Abstract
Glycomics research requires the isolation of glycans from cells for structural characterization and functional studies of the glycans. A method for cell-based microscale isolation and quantification of highly sulfated, moderately sulfated, and nonsulfated glycosaminoglycans (GAGs) was developed using Chinese hamster ovary (CHO) cells. This microscale isolation relies on a mini-strong anion exchange spin column eluted stepwise with different concentrations of sodium chloride solution. Hyaluronic acid, chondroitin sulfate, and heparin were used to optimize the isolation of the endogenous glycosaminoglycans in CHO cells. This method can also be used to determine the presence of nonsulfated GAGs including heparosan, hyaluronic acid, and nonsulfated chondroitin.
Keywords: Chinese hamster ovary cells, Glycosaminoglycans, Anion exchange chromatography, Heparosan, Disaccharide analysis
INTRODUCTION
Glycomics research is currently undergoing rapid development as a result of recent advances in technologies for glycan structural analysis and is beginning to elucidate the structure-activity relationships of glycan–protein interactions.[1–4] The most extensively studied complex glycans are glycosaminoglycans (GAGs), which are involved in many critical biological processes including development, angiogenesis, anticoagulation, axonal growth, cancer, and microbial/viral pathogenesis.[5,6] GAGs include hyaluronic acid (HA), chondroitin sulfate/dermatan sulfate (CS/DS), keratan sulfate (KS), and heparan sulfate/heparin (HS/HP) families. HA is a structurally simple, nonsulfated polysaccharide consisting of alternating sequences of β-(1→4)-glucuronic acid and β-(1→3)-N-acetylglucosamine, of variable molecular weights. In contrast, the CS/DS (uronic acid α- or β-(1→3)-N-acetylgalactosamine-β-(1→4)) and HS/HP (uronic acid α- or β-(1→4)-N-acetylglucosamine-β-(1→4)) families are structurally complex, ranging from chains without sulfo groups (i.e., chondroitin and heparosan), to moderately sulfated chains of one to two sulfo groups/disaccharide repeating unit, to highly sulfated chains of three sulfo groups/disaccharide repeating unit.[7,8] The net charge of a nonsulfated GAG might range from neutral at pH 3 (below the pI of the uronic acid carboxyl group) to −1,000 at pH 7. In contrast, sulfated GAGs are polyanions at virtually all biologically accessible pH values.
Glycomics research requires the isolation of glycans from cells for structural characterization and functional studies. In previous studies, our laboratory has successfully applied anion exchange spin columns for the isolation of HS/HP from serum, cells, and tissues.[9,10] No method has been reported for cell-based microscale isolation of nonsulfated GAGs, such as HA, heparosan, and chondroitin, from biological samples. These nonsulfated GAGs were often simply ignored or underestimated in glycan analysis.
Chinese hamster ovary (CHO) cells are used in industrially important processes, such as the preparation of glycoprotein-based drugs.[11] Suspension CHO cells (CHO-S) are known to produce nonsulfated or moderately sulfated CS/DS and HS/HP GAGs without having measurable amounts of HA.[12] There are a variety of CHO cell lines, and the CHO-S line, capable of growing in suspension culture, is of particular pharmaceutical importance in preparing glycoprotein-based therapeutics and in metabolic engineering studies to produce heparin.[13] We have focused on the challenge of devising a method for the microscale recovery of the total GAG content (including nonsulfated, moderately sulfated, and highly sulfated GAGs) from cultured CHO-S cells containing a complex mixture of proteins, nucleic acids, and lipids. Such a recovery method would be of value in our metabolic engineering studies and directly applicable to glycomics studies of other cultured cells, biological fluids, and tissue samples. In the current study, we report a method for cell-based microscale isolation and quantification of highly sulfated, moderately sulfated, and nonsulfated GAGs using CHO cells. This microscale isolation relied on a mini-strong anion exchange spin column that was eluted stepwise with different concentrations of NaCl. The recovered GAGs were subjected to disaccharide compositional analysis, after 2-aminoacridone (AMAC) tagging, by single-step ultra-performance liquid chromatography (UPLC)-mass spectroscopy (MS).[14]
The carbazole assay is a nonspecific, colorimetric method for the quantification of a purified mixture of GAGs that can be interfered with by impurities such as proteins.[5] Similarly, dye-binding assays are nonspecific methods for the quantification of GAGs, but these methods show widely different response factors for GAGs having different levels of sulfation.[16] Alternative approaches for the quantification of GAGs include specific disaccharide compositional analysis by high-performance liquid chromatography (HPLC),[17,18] capillary electrophoresis,[19] and fluorophore-assisted carbohydrate electrophoresis.[20] These methods are very sensitive and are particularly useful for the microscale quantification of GAGs after digestion with GAG lyases. In our laboratory, reverse-phase ion-pairing (RPIP)-HPLC has been used successfully for the analysis of HS/HP disaccharides.[10] However, the ion-pairing reagents in mobile phase decrease the sensitivity of analytes on electrospray ionization mass and seriously contaminate the MS ion source, making this analytical method difficult to use on shared LC-MS instruments. Several highly sensitive analytical methods have involved labeling GAG-derived disaccharides through their reductive amination with AMAC, 2-aminobenzamide (2-AB), and 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3- propionic acid (BODIPY) fluorophores. These labeled disaccharides are then detected with high sensitivity by HPLC[18] and CE with laser-induced fluorescence detection (CE-LIF).[21,22] Recently, we demonstrated an improved, simplified, and highly sensitive method to analyze 17 AMAC-tagged standard disaccharides from HS/HP, CS/DS, and HA by a single RP-UPLC-MS analysis.[14] The current study examines the combination of microscale GAG recovery from CHO-S cells with this RP-UPLC-MS analysis.
