Abstract
Aims: The aim of this study was to perform combined analyses of six genetic variants for the risk of hypertension. Methods: After a comprehensive literature search for genetic variants involved with the association study of hypertension, we harvested a total of five genes (six variants) for the current meta-analyses. These genes consisted of CYP4A11 (T8590C), RGS2 (1891-1892del TC and G638A), HTR2A (T102C), GNAS (T393C), and HSD3B1 (T→C Leu338). Results: A total of 20 studies among 13,816 cases and 19,248 controls were retrieved for the meta-analyses of six genetic variants. It was shown that the RGS2 1891-1892del TC (OR=1.10, 95% CI=1.02–1.19, p=0.02) polymorphism and the CYP4A11 T8590C (OR=1.19, 95% CI=1.00–1.41, p=0.05) polymorphism were significantly associated with increased risk of hypertension. No association was found between the other four variants and the risk of hypertension. Conclusion: This meta-analysis revealed that the RGS2 1891-1892del TC polymorphism and CYP4A11 T8590C polymorphism were associated with hypertension risk. However, HSD3B1 T→C Leu338, HTR2A T102C, GNAS T393C, and RGS2 G638A polymorphisms were not associated with hypertension risk.
Introduction
Hypertension has been identified as the leading risk factor for mortality and is ranked third among the causes of disability-adjusted life years (Ezzati et al., 2002; Chobanian et al., 2003). In 2000, an estimated 26.4% (972 million) of the world's adult population was recorded as hypertensive. This number is projected to increase by 60% to a total of 1.56 billion by 2025 (Kearney et al., 2005). It is now recognized that small vessel disease plays a role in the pathogenesis of hypertension (Rizzoni et al., 1994).
Hypertension is a complex disease influenced by genes, environmental factors, and their interactions (Harrison et al., 2008).Twin and family studies have suggested that approximately 20%–60% of blood pressure variation could be attributed to genetics (Kurtz and Spence, 1993). Up to now, many candidate genes have been reported to be implicated in the regulation of blood pressure and the susceptibility of hypertension (Halushka et al., 1999; Kohara et al., 2008; Sober et al., 2009). In addition, several genome-wide association studies also identified many susceptibility loci, which were associated with blood pressure and hypertension (Levy et al., 2009; Newton-Cheh et al., 2009).
HSD3B1 is exclusively expressed as 3β-HSD in the placenta and peripheral tissues, including in the mammary gland, prostate, and skin. HSD3B1 is the susceptibility gene for hypertension. In humans, a genetic variation in HSD3B1 can lead to an elevation in plasma aldosterone with a resultant increase in the intravascular volume and blood pressure (Rheaume et al., 1991; Simard et al., 1996). HTR2A encodes the 5HT2a receptor, which is expressed in the peripheral vasculature and has been implicated in hypertension due to its vasoconstrictive effect (Hoyer et al., 1994). GNAS encodes the Gαs subunit of the stimulatory G protein, which is expressed in a tissue-specific manner. The TT genotype of the 393T>C polymorphism of the GNAS1 gene, which is associated with higher Gαs expression levels in different tissues, for example, heart tissue, is also associated with an increased pulse rate during opiate withdrawal (Frey et al., 2005). CYP4A11 acts mainly as an enzyme that converts arachidonic acid (AA) to 20-hydroxyeicosatetraenoic acid (20-HETE), and has a crucial role in the modulation of cardiovascular homeostasis (Zordoky and El-Kadi, 2010). The relationship between the polymorphisms of the CYP4A11 gene and hypertension as well as cerebral infarction has been established (Muthalif et al., 1998; Gainer et al., 2005). RGS2 interacts with the G-subunits of heterotrimeric G proteins, accelerating the rate of GTP hydrolysis and finalizing the intracellular signaling. It may be the most potent GTPase-activating protein for Gqa mediating the action of a wide variety of key neurotransmitters and hormones that are active in the cardiovascular system (Ross and Wilkie, 2000; Tang et al., 2003). From a theoretical standpoint, genetic alterations of RGS2 may contribute to the development of hypertension in humans.
