Abstract
Afferent lymphatic vessels fulfill essential immune functions by transporting leukocytes and lymph-borne antigen to draining lymph nodes (dLNs). An important cell type migrating through lymphatic vessels are dendritic cells (DCs). DCs reside in peripheral tissues like the skin, where they take up antigen and transport it via the lymphatic vascular network to dLNs for subsequent presentation to T cells. As such, DCs play a key role in the induction of adaptive immune responses during infection and vaccination, but also for the maintenance of tolerance. Although the migratory pattern of DCs has been known for long time, interactions between DCs and lymphatic vessels are only now starting to be unraveled at the cellular level. In particular, new tools for visualizing lymphatic vessels in combination with time-lapse microscopy have recently generated valuable insights into the process of DC migration to dLNs. In this review we summarize and discuss current approaches for visualizing DCs and lymphatic vessels in tissues for imaging applications. Furthermore, we review the current state of knowledge about DC migration towards, into and within lymphatic vessels, particularly focusing on the cellular interactions that take place between DCs and the lymphatic endothelium.
Introduction
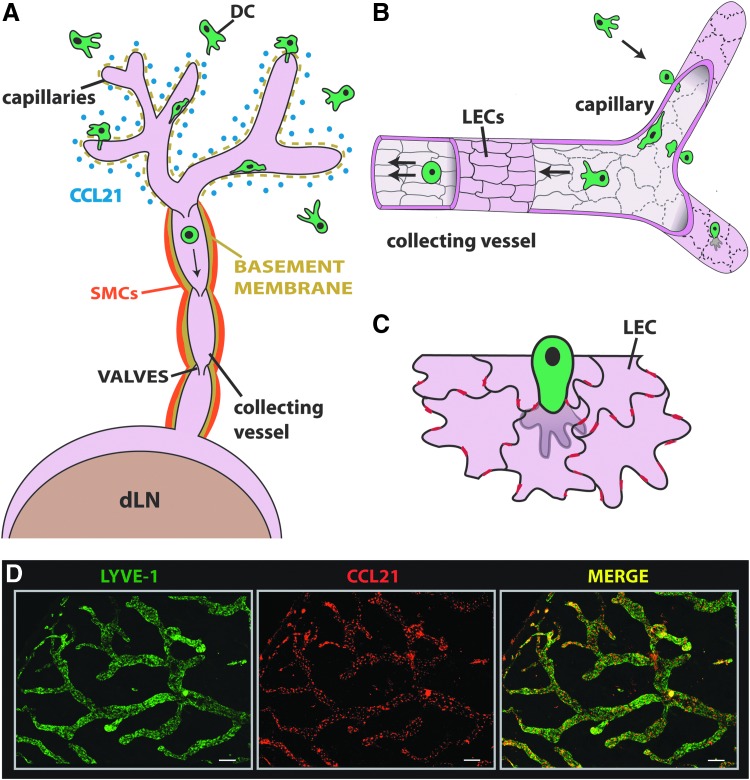
In contrast to leukocyte extravasation from blood vessels, leukocyte migration into afferent lymphatic vessels has been much less well characterized. Afferent lymphatic vessels begin as blind-ended capillaries, which merge into larger collecting vessels and connect with dLNs (Fig. 1A). The functional units of collecting lymphatic vessels are the lymphangions, which span between valves and spontaneously contract to propagate lymph and lymph-borne cells.1,2 At the cellular and molecular level, important differences exist between lymphatic capillaries and collectors: The fluid absorbing lymphatic capillaries are surrounded by a thin, perforated basement membrane but are devoid of smooth muscle cell (SMC) coverage. By contrast, collecting lymphatic vessels are less permeable and are surrounded by a continuous basement membrane and a SMC layer (Fig. 1A).2,3 A further important distinction between these two vessels segments occurs at the level of the cell-cell junctions: Similar to blood vascular endothelial cells (BECs) in blood vessels, lymphatic endothelial cells (LECs) in lymphatic collectors are surrounded by a continuous “zipper-like” lining of junctional adhesion molecules (e.g. CD31, VE-cadherin).4 By contrast, LECs of lymphatic capillaries are oakleaf-shaped and are joined by discontinuous cell junctions with “button”-like accumulations of cell adhesion molecules (Figs 1B and 1C).4 At the sites of such “buttons”, LECs partially overlap and generate loose flaps, through which tissue fluid and leukocytes are thought to enter into lymphatic vessels (F 1C).4,5 Early knowledge about leukocytes migrating through afferent lymphatics has come from lymph canulation studies performed more than 20 years ago in large animals like sheep.6,7 Such experiments have revealed that afferent lymph contains approximately 90% of lymphocytes, in particular CD4+ effector/memory cells, and 1–10% of dendritic cells (DCs).6,7
FIG. 1.
Dendritic cell (DC) migration into afferent lymphatic vessels (LVs). (A) In tissues like the skin LVs begin as blind-ended capillaries, which merge into collecting vessels and connect with dLNs. DC migration into afferent LVs primarily occurs at the level of CCL21-expressing lymphatic capillaries. In contrast to lymphatic collectors, lymphatic capillaries have a highly fenestrated basement membrane and are not surrounded by smooth muscle cells (SMCs). (B) While lymphatic endothelial cells (LECs) in lymphatic collectors are tightly connected by continuous, “zipper-like” cell junctions (solid black lines), lymphatic capillaries display a discontinuous, “button-like” expression of junctional adhesion molecules (dotted black lines). (C) In lymphatic capillaries, adjacent oakleaf-shaped LECs partially overlap, thereby creating open flaps. DCs are thought to transmigrate the lymphatic endothelium by migrating through the flaps (black lines). Red lines represent tight and adherens junctions present between neighboring LECs. (D) The chemokine CCL21 is constitutively expressed by lymphatic vessels and attracts CCR7-expressing DCs. The expression of LYVE-1 (green) and CCL21 (red) was analyzed in tissue whole mounts prepared from murine ear skin. Stainings were performed under PFA-fixed and permeabilizing conditions, as described.66 CCL21 is mainly present in intracellular deposits in LECs. Scale bar: 200 μm.
Similar to lymphatic vessels, DCs are present in most peripheral tissues and are particularly abundant at interfaces between the body and the environment, such as in the skin or in mucosal tissues. DCs function as important immune sentinels and are capable of bridging between the innate and the adaptive immune system.8,9 As their name implies, DCs possess long dendritic processes10 that constantly sample their environment for pathogens. Moreover, they express many pattern recognition receptors that enable them to recognize and respond to pathogens or signs of tissue damage. These stimuli, particularly pathogen-derived molecules, induce a maturation process during which DCs reduce their endocytic activity and upregulate genes involved in antigen presentation and T cell activation, such as major histocompatibility complex (MHC) molecules, co-stimulatory molecules and cytokines.9,11 Furthermore, maturation induces changes in the migratory behavior of DCs: Specifically, DCs down-regulate the expression of inflammatory chemokine receptors but upregulate the CC-chemokine receptor 7 (CCR7).12,13 The latter receptor is responsible for their migration towards lymphatic vessels, which constitutively express the chemokine CCL21 (Fig. 1D).14 Upon arrival in dLNs CCR7 coordinates the entry of DCs into the T cell area,9,15–18 where CCL21 is expressed by fibroblastic reticular cells (FRCs).19 Once within the T cell area, DCs can present antigen to T cells and induce antigen-specific T cell responses.9,15–17 Thus, DCs are essential for inducing an adaptive immune response in the context of infections as well as during vaccination. Moreover, DCs play an important role in the maintenance and induction of peripheral immune tolerance to auto-antigens.20 Given their importance in the immune system, considerable interest exists in modulating DC function and migration, for example in the context of cancer immunotherapy or vaccine design.21,22 DCs present in different organs are not a homogenous population but comprise functionally related cells that can be grouped into multiple subsets.23–25 In mice a common feature of all DCs is their expression CD45, CD11c and constitutive expression of MHC II.24 However, this definition is very broad and in certain tissues may lead to the false inclusion of other leukocytes, particularly from the macrophage lineage.25,26 In the skin, the tissue where DC migration into lymphatic vessels has been best studied, one commonly distinguishes between Langerhans cells (LCs) in the epidermis, and dermal DCs, which can be further subdivided into different subsets, based on their expression of markers like CD11b, langerin and CD103.24 Both LCs and dermal DCs migrate to dLNs in response to pathogen encounter or in response to an inflammatory stimulus, such as during contact sensitization. Interestingly, the kinetics of DC and LC migration greatly differ. Experiments in mice have shown that in response to contact sensitization the arrival of dermal DCs in dLNs peaked 1 day after skin sensitization, whereas most LCs only arrived in the LN several days later.27,28 The functional differences between LCs and the individual dermal DC subsets are only now starting to be unraveled.24,25
Since many new insights into DC migration to dLNs stem from fluorescent time-lapse-microscopy experiments, the transgenic mouse models and other strategies used for visualizing DCs and lymphatic vessels in tissues for imaging applications will be briefly introduced. In the subsequent sections, we will then describe and discuss what is currently known about the process and the cellular interactions occurring during DC migration towards, into and within lymphatic vessels.
