Abstract
Conventional drug therapy and several anti-CD154 mAb-based regimens were tested in the nonhuman primate (NHP) islet allograft model and found to be inadequate because islets were lost to rejection. Short-term therapy with an optimized donor-specific transfusion (DST) + rapamycin (RPM) + anti-CD154 mAb regimen enables immunosuppression drug-free islet allograft function for months following cessation of therapy in the NHP islet allograft model. After a substantial period of drug-free graft function, these allografts slowly and progressively lost function. Pathologic studies failed to identify islet allograft rejection as a destructive islet invasive lymphocytic infiltration of the allograft was not detected. To evaluate the mechanism, immunologic versus nonimmunologic, of the late islet allograft loss in hosts receiving the optimized therapeutic regimen, we performed experiments with islet autografts and studied islet function in NHPs with partial pancreatectomy. The results in both experiments utilizing autologous islet allografts and partially pancreatectomized hosts reinforce the view that the presence of a marginal islet mass leads to slowly progressive nonimmunological islet loss. Long-term clinically successful islet cell transplantation cannot be realized in the absence of parallel improvements in tolerizing regimens and in the preparation of adequate numbers of islets.
Keywords: Allograft, autografts, cynomologlus monkeys, islet transplantation
Introduction
Insulin therapy provides an imperfect solution for the long-term care of patients with type 1 diabetes (T1D). While life expectancy is improved, insulin therapy does not prevent chronic diabetic complications in many T1D patients. In theory, the induction of immune tolerance to islet transplants offers the greatest hope for eluding the complications of T1D and maintenance immunosuppressive therapy for transplantation. We now report that short-term therapy with an optimized regimen consisting of donorspecific transfusion (DST) + rapamycin (RPM) + antiCD154 mAb enables immunosuppressive drug-free islet allograft function for months following cessation of therapy in the nonhuman primate (NHP) islet allograft model. This regimen proved superior to several other therapeutic strategies. The loss of islet function in hosts receiving the optimized DST + RPM + anti-CD154 mAb regimen was far more gradual than in hosts receiving less effective regimens whose grafts were abruptly lost in the early post-transplant period as a consequence of acute cellular rejection. To evaluate the mechanism of this siowly progressive graft loss, we performed experiments in an islet autograft mode!. Despite the absence of rejection inherent in this model, the transplanted islets slowly and progressively lost function. As islet harvesting and transplant procedures are known to lead to islet cell loss (1,2), we also studied islet function in NHPs subjected to partial pancreatectomy. Again slow and progressive deterioration in blood glucose control was the outcome. Our studies suggest that long-term islet function, even in potentially immune tolerant hosts, may be impeded by insidious and progressive nonimmunologic loss of functioning islets in hosts receiving a marginal mass of functioning islets.
Materials and Methods
Animals
Donor and recipient cynomolgus monkeys (Macaca fascicularis) weighing between 3.1 and 9.0 kg were obtained from Charles River/Biomedical Resource, Inc., Houston, TX and quarantined for 6 weeks before study. These monkeys were negative for tests indicating infection with a carrier state for B-Virus, SIV, STLV-1, SRV, TB and Hemagram parasites. NHP care was in accordance with 'good laboratory practice, regulations for nonclinical laboratory studies.' The program and facilities at the Massachusetts General Hospital are fully accredited by the American Association for the Accreditation of Laboratory Animals Care (AAALC).
Induction and management of diabetes
After overnight fasting, monkeys were anesthetized with intra-muscular ketamine 10–15 mg/kg and hydrated with 50–60 mL of normal saline (NS) i.v. Immediately after dilution in 10 mL NS, streptozotocin (STZ) at a dose of 55 mg/kg (Sigma, St. Louis, MO) was given by rapid i.v. injection (3). Additional hydration with 100–150 mL of NS was given i.v. Blood glucose levels were tested three times per day using an Accu-Check blood glucose monitor (Roche, Indianapolis, IN). Each monkey treated with STZ in this series became diabetic with nonfasting blood glucose levels >400 mg/dL on three consecutive days. Diabetic monkeys were then treated with two to three injections of insulin per day (6–15 units a day). Diabetic monkeys were given i.v. NS, twice a week. The following tests were performed weekly upon peripheral blood: a complete blood count (CBC), electrolytes, creatinine, blood urea nitrogen, SGOT, alkaline phosphatase and bilirubin. Serum C-peptide levels were measured by radioimmunoassay (Human C-peptide RIA Kit, Linco Research, Inc., St. Charles, MO). The assay has a 90% cross-reactivity with cynomolgus monkey C-peptide.
Immunosuppressive reagents
Table 1 lists the immunosuppressive reagents utilized in these experiments.
Table 1.
