Abstract
Background:
Osteoporotic fractures commonly occur after low-energy trauma in postmenopausal women with reduced bone quantity documented by low bone mineral density (BMD). Low-energy fractures, however, have also been reported to occur in premenopausal women with normal or near-normal BMD, suggesting the existence of a bone quality abnormality.
Methods:
Bone quality and quantity were evaluated in a cross-sectional study of three groups of premenopausal white females: (1) twenty-five subjects with low-energy fracture(s) and BMD in the normal range (t-scores > −2.0), (2) eighteen subjects with low-energy fracture(s) and BMD in the osteoporotic range (t-scores ≤ −2.5), and (3) fourteen healthy volunteers (controls). Bone quality was assessed with use of Fourier transform infrared spectroscopy and histomorphometry in iliac crest bone samples obtained from all subjects; bone quantity was assessed by dual x-ray absorptiometry and histomorphometry.
Results:
The collagen crosslinking ratio in the non-low-BMD subjects with fractures was 13% greater than the ratio in the low-BMD subjects with fractures and 14% greater than the ratio in the controls (p < 0.001 for both). Cancellous bone volume was 29% greater (p < 0.01) and trabecular separation was 31% less (p < 0.01) in the non-low-BMD subjects with fractures than in the low-BMD subjects with fractures; the values in the non-low-BMD subjects did not differ from those in the controls. Bone turnover did not differ among the groups, and osteomalacia was not present in any subject. Thus, the non-low-BMD subjects with fractures maintained bone quantity, but the collagen crosslinking ratio, a parameter of bone quality, was abnormal. In contrast, the low-BMD subjects with fractures did not have this collagen crosslinking abnormality but did have abnormal bone quantity.
Conclusions:
This study highlights a collagen crosslinking abnormality in patients with low-energy fractures and nonosteoporotic t-scores. Reports have indicated that altered collagen crosslinking is associated with subnormal fracture resistance. A finding of nonosteoporotic bone mass in a patient with low-energy fractures would justify assessment of bone material quality, which currently requires a bone biopsy. Further studies are needed to search for possible noninvasive tests to diagnose abnormal crosslinking. Since no specific therapies for abnormal collagen crosslinking are currently available, studies are also needed to explore novel therapeutic modalities to reverse the underlying collagen crosslinking abnormality.
Level of Evidence:
Prognostic Level III. See Instructions for Authors for a complete description of levels of evidence.
Before the advent of routine measurement of bone mineral density (BMD) by x-ray absorptiometry, osteoporosis was defined as a clinical syndrome in postmenopausal women with low-energy fracture(s) accompanied by low bone mass. Given the ease of use and widespread availability of dual x-ray absorptiometry (DXA), the World Health Organization subsequently defined osteoporosis as a reduction in BMD t-scores of ≥2.5 standard deviations from the mean value in young adults1. This definition is now routinely used worldwide in clinical practice for the diagnosis of osteoporosis. Fractures may also occur, however, with low-energy trauma in premenopausal women who are nonosteoporotic as classified on the basis of their BMD t-scores2-4.
It is easy to understand the occurrence of low-energy fractures in patients with osteoporotic t-scores, but it remains unclear why low-energy fractures occur in premenopausal women with nonosteoporotic t-scores. Factors other than low bone quantity characteristic of osteoporosis must be considered, and chief among these is abnormal bone quality. Bone quality includes material properties and microarchitectural features, which are major contributors to the load-bearing capabilities of bone5-8. Low-energy fractures associated with abnormal bone quality have been reported to occur in premenopausal women with idiopathic osteoporosis9-13. There is limited information evaluating bone quality in premenopausal women with fractures but without osteoporotic BMD or secondary osteoporosis while controlling for the potentially confounding effects of sex or race. The present study was designed to test the hypothesis that, in the absence of bone quantity abnormalities, abnormal bone quality in premenopausal women is associated with low-energy fracture.