Investigating Interactions between Dendritic Cells and Lymphatic Vessels by Time-Lapse Microscopy
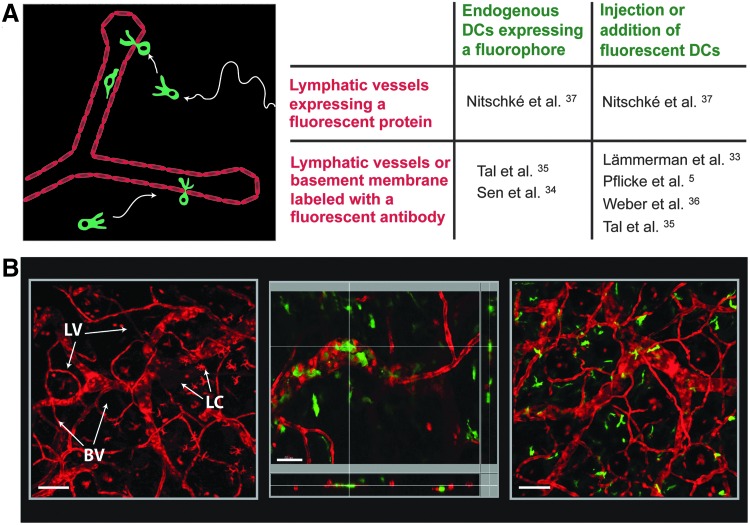
Over the last 10 years advances made in two-photon or confocal microscopy-based imaging have greatly expanded our knowledge about the dynamics of the immune response, and several excellent reviews on this topic exist.29–32 Recently, also the process of DC migration into or within lymphatic vessels has started to be further investigated at the cellular level by real-time imaging.5,33–37 Such studies have either been performed in dermal explants or in the skin of anesthetized mice. Both setups greatly depend on tools to simultaneously visualize lymphatic vessels and DCs in the tissue (Fig. 2A). Current approaches involve imaging endogenously fluorescent DCs in transgenic reporter mice or imaging in vitro-generated fluorescent DCs that are injected into the tissue. Similarly, lymphatic vessels can be visualized by direct labeling with injected fluorescent antibodies or by performing imaging in gene-targeted mice expressing a fluorescent protein in lymphatic vessels (Fig. 2A). In the following, these different approaches will be further described:
FIG. 2.
Visualizing lymphatic vessels (LVs) and dendritic cells (DCs) for time-lapse microscopy experiments. (A) Time-lapse microscopy experiments depend on tools that allow simultaneously visualizing DCs and lymphatic vessels (LVs), labeled in two different colors. The panel summarizes the different approaches that have been undertaken to visualize DCs and lymphatic endothelial cells (LECs) in tissues. These involve the use of gene-targeted reporter mice expressing a fluorescent protein in LECs or DCs, labeling LVs or the surrounding basement membrane with fluorescent antibodies in situ, or working with vitro-generated fluorescent DCs. The references indicate studies, in which these strategies have been applied. (B) Visualizing LVs and DCs in VE-cadherin-Cre x RFP mice.37 These mice express RFP in both blood and lymphatic vessels, as well as in a low percentage of leukocytes.37 Left image: Confocal image taken from an IVM experiment performed in the ear skin of a VE-cadherin-Cre x RFP mouse: LVs can be distinguished from blood vessels (BV) based on morphological differences: Lymphatic capillaries are much larger than capillary blood vessels and often contain characteristic blind ends. Also some epidermal Langerhans cells (LC) are visible. Scale bar: 100 μm. Middle and right image: Two different approaches were followed to incorporate fluorescent DCs into the skin of VE-cadherin-Cre x RFP mice. Middle image: LPS-matured BM-derived YFP+ DCs mice were injected into the ear skin. The panel shows a high-magnification confocal image with z-axis projections, confirming the intralymphatic location of a selected DC. Scale bar: 50 μm. Right image: Alternatively, BM chimeras were generated by reconstituting irradiated VE-cadherin-Cre x RFP mice with BM from CD11c-YFP mice. The panel shows a confocal image taken from an IVM experiment performed in the ear skin. Scale bar: 100 μm.
Visualization of dendritic cells
The most widely used tool for visualizing DCs during intravital microscopy (IVM) have been mice expressing yellow fluorescent protein (YFP) under the control of the CD11c promoter (CD11c-YFP mice).38 In fact, CD11c-YFP mice have been used in several IVM studies investigating interstitial DC migration or DC interactions with dermal lymphatic vessels.34,35,37,39 Also other transgenic mouse strains, e.g. mice expressing Cre recombinase or a fluorescent protein under the control of the CD11c,40 CX3CR141,42 or langerin27,43 promoters, have been successfully used to visualize DCs in IVM. The advantage of a genetic approach is that it allows to study endogenous DCs in the unperturbed tissue environment. However, given the existence of numerous DC subsets,23–25 it needs to be considered that not all DCs in a transgenic reporter mouse might express high levels of the fluorescent protein. Furthermore, in some tissues or mouse models, fluorescent protein expression might not be restricted to DCs but include cells from other lineages.25,26 A further complication might be the insufficient mobilization of endogenous fluorescent DCs into lymphatic vessels: In uninflamed skin only few DCs are typically found within lymphatic vessels. Although the number of DCs migrating into or within lymphatics can be increased by injection of a maturation-inducing stimulus like lipopolysaccharides (LPS),37 intralymphatic cell numbers per field of view typically remain low, what will complicate quantitative analysis. As an alternative to imaging endogenous DCs, time-lapse microscopy has also been performed using in vitro-generated, bone-marrow (BM)-derived DCs that are added to the tissue5,33,36 or injected into the tissue35,37 prior to imaging (Fig. 2A). In this case, DCs are either labeled with a fluorescent dye in vitro or are generated from transgenic BM (e.g. BM from CD11c-YFP mice). Since BM-derived DCs differ from endogenous tissue-resident DCs and need to be introduced into the tissue, this approach is less physiologic. However, a clear advantage of this method is that it allows working with in vitro-matured CCR7hi DCs, what will increase the likelihood of capturing numerous DCs migrating into or within lymphatic vessels during IVM.
