Abstract
Objective
The aim of this study was to assess morphological features of intact adipose tissue (AT) ex vivo from both subcutaneous (s.c.) abdominal and gluteal areas using a novel approach of multiphoton autofluorescence microscopy (MPAM) combined with second harmonic generation microscopy (SHGM), and to assess the relationship between morphological features in the two AT sites and insulin resistance to peripheral glucose disposal.
Method
This study was a cross-sectional evaluation of AT morphology feature and peripheral insulin resistance.
Subjects
Fourteen overweight/obese premenopausal women underwent body composition studies, hyperinsulinemic–euglycemic clamps, and needle biopsy of both the s.c. abdominal and gluteal AT areas. MPAM combined with SHGM was used to measure adipocyte maximal diameter and collagen fiber bundle thickness within a sampled image volume after three-dimensional visualization.
Results
Higher body mass index (BMI) was associated with larger adipocyte diameter in s.c. abdominal, but not gluteal, AT. Higher adipocyte diameter was associated with higher pericellular collagen thickness. Adipocyte diameter in s.c. abdominal, but not gluteal, AT was associated positively with leptin and negatively with adiponectin plasma levels and peripheral glucose disposal rate. The latter correlation was no longer significant after adjustment for collagen thickness.
Conclusion
In overweight/obese premenopausal women, larger adipocyte diameter in s.c. abdominal, but not gluteal, AT associates with low plasma adiponectin and systemic insulin resistance, and suggests that increased collagen thickness (obesity-related scarring) could contribute to these findings.
Introduction
Excessive caloric intake is physiologically met by storage of triglycerides in adipocytes, a key function of adipose tissue (AT), which guarantees maintenance of systemic metabolic homeostasis. However, with increasing overall body fat content, the relative quantity of triglyceride deposition in various AT compartments may vary. Major areas of triglyceride deposition are visceral, subcutaneous (s.c.) abdominal, and s.c. peripheral AT. Mass increase in each of these areas has been associated with different systemic metabolic consequences. For example, whereas increased fat accumulation in both visceral and s.c. abdominal AT has been associated with systemic insulin resistance, accumulation in peripheral s.c. AT has been associated with neutral or protective effects.1–3 Accordingly, upper body fat distribution is a major indicator of risk for metabolic complications of obesity, and lower body fat distribution is an indicator of decreased risk for metabolic complications of obesity.4,5 Therefore, a better understanding of the mechanisms regulating fat distribution seems promising in identifying novel approaches to prevent chronic complications of weight gain, including type 2 diabetes and cardiovascular disease.
Although the specific mechanisms involved in regulating fat distribution pattern continue to be elusive, differences in local new adipocyte recruitment (hyperplasia) and triglyceride storage efficiency (hypertrophy resulting in enlargement rather than number of adipocytes) could account for differential fat distribution pattern in overweight individuals. Recent work has pointed out that weight gain induces prevalently adipocyte hyperplasia in s.c. peripheral AT and prevalently hypertrophy in the s.c. abdominal AT.6 Interestingly, whereas hyperplasia does not appear to induce adverse changes in adipocyte function, adipocyte hypertrophy has been associated with decreased adiponectin, increased leptin, increased inflammation, and adipocyte insulin resistance.7–9 This is thought to contribute to changes in lipids and glucose metabolism, eventually manifesting with development of systemic insulin resistance.
