Abstract
In lymphedema, there is a profound predisposition to infection with bacterial pathogens. It therefore seems appropriate to reconsider our unique functional definition of the lymphatic structures within a circulatory construct. While the lymphatics unquestionably fulfill a vital circulatory function, it seems more appropriate to view this complex network, comprised both of endothelial-lined vessels and of lymphoid tissue, as the nexus between the circulatory and immune systems. Viewed in this fashion, it becomes evident that the complex biology of regional lymphatic disruption is a manifestation of the interplay between these two vital bodily functions.
Experimental lymph stasis in murine model has been utilized to effectively demonstrate the pathological attributes of human lymphedema, namely, inflammation, fat deposition, and fibrosis. Large-scale transcriptional corroborates the role of inflammatory mechanisms. The murine studies have set the stage for subsequent translational investigation of human lymphedema. Many of the gene expression pathways invoked by lymphedema are relevant to the inflammatory response and have provided a pragmatic approach to the successful identification of potentially relevant circulating biomarkers for human lymphedema.
The lymphatic vascular conduit, despite its origin from the embryonic venous Anlage and the prominent role of endothelial cell biology in its structure and function, has eluded comprehension comparable to its arteriovenous counterparts.1–2 Among the functions of the lymphatics, a complex and nearly ubiquitous circulatory constituent, is its well-recognized responsibility for maintaining interstitial fluid homeostasis. Accordingly, a misconception that commonly arises is the assignment of the lymphatics to a functional role as a virtual ‘sewer system’ for the mammalian internal milieu.
Inarguably, the loss of regional lymphatic function is accompanied, at least initially, by an increase in the interstitial fluid volume, clinically described as edema;3 however, in lymphedema, the derangement that accompanies a loss of lymphatic vascular function, that which begins as a freely moveable, ‘pitting’ accumulation of tissue fluid (Fig. 1A) almost always progresses to a profound alteration in the structural biology and function of the integument (Fig. 1B).4 These changes are unique to the lymphatic-derived forms of lymphedema.
FIG. 1.
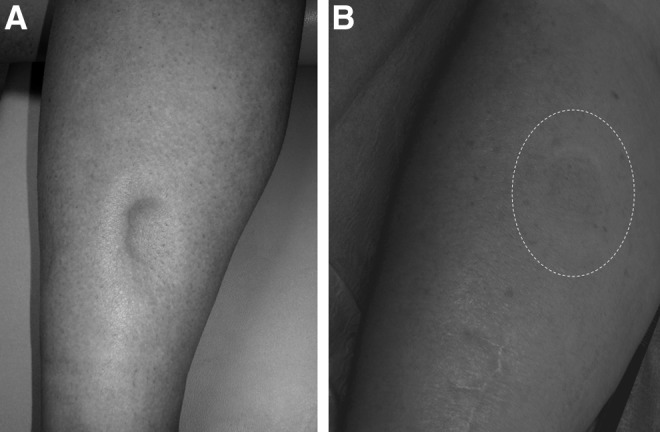
(A) Early in the course of lymphatic dysfunction, the accumulated, protein-rich interstitial fluid remains mobile and can be displaced by the examining finger (‘pitting edema’). (B) In chronic lymphedema, but not in the non-lymphatic forms of edema, structural changes in the tissues ultimately obliterate the ‘pitting’ quality of the edema. Here the examining finger can elicit no change in the external contour of the skin (dotted line).
The consequences of chronic lymph stasis are profound and, as yet, quite incompletely understood. In addition to the accumulation of protein-enriched interstitial fluid, one encounters an early and progressive predisposition to tissue fibrosis. There are local disturbances of microcirculatory flow within the integument, perhaps due to the demonstrable lymph stasis in the arterial wall. The epidermal and dermal components thicken substantially, based upon an increase in cellular number and overall mass, and there is a profound increase in the thickness of the subcutaneous adipose layer of the involved appendage.5 There are deleterious effects on the ligaments and tendons and, most importantly, there is a significant predisposition to recurrent soft-tissue infection (Fig. 2).
