Abstract
Objectives
To estimate the cost-effectiveness of remote monitoring strategies versus usual care for adults recently discharged after a heart failure (HF) exacerbation.
Design
Decision analysis modelling of cost-effectiveness using secondary data sources.
Setting
Acute hospitals in the UK.
Patients
Patients recently discharged (within 28 days) after a HF exacerbation.
Interventions
Structured telephone support (STS) via human to machine (STS HM) interface, (2) STS via human to human (STS HH) contact and (3) home telemonitoring (TM), compared with (4) usual care.
Main outcome measures
The incremental cost per quality-adjusted life year (QALY) gained by each strategy compared to the next most effective alternative and the probability of each strategy being cost-effective at varying willingness to pay per QALY gained.
Results
TM was the most cost-effective strategy in the scenario using these base case costs. Compared with usual care, TM had an estimated incremental cost effectiveness ratio (ICER) of £11 873/QALY, whereas STS HH had an ICER of £228 035/QALY against TM. STS HM was dominated by usual care. Threshold analysis suggested that the monthly cost of TM has to be higher than £390 to have an ICER greater than £20 000/QALY against STS HH. Scenario analyses performed using higher costs of usual care, higher costs of STS HH and lower costs of TM do not substantially change the conclusions.
Conclusions
Cost-effectiveness analyses suggest that TM was an optimal strategy in most scenarios, but there is considerable uncertainty in relation to clear descriptions of the interventions and robust estimation of costs.
Keywords: HEALTH ECONOMICS, STATISTICS & RESEARCH METHODS
Article summary.
Article focus
Decision analysis modelling using secondary data sources to estimate the cost-effectiveness of remote monitoring strategies versus usual care for adults recently discharged after a heart failure exacerbation.
Clinical effectiveness parameters were estimated from a network meta-analysis of the available evidence and costs were estimated using ‘bottom up’ costing methods.
Key messages
Base case cost-effectiveness analyses suggest that telemonitoring during office hours (TM) is the most cost-effective strategy at a threshold of £20 000/QALY, albeit with uncertainty.
Scenario analyses performed (using higher usual care costs, lower TM costs and higher TM costs) do not substantially change the conclusions.
Strengths and limitations of this study
The model uses robust evidence from a recent network-meta-analysis and appropriate sensitivity analyses are presented around other key parameters.
The effectiveness parameters used are a synthesis of heterogeneous estimates and uncertainties still remain about the assumptions made in the bottom-up costing of different scenarios.
Background
Heart failure (HF) is associated with high levels of morbidity and mortality, with the highest risk being immediately after discharge from hospital.1 A total of 20–30% of patients are readmitted within 30 days, rising to 50% at 6 months.2 Patients who are discharged have around 28% risk of mortality within the first year after HF discharge.3 Strategies to slow disease progression are needed for at-risk patients, to improve the prognosis, even among those receiving optimal pharmaceutical therapy.4
Remote monitoring (RM) of indicators of deterioration (eg, weight, arrhythmia, blood pressure, intrathoracic impedance, heart rates during rest and exertion and symptom control) can facilitate early detection of clinically significant changes as well as earlier intervention to restabilise the syndrome, prevent emergency admissions and avoid complications.5 RM can be broadly classified into telemonitoring (TM), in which physiological data are electronically transmitted to a healthcare team, and structured telephone support (STS), that is, the use of phone calls, usually by specialist nurses, to deliver self-care support and/or management.6 For STS, support can be provided by human-to-human contact (HH), or via a human-to-machine interface (HM); that is, STS with an interactive response system (eg, a voice-interactive system). For TM, support can be provided during office hours only or 24 h/ day, 7 days/week (24/7), though few studies have used the latter approach.
The cost-effectiveness of an RM strategy can be estimated by comparing the outcomes and costs associated with the strategy to the next most effective alternative. If these outcomes are estimated as quality-adjusted life years (QALYs), then the incremental cost-effectiveness ratio (ICER), or cost per QALY gained, can be calculated and compared to alternative uses of healthcare funding. In the UK (UK), the National Institute for Health and Clinical Excellence (NICE) typically recommends in favour of funding interventions with an ICER below thresholds of £20 000/QALY, requires considerable clinical benefit to recommend funding interventions between £20 000 and £30 000/QALY, and recommends against funding interventions with an ICER above these thresholds. The aim of this paper is to estimate the incremental cost per QALY of RM strategies compared with usual care, so as to determine which RM strategy should be recommended according to typical NICE thresholds for cost-effectiveness.