Besides adipocyte size, collagen content has also been proposed to contribute in determining AT dysfunction. In mice, an increase in body fat content associates with an increase in Col6a3 gene expression.10 This was associated with AT inflammation and abnormalities in systemic glucose metabolism. On the other hand, absence of Col6a3 production in knockout mice on a high-fat diet was associated with larger adipocytes, less AT inflammation, and better glucose metabolism.10 In the same study, we also reported that in s.c. AT of insulin-resistant Asian Indians, Col6a3 expression was increased, even in absence of obesity. These findings support the view that increased fat deposition in AT induces increased inflammatory response and collagen production, which, in turn, reduces the capacity of adipocytes to expand further. This may contribute to fat redistribution and increased circulating free fatty acid, thus influencing the risk for insulin resistance. Recent observation in AT of lean and obese subjects has suggested that the prevalent collagen types in humans is of type I and III in fibrous bundles and type VI surrounding parenchymal adipocytes.11 An inverse relationship between collagen quantity and adipocyte size was described in omental AT of obese subjects, supporting the view that increased fibrosis may impair adipocyte maturation. The systemic metabolic consequences of this interaction between adipocyte size, AT inflammation, and collagen production has not been elucidated. Furthermore, no studies in humans have thus far evaluated morphological differences that combine measures of adipocyte size and collagen content between s.c. abdominal and gluteal AT. Suboptimal methodology for measurement of adipocyte size and collagen quantity could be a major limitation for quantification of obesity-induced changes in AT, which has complex three-dimensional (3D) architecture. These limitations originate from the fact that single-plane histopathology used to capture a single section within the 3D tissue is not suitable for accurate assessment of the size of cells in AT in 3D space. Similarly, quantification of collagen deposition using histopathology is limited to individual planes. Furthermore, methods that require AT digestion and osmium fixation introduce additional artifacts that diminish the ability to perform precise measurements of adipocyte size.
In this study, we use the nonlinear optical microscopy technique of multiphoton autofluorescence microscopy and second harmonic generation microscopy (MPAM/SHGM) to evaluate the 3D characteristics of s.c. abdominal and peripheral AT. This approach allows using fresh, intact, unfixed, and unstained tissue to compare morphological differences between s.c. abdominal and peripheral AT, specifically allowing measurement of adipocyte size and AT collagen bundle thickness. The combination of MPAM/SHGM has been used in a number of tissues to image cells and other microstructures hundreds of microns in depth with subcellular resolution,12 but it has been applied to adipose tissue only on a limited basis. One previous study demonstrated the feasibility for visualization of adipocytes and collagen in adipose tissue by MPAM/SHGM.13 That study showed adipocytes and collagen could be identified by MPAM and SHGM, respectively, but did not indicate the site of AT origin or status of obesity. Given the complex 3D architecture and makeup of AT and the potential role that the extracellular matrix may play in remodeling and function of this tissue,14 methodologies that provide indication of spatial organization between AT constituents could be highly beneficial to the characterization of intact AT but also be used to better understand the changes associated with obesity and insulin resistance. In the current study, we expand the use of MPAM/SHGM imaging to derive 3D information that can be quantitatively related to metabolic and obesity parameters. Specifically inherent signal contrast from adipocyte autofluorescence and fibrillar collagen SHG were used to quantify cellular and collagen features of AT and to correlate these with measures of obesity [body mass index (BMI)] and insulin resistance (glucose disposal rate during hyperinsulinemic euglycemic clamp). We hypothesized that maximum adipocyte diameter (measure of adipocyte hypertrophy) and collagen content (measure of AT scarring) are higher in s.c. abdominal than s.c. gluteal AT, and that these morphological differences contribute to explain the differential impact of the two AT compartments on systemic measures of insulin resistance (glucose disposal rate during hyperinsulinemic clamps).
Subjects and Methods
The Institutional Review Boards at the University of Texas Medical Branch at Galveston (UTMB) approved the conduct of this study. A total of 14 premenopausal women with BMI >25 kg/m2 were enrolled by public advertisement, and informed consent was obtained from all participants. Subjects with a previous diagnosis of diabetes mellitus or other endocrine disorders, coronary artery disease, or renal insufficiency were excluded. All studies were performed at the Clinical Research Center (CRC) at UTMB. Each subject was provided a eucaloric diet for the duration of 3 days as outpatient. During the first 2 days, body composition studies were performed. On day 3, each subject underwent subcutaneous adipose tissue biopsy followed by an oral glucose tolerance test (OGTT) after overnight fast. On day 4, a hyperinsulinemic–euglycemic clamp was performed.
Body composition studies
Height and weight were measured by standard procedures. Waist and hip circumferences were measured, using a flexible measuring tape with a tension caliper at the extremity (Gulick-Creative Health Product, Inc., Plymouth, MI), midway between the xiphoid and the umbilicus during the midinspiratory phase and at the maximum circumference in the hip area, respectively. The means of three repeat measurements at each site were used for calculations.