FIG. 2.
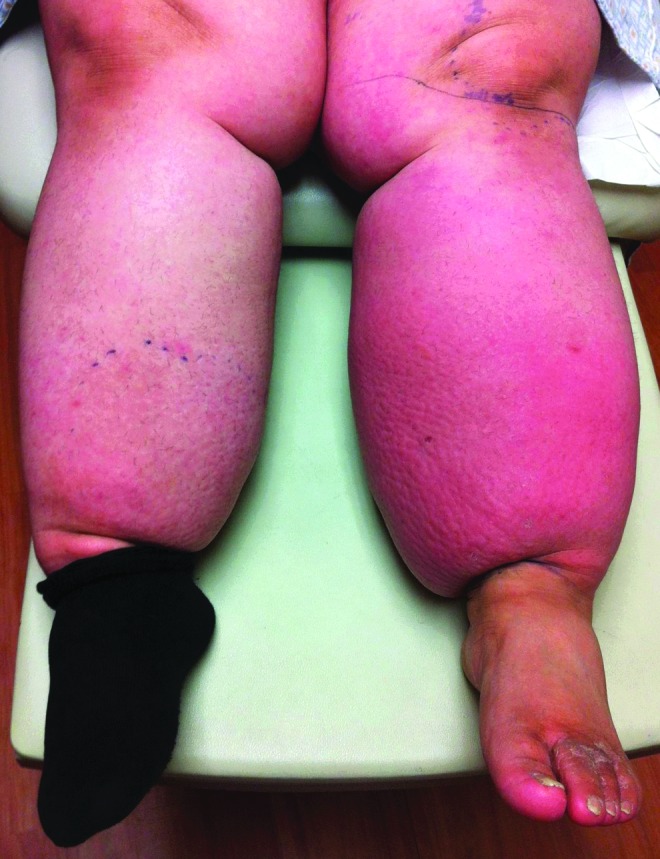
The prominent increase in capillary blood flow to the skin in the left leg (erythema) is a sign of soft-tissue infection complicating chronic lymphedema.
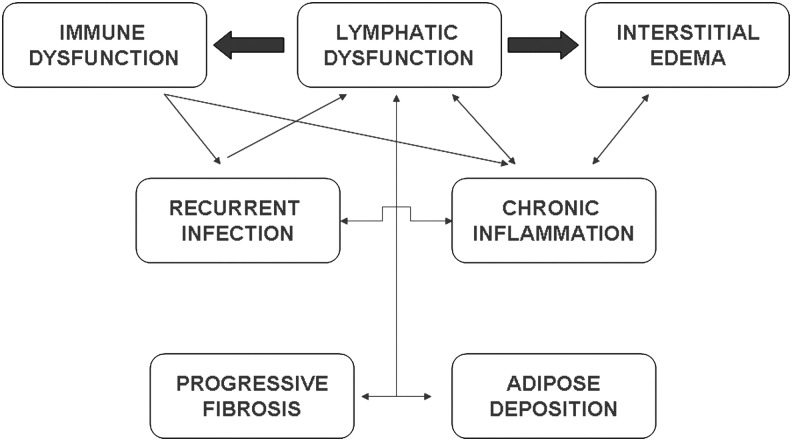
Given this profound predisposition of the lymphedematous limb to infection with bacterial pathogens, it seems appropriate to re-consider our unique functional definition of the lymphatic structures within a circulatory construct. While the lymphatics unquestionably fulfill a vital circulatory function, it seems more appropriate to view this complex network, comprised both of endothelial-lined vessels and of lymphoid tissue, as the nexus between the circulatory and immune systems. Viewed in this fashion, it becomes evident that the complex biology of regional lymphatic disruption is a manifestation of the interplay between these two vital bodily functions (Fig. 3).
FIG. 3.
Schematic depiction of the complex interplay of biological forces in the pathogenesis of chronic lymphedema.