Immunosuppressive reagents
| Abbreviation | Reagent | Source | Dose and timing |
|---|---|---|---|
| MMF | Mycophenolate mofetil CellCept | Roche Laboratories, Nutley, NJ | 20 mg/kg by oral gavage days 0 through 14 |
| ATG | Anti-thymocyte lobulin ATGAM | Pharmacia and Upjohn Co. Kalamazoo, MI | 50 mg/kg i.v. days −2, −1, 0 |
| CsA | Cyclosporine sandimmune | Novartis Pharmaceuticals East Hanover, NJ | 15 mg/kg i.m. days 0 through 28 |
| RPM (low) | Sirolimus rapamycin | Wyeth Pharmaceuticals Philadelphia, PA | 0.5 mg/kg by oral gavage days −2 to 28 |
| RPM | Sirolimus rapamycin | Wyeth Pharmaceuticals Philadelphia, PA | 1.0 mg/kg by oral gavage days −2 to 28 |
| Anti-CD154 (low) | IDEC #103 | IDEC Pharmaceuticals San Diego, CA | 15 mg/kg i.v. 2×/week for 4 weeks |
| Anti-CD154 | IDEC #103 | IDEC Pharmaceuticals San Diego, CA | 25 mg/kg i.v.×2, 20mg/kg ×6, 15 mg/kg ×2 given over 4 weeks |
| DST | Donor lymphocyte infusion | 25000 lymph node lymphocytes | As indicated |
Isolation of donor islets
Donor islets were isolated on the day of transplantation from pancreases coming from one of three sources: distal pancreatectomies on monkeys in our colony that were later used as recipients, total pancreatectomies on healthy monkeys in our colony that were sacrificed for other reasons or total pancreatectomies on healthy monkeys that were sacrificed at Charles River Laboratories (Worcester, MA). The pancreases were subjected to warm ischemia for less then 5 min, then perfused via the pancreatic duct with University of Wisconsin (UW) solution and then transported on ice to the JDRF islet isolation facility at the Joslin Diabetes Center. The cold ischemia time was >1 h. The donor pancreas was distended with Liberase HI (Roche Biochemicals, Indianapolis, IN) and incubated at 37°C in a static digestion chamber for 45 min. The digested tissue was collected and applied to a three-layer discontinuous Euroficoll gradient (layer densities of 1.112,1.096 and 1.060). The pancreatic tissue was bottom-loaded within the 1.112 layer and centrifuged at 900gfor 22 min at 4°C. Three 50 jiL samples were stained with dithizone and counted to assess the total islet cell yield. Samples were also taken for evaluation of DNA content, insulin staining, viability and histology. Finally the total number of islets was calculated as islet equivalents (IEQ) with an average diameter of 150 μm per islet. The islets were infused into the recipient in a 10–20 mL volume suspended in NS (4,5).
Islet transplantation
Recipients were anesthetized using intramuscular ketamine 10–15 mg/kg. Subsequently, anesthesia was maintained with ketamine 10–15 mg/kg i.v. and Valium 1 mg/kg i.v. or i.m. during surgery. A mesenteric venous branch (often the inferior mesenteric vein) was cannulated with a 22-gauge catheter then passed centrally into the portal circulation. Islets were infused into the recipient over a period of 2–3 min. In some cases an aliquot, ca. 5000 IEQ (50 μL volume), was placed under the left kidney capsule. The opening in the capsule containing the islet graft was closed with 5–0 silk sutures (6).
Following islet transplantation, blood glucose levels were checked twice daily. Insulin was not administered unless fasting blood glucose levels were consistently (>two blood glucose readings) above 200 mg/dL. The date of graft failure was defined as the first of 3 consecutive days of fasting blood glucose levels >250 mg/dL unless the blood sugar subsequently normalized without insulin therapy.
Intravenous glucose tolerance test (IVGTT)
Food was withheld for 15 h before testing. The test monkeys were given 0.5 g/kg of glucose in a 25% glucose solution intravenously over 1 min. Blood samples were then collected at 0, 1, 3, 5, 10, 15, 20, 25, 30, 45 and 60 min post-glucose challenge for assessment of blood glucose and C-peptide levels.
Islet transplant biopsy
Ketamine anesthesia for kidney biopsies was given as described above for islet transplantation. A wedge-biopsy (1/8 of an inch) of the islet transplant placed beneath the renal capsule was obtained.
Histology samples
Wedge biopsies of renal tissue bearing islets transplanted under the renal capsule were obtained, and the specimens were coded and randomized enabling blinded analysis of the sections. After blocking in paraffin, serial 4-μm sections were cut through the entire graft site and alternate slides, each with 3–5 sections, were stained with hematoxylin-eosin (H&E) for assessment of the mononuclear leukocytic infiltrate and stained with immunoperoxidase to detect endocrine cells. After sacrifice, autopsies were done. Tissue samples from pancreases, livers, spleen, thymus and kidneys were studied. Tissues were fixed in 10% formalin, embedded in paraffin, sectioned at 4–5 μm, and stained with H&E and by indirect immunoperoxidase staining for insulin and glucagon (7).
Results
Sixteen allogeneic islet transplants were performed in cynomolgus monkey recipients to test the efficacy of potentially tolerizing anti-CD154-based regimens. For comparison, several forms of continuous immunosuppression with conventional immunosuppressive drugs were also studied for efficacy. Three autologous islet transplants and two partial pancreatectomies were also performed. The survival of islet transplants for this entire series is summarized in Tables 2-4.
Table 2.
Conventional immunosuppression
| Monkey no.1
(IEQ/kg) |
Treatment | Survival2
(days) |
|---|---|---|
| 2 (12 000) | None (allogeneic islets) | 5 |
| 3 (15 600) | MMF | 18 |
| 4 (5600) | ATG, MMF and CsA | 14 |
| 5 (5500) | 25 | |
| 6 (10 800) | RPM | 12 |
| 7 (12 600) | RPM and DST | 6 |
Monkey numbers do not indicate the chronological sequence of their islet transplant.
