Abstract
Background
Osteopathic physicians utilize manual medicine techniques called lymphatic pump techniques (LPT) to improve lymphatic flow and enhance immunity. Clinical studies report that LPT enhances antibody responses to bacterial vaccines, shortens duration of cough in patients with respiratory disease, and shortens the duration of intravenous antibiotic therapy and hospital stay in patients with pneumonia. The purpose of this study was to identify if thoracic LPT (Th-LPT) or abdominal LPT (Ab-LPT) would reduce Streptococcus pneumoniae colony-forming units (CFU) in the lungs of rats with acute pneumonia.
Methods and Results
Rats were nasally infected with S. pneumoniae and received either control, sham, Ab-LPT, or Th-LPT once daily for 3 consecutive days. On day 4 post-infection, lungs were removed and bacteria were enumerated. Three daily applications of either Ab-LPT or Th-LPT were able to significantly (p<0.05) reduce the numbers of pulmonary bacteria compared to control and sham. There were no significant differences in the percentage or concentration of leukocytes in blood between groups, suggesting neither Ab-LPT nor Th-LPT release leukocytes into blood circulation.
Conclusions
Our data demonstrate that LPT may protect against pneumonia by inhibiting bacterial growth in the lung; however, the mechanism of protection is unclear. Once these mechanisms are understood, LPT can be optimally applied to patients with pneumonia, which may substantially reduce morbidity, mortality, and frequency of hospitalization.
Introduction
Community-acquired pneumonia is the most common and potentially fatal infectious disease, with Streptococcus pneumoniae remaining the leading causative pathogen.1 While antibiotic treatments have substantially reduced the rate of death from pneumococcal pneumonia, the prevalence of organisms resistant to antimicrobial therapy has increased substantially, raising the possibility that these pharmacological treatments will become less effective for treatment of infectious disease in the future.1,2 Therefore, there is a need to examine the benefits of complementary and alternative medicine (CAM) procedures that may aid in the treatment and prevention of infectious disease.
Manual medicine procedures designed to increase lymph flow or prevent the accumulation of fluid into tissue can be used for the treatment of edema and infectious disease. These procedures include exercise, limb movement, and tissue compression.4–7 Osteopathic physicians utilize manual medicine techniques called lymphatic pump techniques (LPT) to improve lymphatic flow and enhance immunity.7,8 LPT can be applied to the thoracic cage (thoracic pump), abdomen (abdominal pump), feet and legs (pedal pump), and the areas of the spleen and liver.7,8
Clinical studies report that LPT enhances antibody responses to bacterial vaccines,9,10 shortens duration of cough in patients with respiratory disease,11 and shortens the duration of intravenous antibiotic therapy and hospital stay in patients with pneumonia.12 While there are no published reports measuring the effect of LPT on the lymphatic system of humans, studies in animals demonstrate that LPT promotes the uptake of interstitial antigens into the lymphatic system,13 enhances lymph flow,14,15 increases the lymphatic concentration of leukocytes16,17 enhances the lymphatic flux of inflammatory cytokines, chemokines, and reactive oxygen and nitrogen species,18 and reduces bacterial burden in the lungs of rats.19 Collectively, these studies suggest that LPT enhances the lymphatic and immune systems and can protect against respiratory disease; however, the mechanism by which LPT protects against pneumonia has not been quantitatively defined. The purpose of this study was to identify if thoracic LPT (Th-LPT) or abdominal LPT (Ab-LPT) would reduce S. pneumoniae colony-forming units (CFU) in the lungs of rats with acute pneumonia.
Materials and Methods
Animals
This study was approved by the Institutional Animal Care and Use Committee and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication no. 85-23, revised 1996). Male, inbred Fischer 344 rats, free of clinically evident signs of disease, weighing between 200–300 grams, with indwelling jugular vein catheters were used in this study (Charles River Industries, Wilmington, MA).
Infection
Under anesthesia (30 mg/kg ketamine and 5 mg/kg xylazine, Miller Veterinary, Fort Worth, TX), Fischer 344 rats were intranasally inoculated with 1×108 S. pneumoniae (ATCC 6301) CFU in a total of 100 μL phosphate buffer saline (PBS). Following infection, the rats were held vertically for a few seconds to allow for aspiration of the fluid.
