Abstract
Although two thirds of the 120 million people infected with lymph-dwelling filarial parasites have subclinical infections, ∼40 million have lymphedema and/or other pathologic manifestations including hydroceles (and other forms of urogenital disease), episodic adenolymphangitis, lymphedema, and (in its most severe form) elephantiasis. Adult filarial worms reside in the lymphatics and lymph nodes and induce lymphatic dilatation. Progressive lymphatic damage and pathology results primarily from the host inflammatory response to the parasites but also perhaps from the host inflammatory response to the parasite's Wolbachia endosymbiont and as a consequence of superimposed bacterial or fungal infections. This review will attempt to shed light on disease pathogenesis in lymphatic filariasis.
Introduction
Infection with three closely related filarial worms—Wuchereria bancrofti, Brugia malayi and Brugia timori—each causative agents of what is termed lymphatic filariasis (LF), are vector (mosquito)-borne infections with similar life cycles that involve the adult worms living in the afferent lymphatics (and/or the lymph nodes) while their larval progeny, the microfilariae, circulate in the peripheral blood where they are available to infect mosquito vectors when they feed. Lymphatic filarial disease is the second leading parasitic cause of disability with disability-adjusted life years (DALYs) estimated to be 5.549 million.1,2 Bancroftian filariasis, caused by W. bancrofti, is responsible for 90% of those with lymphatic filariasis3 with the remaining 10% being caused by the two Brugian species. As of mid-2013, it has been estimated that LF is endemic in 72 countries in which 120–129 million are currently infected. Of these, ∼40 million have overt disease (hydrocele or lymphedema).
The clinical manifestations of LF are varied, but they most commonly segregate between two major outcomes—those with a subclinical condition associated with circulating microfilaremia and those with significant lymphatic compromise and damage. With the availability of better imaging techniques (e.g., ultrasound, lymphoscintigraphy, MRI, CT), it has become apparent that almost everyone with active infection (e.g., microfilarial positivity) has some degree of lymphatic abnormality that may include: dilatation and tortuosity of lymph vessels with collateralization, increased or abnormal patterns of lymph flow4,5 and urogenital lymphangiectasia.6,7
Overt (and clinically apparent) disease occurs in a significant minority (about 30–40%) of the 120 million individuals with LF. Lymphatic disease in LF is both acute and chronic. The acute manifestations of LF are typically characterized by retrograde adenolymphangitis (ADL)8 with the inguinal, axillary and epitrochlear nodes being most commonly involved. In W. bancrofti infection the lymphatic system of the male urogenital tract is frequently affected and is expressed clinically as funiculitis, epididymitis and/or orchitis.9 Filarial ADL is distinct from what has more recently been termed acute dermatotolymphangitis (DLA), a process characterized by development of a plaque-like lesion of cutaneous or subcutaneous inflammation and accompanied by ascending lymphangitis and regional lymphadenitis. These pathological features are accompanied by systemic signs of inflammation including fever and chills and are thought to result primarily from bacterial and fungal infections superimposed (or caused by) compromised lymphatic function.10