Survival was defined as the first of three consecutive days with fasting blood sugars permanently >250 mg/dL without insulin therapy. (See Materials and Methods.)
Table 4.
Optimized anti-CD154-based immunosuppression
| Monkey no. (IEQ/kg) |
Treatment | Survival (days) |
|---|---|---|
| 14 (17000) | RPM, DST and anti-CD154 | 130 |
| 15 (10800) | (DST on day −7 to −3) | 180 |
| 16 (7400) | 230 | |
| 17 (10200) | RPM, DST and anti-CD154 (DST on day -2; Repeat Rx1 day 100 and day 200) |
260 |
Repeat Rx consisted of rapamycin at 1 mg/kg p.o. qd and anti-CD154 20 mg/kg i.v. 2×/week for a two-week period starting on day 100 and 200.
Rejection was noted in untreated allogeneic islet recipients and in recipients receiving standard immunosuppressive drug
In the only monkey islet allograft recipient not given immunosuppression (monkey 2) rejection as expected was noted soon following transplantation (Table 2). Blood glucose levels normalized within 3 days post-transplantation but became persistently and abnormally high by the 5th post-operative day. Five allogeneic islet transplant recipients (monkeys 3–7) received a variety of conventional immunosuppressive drugs, but the islet transplants were afforded protection from hyperglycemia for only 6–25 days despite ongoing conventional immunosuppressive drug treatment (Table 2 and Figure 1). in each of these recipients, rejection was early (Figure 1) and the pace of graft loss following detection of graft dysfunction was rapid (Figure 2).
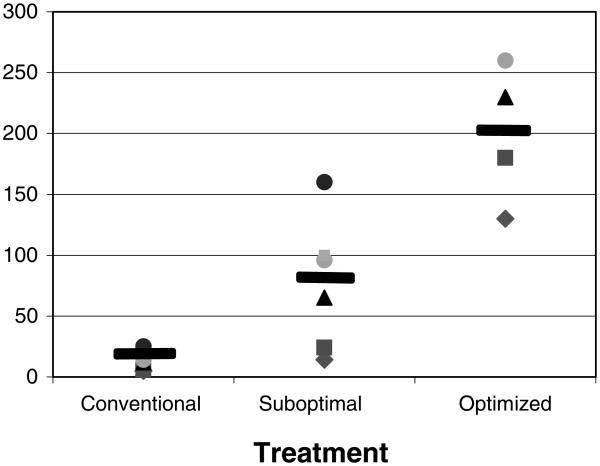
Figure 1. Days to islet graft rejection.
This graph compares graft survival in conventional, suboptimal and optimized regimens.
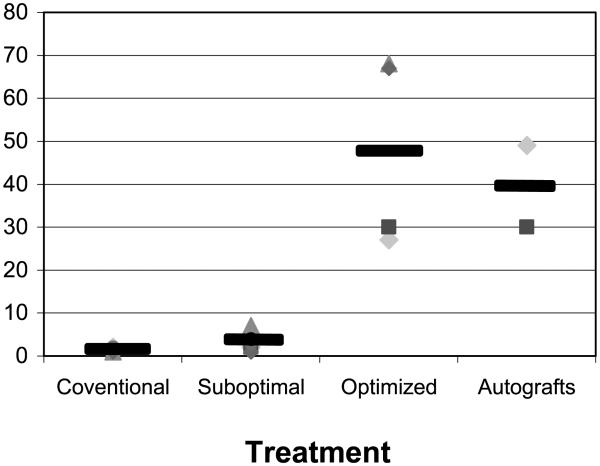
Figure 2. Days from inception to completion of graft failure.
This graph compares the days from inception to completion of graft failure in conventional, suboptimal and optimized treatment groups and in the autografts.
Allogeneic islet graft survival in recipients receiving anti-CD154-based treatments
The clinical course of 10 allogeneic islet transplant recipients receiving treatment with anti-CD154 mAb-based regimens is shown in (Tables 3 and 4). To probe for the possible induction of tolerance, immunosuppressive therapy was stopped within 30 days following islet transplantation in nine recipients monkeys 8–16. The other recipient, monkey 17, was treated with three separate and spaced courses of the RPM and anti-CD154 mAb regimen (as discussed below). This monkey also received DST prior to transplantation.
Table 3.
Suboptimal anti-CD154-based immunosuppression
| Monkey no. (IEQ/kg) |
Treatment | Survival (days) |
|---|---|---|
| 8 (11 500) | RPM (low), anti-CD154 (low)1 |
24 |
| 9 (12 500) | RPM and anti-CD154 | 65 |
| 10 (14 300 + 8200) | 160 | |
| 11 (15 700) | RPM, DST and anti-CD1542 (DST and RPM on day 03) |
14 |
| 12 (20 800) | RPM, DST and anti-CD154 (DST on day −17) |
96 |
| 13 (16 100) | RPM, DST and anti-CD154 (DST on day −2) |
100 |
Anti-CD154 treatment started at the time of transplantation.
When used with DST, the first dose of anti-CD154 was given at the time of the lymphocyte infusion and then continued at the time of transplantation.
Rapamycin was started on day 0, along with DST and anti-CD154. Rapamycin started on day −2 for all other monkeys.