Visualization of lymphatic vessels
Various gene-targeted mice expressing a Cre-recombinase or a fluorophore under an LEC-specific promoter have recently been generated,44–48 and some have already been applied to IVM.47–49 These genetic models undoubtedly represent promising tools, but they have not yet been used to investigate interactions between lymphatic vessels and DCs. Thus far, lymphatic vessels have mainly been visualized in situ by injection of fluorescently labeled anti-LYVE1 antibodies5,33–36 or fluorescent antibodies directed against components of the lymphatic basement membrane.5 On the other hand, our group has recently performed IVM in the ear skin of VE-cadherin-Cre x RFP mice,37 which feature Cre-mediated expression of a red-fluorescent protein (RFP)50 under the control of the VE-cadherin promoter51 in both LECs and BECs. Since lymphatic vessels and blood vessels in tissues can easily be distinguished based on morphologic differences, it is possible to directly use these mice for IVM-based visualization of lymphatic vessels (Fig. 2B). To incorporate DCs into VE-cadherin-Cre x RFP mice, BM-derived DCs from CD11c-YFP mice are injected into the ear skin, where imaging is performed. Alternatively, endogenous DCs can be imaged, by performing IVM in BM chimeras, generated by reconstituting irradiated VE-cadherin-Cre x RFP mice with the BM of CD11c-YFP mice (Fig. 2B).37
Dendritic Cell Migration Toward Afferent Lymphatic Vessels
As mentioned previously, DC maturation induces the upregulation of the CCR7 chemokine receptor in DCs.12,13 CCR7 expression is widely accepted as the most important determinant of DC migration to dLNs.12,17,52 The ligands of the CCR7 receptor are the chemokines CCL21 and CCL19. CCL21 comprises a C-terminal moiety of highly positively charged amino acids, which is responsible for the immobilization of CCL21 on heparan sulfates in the interstitium or on cell surfaces.53–56 By contrast, CCL19 lacks these positively charged C-terminal residues and therefore mainly remains soluble in tissues.54 Interestingly, it was recently shown that DCs that are in direct contact with surface-bound CCL21 can proteolytically cleave off the heparan sulfate-binding moiety, thereby generating a soluble form of CCL21.57 Surface-bound and soluble CCL21 are thought to cooperatively contribute to DC migration: While surface-bound CCL21 enhances cell migration by triggering integrin activation and cell adhesion, soluble CCL21 can form soluble gradients that contribute to DC chemotaxis.57 Lymphatic vessels are the main source of CCL21 in peripheral tissues like the skin (Fig. 1D). In analogy to CCR7-deficiency, antibody-mediated blockade of CCL21 was shown to reduce DC migration to dLNs.14 CCL19 does not appear to be expressed in LECs, but is produced by FRCs in LNs.19,58 Furthermore, CCL19 is expressed by activated DCs and has been suggested to serve as an autocrine sensing mechanism of interstitial flow in DCs.59 However, no reduction in DC migration to dLNs was recently observed in CCL19-deficient mice.60
Interestingly, CCL21 is encoded by two genes in the murine genome. The gene products, CCL21-Leu and CCL21-Ser, differ in only one amino acid in position 65, as well as in the anatomic location where they are expressed.61,62 CCL21-Ser is mainly expressed by high endothelial venules (HEVs) and FRCs in secondary lymphoid organs (SLOs) and helps guiding T cells and DCs into the T cell zone.19,63 By contrast, CCL21-Leu is mainly expressed by lymphatic vessels in peripheral tissues and therefore is the main attractant for DCs towards afferent lymphatics.61,62,64,65 An interesting naturally occurring mutant mouse strain are so-called plt (paucity of lymph node T cells) mice, which have a defect in the production of CCL19 and CCL21-Ser but retain expression of CCL21-Leu in peripheral lymphatics.18,61 Consequently, DCs in plt mice are still able to enter into dermal afferent lymphatics, but their entry and correct positioning in the dLN are impaired.18,19 Immunofluorescent analysis performed in ear skin whole mounts has detected large intracellular deposits of CCL21,35,36,66,67 (Fig. 1D) which appear to form part of the extended trans-Golgi-network in LECs.36 More recently, Weber et al. have achieved to stain extracellular CCL21.36 The authors of this study could demonstrate that CCL21 is immobilized in the interstitium on heparan sulfates, forming a gradient of CCL21 that surrounds dermal lymphatic vessels.36 Performing time-lapse microscopy experiments in murine ear explants this study revealed that DCs within a distance of 100 μm from a lymphatic vessel start to sense CCL21 and increase the velocity and the directedness of their migration towards the lymphatic vessel.36
Experiments performed in vitro and in vivo have revealed that inflammation upregulates CCL21 expression in LECs.66–68 This suggests that increased availability of CCL21 could contribute to the enhancement of DC migration that is typically observed in the context of inflammation.66,68 However, it is still unclear how inflammation affects the in vivo secretion of CCL21 in LECs and the formation of the extracellular CCL21 gradient.36 Besides CCL21, several other chemokines are strikingly upregulated in the context of inflammation.66,69,70 However, DC migration to dLNs in the context of tissue inflammation remains highly dependent on CCR7-signalling, indicating an only minor contribution of inflammation-induced chemokines or other LEC-expressed mediators.66 Nevertheless, besides the CCL21/CCR7 axis, at least two other pathways involved in DC migration to dLN have recently been identified. During tissue inflammation LECs upregulate the expression of CXCL12,66,71 which was shown to enhance the migration of CXCR4-expressing dermal DCs and LCs to dLNs.71 Similarly, the sphingosine-1-phosphate (S1P) system, which is indispensible for T cell egress from LNs into efferent lymphatics,72 appears to be important for DC migration into afferent lymphatics: LECs are thought to be the main source of the high S1P levels present in lymph,44 and maturing DCs have been shown to upregulate S1P receptors and to display chemotaxis towards S1P.73,74 Moreover, S1P receptor 1 (S1P1)-/- DCs as well as DCs in mice treated with the S1P analog FTY720 displayed reduced migration from skin to dLNs.73,74 Thus, although CCL21/CCR7 signaling is of key importance for DC migration to dLNs,17,52,66,68 other chemotactic signaling pathways also contribute, possibly by affecting distinct steps in the migratory process.
Dendritic Cell Migration into Afferent Lymphatic Vessels
Entry of DCs into afferent lymphatic vessels was long thought to be a rather passive process mainly driven by flow. However, recent studies demonstrating the involvement of adhesion molecules and of other LEC-expressed molecules in DC migration to dLNs clearly argue for a molecular regulation of the entry process.52,69,75–77 Whole mount experiments have revealed that the button-like cell junctions in initial lymphatic capillaries generate loose flaps of about 2–3 μm in diameter, through which leukocytes are thought to migrate into the lymphatic vessel lumen4,5 (Fig. 1C). Notably, leukocyte migration through the dense network of the interstitium or through blood vascular endothelium is highly dependent on actomyosin-mediated contraction of the cell body, in particular of the cell's bulky nucleus.78,79 Therefore, it is rather unlikely that lymphatic flaps, which present narrow openings, could be traversed passively, without active cellular contraction of the transmigrating leukocytes. Indeed, in vitro transwell chemotaxis assays performed with T lymphocytes have revealed that pharmacologic inhibition of the Rho-associated protein kinase (ROCK), which is important for actomyosin-mediated nuclear contraction, did not affect lymphocyte migration through large pores but significantly reduced transmigration through narrow pores of 3 μm in diameter.80 The latter finding suggests that also DC transmigration through lymphatic flaps requires active cell migration and actomyosin-mediated nuclear contraction.
In addition to the lymphatic endothelium, also the thin basement membrane surrounding lymphatic capillaries4,5,33 likely represents a barrier for leukocytes. The lymphatic basement membrane is composed of collagen IV, laminin, perlecan and nidogen and is highly fenestrated.5,33 It contains permissive sites, so-called portals, that likely need to be overcome by active cellular squeezing.5 Intriguingly, time-lapse microscopy experiments performed in murine skin explants have shown that the pores present in the basement membrane are physically widened up by the transmigrating DCs.5 After traversing the basement membrane DCs are in direct contact with the lymphatic endothelium. Here, time-lapse microscopy studies have revealed that DCs use the characteristic flaps for their entry into the lymphatic vessels.5 High-resolution imaging of this process in ear explants, in which lymphatics were visualized with fluorescent anti-LYVE-1 antibody, suggest that entry causes deformation of the oakleaf-shaped LECs and an inward bending of the flaps in the direction of the lymphatic vessel lumen, while leaving the button-like junctions intact5 (Fig. 1C). It is currently unclear, whether DCs also enter lymphatic vessels by migrating through the zipper-like junctions of lymphatic collectors. Of note, the basement membrane surrounding lymphatic collectors was shown to be much less fenestrated,5 indicating that access and transmigration into collectors might be more difficult. Interestingly, it was recently reported that in the context of chronic inflammation in the airways and ongoing lymphangiogenesis, the button-like junctions in lymphatic capillaries were replaced by zippers.81 Given that DC migration typically is enhanced in the context of inflammation, the latter finding suggests that migration into lymphatics may also occur across zipper-like junctions. However, it has thus far not been quantitatively investigated how inflammation-induced morphologic changes of the lymphatic network affect leukocyte transmigration.