Associations between single-nucleotide polymorphisms (SNPs) of the above five genes and hypertension have been reported in different ethnic groups (Abe et al., 2002; Yamamoto et al., 2006; Fu et al., 2008; Shimodaira et al., 2010; Kamide et al., 2011). Since the allelic frequencies of genes often differ substantially in different ethnic groups, meta-analysis may help compare the genetic associations in different populations. In the present study, we evaluate the combined contribution of the SNPs in these genes to hypertension susceptibility in different populations using a meta-analysis approach.
Materials and Methods
Publication search and selection criteria
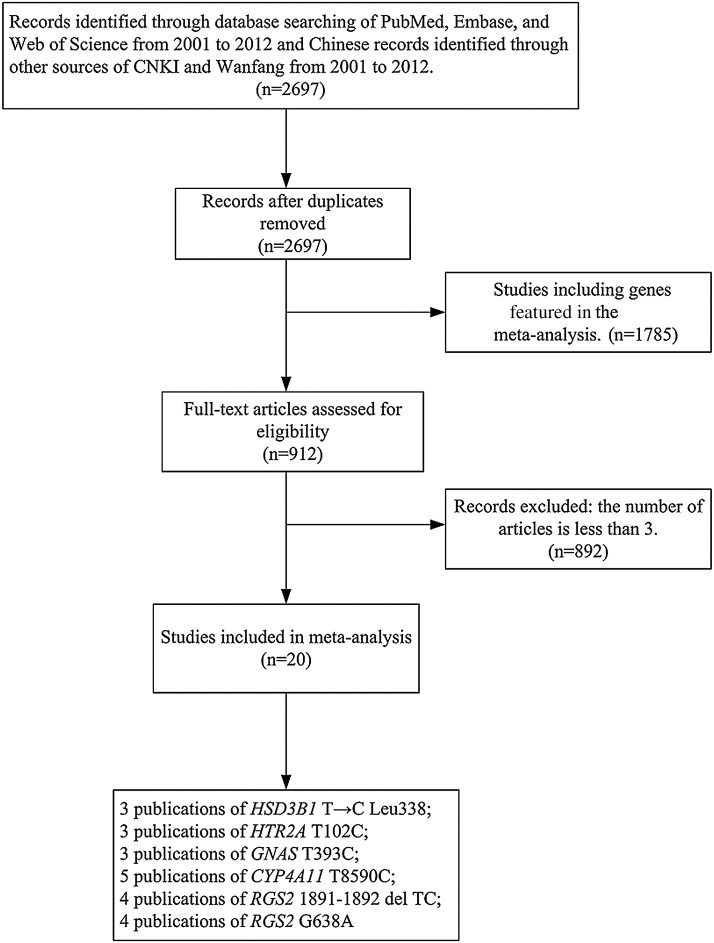
Articles were identified by a search of the following electronic databases: PubMed (www.ncbi.nlm.nih.gov/pubmed), Embase (www.elsevier.com/online-tools/embase), Web of Science (thomsonreuters.com/products_services/science/science_products/a-z/web_of_science), Wanfang data base (www.wanfangdata.com.cn), and China National Knowledge Infrastructure (CNKI; www.cnki.net). The search terms used the keywords and Medical Subject headings: “hypertension,” “polymorphism,” “allele,” “genotype,” and “SNP” published from 2001 to 2012. The publications eligible for the present meta-analyses should be case–control studies on hypertension, addressing the following polymorphisms: T8590C in CYP4A11, 1891-1892del TC and G638A in RGS2, T102C in HTR2A, T393C in GNAS, or T→C Leu338 in HSD3B1 gene. The flow diagram of our meta-analyses is shown in Figure 1.
FIG. 1.
Flow diagram of meta-analyses.
Data extraction
We carefully extracted information from the eligible articles. We read the articles and identified the SNPs that were analyzed in at least three articles and never had a meta-analysis concerning hypertension. The following data were extracted from each article: the PMID, the first author's name, year of publication, ethnic group, number of genotypes, genotyping, and total number of cases and controls.