The chronic and most debilitating sequelae of LF often develop years after initial infection.8 In Bancroftian filariasis, the main clinical features are hydrocele, lymphedema, elephantiasis and chyluria whereas for Brugian filariasis the urogenital areas are commonly spared. The development of pathology is thought to be dependent on the presence of the adult worm. Histologically, the worm elicits little reaction as long as it is alive; however, upon death of the worm, a granulomatous reaction ensues.11,12 In addition, there is endothelial and connective tissue proliferation with tortuosity of the lymphatics and damaged or incompetent valves. This typically results in lymphatic dilatation and subsequently lymphatic dysfunction and compromise, leading to lymphedema. Secondary changes are: 1) associated brawny edema with hardening of tissues; 2) later hyper-pigmentation and hyper-keratosis with wart-like protuberances; 3) redundant skin folds and skin fissuring providing pathways for entry of microorganisms into the lymphatics.13
Pathogenesis of Disease in Lymphatic Filariasis
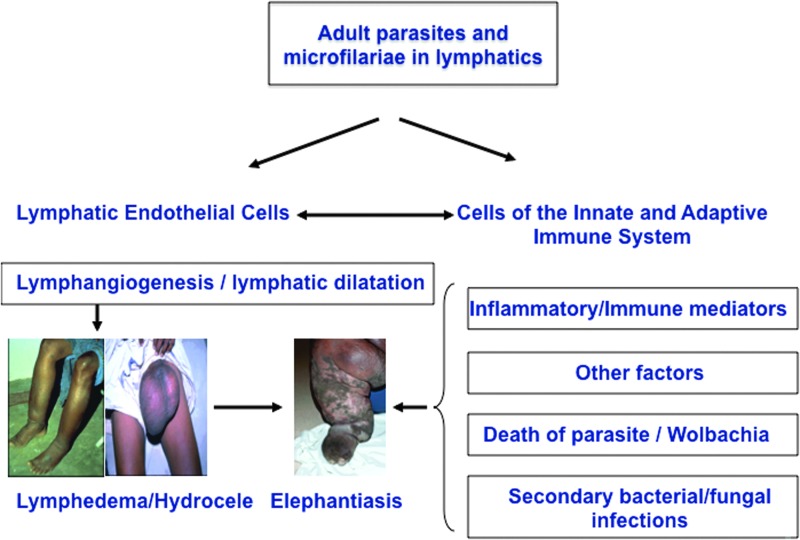
The most severe clinical manifestations of LF are lymphedema and elephantiasis. Although the immune responses to filarial parasites have been well studied with respect to natural history, diagnosis, and treatment, there is a relative paucity of information in terms of the mechanisms underlying development of pathology. The two major independent components of lymphatic filarial disease are lymphangiectasia and inflammatory reactions around the adult worms (Fig. 1). While most infected individuals exhibit lymphangiectasia, clinically apparent lymphedema is not common.5,11 It is also clear that with patent infection, lymphangiectasia develops in the vicinity of adult worm nests.11 Subclinical lymphangiectasia of the lymphatic vessels containing live adult worms have been shown to exhibit distention with no apparent inflammatory reactions in the vessel wall, with little or only a fleeting inflammatory response to living adult parasites.14 Further, that lymphangiectasia is not restricted to the exact segment of lymphatics where the worms reside15,16 suggests that this process is mediated by soluble products excreted or secreted by the parasite that act on the lymphatic endothelial cells. It is also clear that with the advent of adaptive immunity, the host inflammatory response against the dead or dying worm and the subsequent release of parasite products and inflammatory mediators, a stage of irreversible lymphatic dysfunction ensues.12,17,18 In addition, lymphatic dysfunction has been shown to predispose infected individuals to secondary bacterial and fungal infections and trigger inflammatory reactions in the skin and subcutaneous tissue that accelerates the progression of lymphedema and precipitates the development of elephantiasis.19,20 This two-step model of pathogenesis is mirrored in immunodeficient chronically infected and/or immune reconstituted animals in which the development of reversible pathology initially occurs in the absence of immune reconstitution but where fibrosis and cellular hyperplasia occurs once the adaptive immune system is established.21–23
FIG. 1.