RESULTS AND DISCUSSION
Recovery of HA, CS-A, and HP Using Anion Exchange Column
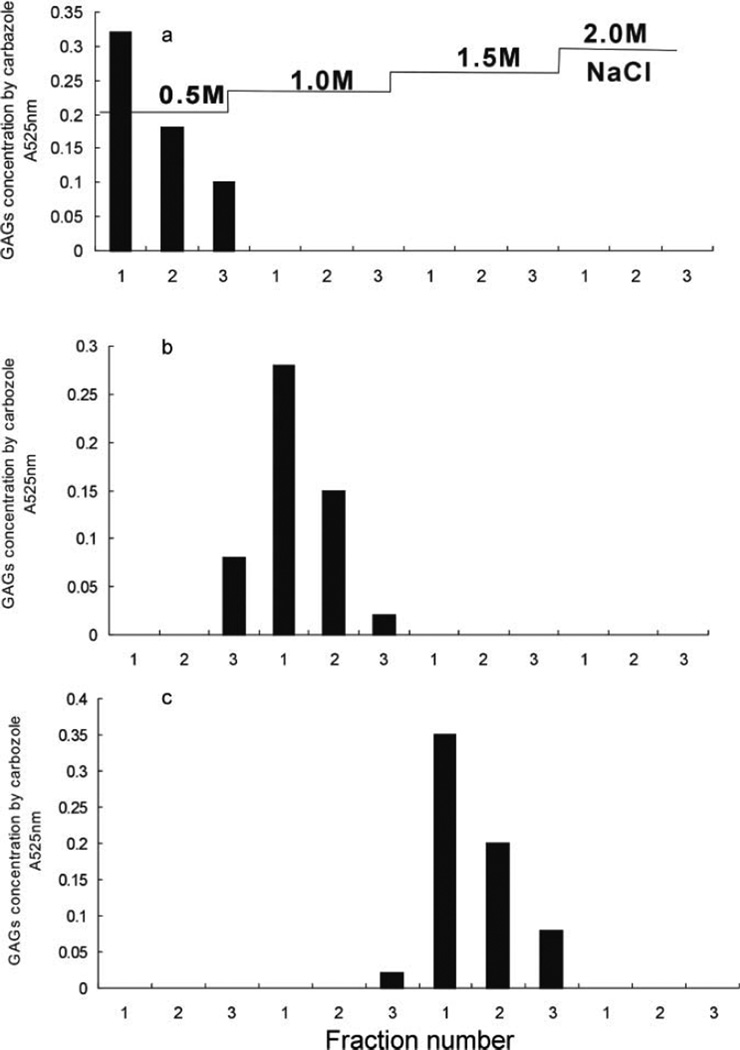
HA (nonsulfated), CS-A (moderately sulfated, ~1 sulfate/disaccharide), and HP (highly sulfated, ~2.7 sulfates/disaccharide) were individually (100 µg of each) dissolved in water and bound to a mini-strong anion exchange spin column. The column was washed by water and sequentially three times with 0.1, 0.2, 0.3, 0.5, 1.0, 1.5, and 2.0 M NaCl. The GAG concentration in each tube was measured by carbazole assay (Fig. 1). Despite an absence of sulfate groups, HA bound to the mini-strong anion exchange spin column. The 100-µg HA that bound to the strong anion exchange spin column was completely eluted by washing the column three times with 200 µL of 0.5 M NaCl. As GAGs with a moderate and high level of sulfate groups, CS-A and HP were released from strong anion exchange columns with 1.0 M and 1.5 M NaCl, respectively. Thus, for the microscale analysis of GAGs, a simple separation procedure, strong anion exchange spin columns can facilitate the separate or combined recovery of HA, CS-A, and HP, GAGs having very different sulfation levels.
Figure 1.
HA, CS-A, and HP recovered using a mini-strong anion exchange column. GAG concentration was determined at 525 nm by carbazole assay. (a) HA. (b) CS-A. (c) HP.
