Editorial
Parallel excitation [1,2] with a multi-element Radio Frequency (RF) transceiver array [3–9] as a contemporary methodology has been advocated for human MR imaging at ultrahigh magnetic fields (7 Tesla and above). In ultrahigh field MRI, the required high operating frequency and thus shortened wavelength of radio frequency waves creates a complex wave behavior and increased phase variation of RF magnetic fields (i.e. B1 fields) in conductive and high dielectric biological samples, such as human body, resulting in inhomogeneous image distribution. The inhomogeneous image distribution consequently leads to difficulties in quantifying the MR signal intensity. With independent phase and amplitude control of each channel of a transceiver array, parallel excitation can be applied to perform B1 shimming to obtain uniform B1 distribution. In MR safety aspect, RF power required to excite the spins increases dramatically at ultrahigh fields compared with that at lower fields, e.g. 1.5T. The high RF excitation power results in high Specific Absorption Rate (SAR) in human body, ultimately increases tissue heating during MRI. It is demonstrated that by using the parallel excitation method, the RF excitation profile can be optimized, providing in a significantly reduced SAR and therefore safer MRI at ultrahigh fields. In fact, the emerging method of parallel excitation has become essential for ultrahigh field MRI in addressing B1 in homogeneity, increased SAR and tissue heating.
Additionally, parallel excitation with a multi-element RF transceiver array has opened a new avenue to selective excitation in MR imaging, providing a fast and efficient approach to perform selective excitation [1,2]. Conventionally a single RF pulse is used in MRI to perform slice selective or multidimensional spatial selective excitation by exciting the nuclei in the area of interest and limiting the electromagnetic signal emitted from imaging object within spatially restricted areas [10–16]. This often requires homogeneous RF field to ensure excitation accuracy. However, as described above it is technically challenging to achieve homogeneous B1 fields with the increase of the magnetic field strength, where the dielectric resonance [17] and the conductivity effect of high dielectric and conductive biological samples [18,19] lead to enlarged B1 field variation [20] even with an intrinsically homogeneous volume coil [4,21,22,23]. This effect becomes more pronounced at ultrahigh field such as 7 Tesla (7T) due to the shortened wavelength of radio frequency (RF) wave [24]. In conventional selective excitations, the pulse width of the required multidimensional RF pulses is usually long, resulting in a long excitation time, especially in applications where a large excitation Field Of View (FOV) is involved. Such long excitation could potentially exacerbate the high SAR at ultrahigh fields. Although special k-space trajectory such as spiral trajectory and iterative pulse design method have been developed to reduce the length of multidimensional RF pulse [25–27], multiple pulse parallel excitation method adopted from parallel imaging is able to significantly reduce the excitation time, providing a whole new approach to selective excitation and more capabilities than conventional single RF pulse excitation [1–3,5,6,8,9,28–30]. Parallel excitation RF pulses were originally designed to shorten the duration of multidimensional spatially selective excitation [1,2,28,31–35]. This capability has been used for both small tip-angle excitation and large tip-angle multidimensional RF pulses [36–38]. By using the multichannel parallel excitation, the pulse width for multidimensional spatial selective excitation can be dramatically reduced. Parallel excitation can also take advantage of independent control of each RF pulse to reduce the RF power and minimize the SAR [2,39–48] during the multidimensional spatial selective excitation. Furthermore, by using multiple independent RF pulses, the phase and amplitude of each pulse can be adjusted to manipulate the excitation profiles to achieve the desired RF field in conductive and high dielectric biological sample [44,49–52]. These capabilities, achieved by utilizing the extra degree of freedom from multiple RF pulse excitation, can provide much more advantages over conventional single RF transmission. Apparently, to design a practically optimal RF pulses for selective excitation using parallel excitation, it is necessary to not only optimize the excitation profile homogeneity, but also minimize the peak RF power to reduce the SAR [27,53,54] in order to ensure the safety during MR examinations.
To enable parallel excitation for B1 shimming, SAR optimization and multiple RF pulse excitation, an MR scanner must be equipped with multi-channel transmitters which can independently control the amplitude and phase of RF pulse on each channel. However, in current system setup, most existing MR scanners used in research institutions and clinical settings are not equipped with the multi-channel transmitters and thus are not capable of implementing this emerging concept of parallel excitation for B1 shimming, SAR optimization and fast selective excitation. In recent years, some parallel transmit systems have been developed to allow parallel excitation applications on existing MR scanners [55–57]. These designs can provide 100 Watts power (or higher, depending on the type of amplifiers used) for each RF excitation channel and can be used for simultaneously multiple RF pulse excitations on commercial MR scanners.
