein expression in lung tissue of treated animals was significantly decreased although the use of NOS inhibitors is expected to result in a decrease in NOS activity rather than NOS expression. In this context, has previously been proposed that nNOS may be a trigger of the pulmonary response the ALI including the up-regulation of iNOS expression (7). It is conceivable that the inhibition of nNOS during the initial phase post-injury inhibited the up-regulation of iNOS expression in the current study.
In contrast to previous experiments (19), this study demonstrated an injury-induced increase in nNOS expression in lung homogenates, possibly indicating that nNOS can also be induced by ALI and bacterial challenge. An alternative explanation is provided by previous studies reporting that circulating neutrophils can express nNOS (38, 39). Therefore, the injury-related increase in neutrophil contents in lung tissue may explain the increase in nNOS protein in the current study. Vice versa, less circulating neutrophils could explain the decrease in nNOS protein in lung homogenates of treated sheep. However, this speculation conflicts with the finding of similar MPO activity in treated and untreated animals and merits further evaluation in future studies.
The present study has several limitations we want to acknowledge. First, only one of innumerable possible combinations of NOS inhibitors has been tested. Future studies will have to clarify if different combinations of NOS inhibitors or administration at different time points (e.g. switching the order of NOS inhibitors) may provide better results. Second, ALI induced by smoke inhalation and subsequent bacterial infection represents only one of many possible causes of ALI. However, the model was chosen because a) this double hit injury reflects a frequent clinical setting in burn patients with smoke inhalation injury (10) and b) it provides the possibility of comparing the current results with the results from previous studies on nNOS and iNOS inhibition using the same animal model (15, 18). Third, the current study design includes a predetermined killing time in order to harvest comparable lung tissue samples. Consequently, it was not possible to obtain survival data. Another limitation of the current study is represented by the fact that histopathology and immunoblotting findings are presented as semiquantitative results. Finally, it would have been favorable to measure NOS activities in lung tissue. For technical reasons, however, this was not possible.
Conclusions
A novel combination treatment consisting of early nNOS and delayed iNOS inhibition shows potential benefit by reducing nitrosative stress in the lung and limiting the degree of airway obstruction in sheep with ALI. However, the combination treatment fails to improve pulmonary function to a greater extent than sole nNOS or iNOS inhibition in previous studies. Evaluation of different NOS inhibitor combinations in different animal models is warranted.
Acknowledgments
Financial Support: This study was supported by grant 0565028Y from the American Heart Association 0565028Y, grants SBI 8450 and SBI 8954 from the Shrines of North America and by grants GM066312 and GM060688 from the National Institutes of Health.
Footnotes
The authors have not disclosed any potential conflicts of interest.
References
- 1.Dancey DR, Hayes J, Gomez M, et al. ARDS in patients with thermal injury. Intensive Care Med. 1999;25(11):1231–1236. doi: 10.1007/pl00003763. [DOI] [PubMed] [Google Scholar]
- 2.Enkhbaatar P, Murakami K, Shimoda K, et al. The inducible nitric oxide synthase inhibitor BBS-2 prevents acute lung injury in sheep after burn and smoke inhalation injury. Am J Respir Crit Care Med. 2003;167(7):1021–1026. doi: 10.1164/rccm.200209-1031PP. [DOI] [PubMed] [Google Scholar]
- 3.Murakami K, Traber DL. Pathophysiological basis of smoke inhalation injury. News Physiol Sci. 2003;18:125–129. doi: 10.1152/nips.01427.2002. [DOI] [PubMed] [Google Scholar]
- 4.Soejima K, Schmalstieg FC, Sakurai H, et al. Pathophysiological analysis of combined burn and smoke inhalation injuries in sheep. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1233–1241. doi: 10.1152/ajplung.2001.280.6.L1233. [DOI] [PubMed] [Google Scholar]
