Abstract
Photodynamic Therapy (PDT) holds great promise for the treatment of head and neck (H&N) carcinomas where repeated loco-regional therapy often becomes necessary due to the highly aggressive and recurrent nature of the cancers. While interstitial light delivery technologies are being refined for PDT of H&N and other cancers, a parallel clinically relevant research area is the formulation of photosensitizers in nanovehicles that allow systemic administration yet preferential enhanced uptake in the tumor. This approach can render dual-selectivity of PDT, by harnessing both the drug and the light delivery within the tumor. To this end, we report on a cell-targeted nanomedicine approach for the photosensitizer silicon phthalocyanine-4 (Pc 4), by packaging it within polymeric micelles that are surface-decorated with GE11-peptides to promote enhanced cell-selective binding and receptor-mediated internalization in EGFR-overexpressing H&N cancer cells. Using fluorescence spectroscopy and confocal microscopy, we demonstrate in vitro that the EGFR-targeted Pc 4-nanoformulation undergoes faster and higher uptake in EGFR-overexpressing H&N SCC-15 cells. We further demonstrate that this enhanced Pc 4 uptake results in significant cell-killing and drastically reduced post-PDT clonogenicity. Building on this in vitro data, we demonstrate that the EGFR-targeted Pc 4-nanoformulation results in significant intra-tumoral drug uptake and subsequent enhanced PDT response, in vivo, in SCC-15 xenografts in mice. Altogether our results show significant promise towards a cell-targeted photodynamic nanomedicine for effective treatment of H&N carcinomas.
Keywords: Epidermal Growth Factor Receptor, Nanomedicine, Photodynamic therapy, peptides, polymeric nanoparticles
Introduction
Head and neck (H&N) cancers are the sixth most common type of cancer and account for approximately 350,000 cancer deaths every year1. These malignancies are highly aggressive, with a mean doubling time of 57 days and loco-regional recurrence in >50% of patients 2. Repetitive treatment of these patients by chemotherapy, radiotherapy and surgery presents very high risk of functional and cosmetic debilitation to H&N tissue 3. Hence there is a significant clinical interest to explore adjunctive and alternative treatment modalities that can minimally invasively yet selectively treat H&N cancers without side-effects and can be repeated as needed. To this end, a treatment modality that holds great promise is Photodynamic Therapy (PDT). In PDT, light-activation of a photosensitizer (PS) leads to formation of highly reactive oxygen species through energy transfer processes, which in turn cause oxidative damage resulting in cell death 4–6. A major advantage of PDT over chemo- or radiotherapy is that both the PS and the irradiating light are mostly inert by themselves and hence confer minimal systemic toxicity or collateral damage 8. The clinical promise of PDT is exhibited by health agency approvals of the PS formulations such as Visudyne® in the US, various other formulations (e.g. Foscan®) in other countries, and several ongoing clinical trials 6,9,10. PDT has shown promise in H&N cancers, but the treatment efficacy is currently fraught with issues involving PS dose and distribution, the drug-light administration interval, tissue oxygenation levels, light dose and light intensity 11–13.
In the context of drug dose and distribution, a significant issue involves phototoxicity arising from the unpredictable systemic distribution of the PS. One blatant example is Foscan®, an ethanol/propylene glycol formulation of the PS temoporfin, which failed to achieve FDA approval for treatment of H&N cancers due to poor tumor selectivity and serious burns arising from lingering skin photosensitivity 14,15. In such formulations involving surfactants and organic excipients, PS accumulation in tumors is not by any controllable mechanism. Hence, after intravenous administration of such PS formulations, there is a ‘waiting period’ (the drug-light interval) when the PS accumulating non-specifically in healthy tissues is expected to clear off, while that accumulated within tumor tissues is expected to be retained due to poor lymphatic drainage 17. Only then the tumor can be photoirradiated for PDT. This mechanism cannot ensure sufficient tumor uptake of the PS and the lack of tumor-selective uptake mechanisms becomes a critical limitation in efficient PDT of H&N cancers where healthy tissues in the light path need to be preserved for functional and cosmetic reasons.
