Abstract
Background
Serious infections of the head and neck cause lymphedema that can lead to airway compromise and oropharyngeal obstruction. Lymphangiogenesis occurs in the head and neck during infection and after immunization. The goal of this project was to develop tools to image lymphatic vessels in living animals and to be able to isolate individual lymphatic endothelial cells in order to quantify changes in single cells caused by inflammation.
Methods
The ProxTom transgenic red-fluorescent reporter mouse was developed specifically for the purpose of imaging lymphatic vessels in vivo. Prox1 is a transcription factor that is necessary for lymphangiogenesis in development and for the maintenance of lymphatics in adulthood. Mice were immunized and their lymphatic vessels in lymph nodes were imaged in vivo. Individual lymphatic endothelial cells were isolated by means of their fluorescence.
Results
The ProxTom transgene has the red-fluorescent reporter td-Tomato under the control of Prox1 regulatory elements. tdTomato was faithfully expressed in lymphatic vessels coincident with endogenous Prox1 expression. We show lymphangiogenesis in vivo after immunization and demonstrate a method for the isolation of lymphatic endothelial cells by their tdTomato red-fluorescence.
Conclusions
The faithful expression of the red-fluorescent reporter in the lymphatic vessels of ProxTom means that these mice have proven utility for in vivo study of lymphatic vessels in the immune response. ProxTom has been made available for distribution from the Jackson Laboratory: http://jaxmice.jax.org/strain/018128.html.
Introduction
The National Institute of Allergy and Infectious Diseases (NIAID) workshop on November 7, 2012 was convened to bring together lymphatic researchers to discuss “Lymphatic Function and the Immune Response to Microbial or Viral Infection”. Five years earlier, in 2007, a similar group of lymphatic biologists met at the National Institutes of Health (NIH) to discuss how best lymphatic vessels could be measured.1
As immunologists, we want to understand how lymphatic vessels (LVs) might direct and influence the ongoing immune response to infection. We already knew that dramatic changes occurr in LVs in the lymph node after immunization, which could affect the subsequent priming to a second antigen.2 But back in 2007, we were frustrated by a lack of tools available for lymphatic research, and in particular, by an absence of tools for in vivo work. The phenotypic drift of primary lymphatic endothelial cells in culture, together with rapid advances in intra-vital microscopy led to the call for a lymphatic reporter mouse. We understood that: If you want to measure something—it helps if you can see it! So, with this in mind, we developed the ProxTom lymphatic- reporter mouse.3 ProxTom has brilliant, red- fluorescent lymphatic vessels and has proven successful for ‘live’ imaging of the head and neck (Fig. 1). (Supplementary videos are available in the online article at www.liebertpub.com/lrb.) We have made ProxTom mice available to the lymphatic research community. ProxTom are being distributed by the Jackson Laboratory, and information on how to obtain them can be found at the following website: http://jaxmice.jax.org/strain/018128.html.
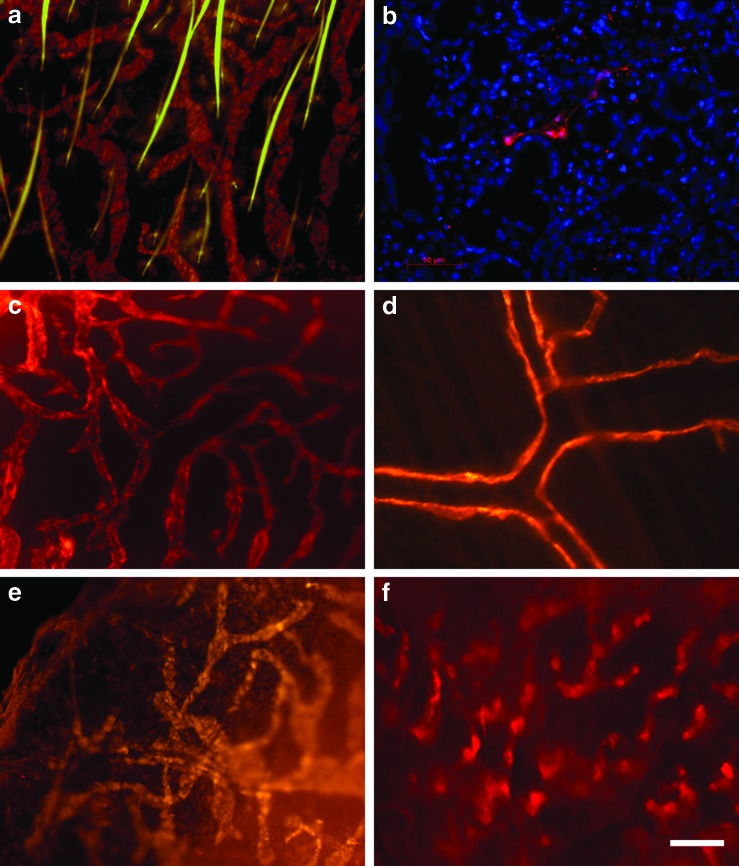
FIG. 1.
