Abstract
Background
Low left ventricular ejection fraction (LVEF) increases risk for both sudden cardiac death (SCD) and for heart failure (HF) death; however, implantable cardioverter‐defibrillators (ICDs) reduce the incidence of SCD, not HF death. Distinguishing individuals at risk for HF death (non‐SCD) versus SCD could improve ICD patient selection.
Objective
This study evaluated whether electrocardiogram (ECG) quantification of myocardial infarction (MI) could discriminate risk for SCD versus non‐SCD.
Methods
Selvester QRS scoring was performed on 995 MADIT‐II trial subjects’ ECGs to quantify MI size. MIs were categorized as small (0–3 QRS points), medium (4–7) or large (≥8). Mortality, SCD and non‐SCD rates in the conventional medical therapy (CMT) arm and mortality and ventricular tachycardia/fibrillation (VT/VF) rates in the ICD arm were analyzed by QRS score group. Both arms were analyzed to determine ICD efficacy by QRS score group.
Results
In the CMT arm, mortality, SCD and non‐SCD rates were similar across QRS score groups (P = 0.73, P = 0.92, and P = 0.77). The ICD arm showed similar rates of mortality (P = 0.17) and VT/VF (P = 0.24) across QRS score groups. ICD arm mortality was lower than CMT arm mortality across QRS score groups with greatest benefit in the large scar group.
Conclusion
Recently, QRS score was shown to be predictive of VT/VF in the SCD‐HeFT population consisting of both ischemic and nonischemic HF and having a maximum LVEF of 35% versus 30% for MADIT‐II. Our study found that QRS score did not add prognostic value in the MADIT‐II population exhibiting relatively more severe cardiac dysfunction.
Keywords: sudden death, heart failure, implantable cardioverter‐defibrillator, electrocardiography, electrophysiology, tachyarrhythmias
The mortality benefit of implantable cardioverter‐defibrillators (ICD) in patients with prior myocardial infarction (MI) and reduced left ventricular ejection fraction (LVEF) demonstrated in Multicenter Automatic Defibrillator Implanta‐tion Trial II (MADIT‐II) and Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT) has resulted in recommendation for broad use of these devices and dramatically increased implantation rates in recent years.1, 2, 3, 4 The high incremental cost‐effectiveness ratio and relatively low rate of appropriate ICD discharge illustrates the need for better risk‐stratification techniques to identify patients for ICD implantation.5, 6
The mortality reduction in MADIT‐II was due to a reduction in sudden cardiac death (SCD) with no effect on heart failure (HF) deaths.7 Prior studies have shown that as HF progresses, the relative risk of total mortality and HF death (non‐SCD) increases while the relative risk of SCD decreases.8, 9, 10 This may explain the U‐shaped pattern in ICD efficacy observed where ICD efficacy was greatest in patients with an intermediate risk of death, but lower in patients at highest risk for death.11 Distinguishing individuals at high risk for non‐SCD from those primarily at risk for SCD may allow for identification of who will benefit most from ICDs.
Analysis of the SCD‐HeFT trial found that quantifying infarct size using the Selvester QRS score was predictive of ventricular tachycardia or ventricular fibrillation (VT/VF) and, when combined with LVEF, was able to separate subgroups of low, medium, and high risk of VT/VF.12 Additionally, several studies have implicated infarct size as a predictor of both heart failure development and worse outcomes.13, 14, 15 The value of the QRS score in predicting SCD versus non‐SCD risk in ICD eligible subjects has not yet been examined.
This study evaluated whether QRS score is useful for risk stratification of ICD eligible patients. We hypothesized that (1) subjects with moderate/large infarcts would have the highest rate of SCD; (2) as MI size increases, the rates of non‐SCD would approach that of VT/VF and/or SCD; and (3) ICDs would confer the greatest mortality benefit in patients with large MIs.
METHODS
Population
MADIT‐II enrolled subjects who were more than 21 years of age with an MI 1 month or more before entry and an LVEF of ≤30% as described previously.1 New York Heart Association (NYHA) class IV patients were excluded. Subjects were randomized in a 3:2 ratio to receive an ICD (N = 742) or conventional medical therapy (CMT, N = 490). We analyzed the ICD and CMT arms independently except for the evaluation of ICD efficacy, for which we utilized data from both arms. The Duke University Health System Institutional Review Board approved this substudy.
ECG Analysis
All MADIT‐II subjects received electrocardiograms (ECGs) before randomization. Standard 12‐lead ECGs were evaluated by a single investigator (ZL) blinded to all clinical data aside from age and gender. ECGs were excluded from analysis if poor quality, missing lead information, ventricular paced rhythm, or indeterminate QRS axis prohibited accurate evaluation. Selvester QRS scoring was performed as previously described.12, 16, 17 Briefly, conduction‐specific QRS‐scoring criteria involving Q‐, R‐ and S‐wave amplitudes, durations, amplitude ratios and notches in 10 of the 12 standard ECG leads (excluding leads III and aVR) were applied. Due to the QRS score's strict assessment of waveform durations, amplitude ratios and QRS notching, ECGs were required to be of high quality to facilitate accurate scoring. ECGs that were difficult to assess to due to quality concerns were adjudicated in conference (with additional scorer GSW) or excluded to poor quality. Interobserver and intraobserver variability was determined by rescoring 10% of the overall sample by the primary scorer and an additional experienced scorer (GSW).