Materials and Methods
Study Design
This cross-sectional study was designed to quantify bone quality and quantity in three groups of premenopausal women: (1) those with low-energy fractures and nonosteoporotic BMD t-scores (the non-low-BMD fracture group), (2) those with low-energy fractures and osteoporotic BMD t-scores (the low-BMD fracture group), and (3) healthy volunteers (the control group). Bone samples for the study were obtained from subjects undergoing iliac crest biopsy for work-up of low-energy fractures at our institution. Bone from the iliac crest serves as a useful model of the skeleton because histological and mechanical changes in this tissue are also associated with histological14 and mechanical15 changes in bone at other skeletal sites. The two primary study groups included premenopausal adult white women with one or more low-energy fractures; those in the non-low-BMD group had a nonosteoporotic BMD as indicated by a t-score of >−2.0 at both the hip and the lumbar spine, and those in the low-BMD group had an osteoporotic BMD as indicated by a t-score of ≤−2.5 at the hip or lumbar spine. Low-energy fractures were defined as those occurring without trauma during normal activities of daily living. Control bone samples were obtained from biopsies performed in healthy premenopausal white women volunteers with BMD t-scores of >−2.0 and no fractures.
Subjects were excluded if they had a diagnosis of osteogenesis imperfecta or other genetic bone disease, histologically proven osteomalacia (osteoid thickness of >20 μm and mineralization lag time of >100 days), hyperparathyroid bone disease or other disorders associated with secondary osteoporosis, chronic kidney disease, abnormal mineral metabolism, Marfan syndrome, endocrine abnormalities, celiac or other gastrointestinal disorders, bariatric procedures, diabetes, Paget disease of bone, amenorrhea, eating disorders, or malignancies. Subjects were also excluded if they had a history of drug or alcohol abuse or of prior use of bisphosphonates, teriparatide, selective estrogen receptor modulators, sex steroids, or any other medications known to alter bone metabolism. The protocol of this institutional review board-approved cross-sectional study adhered to the Declaration of Helsinki.
Bone Mineral Density
Bone mineral density was measured at the hip and at the lumbar spine (L2-L4) in all study subjects with use of DXA (Lunar iDXA; GE Healthcare, Madison, Wisconsin). The coefficient of variation of the BMD measurements was 1.2% at the spine and 0.9% at the hip.
Serum Biochemistry
A renal metabolic panel was obtained and serum alkaline phosphatase was measured by routine laboratory techniques. In addition, serum parathyroid hormone (PTH) levels were measured by radioimmunoassay (Total Intact PTH; Scantibodies, Santee, California), serum calcidiol was measured by liquid chromatography-tandem mass spectrometry (API 3200; AB SCIEX, Framingham, Massachusetts), serum bone-specific alkaline phosphatase was measured by immunocapture enzyme activity assay (Quidel, San Diego, California), serum N-terminal telopeptide was measured by ELISA (enzyme-linked immunosorbent assay) (Osteomark NTX; Inverness Medical Innovations, Waltham, Massachusetts), and serum osteocalcin was measured by ELISA (Quidel).
Mineralized Bone Histology and Bone Histomorphometry
Bone samples, obtained after tetracycline double-labeling16, were processed without mineral removal and were embedded in methylmethacrylate. Serial sections (thicknesses, 4 and 7 μm) were cut and were stained with modified Masson-Goldner trichrome stain. Unstained sections were prepared for fluorescent and polarized light microscopy17.
Histomorphometry was performed at standardized sites in cancellous bone to obtain quantitative static and dynamic parameters reflecting bone structure (cancellous bone volume/tissue volume), microarchitecture (trabecular separation, trabecular thickness), bone turnover (bone formation rate/bone surface area), and mineralization (osteoid thickness, mineralization lag time)18,19. Measurements were made at ×200 magnification (Osteoplan II System; Kontron, Munich, Germany). All measured parameters were defined in accordance with the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research20.
Bone Material (Mineral and Matrix) Properties
Bone material properties were measured with use of a Fourier transform infrared (FTIR) spectrometer (Nexus 670; Thermo Electron, Waltham, Massachusetts) on sections prepared from anterior iliac crest bone samples. A 4-μm-thick undecalcified section was cut from each bone sample and placed between two barium fluoride discs for FTIR analysis21. Infrared spectra were collected from these “sandwiched” bone sections with use of a microscope that was attached to the spectrometer and operated in transmission mode for 200 scans at 4 cm−1 resolution. Three trabeculae were chosen from each section. Trabeculae were evaluated beginning at a distance of five to seven optical fields (at ×200) below the cortex. Spectroscopic measurements were made in the center of each of these three trabeculae. Background scans were used to correct for the spectral contributions of the barium fluoride discs and the methylmethacrylate mount.