- 5.Soejima K, Schmalstieg FC, Traber LD, et al. Role of nitric oxide in myocardial dysfunction after combined burn and smoke inhalation injury. Burns. 2001;27(8):809–815. doi: 10.1016/s0305-4179(01)00051-1. [DOI] [PubMed] [Google Scholar]
- 6.Soejima K, Traber LD, Schmalstieg FC, et al. Role of nitric oxide in vascular permeability after combined burns and smoke inhalation injury. Am J Respir Crit Care Med. 2001;163(3 Pt 1):745–752. doi: 10.1164/ajrccm.163.3.9912052. [DOI] [PubMed] [Google Scholar]
- 7.Traber DL, Hawkins HK, Enkhbaatar P, et al. The role of the bronchial circulation in the acute lung injury resulting from burn and smoke inhalation. Pulm Pharmacol Ther. 2007;20(2):163–166. doi: 10.1016/j.pupt.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Westphal M, Cox RA, Traber LD, et al. Combined burn and smoke inhalation injury impairs ovine hypoxic pulmonary vasoconstriction. Crit Care Med. 2006;34(5):1428–1436. doi: 10.1097/01.CCM.0000215828.00289.B9. [DOI] [PubMed] [Google Scholar]
- 9.Cox RA, Burke AS, Soejima K, et al. Airway obstruction in sheep with burn and smoke inhalation injuries. Am J Respir Cell Mol Biol. 2003;29(3 Pt 1):295–302. doi: 10.1165/rcmb.4860. [DOI] [PubMed] [Google Scholar]
- 10.Shirani KZ, Pruitt BA, Jr, Mason AD., Jr The influence of inhalation injury and pneumonia on burn mortality. Ann Surg. 1987;205(1):82–87. doi: 10.1097/00000658-198701000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakami K, Bjertnaes LJ, Schmalstieg FC, et al. A novel animal model of sepsis after acute lung injury in sheep. Crit Care Med. 2002;30(9):2083–2090. doi: 10.1097/00003246-200209000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 13.Thiemermann C. Nitric oxide and septic shock. Gen Pharmacol. 1997;29(2):159–166. doi: 10.1016/s0306-3623(96)00410-7. [DOI] [PubMed] [Google Scholar]
- 14.Titheradge MA. Nitric oxide in septic shock. Biochim Biophys Acta. 1999;1411(2-3):437–455. doi: 10.1016/s0005-2728(99)00031-6. [DOI] [PubMed] [Google Scholar]
- 15.Enkhbaatar P, Murakami K, Shimoda K, et al. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole attenuates acute lung injury in an ovine model. Am J Phys iol Regul Integr Comp Physiol. 2003;285(2):R366–372. doi: 10.1152/ajpregu.00148.2003. [DOI] [PubMed] [Google Scholar]
- 16.Enkhbaatar P, Traber D. Role of neuronal nitric oxide synthase in cardiopulmonary lesions of sepsis. In: Vincent JL, editor. Yearbook of intensive care and emergency medicine 2004. Berlin: Springer; 2004. [Google Scholar]
- 17.Gocan NC, Scott JA, Tyml K. Nitric oxide produced via neuronal NOS may impair vasodilatation in septic rat skeletal muscle. Am J Physiol Heart Circ Physiol. 2000;278(5):H1480–1489. doi: 10.1152/ajpheart.2000.278.5.H1480. [DOI] [PubMed] [Google Scholar]
- 18.Enkhbaatar P, Murakami K, Traber LD, et al. The inhibition of inducible nitric oxide synthase in ovine sepsis model. Shock. 2006;25(5):522–527. doi: 10.1097/01.shk.0000209525.50990.28. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto I, Abe M, Shibata K, et al. Evaluating the role of inducible nitric oxide synthase using a novel and selective inducible nitric oxide synthase inhibitor in septic lung injury produced by cecal ligation and puncture. Am J Respir Crit Care Med. 2000;162(2 Pt 1):716–722. doi: 10.1164/ajrccm.162.2.9907039. [DOI] [PubMed] [Google Scholar]
- 20.Maybauer DM, Maybauer MO, Traber LD, et al. Effects of severe smoke inhalation injury and septic shock on global hemodynamics and microvascular blood flow in sheep. Shock. 2006;26(5):489–495. doi: 10.1097/01.shk.0000230300.78515.ed. [DOI] [PubMed] [Google Scholar]
- 21.Murakami K, McGuire R, Jodoin J, et al. Aminoguanidine did not attenuate septic changes and acute lung injury in sheep. The 7th World Congress for Microcirculation. 2001;(Suppl):465–469. [Google Scholar]
- 22.Lopez A, Lorente JA, Steingrub J, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32(1):21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 23.Bakker J, Grover R, McLuckie A, et al. Administration of the nitric oxide synthase inhibitor NG-methyl-L-arginine hydrochloride (546C88) by intravenous infusion for up to 72 hours can promote the resolution of shock in patients with severe sepsis: results of a randomized, double-blind, placebo-controlled multicenter study (study no. 144-002) Crit Care Med. 2004;32(1):1–12. doi: 10.1097/01.CCM.0000105118.66983.19. [DOI] [PubMed] [Google Scholar]