ProxTom lymphatic vessels. Lymphatic vessels (LVs) in the skin of the ear are red (tdTomato) and the hairs auto-fluoresce green (a) A LV is seen in between the acini in a section through a ProxTom submandibular salivary gland; nuclei are counterstained with DAPI (blue) (b), LVs in the tongue (c), and in the peritoneum (d). Changes in the appearance of LVs in the skin of the abdomen after skin-painting with oxazolone: untreated skin (e), oxazolone-treated (f ). The white scale bar represents 50 μm. A color version of this figure is available in the online article at www.liebertpub.com/lrb
Our goal is to use ProxTom mice in order to study immune cells interacting with LVs ‘live’ in an ongoing immune response inside lymph nodes and in tertiary lymphoid organs (TLOs).4 We have studied the specialized role of LVs in lymph nodes and TLOs after immunization,2 during infection,5 and in autoimmunity.6,7 TLOs are lymphoid tissue aggregations that occur in chronic inflammation and that share a similar tissue organization with lymph nodes. Like lymph nodes, TLOs have distinct B and T cell zones, high endothelial venules, germinal centers, and LVs.4 One important difference between lymph nodes and TLOs is that lymph nodes have a capsule and a subcapsular sinus, whereas TLOs do not. The lack of a subcapsular sinus in TLOs may affect how lymph, dendritic cells, and antigens move through the TLO. The anatomical difference between lymph nodes and TLOs may have important consequences for antigen priming. ProxTom mice will allow us to directly examine how lymph flows through a TLO and compare it to flow through a lymph node, for the first time in vivo.
More recently, we have become interested in LVs in the head and neck (Fig. 1). The head and neck region is important for lymphatic biologists, not only because the thoracic duct terminates in the neck, but also because 75% of all lymphatic vessel malformations occur here.8 Inflammation of the upper airway can have life-threatening consequences, especially in children. Children have smaller airways and only a slight amount of swelling can significantly obstruct the trachea.
Mycoplasma pulmonis infection of the trachea causes vascular remodeling of LVs, and this ‘inflammatory lymphangiogenesis' is regulated by TNFα9 and its related cytokine lymphotoxin (LTα).10 LTα is an important cytokine that has been studied by us because of its importance for the development of both lymph nodes and TLOs,11 but LTα also contributes to new LV formation. Lymphangiogenesis after Mycoplasma pulmonis infection was much less severe in LTα-deficient mice. Ectopic expression of transgenic LTα under the control of the rat insulin promoter causes TLO to form in the pancreas.6 Using a conditional system to switch on LTα, Mounzer et al.10 showed that the formation of new LVs was the very first step in the process of organizing lymphoid tissues into a TLO.
ProxTom mice will allow us to really begin to understand how LVs function inside lymph nodes and TLOs in inflammation. In the future, we want to understand how LVs initiate TLO development and LVs role in the maintenance of TLO in chronic inflammation.
At the same time as we developed ProxTom, some other transgenic reporter mice were also described: A green-fluorescent lymphatic reporter mouse also driven by the prox1 promoter is on a mixed background12 A third transgenic mouse again using Prox1 to drive a fluorescent mOrange2 reporter is also suitable for in vivo imaging.13 A fourth lymphatic reporter uses the lymphatic receptor VEGFR3-promoter to drive a yellow fluorescent protein.14
Here, we demonstrate the utility of ProxTom mice for ‘live’ lymphatic vessel imaging and for the isolation of pure lymphatic endothelial cells. Supplementary videos are available in the online article at www.liebertpub.com/lrb.