To this end, the ‘targeted nanomedicine’ approach can provide an excellent way to resolve the above-described issues regarding tumor-selective PS delivery and distribution. In this approach, the drug is packaged inside a nanovehicle that protects the drug from rapid plasma destabilization, minimizes uptake into non-specific healthy organs and prevents plasma clearance through the kidney, while allowing preferential accumulation within the tumor stroma via extravasation through the tumor-associated leaky vasculature. This is known as the ‘enhanced permeation and retention’ (EPR) mechanism for passive targeting of tumors, which is responsible for the improved tumor-selectivity of chemotherapy formulations like Doxil® (Doxorubicin in liposomes) and Abraxane® (Paclitaxel in albumin nanoparticles) 18,19. Beyond the passive EPR mechanism, the nanomedicine approach further provides the opportunity to decorate the surface of the nanovehicles with specific molecular motifs that facilitate active binding and internalization of the vehicles (hence their payload) selectively in the cancer cells for improved intracellular drug delivery and therapeutic effect. Research on such cell-targeted active delivery of PS to tumors using surface-functionalized nanovehicles has gained significant momentum in recent years 20–23. Building upon this, here we report on an actively cell-targeted photodynamic nanomedicine formulation focused towards H&N cancers.
Over 90% of the aggressive H&N cancers are Squamous Cell Carcinomas (SCCs) and they express a high level of Epidermal Growth Factor Receptors (EGFRs) 24. EGFR, a glycoprotein member of the ErbB family, consists of an extracellular ligand-binding domain, a hydrophobic transmembrane region, and an intracellular tyrosine kinase (TK) domain. The extracellular domain can bind endogenous EGF family ligands which in turn leads to receptor-internalization and cytoplasmic TK activity, which activates cellular signaling pathways that inhibit apoptosis, promote cell proliferation, trigger angiogenesis and enhance tumor survival and metastatic potential. EGFR upregulation and subsequent signaling activities have been implicated in the aggressiveness and recurrence frequency of this cancer 26,27. Hence, EGFR immunotherapy by monoclonal antibodies (e.g., Cetuximab) and small molecule Tyrosine Kinase inhibitors (e.g., Erlotinib) have become clinically important in these cancers 27–29. We rationalize that conjugating EGFR-targeting ligands on the surface of a PS-loaded nanovehicle can facilitate intracellular PS delivery selectively to H&N SCCs for enhanced PDT. Based on this rationale, we have designed a polymeric micelle based nanomedicine formulation of the second generation photosensitizer silicon phthalocyanine 4 (Pc 4), have decorated the surface of the micellar vehicles with optimum density of an EGFR-targeting peptide ligand GE11 and have investigated the ability of this formulation to enhance the delivery of Pc 4 to EGFR-overexpressing H&N SCC cells in vitro and in vivo for improved PDT (Figure 1). Our results show significant promise of this approach towards a PDT strategy for H&N cancers. Furthermore, because of reported EGFR-upregulation in several other cancer types, we envision that our PDT strategy can be adapted towards treatment of these other cancers.
Figure 1.
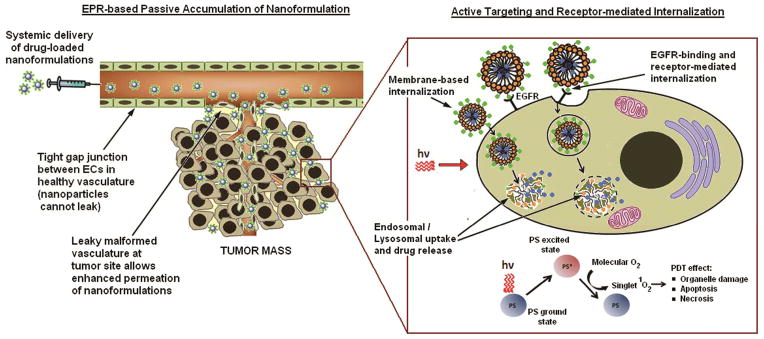
EPR-mediated passive targeting and subsequent receptor-mediated cell-specific active targeting of the drug (e.g. photosensitizer Pc 4) nanoformulation in tumor; the cancer cells can subsequently undergo PDT-induced death upon photoirradiation.