Recovery of HA from Protein Mixture by Strong Anion Exchange Column
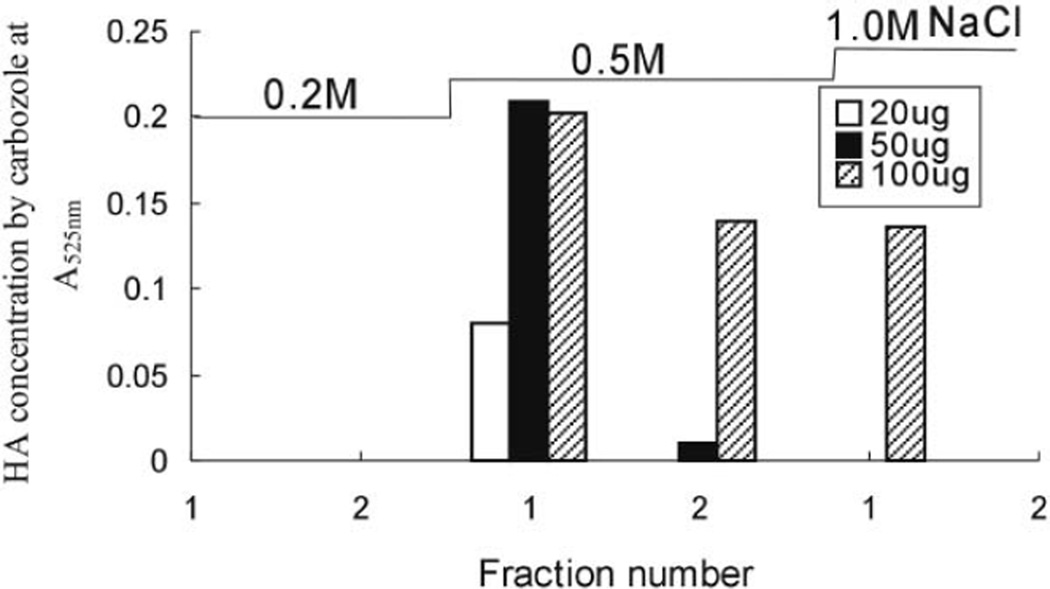
HA (20 µg, 50 µg, and 100 µg) was mixed with the same amounts of bovine serum albumin before using a mini-strong anion exchange column to investigate if the presence of protein in the sample would influence HA recovery. After proteolysis and filtration, mini-strong anion exchange spin column was washed with 8 M urea buffer (2% CHAPS, pH 8.3) to remove peptides, followed by sequential, duplicate washes with 0.1, 0.2, 0.5, and 1.0 M aqueous NaCl solution. The HA concentration in each wash was determined by carbazole assay (Fig. 2). HA bound to the mini-strong anion exchange column and was recovered in the 0.5 M NaCl wash. In the 100-µg HA sample, some HA was also recovered in 1.0 M NaCl wash. The percent recoveries from samples containing 20 µg, 50 µg, and 100 µg of HA were 86%, 79%, and 93%, respectively. Protein concentration, determined by the UV absorbance at 275 nm, showed that ~70% of the bovine serum albumin had been removed by Actinase E proteolysis followed by ultrafiltration through a 10-kDa MWCO membrane and that the remaining protein was removed by washing the mini-strong anion exchange column with 8 M urea buffer, water, and 0.2 M NaCl, suggesting that the presence of protein in a sample would not adversely impact the recovery of GAGs (data not shown).
Figure 2.
HA recovered from an HA and albumin mixture using a mini-strong anion exchange column.
Separation and Analysis of GAGs in CHO-S Cells
While reverse-phase ion-pairing (RPIP)-UPLC coupled to electrospray ionization-mass spectrometry (ESI-MS) has been successfully applied for disaccharide analysis,[10] the presence of nonsulfated GAGs in CHO-S cells has been difficult to establish. This is because nonsulfated GAGs, such as heparosan, chondroitin, and hyaluronic acid, are hard to recover from cell samples, and in disaccharide analysis they elute early, close to the residual salt peak at the solvent front. Moreover, even when such analysis is successful, it is unclear whether the 0SHS and 0SCS disaccharides come from nonsulfated domains within moderately sulfated GAG chains or from GAG chains that are completely devoid of all sulfation.
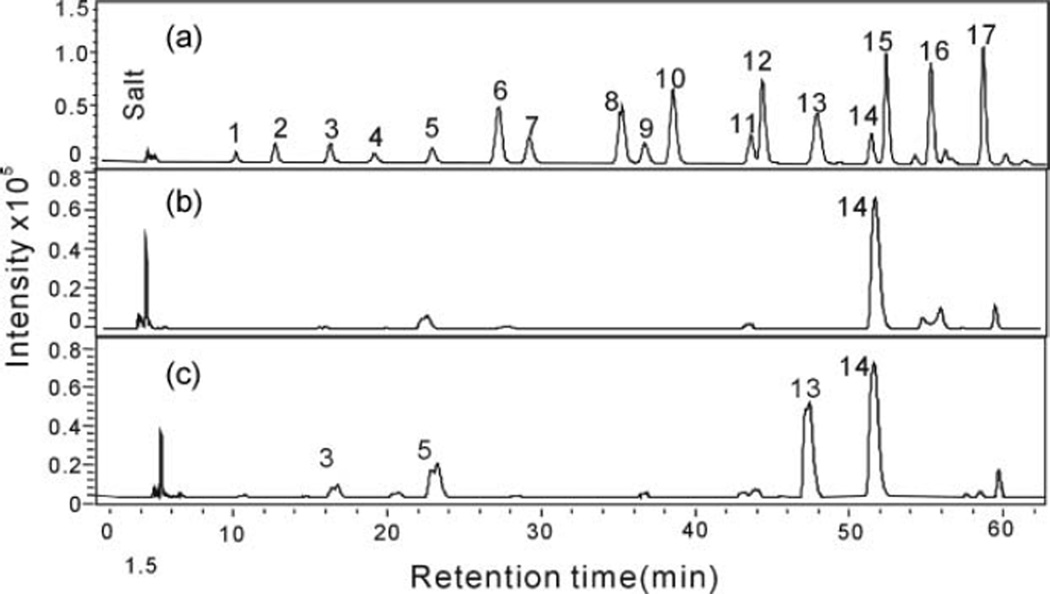
A strong anion exchange spin column was used to separate the nonsulfated, moderately sulfated, and highly sulfated GAGs. The GAGs recovered in the 0.5 M and the 16% NaCl washes were treated with GAG lyases and AMAC labeled, and RP-UPLC-MS analysis was performed. The extracted ion chromatograms (EICs) of the disaccharides obtained from the CHO-S cell GAGs are presented in Figure 3 and their composition in Table 1. Disaccharide analysis of endogenous CHO-S cell GAGs, eluting in the 0.5 M NaCl, showed a single peak corresponding to 0SHS (1.62 µg) consistent with the presence of heparosan chains devoid of all sulfation (Fig. 3b). No 0SHA or 0SCS was observed, consistent with the absence of hyaluronic acid and nonsulfated chondroitin. The endogenous GAGs from CHO-S cells recovered in the 16% NaCl wash showed the presence of three major disaccharides, 0SHS, NSHS, and NS2SHS, and three minor disaccharides, 2SHS, 6S2SHS and TriSHS, from heparan sulfate chains both nonsulfated, moderately sulfated, and highly sulfated sequences (Fig. 3c). Only one type of CS/DS disaccharide was observed corresponding to 4SCS, consistent with CS-A chains of uniform structure. The observed composition in the 16% NaCl wash is consistent with the disaccharide composition previously reported by RPIP-UPLC-MS analysis of CHO-S cells.[10]
Figure 3.