Oral glucose tolerance testing
A standard OGTT with 75 grams of glucose (Tru-Glu100, Fisher Scientific, Pittsburgh, PA) was conducted after 12 hr overnight fasting on day 3. Samples for measurement of glucose were collected at baseline and at 30-min intervals from glucose administration for a total of 150 min duration.
Hyperinsulinemic–euglycemic clamps
On the morning of study day 4, a hyperinsulinemic–euglycemic clamp was performed after an overnight fast. In brief, a primed-continuous infusion of regular insulin (Humulin, Squibb-Novo, Princeton, NJ) was started at a rate of 80 mU/m2 (body surface area)/min, and was continued for 2 hr. On the basis of our previous studies in young normoglycemic subjects, this infusion protocol induces complete suppression of hepatic glucose output, even in the presence of significant systemic insulin resistance.2 The rate of glucose disposal (Rd) was calculated by subtracting the urinary glucose excretion from the rate of appearance (Ra) during the last 40 min of the study.15
AT biopsy
AT was obtained using a 14 gauge×9-cm Temno II biopsy needle (Allegiance) from the abdominal s.c. area in the right lower quadrant 2 cm above and medial to the anterior iliac tuberosity and also from the gluteal area. AT was obtained at the CRC. Fresh AT was kept in Dulbecco's modified Eagle medium (DMEM; Invitrogen, Carlsbad, CA). Prior to imaging, AT was rinsed and then immersed in 1×phosphate-buffered saline (PBS; Mediatech, Manassas, VA) solution in a 35-mm coverglass bottom (#1.5 coverglass) imaging dish (Matek, Inc.). Another aliquot of AT was frozen in liquid nitrogen immediately after collection.
MPM autofluorescence imaging and SHG imaging system
MPAM/SHGM was conducted on a modified imaging system based on a Carl Zeiss Confocal LSM 410 inverted microscope and outfitted with optics designed for ultrafast laser excitation and non-descanned detection of multiphoton emission.16 The excitation source was a femtosecond Ti:Sapphire laser (Tsunami, Spectra Physics/Newport, Irvine CA) having a 5W frequency-doubled Nd:YVO4 pump laser (Millenium, Spectra Physics/Newport, Irvine, CA). The Tsumani laser was operated with a typical pulse width at 140 fs at an 80-MHz repetition rate; the laser wavelength is adjustable from 710 nm to 950 nm. For adipocyte autofluorescence imaging, the laser excitation wavelength was set at 780 nm; for SHG arising from fibrillar collagen, the illumination wavelength was set at 840 nm. The average laser power incident on the AT sample was 20 mW for all trials. An epiconfiguration was used for collection of emitted light and detected using a cooled photomultiplier tube (PMT) placed in a non-descanned configuration (R6060, Hamamatsu, Japan). Broadband fluorescence emission light spanning 450–600 nm was collected at the PMT. For SHG signal detection, a narrow bandpass filter centered at 420 nm with 40-nm bandwidth was used. Two objectives were used in the collection of the images, a 40×, 1.2 numerical aperture (N.A.) water objective providing a 320×320-μm field of view and a 20×, 0.75 N.A. air objective providing a larger (640×640 μm) field of view. The 40×objective was used for collection of images from the first 7 patient samples, and the 20×was used on samples from the second set of samples. A z-stack with total depth of 100 μm was acquired with a step size of 2 μm.
Each biopsy sample was randomly imaged at two regions of interest. ImageJ17 was used to visualize z-stack data and to make measurements of adipocyte diameter. In each z-stack, the maximal diameter for individual adipocytes was measured and correlated with obesity and metabolic parameters. Adipocyte diameter measurements were made manually using the line tool in ImageJ,18 and the mean and median maximum diameters measured for cells within a sampled volume were calculated. Adipocytes along the imaged region borders were excluded from diameter measurements. 3D image reconstructions were made using Imaris software (Bitplane Inc, St. Paul, MN). SHGM images were analyzed to measure the thickness of pericellular collagen bundles present between adipocytes. Where present, the average thickness of collagen fiber bundles was measured along five evenly distributed points along the length of each observed bundle. An average for each sample was obtained by averaging the mean of the individual bundles observed in that sample. Non-pericellular collagen surrounding the AT was excluded from these measurements.