Experimental Section
Materials
The EGFR-targeting GE11 peptide, YHWYGYTPQNVI, was custom synthesized by Abgent Inc. (San Diego, California), with the addition of a cysteine residue on the N terminus to facilitate thioether-mediated conjugated to the PEG block of the PEG-PCL co-polymer that forms the final micellar nanoformulation. The polymer synthesis and characterization, GE11 conjugation and subsequent micelle fabrication have been described previously 30–32. The structures and molecular weights of mPEG-PCL, Mal-PEG-PCL and GE11-functionalized PEG-PCL have been confirmed by 1H NMR and MALDI-TOF mass spectroscopy and have been reported previously 30–32. Finally, confirmation of nanoparticle morphology using Scanning Electron Microscopy (SEM) has been reported previously30–32. For photodynamic treatment, a light-emitting diode array (EFOS, Mississauga, Ontario, Canada) was utilized at a fluence rate of 6 mW/cm2 (λmax=675 nm; width of output peak at half-maximum 24 nm). Post-PDT cell viability was analyzed using a standard (4,5-dimethylthiazol-2-yl)-2,5-diphennyltetrazolium bromide (MTT) assay purchased from Sigma Aldrich (St. Louis, MO). Crystal violet stain for clonogenic assays was also purchased from Sigma Aldrich. Fluorescence probes Hoechst 33342 and Lysotracker Green were purchased from Invitrogen (Carlsbad, CA) and were used as per the manufacturer’s instructions. Pc 4 was donated by the laboratory of Dr. Malcolm Kenney in the CWRU Department of Chemistry.
Cell Culture
The human squamous cell carcinoma of the tongue cell line, SCC-15, was obtained from the American Type Culture Collection (Manassas, VA) and cultured in 1:1 (v/v) Dulbecco’s Modified Eagle’s Medium/Ham’s F12 medium (DME/F12 medium; Fisher Scientific) containing 10% fetal bovine serum, penicillin (50 units/mL), streptomycin (50 μg/mL) and hydrocortisone (400 ng/mL). In our studies, MCF-7 cells were used as a control because they express only minimal levels of EGFR 33. Cultures were maintained in a humidified atmosphere of 5% CO2 and 95% air in a 37°C incubator.
Cellular Uptake of Nanoparticles
SCC-15 cells were plated onto 96-well black-wall/clear-bottom plates at a density of 2 × 104 cells/well in DME/F12 and allowed to grow for 24h. Dilutions of Pc 4 loaded EGFR-targeted micelles were made in medium such that the final concentration given to cells was 125 and 250 nM Pc 4. Controls included Pc 4 in 3% DMF and Pc 4 loaded nontargeted micelles. Cells were then allowed to incubate at 37°C shielded from ambient light. Following various incubation times varying from 2 hours to 24 hours, the supernatant was removed from all the cells followed by a several thorough washings with PBS to remove any extracellular, loosely bound micelles. In order to allow for accurate fluorescence measurements of intracellular Pc 4, 150 μL of DMSO is then added to the cells to release and solubilize Pc 4 from cells. The plates were shaken for 10 min and fluorescence emission was monitored on a microplate reader (Tecan, San Jose, CA).
Uptake and Intracellular Fate of Pc 4-loaded Nanoparticles
In order to monitor the spatio-temporal pattern of cellular uptake of the Pc 4-loaded nanoparticles, confocal microscopy was utilized along with organelle-specific fluorescence probes and the inherent fluorescence of Pc 4. EGFR-targeted and nontargeted Pc 4-loaed micelles were incubated with high EGFR SCC-15 cells and low EGFR MCF-7 cells. All cells were seeded at 50,000 cells/plate (approximately 50% confluence) in 2 mL media and allowed to attach for 24h. Cells were then incubated with Hoechst 33342 (stains nucleus blue) and Lysotracker® Green (stains lysosomes green), as per the manufacturer’s instructions, followed by thorough washing with PBS. Pc 4-loaded nanoparticles were added at a final concentration of 400 nM Pc 4 per well and fluorescence images were taken over time using an Olympus FV1000 Confocal Microscope.