Materials and Methods
Mice
C57BL/6 mice were obtained from Jackson Laboratory. The Yale University Institutional Animal Care and Use Committee approved all animal use. The ProxTom transgene was constructed using the pClasper yeast-bacterial shuttle vector.15 The red-fluorescent reporter tdTomato was expressed under the control of the prox1 promoter in lymphatic vessels.3,16 The experiment details about the development of ProxTom transgenic mice have been published.3
Immunization
ProxTom mice were immunized by skin-painting with 50 μL 4% oxazolone. Four days after painting the hind leg, the popliteal lymph node was surgically exposed and examined in vivo using a two-photon laser scanning microscope.17 The lymphatic vessels of immunized ProxTom lymph nodes were compared to those from unimmunized ProxTom controls.
Flow cytometry
Lymph nodes were harvested 4 days after immunization with oxazolone. Lymphatic endothelial cells were isolated from lymph nodes using a method modified from that described by Halin and Detmar.18 Peripheral lymph nodes were harvested, pooled, and incubated in RPMI+10% FBS+1 mg/mL collagenase IV at 37°C for 50 min. Single cell suspensions were obtained by pipetting the nodes in the dissociation solution every 5 min. Cells were then passed through a cell strainer (100 μm) and spun down to replace dissociation media. Cells were stained with the following antibodies: CD31-FITC (Pharmingen), CD45-APC (Pharmingen), and Podoplanin-biotin-PE (ebioscience). Cells were analyzed by fluorescence activated cell sorting (FACS) using the green laser on an LSRII flow cytometer (BD)
Ex vivo microscopy
Tissues were either photographed immediately, or fixed in periodate-lysine-paraformaldehyde (PLP), embedded, and stored at −80°C. 7 μm-thick frozen sections were cut and sections were counterstained with DAPI (Sigma). Digital images were captured using an Axiocam (Zeiss) camera mounted directly onto an Axioskop fluorescent microscope (Zeiss). Images were analyzed using Axiovision software (Zeiss). Images were saved in the TIFF format. Red, green, and blue images were merged using Photoshop 8.0.
In Vivo Microscopy
Intra-vital images of the ProxTom popliteal lymph nodes were obtained using a Olympus BX61WI fluorescence microscope as described previously.3,17 All images were captured and analyzed with help and expert guidance from David Gonzalez and Ann Haberman from the Yale Imaging Core Centre.
Results
Lymphatic vessels in the head and neck ex vivo
The brilliant red of the tdTomato fluorescent reporter can easily be appreciated in ProxTom LVs.11 tdTomato was stable in tissues for more than 4 hours postmortem. Figure 1 demonstrates the clarity and detail of LVs in the head and neck of ProxTom: LVs in the ear skin (a), LVs in the submandibular salivary gland (b), LVs in the tongue (c), and in the peritoneum (d). Figure 1e and 1f show the changes in the appearance of LVs in the skin after painting with oxazolone. After painting with oxazolone, the skin is thickened and the reticular pattern of the LVs is lost, and the blind-ended lymphatic sacs appear to be dilated (f ).