Methods
Model scope
A Markov model was developed using MS Excel software (Microsoft Corporation) to estimate the cost-effectiveness of RM interventions with usual care for patients discharged in the past 28 days with a HF-related hospitalisation,7 measured as the incremental cost per QALY gained by each strategy compared with the next most effective alternative on the cost-effectiveness frontier. Cost-effectiveness results are estimated as mean values of 10 000 probabilistic sensitivity analysis (PSA) runs, with each PSA run using different estimates for the risks, HRs, costs and utilities sampled from probability distributions representing uncertainty in the parameter estimates. Additionally, the probability that each strategy would be the most cost-effective was calculated at different thresholds for willingness to pay (WTP) for health gain. Cost-effectiveness acceptability curves were constructed by plotting the probability of each strategy being cost-effective against the WTP threshold.
The following strategies postdischarge interventions were tested in the model:
Usual care
STS HH (structured telephone support with human-to-human contact)
STS HM (structured telephone support with human-to-machine interface) and
TM (ie, telemonitoring with transmitted data reviewed by medical staff or medical support provided during office hours)
The clinical effectiveness parameters of these three RM strategies were estimated from a network meta-analysis of the available evidence,8 and costs were estimated using ‘bottom up’ costing methods. It was assumed that the interventions were provided for the 6 months following discharge from the hospital, as the majority of the RM trials included in the network meta-analysis used a 6-month follow-up duration.8 At the end of 6 months, all patients were assumed to receive usual care as per the NICE Clinical Guidelines for the Management of Adults with Chronic Heart Failure,9 irrespective of whether they received intervention or postdischarge usual care during the treatment period.
Model structure
In the Markov model, two different states were considered:
Alive at home
Dead
The Markov model used a monthly cycle length with half-cycle correction and assigned each patient with a monthly probability of death based on the time since discharge and the type of treatment. In each period, the patients who were alive were under the risk of an average number of monthly rehospitalisations, that is, readmissions to a hospital for HF or other causes. Each patient then accrued lifetime QALYs and healthcare costs according to their hospitalisation and treatment status. The model used a 30 year (patient lifetime) horizon, although the impact of each intervention was for the first 6 months after an initial discharge. The costs and QALYs were discounted at an annual discount rate of 3.5% and the economic perspective of the model was that of the NHS in England and Wales. Repeat interventions after repeat hospitalisations were not considered in this model.
Baseline mortality and hospitalisation
The baseline monthly probabilities of death were estimated from Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) study,10 which included 7572 patients with a mean age of 65.5 years followed up for 38 months, as it assessed the influence of non-fatal hospitalisation for heart failure on subsequent mortality. The data from the CHARM study10 showed that the mortality risk was highest immediately after hospital discharge and then decreased over time, as shown in table 1.
Table 1.
Monthly mortality probability vs time since discharge for patients with heart failure (HF) in usual care
| Time since discharge (in months) | Mortality probability per month | Lower 95% CI | Upper 95% CI |
|---|---|---|---|
| 0–1 | 0.04622 | 0.03616 | 0.05891 |
| 1–3 | 0.03306 | 0.02644 | 0.04124 |
| 3–6 | 0.02674 | 0.02166 | 0.03306 |
| 6–12 | 0.02353 | 0.01964 | 0.02831 |
| 12–24 | 0.01866 | 0.01565 | 0.02226 |
| >24 | 0.01467 | 0.01127 | 0.01911 |
The mean numbers of HF-related and other-cause hospitalisations (estimated as all-cause hospitalisations minus the HF-related hospitalisations) were estimated from a meta-analysis of 21 studies (5715 patients, median age 70.7 ranging from 45 to 78 years) reported by Klersy et al.11 The average number of monthly HF-related and all cause rehospitalisations for patients in usual care are as shown in table 2.
Table 2.
Monthly risk of hospitalisations per patient in usual care
Effectiveness of interventions
The effectiveness parameters in the economic model were the HRs for all-cause mortality, all-cause hospitalisations and HF-related hospitalisations for the different interventions (ie, STS HM, STS HM and TM) against standard care. These effectiveness parameters were estimated from a network meta-analysis (NMA) of 21 RM studies8 (total 6317 patients, with the mean age across studies ranging from 57 to 78 years) and applied to the baseline parameters to estimate the hospitalisation and mortality risk parameters for the different interventions. It was assumed that the treatment effectiveness (and costs) lasts only for the treatment duration of 6 months, after which the baseline risks of hospitalisation and mortality are applied.