QRS scores were evaluated as both continuous and categorical predictors. For categorization, QRS scores were classified as low (0–3 points), medium (4–7 points) and high (≥8 points) based on prior studies showing the prognostic value of these strata.18, 19 A dichotomized QRS score (0–1 vs. ≥2 QRS points) was also utilized for comparison to the 0 versus ≥1 dichotomous score used in the previous SCD‐HeFT substudy while still maintaining an adequate sample size.12
Outcomes
The cause of death was adjudicated by a blinded events committee,7 using a modified Hinkle‐Thaler Scheme. Cardiac deaths were subdivided into sudden (SCD), nonsudden (non‐SCD) and unclassified cardiac death.
The ICDs of subjects were interrogated after each shock episode or antitachycardia pacing (ATP) and the rhythm preceding the event was classified according to prespecified criteria.20 Episodes of VT or VF preceding an ICD shock or ATP was the primary end point. All‐cause mortality was the secondary end point.
Statistical Analysis
Wilcoxon rank‐sum and chi‐square statistics were used for comparison of descriptive statistics. Interclass correlation coefficient values are reported for a randomly selected subset (10% of subjects, N = 123). Survival analysis for the relationship between QRS score and overall mortality, SCD, VT/VF and non‐SCD was performed by a log‐rank test for the categorical and dichotomous QRS score and by Cox proportional hazards for the continuous QRS score. Kaplan‐Meier plots were generated for the stratified QRS score for overall mortality, SCD, VT/VF, and non‐SCD for a mean follow‐up of 1.7 years. All survival analyses were performed with Cox proportional hazards controlling for LVEF and subgroup analysis was performed excluding patients with right or left bundle branch block. Two‐sided significance was set at α = 0.05.
RESULTS
Study Population
Of the 1232 subjects included in the MADIT‐II study, 995 (81%) had ECGs of sufficient quality for QRS scoring. Tables 1A and 1B show baseline characteristics of subjects in the different QRS score groups. In both arms, left bundle branch block (LBBB) was more common in the QRS Low and QRS Med groups; whereas, right bundle branch block (RBBB) was seen more frequently in the QRS High group. The mean LVEF was similar across QRS score groups (22.9%, 23.1%, and 22.8% for QRS Low, Med, and High, respectively). After a mean follow‐up of 19.9 months, 81 subjects in the CMT arm died (40 SCD, 20 non‐SCD) and 151 subjects in the ICD arm experienced VT/VF (117 VT, 34 VF).
Table 1.
A. Baseline Characteristics by QRS Score Group for Conventional Medical Therapy Arm: Data Are Presented as Mean ± Standard Deviation or N (%) of the Population
| Clinical Characteristics | Conventional Medical Therapy Arm | |||
|---|---|---|---|---|
| QRS Low (0–3) | QRS Med (4–7) | QRS High (≥8) | P‐ | |
| N = 104 | N = 135 | N = 164 | Value | |
| Age at randomization | 65 ± 11 | 66 ± 10 | 63 ± 11 | 0.066 |
| Body mass index (kg/m2) | 28 ± 5 | 28 ± 5 | 28 ± 5 | 0.701 |
| Systolic blood pressure | 123 ± 20 | 123 ± 19 | 118 ± 16 | 0.012 |
| Diastolic blood pressure | 71 ± 10 | 70 ± 10 | 70 ± 11 | 0.537 |
| Heart rate | 71 ± 12 | 72 ± 12 | 72 ± 13 | 0.951 |
| QRS duration (s) | 0.12 ± 0.03 | 0.12 ± 0.03 | 0.12 ± 0.03 | 0.378 |
| Number of prior hospitalizations | 1.3 ± 1.3 | 1.1 ± 1.2 | 1.1 ± 1.4 | 0.244 |
| MI months before enrollment | 73 ± 69 | 75 ± 72 | 72 ± 69 | 0.877 |
| Blood urea nitrogen | 23 ± 12 | 23 ± 12 | 24 ± 13 | 0.989 |
| Creatinine | 1.3 ± 0.8 | 1.3 ± 0.4 | 1.3 ± 0.5 | 0.402 |
| Female | 22 (21%) | 20 (15%) | 21 (13%) | 0.177 |
| White race | 86 (83%) | 122 (90%) | 139 (85%) | 0.205 |
| Left bundle branch block | 27 (26% | 26 (19%) | 18 (11%) | 0.007 |
| Right bundle branch block | 1 (1%) | 4 (3%) | 21 (13%) | <0.001 |
| Atrial fibrillation | 5 (5%) | 14 (10%) | 15 (9%) | 0.280 |
| CHF NYHA Class II‐IV | 68 (65%) | 82 (61%) | 97 (59%) | 0.543 |
| EF < 25% | 49 (47%) | 65 (48%) | 84 (51%) | 0.837 |
| Diabetes | 42 (40%) | 54 (40%) | 59 (36%) | 0.730 |
Table 1.