- 24.Booke M, Hinder F, McGuire R, et al. Selective inhibition of inducible nitric oxide synthase: effects on hemodynamics and regional blood flow in healthy and septic sheep. Crit Care Med. 1999;27(1):162–167. doi: 10.1097/00003246-199901000-00045. [DOI] [PubMed] [Google Scholar]
- 25.Su CF, Yang FL, Chen HI. Inhibition of inducible nitric oxide synthase attenuates acute endotoxin-induced lung injury in rats. Clin Exp Pharmacol Physiol. 2007;34(4):339–346. doi: 10.1111/j.1440-1681.2007.04553.x. [DOI] [PubMed] [Google Scholar]
- 26.Watson D, Grover R, Anzueto A, et al. Cardiovascular effects of the nitric oxide synthase inhibitor NG-methyl-L-arginine hydrochloride (546C88) in patients with septic shock: results of a randomized, double-blind, placebo-controlled multicenter study (study no. 144-002) Crit Care Med. 2004;32(1):13–20. doi: 10.1097/01.CCM.0000104209.07273.FC. [DOI] [PubMed] [Google Scholar]
- 27.Szabo C, Mitchell JA, Thiemermann C, et al. Nitric oxide-mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol. 1993;108(3):786–792. doi: 10.1111/j.1476-5381.1993.tb12879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thiemermann C, Szabo C, Mitchell JA, et al. Vascular hyporeactivity to vasoconstrictor agents and hemodynamic decompensation in hemorrhagic shock is mediated by nitric oxide. Proc Natl Acad Sci U S A. 1993;90(1):267–271. doi: 10.1073/pnas.90.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer SR, Deyo DJ, Bone HG, et al. Nitric oxide synthase inhibition restores hypoxic pulmonary vasoconstriction in sepsis. Am J Respir Crit Care Med. 1997;156(3 Pt 1):833–839. doi: 10.1164/ajrccm.156.3.9701033. [DOI] [PubMed] [Google Scholar]
- 30.Szabo C. The pathophysiological role of peroxynitrite in shock, inflammation, and ischemia-reperfusion injury. Shock. 1996;6(2):79–88. doi: 10.1097/00024382-199608000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6(8):662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 32.El-Remessy AB, Al-Shabrawey M, Platt DH, et al. Peroxynitrite mediates VEGF's angiogenic signal and function via a nitration-independent mechanism in endothelial cells. Faseb J. 2007;21(10):2528–2539. doi: 10.1096/fj.06-7854com. [DOI] [PubMed] [Google Scholar]
- 33.Szabo C. DNA strand breakage and activation of poly-ADP ribosyltransferase: a cytotoxic pathway triggered by peroxynitrite. Free Radic Biol Med. 1996;21(6):855–869. doi: 10.1016/0891-5849(96)00170-0. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Dawson VL, Dawson TM, et al. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science. 1994;263(5147):687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- 35.Szabo C, Cuzzocrea S, Zingarelli B, et al. Endothelial dysfunction in a rat model of endotoxic shock. Importance of the activation of poly (ADP-ribose) synthetase by peroxynitrite. J Clin Invest. 1997;100(3):723–735. doi: 10.1172/JCI119585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiarugi A, Moskowitz MA. Poly(ADP-ribose) polymerase-1 activity promotes NF-kappaB-driven transcription and microglial activation: implication for neurodegenerative disorders. J Neurochem. 2003;85(2):306–317. doi: 10.1046/j.1471-4159.2003.01684.x. [DOI] [PubMed] [Google Scholar]
- 37.Hassa PO, Hottiger MO. The functional role of poly(ADP-ribose)polymerase 1 as novel coactivator of NF-kappaB in inflammatory disorders. Cell Mol Life Sci. 2002;59(9):1534–1553. doi: 10.1007/s00018-002-8527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenberg SS, Ouyang J, Zhao X, et al. Human and rat neutrophils constitutively express neural nitric oxide synthase mRNA. Nitric Oxide. 1998;2(3):203–212. doi: 10.1006/niox.1998.0176. [DOI] [PubMed] [Google Scholar]
- 39.Saini R, Patel S, Saluja R, et al. Nitric oxide synthase localization in the rat neutrophils: immunocytochemical, molecular, and biochemical studies. J Leukoc Biol. 2006;79(3):519–528. doi: 10.1189/jlb.0605320. [DOI] [PubMed] [Google Scholar]