It should be noted that considerable heterogeneity was identified in the manner in which RM and usual care were performed among the studies included in NMA,8 which resulted in heterogeneity between studies in the estimate of HRs. For example, in RM, there was variation between studies in the type of devices used, parameters monitored and the protocols for triage and follow-up. In particular, the data from Dar et al12 (Home-HF trial conducted in three district general hospitals in West London) appeared to be inconsistent with the data from the remaining studies because it showed a higher incidence of mortality among the TM group than the usual care group. However, the 6-month mortality rate in the usual care group (5.5%) was substantially lower than would be expected in a HF cohort receiving care outside the context of a clinical trial (ie, between 13% and 21%),3 which the authors attributed to the high-quality usual care (provided by a HF nurse specialist and a consultant with an interest in HF). The impact of this study was assessed in sensitivity analyses, and HRs from both the NMAs are presented in table 3.
Table 3.
HRs for interventions versus usual care for all-cause mortality and hospitalisations
| HRs for interventions versus usual care for mortality (all-cause) and hospitalisation (all-cause and HF) from NMA including Home-HF12 study | ||||||
|---|---|---|---|---|---|---|
| Type | All-cause mortality |
HF-hospitalisation |
All-cause hospitalisation |
|||
| R | 95% PrI | HR | 95% PrI | HR | 95% PrI | |
| STS HH | 0.77 | (0.31, 1.86) | 0.77 | (0.50, 1.19) | 0.97 | (0.38, 2.43) |
| STS HM | 0.98 | (0.30, 3.23) | 1.03 | (0.58, 1.77) | 1.06 | (0.31, 3.61) |
| TM | 0.76 | (0.30, 1.91) | 0.95 | (0.59, 1.62) | 0.75 | (0.28, 1.91) |
| HRs for interventions versus usual care for mortality (all-cause) and hospitalisation (all-cause and HF) from NMA excluding Home-HF12 study | ||||||
| STS HH | 0.75 | (0.45, 1.27) | 0.76 | (0.51, 1.13) | 0.96 | (0.42, 2.18) |
| STS HM | 0.98 | (0.49, 1.95) | 1.02 | (0.61, 1.69) | 1.06 | (0.35, 3.22) |
| TM | 0.62 | (0.35, 1.09) | 0.86 | (0.54, 1.38) | 0.67 | (0.26, 1.53) |
HR, hazard ratio; PrI, predictive interval; STS HM, structured telephone support via human to machine interface; STS HH, structured telephone support via human to human contact; TM, telemonitoring
On deciding which of these results are most representative of their setting, the key question for decision-makers relate to the inclusion of the Home-HF study12 in the effectiveness meta-analyses. If one accepts that usual care is best represented by the usual care arm in the Home-HF study,12 which is the only study showing a statistically significant difference in effectiveness of usual care over RM, then the results including Home-HF study12 might be considered more relevant than those without. If, on the other hand, one considers that the performance of usual care is better represented by the other studies and that usual care in Home-HF study12 is not representative of current usual care, then the results excluding Home-HF study12 might be more generalisable. This consideration predominantly affects the HRs around the telemonitoring intervention only and does not impact substantially on the structure telephone service interventions.
Health-related quality of life
A review was conducted to estimate the health-related quality of life (HRQoL) and four studies were found,13–16 all of which reported utilities for recently discharged HF patients under usual care around 0.57 to 0.6. There was no quantified evidence on the extent to which RM improves the HRQoL of the patients in the RM studies included in NMA, and thus the same utility values were used for HF patients in usual care as well as (each of the three) RM strategies in the economic model. The disutility caused by rehospitalisation for HF was estimated as 0.1 based on a study by Yao et al17 who estimated the disutility to be equivalent to the utility of one health state lower in terms of the New York Heart Association (NYHA) class, and this disutility was assumed to last for 1 year. In the absence of evidence regarding the disutility caused by rehospitalisation for other causes (not directly HF-related), it was assumed that there was no disutility caused by rehospitalisation for other causes.
A utility score of 0.58 was applied to the patients for each month in the first year after discharge and a utility score of 0.67 was used after the first year. Any HF-related hospitalisation was assumed to result in a disutility of 0.1 for a whole year, that is, the utility of the patient for that year was 0.67–0.1, that is, 0.57. Within the PSA, the uncertainty in the utility values was represented using a normal distribution using the deterministic values as the mean with an SD of 0.015 and estimated based on the difference between the utilities reported by Capomolla et al13 and Iqbal et al,15 while the disutility was represented using a triangular distribution with (−0.08, 0.11) as the range with −0.1 as the mode.
Costs
The costs used in the model are (1) costs of RM interventions after initial discharge only, (2) costs of usual care and (3) repeat hospitalisation costs. These costs are summarised in table 4 and are described in detail in this section.
Table 4.