Allogeneic islet graft survival in recipients receiving suboptimal anti-CD154-based treatments
In retrospect, monkeys 8–13 received a suboptimal regimen although this was not our purpose (Table 3). In mouse allograft models, the use of RPM as an adjunct to costimulation blockade therapy provides additive benefit (8,9). Two monkeys received a course of RPM + anti-CD154 mAb, one (monkey 8) receiving a low RPM and anti-CD154 mAb dose protocol and the other, monkey 9, a higher dose RPM and anti-CD154 mAb protocol (see Table 3 and Figure 1). The monkey receiving the higher dose protocol experienced a modest period of excellent graft function following cessation of therapy (Table 3). An improved anti-CD154 dosing schedule was recommended by Dr. R. Noelle (Dartmouth Medical School Planover, NH) on the basis of pharmacokinetic studies with serial blood level determinations. This improved RPM + anti-CD154 protocol used in monkey 9–17 utilized high dose anti-CD154 mAb able to saturate the CD154 target molecule (R. Noelle, personal communication, Tables 1-4) and higher RPM doses that proved sufficient to create therapeutic RPM blood levels (1 mg/kg/day) equivalent to those ideally achieved in clinical practice. As DST treatment, like RPM, synergizes with anti-CD154 treatment (10), we tested the efficacy of a regimen consisting of DST + RPM + anti-CD154 mAb. As shown in Tables 3 and 4, seven monkeys underwent islet transplantation and received DST at various times as an adjunct to RPM and anti-CD154 mAb treatment. The results achieved with the suboptimal protocols are summarized in Tables 3 and Figures 1 and 2. DST consisted of an infusion of 25 000 donor lymph node cells and an injection of anti-CD154 mAb at the time of the DST infusion. In one case (monkey 11), the DST was given on the day of transplantation and the islet transplant was lost very quickly (day 14). In all other cases, the DST was administered before the transplant. It is noted that the pace of rejection from the inception of modest hyperglycemia to complete graft failure with extreme hyperglycemia was rapid in the untreated hosts and in hosts receiving conventional drug therapy or suboptimal anti-CD154-based therapy (Figure 2).
Allogeneic islet graft survival in recipients receiving an optimized anti-CD154-based regimen
In this optimized regimen, the interval between DST and transplantation ranged from days —7 to —3. This DST protocol was used in concert with higher dose RPM + anti-CD154 regimen described above. The elements of this more successful regimen include the use of higher doses of RPM and anti-CD154 as well as the timing of DST. This 'optimized' regimen enabled much longer term graft survival following cessation of treatment than noted with the foregoing therapies (Table 4, Figure 1), but graft failure was eventually noted in each recipient (Table 4, Figure 1). It is notable that the pace of graft loss differed from that experienced in the previous treatment groups, as graft dys-function was slowly, not rapidly, progressive (Figure 2). An additional allograft was performed (monkey 17) in a host that was retreated with initial regimen at day 100 post-transplantation. This recipient experienced the longest period of graft function but graft failure was evident by day 260 post-transplantation.
The body weights of the NHPs receiving islet transplants did not change dramatically. The control NHPs and monkeys with suboptimal treatment subjected to acute rejection lost about 10% of their body weight (see monkey 2–13). In contrast, monkeys receiving autografts or the optimized anti-CD154 regimen gained on average 10–15% of their body weight (monkeys 1a, 1b, 1c and 14–17). Body weights were calculated from the day of islet transplant.
In sum, rapid and early rejection was noted during active drug therapy in control untreated NHP recipients and in recipients receiving conventional drug therapy or suboptimal anti-CD154-based therapies (Tables 2 and 3, Figures 1, 2). In contrast prolonged allograft survival, enduring long past the cessation of drug therapy and late, slowly progressive loss of graft function was noted in recipients receiving the most effectively timed DST + optimized RPM + high dose anti-CD154 therapy (Table 4 and Figures 1,2). Finally, untreated allograft recipients or recipients receiving conventional drug therapy or nonoptimized anti-CD154 mAb-based regimens were found to have circulating anti-donor antibodies. In contrast the circulation of hosts with prolonged drug-free graft survival following cessation of optimizes anti-CD154-based treatment contained no or very low levels of anti-donor antibodies (data not shown).
An example of acute islet allograft rejection in a host receiving a suboptimal anti-CD154-based regimen
Acute rapidly progressive islet transplant rejection in a host receiving suboptimal anti-CD154-based therapy was evident in monkey 8 (Table 3, Figures 1, 2). Monkey 8 was treated with RPM (0.5 mg/kg p.o. qd) and anti-CD154 (15 mg/kg) (Table 3). We later learned that this regimen led to subtherapeutic serum levels of RPM (2–8 ng/mL). Although reasonable (ca.180 mg/dL) albeit not ideal blood sugar control was obtained for the first 3 weeks, this recipient became overtly hyperglycemic by day 24 and required insulin therapy thereafter.