Besides experiments performed in tissue explants, DC migration into lymphatic vessels has recently also been visualized by IVM performed in the ear skin or foot pad of anesthetized mice,34,35,37 Sen et al. observed that DCs probed the surface as well as the lumen of lymphatics for several minutes before taking approximately 20–30 minutes to transmigrate.34 Similarly, Tal et al.35 and our group37 reported that DCs interacted with the lymphatic vessels and transmigrated into the lumen within approximately 30–60 minutes. Interestingly, both latter studies observed that occasionally more than one DC sequentially transmigrated through the same location, likely representing the same portal and flap of the lymphatic capillary.5,35,37
Molecules involved in transmigration
A lot of our current knowledge about molecules involved in DC migration across lymphatic endothelium stems from in vitro cell culture experiments. In these experiments, LECs are typically grown to confluence on the upper side of transwells inserts, and DC migration towards a chemotactic stimulus deposited in the lower well is studied over time. Since tissue exit of DCs into lymphatics involves basolateral to luminal migration, some investigators have attempted to better mimic this process by growing LECs on the bottom of the transwell inserts.67,69,75,82 One potential limitation of both in vitro setups is the fact that LECs grown in monolayers form continuous zipper-like cell junctions.83,84 Monolayers therefore more resemble the architecture of lymphatic collectors rather than the button-like arrangement of cell junctions, which give rise to the characteristic flaps present in lymphatic capillaries. Nevertheless, in vitro transmigration assays have identified various molecules, such as the LEC-expressed adhesion molecules CD31,85 CD99,85 VCAM-1,69 ICAM-1,69 L1CAM,75 and CD137,86 that mediate in vitro DC transmigration across lymphatic endothelium, and most of these molecules were also shown to affect this process in vivo.69,75,76 Interestingly, besides LEC-expressed adhesion molecules, also transmural lymph flow was shown to enhance DC transmigration, likely by inducing the upregulation of CCL21 and ICAM-1 in LECs.82
One LEC-expressed adhesion molecule that has received particular interest with regards to its involvement in DC migration is ICAM-1. FITC painting and adoptive transfer experiments had revealed that in ICAM-1-/- mice DC and LC migration from skin to dLNs was reduced, suggesting an involvement of LEC-expressed ICAM-1 in this process.87 More recently, Johnson et al. reported that ICAM-1 and VCAM-1, are upregulated on lymphatic vessels in response to inflammation69 and that these molecules were involved in DC migration to dLNs.69 Since ICAM-1 is a ligand of the DC-expressed integrins Mac-1 (αMβ2) and LFA-1 (αLβ2),88 these data suggested that DC-expressed β2-integrins were involved in DC migration to dLNs. Surprisingly, experiments performed with pan-integrin knock-out DCs did not reveal any integrin-dependence of DC migration into lymphatic vessels or from skin to dLNs under steady-state conditions.33 It is important to note that in absence of inflammatory stimuli lymphatic vessels in the tissue—in contrast to in vitro-cultured LECs—express very low levels of ICAM-1 and VCAM-1.66 Therefore, the apparent discrepancy concerning the role of integrins in DC migration likely is explained by the inflammation-induced upregulation of integrin ligands in LECs, what might modulate the requirement for integrins in DC migration. In support of this explanation, blockade of the DC-expressed integrin LFA-1 was recently shown to reduce DC migration to dLNs from inflamed but not from uninflamed skin.89
Besides LEC-expressed adhesion molecules, also CCL21 has been implicated in the DC transmigration process. In vitro, CCL21 was shown to enhance in vitro DC transmigration across lymphatic endothelium.67 This process was inhibited by blockade of DC-expressed β2-integrins, suggesting that migration across lymphatic endothelium involved integrin-mediated adhesion. Interestingly, in vitro-cultured LECs display CCL21 on their cell surface via heparan sulfates,55,56 suggesting that LEC-bound CCL21 might indeed trigger integrin activation and adhesion in transmigrating DCs.57 However, as outlined above, LECs present in steady-state tissues express virtually no ICAM-1 or VCAM-1. Therefore, the exact role of CCL21, ICAM-1 and integrin activation in DC transmigration across lymphatic endothelium has not yet been conclusively addressed.
Besides adhesion molecules and CCL21 also other LEC-expressed molecules have recently been implicated in DC migration into lymphatic vessels. In particular, the LEC-expressed small transmembrane glycoprotein podoplanin was found to support DC migration to dLNs by interacting with the DC-expressed C-type lectin receptor CLEC-2.77 CLEC-2 deficiency impaired the entry to DCs into lymphatic vessels. The authors further showed that activation of CLEC-2 by binding to surface-expressed podoplanin induced the rearrangement of the actin cytoskeleton in DCs and promoted DC motility,77 Of note, CLEC-2 is also expressed on platelets, and interactions between platelet-derived CLEC-2 and podoplanin were recently shown to mediate blood vascular and lymphatic separation during embryonic development.90,91 Finally, another molecule shown to mediate DC migration to dLNs is the axonal guidance molecule semaphorin 3A (Sema3A),76 which is highly expressed in lymphatic vessels but not in blood vessels.92,93 Takamatsu et al. reported that DC migration to dLNs was significantly reduced in Sema3A-/- mice or in mice deficient in plexin-A1, which together with neuropilin-1 forms the Sema3A receptor on DCs.76 The authors further demonstrated that LEC-derived Sema3A promoted actomyosin-mediated cellular contraction in DCs, thereby enhancing DC transmigration across lymphatic endothelium. The observation that Sema3A-induced cellular contraction was required for DC migration into lymphatic vessels in vivo provides further evidence that the lymphatic vasculature indeed poses a physical barrier to immigrating leukocytes.
Dendtritic Cell Migration within Afferent Lymphatic Vessels
Until recently, it was commonly assumed that DCs and other leukocytes that had transmigrated into the lymphatic vessel lumen were passively transported by lymph flow.65,94,95 This assumption was supported by IVM performed in rat mesentery lymphatics, where freely flowing lymphocytes are rapidly propagated in a pulsatile fashion.96,97 Notably, the peak velocities of lymph flow in collecting mesenteric lymphatic vessels reach several mm/sec97–99—values that are comparable to the blood flow velocities through blood vascular capillaries and venules.100 By contrast the lymph flow velocities in lymphatic capillaries appear to be several orders of magnitude lower, reportedly ranging from 1–30 μm/s.101,102 These findings have raised doubts whether the hydrodynamic forces within lymphatic capillaries are sufficient to support passive transport of flowing leukocytes, as observed in blood vessels or in larger collecting lymphatics. In fact, recent IVM data from three different groups34,35,37 have revealed that most DCs actively migrated within initial lymphatic capillaries and appeared to be only passively propagated by lymph once they reached collecting vessels (Fig. 1B). Performing IVM in the ear skin of VE-cadherin-Cre x RFP mice (Fig. 2B) our group could identify a first molecule, namely ROCK, which is involved in intralymphatic DC migration.37 As previously mentioned, ROCK is required for actomyosin-mediated cellular contraction when leukocytes migrate through narrow pores, such as the interstitial space or across endothelium.78,79 In addition, ROCK was shown to support leukocyte migration by mediating the de-adhesion of integrins at the cellular rear from endothelial-expressed integrin ligands, for example during leukocyte crawling in blood vessels.80,103 Our IVM data have revealed that pharmacologic blockade of ROCK only had a very subtle effect on reducing intralymphatic crawling in steady-state. By contrast, in presence of tissue inflammation, ROCK blockade profoundly reduced the velocity of DC crawling within lymphatic capillaries, likely by inhibiting de-adhesion from inflammation-induced ICAM-1.37 Thus far, no other molecules involved in intralymphatic DC crawling have been identified. However, it is very likely that molecules previously shown to be critical for DC transmigration across lymphatic endothelium also support intralymphatic DC migration. Interesting candidates in this regard could be the previously mentioned Sema3A/plexin-A176 and podoplanin/CLEC-277 signaling pathways that were already shown to modulate DC motility.