Cost parameters used in the economic model
| Costs (in £) per 6 months | Base-case scenario | Low-cost scenario | High-cost scenario | Source |
|---|---|---|---|---|
| UC | £161 | – | £592 | TEN-HMS,18 Clinical opinion |
| STS HM | £715 | £623 | £794 | Clinical opinion |
| STS HH | £1075 | £1051 | £1152 | Clinical opinion |
| TM | £1051 | £801 | £1288 | Clinical opinion |
| Usual care costs (per month) after 6 month intervention duration | ||||
| UC after 6 months | £8.23 | – | – | NICE HF guidelines9 |
| Hospitalisation costs | Estimate | Lower 95% CI | Upper 95% CI | |
| HF-related hospitalisations* | £2514.49 | £1857 | £2809 | NHS Reference Costs for 201121 |
| Other-cause hospitalisations† | £1529.79 | £1129 | £1709 | NHS Reference Costs for 201121 |
The RM studies did not report clearly or in detail what was involved in the usual postdischarge care or RM, thus making it difficult to accurately determine the costs.8 Owing to the variation involved in the RM interventions and usual care, cost scenarios were developed for each RM classification (ie, STS HM, STS HH and TM) and usual care. These costs were estimated using bottom-up costing methods for a typical health organisation of 250 HF patients (estimated based on the median size of the NHS Foundation Trusts in the UK) for a period of 6 months. Furthermore, it was assumed that, after 6 months, all patients would receive usual care as recommended in NICE clinical guidelines for the management of adults with CHF,9 irrespective of whether they received the remote monitoring intervention or postdischarge usual care during the intervention period.
It was assumed that the usual postdischarge care was the same as that described in the TEN-HMS study18 and the usual care costs were estimated by applying the hourly NHS staff rates from PSSRU 201119 to the resource use data in the TEN-HMS study.18 A high cost usual postdischarge care scenario was also developed based on discussions with the clinical expert group (AB, AAM, JC and MRC).
The total costs of RM interventions were broken down into the costs of the device, monitoring costs and medical care costs.8 The costs of the RM devices were elicited from an expert advisory group. The monitoring costs were estimated using activity-based costing for the resources spent by the staff on triage and follow-up based on evidence from the literature.20 The costs of medical care for the usual care arm, STS and TM arms were estimated by applying the hourly NHS staff rates from PSSRU 201119 to their respective medical care resource use data reported in the TEN-HMS study.18
The mean inpatient admission cost for HF-related hospitalisations was calculated from the weighted average of the costs for the HRG ‘Heart Failure or Shock” (EB03H, EB03I) based on the data obtained from the NHS Reference Costs for 2011.21 For hospital admissions for any cause other than HF, it was assumed that these costs were the same as the mean cost of hospital admission for the general population. This was estimated as a weighted average of elective inpatient admissions and non-elective inpatient admissions (including short and long stay) based on the data from the NHS Reference Costs for 2011.21
Results
The results of the cost-effectiveness analysis using base case costs are presented in table 5 for both estimates of effectiveness, including and excluding data from the Home-HF study,12 to address the uncertainty in the effectiveness evidence. Results are also presented for five cost scenarios (higher usual care cost scenario, lower cost scenario of TM, higher cost scenario of TM, lower STS cost scenario and higher STS cost scenario) and the 12 month intervention duration scenario in online supplementary tables S1 and S2, respectively.
Table 5.
Summary of the economic analysis results using base case costs
| Usual care | STS HM | STS HH | TM | |
|---|---|---|---|---|
| Total costs | ||||
| Including Home-HF12 | £8478 | £8965 | £9574 | £9437 |
| Excluding Home-HF12 | £8478 | £9087 | £9658 | £9665 |
| Total QALYs | ||||
| Including Home-HF12 | 2.4137 | 2.3633 | 2.4950 | 2.4944 |
| Excluding Home-HF12 | 2.4137 | 2.4043 | 2.5230 | 2.5847 |
| ICERs | ||||
| Including Home-HF12 | Dominated | £228 035* | £11 873 | |
| Excluding Home-HF12 | Dominated | Extendedly dominated | £6942* | |
| Probability of cost-effectiveness (%) | ||||
| Including Home-HF12 | 6 | 19 | 35 | 40 |
| Excluding Home-HF12 | 1 | 7 | 19 | 73 |
*Last strategy in the cost-effectiveness frontier.
ICERs, incremental cost-effectiveness ratios; QALYs, quality adjusted life years; STS HH, structured telephone support via human to human contact; STS HM, structured telephone support via human to machine interface; TM, telemonitoring.