Evaluation of In vitro PDT efficacy
In vitro PDT efficacy of the EGFR-targeted Pc 4-nanoformulation was evaluated using a standard MTT assay. SCC-15 cells were plated in 96-well flat-bottomed plates at a density of 2×104 cells per well. Cells were incubated for 24 hours with targeted Pc 4-loaded micelles with final effective Pc 4 concentrations of 200 nM, 400 nM and 800 nM. Separate plates were prepared and shielded from ambient light for testing “dark toxicity” while PDT plates were photoirradiated for 400 seconds, as this has been demonstrated previously as the optimal irradiation time 32. Both sets of plates were then incubated at 37°C for 24h followed by incubation with MTT for four hours at 37°C to allow for conversion of yellow MTT to purple formazan by live cells. Formazan was then quantified by absorption at 40 nm on a microplate reader (BioTek, Winooski, Vermont). Measurements for treated cells were normalized to that of controls in order to report cell viability. We have previously reported using model EGFR-overexpressing versus low EGFR cells that, without targeting there is very low uptake of the Pc 4 formulation in the cells at shorter incubation periods and correspondingly there is low cell-killing upon PDT 31,32. Hence in the current manuscript, we have only reported the PDT effect on EGFR-overexpressing H&N SCC-15 cells with our EGFR-targeted Pc 4 nanoformulation, incubated for 24 hours.
Clonogenic Cell Survival Assay
Clonogenic assays measure the ability of a cell to re-grow into a colony following cell-damaging treatment. The assay determines the surviving fraction (SF) which is defined as:
The plating efficiency is determined by dividing the number of colonies counted by the number of cells plated for the control cells. For this assay, cells were seeded in triplicate into T25 cm2 flasks and allowed to grow overnight to approximately 80% confluence. The next day, ‘control’ group cells were replaced with fresh media while ‘treated’ group cells were incubated with Pc 4 loaded targeted micelles at a final concentration of 400 nM Pc 4. Following a 24 hour incubation period, the treated cells were photoirradiated as described previously for 400 seconds. After a 24 hour post-PDT incubation time, cells were harvested by trypisinization, counted using a hemocytometer and diluted such that 1000 cells were seeded in triplicate into 60 mm petri dishes. The plates were incubated for 2 weeks after which the cells were fixed with 10% formalin and stained with crystal violet. The number of colonies consisting of more than 50 cells was counted.
Evaluation of In vivo PDT Efficacy
The in vivo pilot PDT studies (three mice per group) were carried out according to animal care and use protocols approved by the University of Pittsburgh Institutional Animal Care and Use Committee. For this, a human tongue carcinoma xenograft tumor model was used to determine in vivo effect of targeted versus nontargeted Pc 4-loaded nanoparticles. Tumor growth was initiated in SCID mice (female, 4–6 weeks of age) by subcutaneous injection of SCC-15 cells on the right flank. Intraperitoneal injection of Nembutal (50 mg/kg) was used to anesthetize mice and fur was removed from the skin over the tumor by application of Nair (Church & Dwight, Co., Princeton, NJ) 24 hours before Pc 4 dosing. Targeted and nontargeted Pc 4-loaded nanoformulations (at total dose of 2 mg Pc 4/kg mouse body weight) were administered to the mice at 0.01 mL/g by bolus tail vein injection on day 0. Following dosing, the mice were housed under a Lee Dark Green filter #124 (Baltimore Stage Lighting, Balmore, MD) to shield them from ambient light except that between 450–600 nm. Intra-tumoral uptake of Pc 4 was determined using an Optical Pharmacokinetics System (OPS) built by Optimum Technologies, Inc. (Southbridge, MA) which includes a pulsed xenon lamp (Perkin-Elmer, Waltham, MA), a linear array CCD detector and a spectrometer (Ocean Optics, Dunedin, FL). The optical probe contains a source fiber and a light collecting fiber. To take measurements, the probe is placed on the surface of the tumor tissue and triplicate measurements are collected. Measurements consist of a reflectance intensity recorded between 450 nm and 850 nm. For comparison, standards of each formulation were prepared at final Pc 4 concentrations between 0.1 and 30 μM in 96-well black plates and OPS measurements were taken on each well. A detailed description of the OPS-based method for determination of tumor drug concentration, has been reported previously 34. Photoirradiation at 672 nm was carried out using a two-beam split diode laser (Intense Ltd., North Brunswick, NJ) with equal power in each beam and a bifurcated optical fiber assembly (Ocean Optics, Dunedin, FL). Uniformly distributed irradiation was obtained by connecting a microlens fiber (Pioneer Optics, Bloomfield, CT) to the end of the fiber assembly. A Thorslabs PM100 power meter (Newton, NJ) with an integrating sphere detector was used to measure photoirradiation power. The total fluence was 150 J/cm2 and the total fluence rate on the tumor surface was kept constant at 132 mW/cm2. Two days after tail vein injection, mice were anesthetized with Nembutal and placed in a special restraint system which allowed for unobstructed photoirradiation of the tumor while protecting healthy tissue. Bilateral beam placement allowed for the laser to encompass the full tumor throughout the photoirradiation duration. This method also confers minimal light exposure to nearby internal organs which may have residual Pc 4. Tumor volumes were measured twice weekly using calipers. Tumor growth was normalized to the original tumor volume 3 days prior to formulation injection.