B. Baseline Characteristics by QRS Score Group for ICD Arm: Data Are Presented as Mean ± Standard Deviation or N (%) of the Population
| Clinical Characteristics | ICD Arm | |||
|---|---|---|---|---|
| QRS Low (0–3) | QRS Med (4–7) | QRS High (≥8) | P‐ | |
| N = 257 | N = 363 | N = 375 | Value | |
| Age at randomization | 65 ± 9 | 64 ± 11 | 63 ± 11 | 0.093 |
| Body mass index (kg/m2) | 27 ± 5 | 28 ± 5 | 29 ± 6 | 0.105 |
| Systolic blood pressure | 126 ± 17 | 122 ± 19 | 121 ± 19 | 0.013 |
| Diastolic blood pressure | 71 ± 12 | 71 ± 11 | 71 ± 11 | 0.849 |
| Heart rate | 74 ± 13 | 73 ± 14 | 71 ± 13 | 0.027 |
| QRS duration (s) | 0.12 ± 0.03 | 0.12 ± 0.03 | 0.12 ± 0.03 | 0.081 |
| Number of prior hospitalizations | 1.4 ± 1.6 | 1.1 ± 1.9 | 1.1 ± 1.2 | 0.234 |
| MI months before enrollment | 73 ± 74 | 87 ± 86 | 84 ± 77 | 0.333 |
| Blood urea nitrogen | 24 ± 14 | 23 ± 12 | 22 ± 12 | 0.135 |
| Creatinine | 1.3 ± 0.6 | 1.2 ± 0.4 | 1.2 ± 0.4 | 0.338 |
| Female | 49 (19%) | 65 (18%) | 56 (15%) | 0.469 |
| White race | 203 (79%) | 327 (90%) | 319 (85%) | 0.010 |
| Left bundle branch block | 44 (17%) | 98 (27%) | 41 (11%) | <0.001 |
| Right bundle branch block | 13 (5%) | 22 (6%) | 56 (15%) | <0.001 |
| Atrial fibrillation | 23 (9%) | 33 (9%) | 30 (8%) | 0.935 |
| CHF NYHA Class II‐IV | 182 (71%) | 240 (66%) | 233 (62%) | 0.216 |
| EF < 25% | 121 (47%) | 182 (50%) | 188 (50%) | 0.772 |
| Diabetes (F3AQ3) | 108 (42%) | 116 (32%) | 124 (33%) | 0.145 |
Bolded p‐values represent statistically significant p‐values (p < 0.05).
QRS Scoring
Of the 995 subjects evaluated, 40 RBBB (4.0%), 108 left anterior fascicular block (LAFB, 10.9%), 59 LAFB + RBBB (5.9%), 131 left ventricular hypertrophy (LVH, 13.2%), 161 LBBB (16.2%) and 496 without confounders (49.9%). The median QRS score was 6 with an interquartile range of 3–9. The QRS Low, Med. and High categories included 257 (25.8%), 363 (36.4%) and 375 (37.7%) subjects, respectively.
The intra‐ and interobserver intraclass correlation coefficients for QRS score were 0.95 and 0.89, respectively. The intra‐ and interobserver agreement on QRS score classification (low, medium, or high) was 86% and 79%, respectively. Upon rescoring, the mean absolute differences in QRS score for the original and second scorer were 0.93 and 1.42 points, respectively.
CMT Arm
In the CMT arm, there were 23, 25, and 33 deaths in the QRS Low, Med, and High groups, respectively (Table 2). No association was found between the continuous QRS score and overall risk of mortality (Table 3A, P = 0.18). Similarly, there was no difference in overall probability of death among the different QRS score groups (Fig. 1A, P = 0.73). Surprisingly, the dichotomized QRS score (0–1 vs. ≥ 2 points) showed increased probability of death among subjects with QRS scores of 0–1 compared to those with scores ≥ 2 (Table 3B, HR = 2.03, P = 0.04). All analyses controlled for LVEF but univariate analyses showed similar trends (data not shown). Due to the higher prevalence of LBBB in the QRS Low group and the higher prevalence of RBBB in the QRS High group (Tables 1A and 1B), analysis was repeated excluding subjects with either LBBB or RBBB but categorical QRS score remained nonpredictive of mortality (P = 0.34).
Table 2.