Pathogenesis of lymphatic filarial disease (lymphedema, hydrocele, elephantiasis). Live filarial parasites and/or their products have a direct effect on lymphatic endothelial cells and on the cells of the innate and adaptive immune system. The interplay among inflammatory/ immune mediators, attrition of the parasites, Wolbachia and other factors contribute to pathogenesis and development of filarial disease. Secondary microbial infections further aggravate the pathology. A color version of this figure is available in the online article at www.liebertpub.com/lrb
The importance of pro-inflammatory cytokines, possibly of innate origin, in the pathogenesis of lymphedema, has been strengthened by a series of studies in humans with chronic pathology, either in early or late stages of lymphedema. Studies have shown that individuals with chronic lymphatic pathology have elevated levels of C-reactive protein,24 pro-inflammatory cytokines such as TNF-α, IL-6 and soluble TNF receptor,25,26 endothelin-1 and IL-2,27 as well as IL-8, MIP-1α, MIP-1β, MCP-1, TARC and IP-1028 in the peripheral circulation. Very few studies have actually examined the inflammatory milieu within the affected lymphatics; one study has described elevated levels of gamma-globulins, α-1 acid glycoprotein and IL-1β in the lymph fluid.29
Since the endothelium appears to be closely associated with pathogenesis of lymphatic disease, studies targeting the interaction between endothelial cells (vascular or lymphatic) and filarial parasites have been performed. The anatomical changes in the architecture of lymphatics that range from lymphangiectasia and granulomatous responses to the development of collaterals suggests that active lymphatic remodeling involving endothelial cell growth, migration and proliferation is an important feature of early disease.30,31 Indeed, although earlier studies using blood vascular endothelial cells failed to demonstrate an effect of soluble somatic filarial antigens,32 a more recent study suggests that live filarial parasites (and their excretory/ secretory products) induce activation, proliferation and tube formation in lymphatic endothelial cells.31 Moreover, only serum from patently infected or diseased individuals was shown to induce significant lymphatic endothelial cell (LEC) proliferation.33
Differentiation of LEC into tube-like networks was found to be associated with significantly increased levels of matrix metalloproteinases (MMPs) and inhibition of their endogenous inhibitors—TIMPs (tissue inhibitors of MMPs).33 Global gene expression analysis revealed alterations in genes involved in junction adherence pathways that decreased trans-endothelial transport, implicating parasite induced alterations in normal physiology of the lymphatic endothelium.33 Other studies have implicated the vascular endothelial growth factor (VEGF) family in lymphangiogenesis.34,35 Other angiogenic factors such as angiopoietins-1 and -2 are also found at elevated levels in individuals with filarial-induced pathology.36
A major factor involved in the initiation of the pro-inflammatory response and the increased production of VEGF-A and -C might be the endosymbiont, Wolbachia, present in most filarial nematodes (including W. bancrofti and the 2 Brugia spp).34 It has been known for several decades that filarial parasites harbor the intracellular rickettsial-like endosymbiotic bacteria.37 Indeed, the interaction of Wolbachia and its products with the pattern-recognition receptor TLR4 was thought to be responsible for the production of cytokines such as TNF-α and IL-1β.38 More recently, it has been demonstrated that the increased levels of VEGF-C and sVEGF-R3 (observed in lymphedema patients) were reduced following doxycycline treatment (a regimen that eliminates Wolbachia) and that there was improvement in lymphedema.35 Not all studies, however, favor a role for Wolbachia in inducing lymphangiogenesis36,39 It is however clear that the interaction between the filariae and TLR does play an important role in the pathogenesis of filarial disease.40,41 These data strongly suggest an important association between pattern recognition pathway signaling and lymphangiogenesis.
Persistent immune activation is associated with elevations of circulating microbial products, acute-phase proteins, and the so-called microbial translocation molecules.42 Translocation of microbial products from the lumen of the intestine into the periphery is thought to contribute to induction of inflammation by stimulating immune effector cells directly through their pattern recognition receptors42; however, intra- and peri-lymphatic damage—an underlying feature of filarial disease—might also contribute to the presence of microbial translocation products in the bloodstream. Indeed, we have shown that increased circulating levels of LPS (which serves as a marker for microbial translocation) and decreased levels of LPS-binding protein (LBP) are characteristic features of filarial lymphatic pathology43 that in turn appear to cause immune activation. Since filarial lymphedema is known to be associated with increased bacterial and fungal loads in the lymphatics, our studies reveal that these damaged lymphatics may serve as a potential nidus for bacterial translocation through leaky lymphatic endothelium.