Established parameters reflecting bone quality were determined22. Specifically, the mineral-to-matrix ratio was obtained by dividing the area under the phosphate (mineral) peak (900 to 1200 cm−1) by the area under the amide I (matrix) peak (1590 to 1720 cm−1) after baseline correction of both peaks (see Appendix). The carbonate-to-phosphate ratio (i.e., the amount of carbonate substituted in the hydroxyapatite crystal) was obtained by dividing the area under the carbonate peak (850 to 890 cm−1) by the area under the phosphate peak. Crystallinity, a measure of crystal size and perfection, was obtained by dividing the area under the 1020 cm−1 peak by the area under the 1030 cm−1 peak23. The collagen crosslinking ratio, a measure of collagen maturity, was the ratio of the areas under the 1660 cm−1 (mature crosslinks) and 1690 cm−1 (immature crosslinks) peaks24. The coefficient of variation was 4.3% for the mineral-to-matrix ratio, 2.0% for the carbonate-to-phosphate ratio, 1.7% for the crystallinity, and 4.1% for the crosslinking ratio.
Data Analyses
Data were tested for normality with use of the Kolmogorov-Smirnov test and for equality of variances with use of the Levene test. Multiple-group comparisons were performed with use of analysis of variance (ANOVA) with Scheffe post-hoc correction. Two-group comparisons were made with use of the Student t test. Univariate analyses (Pearson tests) were used to determine whether BMD and age were correlated with the material and histomorphometric parameters of bone. A p value of <0.05 was considered significant.
Source of Funding
This study was supported by the National Institutes of Health (1RO1AR061578-01A1) and the Kentucky Nephrology Research Trust.
Results
Subject Characteristics and Biochemical Results
Fifty-seven premenopausal adult female white subjects met the selection criteria and were included in the study; twenty-five were in the non-low-BMD fracture group, eighteen were in the low-BMD fracture group, and fourteen were healthy volunteers (controls).
Subjects in the non-low-BMD group first presented with a mean of 3.6 low-energy fractures during adulthood compared with 1.4 low-energy fractures in the low-BMD group (p < 0.05). A hallmark of these low-energy fractures was that subjects were unable to identify a specific mechanical event associated with the fracture. The number of patients who sustained fractures in particular bones differed between the two fracture groups (Table I). Nondisplaced metatarsal fractures (Fig. 1) were the most common fractures in the non-low-BMD group (experienced by 56% of the subjects), whereas spinal fractures were the most common fractures in the low-BMD group (experienced by 28% of the subjects) (Table I). No atypical femoral fractures occurred in any of the study subjects.
TABLE I.
Number of Patients with Low-Energy Fractures According to Bone Site
| Bone Site | Non-Low-BMD Group (N = 25) | Low-BMD Group (N = 18) |
| Metatarsal | 14 | 3 |
| Tibia | 7 | 1 |
| Femoral neck | 6 | 3 |
| Spine | 3 | 5 |
| Pelvis | 3 | 1 |
| Wrist | 2 | 1 |
| Calcaneus | 2 | 0 |
| Rib | 1 | 3 |
| Forearm | 1 | 1 |
| Talus | 1 | 0 |
| Metacarpal | 1 | 0 |
Fig. 1.
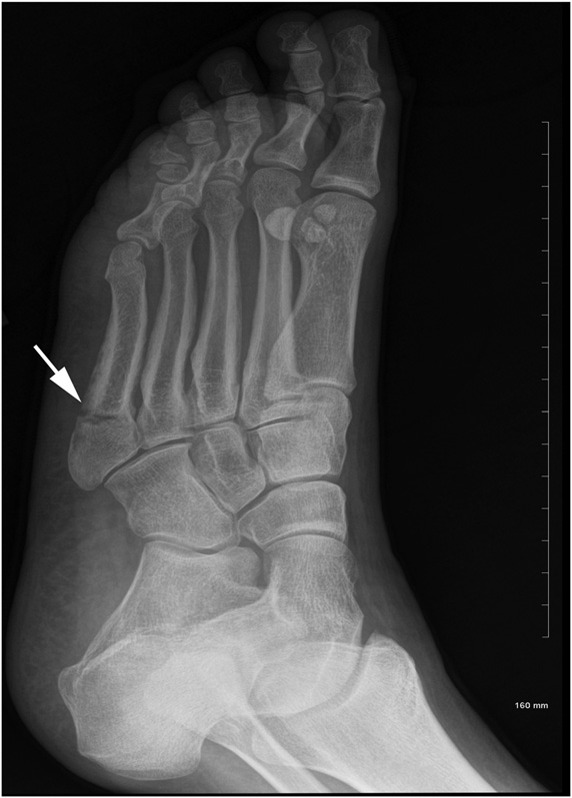
Oblique radiograph of a nondisplaced transverse fracture of the proximal fifth metatarsal (arrow) in a premenopausal subject with non-low BMD.