Statistical analysis
All statistical analyses were performed using the REVMAN software (version 5.0; Cochrane Collaboration, Oxford, United Kingdom). In our meta-analysis, we used Cochran's Q and the inconsistency index (I2) statistic to measure heterogeneity (Lau et al., 1997). Substantial heterogeneity was defined when I2 is greater than or equal to 50%. The OR with 95% CI was used to estimate the strength of association between genetic variants and hypertension. The combined ORs and the corresponding 95% CIs were based on either the fixed-effect (I2<50%) or random-effect model (I2≥50%) (Mantel and Haenszel, 1959; DerSimonian and Laird, 1986; DerSimonian and Kacker, 2007). The Z-test was used to determine the pooled OR. A p<0.05 was considered as significant.
Results
As shown in Figure 1, our initial search for the genetic studies of hypertension retrieved 2697 articles from PubMed, Embase, Web of Science, CNKI, and Wanfang from 2001 to 2012. Among them, 1785 studies have addressed genes reported in previous meta-analyses and thus they were excluded in further analysis. A total of 892 articles were again filtered out because they did not meet the criteria that the chosen articles must accumulate at least three independent genotypic datasets for the same genetic variants so that this study would be statistically significant. At last, 20 case–control studies of 6 SNPs from 20 articles were included in the current meta-analyses (Table 1).
Table 1.
Characteristics of the Six Genetic Variants Associated with Hypertension
| Gene | SNP | Year | Author | Ethnic group | Genotype (case/control) | Allele (case/control) | |||
|---|---|---|---|---|---|---|---|---|---|
| HSD3B1 | T→C Leu338 | TT | TC | CC | T | C | |||
| 2002 | Rosmond et al. | Swedish | 4/26 | 20/68 | 15/21 | 28/120 | 50/110 | ||
| 2004 | Speirs et al. | Australian | 20/60 | 71/109 | 32/61 | 111/229 | 135/231 | ||
| 2010 | Shimodaira et al. | Japanese | 122/129 | 114/133 | 39/24 | 358/391 | 192/181 | ||
| HTR2A | T102C | CC | CT | TT | C | T | |||
| 2001 | Liolitsa et al. | British | 124/87 | 172/174 | 46/58 | 420/348 | 264/290 | ||
| 2004 | Yu et al. | Chinese | 34/31 | 68/67 | 96/66 | 136/129 | 260/199 | ||
| 2006 | Yamamoto et al. | Japanese | 298/467 | 517/899 | 287/500 | 1113/1833 | 1091/1899 | ||
| GNAS | T393C | TT | TC | CC | T | C | |||
| 2002 | Abe et al. | Japanese | 297/532 | 486/904 | 184/404 | 1080/1968 | 854/1712 | ||
| 2003 | Chen et al. | Japanese | 238/500 | 342/776 | 119/333 | 818/1776 | 580/1442 | ||
| 2004 | Yamamoto et al. | Japanese | 87/187 | 126/254 | 54/113 | 300/628 | 234/480 | ||
| CYP4A11 | T8590C | TT | CT | CC | T | C | |||
| 2005 | Gainer et al. | American | 126/152 | 64/41 | 5/4 | 316/345 | 74/49 | ||
| 2005 | Mayer et al. | German | 481/574 | 149/164 | 19/10 | 1111/1312 | 187/184 | ||
| 2006 | Mayer et al. | German | 152/250 | 68/79 | 8/3 | 372/579 | 84/85 | ||
| 2008 | Fu et al. | Japanese | 179/133 | 122/64 | 3/10 | 480/330 | 128/84 | ||
| 2008 | Sugimoto et al. | Japanese | 325/326 | 157/153 | 13/15 | 807/805 | 183/183 | ||
| RGS2 | 1891-1892del TC | II | ID | DD | I | D | |||
| 2005 | Yang et al. | Japanese | 257/420 | 391/513 | 123/169 | 905/1353 | 637/851 | ||
| 2008 | Zhao et al. | Chinese | 690/758 | 1229/1168 | 474/411 | 2609/2684 | 2177/1990 | ||
| 2010 | Li et al. | Xinjiang Kazak | 177/227 | 214/215 | 53/47 | 568/669 | 320/309 | ||
| 2011 | Kamide et al. | Japanese | 300/646 | 410/862 | 124/272 | 1010/2154 | 658/1404 | ||
| RGS2 | G638A | GG | GA | AA | G | A | |||
| 2005 | Yang et al. | Japanese | 174/220 | 389/554 | 208/328 | 737/994 | 805/1210 | ||
| 2008 | Kohara et al. | Japanese | 603/663 | 1520/1837 | 1104/1224 | 2726/3163 | 3728/4285 | ||
| 2009 | Ruixing et al. | Chinese | 46/165 | 118/446 | 93/254 | 210/776 | 304/954 | ||
| 2011 | Kamide et al. | Japanese | 174/369 | 413/890 | 243/519 | 761/1628 | 899/1928 | ||
SNP, single-nucleotide polymorphism.