Apart from systemic immune activation, progressive fibrosis and extracellular matrix remodeling is another salient feature of filarial pathology. Recent data suggest that an increase in circulating levels of MMPs and TIMPs is characteristic of the filarial disease process and that that altered ratios of MMP/TIMP are an important underlying factor in the pathogenesis of tissue fibrosis in filarial lymphatic disease.44 Thus, filarial pathology arises out of a complex early interplay between the parasite and the host's innate responses and its tissue homeostasis.
There have been a large number of studies that have implicated a role for the adaptive immune systems in mediating pathology in LF.45–52 Although beyond the scope of this review the current hypothesis related to patholgenesis of disease in LF focuses on the failure to regulate (through IL-10, regulatory T cells) the antigen specific pro-inflammatory responses that may mediate pathology in LF.53
Host genetics are known to play an important role in susceptibility to infection and disease in a variety of infectious diseases. Similarly, in lymphatic filariasis, the pathogenesis of lymphedema and hydrocele might be influenced by host genetic factors. Although the cause of differential susceptibility to clinical expression of filarial infection has been only addressed in a few studies, early studies implicated the major histocompatibility complex (MHC).54,55 However, analysis of class II HLA loci, namely, DQA, DQB and DRB failed to identify an association with filarial infection nor outcomes within the infected group.56 Two studies in Haiti examining genetic associations within families have suggested a genetic basis for developing pathology in LF.57,58 A case-control study examining the role of VEGF-A SNPs in hydrocele revealed that a VEGF-A gene polymorphism in −460C/T was significantly associated with higher levels of plasma VEGF-A as well as the development of hydrocele.59 A recent study has implicated polymorphisms of endothelin-1 and TNFR II with the development of chronic disease.60 Future studies utilizing genome-wide scans as well as candidate gene approaches to identify loci and genes associated with pathogenesis should shed more light on the role of genetic factors in development of disease.
Conclusions
A characteristic feature of all parasite infections is that complete elimination of all parasites is rarely achieved, presumably since sterilizing immunity might necessitate host deleterious immune responses. Therefore, immune-mediated pathology is often associated with disease manifestation in many parasitic infections. The optimal host response is one that balances parasite control at levels at which the parasite load can be tolerated and leads to maintenance of immune homeostasis without irreparable tissue damage. Filarial infections are a classical example of host-parasite interactions resulting in an immune system-parasite homeostatic balance, which can fail (albeit rarely). However, in the rare instances of failure, the effects are of a debilitating and devastating nature, in large part due to exuberant host immune responses.
Author Disclosure Statement
The author has no conflicts of interest to declare.
This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
References
- 1.Fenwick A. The global burden of neglected tropical diseases. Public Health. 2012 Mar;126:233–236. doi: 10.1016/j.puhe.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Global programme to eliminate lymphatic filariasis. Releve epidemiologique hebdomadaire/Section d'hygiene du Secretariat de la Societe des Nations=Weekly epidemiological record/Health Section of the Secretariat of the League of Nations. 2007 Oct 19;82:361–380. [PubMed] [Google Scholar]