In the analysis using base case costs, TM is the most cost-effective strategy at a threshold of £20 000/QALY in both analyses, that is, including and excluding the Home-HF study.12 TM is also the most effective strategy (ie, highest QALYs gained) in the analyses that excluded the Home-HF study,12 but not in the analyses that included the Home-HF study,12 with STS HH providing the highest number of expected QALYs. However, the additional QALYs gained by STS HH are not worth the additional costs of the strategy as seen in the ICERs (against TM) greater than the threshold of £20 000/QALY.
In the analyses that included the Home-HF study,12 there is only a 40% chance of TM being cost-effective at the threshold of £20 000/QALY, as shown in figure 1. Excluding the Home-HF study,12 the probability that TM during office hours is cost-effective increases to 73% (figure 2).
Figure 1.
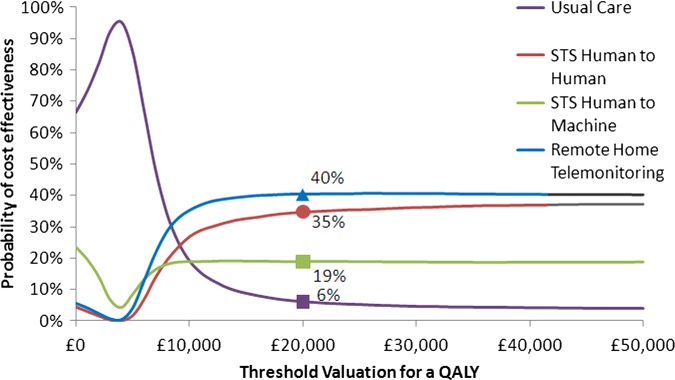
Cost effectiveness acceptability curve for base case economic analysis using effectiveness data including the Home-HF study.12
Figure 2.
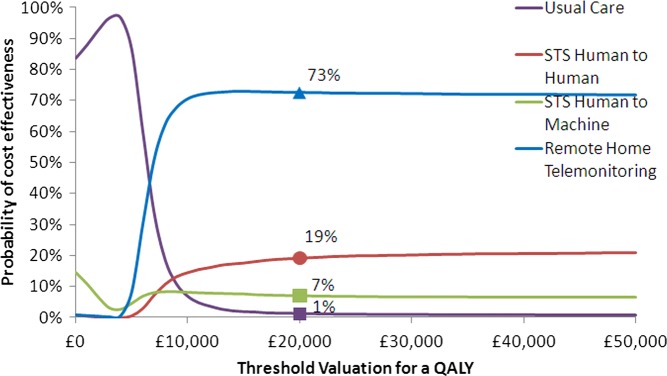
Cost effectiveness acceptability curve for economic analysis using effectiveness data excluding the Home-HF study.12
Scenario analyses performed using higher costs of usual care, higher costs of STS HH and lower costs of TM do not substantially change the conclusions. TM was estimated to be the most cost-effective strategy in all these scenarios. Scenario analysis performed using higher costs of TM (£215/month) suggested that TM is dominated by STS HH. This is because a small change in the difference between costs of TM and STS HH led to a marked change in ICERs, given the small difference (0.0021 QALYs) in expected QALYs between STS HH and TM in the analyses that included the Home-HF study.12 However, the same scenario analysis (ie, higher cost of TM of £215/month) that excluded the data from the Home-HF study12 suggested that TM is still the most cost-effective strategy with an ICER of £8223/QALY against usual care (STS HH is extendedly dominated by a combination of usual care and TM). This is due to the much higher difference in the expected QALYs between STS HH and TM (0.0602 QALYs), where a small change in the difference between costs of TM and STS HH cannot lead to a marked change in the ICER. The threshold analysis suggested that the monthly cost of TM has to be higher than £390/month to have an ICER greater than £20 000/QALY against STS HH. The ICER of TM against usual care, at this monthly cost of £390, is £13 357/QALY.
Scenario analyses performed using different estimates of the disutility for HF-related and other cause hospitalisations produced results similar to that in the base case analysis, that is, the results are robust to the variations in the disutility of hospitalisations.
Scenario analysis using a 12-month treatment duration produced similar results as in the 6-month treatment duration scenarios. TM for 12 months was also cost-effective when compared with TM for 6 months with an ICER of £14 066/QALY. However, treating 2×N patients using TM for 6 months was cost-effective with it being dominant against a combination of treating N patients using TM for 12 months with the rest of the N patients under usual care.