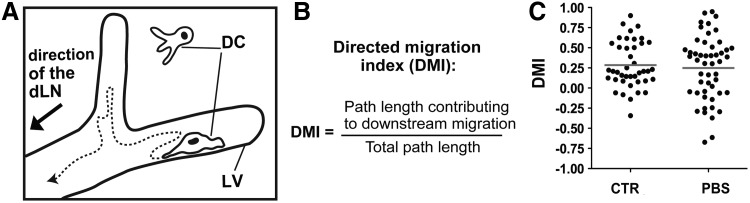
Our IVM experiments revealed that albeit DCs displayed a net forward movement in the direction the dLN, DCs frequently changed direction to migrate for some time against the presumed lymph flow (Fig. 3A). To determine the propensity of DCs to migrate in the downstream direction of the dLN, we calculated a “directed migration index” (DMI), by dividing the path length contributing to the DC's downstream migration by the entire path length the cell had migrated during a given time-interval (Fig. 3B).37 This analysis revealed that only approximately 30% of the total DC migratory activity accounted for DC movement in the presumed downstream direction of the dLN (Figs. 3B and 3C). IVM generally requires the immobilization of animals and anesthesia, both of which can reduce lymph flow.104,105 To investigate whether DCs displayed more migration in downstream direction under conditions of high lymph flow, imaging was also performed upon injection of PBS into the ear. Under these conditions, many cells started to migrate more directly within lymphatic vessels,37 as previously reported.35 However, in addition to more directed downstream-migration, also increased migration in upstream direction was observed, resulting in no difference in the net DMI, i.e., in the DCs' propensity to migrate in downstream direction of the dLN (Fig. 3C and37). Overall, these findings suggest that DC migration in afferent lymphatic vessels occurs in a semi-directed fashion, but the stimulus that determines downstream migration is still unclear.
FIG. 3.
Dendritic cells (DCs) actively migrate within lymphatic vessels (LVs) and display a pattern of semidirected migration. (A) Schematic representation of a DC migrating within a LV: The dotted line exemplifies the typical path of a DC migrating in semi direction towards the dlN. (B) To determine the propensity of DCs to migrate in the downstream direction of the presumed lymph flow, a “directed migration index” (DMI) was calculated. 0<DMI≤1: migration in direction of presumed lymph flow.−1≤DMI<0: migration in the opposite, upstream direction. (C) Pooled data from 10 different experiments revealed a DMI of 0.28 (“CTR”), indicating semi-directed DC migration in downstream direction. Notably, the DMI was not increased after experimentally elevating the lymph flow by injecting PBS into the ear tissue (“PBS”). Dots represent individual cells. Panel (C) was originally published in Blood.37 Differential requirement for ROCK in dendritic cell migration within lymphatic capillaries in steady-state and inflammation. Blood; 2012:120:2249–2258. © the American Society of Hematology.
Conclusion
New tools for visualizing the lymphatic vasculature and cutting-edge imaging technologies now make it possible to simultaneously visualize lymphatic vessels and DCs in living tissues and to study interactions between these two cell types in real time. This approach has provided valuable new insights into the process of DC migration towards, across and within lymphatic capillaries. One of the most recent findings made by IVM has been that intralymphatic DCs are not immediately drained by the lymph flow but crawl within lymphatic capillaries and hence continue to interact for long time with the lymphatic endothelium. This indicates that leukocyte migration within lymphatic vessels is more complex than previously anticipated and that similar steps as in the well-studied extravasation from blood vessels106,107 might be involved in this process, namely, crawling, rolling, free flow—albeit in reverse order. Furthermore, it remains to be investigated whether some DCs that have entered into lymphatic vessels might exit the vessel again before reaching the dLN. The finding that DCs migrate in a semi-directed fashion within lymphatic vessels opens up the intriguing possibility that active intralymphatic DC migration might have biologic effects that reach beyond the mere propagation of DCs to downstream vessel segments. For example, it is possible that LECs provide further maturation and differentiation signals to interacting and crawling DCs. In fact, it was recently shown that in the context of sterile tissue inflammation LECs can directly influence the maturation phenotype and function of DCs by a Mac-1/ICAM-1 dependent mechanism.108 Moreover, DCs crawling within lymphatic vessels might confer survival and differentiation signals to the lymphatic endothelium and help to maintain the characteristic phenotype and function of afferent lymphatic vessels. Interestingly, it was recently reported that DCs that have migrated to dLNs exert a similar effect on HEVs.109,110 Furthermore, the observation that DCs crawl within lymphatic vessels raises the possibility that many molecules that were previously thought to only mediate DC transmigration across lymphatic endothelium are in fact also involved in intralymphatic DC migration. Clearly, further research is warranted to better understand the molecular control and biologic relevance of DC interactions with lymphatic endothelium.
Conflict of Interest Disclosures:
The authors declare no competing financial interests.
Dr. Halin received financial support from the Swiss National Science Foundation (grant 310030_138330), ETH Zurich (grant 0-20566-09) and the Novartis Foundation of Medical and Biologic Research (Switzerland).
References
- 1.Tammela T. Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 2.Schulte-Merker S. Sabine A. Petrova T. V. Lymphatic vascular morphogenesis in development, physiology, and disease. The Journal of Cell Biology. 2011;193:607–618. doi: 10.1083/jcb.201012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makinen T. Adams R. H. Bailey J. Lu Q. Ziemiecki A. Alitalo K. Klein R. Wilkinson G. A. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes & Development. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baluk P. Fuxe J. Hashizume H. Romano T. Lashnits E. Butz S. Vestweber D. Corada M. Molendini C. Dejana E. McDonald D. M. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pflicke H. Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J Exp Med. 2009;206:2925–2935. doi: 10.1084/jem.20091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bujdoso R. Hopkins J. Dutia B. M. Young P. McConnell I. Characterization of sheep afferent lymph dendritic cells and their role in antigen carriage. J. Exp. Med. 1989;170:1285–1302. doi: 10.1084/jem.170.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young A. J. The physiology of lymphocyte migration through the single lymph node in vivo. Seminars in Immunology. 1999;11:73–83. doi: 10.1006/smim.1999.0163. [DOI] [PubMed] [Google Scholar]
- 8.Banchereau J. Steinman R. M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 9.Ueno H. Klechevsky E. Morita R. Aspord C. Cao T. Matsui T. Di Pucchio T. Connolly J. Fay J. W. Pascual V. Palucka A. K. Banchereau J. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–142. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 10.Steinman R. M. Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sallusto F. Cella M. Danieli C. Lanzavecchia A. Dendritic cells use macropinocytosis, the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sallusto F. Schaerli P. Loetscher P. Schaniel C. Lenig D. Mackay C. R. Qin S. Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 1998;28:2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 13.Sozzani S. Allavena P. D'Amico G. Luini W. Bianchi G. Kataura M. Imai T. Yoshie O. Bonecchi R. Mantovani A. Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. J Immunol. 1998;161:1083–1086. [PubMed] [Google Scholar]