- 3.Lymphatic filariasis: the disease and its control. Fifth report of the WHO Expert Committee on Filariasis. World Health Organ Tech Rep Ser. 1992;821:1–71. [PubMed] [Google Scholar]
- 4.Freedman DO. de Almeida Filho PJ. Besh S. Maia e Silva MC. Braga C. Maciel A. Lymphoscintigraphic analysis of lymphatic abnormalities in symptomatic and asymptomatic human filariasis. J Infect Dis. 1994 Oct;170:927–933. doi: 10.1093/infdis/170.4.927. [DOI] [PubMed] [Google Scholar]
- 5.Freedman DO. de Almeido Filho PJ. Besh S, et al. Abnormal lymphatic function in presymptomatic bancroftian filariasis. J Infect Dis. 1995 Apr;171(4):997–1001. doi: 10.1093/infdis/171.4.997. [DOI] [PubMed] [Google Scholar]
- 6.Noroes J. Addiss D. Amaral F. Coutinho A. Medeiros Z. Dreyer G. Occurrence of living adult Wuchereria bancrofti in the scrotal area of men with microfilaraemia. Trans R Soc Trop Med Hyg. 1996 Jan-Feb;90:55–56. doi: 10.1016/s0035-9203(96)90478-2. [DOI] [PubMed] [Google Scholar]
- 7.Noroes J. Addiss D. Santos A. Medeiros Z. Coutinho A. Dreyer G. Ultrasonographic evidence of abnormal lymphatic vessels in young men with adult Wuchereria bancrofti infection in the scrotal area. J Urol. 1996 Aug;156:409–412. doi: 10.1097/00005392-199608000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Partono F. The spectrum of disease in lymphatic filariasis. Ciba Found Symp. 1987;127:15–31. doi: 10.1002/9780470513446.ch3. [DOI] [PubMed] [Google Scholar]
- 9.Pani SP. Srividya A. Clinical manifestations of bancroftian filariasis with special reference to lymphoedema grading. Indian J Med Res. 1995 Sep;102:114–118. [PubMed] [Google Scholar]
- 10.Dreyer G. Medeiros Z. Netto MJ. Leal NC. de Castro LG. Piessens WF. Acute attacks in the extremities of persons living in an area endemic for bancroftian filariasis: differentiation of two syndromes. Trans R Soc Trop Med Hyg. 1999 Jul-Aug;93:413–417. doi: 10.1016/s0035-9203(99)90140-2. [DOI] [PubMed] [Google Scholar]
- 11.Dreyer G. Noroes J. Figueredo-Silva J. Piessens WF. Pathogenesis of lymphatic disease in bancroftian filariasis: a clinical perspective. Parasitol Today. 2000 Dec;16:544–548. doi: 10.1016/s0169-4758(00)01778-6. [DOI] [PubMed] [Google Scholar]
- 12.Figueredo-Silva J. Noroes J. Cedenho A. Dreyer G. The histopathology of bancroftian filariasis revisited: the role of the adult worm in the lymphatic-vessel disease. Ann Trop Med Parasitol. 2002 Sep;96:531–541. doi: 10.1179/000349802125001348. [DOI] [PubMed] [Google Scholar]
- 13.Olszewski WL. Jamal S. Manokaran G. Lukomska B. Kubicka U. Skin changes in filarial and non-filarial lymphoedema of the lower extremities. Trop Med Parasitol. 1993 Mar;44:40–44. [PubMed] [Google Scholar]
- 14.Dreyer G. Noroes J. Addiss D. Santos A. Medeiros Z. Figueredo-Silva J. Bancroftian filariasis in a paediatric population: an ultrasonographic study. Trans R Soc Trop Med Hyg. 1999 Nov-Dec;93:633–636. doi: 10.1016/s0035-9203(99)90078-0. [DOI] [PubMed] [Google Scholar]
- 15.Amaral F. Dreyer G. Figueredo-Silva J, et al. Live adult worms detected by ultrasonography in human Bancroftian filariasis. Am J Trop Med Hyg. 1994 Jun;50:753–757. doi: 10.4269/ajtmh.1994.50.753. [DOI] [PubMed] [Google Scholar]