Recent advances in the investigation of both experimental and human clinical lymphatic dysfunction underscore the central role of inflammation. In both murine experimental lymph stasis and in human post-lymphadenectomy lymphedema, there is a very early upregulation of endogenous danger signals in the soft tissues; it is conjectured that this early expression of endogenous danger signals contributes to the process of inflammatory lymphangiogenesis.6
Similarly, experimental lymph stasis in murine models have been utilized to effectively demonstrate the pathological attributes of human lymphedema, namely, inflammation, fat deposition, and fibrosis.7–9 It is becoming increasingly clear that these protean processes are interrelated in the pathogenesis of the tissue response to lymphatic dysfunction, but much remains to be explored.
Given the relevance of the murine tail lymphedema model to the human disease,10 we have previously used this model to explore the inflammatory manifestations of this form of experimental lymphatic insufficiency.11 Large-scale transcriptional profiling provided corroboration of these inflammatory mechanisms, when normal skin expression profiles were compared to those of the lymphedema counterparts: pathway analysis disclosed significant upregulation of a number of relevant biological pathways, including defense/immunity protein activity; complement activity; response to pathogen; immune response; response to stress, and humoral immune response, as well as those related to extracellular matrix and lipid metabolism and biosynthesis.
These murine studies have set the stage for subsequent translational investigation of the human disease of lymphedema, with the suggestion that further analysis of such pathway activation might lead to the identification of circulating biomarkers relevant to disease activation and progression.12
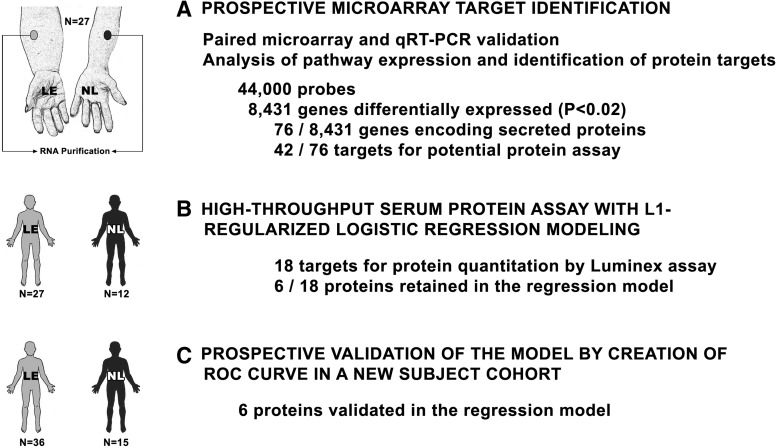
In the prior transcriptional profiling of the murine model, we identified a remarkably small number of specific pathways with altered expression in lymphedema. For the human investigation, we used a similar, microarray-based transcriptomic analysis of human skin for unbiased, a priori prospective candidate identification, and subsequently validated these candidates through direct serum assay of the identified protein products of upregulated genes (Fig. 4). Many of the gene expression pathways invoked by lymphedema are relevant to the inflammatory response (Fig. 5).
FIG. 4.
Trasncriptomic identification and validation of potential circulating biomarkers in human lymphatic vascular insufficiency. The final results were analyzed through plotting a receiver operating characteristic (ROC) curve. Reprinted with permission from (12).
FIG. 5.
Gene expression pathways invoked by human lymphedema.
For the identification of potentially relevant circulating biomarkers for human lymphedema we focused our attention on genes with corresponding secreted protein products and took these candidates forward to a protein multiplex assay applied to diseased and normal subjects. We developed a logistic regression-based model on an eventual group of six proteins and validated our system on a separate cohort of study subjects. With this approach, we succesfully developed an accurate bioassay utilizing proteins representing four central pathogenetic modalities of the disease: lymphangiogenesis (basic fibroblast growth factor [FGFb]); inflammation (interleukin-4, interleukin-10 and tissue necrosis factor [TNFb]), fibrosis (transforming growth factor beta [TGFβ]) and lipid metabolism (leptin), suggesting that these proteins are directly related to the pathogenesis of the tissue pathology accompanying the lymphatic vascular insufficiency. Further studies are warranted to determine whether this newly-identified biomarker panel will possess utility as an instrument for in vitro diagnosis of early and latent disease, with potential applicability to risk stratification, quantitation of disease burden, and response to therapy.