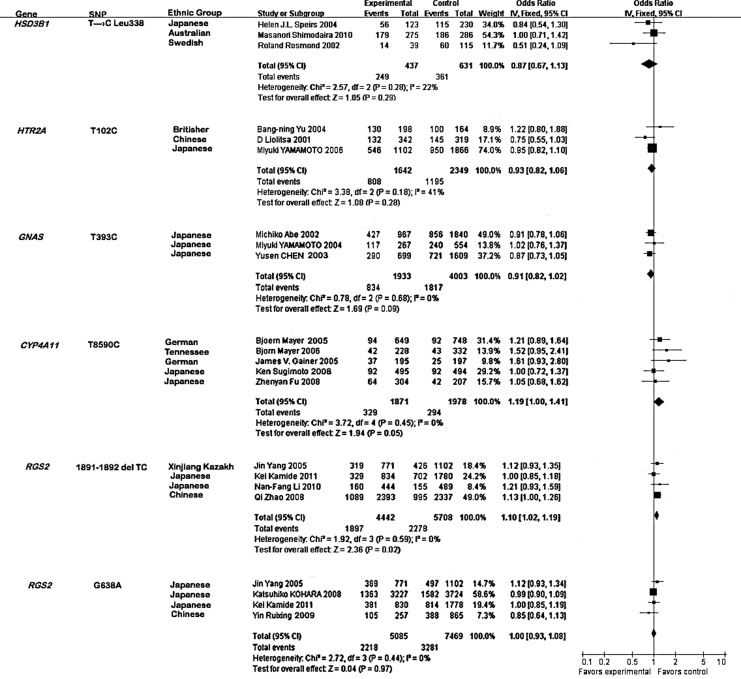
No evidence of statistical heterogeneity was observed for four SNPs (Fig. 2), including T→C Leu 338 of HSD3B1 (I2=22%), T102C of HTR2A (I2=41%), T393C of GNAS (I2=0%), and G638A of RGS2 (I2=0%). No visual bias was shown in the meta-analyses of these four SNPs (Fig. 3). Meta-analysis of GNAS T393C polymorphism included three studies (Abe et al., 2002; Chen et al., 2003; Yamamoto et al., 2004) among 1933 hypertension cases and 4003 controls. As shown in Figure 2, our result indicated that 1891-1892del TC of the RGS2 gene was significantly associated with hypertension risk in the Japanese, Chinese, and Xinjiang Kazakh populations (the overall OR=1.10, 95% CI=1.02–1.19, p=0.02). Meta-analysis of T8590C of CYP4A11 among 1871 hypertension cases and 1978 controls (Gainer et al., 2005; Mayer et al., 2005, 2006; Fu et al., 2008; Sugimoto et al., 2008) indicated that the CYP4A11 T8590C polymorphism was significantly associated with hypertension risk in the Tennessee, Augsburg, Japanese, and German populations (Fig. 2, the overall OR=1.19, 95% CI=1.00–1.41, p=0.05). For the other four SNPs, our meta-analyses were unable to find significant associations of them with hypertension.
FIG. 2.
Forest plot for the six polymorphisms associated with the risk of hypertension.
FIG. 3.