- 16.Dreyer G. Amaral F. Noroes J. Medeiros Z. Ultrasonographic evidence for stability of adult worm location in bancroftian filariasis. Trans R Soc Trop Med Hyg. 1994 Sep-Oct;88:558. doi: 10.1016/0035-9203(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 17.Connor DH. Palmieri JR. Gibson DW. Pathogenesis of lymphatic filariasis in man. Z Parasitenkd. 1986;72:13–28. doi: 10.1007/BF00927731. [DOI] [PubMed] [Google Scholar]
- 18.von Lichtenberg F. The Wellcome Trust lecture. Inflammatory responses to filarial connective tissue parasites. Parasitology. 1987;94(Suppl):S101–122. doi: 10.1017/s003118200008584x. [DOI] [PubMed] [Google Scholar]
- 19.Olszewski WL. Jamal S. Manokaran G, et al. Bacteriologic studies of skin, tissue fluid, lymph, and lymph nodes in patients with filarial lymphedema. Am J Trop Med Hyg. 1997 Jul;57:7–15. doi: 10.4269/ajtmh.1997.57.7. [DOI] [PubMed] [Google Scholar]
- 20.Shenoy RK. Kumaraswami V. Suma TK. Rajan K. Radhakuttyamma G. A double-blind, placebo-controlled study of the efficacy of oral penicillin, diethylcarbamazine or local treatment of the affected limb in preventing acute adenolymphangitis in lymphoedema caused by brugian filariasis. Ann Trop Med Parasitol. 1999 Jun;93:367–377. doi: 10.1080/00034989958366. [DOI] [PubMed] [Google Scholar]
- 21.Vickery AC. Albertine KH. Nayar JK. Kwa BH. Histopathology of Brugia malayi-infected nude mice after immune-reconstitution. Acta Trop. 1991 Apr;49:45–55. doi: 10.1016/0001-706x(91)90029-j. [DOI] [PubMed] [Google Scholar]
- 22.Vincent AL. Vickery AC. Lotz MJ. Desai U. The lymphatic pathology of Brugia pahangi in nude (athymic) and thymic mice C3H/HeN. J Parasitol. 1984 Feb;70:48–56. [PubMed] [Google Scholar]
- 23.Nelson FK. Greiner DL. Shultz LD. Rajan TV. The immunodeficient scid mouse as a model for human lymphatic filariasis. J Exp Med. 1991 Mar 1;173:659–663. doi: 10.1084/jem.173.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lal RB. Dhawan RR. Ramzy RM. Farris RM. Gad AA. C-reactive protein in patients with lymphatic filariasis: increased expression on lymphocytes in chronic lymphatic obstruction. J Clin Immunol. 1991 Jan;11:46–53. doi: 10.1007/BF00918794. [DOI] [PubMed] [Google Scholar]
- 25.Das BK. Sahoo PK. Ravindran B. A role for tumour necrosis factor-alpha in acute lymphatic filariasis. Parasite Immunol. 1996 Aug;18:421–424. doi: 10.1046/j.1365-3024.1996.d01-126.x. [DOI] [PubMed] [Google Scholar]
- 26.Satapathy AK. Sartono E. Sahoo PK, et al. Human bancroftian filariasis: immunological markers of morbidity and infection. Microbes Infect. 2006 Aug;8:2414–2423. doi: 10.1016/j.micinf.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 27.el-Sharkawy IM. Haseeb AN. Saleh WA. Serum levels of endothelin-1 (ET-1), interleukin-2 (IL-2) and amino-terminal propeptide type III procollagen (PIII NP) in patients with acute and chronic filariasis. J Egypt Soc Parasitol. 2001 Apr;31:169–176. [PubMed] [Google Scholar]
- 28.Babu S. Blauvelt CP. Kumaraswami V. Nutman TB. Chemokine receptors of T cells and of B cells in lymphatic filarial infection: a role for CCR9 in pathogenesis. J Infect Dis. 2005 Mar 15;191:1018-1–026. doi: 10.1086/427658. [DOI] [PubMed] [Google Scholar]
- 29.Olszewski WL. Jamal S. Lukomska B. Manokaran G. Grzelak I. Immune proteins in peripheral tissue fluid-lymph in patients with filarial lymphedema of the lower limbs. Lymphology. 1992 Dec;25:166–171. [PubMed] [Google Scholar]
- 30.Witte MH. Way DL. Witte CL. Bernas M. Lymphangiogenesis: mechanisms, significance and clinical implications. EXS. 1997;79:65–112. doi: 10.1007/978-3-0348-9006-9_5. [DOI] [PubMed] [Google Scholar]