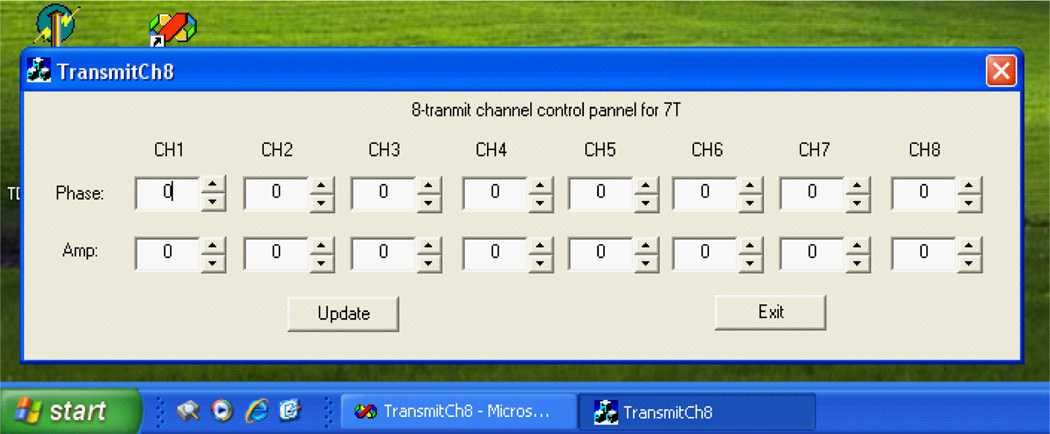
In practice, it is desired to have a multi-channel transmitter system which is easy to be integrated to the existing MR scanners. In this research endeavor, a PC controlled 8-channel transmit circuit with independent phase and amplitude for each channel for 7T MR scanner has been proposed [57]. In this design, the phase and amplitude of each channel are adjusted by voltage control phase shifter and attenuator respectively. Both control voltages are communicated through a PC via a 16-channel Digital Analog Converter (DAC). The input of this circuit can be the RF pulse generated by the signal generator of the MR spectrometer. Through this phase and amplitude control circuit, the output signal of each channel can be amplified for spin excitation by RF amplifiers which could be either regular RF amplifiers or on-coil MOSFET amplifiers [58,59]. Figure 1 shows the block diagram of this multi-channel transmitter circuit design. The PC is utilized to send out digital voltages which determine the phase shift and amplitude change of each channel. The circuit board of the 8-channel transmit system is shown in Figure 2. A Graphic User Interface (GUI) for PC was also developed to facilitate the control of the output voltages of the DAC (Figure 3).
Figure 1.
Block diagram of the multi-channel transmit circuit. The personal computer (PC) is utilized to send out digital voltages which determine how many degree and dB the phase and the amplitude of each channel are to be adjusted respectively. These digital voltages are converted to analog voltages using the 12-bit DAC. The outputs of the DAC are connected to the control pins of the voltage variable phase shifters and attenuators respectively (yellow lines) to control the phase and amplitude of the 8-transmit channels. Thus the output RF pulse of each transmit channel can be with independent phase and amplitude. The output of each channel is then amplified for spin excitation by a regular RF amplifier or an on-coil MOSFET amplifier.
Figure 2.
The circuit board of the 8-channel transmits system (low power part) with independent phase and amplitude control.
Figure 3.
The graphic user interface (GUI) for Windows system on PC for multi-channel transmit control, providing a user-friendly operation.
We utilized the benchmark signal to test its performance. A 298MHz sine waveform was output to the 8-channel transmit circuit and an oscilloscope was used to display the output waveforms from the circuit. The phases and amplitude varying with the control voltages were plotted in Figure 4, demonstrating the sufficient dynamic range of the design for MR applications. Several samples of the pulse waveforms with different phases and amplitudes are shown in Figure 5. By synchronizing the clock signal to the excitation signal of MRI scanner, this design should be readily integrated to existing MR scanner.
Figure 4.
Phase (upper insert) and amplitude (low insert) varies with phase control voltage and amplitude control voltage at 4 different power attenuations, respectively. When the phase control voltage varies from 0V to 12V, the pulse phase can be shifted from −30° to 380°. When the voltage varies from 0V to 10V the output power can be attenuated from 31dB to 5dB.