Disaccharide analysis, based on EIC, of endogenous GAGs present in CHO-S cells. (a) Seventeen standard disaccharides from HS/HP, CS, and HA: 1. Tri SHS, 2. NS6SHS, 3. NS2SHS, 4. Tri SCS, 5. NSHS, 6. SBCS, 7. 2S6SHS, 8. SDCS, 9. 6SHS, 10. SECS, 11. 2SHS, 12. 2SCS, 13. 4SCS, 14. 0SHS, 15. 6SCS, 16. 0SHA, 17. 0SCS. (b) Endogenous GAGs in CHO-S cells recovered in 0.5M NaCl wash. (c) Endogenous GAGs in CHO-S cells recovered in 16% NaCl wash.
Table 1.
Disaccharide composition of GAGs from CHO-S cells
| CSa | HS/HPa | ||||||
|---|---|---|---|---|---|---|---|
| Sample resource | 4SCS | TriSHS | 4SHS | NSHS | 6SHS | 2SHS | OSHS |
| GAGs eluted by 0.5 M NaCl | 28.8 ± 4.6b | ||||||
| GAGs eluted by 16% NaCl | 9.2 ± 0.8 | 1.5 ± 0.9 | 9.3 ± 0.8 | 17.9 ± 1.1 | 0.64 ± 0.2 | 0.73 ± 0.1 | 33.9 ± 1.7 |
Calculated based on all the disaccharides afforded from HS/HP and CS corresponding to 100%.
Values are mean ± SD from triplicate experiments.
Surprisingly, we recovered heparosan in the 0.5 M NaCl wash, demonstrating that 29% of all the GAGs (0.52 µg CS, 3.48 µg HS, and 1.62 µg heparosan) in CHO-S cells were composed of heparosan, HS chains devoid of all sulfation. HS/HP is biosynthesized through a similar pathway in all eukaryotic cells. Chain elongation of heparosan on the tetrasaccharide linkage region on a core protein takes place in the Golgi, after which this linear homocopolymer is sequentially modified through the action of N-deacetylase/N-sulfotransferase (NDST), C-5 epimerase (C5 Epi), and 2-,6- and 3-O-sulfotransferases (OSTs).[23] Apparently, in the case of CHO-S cells, no chain modification takes place on nearly a third of these chains. Current research in our laboratory is aimed at metabolic engineering of the HS biosynthetic pathway to produce secreted CHO cell HP. Metabolic engineering has focused on stably inserting selected genes encoding for more extensive chain modification steps, such as NDST-2 and 3OST-1, favoring the formation of anticoagulant HP in CHO cells.[13] The results of the current study demonstrate that a major fraction of chains in the CHO-S cell line escape all modification reactions. This suggests that these chains occupy Golgi compartments devoid of modification enzymes and that more extensive metabolic engineering combined with pathway balancing may be required for the successful production of HP in CHO cell culture. The separation and quantification of heparosan from cultured CHO cells will aid in monitoring the progress of these metabolic engineering studies.
Recovery of HA, CS-A, and HP in CHO-S Cells by Mini-Strong Anion Exchange Columns
In control experiments, added pure HA, CS-A, and HP (2 µg of each) were digested exhaustively with chondroitinases and heparinases, AMAC labeled, and analyzed by RP-UPLC-MS. Disaccharide analysis revealed that 71.2%, 62.4%, and 74.6% of the added HA, CS-A, and HP, respectively, had been recovered. The loss was mainly attributed to incomplete enzymatic digestion and incomplete recovery of disaccharides by MWCO spin column used to remove the GAG lyases. Next, CHO-S cells were used as the background for the evaluation of the recovery of added HA (nonsulfated), CS-A (moderately sulfated, ~1 sulfate/disaccharide), and HP (highly sulfated, ~2.7 sulfates/disaccharide) from a complex mixture. It should be noted that our analysis of CHO-S GAGs (Table 1) established the absence of 0SHA, 0SCS, and 6SCS containing GAGs with only very small amounts of TriSHS containing GAGs. Since these disaccharides are prominent in the added HA, CS-A, and HP, their presence serves as internal standards allowing us to calculate the recovery of HA (nonsulfated GAG), CS-A (moderately sulfated GAG), and HP (highly sulfated GAG).