Funnel plot for the six genetic variants involved in the meta-analyses.
Discussion
The present meta-analyses encompassed 13,816 hypertension cases and 19,248 controls for the association analyses of five genes. The meta-analysis of RGS2 1891-1892del TC indicates a significant contribution of this polymorphism to the risk of hypertension. In addition, our results conclude that the CYP4A11 T8590C polymorphism is moderately associated with hypertension risk, which is in accordance with previous observations (Gainer et al., 2005; Mayer et al., 2005, 2006; Fu et al., 2008; Sugimoto et al., 2008). Under a moderate risk of hypertension (OR=1.2), power analysis showed that there was a lack of power for T→C Leu338 of HSD3B1 (51.3%). This may partly explain why we cannot observe a positive association for T→C Leu338 of the HSD3B1 gene.
Signaling by G-protein-coupled neurotransmitter receptors governs both blood pressure and electrolyte and fluid balance by the kidney (Takahashi and Smithies, 1999; Lifton et al., 2001). The regulator of G protein signaling (RGS) proteins is important in regulating signaling cascades initiated by G-protein-coupled receptors (GPCRs) activation (De Vries et al., 2000). RGS proteins facilitate the intrinsic inactivation of guanosine triphosphatase activity of G-protein α-subunits, and thereby serve as effector channel blockers. RGS2 is unique among the RGS proteins in its apparent selectivity toward Gpa, which mediates the action of mouse physiological vasoconstrictors, including norepinephrine, angiotensin II, endothelin-1, and thrombin. RGS2 also attenuates Gi- and Gs-mediated pathways (Ingi et al., 1998; Sinnarajah et al., 2001), suggesting that it can affect blood pressure via other physiologically important agonists such as serotonin, dopamine, and bradykinin.
The mechanisms by which the common SNP 1891-1892TC I/D of the RGS2 gene might contribute to hypertension are unknown. The 1891-1892TC I/D is located in an intronic region (Riddle et al., 2006). Although functions of introns are largely unknown, introns contain several short sequences important for efficient splicing by the spliceosome (Trapnell et al., 2009). Further investigation is critically needed to determine whether 1891-1892TC I/D could change RGS2 function. In addition, 1891-1892TC I/D may be a mere genetic marker and it may be in linkage disequilibrium with other functional polymorphisms that play important roles in the pathogenesis of hypertension. Therefore, a greater density of genotyping around the RGS2 or 1891-1892TC I/D site is needed.
CYP4A11 converts AA to 20-HETE, which acts in either a prohypertensive or antihypertensive manner depending on whether it is expressed at renovascular or tubular sites in the kidney, respectively. In the renal tubule, 20-HETE blocks sodium transport and acts primarily as a natriuretic, antihypertensive substance. In the renal vasculature, 20-HETE has vasoconstricting and prohypertensive effects (McGiff and Quilley, 2001; Roman, 2002). Both animal and human studies (Carroll and McGiff, 2000; McGiff and Quilley, 2001; Laffer et al., 2003a, 2003b) indicate that the CYP4A11 gene is a candidate gene of hypertension. A variant of the human CYP4A11 (T8590C) encodes for a monooxygenase with a reduced 20-HETE synthase activity. The association of the T8590C variant with hypertension supports its role as a polygenic determinant of blood pressure control in humans. The results obtained from the large population database further suggest that the relevance of the variant may vary according to hypertension comorbidity (Gainer et al., 2005).
CYP4A11 T8590C is a risk factor of hypertension in the Tennessee, Augsburg, Japanese, and German populations. However, the effects of the CYP4A11 T8590C polymorphism on the four ethnic groups are different, due to the hereditary, environmental, climatical, and dietetical diversities. The hypertension meta-analysis of the CYP4A11 T8590C polymorphism in the Japanese population shows OR of 1.05 (0.68, 1.62) and 1.00 (0.72, 1.37), suggesting that the risk in the Japanese population is relatively small, compared to the Augsburg population (OR=1.21, 95% CI=0.89–1.64), the Tennessee population (OR=1.52, 95% CI=0.95–2.41), and the German population (OR=1.61, 95% CI=0.93–2.80, Fig. 2).