Discussion
The results of the base case cost-effectiveness analyses suggest that TM is expected to be the most cost-effective strategy at a threshold of £20 000/QALY. However, there is uncertainty involved in suggesting that TM is the most probable cost-effective strategy and. in particular, there is greater uncertainty when data from the Home-HF study12 is included than when it is excluded. For decision-makers, the key question is whether the usual care arm in their local setting is similar to the usual care arm in the Home-HF study.12 If so, then the results including the Home-HF study12 might be considered more relevant and if not, the results excluding the Home-HF study12 might be considered more relevant. The scenario analysis performed (using higher usual care costs, lower TM costs, higher TM costs and higher STS costs) did not substantially change the conclusions. TM was estimated to be the most cost-effective strategy in all these scenarios. Furthermore, TM for 12 months was also cost-effective when compared with TM for 6 months, which suggests that it is cost-effective to keep the patients on TM beyond 6 months. In situations with a limited number of TM devices, assuming a homogeneous patient group, it is cost-effective to treat all patients using TM for 6 months than using TM for 12 months on half the patients with the other half of the patients under usual care.
There have been two cost-effectiveness analyses studies of RM in HF, but neither considered the different RM approaches separately.11 22 The analysis reported by Miller et al22 was based on a single trial of STS,23 whereas Klersy et al11 included data from a meta-analysis of a wide range of RM studies. Miller et al22 estimated that STS compared with usual care had an ICER around $43 650/QALY; this study did not include a PSA, but univariate sensitivity analyses (ICER varying from $28 691 to $129 738 per QALY) was performed. Klersy et al11 focused mainly on the effectiveness rather than the costs and used a time horizon of 1 year. A budget impact analysis was presented and the different diagnosis-related group (DRG) reimbursement tariff groups, as a proxy for hospitalisation costs, were considered with cost savings per patient ranging between €306.8 and €992.94. However, other costs such as RM costs and outpatient visit costs were not considered. The authors performed scenario analyses using different DRG costs as part of the budget analysis to address the uncertainty in the hospitalisation costs, but neither deterministic sensitivity analysis nor PSA was performed.
The Whole systems demonstrator (WSD) programme,24 a randomised controlled trial of telehealth that included over 6000 patients, analysed costs and outcomes for 965 patients monitored for 12 months: 534 receiving telehealth and 431 receiving usual care. Cost-effectiveness analysis estimated the ICER at £92 000/QALY when telehealth was added to usual care for people with chronic conditions (diabetes, HF and chronic obstructive pulmonary disease (COPD)). However, this trial-based evaluation (with a time horizon of 1 year) potentially underestimates the health benefits as it does not include the long-term QALYs gained from the reduction in mortality. Furthermore, the WSD analysis included all patients with chronic conditions (diabetes, HF and COPD), whereas the population under consideration in our analysis included patients who had been recently discharged with HF. The cost-effectiveness results for HF patients are not yet publicly available in a peer-reviewed journal, and thus it is difficult to compare the results of the current analysis with the results of the WSD analysis.
The analysis reported in this paper also has some limitations that need to be taken into account. Any modelling process involves simplifications and assumptions that may not accurately reflect clinical practice. Owing to the lack of detail provided in research studies included in the NMA concerning the components of RM packages and usual care (eg, communication protocols, routine staff visits and resources used), scenarios for different RM classifications were developed and their costs were estimated using bottom-up costing methods. Although the users can decide which of these analyses is most representative of their setting, uncertainties still remain about the assumptions made in the estimation of these costs. However, it should be noted that the monthly costs estimated were similar to those reported in the WSD. Implementation costs (such as set-up costs, staff training costs, service reconfiguration costs, costs for dual running of usual care and RM services) were not included in the model but are often a consideration for the health organisations.
RM interventions included in the NMA were heterogeneous in terms of monitored parameters and selection criteria for HF; this was the case even within specific types of RM (STS HH, STS HM and TM) and is reflected in the uncertainty of the effectiveness parameters. A limitation of the analyses is that the effectiveness parameters remained the same for the different cost scenarios, whereas in reality there might be some correlation between the costs and effectiveness of different RM strategies. Also, it was assumed that the effectiveness and costs of the interventions are constant over time, irrespective of the duration of deployment. Furthermore, as the analysis is not severity specific, it assumes that the interventions are equally effective in different severity groups within the population under consideration (ie, patients who are recently discharged with HF). Repeat interventions after repeat hospital admissions for heart failure are not modelled and the mortality risk is not reset for patients who are readmitted as this would need detailed evidence on baseline risks and effectiveness in different patient groups. As this detailed evidence is not available, the cohort model uses evidence about the overall average of the patients to estimate the cost-effectiveness.