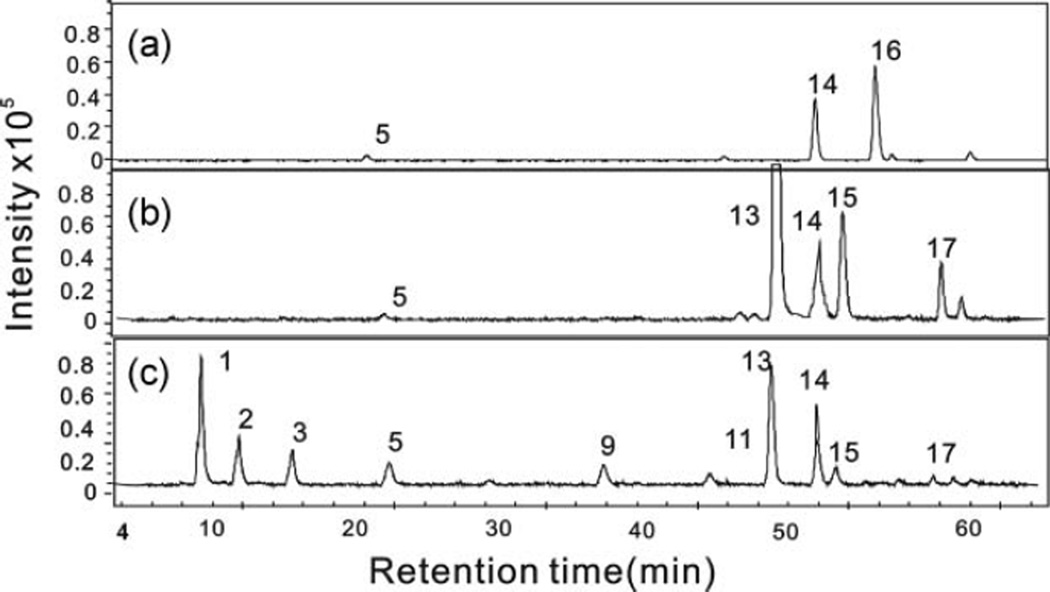
After strong anion exchange spin column, followed by chondroitinase and heparinase digestion and AMAC labeling, the EICs were obtained in the RP-UPLC–MS disaccharide analysis of exogenous HA, CS-A, and HP added to CHO-S cells and percent recovery are shown in Figure 4 and Table 2. The 0.5 M NaCl wash of cell samples containing 2 µg of added HA gave RP-UPLC-MS analyses showing two expected disaccharide peaks, corresponding to 0SHS and 0SHA, associated with the endogenous heparosan and exogenous HA (Fig. 4a), consistent with the anticipated weak binding of HA to the strong anion exchange spin column. No 0SHA was observed in the disaccharide analysis of GAGs eluting in the 16% NaCl wash; instead, the disaccharide analysis of the 16% NaCl wash showed the presence of HS/HP and CS/DS disaccharides in the CHO-S cell samples. As shown in Figure 4b, 4SCS, 6SCS, and 0SCS concentration in CHO cells increased in GAGs eluted by 16% NaCl from the CS-A/CHO cell mixture. After addition of HP in CHO cells, TriSHS, NS6SHS, NS2SHS, 2S6SHS, 6SHS, and 2SHS peaks from heparin appeared in GAGs eluted by 16% NaCl and the concentration of NSHS and 0SHS increased (Fig. 4c).
Figure 4.
Disaccharide analysis, based on EIC, of HA, CS-A, and HP GAGs added to CHO-S cells. (a) HA recovered from HA/CHO-S cell mixture in 0.5 M NaCl wash. (b) CS-A recovered from CS-A/CHO-S cell mixture in 16% NaCl wash. (c) HP recovered from HP/CHO-S cell mixture in 16% NaCl wash.
Table 2.
Recovery percent (%, based on the data from disaccharide analysis) of HA, CS-A, and HP added to CHO-S cells
| GAGs recovered from cells | GAGs recovered from buffer (control) | ||||
|---|---|---|---|---|---|
| GAGs | Exogenous GAG amount (µg) |
Recovery rate (%) of SAX spin column isolation |
Recovery rate (%) based on disaccharide analysis |
Recovery rate (%) of SAX spin column isolation |
Recovery rate (%) based on disaccharide analysis |
| HA | 2 | 49.7 ± 4.5a | 35.4 ± 6.5 | 74.7 ± 3.6 | 53.2 ± 4.3 |
| 5 | 45.2 ± 3.6 | 32.2 ± 4.3 | |||
| CS-A | 2 | 48.5 ± 5.2 | 30.3 ± 5.4 | 69.2 ± 4.5 | 43.2 ± 3.5 |
| 5 | 58.9 ± 4.3 | 36.8 ± 4.5 | |||
| HP | 2 | 47.5 ± 3.5 | 35.4 ± 3.5 | 61.1 ± 4.8 | 45.6 ± 6.3 |
| 5 | 53.3 ± 4.2 | 39.8 ± 4.8 | |||
Values are mean ± SD from triplicate experiments.