Leukocyte enumeration
Blood sample were collected via cardiac puncture into EDTA coated vacutainer blood test tubes (Fisher, Pittsburg, PA). Total leukocytes and a differential leukocyte count in blood samples were enumerated using the Hemavet 950 (Drew Scientific, Waterbury, CT).
Bacterial enumeration
S. pneumoniae stocks were stored at −80°C in Brain Heart Infusion (BHI) broth containing 10% glycerol until use. Bacteria were cultured on 5% sheep blood agar plates (Fisher) and incubated overnight at 37°C and 5% CO2. The bacteria were collected by washing the plates twice with sterile PBS, and diluted to the appropriate optical density at 600 nm to achieve the desired inoculum for infection. The CFU in the suspension was determined retrospectively by serial dilution and plating on sheep blood agar plates. For enumeration of bacteria in pulmonary tissue, lungs were removed and minced with sterile scissors in 5 mL sterile PBS. The lung tissue was then homogenized for 25 sec using a tissue homogenizer (Kinematica Inc., Bohemia, NY). Ten-fold (1:10 to 1:1,000,000) serial dilutions were made in a 96-well plate. Twenty μL of each dilution was plated onto trypticase soy agar (TSA) with 5% sheep blood plates in duplicates. The plates were incubated overnight at 37°C with 5% CO2 and CFU were counted after 18 hours.
Lymphatic pump treatments
At days one, two and three post-infection, rats received control, sham, Ab-LPT, or Th-LPT. Control rats received no treatment or anesthesia. During sham, rats were anesthetized via intravenous injection with 10 mg/kg propofol (Miller Veterinary, Fort Worth, TX) and the operator contacted the rat for 4 min in a manner similar to LPT; however no compressions were made.
During Ab-LPT, rats were anesthetized and the operator contacted the abdomen of the rat with the thumb on one side and index finger and middle finger on the other side of the medial sagittal plane. The fingers were placed bilaterally caudal to the ribs. Sufficient pressure was exerted medially and cranially to compress the lower ribs until significant resistance was met against the diaphragm, then the pressure was released. Compressions were administered at a rate of approximately 1/sec for the duration of the 4 min of treatment as previously described.17
Under anesthesia, Th-LPT was applied similar to a method previously described.13 With the rat supine, 1 min of gentle massage was performed, followed by 3 min of Th-LPT. Massage was applied to the thoracic cage and diaphragm area to stretch the restricted tissue. For Th-LPT, the operator's index fingers contacted the lateral aspect of the lower ribs, and bilateral finger pressure was applied for approximately 1 per second for 20 sec. This pressure was released for 10 sec to allow for inspiration. This cycle was repeated over 3 min.
Results
Ab-LPT and Th-LPT reduce S. pneumoniae CFU in the lungs
To determine if Ab-LPT or Th-LPT would decrease S. pneumoniae CFU in the lung, rats were nasally infected with 1×108 S. pneumoniae CFU as previously described.10,19 Twenty-four hours after infection, rats were divided into control, sham, and abdominal LPT (Ab-LPT) or thoracic LPT (Th-LPT) groups. For 3 consecutive days, the control group received no treatment or anesthesia, the sham group received 4 min of light touch (under anesthesia), and the Ab-LPT and Th-LPT groups received 4 min of LPT (under anesthesia).
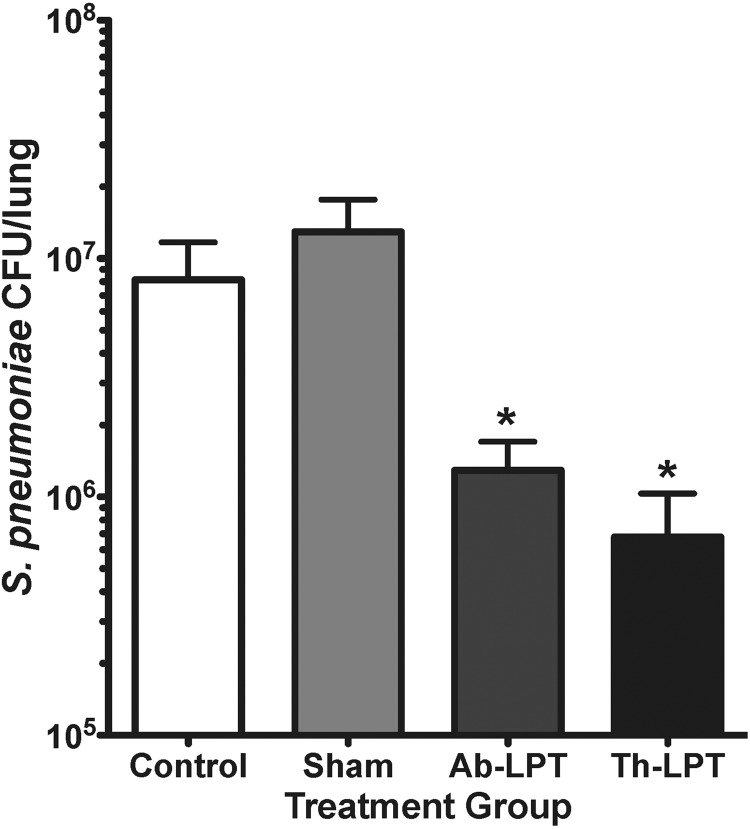
Three daily applications of either Ab-LPT or Th-LPT were able to significantly (p<0.05) reduce the numbers of pulmonary bacteria compared to control and sham (Fig. 1). There was no statistical difference between Ab-LPT or Th-LPT, suggesting both treatments inhibit bacterial growth.