Biochemical measurements
Cholesterol and triglycerides were measured by enzymatic methods.15 High-density lipoprotein cholesterol (HDL-C) was determined in the supernatant after precipitating apolipoprotein B–containing lipoproteins using heparin-manganese chloride. Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation. Adiponectin, leptin, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) were measured using multiplex immunoassays (Millipore, Billerica, MA). High-sensitivity C-reactive protein (hsCRP) was measured by a highly sensitive nephelometric assay using a monoclonal antibody to CRP coated on polystyrene beads (Dade Behring, Newark, DE).
Statistical analysis and calculations
After conversion to a SAS (SAS v. 9.1; SAS Institute, Cary, NC) database, all variables were examined using plots, summary statistics, and tests of normality for continuous variables. All variables except gender demonstrated varying amounts of asymmetry. To account for asymmetry in the small number of samples, Spearman correlations were estimated.
Results
A total of 14 women volunteered for this study. Their age range was 22–45 years and they were all premenopausal. BMI range was between 26 and 61 kg/m2. The general characteristics of the study group are summarized in Table 1. None of the participants had a history of glucose intolerance or of taking medication affecting glycemic, lipid, or blood pressure control. However, 3 subjects were found to have fasting plasma glucose (FPG) above 126 mg/dL. The remaining 11 volunteers had a FPG value below 100 mg/dL.
Table 1.
General Characteristics of the Study Subjects
| Variable | |
|---|---|
| n | 14 |
| Age (years) | 34±8 |
| BMI (kg/m2) | 38±11 |
| Waist circumference (cm) | 44±8 |
| Hip circumference (cm) | 50±8 |
| Systolic blood pressure (mmHg) | 124±19 |
| Diastolic blood pressure (mmHg) | 77±8 |
| Fasting glucose (mg/dL) | 97±16 |
| Plasma total cholesterol (mg/dL) | 164±26 |
| Plasma LDL-C (mg/dL) | 96±27 |
| Plasma HDL-C (mg/dL) | 45±8 |
| Plasma triglyceride (mg/dL) | 115±42 |
| Rd value (mg/min per kg body weight) | 5.1±2.5 |
| Adiponectin (μg/mL) | 11±8 |
| Leptin (ng/mL) | 40±18 |
| IL-6 (pg/mL) | 11.8±18.7 |
| TNF-α (pg/mL) | 3.2±1.6 |
| hsCRP (μg/mL) | 2.8±1.4 |
| HOMA-IR | 1.0±09 |
Data are presented as mean±standard deviation.
BMI, body mass index; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; Rd value, rate of glucose disposal; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; hsCRP, high-sensitivity C-reactive protein; HOMA-IR, homeostasis model assessment of insulin resistance.
Figure 1 illustrates the ability of MPAM/SHGM techniques to visualize the architecture of abdominal and gluteal adipocytes and fibrillar collagen bundles in fresh AT biopsies samples based on autofluorescence and SHG. The SHG image reconstructions shown in the insert in Fig. 1, D and H, demonstrate it is feasible to assess collagen distribution in 3D space in AT.
FIG. 1.
Multiphoton autofluorescence microscopy (MPAM) and second harmonic generation (SHG) images illustrating adipocyte and collagen imaged in fresh adipose tissue and measured for quantitative evaluation of cell size and bundle thickness, respectively. (A) Abdominal adipose tissue (AT) multiphoton autofluorescence microscopy (MPAM) image. (B) SHG image from same sample depicting only fibrillar collagen in the same field of view. (C) Abdominal AT autofluorescence (green) and SHG (yellow) image overlay. (D) Three-dimensional (3D) reconstruction of SHG for abdominal AT. (E) Gluteal AT MPAM image. (F) Gluteal AT SHG image from the same sample. (G) Gluteal AT autofluorescence (green) and SHG (yellow, few) image overlay. (H) 3D reconstruction of SHG for gluteal AT.
We first evaluated the correlation between adipocyte size in each of the s.c. AT compartments studied and systemic insulin resistance (Rd value) measured by the hyperinsulinemic–euglycemic clamps. Figure 2 shows that, while increasing s.c. abdominal maximum adipocyte diameter was strongly associated with decreasing Rd value (r=0.62, P=0.01), there was no correlation between s.c. peripheral maximum adipocyte diameter and Rd value (Fig. 2A,B).