The percent recovery of the 2 and 5 µg of added HA from the CHO cell/HA mixture in the form of 0SHA was 35% and 32%, respectively. In HA control experiments without CHO-S cells, the percent recovery of 0SHA from 2 µg HA, after all the same procedures, was 53.2%. The percent recovery of SAX spin column isolation from samples containing 2 and 5 µg of added HA was calculated as 50% and 45%, respectively. The recovery of SAX spin column isolation CS-A and heparin was 59% and 53%, respectively. The low GAGs recovery from CHO samples might be due to the multiple-step treatment procedure, including protein removal and desalting by membrane ultrafiltration, enzymatic disaccharide preparation, and purification. Our previous experiments showed that 100% of disaccharides were not able to pass through the ultrafiltration membrane (MWCO 10 kDa) even after five times washes. Despite the reduction in GAG recovery in the presence of cells, the percent recovery was still sufficient for reliable microscale analysis.
CONCLUSION
In summary, all GAG chains can be recovered and quantified from cultured cells using a stepwise procedure involving protease digestion and strong anion exchange chromatography, followed by centrifugal desalting, enzymatic digestion, AMAC labeling, and RP-UPLC-MS disaccharide analysis. The recovered AMAC-labeled disaccharides are free of most impurities and are of sufficient purity for accurate quantification. This method also demonstrates, for the first time, that heparosan, a nonsulfated form of HS, is a major GAG component in CHO-S cells.
EXPERIMENTAL
Materials and Methods
Heparin (HP) sodium salt from pig intestinal and chondroitin sulfate A (CS-A) sodium salt from whale cartilage were from Celsus Laboratories (Cincinnati, OH, USA). Hyaluronic acid (HA) sodium salt was provided by Prof. Toshihiko Toida (Chiba University, Japan). Vivapure Q Mini H spin columns were from Sartoriou Stedim Biotech (Bohemia, NY, USA). The eight unsaturated HS/HP disaccharide standards unsaturated heparin/HS disaccharides standards (Di-0S, ΔUA-GlcNAc; Di-NS, ΔUA-GlcNS; Di-6S, ΔUA-GlcNAc6S; Di-UA2S, ΔUA2S-GlcNAc; Di-UA2SNS, ΔUA2S-GlcNS; Di-NS6S, ΔUA-GlcNS6S; Di-UA2S6S, ΔUA2S-GlcNAc6S; and Di-triS, ΔUA2S-GlcNS6S) and eight unsaturated CS/DS disaccharides standards (Di-0S, ΔUA-GalNAc [where ΔUA is Δ-deoxy-L-threo-hex-4-enopyranosyl uronic acid]; Di-4S, ΔUA-GalNAc4S; Di-6S, ΔUA-GalNAc6S; Di-UA2S, ΔUA2S-GalNAc; Di-diSB, ΔUA2S-GalNAc4S; Di-diSD, ΔUA2S-GalNAc6S; Di-diSE, ΔUA-GalNAc4S6S; Di-triS, ΔUA2S-GalNAc4S6S) were obtained from Seikagaku Corporation (Japan). One unsaturated disaccharide from HA, 0SHA, was from Iduron Co (Manchester, UK). AMAC and NaCNBH3 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Actinase E was from Kaken Biochemicals (Tokyo, Japan). Chondroitin lyase ABC from Proteus vulgaris and chondroitin lyase ACII from Arthrobacter aurescens was from Seikagaku Corporation (Tokyo, Japan). Recombinant Flavobacterial heparin lyase I, II, and III were expressed in our laboratory using Escherichia coli strains, provided by Professor Jian Liu (University of North Carolina, College of Pharmacy, Chapel Hill, NC, USA).
CHO-S Cell Culture
CHO-S cells were grown in CD–CHO medium supplemented with 2% HT (hypoxanthine/thymidine mixture, Gibco–Invitrogen, Carlsbad, CA, USA) and 8 mM Glutamax (Gibco–Invitrogen, Carlsbad, CA, USA). The cells were incubated in a 5% CO2 and 37°C incubator. For routine maintenance, cells were seeded at 2 × 105 cells/mL fresh media and cells were subcultured every 3 to 4 days. Cell viability was measured with hemocytometry and the trypan blue exclusion method.
Recovery of HA, CS-A, and HP by Strong Anion Exchange Column
HA, CS-A, and HP (100 µg) were individually dissolved into 200-µL aliquots of water. After a Vivapure MINI Q H spin column was pre-equilibrated with 200 µL of water, the sample was bound on the columns by centrifugation at 700 × g.[9] The column was eluted by washing three times with 200 µL of water followed by three washes with 0.1, 0.2, 0.3, 0.5, 1.0, 1.5, and 2.0 M NaCl solution. The content of GAG collected in each tube was estimated by carbazole assay.[15]
Recovery of HA from Protein by Strong Anion Exchange Column
HA samples (20 µg, 50 µg, or 100 µg) were individually mixed with bovine serum albumin (20 µg, 50 µg, or 100 µg). The samples dissolved in 1 mL water were individually proteolyzed with 5 mg/mL Actinase E for 20 h at 55°C. The enzymatic products were filtered using an YM-10kDa MWCO centrifugal filter (Millipore, Billerica, MA, USA) to remove peptides. Samples from the top level of the filtration membrane were collected and lyophilized. The Vivapure MINI Q H spin column was pre-equilibrated with 8 M urea (2% CHAPS, pH 8.3), and after the digested sample was dissolved in 8 M urea buffer, the sample was bound on the column by centrifugation at 700 × g. After washing twice with 8 M urea buffer (2% CHAPS at pH 8.3), HA was eluted by washing twice with 200 µL of 0.2 M, 0.5 M, and 1.0 M NaCl solution. The content of HA in each tube was estimated by carbazole assay.[15] The percent recovery of HA (%) = [A525nm/Ain525nm] × 100 (A525nm: absorbance of HA by carbazole assay; Ain525nm: initial absorbance of HA in 200 µL by carbazole assay).