- 31.Bennuru S. Nutman TB. Lymphatics in human lymphatic filariasis: in vitro models of parasite-induced lymphatic remodeling. Lymphat Res Biol. 2009 Dec;7:215–219. doi: 10.1089/lrb.2009.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao UR. Zometa CS. Vickery AC. Kwa BH. Nayar JK. Sutton ET. Effect of Brugia malayi on the growth and proliferation of endothelial cells in vitro. J Parasitol. 1996 Aug;82:550–556. [PubMed] [Google Scholar]
- 33.Bennuru S. Nutman TB. Lymphangiogenesis and lymphatic remodeling induced by filarial parasites: implications for pathogenesis. PLoS Pathog. 2009 Dec;5:e1000688. doi: 10.1371/journal.ppat.1000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfarr KM. Debrah AY. Specht S. Hoerauf A. Filariasis and lymphoedema. Parasite Immunol. 2009 Nov;31:664–672. doi: 10.1111/j.1365-3024.2009.01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Debrah AY. Mand S. Specht S, et al. Doxycycline reduces plasma VEGF-C/sVEGFR-3 and improves pathology in lymphatic filariasis. PLoS Pathog. 2006 Sep;2:e92. doi: 10.1371/journal.ppat.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennuru S. Maldarelli G. Kumaraswami V. Klion AD. Nutman TB. Elevated levels of plasma angiogenic factors are associated with human lymphatic filarial infections. Am J Trop Med Hyg. 2010 Oct;83:884–890. doi: 10.4269/ajtmh.2010.10-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLaren DJ. Worms MJ. Laurence BR. Simpson MG. Micro-organisms in filarial larvae (Nematoda) Trans R Soc Trop Med Hyg. 1975;69:509–514. doi: 10.1016/0035-9203(75)90110-8. [DOI] [PubMed] [Google Scholar]
- 38.Taylor MJ. Cross HF. Bilo K. Inflammatory responses induced by the filarial nematode Brugia malayi are mediated by lipopolysaccharide-like activity from endosymbiotic Wolbachia bacteria. J Exp Med. 2000 Apr 17;191:1429–1436. doi: 10.1084/jem.191.8.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esterre P. Plichart C. Huin-Blondey MO. Nguyen LN. Soluble cellular adhesion molecules, selectins, VEGF and endothelin-1 in patients with Wuchereria bancrofti infection and association with clinical status. Parasite Immunol. 2005 Jan-Feb;27:9–16. doi: 10.1111/j.1365-3024.2005.00732.x. [DOI] [PubMed] [Google Scholar]
- 40.Venugopal PG. Nutman TB. Semnani RT. Activation and regulation of Toll-Like Receptors (TLRs) by helminth parasites. Immunol Res. 2009;43:252–263. doi: 10.1007/s12026-008-8079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Babu S. Bhat SQ. Pavan Kumar N, et al. Filarial lymphedema is characterized by antigen-specific Th1 and th17 proinflammatory responses and a lack of regulatory T cells. PLoS Negl Trop Dis. 2009;3:e420. doi: 10.1371/journal.pntd.0000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brenchley JM. Douek DC. Microbial translocation across the GI tract. Annu Rev Immunol. 2012;30:149–173. doi: 10.1146/annurev-immunol-020711-075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anuradha R. Circulating microbial products, acute phase proteins as markers of pathogenesis in lymphatic filarial disease. PLoS Pathog. 2012 doi: 10.1371/journal.ppat.1002749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anuradha R. Altered circulating levels of matrix metallproteinases, inhibitors associated with elevated Type 2 cytokines in lymphatic filarial disease. PLoS Negl Trop Dis. 2012 doi: 10.1371/journal.pntd.0001681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freedman DO. Horn TD. Maia e Silva CM. Braga C. Maciel A. Predominant CD8+ infiltrate in limb biopsies of individuals with filarial lymphedema and elephantiasis. Am J Trop Med Hyg. 1995 Dec;53:633–638. [PubMed] [Google Scholar]