FIG. 2.
The relationships between glucose disposal rate during hyperinsulinemic–euglycemic clamp (Rd value) and maximal adipocyte diameter is illustrated for subcutaneous (s.c.) abdominal and s.c. gluteal adipose tissue measured by multiphoton autofluorescence microscopy. Results from Spearman rank correlation analysis are reported for each panel. BMI, body mass index.
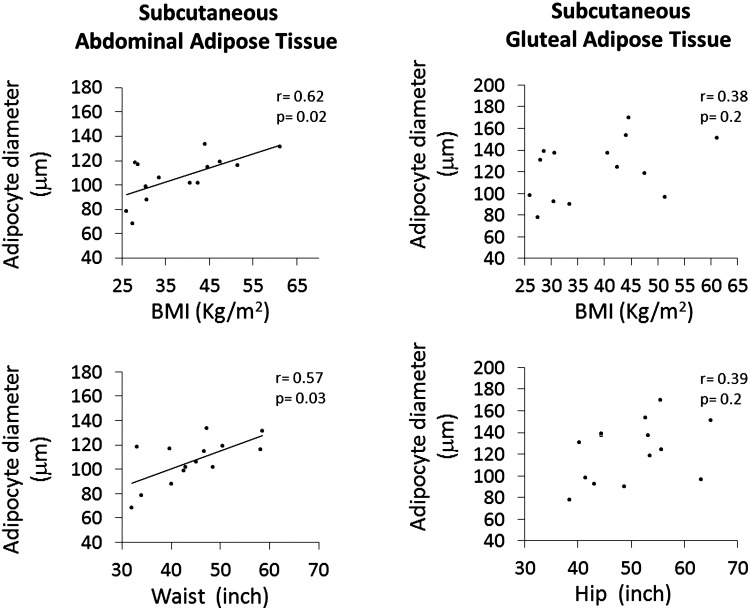
Because increase in BMI is expected to associate with increasing adipocyte size (hypertrophy) and/or increasing number (hyperplasia), we evaluated the correlations between BMI and adipocyte size in the two s.c. AT compartments, separately. As shown in Fig. 3, increasing BMI was significantly correlated with maximum adipocyte diameter of the s.c. abdominal (Spearman r=0.62 and P=0.018) but not gluteal AT (Spearman r=0.38 and P=0.17). Furthermore, increasing waist circumference was correlated with increasing maximum adipocyte diameter of the s.c. abdominal AT (Spearman r=0.57 and P=0.03), a finding consistent with a predominant hypertrophic response to abdominal fat gain. On the other hand, increasing hip circumference was not associated with increase in maximum adipocyte diameter from the s.c. peripheral AT, thus suggesting preferentially hyperplastic response to peripheral fat during weight gain.
FIG. 3.
The relationships between adipocyte size of each subcutaneous (s.c.) adipose tissue (AT) compartments and measures of obesity and fat distribution were determined using Spearman rank correlation analysis.
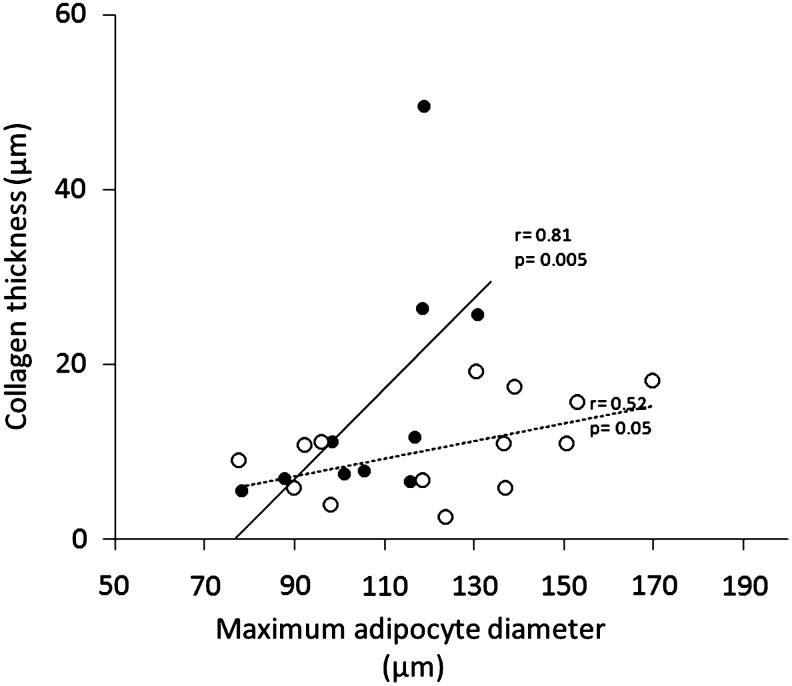
Because an increase in BMI has been previously associated with increased AT collagen expression, we measured AT collagen thickness from each fresh biopsy tissue in concomitance with maximum adipocyte diameter (Fig. 4). The two morphological measures were positively correlated in the s.c. abdominal AT (r=+0.81, P=0.005) but only showed a trend in the s.c. peripheral AT (r=+0.52; P=0.058). The slopes of the two correlation lines were significantly different [analysis of covariance (ANCOVA) P=0.026), with a much larger increase in collagen thickness within the s.c. abdominal than s.c. peripheral AT in association with increasing abdominal adipocyte circumference (Fig. 4). Because of these associations, we performed a Spearman correlation analysis between s.c. abdominal adipocyte size and Rd value, after adjustment for the covariate collagen thickness. The results show significant attenuation of the correlation depicted in Fig. 2A (r=−0.40, P=0.28).
FIG. 4.
The relationship between adipocyte size and collagen thickness are reported from Spearman rank correlation analysis. Analysis of covariance (ANCOVA) P=0.026 for difference in slope. (•) Subcutaneous abdominal; (○) subcutaneous peripheral.