FIG. 1.
Abdominal and thoracic LPT protect against bacterial pneumonia. On day zero, rats were nasally infected with 1×108 S. pneumoniae CFU. Control, sham, Ab-LPT, or Th-LPT were applied once daily for days 1 through 3 post-infection. At day 4 post-infection, lungs were removed and S. pneumoniae CFU were quantified. Data are means±SE of CFU in lung homogenates' *p<0.05 compared to control and sham treatment. N=8–13 rats per group.
Ab-LPT and Th-LPT do not alter blood leukocytes
To determine if either Ab-LPT or Th-LPT increases blood leukocytes or changes the composition of leukocytes in the blood, rats were infected and received treatments as described above, and blood was measured for the percentage and concentration of total white blood cells (WBC), lymphocytes (LY), polymorphonuclear cells (PMN), and monocytes (MO) at day 4 post-infection. There were no significant differences in the percentage (Table 1) or concentration (Table 2) of leukocytes between groups, suggesting neither Ab-LPT nor Th-LPT alter the concentration of the blood leukocyte populations measured.
Table 1.
The Effect of Abdominal and Thoracic LPT on Blood Leukocyte Percentages
| %LY | %PMN | %MO | |
|---|---|---|---|
| Control | 52±3.2 | 42±3.4 | 5.4±0.4 |
| Sham | 38±3.5 | 58±3.2 | 3.4±0.3 |
| Ab-LPT | 45±3.6 | 39±2.4 | 4.7±0.7 |
| Th-LPT | 55±2.5 | 50±4.3 | 4.8±0.2 |
On day zero, rats were nasally infected with 1×108 CFU of S. pneumoniae and received control, sham, Ab-LPT, or Th-LPT treatment once daily for days 1 through 3. On day 4, blood was collected and analyzed for leukocyte concentrations. Data are means±SE the percentage of leukocytes. N=8–13 rats per group.
Table 2.
The Effect of Abdominal and Thoracic LPT on Blood Leukocyte Concentrations
| WBC/mL | LY/mL | PMN/mL | MO/mL | |
|---|---|---|---|---|
| Control | 4.8±0.4 | 2.5±0.3 | 2.0±0.2 | 0.3±.01 |
| Sham | 5.0±0.5 | 2.6±0.3 | 2.9±0.4 | 0.2±.03 |
| Ab-LPT | 4.0±0.6 | 2.4±0.2 | 2.0±0.3 | 0.2±.03 |
| Th-LPT | 5.7±0.4 | 3.1±0.2 | 2.3±0.3 | 0.3±.02 |
On day zero, rats were nasally infected with 1×108 CFU of S. pneumoniae and received control, sham, Ab-LPT, or Th-LPT treatment once daily for days 1 through 3. On day 4, blood was collected and analyzed for leukocyte concentrations. Data are means±SE the concentration of cells (×106) per mL. N=8–13 rats per group.
Discussion
In this study, we found both thoracic and abdominal LPT significantly reduced S. pneumoniae bacteria in the lungs. While we did not identify the mechanism responsible for this protection, previous studies have shown that LPT promotes the uptake of interstitial antigens,13 enhances thoracic duct lymph flow,14 increases the concentration of leukocytes in thoracic15 and mesenteric lymph,16 and enhances the flux of inflammatory mediators in lymph.18 By enhancing the lymphatic drainage of pathogens or antigens, LPT may facilitate the activation of specific lymphocytes within secondary lymphoid tissues. In addition, by increasing leukocytes and inflammatory mediators in lymphatic circulation, LPT may facilitate leukocyte encounters with pathogens and boost immunity. Both mechanisms could potentially enhance immunity to infectious disease.