The BMD values in the groups were consistent with those defined by the inclusion criteria. Subjects in the two fracture groups were younger than the controls, and no differences were detected among the three groups with respect to serum concentrations of calcium, phosphorus, creatinine, glucose, sodium, alkaline phosphatase, parathyroid hormone, calcidiol, bone-specific alkaline phosphatase, N-terminal telopeptide, or osteocalcin (Table II).
TABLE II.
Subject Characteristics and Biochemical Results
| Non-Low BMD (Group 1, N = 25)* | P Value, 1 vs. 2 | Low BMD (Group 2, N = 18)* | P Value, 2 vs. 3 | Controls (Group 3, N = 14)* | P Value, 1 vs. 3 | |
| BMD, total hip (t-score) | −0.42 ± 0.97 | 0.001 | −2.28 ± 0.79 | 0.001 | −0.67 ± 0.98 | >0.1 |
| BMD, lumbar spine (t-score) | −0.53 ± 0.97 | 0.001 | −2.79 ± 0.85 | 0.001 | −0.53 ± 0.92 | >0.1 |
| Age (yr) | 37.2 ± 8.6 | >0.1 | 40.7 ± 9.9 | 0.001 | 52.6 ± 3.5 | 0.001 |
| Serum analysis | ||||||
| Calcium (mg/dL) | 9.43 ± 0.26 | >0.1 | 9.37 ± 0.37 | >0.1 | 9.27 ± 0.39 | >0.1 |
| Phosphorus (mg/dL) | 3.45 ± 0.54 | >0.1 | 3.54 ± 0.62 | >0.1 | 3.64 ± 0.55 | >0.1 |
| Creatinine (mg/dL) | 0.77 ± 0.08 | >0.1 | 0.72 ± 0.13 | 0.074 | 0.85 ± 0.16 | >0.1 |
| Glucose (mg/dL) | 88.7 ± 8.4 | >0.1 | 93.7 ± 7.63 | >0.1 | 92.8 ± 10.5 | >0.1 |
| Sodium (mmol/L) | 139 ± 1.71 | >0.1 | 138 ± 1.95 | >0.1 | 137 ± 4.47 | >0.1 |
| Alkaline phosphatase (U/L) | 65.3 ± 21.3 | >0.1 | 70.8 ± 25.4 | >0.1 | 91.1 ± 44.4 | >0.1 |
| Parathyroid hormone (pg/mL) | 31.1 ± 18.5 | >0.1 | 29.7 ± 15.7 | >0.1 | 28.4 ± 10.5 | >0.1 |
| Calcidiol (ng/mL) | 35.1 ± 11.8 | >0.1 | 36.9 ± 13.7 | >0.1 | 42.6 ± 10.7 | >0.1 |
| Bone-specific alkaline phosphatase (μg/L) | 14.2 ± 6.61 | >0.1 | 19.9 ± 8.12 | 0.068 | 12.1 ± 4.38 | >0.1 |
| N-terminal telopeptide (nM bone collagen equivalent) | 12.6 ± 6.44 | >0.1 | 14.8 ± 9.22 | >0.1 | 10.8 ± 4.11 | >0.1 |
| Osteocalcin (ng/mL) | 17.6 ± 8.85 | >0.1 | 20.6 ± 6.17 | >0.1 | 14.7 ± 7.06 | >0.1 |
The values are given as the mean and the standard deviation.
Histomorphometric Parameters of Bone Structure, Microarchitecture, Turnover, and Mineralization
Cancellous bone volume was 29% greater (p < 0.01) and trabecular separation was 31% less (p < 0.01) in the non-low-BMD subjects with fractures than in the low-BMD subjects with fractures; the values in the non-low-BMD subjects did not differ from those in the controls (Figs. 2 and 3). Trabecular thickness did not differ significantly among the three groups (see Appendix).
Fig. 2.