Event Rates by QRS Score Category: Event Rates for Each Arm are Reported as N (% Population at Risk)
| Event Rates | QRS Low (0–3) | QRS Med (4–7) | QRS High (≥8) |
|---|---|---|---|
| CMT | |||
| Mortality | 23 (22.1%) | 25 (18.5%) | 33 (20.1%) |
| SCD | 10 (10.2%) | 12 (9.0%) | 18 (11.3%) |
| Non‐SCD | 6 (6.1%) | 6 (4.5%) | 8 (5.0%) |
| ICD | |||
| Mortality | 28 (18.2%) | 33 (14.5%) | 24 (11.4%) |
| VT/VF | 45 (30.8%) | 49 (22.3%) | 57 (27.5%) |
| VT | 35 (24.0%) | 34 (15.5%) | 48 (23.2%) |
| VF | 10 (6.8%) | 15 (6.8%) | 9 (4.4%) |
Table 3.
A. Cox Proportional Hazard Ratios for Continuous QRS Score: Cox‐Proportional Hazards for Continuous QRS Score are Reported for End Points in Each Arm*
| Event Type | Hazard Ratio | 95% Confidence Interval | P‐Value |
|---|---|---|---|
| CMT | |||
| Mortality | 0.96 | 0.91–1.02 | 0.176 |
| SCD | 0.96 | 0.88–1.03 | 0.247 |
| Non‐SCD | 0.96 | 0.86–1.07 | 0.426 |
| ICD | |||
| Mortality | 0.94 | 0.89–0.99 | 0.025 |
| VT/VF | 0.98 | 0.94–1.03 | 0.450 |
| VT | 1.00 | 0.96–1.05 | 0.960 |
| VF | 0.95 | 0.87–1.03 | 0.229 |
Figure 1.
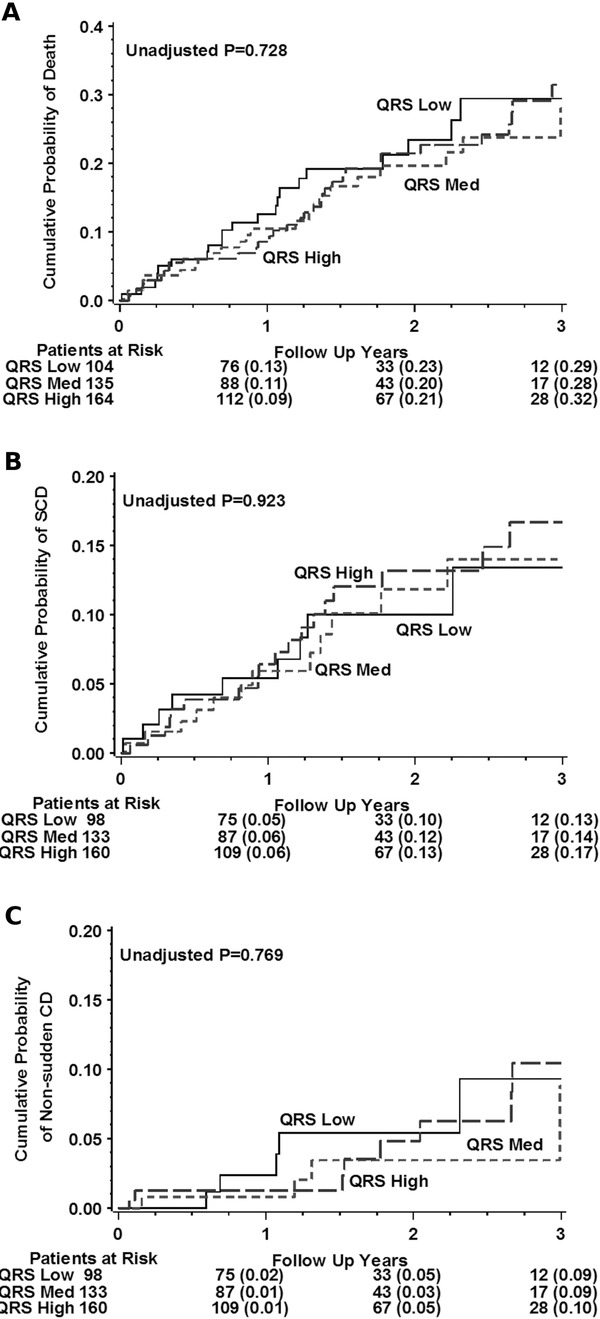
Event rates by QRS score in conventional medical therapy (CMT) arm. Kaplan‐Meier plots illustrating the cumulative probability of death (A), SCD (B), and non‐SCD (C) by categorical QRS score in the CMT arm. QRS Low includes subjects with QRS scores between 0–3, QRS Med 4–7, and QRS High ≥ 8.
Table 3.
B. Cox Proportional Hazard Ratios for Dichotomous (0–1 vs. ≥2) QRS Score: Cox‐Proportional Hazards for Dichoromous QRS Score are Reported for End Points in Each Arm*
| Event Type | Hazard Ratio | 95% Confidence Interval | P‐Value |
|---|---|---|---|
| CMT | |||
| Mortality | 2.03 | 1.04–3.95 | 0.037 |
| SCD | 2.00 | 0.78–5.12 | 0.150 |
| Non‐SCD | 1.85 | 0.43–8.07 | 0.411 |
| ICD | |||
| Mortality | 1.71 | 0.94–3.08 | 0.077 |
| VT/VF | 1.53 | 0.93–2.51 | 0.094 |
| VT | 1.23 | 0.69–2.18 | 0.492 |
| VF | 1.62 | 0.63–4.20 | 0.317 |
*All analyses adjusted for EF <25%.