It is important to mention that while we have shown Ab-LPT enhances the concentration of lymphatic leukocytes and inflammatory mediators,15–19 the effect of Th-LPT on lymphatic immune cells has not been measured. However, Th-LPT has been shown to increase the lymphatic uptake of antigen in rats13 and thoracic duct lymph flow in dogs.14 Furthermore, in humans, Th-LPT enhanced vaccine-specific antibody responses.9,10 Collectively, these data demonstrate that Th-LPT enhances the lymphatic and immune systems. Therefore, similar to Ab-LPT, Th-LPT likely enhances the release of immune cells into lymphatic circulation, which may enhance protection against infectious disease; however further experimentation is needed to support this theory. It is also possible that a different mechanism is responsible for the protection offered by Th-LPT.
LPT may also assist pulmonary function. In support, Th-LPT enhanced the clearance of the tracheobronchial tissues, increased sputum production, and shortened the duration of cough in patients with lower respiratory tract disease.11 In addition, similar manual medicine techniques, such as chest physiotherapy, are used to increase sputum production in patients with cystic fibrosis5 and chronic obstructive pulmonary disease21,22 and are shown to improve mucociliary clearance rates and increase expiratory flow rate and intrapleural pressure.21 Future studies using animal models could measure the effect of LPT on pulmonary function and define whether this mechanism aids the clearance of bacteria from the lungs during pneumonia.
While we found that both LPT significantly reduced S. pneumoniae in the lungs, neither technique significantly increased leukocyte concentrations in blood. Thus, blood leukocyte concentrations may not accurately reflect changes in leukocyte mobilization via the lymph during LPT. Importantly, in clinical studies, not all positive clinical outcomes using LPT were associated with increased blood leukocytes.9–12 Our data support these findings and suggest that while LPT enhances the clearance of S. pneumoniae bacteria, a net increase in circulating leukocytes may not be detected.
Our data suggest that LPT may protect against pneumonia by inhibiting bacterial growth in the lung; however, there are still several questions unanswered. For example, 1) does LPT enhance pulmonary immunity by redistributing lymph-borne cells and inflammatory mediators to the lung, 2) does LPT enhance the delivery of pharmaceutical agents from the lymph or blood to the lung, 3) does LPT enhance pulmonary function? Once these mechanisms are understood, LPT can be optimally applied to patients with pneumonia, which may substantially reduce morbidity, mortality, and hospitalization.
Acknowledgments
The authors thank the Osteopathic Heritage Foundation for their continued support of the Basic Science Research Chair (LMH).
Author Disclosure Statement
No competing financial interests exist.
This study was funded by a National Institutes of Health Grant R01 AT004361 (LMH).
References
- 1.Fuller JD. McGeer A. Low DE. Drug-resistant pneumococcal pneumonia: Clinical relevance and approach to management. Eur J Clin Microbiol Infect Dis. 2005;12:780–788. doi: 10.1007/s10096-005-0059-x. [DOI] [PubMed] [Google Scholar]
- 2.Yoshikawa TT. Antimicrobial resistance and aging: Beginning of the end of the antimicrobial era. J Am Geriat Soc. 2002;50:S226–S229. doi: 10.1046/j.1532-5415.50.7s.2.x. [DOI] [PubMed] [Google Scholar]
- 3.Patterson MM. The coming influenza pandemic: Lessons from the past for the future. J Am Osteopath Assoc. 2005;105:498–500. [PubMed] [Google Scholar]
- 4.Coates G. O'Brodovich H. Goeree GH. Hindlimb and lung lymph flows during prolonged exercise. J Appl Physiol. 1993;2:633–638. doi: 10.1152/jappl.1993.75.2.633. [DOI] [PubMed] [Google Scholar]
- 5.Pisi G. Chetta A. Airway clearance therapy in cystic fibrosis patients. Review. Acta Biomed. 2009;2:102–106. [PubMed] [Google Scholar]