There are some limitations in our study and the results should be interpreted with caution. First, such a result could be owing to a small number of studies that may lead to insufficient statistical power to find a slight effect or a fluctuated risk estimate. Second, the inconsistent conclusion of this analysis may be related to case selection, regional difference, diet and lifestyle of cases, genetic background, and other factors. Third, the positive results reported in the studies may lead to publication bias and heterogeneity in the results of different populations.
In conclusion, we identified significant associations between two SNPs (1891-1892del TC of the RGS2 gene and T8590C of the CYP4A11 gene) and hypertension. Meta-analysis among 10,150 samples confirmed that the RGS2 1891-1892del TC is a risk factor of hypertension in the Japanese, Chinese, and Xinjiang Kazakh populations. Another meta-analysis among 3849 samples suggested that CYP4A11 T8590C is a risk factor of hypertension in the Tennessee, Augsburg, Japanese, and German populations.
Acknowledgments
The research was supported by the grants from the National Natural Science Foundation of China (31100919), Natural Science Foundation of Zhejiang Province (LR13H020003), K.C. Wong Magna Fund in Ningbo University, and Ningbo social development research projects (2012C50032).
Author Disclosure Statement
No competing financial interests exist.
References
- Abe M, et al. Association of GNAS1 gene variant with hypertension depending on smoking status. Hypertension. 2002;40:261–265. doi: 10.1161/01.hyp.0000028490.77489.0c. [DOI] [PubMed] [Google Scholar]
- Carroll MA. McGiff JC. A new class of lipid mediators: cytochrome P450 arachidonate metabolites. Thorax. 2000;55(Suppl 2):S13–S16. doi: 10.1136/thorax.55.suppl_2.S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. Association of the GNAS1 gene variant with hypertension is dependent on alcohol consumption. Hypertens Res. 2003;26:439–444. doi: 10.1291/hypres.26.439. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- DerSimonian R. Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- DerSimonian R. Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- De Vries L, et al. The regulator of G protein signaling family. Annu Rev Pharmacol Toxicol. 2000;40:235–271. doi: 10.1146/annurev.pharmtox.40.1.235. [DOI] [PubMed] [Google Scholar]
- Ezzati M, et al. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- Frey UH, et al. The T393C polymorphism of the G alpha s gene (GNAS1) is a novel prognostic marker in bladder cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:871–877. doi: 10.1158/1055-9965.EPI-04-0720. [DOI] [PubMed] [Google Scholar]
- Fu Z, et al. A haplotype of the CYP4A11 gene associated with essential hypertension in Japanese men. J Hypertens. 2008;26:453–461. doi: 10.1097/HJH.0b013e3282f2f10c. [DOI] [PubMed] [Google Scholar]
- Gainer JV, et al. Functional variant of CYP4A11 20-hydroxyeicosatetraenoic acid synthase is associated with essential hypertension. Circulation. 2005;111:63–69. doi: 10.1161/01.CIR.0000151309.82473.59. [DOI] [PubMed] [Google Scholar]
- Halushka MK, et al. Patterns of single-nucleotide polymorphisms in candidate genes for blood-pressure homeostasis. Nat Genet. 1999;22:239–247. doi: 10.1038/10297. [DOI] [PubMed] [Google Scholar]
- Harrison M. Maresso K. Broeckel U. Genetic determinants of hypertension: an update. Curr Hypertens Rep. 2008;10:488–495. doi: 10.1007/s11906-008-0091-1. [DOI] [PubMed] [Google Scholar]
- Hoyer D, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Ingi T, et al. Dynamic regulation of RGS2 suggests a novel mechanism in G-protein signaling and neuronal plasticity. J Neurosci. 1998;18:7178–7188. doi: 10.1523/JNEUROSCI.18-18-07178.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamide K, et al. Association of intima-media thickening of carotid artery with genetic polymorphisms of the regulator of G-protein signaling 2 gene in patients with hypertension and in the general population. Hypertens Res. 2011;34:740–746. doi: 10.1038/hr.2011.25. [DOI] [PubMed] [Google Scholar]