Maximum adipocyte diameter measured by MPAM in the s.c. abdominal but not in the s.c. peripheral region was significantly correlated with plasma leptin concentrations (r=+0.73, P=0.003 and r=+0.34, P =0.2, respectively). Similarly, maximum adipocyte diameter in the s.c. abdominal but not in the s.c. gluteal region was significantly correlated with plasma adiponectin concentrations (r=−0.61, P=0.02 and r=−0.02, P=0.9, respectively). Other adipokines, including hsCRP, IL-6, TNF-α, monocyte chemotactic protein-1 (MCP-1), and plasminogen activator inhibitor-1 (PAI-1), were not correlated with maximum adipocyte diameter or collagen thickness in neither of the two AT compartments (r values>0.05 for all examined correlations).
Discussion
This study shows that in overweight and obese premenopausal women: (1) Increasing regional adiposity (waist and hip) is associated with larger increase in adipocyte size of s.c. abdominal than s.c. gluteal AT; (2) increasing adipocyte size is associated with higher increase in collagen thickness of s.c. abdominal than and gluteal AT; and (3) increasing adipocyte size in s.c. abdominal but not gluteal AT is correlated with biomarkers of adipose tissue dysfunction and systemic insulin resistance.
In this study, we demonstrated that the 3D imaging capabilities of MPAM and SHGM can be used to determine maximal adipocyte diameter as well as thickness of pericellular collagen bundles in fresh tissue samples. Using this approach, we avoid distortion of the adipocyte dimension related to tissue fixation/staining and we also avoid the measurement bias related to single-slice measurements of multiple adipocytes at different orientation planes. Another advantage of imaging by MPAM/SHG is that pericellular collagen imaging provided by SHG can be used to quantitatively assess the degree of obesity-induced tissue remodeling in AT.
Increase in adipocyte size is commonly viewed as a potential determinant of AT dysfunction and metabolic complications of obesity. Recent work by Tchoukalova et al.6 has shown lower body adipocyte hyperplasia versus abdominal s.c. adipocyte hypertrophy in response to overfeeding and total body fat increase in lean men and women. In that study, these morphological data were not evaluated in their relationship with metabolic parameters. The results of our study support the hypothesis that also in obese premenopausal women, higher BMI and regional adiposity are characterized by hypertrophic response in the s.c. abdominal adipocytes and hyperplasia in s.c. gluteal (lower body) adipocytes. Because adipocyte hypertrophy likely expresses reduced ability to mobilize new adipocytes, higher adipocyte oxidative stress, and redistribution of fat to alternative AT areas or lean tissues, our findings in s.c. abdominal adipose tissue support a role of this AT compartment on metabolic complications of obesity.