- 14.Saeki H. Moore A. M. Brown M. J. Hwang S. T. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J. Immunol. 1999;162:2472–2475. [PubMed] [Google Scholar]
- 15.Mempel T. R. Henrickson S. E. von Andrian U. H. T cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 16.Braun A. Worbs T. Moschovakis G. L. Halle S. Hoffmann K. Bolter J. Munk A. Forster R. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat Immunol. 2011;12:879–887. doi: 10.1038/ni.2085. [DOI] [PubMed] [Google Scholar]
- 17.Forster R. Schubel A. Breitfeld D. Kremmer E. Renner-Muller I. Wolf E. Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 18.Gunn M. D. Kyuwa S. Tam C. Kakiuchi T. Matsuzawa A. Williams L. T. Nakano H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 1999;189:451–460. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luther S. A. Tang H. L. Hyman P. L. Farr A. G. Cyster J. G. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12694–12699. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maldonado R. A. von Andrian U. H. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palucka K. Banchereau J. Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity. 2010;33:464–478. doi: 10.1016/j.immuni.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palucka K. Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashimoto D. Miller J. Merad M. Dendritic cell, macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helft J. Ginhoux F. Bogunovic M. Merad M. Origin, functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol Rev. 2010;234:55–75. doi: 10.1111/j.0105-2896.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 25.Satpathy A. T. Wu X. Albring J. C. Murphy K. M. Re(de)fining the dendritic cell lineage. Nat Immunol. 2012;13:1145–1154. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hume D. A. Macrophages as APC and the dendritic cell myth. Journal of Immunology. 2008;181:5829–5835. doi: 10.4049/jimmunol.181.9.5829. [DOI] [PubMed] [Google Scholar]
- 27.Kissenpfennig A. Henri S. Dubois B. Laplace-Builhe C. Perrin P. Romani N. Tripp C. H. Douillard P. Leserman L. Kaiserlian D. Saeland S. Davoust J. Malissen B. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Shklovskaya E. Roediger B. Fazekas de St Groth B. Epidermal, dermal dendritic cells display differential activation, migratory behavior while sharing the ability to stimulate CD4+ T cell proliferation in vivo. J Immunol. 2008;181:418–430. doi: 10.4049/jimmunol.181.1.418. [DOI] [PubMed] [Google Scholar]
- 29.Cavanagh L. L. Weninger W. Dendritic cell behaviour in vivo: lessons learned from intravital two-photon microscopy. Immunol Cell Biol. 2008;86:428–438. doi: 10.1038/icb.2008.25. [DOI] [PubMed] [Google Scholar]
- 30.Bousso P. Moreau H. D. Functional immunoimaging: the revolution continues. Nat Rev Immunol. 2012;12:858–864. doi: 10.1038/nri3342. [DOI] [PubMed] [Google Scholar]
- 31.Li J. L. Goh C. C. Keeble J. L. Qin J. S. Roediger B. Jain R. Wang Y. Chew W. K. Weninger W. Ng L. G. Intravital multiphoton imaging of immune responses in the mouse ear skin. Nat Protoc. 2012;7:221–234. doi: 10.1038/nprot.2011.438. [DOI] [PubMed] [Google Scholar]
- 32.Germain R. N. Robey E. A. Cahalan M. D. A decade of imaging cellular motility and interaction dynamics in the immune system. Science. 2012;336:1676–1681. doi: 10.1126/science.1221063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lammermann T. Bader B. L. Monkley S. J. Worbs T. Wedlich-Soldner R. Hirsch K. Keller M. Forster R. Critchley D. R. Fassler R. Sixt M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 34.Sen D. Forrest L. Kepler T. B. Parker I. Cahalan M. D. Selective and site-specific mobilization of dermal dendritic cells and Langerhans cells by Th1- and Th2-polarizing adjuvants. Proc Natl Acad Sci U S A. 2010;107:8334–8339. doi: 10.1073/pnas.0912817107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tal O. Lim H. Y. Gurevich I. Milo I. Shipony Z. Ng L. G. Angeli V. Shakhar G. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J Exp Med. 2011;208:2141–2153. doi: 10.1084/jem.20102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber M. Hauschild R. Schwarz J. Moussion C. de Vries I. Legler D. F. Luther S. A. Bollenbach T. Sixt M. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339:328–332. doi: 10.1126/science.1228456. [DOI] [PubMed] [Google Scholar]
- 37.Nitschke M. Aebischer D. Abadier M. Haener S. Lucic M. Vigl B. Luche H. Fehling H. J. Biehlmaier O. Lyck R. Halin C. Differential requirement for ROCK in dendritic cell migration within lymphatic capillaries in steady-state and inflammation. Blood. 2012;120:2249–2258. doi: 10.1182/blood-2012-03-417923. [DOI] [PubMed] [Google Scholar]
- 38.Lindquist R. L. Shakhar G. Dudziak D. Wardemann H. Eisenreich T. Dustin M. L. Nussenzweig M. C. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 39.Ng L. G. Hsu A. Mandell M. A. Roediger B. Hoeller C. Mrass P. Iparraguirre A. Cavanagh L. L. Triccas J. A. Beverley S. M. Scott P. Weninger W. Migratory dermal dendritic cells act as rapid sensors of protozoan parasites. PLoS Pathog. 2008;4:e1000222. doi: 10.1371/journal.ppat.1000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chieppa M. Rescigno M. Huang A. Y. Germain R. N. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller A. J. Kaiser P. Dittmar K. E. Weber T. C. Haueter S. Endt K. Songhet P. Zellweger C. Kremer M. Fehling H. J. Hardt W. D. Salmonella gut invasion involves TTSS-2-dependent epithelial traversal, basolateral exit, and uptake by epithelium-sampling lamina propria phagocytes. Cell Host Microbe. 2012;11:19–32. doi: 10.1016/j.chom.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 42.Xu C. Shen Y. Littman D. R. Dustin M. L. Velazquez P. Visualization of mucosal homeostasis via single- and multiphoton intravital fluorescence microscopy. J Leukoc Biol. 2012;92:413–419. doi: 10.1189/jlb.0711344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bachy V. Hervouet C. Becker P. D. Chorro L. Carlin L. M. Herath S. Papagatsias T. Barbaroux J. B. Oh S. J. Benlahrech A. Athanasopoulos T. Dickson G. Patterson S. Kwon S. Y. Geissmann F. Klavinskis L. S. Langerin negative dendritic cells promote potent CD8+ T-cell priming by skin delivery of live adenovirus vaccine microneedle arrays. Proc Natl Acad Sci U S A. 2013;10:3041–3046. doi: 10.1073/pnas.1214449110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pham T. H. Baluk P. Xu Y. Grigorova I. Bankovich A. J. Pappu R. Coughlin S. R. McDonald D. M. Schwab S. R. Cyster J. G. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med. 2009;207:17–27. doi: 10.1084/jem.20091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi I. Chung H. K. Ramu S. Lee H. N. Kim K. E. Lee S. Yoo J. Choi D. Lee Y. S. Aguilar B. Hong Y. K. Visualization of lymphatic vessels by Prox1-promoter directed GFP reporter in a bacterial artificial chromosome-based transgenic mouse. Blood. 2011;117:362–365. doi: 10.1182/blood-2010-07-298562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bazigou E. Lyons O. T. Smith A. Venn G. E. Cope C. Brown N. A. Makinen T. Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. J Clin Invest. 2011;121:2984–2992. doi: 10.1172/JCI58050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagerling R. Pollmann C. Kremer L. Andresen V. Kiefer F. Intravital two-photon microscopy of lymphatic vessel development, function using a transgenic Prox1 promoter-directed mOrange2 reporter mouse. Biochemical Society Transactions. 2011;39:1674–1681. doi: 10.1042/BST20110722. [DOI] [PubMed] [Google Scholar]