Bolded p‐values represent statistically significant p‐values (p < 0.05).
There were 10 SCDs in the QRS Low group, 12 SCDs in the QRS Med group and 18 SCDs in the QRS High group (Table 2). Continuous QRS score was not associated with risk of SCD (Table 3A, P = 0.25). There was no difference in the probability of SCD based on QRS score group (Fig. 1B P = 0.92). Dichotomized QRS Score showed no difference in the probability of SCD (Table 3B, P = 0.15).
The QRS Low, Med, and High groups had 6, 6, and 8 non‐SCDs, respectively (Table 2). Continuous QRS score was not associated with increased risk of non‐SCD (Table 3A, P = 0.43). There was also no difference in the probability of non‐SCD based on QRS score classification (Fig. 1C, P = 0.77).
The average QRS score for subjects with SCD was 6.3 ± 4.0 whereas the average QRS score for those with non‐SCD was 6.5 ± 4.0. The average QRS score for those who survived throughout follow‐up was 7.0 ± 4.3.
ICD Arm
In the ICD arm, there were 28, 33, and 24 deaths in the QRS Low, Med, and High groups respectively (Table 2). Cox proportional hazard for mortality by continuous QRS score showed decreased risk of death with increasing QRS score (Table 3A, HR = 0.94, P = 0.03). However, this finding was not borne out by the categorical analysis wherein all three QRS score categories demonstrated similar rates of mortality (Fig. 2A, P = 0.17). Moreover, the dichotomous QRS score also showed similar rates of death (Table 3B, P = 0.08). Nevertheless, the continuous QRS score mortality relationship remained significant after removing LBBB and RBBB patients (HR = 0.93, P = 0.047).
Figure 2.
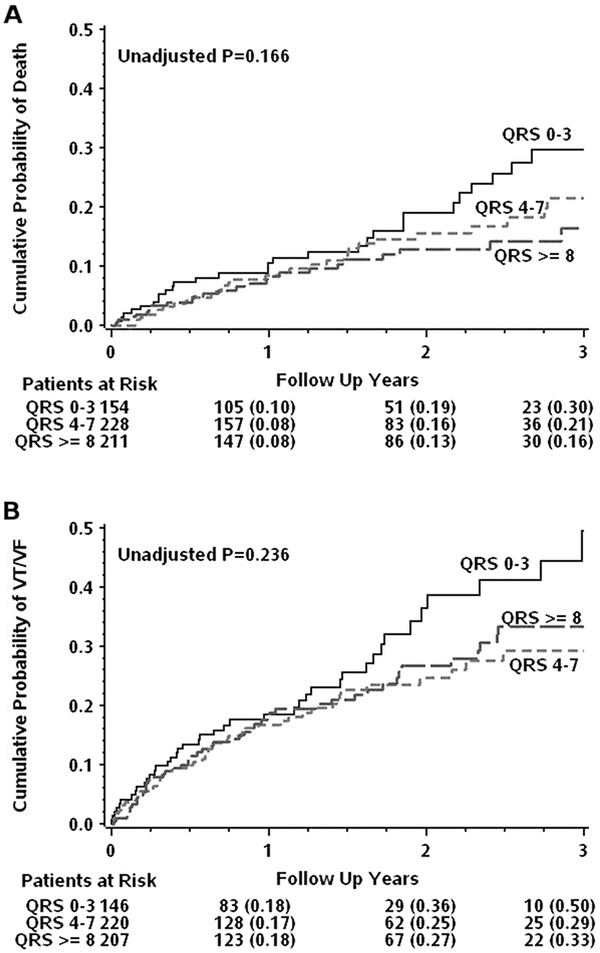
Event rates by QRS score in implantable cardioverter defibrillator (ICD) arm. Kaplan‐Meier plots illustrating the cumulative probability of death (A) and VT/VF (B) by categorical QRS Score in the ICD arm. QRS Low includes subjects with QRS scores between 0–3, QRS Med 4–7 and QRS High ≥ 8.
VT/VF occurred in 45 subjects in the QRS Low group, 49 in the QRS Med group and 57 in the QRS High group (Table 2). Cox proportional hazard by continuous QRS score was not significant for occurrence of VT/VF (Table 3A, P = 0.45). Neither categorical (Fig. 2B, P = 0.24) nor dichotomous (Table 3B, P = 0.09) QRS score demonstrated different rates of VT/VF. Considering the rates of appropriate ICD discharge for VT or VF independently also showed similar trends across continuous (Table 3A), categorical (Table 2) and dichotomous QRS score (Table 3B).