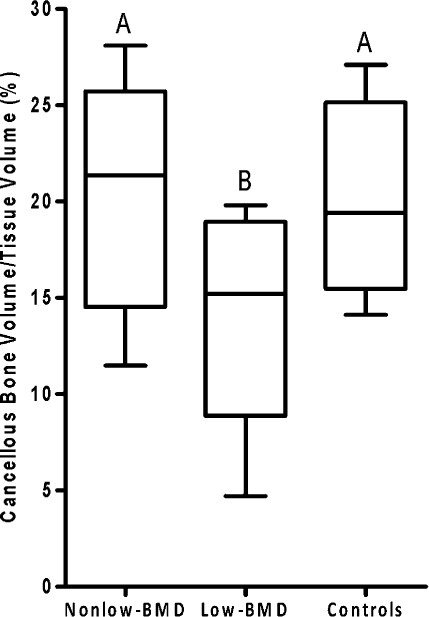
Box plots of cancellous bone volume/tissue volume in bone from subjects with non-low BMD (t-score > −2.0) and low-energy fractures, subjects with low BMD (t-score ≤ −2.5) and low-energy fractures, and healthy volunteers (controls). Box plots labeled with the same letters do not differ significantly. The bottom and top of the box represent the interquartile range (25% to 75%), the line within the box denotes the median (50%), and the upper and lower bounds of the error bars denote the range.
Fig. 3.
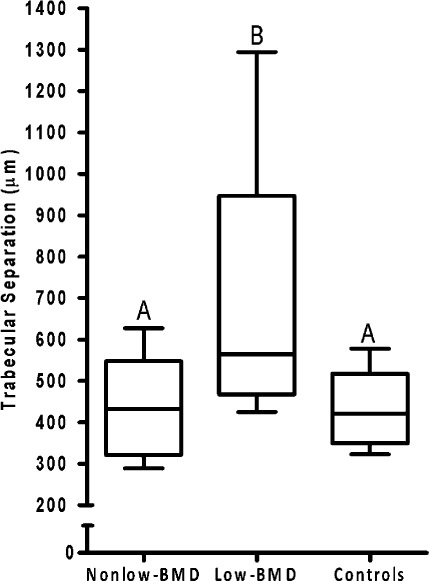
Box plots of trabecular separation in bone from subjects with non-low BMD (t-score > −2.0) and low-energy fractures, subjects with low BMD (t-score ≤ −2.5) and low-energy fractures, and healthy volunteers (controls). Box plots labeled with the same letters do not differ significantly. The bottom and top of the box represent the interquartile range (25% to 75%), the line within the box denotes the median (50%), and the upper and lower bounds of the error bars denote the range.
Bone turnover and bone mineralization parameters did not different significantly among the three groups (see Appendix). None of the measured histomorphometric parameters were correlated with BMD or age.
Bone Material (Mineral and Matrix) Properties
The mean collagen crosslinking ratio in the non-low-BMD group was 13% greater (p < 0.001) that that in the low-BMD group and 14% greater (p < 0.001) than that in the controls (Fig. 4). The collagen crosslinking ratio did not differ significantly between the low-BMD group and the controls. No differences were observed among the three groups with respect to any other measured bone mineral parameter (see Appendix). None of the measured mineral or matrix properties were correlated with BMD, age, or any histomorphometric parameter.
Fig. 4.
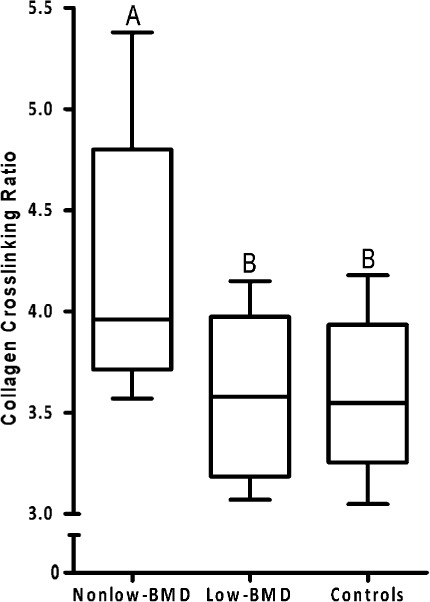
Box plots of the collagen crosslinking ratio in bone from subjects with non-low BMD (t-score > −2.0) and low-energy fractures, subjects with low BMD (t-score ≤ −2.5) and low-energy fractures, and healthy volunteers (controls). Box plots labeled with the same letters do not differ significantly. The bottom and top of the box represent the interquartile range (25% to 75%), the line within the box denotes the median (50%), and the upper and lower bounds of the error bars denote the range.