Some of the assumptions above may not hold true in reality, and therefore further research is needed to address these issues. Given the complex nature of RM interventions, new research should seek to examine the ‘active ingredients’ of RM and identify patient subgroups that can benefit most from the intervention, as well as patients in whom these interventions are unlikely to be effective. In addition, usual care ought to be more robustly determined, reflecting best practice as defined in the current guidelines, although this is not commonly the case. Furthermore, to aid robust cost-effectiveness estimations, the costs associated with usual care and RM interventions need to be reported in detail (including the costs of HF treatment pathways) and QoL needs to be reported with observations at specific time points in order to estimate the difference in the utility of the patients between the RM and usual care groups. Future studies should provide greater detail on reconfiguration and set-up costs and link more clearly with the financial impact (eg, cost variation with scale and over time) on provider organisations. Wider adaptation of RM in the NHS can be facilitated by providing financial impact data (eg, set-up costs, quarterly costs of service, costs of reconfiguration) along with the long-term cost-effectiveness information.
The results of the current analysis have important implications for the healthcare systems facing rising demand from emergency admissions. HF is a leading cause of hospitalisation in the UK, with 58 164 admissions recorded for HF (as first diagnoses) between April 2009 and March 2010 in England and Wales.25 The cost of inpatient bed days for HF alone has been estimated at £563 million,26 with around 90% of HF admissions to emergency departments,27 lasting a median of 9 days.25 The evidence shows that use of RM could substantially reduce HF admissions, with an associated reduction in pressure on acute beds, and consequent cost savings. Furthermore, the use of TM might allow the potential transfer from emergency admission to elective admission, that is, scheduling admissions of patients directly (not via A&E) to either a ward or to a day unit for offloading, leading to major resource savings, with less patient disutility.
Indeed, the Department of Health (DH) recognised this potential and launched an initiative, ‘3 Million Lives” (3ML), to help at least three million people with long-term conditions and/or social care to benefit from the use of telehealth and telecare services.28 A concordat has been entered into by the DH and the telehealth and telecare industry to work together to accelerate the use of TM.29 Thus far, seven pathfinder sites have agreed contracts with the industry to ensure that 100 000 people benefit from the technology in 2013.30 Implemented effectively as part of a whole system redesign of care, TM may alleviate the pressure on long-term NHS costs and improve people's quality of life through better self-care in the home setting.
Supplementary Material
Acknowledgments
The authors would like to thank Mr Tim Ellis, Research Fellow, University of Sheffield; Professor Mark Hawley, Professor of Health Services Research, University of Sheffield; Hazel Marsh, Research Nurse, Barnsley Hospital NHS Foundation Trust and Dr Rachel O'Hara, Lecturer in Public Health, University of Sheffield for providing clinical and TM expertise. They would also like to thank Dr Lizzie Coates for peer reviewing the draft paper.
Footnotes
Contributors: PT developed the decision analytical model, undertook the analysis and drafted the paper. HB assisted in parameter searches, model development and analysis. AB provided expert modelling advice. AP was responsible for conception and design; he undertook the systematic review along with TG and provided data for the meta-analysis. JWS and JW provided the meta-analysis results and statistical input. AB, AAM, JC and MRC provided expert clinical input. RW developed and undertook the literature searches. All authors commented on the draft/final paper.
Funding: The project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme (number 09/107/01) and sponsored by the University of Sheffield. The study funders had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The researchers were independent of the study funders.
Competing interests: MRC's salary is supported by the NIHR Biomedical Research Unit at the Royal Brompton Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Riley JP, Cowie MR. Telemonitoring in heart failure. Heart 2009;95:1964–8 [DOI] [PubMed] [Google Scholar]
- 2.Hasan A, Paul V. Telemonitoring in chronic heart failure. Eur Heart J 2011;32:1457–64 [DOI] [PubMed] [Google Scholar]
- 3.2012. National Heart Failure Audit April 2010 to March 2011. http://yhhiec org uk/wp-content/uploads/2011/10/11070604_Tele_Moni_Workbk pdf; http://www.ucl.ac.uk/nicor/audits/heartfailure/additionalfiles/pdfs/annualreports/annual11.pdf (accessed Mar 2012)
- 4.McKelvie RS. Heart failure. Clin Evid 2011;2011:0204. [PMC free article] [PubMed] [Google Scholar]