The average QRS score for subjects who experienced VT/VF was 6.3 ± 4.2 whereas the average QRS score for those who survived throughout follow‐up without a VT/VF event was 6.5 ± 4.0.
QRS Score and ICD Efficacy
To assess categorical QRS score as a predictor of ICD efficacy, we determined the risk of death between the ICD and CMT arms by QRS score group (Table 4). ICD implantation was associated with a lower risk of death in all three QRS score groups; but, this reduction of risk only reached statistical significance in the QRS High group (HR = 0.56, P = 0.03). However, interaction analysis comparing the mortality benefit of ICD arm assignment between the QRS High group and the QRS Med and QRS Low groups showed no significant difference (P = 0.47, P = 0.39). The interaction of ICD implantation and continuous QRS score was also not significant (P = 0.46). The previous SCD‐HeFT substudy found QRS score combined with LVEF resulted in a larger discriminative power for risk of VT/VF.12 However, the combination of LVEF with the dichotomous QRS score (both as 0–1 vs. ≥2 QRS points and 0 vs. ≥1 QRS point) as a predictor of VT/VF or SCD for the MADIT‐II cohort was unable to discriminate subgroups of differential risk (data not shown).
Table 4.
Mortality Hazard Ratio for ICD Arm Assignment versus CMT Arm Assignment by QRS Score
| QRS Score Group | Hazard Ratio | 95% Confidence Interval | P‐Value |
|---|---|---|---|
| QRS Low (0–3) | 0.78 | 0.45–1.36 | 0.39 |
| QRS Med (4–7) | 0.74 | 0.44–1.24 | 0.25 |
| QRS High (≥8) | 0.56 | 0.33–0.95 | 0.03 |
Bolded p‐values represent statistically significant p‐values (p < 0.05).
Previous studies have shown that inferior/posterior MI is associated with higher risk of all‐cause mortality than anterior/lateral MI.21 However, controlling for infarction location (anterior/lateral vs. inferior/posterior) did not significantly affect the predictive power of the QRS score (data not shown).
DISCUSSION
QRS scores were similar among patients who experienced VT/VF, SCD, and non‐SCD demonstrating that QRS score alone is unable to discriminate between individuals at risk for SCD versus HF death in the MADIT‐II population. Higher QRS score was associated with decreased mortality risk in both the CMT arm (by dichotomous score) and the ICD arm (by continuous score) and the QRS High group demonstrated the largest mortality benefit; however, QRS score was unable to identify subgroups that would be inappropriate candidates for ICD implantation.
Arrhythmia and SCD Risk
This study tested the hypothesis that higher QRS score would be associated with increased incidence of SCD in the CMT arm of the MADIT‐II trial; and higher QRS score would be associated with appropriate ICD discharge for VT or VF in the ICD arm. Our results show that subjects experienced similar risk of SCD, VT and VF across different QRS scores. None of the three considerations of the QRS score (continuous, categorical, or dichotomous) were able to identify subjects at particularly high or low risk for SCD or ventricular arrhythmias.
Higher QRS score was shown to be associated with appropriate ICD discharge for VT or VF in the SCD‐HeFT population.12 One possible explanation for the differences in the results of our study and the SCD‐HeFT substudy may stem from the subject population differences. SCD‐HeFT included subjects with LVEFs ≤ 35% due to either ischemic (52%) or nonischemic (48%) etiology, whereas MADIT‐II enrolled subjects with LVEFs ≤ 30% and exclusively due to ischemic etiology.1, 2 While the average LVEFs of MADIT‐II were slightly lower than SCD‐HeFT (23% vs. 25%) and the median QRS score was slightly higher (6 vs. 5), at 5 years of follow‐up the total mortality rate was higher in MADIT‐II than SCD‐HeFT (43% vs. 36% in the CMT arm and 33% vs. 29% in the ICD arm)2, 22 suggesting that the MADIT‐II population included more seriously ill subjects than SCD‐HeFT. It may be that in a population with more severe post‐MI LV dysfunction, QRS score fails to contribute incremental prognostic value. The MADIT‐II trial also did not include subjects with nonischemic heart failure who may have different determinants of arrhythmia risk; however, subgroup analysis of the ischemic subjects in SCD‐HeFT demonstrated a similar relationship between QRS score and VT/VF as in the nonischemic subjects.12
The median follow‐up in SCD‐HeFT was much longer than that of MADIT‐II (45.5 months and 20 months, respectively).1, 2 The separation in the Kaplan‐Meier plots between the scar absent and scar present group of the SCD‐HeFT substudy becomes most pronounced after 24 months of follow‐up.12 It is possible that the follow‐up period in MADIT‐II was not sufficient to detect a difference in long‐term event risk. The median time between index MI and randomization may influence the risk of VT/VF and this duration was also slightly different between SCD‐HeFT and MADIT‐II (52 months vs. 60 months respectively);23, 24 however, a recent study of SCD‐HeFT demonstrated that ICD benefit did not vary with time from MI to implantation.23 Additional analyses of the QRS score's prognostic value in a population like SCD‐HeFT could help to clarify some of these issues.