Discussion
The novel result of this study is the greater collagen crosslinking ratio, a bone quality parameter, in non-low-BMD subjects with low-energy fractures. It is important to note that such fractures in the non-low-BMD subjects could not be attributed to abnormal bone structure or microarchitecture (including lower cancellous bone volume, thinner trabeculae, or greater trabecular separation). In contrast, such fractures in the low-BMD group could be attributed to reduced bone quantity, and the subjects in this group did not have the material quality abnormality observed in the non-low-BMD group. These findings confirmed our hypothesis that low-energy fractures in premenopausal women with nonosteoporotic BMD are associated with an abnormality in bone quality evidenced by increased collagen crosslinking.
Clinically, patients who sustain low-energy fractures are considered osteoporotic regardless of their BMD t-score; however, the present findings of a different fracture distribution and of greater collagen crosslinking in the non-low-BMD group compared with the low-BMD group suggests that these two groups manifest different disease entities. One disease entity (seen in the low-BMD group) is attributable to abnormal bone quantity; the other (seen in the non-low-BMD group) is attributable to abnormal bone quality as manifested by abnormal collagen crosslinking. Reduced bone quantity is known to diminish bone fracture resistance, as demonstrated by the finding that spinal fractures, a typical manifestation of classic osteoporosis, were the most prevalent fractures in the low-BMD group. The most prevalent fracture site in the non-low-BMD group was the metatarsals, an uncommon site in classic osteoporosis.
Crosslinking is an important structural feature that affects mechanical performance. The types and extent of collagen crosslinking in bone have only recently been appreciated. Crosslinks alter the mechanical properties of bone25,26. Collagen crosslinking abnormalities have been linked to altered bone biomechanics and diminished fracture resistance in both animal25,27 and clinical studies9,13,28-30. In Wistar rats, beta-aminopropionitrile administered to inhibit lysyl oxidase and thereby induce increased collagen crosslinking resulted in a 27% increase in the collagen crosslinking ratio and a 14% decrease in lumbar bone stiffness25. The present study, however, did not have the ability to establish a cause-and-effect relationship between changes in the collagen crosslinking ratio and reduced bone strength. Abnormally high collagen crosslinking has also been observed in diabetic Wistar Bonn/Kobori rats whose femora had diminished mechanical competence27, but the results of the present study cannot be explained by diabetes since diabetes was an exclusion criterion and morning blood glucose levels were normal. Misof et al. studied premenopausal women, regardless of BMD, who had fragility fractures and compared them with premenopausal women with low BMD and no fractures13. They found an increased collagen crosslinking ratio in subjects with fragility fractures and a significantly lower BMD when subjects with and without fractures were combined and compared with normal controls. The present study separated premenopausal women with fractures into two groups: those with osteoporotic BMD t-scores and those with nonosteoporotic t-scores. The findings showed that an increased collagen crosslinking ratio was associated with the occurrence of low-energy fractures in premenopausal women despite nonosteoporotic BMD that was not significantly different from that in normal controls. Thus, our design controlled for BMD and thereby isolated the effects of alteration in the collagen crosslinking ratio.
The greater collagen crosslinking ratio in the non-low-BMD group cannot be explained by lower bone turnover because turnover did not differ significantly between the two fracture groups, nor can it be attributed to age because no associations between age and collagen crosslinking were found. A recent study showed an association between chronic hyponatremia and fractures in subjects with nonosteoporotic BMD31. Hyponatremia, however, was not observed in non-low-BMD subjects in the present study and there were no differences in serum sodium among the three study groups.
The present study was limited to white women; men and non-white women were excluded to focus on individuals at greatest risk for low-energy fracture. The external validity of these findings will be enhanced by additional data obtained from men and from women of other races.
In conclusion, the key finding of this study confirmed the hypothesis that, in the absence of osteoporotic t-scores, an abnormality in a particular bone quality (the collagen crosslinking ratio) is associated with low-energy fractures in premenopausal women. A finding of nonosteoporotic bone mass with low-energy fractures would justify assessment of bone material quality, which currently requires a bone biopsy. Further studies are needed to search for possible noninvasive tests to diagnose abnormal collagen crosslinking. Since no specific therapies for abnormal collagen crosslinking are available at this time, studies are also needed to explore novel therapeutic modalities to reverse the underlying collagen crosslinking abnormality.
Appendix
Tables comparing properties of bone among the groups and a figure showing a typical FTIR spectrum of bone are available with the online version of this article as a data supplement at jbjs.org.