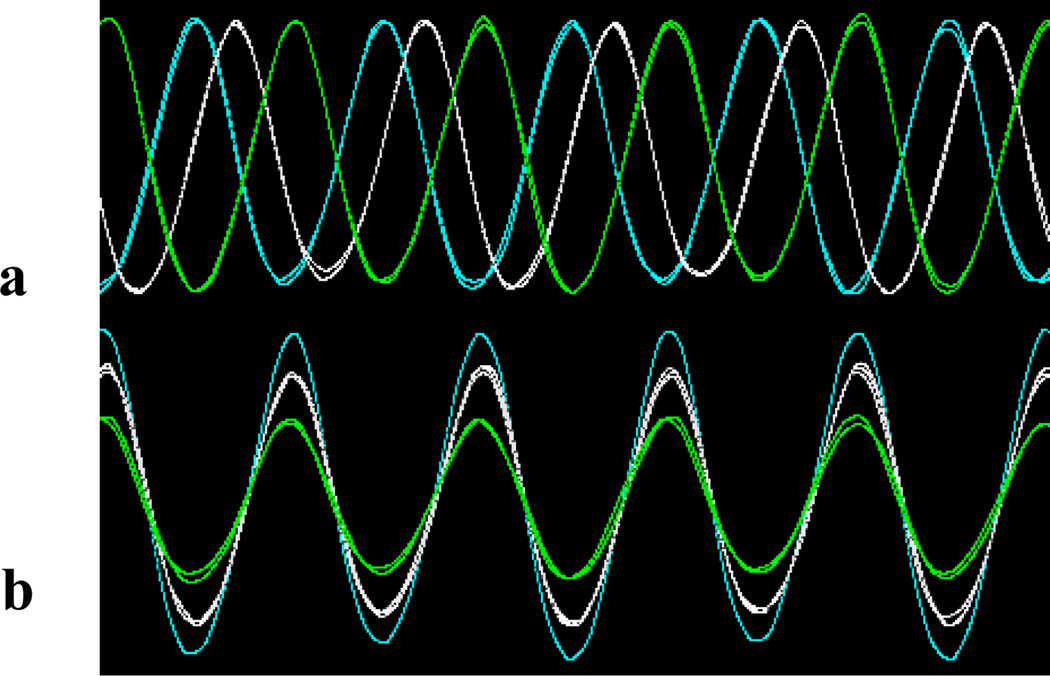
Figure 5.
Samples of waveform with different phases (a) or amplitudes (b) output from the multi-channel transmit circuit.
This PC controlled 8-channel transmit circuit design provides a comparatively simple way to enable parallel excitation applications for the existing MR scanners that are not equipped with multi-channel transmitter systems. With this, parallel excitation for B1 shimming, SAR optimization and fast selective excitation can be performed.
Acknowledgments
This work was partially supported by NIH grants EB004453 and EB008699, and a QB3 Research Award.
References
- 1.Katscher U, Börnert P, Leussler C, van den Brink JS. Transmit SENSE. Magn Reson Med. 2003;49:144–150. doi: 10.1002/mrm.10353. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Y. Parallel excitation with an array of transmit coils. Magn Reson Med. 2004;51:775–784. doi: 10.1002/mrm.20011. [DOI] [PubMed] [Google Scholar]
- 3.Adriany G, Van de Moortele PF, Wiesinger F, Moeller S, Strupp JP, et al. Transmit and receive transmission line arrays for 7 Tesla parallel imaging. Magn Reson Med. 2005;53:434–445. doi: 10.1002/mrm.20321. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Ugurbil K, Chen W. A microstrip transmission line volume coil for human head MR imaging at 4T. J Magn Reson. 2003;161:242–251. doi: 10.1016/s1090-7807(03)00004-1. [DOI] [PubMed] [Google Scholar]
- 5.Wu B, Wang C, Kelley DA, Xu D, Vigneron DB, et al. Shielded microstrip array for 7T human MR imaging. IEEE Trans Med Imaging. 2010;29:179–184. doi: 10.1109/TMI.2009.2033597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu B, Wang C, Krug R, Kelley DA, Xu D, et al. 7T human spine imaging arrays with adjustable inductive decoupling. IEEE Trans Biomed Eng. 2010;57:397–403. doi: 10.1109/TBME.2009.2030170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Xie Z, Pang Y, Vigneron D, Zhang X. ICE decoupling technique for RF coil array designs. Med Phys. 2011;38:4086–4093. doi: 10.1118/1.3598112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu B, Wang C, Lu J, Pang Y, Nelson SJ, et al. Multi-channel microstrip transceiver arrays using harmonics for high field MR imaging in humans. IEEE Trans Med Imaging. 2012;31:183–191. doi: 10.1109/TMI.2011.2166273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu B, Zhang X, Wang C, Li Y, Pang Y, et al. Flexible transceiver array for ultrahigh field human MR imaging. Magn Reson Med. 2012 doi: 10.1002/mrm.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pauly J, Nishimura D, Macovski A. A linear class of large-tip-angle selective excitation pulses. J Magn Reson. 1989;82:571–587. [Google Scholar]
- 11.Pauly J, Nishimura D, Macovski A. A k-space analysis of small-tip-angle excitation. J Magn Reson. 1989;81:43–56. doi: 10.1016/j.jmr.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Hardy CJ, Cline HE. Broadband nuclear magnetic resonance pulses with two-dimensional spatial selectivity. J Appl Phys. 1989;66:1513–1516. [Google Scholar]
- 13.Yuan J, Madore B, Panych LP. Spatially varying fat-water excitation using short 2DRF pulses. Magn Reson Med. 2010;63:1092–1097. doi: 10.1002/mrm.22223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau AZ, Chen AP, Hurd RE, Cunningham CH. Spectral-spatial excitation for rapid imaging of DNP compounds. NMR Biomed. 2011;24:988–996. doi: 10.1002/nbm.1743. [DOI] [PubMed] [Google Scholar]