Inflammatory response to adipocyte hypertrophy and increased oxidative stress includes macrophage recruitment at first, followed by collagen production and scarring. Our study did not find any evidence of obesity-related systemic inflammation, as measured by correlations between BMI and plasma concentrations of adipokines involved in inflammatory response, including IL-6, TNF-α, and hsCRP. This could be due to a number of factors, including lack of lean subjects with consequent small data variability, small sample size, as well as cross-sectional study design during stable weight, when inflammatory flare may have resolved. However, scarring of AT was evident from the high correlation between adipocyte size and collagen thickness. This was clearly more apparent for the s.c. abdominal AT, thus suggesting that a much larger inflammatory response in s.c. abdominal than in s.c. gluteal could have occurred during the weight gain phase of our overweight/obese volunteers. Recent literature suggests that higher collagen content in adipose tissue is associated with decreased adipogenic potential.10,11 Dysregulation of adipocyte maturation is being recognized as a major determinant of fat redistribution, ectopic fat deposition (fatty liver), and systemic insulin resistance.20,21 Adiponectin promotes adipogenesis, thus the inverse association between collagen thickness and plasma adiponectin concentrations suggests that differences in collagen thickness between s.c. abdominal and s.c. gluteal AT could contribute to the strength of the association observed between s.c. abdominal AT and systemic insulin resistance. This view is supported by the attenuated correlation between s.c. abdominal maximum adipocyte diameter and Rd value after statistical adjustment for collagen thickness. Taken together, our results in s.c. abdominal AT support the hypothesis that this AT depot plays a role in metabolic complications of obesity. Although the contribution of increased fat storage in s.c. abdominal adipose tissue to worsening insulin resistance has been a matter of intense debate, its importance had been pointed out by our earlier studies in normoglycemic volunteers and has been subsequently confirmed by other independent investigators.1–3,19 On the other hand, fat deposition in s.c. peripheral AT has been linked to the protective effect on metabolic complications of obesity.5 The findings of our study are in agreement with this view. The small increase in adipocyte size of s.c. gluteal AT with increasing hip circumference suggests that the ability to mobilize new adipocytes during weight gain could be more efficient in this AT compartment, thus reducing the need for adipocyte hypertrophy in response to increasing fat deposition. This will limit development of adipocyte dysfunction, such as high leptin, low adiponectin, and increased nonesterifed fatty acid (NEFA) release, in s.c. peripheral AT. The lack of plasma adiponectin changes with increasing s.c. peripheral adipocyte size likely contributes to maintain adequate preadipocyte differentiation to mature adipocytes and decreased inflammatory response as well. This latter view is supported by our findings depicted in Fig. 4 of smaller increase in collagen thickness with increasing adipocyte size of s.c. peripheral AT.
In conclusion, our cross-sectional data in premenopausal overweight/obese females show that, with increasing BMI, larger adipocyte diameter as measured by MPAM in s.c. abdominal but not gluteal AT associates with measures of AT dysfunction and systemic insulin resistance. Finally, our data suggest that increased collagen thickness observed with increasing s.c. abdominal adipocyte diameter (obesity-related scarring) could contribute to the known association between central obesity and insulin resistance.
Acknowledgments
G.V. carried out experiments and contributed to data interpretation; M.C. carried out experiments and contributed to data interpretation; Y.J. carried out experiments; H.D. carried out experiments; M.M. conceived experiments and contributed to data interpretation; N.A. conceived the experiments, analyzed the data, and contributed to data interpretation. All authors were involved in writing the manuscript and had final approval of the submitted and published versions.
We acknowledge the contribution of Geetika Saraf (Division of Endocrinology) and Elena Sbrana, PhD (Department of Pathology) for technical support. We acknowledge the Institute for Translational Sciences (ITS), Key Resources Clinical Research Center (CRC), Biostatistics, Epidemiology and Research Design (BERD), Department of Pathology at the University of Texas Medical Branch at Galveston, for support in conduction of this study.
This work was supported by National Institutes of Health (NIH) grant R01 DK072158 from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and in part by CTSA-NIH 1UL1RR029876 from the National Center for Research Resources (NCRR) and Shriners' Hospitals grant 71007.