- 5.Anker SD, Koehler F, Abraham WT. Telemedicine and remote management of patients with heart failure. [Review]. Lancet 2011;378:731–9 [DOI] [PubMed] [Google Scholar]
- 6.Inglis SC, Clark RA, McAlister FA, et al. Structured telephone support or telemonitoring programmes for patients with chronic heart failure. Cochrane Database Syst Rev 2010;(8):CD007228. [DOI] [PubMed] [Google Scholar]
- 7.Pandor A, Thokala P, Gomersall T, et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess 2013;17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandor A, Pandor A, Gomersall T, et al. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta-analysis. Heart Published Online First: 16 May 2013 doi:10.1136/heartjnl-2013-303811 [DOI] [PubMed] [Google Scholar]
- 9.National Clinical Guideline Centre Chronic heart failure: the management of chronic heart failure in adults in primary and secondary care. London: National Clinical Guideline Centre, 2012 [Google Scholar]
- 10.Solomon SD, Dobson J, Pocock S, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation 2007;116:1482–7 [DOI] [PubMed] [Google Scholar]
- 11.Klersy C, De SA, Gabutti G, et al. Economic impact of remote patient monitoring: an integrated economic model derived from a meta-analysis of randomized controlled trials in heart failure. Eur J Heart Failure 2011;13:450–9 [DOI] [PubMed] [Google Scholar]
- 12.Dar O, Riley J, Chapman C, et al. A randomized trial of home telemonitoring in a typical elderly heart failure population in North West London: results of the Home-HF study. Eur J Heart Failure 2009;11:319–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capomolla S, Pinna GD, La Rovere MT, et al. Heart failure case disease management program: a pilot study of home telemonitoring versus usual care. Eur Heart J 2004;6(Suppl F):F91–8 [Google Scholar]
- 14.Calvert MJ, Shankar A, McManus RJ, et al. Evaluation of the management of heart failure in primary care. Fam Pract 2009;26:145–53 [DOI] [PubMed] [Google Scholar]
- 15.Iqbal J, Francis L, Reid J, et al. Quality of life in patients with chronic heart failure and their carers: a 3-year follow-up study assessing hospitalization and mortality. Eur J Heart Failure 2010;12:1002–8 [DOI] [PubMed] [Google Scholar]
- 16.Miller LC, Cox KR, Miller LC, et al. Case management for patients with heart failure: a quality improvement intervention. J Gerontol Nurs 2005;31:20–8 [DOI] [PubMed] [Google Scholar]
- 17.Yao G, Freemantle N, Flather M, et al. Long-term cost-effectiveness analysis of nebivolol compared with standard care in elderly patients with heart failure: an individual patient-based simulation model. Pharmacoeconomics 2008;26:879–89 [DOI] [PubMed] [Google Scholar]
- 18.Cleland JG, Louis AA, Rigby AS, et al. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: The Trans-European Network-Home-Care Managments System (TEN-HMS) study. J Am Coll Cardiol 2005;45:1654–64 [DOI] [PubMed] [Google Scholar]
- 19.PSSRU Unit costs of health and social care 2011. 2011
- 20.Boyne J, Vrijhoef HJ, Nieman FH, et al. Telemonitoring in patients with heart failure: results from a multicenter randomized controlled trial (the tehaf study). J Am Coll Cardiol 2011;57(14 Suppl 1):E389 [Google Scholar]
- 21.Department of Health NHS reference costs 2007–08. 2009. London, UK, 2009 [Google Scholar]
- 22.Miller G, Randolph S, Forkner E, et al. Long-term cost-effectiveness of disease management in systolic heart failure. Med Deci Making 2009;29:325–33 [DOI] [PubMed] [Google Scholar]
- 23.Galbreath AD, Krasuski RA, Smith B, et al. Long-term healthcare and cost outcome of disease management in a large, randomized, community-based population with heart failure. Circulation 2004;110:3518–26 [DOI] [PubMed] [Google Scholar]
- 24.Henderson C, Knapp M, Fernandez J-L, et al. Cost effectiveness of telehealth for patients with long term conditions (Whole Systems Demonstrator telehealth questionnaire study): nested economic evaluation in a pragmatic, cluster randomised controlled trial. BMJ 2013;346:f1035. [DOI] [PubMed] [Google Scholar]
- 25.NHS information centre National Heart Failure Audit. 2010. http://www npc nhs uk/rapidreview/?p=2479 2010, http://www.npc.nhs.uk/rapidreview/?p=2479
- 26.Cleland JG, McDonagh T, Rigby AS, et al. The national heart failure audit for England and Wales 2008–2009. Heart 2011;97:876–86 [DOI] [PubMed] [Google Scholar]
- 27.HES Online Hospital Episode Statistics 2008–2009. 2012. http://www.hesonline.nhs.uk
- 28.3millionlives: Improving your access to telehealth and telecare. 12 A.D. http://3millionlives.co.uk/ [Google Scholar]
- 29.A concordat between the Department of Health and the telehealth and telecare industry . 12 A.D. http://3millionlives.co.uk//wp-content/uploads/2012/03/Concordat-FINAL.pdf [Google Scholar]
- 30.100,000 people with long-term conditions to benefit from technology in 2013. 12 A.D. http://3millionlives.co.uk/wp-content/uploads/2012/11/14-November-2012-press-release.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