- 6.McGeown JG. McHale NG. Thornbury KD. Effects of varying patterns of external compression on lymph flow in the hindlimb of anesthetized sheep. J Physiol. 1998;397:449–457. doi: 10.1113/jphysiol.1988.sp017011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Degenhardt BF. Kuchera ML. Update on osteopathic medical concepts and the lymphatic system. J Am Osteopath Assoc. 1996;96:97–100. doi: 10.7556/jaoa.1996.96.2.97. [DOI] [PubMed] [Google Scholar]
- 8.Seffinger MA. King HH. Ward RC. Jones JM. Rogers FJ. Osteopathic philosophy. In: Ward RC, editor. Foundations for osteopathic medicine. Philadelphia: Lippincott Williams and Wilkins; 2003. pp. 3–12. [Google Scholar]
- 9.Jackson KM. Steele TF. Dugan EP. Kukulka G. Blue W. Roberts A. Effect of lymphatic and splenic pump techniques on the antibody response to Hepatitis B vaccine: A pilot study. J Am Osteopath Assoc. 1998;98:155–160. [PubMed] [Google Scholar]
- 10.Measel JW., Jr. The effect of lymphatic pump on the immune response: Preliminary studies on the antibody response to pneumococcal polysaccharide assayed by bacterial agglutination and passive hemagglutination. J Am Osteopath Assoc. 1982;82:28–31. [PubMed] [Google Scholar]
- 11.Allen TW. Pence TK. The use of the thoracic pump in treatment of lower respiratory tract disease. J Am Osteopath Assoc. 1967;4:408–411. [PubMed] [Google Scholar]
- 12.Noll DR. Degenhardt BF. Morely TF, et al. Efficacy of osteopathic manipulation as an adjunctive therapy for hospitalized patients with pneumonia: A randomized controlled trial. Osteopath Med Prim Care. 2010;4:2. doi: 10.1186/1750-4732-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dery M. Winterson B. Yonuschot G. The effect of lymphatic pump manipulation on the healthy and injured rat. Lymphology. 2000;33:58–61. [PubMed] [Google Scholar]
- 14.Knott EM. Tune JD. Stoll ST. Downey HF. Increased lymphatic flow in the thoracic duct during manipulative intervention. J Am Osteopath Assoc. 2005;105:447–456. [PubMed] [Google Scholar]
- 15.Hodge LM. King HH. Williams AG, et al. Abdominal lymphatic pump treatment increases leukocyte count and flux in thoracic duct lymph. Lymphat Res Biol. 2007;2:127–132. doi: 10.1089/lrb.2007.1001. [DOI] [PubMed] [Google Scholar]
- 16.Hodge LM. Bearden MK. Schander A. Huff JB. Williams A. King HH. Downey HF. Abdominal lymphatic pump treatment mobilizes leukocytes from the gastrointestinal associated lymphoid tissue into lymph. Lymphat Res Biol. 2010;2:103–110. doi: 10.1089/lrb.2009.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huff JB. Schander A. Downey HF. Hodge LM. Lymphatic pump treatment enhances the lymphatic release of lymphocytes. Lymphat Res Biol. 2010;3:165–169. doi: 10.1089/lrb.2010.0009. (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schander A. Downey HF. Hodge LM. Lymphatic pump manipulation mobilizes inflammatory mediators into lymphatic circulation. Exp Biol Med. 2012;1:58–63. doi: 10.1258/ebm.2011.011220. [DOI] [PubMed] [Google Scholar]
- 19.Hodge LM. Osteopathic lymphatic pump treatments to enhance immunity and treat pneumonia. Int J Osteopath Med. 2012;15:13–21. doi: 10.1016/j.ijosm.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Propst-Graham KL. Preheim LC. Vander Top EA. Snitily MU. Gentry-Nielsen MJ. Cirrhosis-induced defects in innate pulmonary defences against Streptococcus pneumoniae. BMC Microbiol. 2007;7:94. doi: 10.1186/1471-2180-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Schans CP. Forced expiratory manoeuvres to increase transport of bronchial mucus: A mechanistic approach. Monaldi Arch Chest Dis. 1997;4:367–370. [PubMed] [Google Scholar]
- 22.Varekojis SM. Douce FH. Flucke RL, et al. A comparison of the therapeutic effectiveness of and preference for postural drainage and percussion, intrapulmonary percussive ventilation, and high-frequency chest wall compression in hospitalized cystic fibrosis patients. Respir Care. 2003;24:8. [PubMed] [Google Scholar]