Author Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Goodpaster BH. Thaete FL. Simoneau JA, et al. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 2.Abate N. Garg A. Peshock RM, et al. Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest. 1995;96:88–98. doi: 10.1172/JCI118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abate N. Insulin resistance and obesity. The role of fat distribution pattern. Diabetes Care. 1996;19:292–294. doi: 10.2337/diacare.19.3.292. [DOI] [PubMed] [Google Scholar]
- 4.Seidell JC. Pérusse L. Després JP, et al. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: The Quebec Family Study. Am J Clin Nutr. 2001;74:315–321. doi: 10.1093/ajcn/74.3.315. [DOI] [PubMed] [Google Scholar]
- 5.Snijder MB. Dekker JM. Visser M, et al. Hoorn study. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: The Hoorn study. Diabetes Care. 2004;27:372–377. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 6.Tchoukalova YD. Votruba SB. Tchkonia T, et al. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci USA. 2010;107:18226–18231. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammarstedt A. Graham TE. Kahn BB. Adipose tissue dysregulation and reduced insulin sensitivity in non-obese individuals with enlarged abdominal adipose cells. Diabetol Metab Syndr. 2012;4:42. doi: 10.1186/1758-5996-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couillard C. Mauriège P. Imbeault P, et al. Hyperleptinemia is more closely associated with adipose cell hypertrophy than with adipose tissue hyperplasia. Int J Obes Relat Metab Disord. 2000;24:782–788. doi: 10.1038/sj.ijo.0801227. [DOI] [PubMed] [Google Scholar]
- 9.Sjöholm K. Lundgren M. Olsson M, et al. Association of serum amyloid A levels with adipocyte size and serum levels of adipokines: Differences between men and women. Cytokine. 2009;48:260–266. doi: 10.1016/j.cyto.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Khan T. Muise ES. Iyengar P, et al. Metabolic dysregulation and adipose tissue fibrosis: Role of collagen VI. Mol Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Divoux A. Tordjman J. Lacasa D, et al. Fibrosis in human adipose tissue: Composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59:2817–2825. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zipfel WR. Williams RM. Christie R, et al. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc Natl Acad Sci USA. 2003;100:7075–7080. doi: 10.1073/pnas.0832308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Z. Zhuo S. Chen J, et al. Multiphoton microscopic imaging of adipose tissue based on second-harmonic generation and two-photon excited fluorescence. Scanning. 2008;30:452–456. doi: 10.1002/sca.20130. [DOI] [PubMed] [Google Scholar]
- 14.Divoux A. Clément K. Architecture and the extracellular matrix: The still unappreciated components of the adipose tissue. Obes Rev. 2011;12:e494–e503. doi: 10.1111/j.1467-789X.2010.00811.x. [DOI] [PubMed] [Google Scholar]
- 15.Chandalia M. Abate N. Garg A, et al. 1999 Relationship between generalized and upper body obesity to insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999;84:2329–2335. doi: 10.1210/jcem.84.7.5817. [DOI] [PubMed] [Google Scholar]
- 16.Sun J. Shilagard T. Bell B, et al. In vivo multimodal nonlinear optical imaging of mucosal tissue. Optics Express. 2004;12:2478–2486. doi: 10.1364/opex.12.002478. [DOI] [PubMed] [Google Scholar]
- 17.Schneider CA. Rasband WS. Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasband WS. ImageJ. US. National Institutes of Health; Bethesda, MD: 1997–2012. [Mar 25;2013 ]. [Google Scholar]
- 19.Chandalia M. Lin P. Seenivasan T, et al. Insulin resistance and body fat distribution in South Asian men compared to Caucasian men. PLoS One. 2007;2:e812. doi: 10.1371/journal.pone.0000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan W. Ciociola E. Saraf M, et al. Metabolic consequences of ENPP1 overexpression in adipose tissue. Am J Physiol Endocrinol Metab. 2011;301:E901–E911. doi: 10.1152/ajpendo.00087.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abate N. Chandalia M. Role of subcutaneous adipose tissue in metabolic complications of obesity. Metab Syndr Relat Disord. 2012;10:319–320. doi: 10.1089/met.2012.1502. [DOI] [PubMed] [Google Scholar]