Abstract
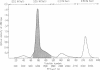
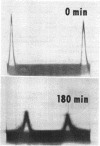
Filtrates from cultures of a psychrophilic Pseudomonas species, which inactivate serum inhibitors of certain viral hemagglutinins, were shown to contain both lecithinase (phospholipase C) and a proteolytic enzyme with elastase activity. The bacterium was cultivated under conditions favoring production of the respective enzymes, and the enzymes were purified by ammonium sulfate precipitation followed by column chromatography or by gel filtration. The elastase was obtained in crystalline form and was recrystallized. It has properties similar to those of a number of other bacterial elastases but is more heat-labile than most. Although a high degree of purification was achieved for the lecithinase, as evidenced by an increase in specific activity, it was not obtained in crystalline form. Partially purified preparations of the lecithinase had extremely high activity compared to that of commercial preparations of phospholipase C from Clostridium welchii.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRINK N. G., LEWIS U. J., WILLIAMS D. E. Pancreatic elastase: purification, properties, and function. J Biol Chem. 1956 Oct;222(2):705–720. [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- LIU P. V. Identification of pathogenic pseudomonads by extracellular antigens. J Bacteriol. 1961 Jan;81:28–35. doi: 10.1128/jb.81.1.28-35.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu P. V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. II. Effects of lecithinase and protease. J Infect Dis. 1966 Feb;116(1):112–116. doi: 10.1093/infdis/116.1.112. [DOI] [PubMed] [Google Scholar]
- MANDL I., COHEN B. B. Bacterial elastase. I. Isolation, purification and properties. Arch Biochem Biophys. 1960 Nov;91:47–53. doi: 10.1016/0003-9861(60)90453-7. [DOI] [PubMed] [Google Scholar]
- MANDL I., KELLER S., COHEN B. Microbial elastases. A comparative study. Proc Soc Exp Biol Med. 1962 Apr;109:923–925. doi: 10.3181/00379727-109-27379. [DOI] [PubMed] [Google Scholar]
- MILLER G. L., GOLDER R. H. Buffers of pH 2 to 12 for use in electrophoresis. Arch Biochem. 1950 Dec;29(2):420–423. [PubMed] [Google Scholar]
- MORIHARA K. PRODUCTION OF ELASTASE AND PROTEINASE BY PSEUDOMONAS AERUGINOSA. J Bacteriol. 1964 Sep;88:745–757. doi: 10.1128/jb.88.3.745-757.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORIHARA K., TSUZUKI H., OKA T., INOUE H., EBATA M. PSEUDOMONAS AERUGINOSA ELASTASE. ISOLATION, CRYSTALLIZATION, AND PRELIMINARY CHARACTERIZATION. J Biol Chem. 1965 Aug;240:3295–3304. [PubMed] [Google Scholar]
- Macfarlane M. G., Knight B. C. The biochemistry of bacterial toxins: The lecithinase activity of Cl. welchii toxins. Biochem J. 1941 Sep;35(8-9):884–902. doi: 10.1042/bj0350884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara H., Sasaki R., Singer A., Jukes T. H. Specific nature of hydrolysis of insulin and tobacco mosaic virus protein by thermolysin. Arch Biochem Biophys. 1966 Aug;115(2):324–331. doi: 10.1016/0003-9861(66)90282-7. [DOI] [PubMed] [Google Scholar]
- McClung L. S., Toabe R. The Egg Yolk Plate Reaction for the Presumptive Diagnosis of Clostridium sporogenes and Certain Species of the Gangrene and Botulinum Groups. J Bacteriol. 1947 Feb;53(2):139–147. doi: 10.1128/jb.53.2.139-147.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H. Substrate specificity of elastolytic and nonelastolytic proteinases from Pseudomonas aeruginosa. Arch Biochem Biophys. 1966 Apr;114(1):158–165. doi: 10.1016/0003-9861(66)90317-1. [DOI] [PubMed] [Google Scholar]
- SACHAR L. A., WINTER K. K., SICHER N., FRANKEL S. Photometric method for estimation of elastase activity. Proc Soc Exp Biol Med. 1955 Nov;90(2):323–326. doi: 10.3181/00379727-90-22022. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., GILFILLAN R. F., BARDAWIL W. A. A plate assay for elastase. Nature. 1960 Oct 22;188:322–323. doi: 10.1038/188322b0. [DOI] [PubMed] [Google Scholar]
- SCHMIDT N. J., DENNIS J., HOFFMAN M. N., LENNETTE E. H. INHIBITORS OF ECHOVIRUS AND REOVIRUS HEMAGGLUTINATION. II. SERUM AND PHOSPHOLIPID INHIBITORS. J Immunol. 1964 Sep;93:377–386. [PubMed] [Google Scholar]
- SCHMIDT N. J., DENNIS J., LENNETTE E. H. STUDIES ON FILTRATES FROM CULTURES OF A PSYCHROPHILIC PSEUDOMONAS SP. WHICH INACTIVATE NONSPECIFIC SERUM INHIBITORS FOR CERTAIN HEMAGGLUTINATING VIRUSES. J Immunol. 1964 Jul;93:140–147. [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H., Dennis J. Hemagglutination-inhibiting antibody responses in human infections with group B coxsackieviruses. J Immunol. 1966 Feb;96(2):311–318. [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H., King C. J. Neutralizing, hemagglutination-inhibiting and group complement-fixing antibody responses in human adenovirus infections. J Immunol. 1966 Jul;97(1):64–74. [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]