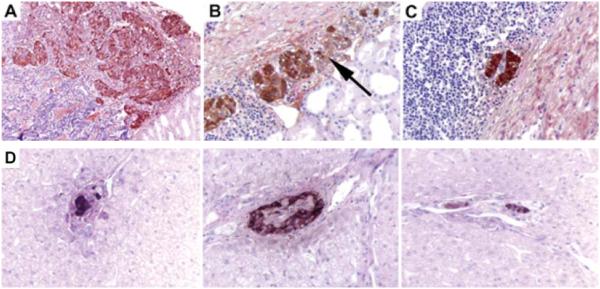
An aliquot of allogeneic islets was placed under the kidney capsule in monkey 8 while the remaining islets were injected into the portal vein. This dual placement was done to enable the histological examination of the islet transplants at various intervals after transplantation. Histologic analysis of islets observed within the kidney and liver biopsies of monkey 8 on days 19, 33 and 45 was conducted (see Figure 3 A-D). On day 19, shortly before the onset of obvious clinical rejection, a lymphocytic infiltration surrounding and invading the subrenal capsular implanted islets was detected, although the islets still showed substantial insulin staining (Figure 3A). Two weeks later (day 33), invasion of the islets by lymphocytes (isletitis) was noted and only slight residual insulin staining was detected (Figure 3B). Figure 3C and D shows the morphology of subrenal capsule and liver placed islets obtained from monkey 8 at the time of sacrifice on day 45. The islets in the liver were small in portal veins and often encapsulated by endothelium. Some showed isletitis. In the kidney, only tiny residual islets were present embedded in inflammation.
Figure 3. Histologic findings during acute rejection at days 15, 33 and 45 from monkey 8.
A, B and C shows islets in under the renal capsule and D shows islets in the liver at day 45. (A) Islets with insulin staining day 19, 400x. (B) Islets with insulin staining, day 33, 400x. Arrow identifies isletitis. (C) Residual islet (kidney) with insulin, day 45, 400x. (D) Isolated islets with Insulin staining in the liver, day 45, 400x.
In recipients receiving DST + optimized anti-CD154 and RPM doses allograft dysfunction was late occurring, slowly progressive, and graft loss was not associated with inflammation and isletitis that is typical of rejection. Instead, lymphocytes surround but do not invade the islets (Figure 4). Noninvasive inflammation was the morphologic pattern noted in recipients 14–17, and graft loss in these recipients occurred long after cessation of active treatment. Moreover, as mentioned, graft failure occurred late and was slowly progressive (see Table 4 and Figures 1 and 2).
Figure 4. Histologic findings in the liver after prolonged survival in monkey 16.
Islets with insulin staining in the liver at day 301, 400x.
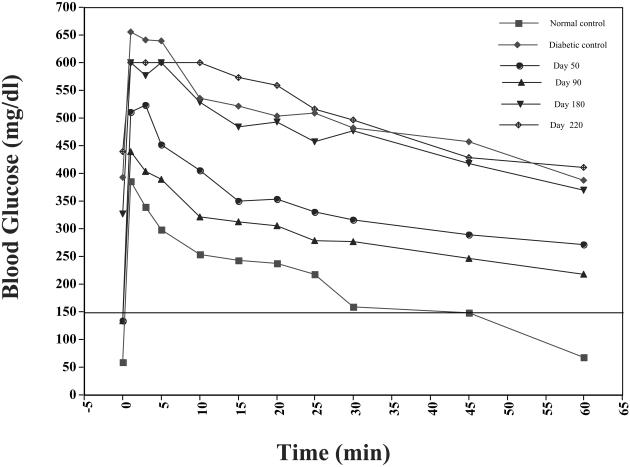
To test the hypothesis that the late occurring gradual loss of graft function may have a nonimmunological basis, we performed a series of autologous islet transplants (Table 5).Monkey 1a was subjected to a partial pancreatectomy followed by administration of STZ. This monkey was rescued with an autologous islet transplant (5000 IEQ/kg). Immunosuppression was not given to this recipient (see Table 5). Blood glucose levels normalized within 4 days of transplantation and remained normal for 100 days, at which point the monkey was sacrificed. Large insulin and glucagon immunocytochemistry stain positive islets were detected. Two additional autografts (monkeys 1b and 1c) were performed and slowly progressive deterioration of fasting blood glucose was eventually observed in each. We observed delayed slow and progressive deterioration in the response to glucose challenge at 90 and 180 days post-transplantation (Figure 5). Each monkey became diabetic 150–180 days post-transplantation (Figure 2).
Table 5.
Autograft survival
| Monkey no.1
(IEQ/kg) |
Treatment | Survival2
(days) |
|---|---|---|
| 1a (5000) | None (autologous islets) | >1003 |
| 1b (5000) | 180 | |
| 1c (5000) | 150 |
Monkey numbers do not indicate the chronological sequence of their islet transplant.
Survival was defined as the first of three consecutive days with fasting blood sugars permanently >250 mg/dL without insulin therapy. (See Materials and Methods.).
This animal was sacrificed with normal blood sugars.
Figure 5.
Intravenous tolerance tests in monkey 1b that received an autograft.
In contrast to the pathologic changes noted with acutely rejected islets which are easy to find as lymphocytic aggregates persist at the site of the transplant for a long time after the islets have lost function, lymphocyte aggregates were not detected (pathology not shown). Moreover, islets were not detected by histological analysis of the liver in each of the diabetic autograft recipients (50 sections/levels cut per liver). This is in agreement with previous reports showing that monkeys and dogs transplanted with autografts develop diabetes in concert with apparent total loss of the transplanted islets (11–13).