Isolating and quantifying ProxTom endothelial cells by flow cytometry
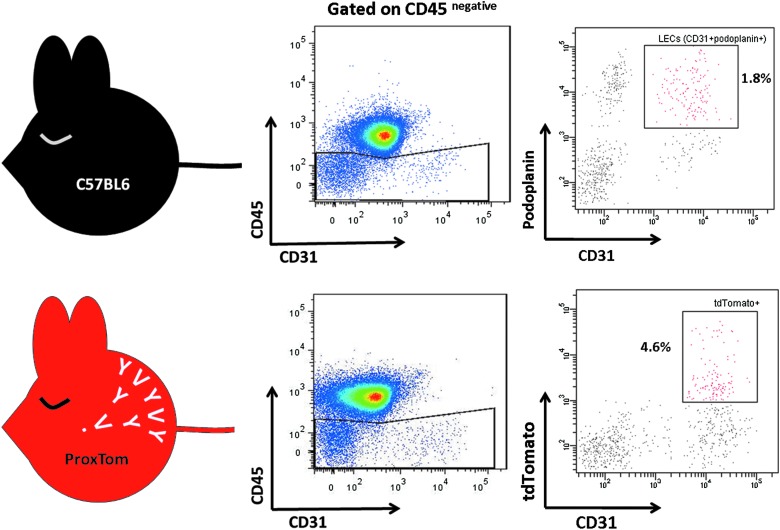
We isolated ProxTom lymphatic endothelial cells (LEC) from lymph nodes and sorted them by FACS, yielding a pure population of LECs without the use of beads or antibodies (Fig. 2). ProxTom LECs sorted by their lack of CD45 and presence of CD31 and tdTomato fluorescence can be further analyzed for mRNA expression even down to a single-cell level. The fluorochrome tdTomato excites at 554 nm and emits at 581 nm and can be detected using a 532 nm green laser.16 To demonstrate the accuracy of LEC isolation using tdTomato fluorescence, we compared LECs isolated from a ProxTom lymph node to LECs from a C57BL/6 lymph node isolated using anti-CD45, anti-podoplanin and anti-CD31 antibodies (Fig. 2). The ratio of LEC to blood endothelial cells was comparable between both mice strains. Taking the sorted tdTomatopositive cells and staining them for podoplanin revealed that tdTomatopositive cells expressed podoplanin (78%) and also the lymphatic marker LYVE-1 (69%). We demonstrate that LECs can be efficiently sorted from ProxTom lymph nodes without manipulation or prior incubation with antibodies or beads. This protocol will allow for efficient sorting of individual ProxTom LEC for the analysis of mRNA and gene expression.
FIG. 2.
Isolation of ProxTom lymphatic endothelial cells by flow cytometry. ProxTom lymphatic endothelial cells (LECs) can be isolated from lymph nodes, and purified by flow cytometry. Lymph node LECs are CD45negative, CD31high, and podoplaninpositive. ProxTom LECs are CD45negative, CD31high, and tdTomatopositive.
Lymphangiogenesis in a lymph node after immunization visualised in vivo
We wanted to understand LV function in resting and inflamed lymph nodes in vivo because we are interested in how the proliferation of LVs in lymph nodes and TLOs affects responses to antigens. We compared an unimmunized lymph node to an immunized lymph node 4 days after immunization with oxazolone. Figure 3 shows a 3D picture of a popliteal lymph node from a ProxTom mouse that was imaged in vivo. Compared to the resting, unimmunized lymph node (Fig. 3a), a massive lymphangiogenesis has occurred after oxazolone treatment (Fig. 3b). After immunization, the lymphatic network appeared thickened and LVs extended deeply into the T cell zone. Conversely, the B cell follicles stood out because LVs did not extend into the B cell zone after immunisation.
FIG. 3.
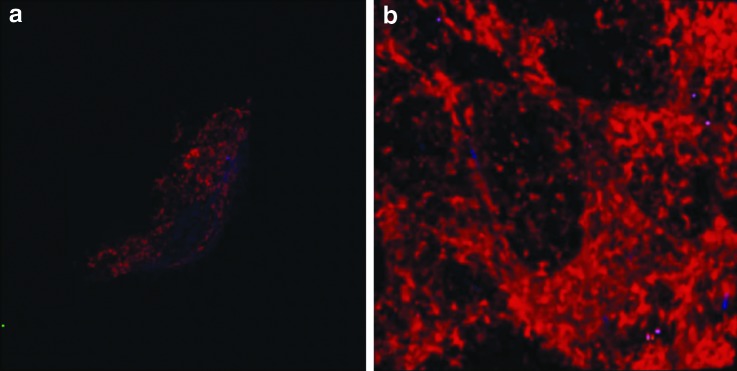
Lymphangiogenesis in vivo in the lymph node after immunization. Lymphangiogenesis occurs in the popliteal lymph node after immunisation of the mouse foot with 4% oxazolone. Figure 3 shows an unimmunized lymph node (a) and a lymph node 4 days after immunization (b). After immunization, the lymphatic network appears denser and extends more deeply into the T cell zone but not into the B cell area. LVs are relatively sparse within the B cell follicles. A color version of this figure is available online at www.liebertpub.com/lrb
Conclusions and Future Directions
The ProxTom transgenic mice described here will be an extremely valuable tool for understanding LV function both at rest and in inflammation. We have demonstrated that ProxTom mice are suitable for the ‘live’ imaging of LVs in lymph nodes during inflammation and we are now using ProxTom mice to study leukocyte migration ‘live’ through LVs in lymph nodes.