Supplementary Material
Disclosure of Potential Conflicts of Interest
Tables comparing properties of bone among the groups and a figure showing a typical FTIR spectrum of bone
Acknowledgments
Note: The authors thank Lisa DeGnore, MD, of Kentucky Orthopaedic and Hand Surgeons and Veronica Vasicek, MD, of Bluegrass Orthopaedics and Hand Care (Lexington, KY) for referring patients with low-energy fractures.
Footnotes
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. None of the authors, or their institution(s), have had any financial relationship, in the thirty-six months prior to submission of this work, with any entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. Also, no author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO study group. World Health Organ Tech Rep Ser. 1994;843:1-129 [PubMed] [Google Scholar]
- 2.Donovan MA, Dempster D, Zhou H, McMahon DJ, Fleischer J, Shane E. Low bone formation in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2005 Jun;90(6):3331-6 Epub 2005 Mar 22 [DOI] [PubMed] [Google Scholar]
- 3.Moreira Kulak CA, Schussheim DH, McMahon DJ, Kurland E, Silverberg SJ, Siris ES, Bilezikian JP, Shane E. Osteoporosis and low bone mass in premenopausal and perimenopausal women. Endocr Pract. 2000 Jul-Aug;6(4):296-304 [DOI] [PubMed] [Google Scholar]
- 4.Khosla S, Lufkin EG, Hodgson SF, Fitzpatrick LA, Melton LJ., 3rd Epidemiology and clinical features of osteoporosis in young individuals. Bone. 1994 Sep-Oct;15(5):551-5 [DOI] [PubMed] [Google Scholar]
- 5.Seeman E, Delmas PD. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med. 2006 May 25;354(21):2250-61 [DOI] [PubMed] [Google Scholar]
- 6.Felsenberg D, Boonen S. The bone quality framework: determinants of bone strength and their interrelationships, and implications for osteoporosis management. Clin Ther. 2005 Jan;27(1):1-11 [DOI] [PubMed] [Google Scholar]
- 7.Paschalis EP, Mendelsohn R, Boskey AL. Infrared assessment of bone quality: a review. Clin Orthop Relat Res. 2011 Aug;469(8):2170-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burr DB. Bone quality: understanding what matters. J Musculoskelet Neuronal Interact. 2004 Jun;4(2):184-6 [PubMed] [Google Scholar]
- 9.Paschalis EP, Shane E, Lyritis G, Skarantavos G, Mendelsohn R, Boskey AL. Bone fragility and collagen cross-links. J Bone Miner Res. 2004 Dec;19(12):2000-4 Epub 2004 Aug 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen A, Dempster DW, Recker RR, Stein EM, Lappe JM, Zhou H, Wirth AJ, van Lenthe GH, Kohler T, Zwahlen A, Müller R, Rosen CJ, Cremers S, Nickolas TL, McMahon DJ, Rogers H, Staron RB, LeMaster J, Shane E. Abnormal bone microarchitecture and evidence of osteoblast dysfunction in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2011 Oct;96(10):3095-105 Epub 2011 Aug 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen A, Liu XS, Stein EM, McMahon DJ, Rogers HF, Lemaster J, Recker RR, Lappe JM, Guo XE, Shane E. Bone microarchitecture and stiffness in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2009 Nov;94(11):4351-60 Epub 2009 Oct 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen A, Recker RR, Lappe J, Dempster DW, Cremers S, McMahon DJ, Stein EM, Fleischer J, Rosen CJ, Rogers H, Staron RB, Lemaster J, Shane E. Premenopausal women with idiopathic low-trauma fractures and/or low bone mineral density. Osteoporos Int. 2012 Jan;23(1):171-82 Epub 2011 Mar 02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Misof BM, Gamsjaeger S, Cohen A, Hofstetter B, Roschger P, Stein E, Nickolas TL, Rogers HF, Dempster D, Zhou H, Recker R, Lappe J, McMahon D, Paschalis EP, Fratzl P, Shane E, Klaushofer K. Bone material properties in premenopausal women with idiopathic osteoporosis. J Bone Miner Res. 2012 Dec;27(12):2551-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meunier P, Courpron P, Edouard C, Bernard J, Bringuier J, Vignon G. Physiological senile involution and pathological rarefaction of bone. Quantitative and comparative histological data. Clin Endocrinol Metab. 1973 Jul;2(2):239-56 [DOI] [PubMed] [Google Scholar]