- 15.Larson PE, Kerr AB, Chen AP, Lustig MS, Zierhut ML, et al. Multiband excitation pulses for hyperpolarized 13C dynamic chemical-shift imaging. J Magn Reson. 2008;194:121–127. doi: 10.1016/j.jmr.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pang Y, Shen GX. Improving excitation and inversion accuracy by optimized RF pulse using genetic algorithm. J Magn Reson. 2007;186:86–93. doi: 10.1016/j.jmr.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim TS, Lee R, Abduljalil AM, Baertlein BA, Robitaille PM. Dielectric resonances and B(1) field inhomogeneity in UHFMRI: computational analysis and experimental findings. Magn Reson Imaging. 2001;19:219–226. doi: 10.1016/s0730-725x(01)00300-9. [DOI] [PubMed] [Google Scholar]
- 18.Collins CM, Liu W, Schreiber W, Yang QX, Smith MB. Central brightening due to constructive interference with, without, and despite dielectric resonance. J Magn Reson Imaging. 2005;21:192–196. doi: 10.1002/jmri.20245. [DOI] [PubMed] [Google Scholar]
- 19.Yang QX, Wang J, Zhang X, Collins CM, Smith MB, et al. Analysis of wave behavior in lossy dielectric samples at high field. Magn Reson Med. 2002;47:982–989. doi: 10.1002/mrm.10137. [DOI] [PubMed] [Google Scholar]
- 20.Saekho S, Yip CY, Noll DC, Boada FE, Stenger VA. Fast-kz three-dimensional tailored radiofrequency pulse for reduced B1 inhomogeneity. Magn Reson Med. 2006;55:719–724. doi: 10.1002/mrm.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Ugurbil K, Sainati R, Chen W. An inverted-microstrip resonator for human head proton MR imaging at 7 tesla. IEEE Trans Biomed Eng. 2005;52:495–504. doi: 10.1109/TBME.2004.842968. [DOI] [PubMed] [Google Scholar]
- 22.Vaughan JT, Garwood M, Collins CM, Liu W, DelaBarre L, et al. 7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in head images. Magn Reson Med. 2001;46:24–30. doi: 10.1002/mrm.1156. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Zhu XH, Chen W. Higher-order harmonic transmission-line RF coil design for MR applications. Magn Reson Med. 2005;53:1234–1239. doi: 10.1002/mrm.20462. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Ugurbil K, Chen W. Microstrip RF surface coil design for extremely high-field MRI and spectroscopy. Magn Reson Med. 2001;46:443–450. doi: 10.1002/mrm.1212. [DOI] [PubMed] [Google Scholar]
- 25.Schröder C, Börnert P, Aldefeld B. Spatial excitation using variable-density spiral trajectories. J Magn Reson Imaging. 2003;18:136–141. doi: 10.1002/jmri.10334. [DOI] [PubMed] [Google Scholar]
- 26.Stenger VA, Boada FE, Noll DC. Variable-density spiral 3D tailored RF pulses. Magn Reson Med. 2003;50:1100–1106. doi: 10.1002/mrm.10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yip CY, Fessler JA, Noll DC. Iterative RF pulse design for multidimensional, small-tip-angle selective excitation. Magn Reson Med. 2005;54:908–917. doi: 10.1002/mrm.20631. [DOI] [PubMed] [Google Scholar]
- 28.Grissom W, Yip CY, Zhang Z, Stenger VA, Fessler JA, et al. Spatial domain method for the design of RF pulses in multicoil parallel excitation. Magn Reson Med. 2006;56:620–629. doi: 10.1002/mrm.20978. [DOI] [PubMed] [Google Scholar]