Data analysis
Statistical analyses were performed using Minitab (Minitab, State College, Pennsylvania). Analyses of variance (ANOVA), post-hoc Tukey’s tests and Student’s t-tests were used to analyze the data from MTT, clonogenic and in vivo assays, where applicable. Significance was reported for p< 0.05.
Results
Uptake of EGFR-targeted Pc 4 nanoformulation in H&N SCC cells in vitro
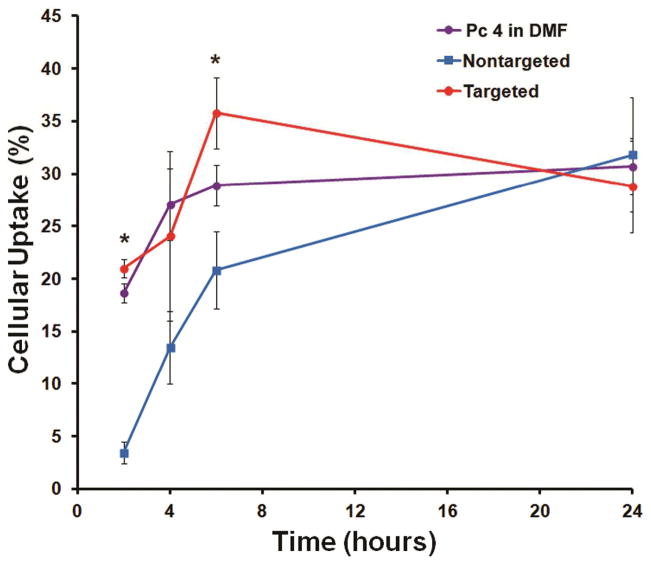
EGFR-targeted versus non-targeted Pc 4 nanoformulations were used to monitor cellular uptake of Pc 4 as a function of time in H&N SCC-15 cells. As described in the Methods section, cells were incubated with the various Pc 4 formulations, followed by subsequent measurement of intracellular Pc 4 which was then expressed as percent uptake. A solution of Pc 4 in 3% DMF was used as a control as this DMF-based Pc 4 solution is known to permeate into cells rapidly 35. The intracellular uptake of Pc 4 was monitored for incubation times of 2, 4, 6 and 24 hours. The SCC-15 cell line is well-known for its high EGFR expression, particularly among H&N SCC lines 36. As evident from the results shown in Figure 2, the EGFR-targeted nanoformulation is much faster rate compared to the nontargeted formulation within the first 8 hrs. This, in translation in vivo, may lead to higher PS accumulation in the tumor within short periods of time and allow for enhanced PDT effect. The higher intracellular uptake of the EGFR-targeted Pc 4 nanoformulation is likely because the binding of the GE11-decorated Pc 4 nanoformulation to the EGFRs triggers rapid receptor-mediated internalization. The in vitro results suggest that the targeted nanoformulation reaches a state of maximum uptake around the 6 hr incubation period, with about 15% higher Pc 4 internalization compared to the nontargeted formulation. With longer incubation periods > 6 hrs, the statistical differences between the targeted and non-targeted formulation conditions seem to get reduced, possibly because at such long incubation periods, nonspecific processes of internalization such as membrane fusion and pinocytosis overlap with the active receptor-mediated processes 31.
Figure 2.
Intracellular uptake of Pc 4 formulations expressed as a percentage of total Pc 4 amount incubated with the EGFR-expressing SCC 15 cells; the EGFR-targeted Pc 4 formulation renders faster and higher uptake of Pc 4 in the cells.