Recovery of HA, CS-A, and HP from CHO-S Cells by Strong Anion Exchange Column[10]
CHO-S cells (1 × 107) was freeze-dried overnight and incubated with actinase E (2 mg/mL) in 1 mL solution at 55°C for 20 h. After removing the particulates from the resulting solutions by a 0.22-µm membrane, the peptides were removed by a Microcon Centrifugal Filter Units YM-10 (10 k MWCO) by centrifuging at 10,000 × g. Then residual GAGs on membrane were collected and purified by Vivapure MINI Q H spin column by eluting with three 300-µL washes each of water, 0.2 M, 0.5 M, and 16% NaCl.
Another HA, CS-A, and HP (2 µg or 5 µg) sample was individually mixed with CHO-S cells (1 × 107) and the same procedure was applied to separate HA, CS-A, HP, and endogenous GAGs in CHO-S cells by Vivapure MINI Q H spin column after proteolysis and filtration to remove peptides. The 0.5 M and 16% aqueous NaCl washes were each recovered, desalted, and lyophilized for disaccharide analysis. HA, CS-A, and HP (2 µg) without cells were treated similarly to control experiments. The percent recovery of HA (CS-A and HP) (%) = total amount of disaccharides from HA (CS-A and HP)/initial amount of HA (CS-A and HP).
Preparation of Disaccharides
HA, CS-A, and endogenous CS/DS GAGs from CHO-S cells were converted to disaccharides by enzymatic treatment with chondroitin lyase ABC (10 m-units) and chondroitin lyase ACII (10 m-units) for 10 h at 37°C. After boiling the chondroitin lyases at 100°C for 2 min to inactivate and cooling to rt, the HS/HP in GAGs were converted to disaccharides by enzymatic treatment with a mixture of heparin lyase I, II, and III (10 mU each) for 10 h at 37°C. All the disaccharides were recovered using a 30-kDa MWCO spin column (Millipore, USA) and freeze-dried. Disaccharide obtained by digestion of 2 µg HA with chondroitin lyase ABC (10 m-units) and chondroitin lyase ACII (10 m-units) at 37°C for 10 h was used as a control experiment.
Derivatization of Unsaturated Disaccharides with AMAC
AMAC solution (10 µL of 0.1 M AMAC in acetic acid/dimethylsulfoxide 3/17 (v/v)) was added to the recovered, freeze-dried disaccharides recovered from HA and CHO cells and incubated at rt for 15 min.[18] Next, 10 µL of 1 M NaBH3 CN was added to the reaction mixture and incubated at 45°C for 4 h. After centrifugation for 5 min at 10,000 × g, the supernatant containing AMAC-tagged disaccharide was collected for RP-UPLC-MS analysis using a mixture of 17 disaccharides (25 ng/µL each disaccharide) as a standard.
RP-UPLC-MS Analysis
LC-MS analyses were performed on an Agilent 1200 LC/MSD instrument (Agilent Technologies, Wilmington, DE) equipped with a 6300 ion-trap. The column used was an Acquity UPLC BEH C18 column (2.1 × 150 mm, 1.7 µm,Waters, Milford, MA, USA) at 45°C. For the dual ammonium acetate and methanol gradient, eluent A was 80 mM ammonium acetate solution and eluent B was methanol. Solution A and 12% solution B were flowed (95 µL/min) through the column for 15 min followed by linear gradients of 12% to 15% solution B from 15 to 30 min, 15% to 30% solution B from 30 to 60 min, and 30% to 100% solution B from 60 to 62 min.
Calibration
Quantification analysis of AMAC-labeled disaccharides was performed using calibration curves constructed by separation of increasing amounts of 17 AMAC-labeled unsaturated disaccharide standards (5, 10, 25, 50, 100 ng/each disaccharide). Linearity was assessed based on the amount of disaccharide and EIC. All analyses were performed in triplicate.
ACKNOWLEDGMENTS
The work was supported by grants from the National Institutes of Health in the forms of grants GM38060, GM090127, HL096972, and HL101721 and the New York State Stem Cell Foundation N08G-264. Xue Zhao was supported by the China Scholarship Council.