The set up that we use for in vivo imaging of the popliteal node described here, is by necessity, invasive. A new, noninvasive technique for imaging lymph nodes has recently been described that could also be used in ProxTom mice; Gibson et al.19 transplanted a lymph node underneath the skin of a mouse's pinna, which made it accessible for repeated, noninvasive, in vivo imaging. The beauty of this ectopic lymph node system is that it allows for longitudinal in vivo imaging of the LVs throughout the course of an inflammatory response, from its initiation through to its resolution.
Now that we have lymphatic reporter mice it will be important to look to the future and develop clinically relevant mouse models. At least two new models are required: LV malformations and lymphedema model, because we need to understand not only how LVs proliferate, but also how they involute. An understanding of LV proliferation will help provide methods to stimulate LV growth after damage caused by infections such as filariasis or after surgery and radiotherapy. The ability to control the involution of LVs may be important in controlling tumor metastasis and to shrink head and neck lymphatic malformations that obstruct the oropharynx.
One interesting find from our study of ProxTom mice as we previously reported by us and others3 was that Prox1 (the master regulator of lymphatic specification) was expressed in the central nervous system (CNS). The CNS is a tissue devoid of lymphatics, and yet we were able to confirm that Prox1 is expressed in the dentate gyrus of the brain and also we reported prox1 expression in the neuroendocrine cells of the adrenal medulla for the first time. In order to understand what drives LV proliferation in lymphatic head and neck malformations, we will need to study the CNS. Valuable information might be gleaned by studying prox1positive neurons that have switched off lymphatic differentiation. This would be straightforward to do using ProxTom mice to isolate a highly pure population of prox1positive neurons from the CNS by FACS and compare their gene expression to a sorted population of LECs.
One advantage of ProxTom mice over other fluorescent reporters12 is that ProxTom is on the C57BL/6 background. Many knock-out mice deficient in genes relevant for infection and autoimmunity are on the C57BL/6 background and for this reason, ProxTom were made directly onto C57BL/6. This removes the need to backcross mice for multiple generations and allows one to take advantage of the many knock-out and transgenic mice on this background.
Here, we show the faithful expression of the red-fluorescent tdTomato in the LVs of ProxTom reporter mice. We demonstrate the remarkable changes occurring to LVs in vivo in the lymph node after immunization and show how a pure population of LVs can be isolated by FACS. ProxTom mice have proven utility for the study of LVs in models of infection and inflammation in vivo. ProxTom has now been made available for distribution from the Jackson Laboratory and information on how to obtain them can be found at the following website: http://jaxmice.jax.org/strain/018128.html.
Supplementary Material
Acknowledgments
The authors thank David Gonzalez and Ann Haberman for their help and guidance.
Author Disclosure Statement
No conflicting financial interests exist.
LAT was supported by Lymphoma Leukaemia Research 09018, 11001, and Brown-Coxe Fellowships; KLB was supported by Juvenile Diabetes Research Fund 44-2008-912. Work in NHR's laboratory was supported by NIH DK 57331, NIH ACE IU19A1082713-01, R21HL098711, and JDRF 4-2007-1059. The 2-photon microscope is part of the Yale Rheumatologic Diseases Core Centre and is supported by NIH P30 AR053495.