Partially pancreatectomized hosts develop slowly deteriorating islet function
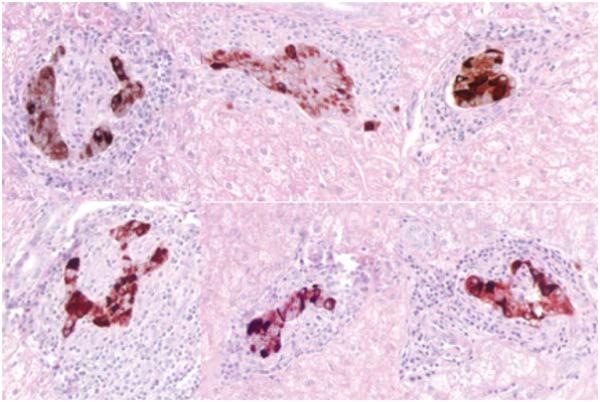
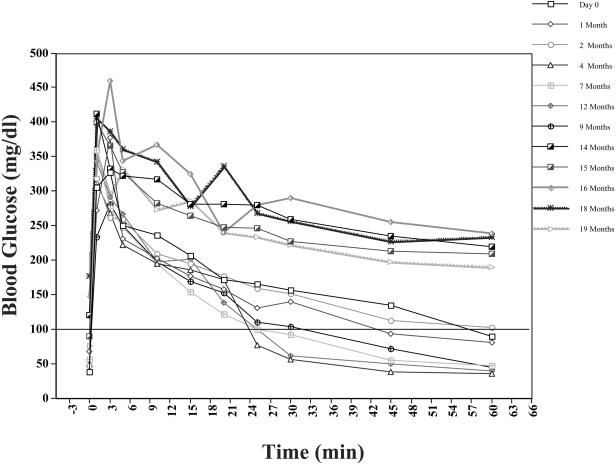
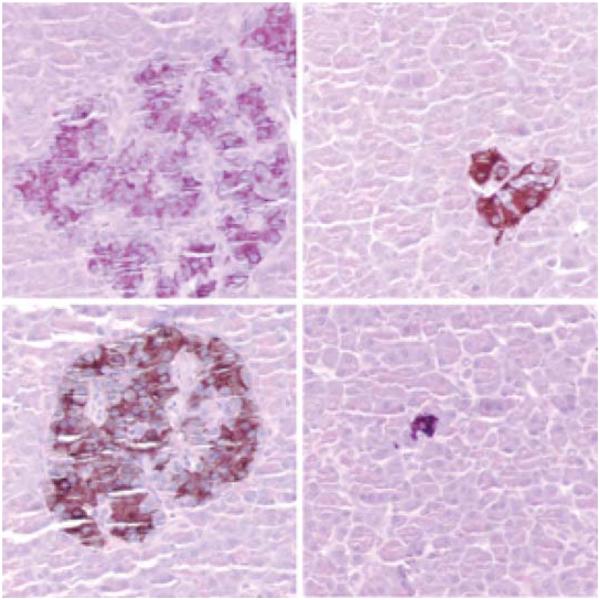
To further study the effect of marginal islet mass, partial pancreatectomies (75–80%) were performed in two monkeys. Blood glucose levels in the partially pancreatectomized monkeys were monitored 2–3 times a week, and an IVGTT was performed every 4 weeks. In both monkeys, the IVGTT became abnormal 13 months post-partial pancreatectomy with progressive metabolic deterioration thereafter (Figure 6). By 14 months post-partial pancreatectomy, nonfasting blood glucose levels ranged from 200 to 300 mg/dL. These monkeys were sacrificed at 19 months post-partial pancreatectomy. Their pancreases contained small numbers of islets that stained positive for insulin and glucagon by immunocytochemistry (Figure 7).
Figure 6.
Intravenous tolerance tests in a monkey with a partial pancreatectomy.
Figure 7. Histological findings in the islets after a partial pancreatectomy.
Islets identified with insulin staining, 400x. Some islets appear slightly hypertrophic and some appear small.
Discussion
In our study a 4-week optimized regimen of DST, RPM and anti-CD154 mAb treatment led to prolonged drug-free islet allograft survival but permanent engraftment was not achieved (Table 4). After substantial period of drug-free graft function several grafts slowly and progressively lost function. Even the institution of repetitive courses of therapy failed to produce permanent engraftment. Pathologic studies failed to reveal the classical hallmarks of islet allograft rejection, i.e. a destructive islet invasive lymphocytic infiltration was not detected in these late failing allografts. A T-cell rich collection of lymphocytes surrounds but does not invade islet allografts in tolerant hosts (14). The pattern of noninvasive inflammation was noted only in hosts receiving the optimized anti-CD154-based regimen long following cessation of therapy at the advent of graft dysfunction (Figure 4). Moreover, serial analysis of anti-alloantibody formation and the failure to detect a robust anti-donor antibody response in the optimally treated group suggests that nonimmunologic mechanisms may have caused or contributed to graft failure in those monkeys with late, slow inexorably progressive allograft dysfunction. While these data are not definitive, they suggest that nonimmune mechanisms contribute to or may even cause the late graft failure observed in the hosts with optimized anti-CD154-based therapy.