- Kearney PM, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- Kohara K, et al. Identification of hypertension-susceptibility genes and pathways by a systemic multiple candidate gene approach: the millennium genome project for hypertension. Hypertens Res. 2008;31:203–212. doi: 10.1291/hypres.31.203. [DOI] [PubMed] [Google Scholar]
- Kurtz TW. Spence MA. Genetics of essential hypertension. Am J Med. 1993;94:77–84. doi: 10.1016/0002-9343(93)90124-8. [DOI] [PubMed] [Google Scholar]
- Laffer CL, et al. Differential regulation of natriuresis by 20-hydroxyeicosatetraenoic acid in human salt-sensitive versus salt-resistant hypertension. Circulation. 2003a;107:574–578. doi: 10.1161/01.cir.0000046269.52392.14. [DOI] [PubMed] [Google Scholar]
- Laffer CL, et al. 20-HETE and furosemide-induced natriuresis in salt-sensitive essential hypertension. Hypertension. 2003b;41(3 Pt 2):703–708. doi: 10.1161/01.HYP.0000051888.91497.47. [DOI] [PubMed] [Google Scholar]
- Lau J. Ioannidis JP. Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- Levy D, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li NF, et al. Association of genetic variations of regulator of G-protein signaling 2 with hypertension in the general Xinjiang Kazakh population. Clin Exp Hypertens. 2010;32:256–261. doi: 10.3109/10641960903265253. [DOI] [PubMed] [Google Scholar]
- Lifton RP. Gharavi AG. Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- Liolitsa D. Powell JF. Prince M, et al. Association study of the 5-HT(2A) receptor gene polymorphism, T102C and essential hypertension. J Hum Hypertens. 2001;15:335–339. doi: 10.1038/sj.jhh.1001177. [DOI] [PubMed] [Google Scholar]
- Mantel N. Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- Mayer B, et al. Association of a functional polymorphism in the CYP4A11 gene with systolic blood pressure in survivors of myocardial infarction. J Hypertens. 2006;24:1965–1970. doi: 10.1097/01.hjh.0000244944.34546.8e. [DOI] [PubMed] [Google Scholar]
- Mayer B, et al. Association of the T8590C polymorphism of CYP4A11 with hypertension in the MONICA Augsburg echocardiographic substudy. Hypertension. 2005;46:766–771. doi: 10.1161/01.HYP.0000182658.04299.15. [DOI] [PubMed] [Google Scholar]
- McGiff JC. Quilley J. 20-Hydroxyeicosatetraenoic acid and epoxyeicosatrienoic acids and blood pressure. Curr Opin Nephrol Hypertens. 2001;10:231–237. doi: 10.1097/00041552-200103000-00012. [DOI] [PubMed] [Google Scholar]
- Muthalif MM, et al. 20-Hydroxyeicosatetraenoic acid mediates calcium/calmodulin-dependent protein kinase II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1998;95:12701–12706. doi: 10.1073/pnas.95.21.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton-Cheh C, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheaume E, et al. Structure and expression of a new complementary DNA encoding the almost exclusive 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase in human adrenals and gonads. Mol Endocrinol. 1991;5:1147–1157. doi: 10.1210/mend-5-8-1147. [DOI] [PubMed] [Google Scholar]
- Riddle EL, et al. Polymorphisms and haplotypes of the regulator of G protein signaling-2 gene in normotensives and hypertensives. Hypertension. 2006;47:415–420. doi: 10.1161/01.HYP.0000200714.81990.61. [DOI] [PubMed] [Google Scholar]
- Rizzoni D, et al. Vascular structural and functional alterations before and after the development of hypertension in SHR. Am J Hypertens. 1994;7:193–200. doi: 10.1093/ajh/7.2.193. [DOI] [PubMed] [Google Scholar]
- Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Rosmond R. Chagnon M. Bouchard C, et al. Polymorphism in exon 4 of the human 3 beta-hydroxysteroid dehydrogenase type I gene (HSD3B1) and blood pressure. Biochem Biophys Res Commun. 2002;293:629–632. doi: 10.1016/S0006-291X(02)00234-6. [DOI] [PubMed] [Google Scholar]
- Ross EM. Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- Ruixing Y. Jinzhen W. Weixiong L, et al. The environmental and genetic evidence for the association of hyperlipidemia and hypertension. J Hypertens. 2009;27:251–258. doi: 10.1097/HJH.0b013e32831bc74d. [DOI] [PubMed] [Google Scholar]
- Shimodaira M, et al. Association of HSD3B1 and HSD3B2 gene polymorphisms with essential hypertension, aldosterone level, and left ventricular structure. Eur J Endocrinol. 2010;163:671–680. doi: 10.1530/EJE-10-0428. [DOI] [PubMed] [Google Scholar]
- Simard J, et al. Molecular biology and genetics of the 3 beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene family. J Endocrinol. 1996;150(Suppl):S189–S207. [PubMed] [Google Scholar]
- Sinnarajah S, et al. RGS2 regulates signal transduction in olfactory neurons by attenuating activation of adenylyl cyclase III. Nature. 2001;409:1051–1055. doi: 10.1038/35059104. [DOI] [PubMed] [Google Scholar]
- Sober S, et al. Targeting 160 candidate genes for blood pressure regulation with a genome-wide genotyping array. PLoS One. 2009;4:e6034. doi: 10.1371/journal.pone.0006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speirs HJ. Katyk K. Kumar NN, et al. Association of G-protein-coupled receptor kinase 4 haplotypes, but not HSD3B1 or PTP1B polymorphisms, with essential hypertension. J Hypertens. 2004;22:931–936. doi: 10.1097/00004872-200405000-00014. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, et al. A polymorphism regulates CYP4A11 transcriptional activity and is associated with hypertension in a Japanese population. Hypertension. 2008;52:1142–1148. doi: 10.1161/HYPERTENSIONAHA.108.114082. [DOI] [PubMed] [Google Scholar]
- Takahashi N. Smithies O. Gene targeting approaches to analyzing hypertension. J Am Soc Nephrol. 1999;10:1598–1605. doi: 10.1681/ASN.V1071598. [DOI] [PubMed] [Google Scholar]
- Tang KM, et al. Regulator of G-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure. Nat Med. 2003;9:1506–1512. doi: 10.1038/nm958. [DOI] [PubMed] [Google Scholar]
- Trapnell C. Pachter L. Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, et al. Association of a GNAS1 gene variant with hypertension and diabetes mellitus. Hypertens Res. 2004;27:919–924. doi: 10.1291/hypres.27.919. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, et al. Interaction between serotonin 2A receptor and endothelin-1 variants in association with hypertension in Japanese. Hypertens Res. 2006;29:227–232. doi: 10.1291/hypres.29.227. [DOI] [PubMed] [Google Scholar]
- Yang J, et al. Genetic variations of regulator of G-protein signaling 2 in hypertensive patients and in the general population. J Hypertens. 2005;23:1497–1505. doi: 10.1097/01.hjh.0000174606.41651.ae. [DOI] [PubMed] [Google Scholar]
- Yu BN. Wang A. Zhou G. T102C genetic polymorphism of the 5-HT2A receptor in Chinese hypertensive patients and healthy controls. Clin Exp Pharmacol Physiol. 31:847–849. doi: 10.1111/j.1440-1681.2004.04124.x. [DOI] [PubMed] [Google Scholar]
- Zhao Q, et al. Interactions among genetic variants from contractile pathway of vascular smooth muscle cell in essential hypertension susceptibility of Chinese Han population. Pharmacogenet Genomics. 2008;18:459–466. doi: 10.1097/FPC.0b013e3282f97fb2. [DOI] [PubMed] [Google Scholar]
- Zordoky BN. El-Kadi AO. Effect of cytochrome P450 polymorphism on arachidonic acid metabolism and their impact on cardiovascular diseases. Pharmacol Ther. 2010;125:446–463. doi: 10.1016/j.pharmthera.2009.12.002. [DOI] [PubMed] [Google Scholar]