- 29.Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR phased array. Magn Reson Med. 1990;16:192–225. doi: 10.1002/mrm.1910160203. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Pang Y, Vigneron D, Glenn O, Xu D, et al. Investigation of multi-channel phased array performance for fetal MR imaging on 1.5T clinical MR system. Quant Imaging Med Surg. 2011;1:24–30. doi: 10.3978/j.issn.2223-4292.2011.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma C, Xu D, King KF, Liang ZP. Joint design of spoke trajectories and RF pulses for parallel excitation. Magn Reson Med. 2011;65:973–985. doi: 10.1002/mrm.22676. [DOI] [PubMed] [Google Scholar]
- 32.Wu X, Vaughan JT, Ugurbil K, Van de Moortele PF. Parallel excitation in the human brain at 9.4 T counteracting k-space errors with RF pulse design. Magn Reson Med. 2010;63:524–529. doi: 10.1002/mrm.22247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Setsompop K, Wald LL, Alagappan V, Gagoski B, Hebrank F, et al. Parallel RF transmission with eight channels at 3 Tesla. Magn Reson Med. 2006;56:1163–1171. doi: 10.1002/mrm.21042. [DOI] [PubMed] [Google Scholar]
- 34.Heilman JA, Derakhshan JD, Riffe MJ, Gudino N, Tkach J, et al. Parallel excitation for B-field insensitive fat-saturation preparation. Magn Reson Med. 2012 doi: 10.1002/mrm.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pang Y, Zhang X. Precompensation for mutual coupling between array elements in parallel excitation. Quant Imaging Med Surg. 2011;1:4–10. doi: 10.3978/j.issn.2223-4292.2011.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grissom WA, Yip CY, Wright SM, Fessler JA, Noll DC. Additive angle method for fast large-tip-angle RF pulse design in parallel excitation. Magn Reson Med. 2008;59:779–787. doi: 10.1002/mrm.21510. [DOI] [PubMed] [Google Scholar]
- 37.Xu D, King KF, Zhu Y, McKinnon GC, Liang ZP. A noniterative method to design large-tip-angle multidimensional spatially-selective radio frequency pulses for parallel transmission. Magn Reson Med. 2007;58:326–334. doi: 10.1002/mrm.21314. [DOI] [PubMed] [Google Scholar]
- 38.Setsompop K, Alagappan V, Zelinski AC, Potthast A, Fontius U, et al. High-flip-angle slice-selective parallel RF transmission with 8 channels at 7 T. J Magn Reson. 2008;195:76–84. doi: 10.1016/j.jmr.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graesslin I, Niemann M, Harvey P, Vernickel P. SAR and RF power reduction with parallel excitation using non-Cartesian trajectories. MAGMA. 2005;18:S251. [Google Scholar]
- 40.Katscher U, Börnert P. Parallel RF transmission in MRI. NMR Biomed. 2006;19:393–400. doi: 10.1002/nbm.1049. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Feng K, McDougall MP, Wright SM, Ji J. Reducing SAR in parallel excitation using variable-density spirals: a simulation-based study. Magn Reson Imaging. 2008;26:1122–1132. doi: 10.1016/j.mri.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Ji JX. Minimal-SAR RF pulse optimization for parallel transmission in MRI. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:5774–5777. doi: 10.1109/IEMBS.2008.4650526. [DOI] [PubMed] [Google Scholar]
- 43.Zelinski AC, Angelone LM, Goyal VK, Bonmassar G, Adalsteinsson E, et al. Specific absorption rate studies of the parallel transmission of inner-volume excitations at 7T. J Magn Reson Imaging. 2008;28:1005–1018. doi: 10.1002/jmri.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yip CY, Grissom WA, Fessler JA, Noll DC. Joint design of trajectory and RF pulses for parallel excitation. Magn Reson Med. 2007;58:598–604. doi: 10.1002/mrm.21262. [DOI] [PubMed] [Google Scholar]