- 48.Truman L. A. Bentley K. L. Smith E. C. Massaro S. A. Gonzalez D. G. Haberman A. M. Hill M. Jones D. Min W. Krause D. S. Ruddle N. H. ProxTom lymphatic vessel reporter mice reveal Prox1 expression in the adrenal medulla, megakaryocytes, and platelets. Am J Pathol. 2012;180:1715–1725. doi: 10.1016/j.ajpath.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sabine A. Agalarov Y. Maby-El Hajjami H. Jaquet M. Hagerling R. Pollmann C. Bebber D. Pfenniger A. Miura N. Dormond O. Calmes J. M. Adams R. H. Makinen T. Kiefer F. Kwak B. R. Petrova T. V. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev Cell. 2012;22:430–445. doi: 10.1016/j.devcel.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 50.Luche H. Weber O. Nageswara Rao T. Blum C. Fehling H. J. Faithful activation of an extra-bright red fluorescent protein in “knock-in” Cre-reporter mice ideally suited for lineage tracing studies. Eur J Immunol. 2007;37:43–53. doi: 10.1002/eji.200636745. [DOI] [PubMed] [Google Scholar]
- 51.Alva J. A. Zovein A. C. Monvoisin A. Murphy T. Salazar A. Harvey N. L. Carmeliet P. Iruela-Arispe M. L. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–767. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 52.Ohl L. Mohaupt M. Czeloth N. Hintzen G. Kiafard Z. Zwirner J. Blankenstein T. Henning G. Forster R. CCR7 governs skin dendritic cell migration under inflammatory, steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 53.Hirose J. Kawashima H. Swope Willis M. Springer T. A. Hasegawa H. Yoshie O. Miyasaka M. Chondroitin sulfate B exerts its inhibitory effect on secondary lymphoid tissue chemokine (SLC) by binding to the C-terminus of SLC. Biochim Biophys Acta. 2002;1571:219–224. doi: 10.1016/s0304-4165(02)00232-5. [DOI] [PubMed] [Google Scholar]
- 54.de Paz J. L. Moseman E. A. Noti C. Polito L. von Andrian U. H. Seeberger P. H. Profiling heparin-chemokine interactions using synthetic tools. ACS Chem Biol. 2007;2:735–744. doi: 10.1021/cb700159m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin X. Truty J. Lawrence R. Johns S. C. Srinivasan R. S. Handel T. M. Fuster M. M. A critical role for lymphatic endothelial heparan sulfate in lymph node metastasis. Mol Cancer. 2010;9:316. doi: 10.1186/1476-4598-9-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bao X. Moseman E. A. Saito H. Petryniak B. Thiriot A. Hatakeyama S. Ito Y. Kawashima H. Yamaguchi Y. Lowe J. B. von Andrian U. H. Fukuda M. Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes. Immunity. 2010;33:817–829. doi: 10.1016/j.immuni.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schumann K. Lammermann T. Bruckner M. Legler D. F. Polleux J. Spatz J. P. Schuler G. Forster R. Lutz M. B. Sorokin L. Sixt M. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity. 2010;32:703–713. doi: 10.1016/j.immuni.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 58.Link A. Vogt T. K. Favre S. Britschgi M. R. Acha-Orbea H. Hinz B. Cyster J. G. Luther S. A. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 59.Shields J. D. Fleury M. E. Yong C. Tomei A. A. Randolph G. J. Swartz M. A. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11:526–538. doi: 10.1016/j.ccr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 60.Britschgi M. R. Favre S. Luther S. A. CCL21 is sufficient to mediate DC migration, maturation and function in the absence of CCL19. Eur J Immunol. 2010;40:1266–1271. doi: 10.1002/eji.200939921. [DOI] [PubMed] [Google Scholar]
- 61.Vassileva G. Soto H. Zlotnik A. Nakano H. Kakiuchi T. Hedrick J. A. Lira S. A. The reduced expression of 6Ckine in the plt mouse results from the deletion of one of two 6Ckine genes. J Exp Med. 1999;190:1183–1188. doi: 10.1084/jem.190.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen S. C. Vassileva G. Kinsley D. Holzmann S. Manfra D. Wiekowski M. T. Romani N. Lira S. A. Ectopic expression of the murine chemokines CCL21a and CCL21b induces the formation of lymph node-like structures in pancreas, but not skin, of transgenic mice. J Immunol. 2002;168:1001–1008. doi: 10.4049/jimmunol.168.3.1001. [DOI] [PubMed] [Google Scholar]
- 63.Bajenoff M. Glaichenhaus N. Germain R. N. Fibroblastic reticular cells guide T lymphocyte entry into and migration within the splenic T cell zone. Journal of Immunology. 2008;181:3947–3954. doi: 10.4049/jimmunol.181.6.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forster R. Braun A. Worbs T. Lymph node homing of T cells and dendritic cells via afferent lymphatics. Trends Immunol. 2012;33:271–280. doi: 10.1016/j.it.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 65.Randolph G. J.. Angeli V.. Swartz M. A.. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 66.Vigl B. Aebischer D. Nitschke M. Iolyeva M. Rothlin T. Antsiferova O. Halin C. Tissue inflammation modulates gene expression of lymphatic endothelial cells, dendritic cell migration in a stimulus-dependent manner. Blood. 2011;118:205–215. doi: 10.1182/blood-2010-12-326447. [DOI] [PubMed] [Google Scholar]
- 67.Johnson L. A. Jackson D. G. Inflammation-induced secretion of CCL21 in lymphatic endothelium is a key regulator of integrin-mediated dendritic cell transmigration. International Immunology. 2010;22:839–849. doi: 10.1093/intimm/dxq435. [DOI] [PubMed] [Google Scholar]
- 68.MartIn-Fontecha A. Sebastiani S. Hopken U. E. Uguccioni M. Lipp M. Lanzavecchia A. Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson L. A. Clasper S. Holt A. P. Lalor P. F. Baban D. Jackson D. G. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006;203:2763–2777. doi: 10.1084/jem.20051759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang S. Lee S. P. Kim K. E. Kim H. Z. Memet S. Koh G. Y. Toll-like receptor 4 in lymphatic endothelial cells contributes to LPS-induced lymphangiogenesis by chemotactic recruitment of macrophages. Blood. 2009;113:2605–2613. doi: 10.1182/blood-2008-07-166934. [DOI] [PubMed] [Google Scholar]
- 71.Kabashima K. Shiraishi N. Sugita K. Mori T. Onoue A. Kobayashi M. Sakabe J. Yoshiki R. Tamamura H. Fujii N. Inaba K. Tokura Y. CXCL12-CXCR4 engagement is required for migration of cutaneous dendritic cells. Am J Pathol. 2007;171:1249–1257. doi: 10.2353/ajpath.2007.070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cyster J. G. Schwab S. R. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol. 2012;30:69–94. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- 73.Czeloth N. Bernhardt G. Hofmann F. Genth H. Forster R. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J Immunol. 2005;175:2960–2967. doi: 10.4049/jimmunol.175.5.2960. [DOI] [PubMed] [Google Scholar]
- 74.Rathinasamy A. Czeloth N. Pabst O. Forster R. Bernhardt G. The origin, maturity of dendritic cells determine the pattern of sphingosine 1-phosphate receptors expressed, required for efficient migration. Journal of Immunology. 2010;185:4072–4081. doi: 10.4049/jimmunol.1000568. [DOI] [PubMed] [Google Scholar]
- 75.Maddaluno L. Verbrugge S. E. Martinoli C. Matteoli G. Chiavelli A. Zeng Y. Williams E. D. Rescigno M. Cavallaro U. The adhesion molecule L1 regulates transendothelial migration and trafficking of dendritic cells. J Exp Med. 2009;206:623–635. doi: 10.1084/jem.20081211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takamatsu H. Takegahara N. Nakagawa Y. Tomura M. Taniguchi M. Friedel R. H. Rayburn H. Tessier-Lavigne M. Yoshida Y. Okuno T. Mizui M. Kang S. Nojima S. Tsujimura T. Nakatsuji Y. Katayama I. Toyofuku T. Kikutani H. Kumanogoh A. Semaphorins guide the entry of dendritic cells into the lymphatics by activating myosin II. Nat Immunol. 2010;11:594–600. doi: 10.1038/ni.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Acton S. E. Astarita J. L. Malhotra D. Lukacs-Kornek V. Franz B. Hess P. R. Jakus Z. Kuligowski M. Fletcher A. L. Elpek K. G. Bellemare-Pelletier A. Sceats L. Reynoso E. D. Gonzalez S. F. Graham D. B. Chang J. Peters A. Woodruff M. Kim Y. A. Swat W. Morita T. Kuchroo V. Carroll M. C. Kahn M. L. Wucherpfennig K. W. Turley S. J. Podoplanin-Rich Stromal Networks Induce Dendritic Cell Motility via Activation of the C-type Lectin Receptor CLEC-2. Immunity. 2012;37:276–289. doi: 10.1016/j.immuni.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vicente-Manzanares M. Ma X. Adelstein R. S. Horwitz A. R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nourshargh S. Hordijk P. L. Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- 80.Soriano S. F. Hons M. Schumann K. Kumar V. Dennier T. J. Lyck R. Sixt M. Stein J. V. In vivo analysis of uropod function during physiological T cell trafficking. J Immunol. 2011;187:2356–2364. doi: 10.4049/jimmunol.1100935. [DOI] [PubMed] [Google Scholar]