References
- 1.http://www.nhlbi.nih.gov/meetings/workshops/lymph-res.html http://www.nhlbi.nih.gov/meetings/workshops/lymph-res.html
- 2.Liao S. Ruddle NH. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J Immunol. 2006;177:3369–3379. doi: 10.4049/jimmunol.177.5.3369. [DOI] [PubMed] [Google Scholar]
- 3.Truman LA. Bentley KL. Smith EC, et al. ProxTom lymphatic vessel reporter mice reveal Prox1 expression in the adrenal medulla, megakaryocytes, and platelets. Am J Pathol. 2012;180:1715–1725. doi: 10.1016/j.ajpath.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stranford S. Ruddle NH. Follicular dendritic cells, conduits, lymphatic vessels and high endothelial venules in tertiary lymphoid organs: Parallels with lymph node stroma. Front Immunol. 2012;3:350. doi: 10.3389/fimmu.2012.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shomer NH. Fox JG. Juedes AE. Ruddle NH. Helicobacter-induced chronic active lymphoid aggregates have characteristics of tertiary lymphoid tissue. Infect Immun. 2003;71:3572–3577. doi: 10.1128/IAI.71.6.3572-3577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Picarella DE. Kratz A. Li CB. Ruddle NH. Flavell RA. Insulitis in transgenic mice expressing tumor necrosis factor beta (lymphotoxin) in the pancreas. Proc Natl Acad Sci USA. 1992;89:10036–10040. doi: 10.1073/pnas.89.21.10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuerten S. Schickel A. Kerkloh C, et al. Tertiary lymphoid organ development coincides with determinant spreading of the myelin-specific T cell response. Acta Neuropathol. 2012;124:861–873. doi: 10.1007/s00401-012-1023-3. [DOI] [PubMed] [Google Scholar]
- 8.Faul JL. Berry GJ. Colby TV, et al. Thoracic lymphangiomas, lymphangiectasis, lymphangiomatosis, and lymphatic dysplasia syndrome. Am J Resp Crit Care Med. 2000;161:1037–1046. doi: 10.1164/ajrccm.161.3.9904056. [DOI] [PubMed] [Google Scholar]
- 9.Baluk P. Yao LC. Feng J. Romano T. Jung SS. Schreiter JL. Yan L. Shealy DJ. McDonald DM. TNF-alpha drives remodelling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Invest. 2009;119:2954–2964. doi: 10.1172/JCI37626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mounzer RH. Svendsen OS. Baluk P, et al. Lymphotoxin-alpha contributes to lymphangiogenesis. Blood. 2010;116:2173–2182. doi: 10.1182/blood-2009-12-256065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruddle NH. Lymphoid neo-organogenesis: Lymphotoxin's role in inflammation and development. Immunol Res. 1999;19:119–125. doi: 10.1007/BF02786481. [DOI] [PubMed] [Google Scholar]
- 12.Choi I. Chung HK. Ramu S, et al. Visualization of lymphatic vessels by Prox1-promoter directed GFP reporter in a bacterial artificial chromosome-based transgenic mouse. Blood. 2011;117:362–365. doi: 10.1182/blood-2010-07-298562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hägerling R. Pollmann C. Kremer L. Andresen V. Kiefer F. Intravital two-photon microscopy of lymphatic vessel development and function using a transgenic Prox1 promoter-directed mOrange2 reporter mouse. Biochem Soc Trans. 2011;39:1674–1681. doi: 10.1042/BST20110722. [DOI] [PubMed] [Google Scholar]
- 14.Martínez-Corral I. Olmeda D. Diéguez-Hurtado R. Tammela T. Alitalo K. Ortega S. In vivo imaging of lymphatic vessels in development, wound healing, inflammation, and tumor metastasis. Proc Natl Acad Sci USA. 2012;109:6223–6228. doi: 10.1073/pnas.1115542109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bentley KL. Shashikant CS. Wang W. Ruddle NH. Ruddle FH. A yeast-based recombinogenic targeting toolset for transgenic analysis of human disease genes. Ann NY Acad Sci. 2010;1207:E58–E68. doi: 10.1111/j.1749-6632.2010.05712.x. [DOI] [PubMed] [Google Scholar]
- 16.Shaner NC. Campbell RE. Steinbach PA. Giepmans BN. Palmer AE. Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 17.Mempel TR. Scimone ML. Mora JR. von Andrian UH. In vivo imaging of leukocyte trafficking in blood vessels and tissues. Curr Opin Immunol. 2004;16:406–417. doi: 10.1016/j.coi.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Halin C. Detmar M. Inflammation, angiogenesis, and lymphangiogenesis. Methods Enzymol. 2008;445:1–25. doi: 10.1016/S0076-6879(08)03001-2. [DOI] [PubMed] [Google Scholar]
- 19.Gibson VB. Benson RA. Bryson KJ, et al. A novel method to allow non-invasive, longitudinal imaging of the murine immune system in vivo. Blood. 2012;119:2545–2551. doi: 10.1182/blood-2011-09-378356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.