It is now well appreciated that islet transplants are subject to nonimmunologic destructive processes. For example, a thrombotic/inflammatory reaction is elicited when isolated islets of Langerhans come in contact with ABO-compatible blood (1,2). It seems likely that ischemia-reperfusion injury occurs during islet harvesting that is exacerbated by transplantation until revascularization is complete. Testing for the presence of these specific nonimmunologic mechanisms of graft failure is pertinent and of active investigative interest but beyond the scope of this study. The islet harvesting and transplant procedures lead to islet cell loss and may leave the recipient with a marginal mass of functioning islets. To distinguish between immunologic versus nonimmunologic injury in late islet allograft loss, we performed experiments with islet autografts. Islet autografts after periods of excellent function also slowly and progressively fail. Nonimmunologic graft failure is clinically relevant and could be related to either marginal islet mass or intra-portal placement.
To further probe the potential role that transplantation of a marginal mass of functioning islets may play in the absence of both an immune attack and the problems associated with harvesting and transplanting islets, we studied islet function in NHPs subjected to partial pancreatectomy (75— 80%). Obviously and as in the autograft model, rejection cannot occur. Unlike the islet model, the problems associated with the islet harvesting, preservation and transplantation procedures are avoided. Thus the partial pancreatectomized system is a purer marginal mass model. Note that a common feature of graft loss in the drug-free hosts following application of an optimized treatment regimen and in autologous islet recipients and in partially pancreatectomized hosts is slowly progressive graft failure. This pattern of gradual progressive graft dysfunction differed from the abrupt and early graft failure noted in recipients that acutely reject their allografts because they did not receive an optimized anti-CD154-based regimen (Figure 2).
In all instances, graft failure in the autograft recipients and the partially pancreatectomized hosts occurred in the absence of invasive isletitis. The results noted in these experiments reenforce the view that the presence of a marginal islet mass leads to nonimmunological islet loss, perhaps as a consequence of metabolic exhaustion (15-17). This mechanism is especially important in the hosts that gain weight following transplantation of marginal islet cell mass (13).
Using beagles as a large animal model Alejandro et al. (11) studied the viability and functional capacity of islet autografts placed into a portal circulation. Twenty percent of the dogs sustained normal levels of fasting blood glucose for >15 months post-transplant. Slowly progressive metabolic deterioration and loss of beta cells below the minimal threshold required to maintain normal fasting blood glucose levels were noted (11). Similar results were reported in other studies of canine autografts (12).
To achieve permanent engraftment, tolerance-inducing protocols are a necessity in addition to an adequate supply of islets. Conventional immunosuppressive drug therapies did not protect islet allografts from acute cellular rejection. Of several potentially tolerance inducing regimens, a high dose anti-CD154-based protocol that included DST and RPM as adjunctive measures was particularly potent in enabling drug-free islet allograft function (Table 4). In previous investigations using murine models, we have noted that the pairing of RPM with anti-CD154 mAb is synergistic and can, in some models, produce transplant tolerance (8). The addition of RPM to anti-CD154 acts to exaggerate the selective apoptotic death of donor reactive T cells (8,9). Although, total and persistent depletion is not discerned, the loss of many donor reactive alloaggressive T cells facilitates induction of tolerance (8,9). This tolerant state is dependent upon the presence of CD25+ T regs (18). As the addition of DST to anti-CD154-based therapy leads to amplified donor reactive T-reg activity (18), it may not be surprising that DST + RPM + anti-CD154 was the most potent therapy tested in this study. The loss of effector T cells through apoptosis and DST-induced increases in T-reg activity likely accounts for the superior potency of this regimen given over a longer time period which has also been used to good advantage by Kirk and his colleagues in NHP skin and renal allograft models (19,20). Some skin and all renal allograft recipients experienced prolonged graft survival following cessation of DST, RPM and anti-CD154 mAb (19,20).
The poor long-term outcome in glucose homeostasis in recipients of autologous islets as well as partial pancreatectomized hosts emphasize the problems that are associated with a marginal of islet cell. As the islet cell mass in the partially pancreatectomized host is not threatened by either rejection or problems that attend islet transplantation, it is most interesting that slowly progressive loss of islet function is evident. In each circumstance in which the host harbors a marginal mass of islets barely able to maintain glucose homeostasis, slow and progressive failure of the residual beta cell mass is the inevitable outcome.
Is nonimmunological loss of islet function a problem clinically? Yes, First 40% of human islet autografts gradually lose (21) function after sustained periods of excellent function. Second, an unexpectedly high rate of late islet allograft failure is also noted in the clinic among islets even after successful engraftment at 1 year (15). The mechanism of graft loss is poorly understood. Moreover, islet autografts in pre-clinical (11-13) and clinical (21-23) conditions have a very significant failure rate. The rate of autograft failure is particularly high when used to treat patients with pancreatitis, harvesting and transplantation of autologous islets are also subject to a substantial rate of failure. Taken together, these data suggest that islet transplants can and do fail as a result of nonimmunological mechanisms. Islet transplants appear particularly vulnerable when a marginal islet cell mass is present. In short the promise of islet cell transplantation in the clinic cannot be realized in the absence of parallel improvements in tolerizing regimens and in the preparation of adequate numbers of islets that are resistant to nonimmunologic mechanisms of graft failure.