Non‐SCD Risk
This study also tested the hypothesis that subjects with very high QRS scores were more likely to experience non‐SCD than those with lower QRS scores. These data demonstrate that the incidence of non‐SCD was similar across the low, medium, and high QRS score groups. Additionally, analysis of ICD efficacy demonstrated that subjects with high QRS scores tended to have the largest mortality benefit. Only 4% of subjects included in this study were NYHA class IV with the majority (57%) being class II or III. This population may not have included enough subjects with severe HF such that their QRS scores would be predictive of their risk of non‐SCD.
QRS Score as a Tool for Risk Stratification
Increasing QRS score was not associated with increased risk of overall mortality in the CMT arm, but was associated with decreased risk of overall mortality in the ICD arm. ICD implantation appeared to confer mortality benefit in all three categorical QRS score groups but only reached statistical significance in the high QRS score group. These results suggest that ICD benefit may be highest in subjects with high QRS scores but do not imply that subjects with lower QRS scores fail to benefit. Additionally, increasing QRS score was associated with increased risk of total mortality in the ICD arm of the SCD‐HeFT substudy, further challenging the interpretation of these results.12 In summary, QRS score alone does not appear to be sufficient for excluding patients for ICD implantation from the broad group meeting MADIT‐II criteria.
Limitations
One limitation of this study is the method by which mortality subtype end points in the CMT arm were classified. Each death was classified by an end point review committee based on the modified Hinkle‐Thaler scheme; however, subjects in the CMT arm were not monitored for arrhythmias, and their cause of death cannot be certain. The accuracy of mode of death classification is known to be imperfect.25 The uncertainty inherent in any classification method may have caused some deaths to be falsely identified as SCD; however, the Hinkle‐Thaler criteria were specifically designed to provide more accurate discrimination between arrhythmic death and sudden death from other causes.26, 27
The ECGs that were analyzed were often photocopies that limited the precision of the analysis. This limitation in quality resulted in the exclusion of a large portion of the population (19%) from analysis. However, comparison of baseline characteristics of included and excluded patients were largely similar (online supplement). The difference in average QRS score between the SCD and non‐SCD group was 0.2 points (6.3 vs. 6.5 points) which is within the margin of error determined by inter and intraobserver agreement measurements. It is possible that more precise scar estimates would be better able to discriminate a true difference between these two groups. Separate efforts are in progress to generate an automated version of this score to achieve a more precise and even more consistent result.
Lastly, the QRS score groups analyzed in this study were imbalanced with respect to RBBB and LBBB prevalence. However, while RBBB was more common in the higher QRS score groups and LBBB was more common in the lower QRS score groups, analyses including and excluding these subjects showed similar results.
CONCLUSIONS
The results of this study suggest that the role for QRS scoring in aiding risk stratification for ICD implantation needs to be evaluated further. This clinical tool is inexpensive and can be performed on the widely available ECG. Automation of the QRS scoring process could make it an appealing tool to aid in clinical decision‐making. Further studies determining populations in which this tool can best serve will potentially aid clinicians in selecting appropriate candidates for ICD therapy.
Supporting information
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Table S1. Online Supplement: Baseline characteristics of patients included and excluded from the present study: Data are presented as mean ± standard deviation or as N (%) of the population.
Acknowledgments
Zak Loring thanks Dr. James P. Daubert and Dr. Galen Wagner for their ongoing mentorship and support. This ongoing research is supported in part by Duke University's CTSA grant TL1RR024126 from NCRR/NIH. The authors thank the MADIT‐II Investigators for collecting the original data for this study.