Markers of disease are likely to be important, since late activating events often precipitate progression from latent disease in humans. Thus, identification of relevant pathways opens the door to more extensive insights into the pathogenesis of disease and is likely to lead to enhanced diagnostic and therapeutic approaches, not only to lymphedema, but to the broad category of lymphatic function in health and disease. The results discussed here suggest that many of these findings are likely to be applicable to the question of the role of lymphatic function in the immune response to a variety of infectious agents.
References
- 1.Rockson SG. Lymphatic biology in health and disease: an NIH perspective. Lymphat Res Biol. 2009;7:181. doi: 10.1089/lrb.2009.7401. [DOI] [PubMed] [Google Scholar]
- 2.Rockson SG. Current concepts and future directions in the diagnosis and management of lymphatic vascular disease. Vasc Med. 2010;15:223–231. doi: 10.1177/1358863X10364553. [DOI] [PubMed] [Google Scholar]
- 3.Rockson SG. Causes and consequences of lymphatic disease. Ann N Y Acad Sci. 2010;1207(Suppl 1):E2–E6. doi: 10.1111/j.1749-6632.2010.05804.x. [DOI] [PubMed] [Google Scholar]
- 4.Rockson SG. The unique biology of lymphatic edema. Lymphat Res Biol. 2009;7:97–100. doi: 10.1089/lrb.2009.7202. [DOI] [PubMed] [Google Scholar]
- 5.Brorson H. Ohlin K. Olsson G. Nilsson M. Adipose tissue dominates chronic arm lymphedema following breast cancer: an analysis using volume rendered CT images. Lymphat Res Biol. 2006;4:199–210. doi: 10.1089/lrb.2006.4404. [DOI] [PubMed] [Google Scholar]
- 6.Zampell JC. Yan A. Avraham T. Andrade V. Malliaris S. Aschen S. Rockson SG. Mehrara BJ. Temporal and spatial patterns of endogenous danger signal expression after wound healing and in response to lymphedema. Am J Physiol Cell Physiol. 2011;300:C1107–C1121. doi: 10.1152/ajpcell.00378.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avraham T. Daluvoy S. Zampell J. Yan A. Haviv YS. Rockson SG. Mehrara BJ. Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair. Am J Pathol. 2010;177:3202–3214. doi: 10.2353/ajpath.2010.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zampell JC. Aschen S. Weitman ES. Yan A. Elhadad S. De Brot M. Mehrara BJ. Regulation of adipogenesis by lymphatic fluid stasis: part I. Adipogenesis, fibrosis, and inflammation. Plast Reconstr Surg. 2012;129:825–834. doi: 10.1097/PRS.0b013e3182450b2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aschen S. Zampell JC. Elhadad S. Weitman E. De Brot M. Mehrara BJ. Regulation of adipogenesis by lymphatic fluid stasis: part II. Expression of adipose differentiation genes. Plast Reconstr Surg. 2012;129:838–847. doi: 10.1097/PRS.0b013e3182450b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider M. Ny A. Ruiz de Almodovar C. Carmeliet P. A new mouse model to study acquired lymphedema. PLoS Med. 2006;3:e264. doi: 10.1371/journal.pmed.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabibiazar R. Cheung L. Han J. Swanson J. Beilhack A. An A. Dadras SS. Rockson N. Joshi S. Wagner R. Rockson SG. Inflammatory manifestations of experimental lymphatic insufficiency. PLoS Med. 2006;3:e254. doi: 10.1371/journal.pmed.0030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin S. Kim J. Lee MJ. Roche L. Yang NL. Tsao PS. Rockson SG. Prospective transcriptomic pathway analysis of human lymphatic vascular insufficiency: identification and validation of a circulating biomarker panel. PLoS One. 2012;7:e52021. doi: 10.1371/journal.pone.0052021. [DOI] [PMC free article] [PubMed] [Google Scholar]