- 46.Freedman DO. Nutman TB. Ottesen EA. Protective immunity in bancroftian filariasis. Selective recognition of a 43-kD larval stage antigen by infection-free individuals in an endemic area. J Clin Invest. 1989 Jan;83:14–22. doi: 10.1172/JCI113850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freedman DO. Parker-Cook S. Maia e Silva MC. Braga C. Maciel A. Very late antigen-4/vascular cell adhesion molecule-1 (VLA-4/VCAM-1) pathway is involved in the transendothelial migration of lymphocytes in bancroftian filariasis. J Immunol. 1996 Apr 15;156:2901–2908. [PubMed] [Google Scholar]
- 48.Freedman DO. Plier DA. de Almeida A, et al. Biased TCR repertoire in infiltrating lesional T cells in human Bancroftian filariasis. J Immunol. 1999 Feb 1;162:1756–1764. [PubMed] [Google Scholar]
- 49.Nutman TB. Kumaraswami V. Ottesen EA. Parasite-specific anergy in human filariasis. Insights after analysis of parasite antigen-driven lymphokine production. J Clin Invest. 1987 May;79:1516–1523. doi: 10.1172/JCI112982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piessens WF. Partono F. Hoffman SL, et al. Antigen-specific suppressor T lymphocytes in human lymphatic filariasis. N Engl J Med. 1982 Jul 15;307:144–148. doi: 10.1056/NEJM198207153070302. [DOI] [PubMed] [Google Scholar]
- 51.King CL. Mahanty S. Kumaraswami V, et al. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J Clin Invest. 1993 Oct;92:1667–1673. doi: 10.1172/JCI116752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Almeida AB. Silva MC. Braga C. Freedman DO. Differences in the frequency of cytokine-producing cells in antigenemic and nonantigenemic individuals with bancroftian filariasis. Infect Immun. 1998 Apr;66:1377–1383. doi: 10.1128/iai.66.4.1377-1383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Babu S. Nutman TB. Immunopathogenesis of lymphatic filarial disease. Seminars in immunopathology. 2012 Nov;34:847–861. doi: 10.1007/s00281-012-0346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan SH. Dissanayake S. Mak JW, et al. HLA and filariasis in Sri Lankans and Indians. Southeast Asian J Trop Med Public Health. 1984 Sep;15:281–286. [PubMed] [Google Scholar]
- 55.Yazdanbakhsh M. Sartono E. Kruize YC, et al. HLA and elephantiasis in lymphatic filariasis. Hum Immunol. 1995 Sep;44:58–61. doi: 10.1016/0198-8859(95)00059-d. [DOI] [PubMed] [Google Scholar]
- 56.Choi EH. Zimmerman PA. Foster CB, et al. Genetic polymorphisms in molecules of innate immunity and susceptibility to infection with Wuchereria bancrofti in South India. Genes Immun. 2001 Aug;2:248–253. doi: 10.1038/sj.gene.6363767. [DOI] [PubMed] [Google Scholar]
- 57.Cuenco KT. Halloran ME. Lammie PJ. Assessment of families for excess risk of lymphedema of the leg in a lymphatic filariasis-endemic area. Am J Trop Med Hyg. 2004 Feb;70:185–190. [PubMed] [Google Scholar]
- 58.Cuenco KT. Halloran ME. Louis-Charles J. Lammie PJ. A family study of lymphedema of the leg in a lymphatic filariasis-endemic area. Am J Trop Med Hyg. 2004 Feb;70:180–184. [PubMed] [Google Scholar]
- 59.Debrah AY. Mand S. Toliat MR, et al. Plasma vascular endothelial growth Factor-A (VEGF-A) and VEGF-A gene polymorphism are associated with hydrocele development in lymphatic filariasis. Am J Trop Med Hyg. 2007 Oct;77:601–608. [PubMed] [Google Scholar]
- 60.Panda AK. Sahoo PK. Kerketta AS. Kar SK. Ravindran B. Satapathy AK. Human lymphatic filariasis: genetic polymorphism of endothelin-1 and tumor necrosis factor receptor II correlates with development of chronic disease. J Infect Dis. 2011 Jul 15;204:315–322. doi: 10.1093/infdis/jir258. [DOI] [PubMed] [Google Scholar]