REFERENCES
- 1. Moss A, Zareba W, Hall W, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877. [DOI] [PubMed] [Google Scholar]
- 2. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 3. ACC/AHA/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation 2008;117:e350–408. [DOI] [PubMed] [Google Scholar]
- 4. Sesselberg HW, Moss AJ, McNitt S, et al. Ventricular arrhythmia storms in postinfarction patients with implantable defibrillators for primary prevention indications: A MADIT‐II substudy. Heart Rhythm 2007;4:1395–1402. [DOI] [PubMed] [Google Scholar]
- 5. Zwanziger J, Hall WJ, Dick AW, et al. The cost effectiveness of implantable cardioverter‐defibrillators: results from the Multicenter Automatic Defibrillator Implantation Trial (MADIT)‐II. J Am Coll Cardiol 2006;47:2310–2318. [DOI] [PubMed] [Google Scholar]
- 6. Myerburg R. Implantable cardioverter‐defibrillators after myocardial infarction. New Engl J Med 2008;359:2245. [DOI] [PubMed] [Google Scholar]
- 7. Greenberg H, Case RB, Moss AJ, et al. Analysis of mortality events in the Multicenter Automatic Defibrillator Implantation Trial (MADIT‐II). J Am Coll Cardiol 2004;43:1459–1465. [DOI] [PubMed] [Google Scholar]
- 8. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT‐HF). Lancet 1999;353:2001–2007. [PubMed] [Google Scholar]
- 9. Wu KC. MRI with late gadolinium enhancement as a predictor of ventricular arrhythmias. Curr Cardiovasc Imaging Rep 2009;2:116–123. [Google Scholar]
- 10. Bello D, Einhorn A, Kaushal R, et al. Cardiac magnetic resonance imaging: Infarct size is an independent predictor of mortality in patients with coronary artery disease. Magn Reson Imaging 2011;29:50–56. [DOI] [PubMed] [Google Scholar]
- 11. Goldenberg I, Vyas AK, Hall WJ, et al. Risk stratification for primary implantation of a cardioverter‐defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol 2008;51:288–296. [DOI] [PubMed] [Google Scholar]
- 12. Strauss DG, Poole JE, Wagner GS, et al. An ECG index of myocardial scar enhances prediction of defibrillator shocks: An analysis of the Sudden Cardiac Death in Heart Failure Trial. Heart Rhythm 2011;8:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Connor CM, Hathaway WR, Bates ER, et al. Clinical characteristics and long‐term outcome of patients in whom congestive heart failure develops after thrombolytic therapy for acute myocardial infarction: Development of a predictive model. Am Heart J 1997;133:663–673. [DOI] [PubMed] [Google Scholar]
- 14. Ertl G, Gaudron P, Neubauer S, et al. Cardiac dysfunction and development of heart failure. Euro Heart J 1993;14:33–37. [DOI] [PubMed] [Google Scholar]
- 15. Thomas KL, Velazquez EJ. Therapies to prevent heart failure post‐myocardial infarction. Curr Heart Fail Rep 2005;2:174–182. [DOI] [PubMed] [Google Scholar]
- 16. Strauss DG, Selvester RH. The QRS complex–a biomarker that “images” the heart: QRS scores to quantify myocardial scar in the presence of normal and abnormal ventricular conduction. J Electrocardiol 2009;42:85–96. [DOI] [PubMed] [Google Scholar]
- 17. Strauss DG, Selvester RH, Lima JA, et al. ECG quantification of myocardial scar in cardiomyopathy patients with or without conduction defects: Correlation with cardiac magnetic resonance and arrhythmogenesis. Circ Arrhythm Electrophysiol 2008;1:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bounous E, Jr. , Califf R, Harrell F, Jr. , et al. Prognostic value of the simplified Selvester QRS score in patients with coronary artery disease. J Am Coll Cardiol 1988;11:35. [DOI] [PubMed] [Google Scholar]
- 19. Jones M, Anderson K, Wilson P, et al. Prognostic use of a QRS scoring system after hospital discharge for initial acute myocardial infarction in the Framingham cohort. Am J Cardiol 1990;66:546–550. [DOI] [PubMed] [Google Scholar]
- 20. Daubert JP, Zareba W, Cannom DS, et al. Inappropriate implantable cardioverter‐defibrillator shocks in MADIT II: Frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol 2008;51:1357–1365. [DOI] [PubMed] [Google Scholar]
- 21. Gomez J, Zareba W, Moss A, et al. Prognostic value of location and type of myocardial infarction in the setting of advanced left ventricular dysfunction. Am J Cardiol 2007;99:642–646. [DOI] [PubMed] [Google Scholar]
- 22. Goldenberg I, Gillespie J, Moss AJ, et al. Long‐term benefit of primary prevention with an implantable cardioverter‐defibrillator: An extended 8‐year follow‐up study of the Multicenter Automatic Defibrillator Implantation Trial II. Circulation 2010;122:1265–1271. [DOI] [PubMed] [Google Scholar]
- 23. Piccini JP, Al‐Khatib SM, Hellkamp AS, et al. Mortality benefits from implantable cardioverter‐defibrillator therapy are not restricted to patients with remote myocardial infarction: An analysis from the Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT). Heart Rhythm 8:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilber DJ, Zareba W, Hall WJ, et al. Time dependence of mortality risk and defibrillator benefit after myocardial infarction. Circulation 2004;109:1082–1084. [DOI] [PubMed] [Google Scholar]
- 25. Pratt CM, Greenway PS, Schoenfeld MH, et al. Exploration of the precision of classifying sudden cardiac death: Implications for the interpretation of clinical trials. Circulation 1996;93:519–524. [DOI] [PubMed] [Google Scholar]
- 26. Torp Pedersen C, Køber L, Elming H, et al. Classification of sudden and arrhythmic death. Pacing Clin Electrophysiol 1997;20:2545–2552. [DOI] [PubMed] [Google Scholar]
- 27. Hinkle LE, Jr. , Thaler HT. Clinical classification of cardiac deaths. Circulation 1982;65:457–464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Table S1. Online Supplement: Baseline characteristics of patients included and excluded from the present study: Data are presented as mean ± standard deviation or as N (%) of the population.