Acknowledgment
We thank Vaja Tchipashivili from the Joslin Diabetes Center for providing all of the monkey islets and Luba Zachachin for expert assistance with tissue processing. This work was funded in part by the Juvenile Diabetes Research Foundation Center for Islet Transplantation at Harvard Medical School and by NIH P01 DK53087 and by NIH 5 RO1 AI50987.
References
- 1.Johansson H, Lukinius A, Moberg L, et al. Tssue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54:1755–1762. doi: 10.2337/diabetes.54.6.1755. [DOI] [PubMed] [Google Scholar]
- 2.Bennet W, Groth CG, Larsson R, Nilsson B, Korsgen O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci. 2000;2:125–133. doi: 10.1517/03009734000000059. [DOI] [PubMed] [Google Scholar]
- 3.Koulmanda M, Qipo A, Chebrolu S, O'Neil J, Auchincloss H, Smith RN. The effect of low versus high dose of streptozotocin in cynomolgus monkeys (Macaca fascilularis) Am J Transplant. 2003;3:267–272. doi: 10.1034/j.1600-6143.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 4.Ricordi C, Gray DW, Hering BJ, et al. Islet isolation assessment in man and large animals. Acta Diabetol Lat. 1990;27:185–195. doi: 10.1007/BF02581331. [DOI] [PubMed] [Google Scholar]
- 5.O'Neil JJ, Tchipashvili V, Parent RJ, et al. A simple and cost-effective method for the isolation of islets from nonhuman primates. Cell Transplant. 2003;12:883–890. doi: 10.3727/000000003771000110. [DOI] [PubMed] [Google Scholar]
- 6.Gray DW. Islet isolation and transplantation techniques in the primate. Surg Gynecol Obstet. 1990;170:225–232. [PubMed] [Google Scholar]
- 7.Kawai T, Sogawa H, Koulmanda M, et al. Long-term islet allograft function in the absence of chronic immunosuppression: a case report of a nonhuman primate previously made tolerant to a renal allograft from the same donor. Transplantation. 2001;72:351–354. doi: 10.1097/00007890-200107270-00036. [DOI] [PubMed] [Google Scholar]
- 8.Wells AD, Li XC, Li Y, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 10.Parker DC, Greiner DL, Phillips NE, et al. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc Nat! Acad Sci USA. 1995;92:9560–9564. doi: 10.1073/pnas.92.21.9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alejandro R, Cutfield RG, Shienvold FL, et al. Natural history of intrahepatic canine islet cell autografts. J Clin Invest. 1986;78:1339–1348. doi: 10.1172/JCI112720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warnock GL, Cattral MS, Rajotte RV. Normoglycemia after implantation of purified islet cells in dogs. Can J Surg. 1988;31:421–426. [PubMed] [Google Scholar]
- 13.Gray DW, Warnock GL, Sutton R, Peters M, McShane P, Morris PJ. Successful autotransplantation of isolated islets of Langerhans in the cynomolgus monkey. Br J Surg. 1986;73:850–853. doi: 10.1002/bjs.1800731029. [DOI] [PubMed] [Google Scholar]
- 14.Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol. 2004;4:259–268. doi: 10.1038/nri1332. [DOI] [PubMed] [Google Scholar]
- 15.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 16.Wahoff DC, Papalois BE, Najarian JS, et al. Autologous islet transplantation to prevent diabetes after pancreatic resection. Ann Surg. 1995;222:562–575. doi: 10.1097/00000658-199522240-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kauffman DB, Morel P, Field MJ, Munn SR, Sutherland DE. Purified canine islet autografts. Functional outcome as influenced by islet numbers and implantation site. Transplantation. 1990;50:385–391. [PubMed] [Google Scholar]
- 18.Sanchez-Fueyo A, Tian J, Picarella D, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4:1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 19.Preston EH, Xu H, Dhanireddy KK, et al. IDEC-131 (anti-CD 154), sirolimus and donor-specific transfusion facilitate operational tolerance in non-human primates. Am J Transplant. 2005;5:1032–1041. doi: 10.1111/j.1600-6143.2005.00796.x. [DOI] [PubMed] [Google Scholar]
- 20.Xu H, Montgomery SP, Preston EH, et al. Studies investigating pretransplant donor-specific blood transfusion, rapamycin, and the CD154-specific antibody IDEC-131 in a nonhuman primate model of skin allotransplantation. J Immunol. 2003;170:2776–2782. doi: 10.4049/jimmunol.170.5.2776. [DOI] [PubMed] [Google Scholar]
- 21.Oberhoizer J, Triponez F, Lou J, Morel P. Clinical islet transplantation: a review. Ann N Y Acad Sci. 1999;875:189–199. doi: 10.1111/j.1749-6632.1999.tb08503.x. [DOI] [PubMed] [Google Scholar]
- 22.Watkins JG, Krebs A, Rossi RL. Pancreatic autotransplantation in chronic pancreatitis. World J Surg. 2003;27:1235–1240. doi: 10.1007/s00268-003-7243-8. [DOI] [PubMed] [Google Scholar]
- 23.Johnson PR, White SA, Robertson GS, et al. Pancreatic islet auto-transplantation combined with total pancreatectomy for the treatment of chronic pancreatitis—the Leicester experience. J Mol Med. 1999;77:130–132. doi: 10.1007/s001090050320. [DOI] [PubMed] [Google Scholar]