Monitoring intracellular uptake and distribution of Pc 4 nanoformulations using confocal microscopy
Confocal microscopy provided a way to monitor the intracellular uptake and distribution of Pc 4 within the short incubation period, and confirm that the EGFR-targeted Pc 4 nanoformulation indeed undergoes much faster uptake compared to the non-targeted analog. For these studies, the fluorescence probes used were Lysotracker Green (green emission, λem=511 nm) which localizes to lysosomes and late endosomes, Hoechst (blue emission, λem=460 nm) which stains cell nuclei, and inherent fluorescence of Pc 4 for the nanoformulations (red emission, λem=675 nm). Representative images from our studies are shown in Figure 3. As shown in the Figure, three conditions were tested, namely, incubating EGFR-targeted Pc 4 nanoformulation on EGFR-overexpressing SCC-15 cells, incubating this targeted nanoformulation on a low-EGFR cell line (MCF-7 in this case) and incubating the non-targeted formulation with the SCC-15 cells. As evident from the Figure the EGFR-targeted formulation bound to the SCC-15 cell membrane very rapidly (within 5 minutes of incubation), most likely due to active interactions between the GE11 peptides on the nanovehicle surface with the high EGFR expression on the SCC-15 cell surface. The yellow co-localization of the red Pc 4 fluorescence and green Lysotracker fluorescence at 10 minutes of incubation and beyond, in these cells, suggests that the EGFR-targeted nanoformulation gets taken up into late stage endosomes and lysosomes via receptor-mediated endocytosis. The intensity of Pc 4 (red) fluorescence in this ‘targeted’ test group increased with incubation time, suggesting increased lysosomal uptake of the nanoformulation followed by possible release of Pc 4 into the cytoplasmic compartment. This release is possibly a combined effect of low pH-catalyzed lysosomal degradation of the acid-labile micellar polymer of the nanovehicle and photo-triggered rupture of the lysosomal vesicles due to the action of the confocal laser light with the lysosome-trapped Pc 4 30,37,38. These results indicate that the EGFR-targeted nanoformulation can undergo receptor-mediated rapid internalization in EGFR-overexpressing SCC-15 cells and subsequently result in rapid cytoplasmic release of Pc 4 from the internalized vehicles. This is important in the context of Pc 4 partitioning into mitochondria, endoplasmic reticulum and the Golgi which are the intracellular targets for Pc 4-PDT 8,39,40.
Figure 3.
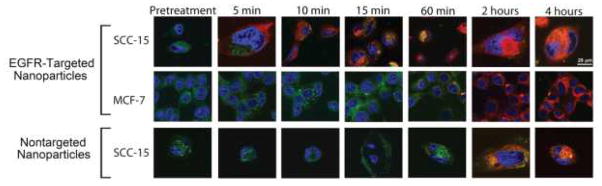
Representative confocal microscopy images showing uptake and intracellular fate of targeted and nontargeted Pc 4 nanoformulations in EGFR-overexpressing cells (SCC-15) and low-EGFR cells (MCF-7); all cells are stained with Lysotracker ® lysosomal stain (green) and Hoechst nuclear stain (blue), while Pc 4 is detected by its inherent red fluorescence; EGFR-targeted Pc 4-nanoformulation shows significantly enhanced rapid uptake and intracellular distribution in EGFR-overexpressing SCC15 cells.
In contrast, it takes much longer incubation time for the EGFR-targeted nanoparticles to get effectively taken up in low-EGFR cells (e.g. MCF-7), as shown in the second row of images in Figure 3. The low levels of EGFR expression in these cells allow for much reduced extent of receptor-mediated active internalization and the uptake processes are more likely attributed to slower processes like membrane fusion and pinocytosis 41. In our in vitro confocal experiments, the Pc 4 (red) fluorescence was detectable in these cells only around the 60 min incubation point and beyond. Also, the co-localization of this red fluorescence with lysosomal (green) fluorescence was minimal, suggesting that the Pc 4 nanoformulation uptake was possibly not solely mediated by receptor-mediated processes. Our second control group shown in the third row of Figure 3, is for non-targeted Pc 4 nanoformulation incubated with the EGFR-overexpressing SCC-15 cells. Due to the absence of the EGFR-targeting moiety (GE11 peptide) on the surface of these nanovehicles, the formulation cannot undergo as much specific receptor-mediated internalization, and the cellular uptake is once again largely via slower processes like membrane fusion and pinocytosis. Hence for this group, the Pc 4 (red) fluorescence is seen within the cells only around 60 min and beyond.