- 45.Wu X, Akgün C, Vaughan JT, Andersen P, Strupp J, et al. Adapted RF pulse design for SAR reduction in parallel excitation with experimental verification at 9.4T. J Magn Reson. 2010 doi: 10.1016/j.jmr.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 46.Lee D, Lustig M, Grissom WA, Pauly JM. Time-optimal design for multidimensional and parallel transmit variable-rate selective excitation. Magn Reson Med. 2009;61:1471–1479. doi: 10.1002/mrm.21950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu Y, Alon L, Deniz CM, Brown R, Sodickson DK. System and SAR characterization in parallel RF transmission. Magn Reson Med. 2012;67:1367–1378. doi: 10.1002/mrm.23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deniz CM, Alon L, Brown R, Sodickson DK, Zhu Y. Specific absorption rate benefits of including measured electric field interactions in parallel excitation pulse design. Magn Reson Med. 2012;67:164–174. doi: 10.1002/mrm.23004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Z, Yip CY, Grissom W, Noll DC, Boada FE, et al. Reduction of transmitter B1 inhomogeneity with transmit SENSE slice-select pulses. Magn Reson Med. 2007;57:842–847. doi: 10.1002/mrm.21221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boskamp EB, Lee RF. Whole Body LPSA transceive array with optimized transmit homogeneity. Proc Intl Soc Mag Reson Med. 2002 [Google Scholar]
- 51.Metzger GJ, Snyder C, Akgun C, Vaughan T, Ugurbil K, et al. Local B1+ shimming for prostate imaging with transceiver arrays at 7T based on subject-dependent transmit phase measurements. Magn Reson Med. 2008;59:396–409. doi: 10.1002/mrm.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaughan JT, Adriany G, Snyder CJ, Tian J, Thiel T, et al. Efficient high-frequency body coil for high-field MRI. Magn Reson Med. 2004;52:851–859. doi: 10.1002/mrm.20177. [DOI] [PubMed] [Google Scholar]
- 53.Conolly S, Nishimura D, Macovski A. Optimal control solutions to the magnetic resonance selective excitation problem. IEEE Trans Med Imaging. 1986;5:106–115. doi: 10.1109/TMI.1986.4307754. [DOI] [PubMed] [Google Scholar]
- 54.Murdoch JB, Lent AH, Kritzer MR. Computer-optimized narrowband pulses for multislice imaging. J Magn Reson. 1987;74:226–263. [Google Scholar]
- 55.Hollingsworth NA, Feng K, Chang C-W, Wright SM, McDougall MP. Development of a 64 Channel Parallel Transmit System. Proceedings of Proceeding in International Society of Magnetic Resonance in Medicine; Honolulu. 2009. [Google Scholar]
- 56.Zhu Y, Chu X, Cao C, Fiveland E, Giaquinto R, et al. Highly Distributed RF Transmission with a 32-Channel Parallel Transmit System. Proceedings of Proceeding in International Society of Magnetic Resonance in Medicine; Honolulu. 2009. [Google Scholar]
- 57.Pang Y, Xie Z, Wu B, Wang C, Vigneron DB, et al. PC controlled 8-transmit channel circuit with independent phase and amplitude control for 7T. Proceedings of Proceeding in International Society of Magnetic Resonance in Medicine; Honolulu. 2009. [Google Scholar]
- 58.Heilman JA, Riffe MJ, Heid O, Griswold MA. High power, high efficiency on-coil current-mode amplifier for parallel transmission arrays. Proceedings of Proceeding in International Society of Magnetic Resonance in Medicine; Berlin. 2007. [Google Scholar]
- 59.Hoult DI, Kolansky G. A 500 W, broadband, non-magnetic RF MOSFET amplifier for MRI use. Proceedings of Proceeding in International Society of Magnetic Resonance in Medicine; Toronto. 2008. [Google Scholar]