- 15.Mosekilde L, Viidik A, Mosekilde L. Correlation between the compressive strength of iliac and vertebral trabecular bone in normal individuals. Bone. 1985;6(5):291-5 [DOI] [PubMed] [Google Scholar]
- 16.Monier-Faugere MC, Langub MC, Malluche HH. Bone biopsies: a modern approach. In: Avioli LV, Krane SM, editors. Metabolic bone disease and clinically related disorders. San Diego: Academic Press; 1998. p 237-73 [Google Scholar]
- 17.Malluche H, Faugere M. Atlas of mineralized bone histology. New York: Karger; 1986 [Google Scholar]
- 18.Manaka RC, Malluche HH. A program package for quantitative analysis of histologic structure and remodeling dynamics of bone. Comput Programs Biomed. 1981 Sep-Dec;13(3-4):191-201 [DOI] [PubMed] [Google Scholar]
- 19.Malluche HH, Sherman D, Meyer W, Massry SG. A new semiautomatic method for quantitative static and dynamic bone histology. Calcif Tissue Int. 1982 Sep;34(5):439-48 [DOI] [PubMed] [Google Scholar]
- 20.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR; Report of the ASBMR Histomorphometry Nomenclature Committee Bone histomorphometry: standardization of nomenclature, symbols, and units. J Bone Miner Res. 1987 Dec;2(6):595-610 [DOI] [PubMed] [Google Scholar]
- 21.Malluche HH, Porter DS, Monier-Faugere MC, Mawad H, Pienkowski D. Differences in bone quality in low- and high-turnover renal osteodystrophy. J Am Soc Nephrol. 2012 Mar;23(3):525-32 Epub 2011 Dec 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faibish D, Gomes A, Boivin G, Binderman I, Boskey A. Infrared imaging of calcified tissue in bone biopsies from adults with osteomalacia. Bone. 2005 Jan;36(1):6-12 Epub 2004 Nov 24 [DOI] [PubMed] [Google Scholar]
- 23.Gadaleta SJ, Paschalis EP, Betts F, Mendelsohn R, Boskey AL. Fourier transform infrared spectroscopy of the solution-mediated conversion of amorphous calcium phosphate to hydroxyapatite: new correlations between X-ray diffraction and infrared data. Calcif Tissue Int. 1996 Jan;58(1):9-16 [DOI] [PubMed] [Google Scholar]
- 24.Paschalis EP, Verdelis K, Doty SB, Boskey AL, Mendelsohn R, Yamauchi M. Spectroscopic characterization of collagen cross-links in bone. J Bone Miner Res. 2001 Oct;16(10):1821-8 [DOI] [PubMed] [Google Scholar]
- 25.Paschalis EP, Tatakis DN, Robins S, Fratzl P, Manjubala I, Zoehrer R, Gamsjaeger S, Buchinger B, Roschger A, Phipps R, Boskey AL, Dall’Ara E, Varga P, Zysset P, Klaushofer K, Roschger P. Lathyrism-induced alterations in collagen cross-links influence the mechanical properties of bone material without affecting the mineral. Bone. 2011 Dec;49(6):1232-41 Epub 2011 Sep 02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banse X, Sims TJ, Bailey AJ. Mechanical properties of adult vertebral cancellous bone: correlation with collagen intermolecular cross-links. J Bone Miner Res. 2002 Sep;17(9):1621-8 [DOI] [PubMed] [Google Scholar]
- 27.Saito M, Fujii K, Mori Y, Marumo K. Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos Int. 2006 Oct;17(10):1514-23 Epub 2006 Jun 13 [DOI] [PubMed] [Google Scholar]
- 28.Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010 Feb;21(2):195-214 [DOI] [PubMed] [Google Scholar]
- 29.Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int. 2006;17(3):319-36 Epub 2005 Dec 09 [DOI] [PubMed] [Google Scholar]
- 30.Burr DB. The contribution of the organic matrix to bone’s material properties. Bone. 2002 Jul;31(1):8-11 [DOI] [PubMed] [Google Scholar]
- 31.Hoorn EJ, Rivadeneira F, van Meurs JB, Ziere G, Stricker BH, Hofman A, Pols HA, Zietse R, Uitterlinden AG, Zillikens MC. Mild hyponatremia as a risk factor for fractures: the Rotterdam Study. J Bone Miner Res. 2011 Aug;26(8):1822-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure of Potential Conflicts of Interest
Tables comparing properties of bone among the groups and a figure showing a typical FTIR spectrum of bone