- 81.Yao L. C. Baluk P. Feng J. McDonald D. M. Steroid-Resistant Lymphatic Remodeling in Chronically Inflamed Mouse Airways. Am J Pathol. 2010;176:1525–1541. doi: 10.2353/ajpath.2010.090909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miteva D. O. Rutkowski J. M. Dixon J. B. Kilarski W. Shields J. D. Swartz M. A. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ Res. 2010;106:920–931. doi: 10.1161/CIRCRESAHA.109.207274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dunworth W. P. Fritz-Six K. L. Caron K. M. Adrenomedullin stabilizes the lymphatic endothelial barrier in vitro and in vivo. Peptides. 2008;29:2243–2249. doi: 10.1016/j.peptides.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kriehuber E. Breiteneder-Geleff S. Groeger M. Soleiman A. Schoppmann S. F. Stingl G. Kerjaschki D. Maurer D. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J Exp Med. 2001;194:797–808. doi: 10.1084/jem.194.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Torzicky M. Viznerova P. Richter S. Strobl H. Scheinecker C. Foedinger D. Riedl E. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31), CD99 are critical in lymphatic transmigration of human dendritic cells. J Invest Dermatol. 2012;132:1149–1157. doi: 10.1038/jid.2011.420. [DOI] [PubMed] [Google Scholar]
- 86.Teijeira A. Palazon A. Garasa S. Marre D. Auba C. Rogel A. Murillo O. Martinez-Forero I. Lang F. Melero I. Rouzaut A. CD137 on inflamed lymphatic endothelial cells enhances CCL21-guided migration of dendritic cells. FASEB J. 2012;26:3380–3392. doi: 10.1096/fj.11-201061. [DOI] [PubMed] [Google Scholar]
- 87.Xu H. Guan H. Zu G. Bullard D. Hanson J. Slater M. Elmets C. A. The role of ICAM-1 molecule in the migration of Langerhans cells in the skin and regional lymph node. Eur. J.Immunol. 2001;31:3085–3093. doi: 10.1002/1521-4141(2001010)31:10<3085::AID-IMMU3085>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hynes R. O. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 89.Teijeira A. Garasa S. Pelaez R. Azpilikueta A. Ochoa C. Marre D. Rodrigues M. Alfaro C. Auba C. Valitutti S. Melero I. Rouzaut A. Lymphatic endothelium forms Integrin-engaging 3D structures during DC transit across inflamed lymphatic vessels. J Invest Dermatol. 2013;133:2276–2285. doi: 10.1038/jid.2013.152. [DOI] [PubMed] [Google Scholar]
- 90.Bertozzi C. C. Schmaier A. A. Mericko P. Hess P. R. Zou Z. Chen M. Chen C. Y. Xu B. Lu M. M. Zhou D. Sebzda E. Santore M. T. Merianos D. J. Stadtfeld M. Flake A. W. Graf T. Skoda R. Maltzman J. S. Koretzky G. A. Kahn M. L. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010;116:661–670. doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Uhrin P. Zaujec J. Breuss J. M. Olcaydu D. Chrenek P. Stockinger H. Fuertbauer E. Moser M. Haiko P. Fassler R. Alitalo K. Binder B. R. Kerjaschki D. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 2010;115:3997–4005. doi: 10.1182/blood-2009-04-216069. [DOI] [PubMed] [Google Scholar]
- 92.Bouvree K. Brunet I. Del Toro R. Gordon E. Prahst C. Cristofaro B. Mathivet T. Xu Y. Soueid J. Fortuna V. Miura N. Aigrot M. S. Maden C. H. Ruhrberg C. Thomas J. L. Eichmann A. Semaphorin3A, Neuropilin-1, and PlexinA1 are required for lymphatic valve formation. Circ Res. 2012;111:437–445. doi: 10.1161/CIRCRESAHA.112.269316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jurisic G. Maby-El Hajjami H. Karaman S. Ochsenbein A. M. Alitalo A. Siddiqui S. S. Ochoa Pereira C. Petrova T. V. Detmar M. An unexpected role of semaphorin3a-neuropilin-1 signaling in lymphatic vessel maturation and valve formation. Circ Res. 2012;111:426–436. doi: 10.1161/CIRCRESAHA.112.269399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alvarez D. Vollmann E. H. von Andrian U. H. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martin-Fontecha A. Lanzavecchia A. Sallusto F. Dendritic cell migration to peripheral lymph nodes. Handb Exp Pharmacol. 2009:31–49. doi: 10.1007/978-3-540-71029-5_2. [DOI] [PubMed] [Google Scholar]
- 96.Galanzha E. I. Tuchin V. V. Zharov V. P. In vivo integrated flow image cytometry and lymph/blood vessels dynamic microscopy. J Biomed Opt. 2005;10:054018. doi: 10.1117/1.2060567. [DOI] [PubMed] [Google Scholar]
- 97.Dixon J. B. Zawieja D. C. Gashev A. A. Cote G. L. Measuring microlymphatic flow using fast video microscopy. J Biomed Opt. 2005;10:064016. doi: 10.1117/1.2135791. [DOI] [PubMed] [Google Scholar]
- 98.Dixon J. B. Greiner S. T. Gashev A. A. Cote G. L. Moore J. E. Zawieja D. C. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation. 2006;13:597–610. doi: 10.1080/10739680600893909. [DOI] [PubMed] [Google Scholar]
- 99.Akl T. J. Nagai T. Cote G. L. Gashev A. A. Mesenteric lymph flow in adult and aged rats. Am J Physiol Heart Circ Physiol. 2011;301:H1828–1840. doi: 10.1152/ajpheart.00538.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Popel A. S. Johnson P. C. Microcirculation and Hemorheology. Annu Rev Fluid Mech. 2005;37:43–69. doi: 10.1146/annurev.fluid.37.042604.133933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berk D. A. Swartz M. A. Leu A. J. Jain R. K. Transport in lymphatic capillaries. II. Microscopic velocity measurement with fluorescence photobleaching. Am J Physiol. 1996;270:H330–337. doi: 10.1152/ajpheart.1996.270.1.H330. [DOI] [PubMed] [Google Scholar]
- 102.Swartz M. A. Berk D. A. Jain R. K. Transport in lymphatic capillaries. I. Macroscopic measurements using residence time distribution theory. Am J Physiol. 1996;270:H324–329. doi: 10.1152/ajpheart.1996.270.1.H324. [DOI] [PubMed] [Google Scholar]
- 103.Smith A. Bracke M. Leitinger B. Porter J. C. Hogg N. LFA-1-induced T cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. J Cell Sci. 2003;116:3123–3133. doi: 10.1242/jcs.00606. [DOI] [PubMed] [Google Scholar]
- 104.Quin J. W. Shannon A. D. The effect of anaesthesia and surgery on lymph flow, protein and leucocyte concentration in lymph of the sheep. Lymphology. 1975;8:126–135. [PubMed] [Google Scholar]
- 105.Schmid-Schonbein G. W. Microlymphatics and lymph flow. Physiol Rev. 1990;70:987–1028. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- 106.von Andrian U. H. Mackay C. R. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 107.Luster A. D. Alon R. von Andrian U. H. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 108.Podgrabinska S. Kamalu O. Mayer L. Shimaoka M. Snoeck H. Randolph G. J. Skobe M. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J Immunol. 2009;183:1767–1779. doi: 10.4049/jimmunol.0802167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moussion C. Girard J. P. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature. 2011;479:542–546. doi: 10.1038/nature10540. [DOI] [PubMed] [Google Scholar]
- 110.Girard J. P. Moussion C. Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12:762–773. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]