In vitro PDT efficacy on SCC-15 cells with EGFR-targeted Pc 4 nanoformulation
We have previously demonstrated on model EGFR-overexpressing cell lines (A431) that PDT-induced cell killing is significantly enhanced when an EGFR-targeted nanoformulation is used to deliver the photosensitizer 31. Building on these findings, here we have studied the PDT-induced cell-killing effect of the EGFR-targeted Pc 4 nanoformulation on the SCC-15 cells at increasing Pc 4 concentrations. For all these studies a photoirradiation power of 6 mW/cm2 was used. Figure 4 shows results of the post-PDT cell viability assays (MTT) where cells were incubated with the EGFR-targeted Pc 4 nanoformulations at total Pc 4 doses of 200, 400 and 800 nM, followed by photoirradiation. As evident from the data, dark conditions (no photoirradiation) confer minimal cytotoxicity, while even at low (200 nM) Pc 4 dose in nanoformulation, significant cell death (p<0.001) is induced upon photoirradiation. It was found that the highest cell death occurred with the 400 nM Pc 4 dose and subsequent increase to 800 nM Pc 4 did not significantly enhance this effect. Hence for subsequent clonogenic studies the 400 nM Pc 4 dose in EGFR-targeted nanoformulation was used on the SCC-15 cells.
Figure 4.
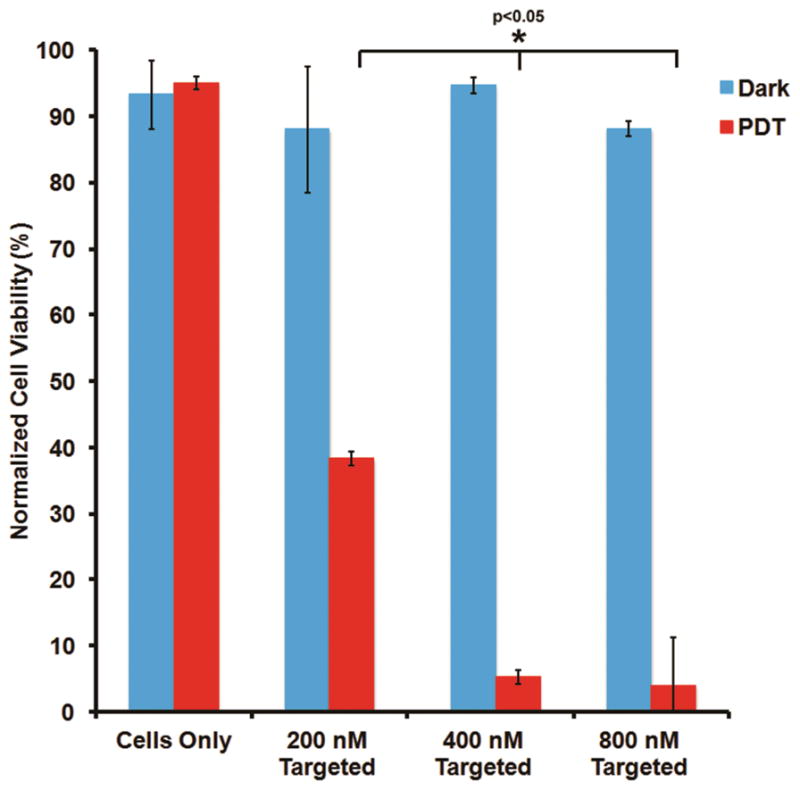
SCC 15 cell response to PDT (post-PDT viability) with various doses of Pc 4 delivered via the EGFR-targeted nanoformulation; without photoirradiation there is minimal cell death (no dark toxicity), while with photoirradiation significant cell killing was observed.