REFERENCES
- 1.Turnbull JE, Field RA. Emerging glycomics technologies. Nat. Chem. Biol. 2007;3:74–77. doi: 10.1038/nchembio0207-74. [DOI] [PubMed] [Google Scholar]
- 2.Guimond S, Puvirajesinghe TM, Skidmore MA, Kalus I, Dierks T, Yates EA, Turnbull JE. Rapid purification and high sensitivity analysis of heparan sulfate from cells and tissues: toward glycomics profiling. J. Biol. Chem. 2009;284:25714–25722. doi: 10.1074/jbc.M109.032755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ly M, Laremore TN, Linhardt RJ. Proteoglycomics: recent progress and future challenges. Omics. 2010;14:389–399. doi: 10.1089/omi.2009.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capila I, Linhardt RJ. Heparin-protein interactions. Angew Chemie Int. Ed. 2002;41:390–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 5.Lin X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development. 2004;131:6009–6021. doi: 10.1242/dev.01522. [DOI] [PubMed] [Google Scholar]
- 6.Linhardt RJ, Toida T. Role of glycosaminoglycans in cellular communication. Acc. Chem. Res. 2004;37:431–438. doi: 10.1021/ar030138x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang F, Zhang Z, Linhardt RJ. Glycosaminoglycans. In: Cummings RD, Pierce M, editors. Handbook of Glycomics: Glycoconjugate Structural Analysis. London: Academic Press/Elsevier; 2009. pp. 59–80. [Google Scholar]
- 8.Volpi N, Linhardt RJ. High-performance liquid chromatography-mass spectrometry for mapping and sequencing glycosaminoglycan-derived oligosaccharides. Nat. Protoc. 2010;5:993–1004. doi: 10.1038/nprot.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang FM, Sun PL, Mu E, Chi LL, Sakai S, Toida T, Zhang HF, Mousa S, Linhardt RJ. Microscale isolation and analysis of heparin from plasma using an anion-exchange spin column. Anal. Biochem. 2006;353:284–286. doi: 10.1016/j.ab.2006.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang B, Weyers A, Baik JY, Sterner E, Sharfstein S, Mousa S, Zhang FM, Dordick J, Linhardt RJ. Ultra-performance ion-pairing liquid chromatography with on-line electrospray ion trap mass spectrometry for heparin disaccharide analysis. Anal. Biochem. 2011;415:59–66. doi: 10.1016/j.ab.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hossler P, Khattak SF, Li ZJ. Optimal and consistent protein glycosylation in mammalian cell culture. Glycobiology. 2009;19:936–949. doi: 10.1093/glycob/cwp079. [DOI] [PubMed] [Google Scholar]
- 12.Kraemer PM, Barnhart BJ. Elevated cell-surface hyaluronate in substrate-attached cells. Exp Cell Res. 1978;114:153–157. doi: 10.1016/0014-4827(78)90047-2. [DOI] [PubMed] [Google Scholar]
- 13.Baik JY, Gasimli L, Yang B, Datta P, Zhang F, Glasse CA, Esko JD, Linhardt RJ, Sharfstein ST. Metabolic engineering of Chinese hamster ovary cells: towards a bioengineered heparin. Metab. Eng. 2012;14:81–90. doi: 10.1016/j.ymben.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang B, Chang Y, Weyers AM, Sterner E, Linhardt RJ. Disaccharide analysis of glycosaminoglycan mixtures by ultra-performance liquid chromatography-mass spectrometry. J. Chromatogr. A. 2012;1225:91–98. doi: 10.1016/j.chroma.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cesaretti M, Luppi E, Maccari F, Volpi N. A 96-well assay for uronic acid carbazole reaction. Carbohydr. Polym. 2003;54:59–61. [Google Scholar]
- 16.Grant AC, Linhardt RJ, Fitzgerald GL, Park JJ, Langer R. Metachromatic activity of heparin and heparin fragments. Anal. Biochem. 1984;137:25–32. doi: 10.1016/0003-2697(84)90341-5. [DOI] [PubMed] [Google Scholar]
- 17.Chun LE, Koob TJ, Eyre DR. Quantitation of hyaluronic acid in tissues by ion-pair reverse-phase high-performance liquid chromatography of oligosaccharide cleavage products. Anal. Biochem. 1988;171:197–206. doi: 10.1016/0003-2697(88)90142-x. [DOI] [PubMed] [Google Scholar]
- 18.Ambrosius M, Kleesiek K, Götting C. Quantitative determination of the glycosaminoglycan Δ-disaccharide composition of serum, platelets and granulocytes by reversed-phase high-performance liquid chromatography. J. Chromatogr. A. 2008;1201:54–60. doi: 10.1016/j.chroma.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Lamari F, Karamanos NK. State-of-the-art of capillary electrophoresis with application to the area of glycoconjugates. Biomed. Chromatogr. 1999;13:501–506. doi: 10.1002/(SICI)1099-0801(199912)13:8<501::AID-BMC956>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Calabro A, Hascall VC, Midura RJ. Adaptation of FACE methodology for microanalysis of total hyaluronan and chondroitin sulfate composition from cartilage. Glycobiology. 2000;10:283–293. doi: 10.1093/glycob/10.3.283. [DOI] [PubMed] [Google Scholar]
- 21.Volpi N, Maccari F, Linhardt RJ. Quantitative capillary electrophoresis determination of oversulfated chondroitin sulfate as a contaminant in heparin preparations. Anal. Biochem. 2009;388:140–145. doi: 10.1016/j.ab.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eldridge SL, Higgins LA, Dickey BJ, Larive CK. Insights into the capillary electrophoresis separation of heparin disaccharides from nuclear magnetic resonance, pKa, and electrophoretic mobility measurements. Anal. Chem. 2009;81:7406–7415. doi: 10.1021/ac901218q. [DOI] [PubMed] [Google Scholar]
- 23.Lindhal U, Feingold DS, Roden L. Biosynthesis of heparin. Trends Biochem. Sci. 1986;11:221–225. [Google Scholar]