Clonogenic Cell Survival Assay
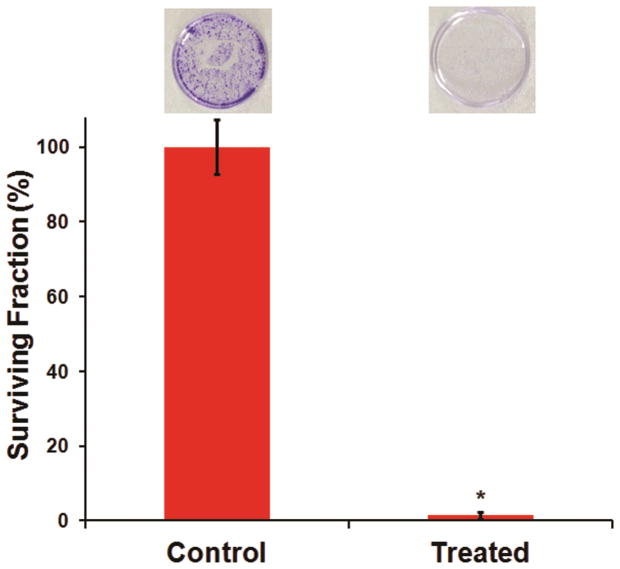
The MTT assay can provide only partial information about the efficacy of anti-cancer treatment. For confirmation of treatment efficacy, it is necessary to establish that even if some cells may remain viable after PDT, their ability to further divide and proliferate become compromised. In translation in vivo, this would imply that post-PDT the tumor re-growth will be severely impaired 42. Therefore, we have also carried out clonogenic studies to assess the ability of the remaining viable SCC-15 cells to re-grow and form colonies post-PDT. Our quantitative data from these studies, along with representative pictures of corresponding colony formations in cell-culture dishes, are shown in Figure 5. As evident from the data, incubation of the SCC-15 cells with the EGFR-targeted Pc 4 nanoformulation at 400 nM Pc 4 dose, followed by photoirradiation to render PDT, resulted in a drastic loss of cell clonogenicity. The ‘control’ cells, which were not photoirradiated, were able to grow colonies effectively. These results establish the high PDT efficacy of our approach.
Figure 5.
Results from clonogenic assays to evaluate PDT response with 400 nM Pc 4 delivered via EGFR-targeted nanoformulation; without photoirradiation, the cells efficiently grew and formed colonies, while upon photoirradiation, the re-growth and colony-forming capability were significantly reduced.
In vivo PDT Efficacy
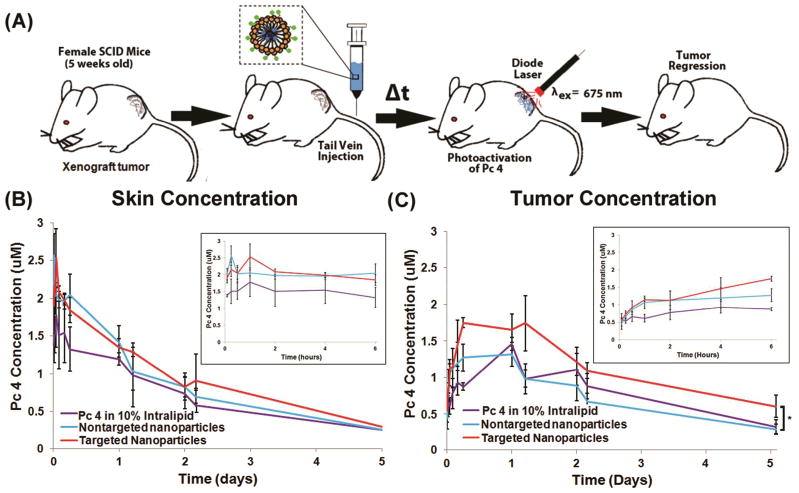
Driven by the successful PDT effect of the EGFR-targeted Pc 4 nanoformulation delivery in vitro, the targeted versus the non-targeted Pc 4 nanoformulations were tested for PDT response in subcutaneous SCC-15 xenografts in mice. Figure 6A shows a schematic of the experimental setup for the study. Figure 6B shows the Pc 4 uptake and retention in the skin while Figure 6C shows uptake and retention in the tumor, following intravenous administration of EGFR-targeted versus non-targeted Pc 4 formulation, compared to administration of Pc 4 formulated in 10% Intralipid, all at the same drug dose. The skin and tumoral uptake during the first 6 hours are shown in the magnified insets in the top right corners of 6B and 6C. As evident from the data, all the formulations show some uptake in the skin from systemic distribution immediately following intravenous administration, but the Pc 4 concentration in the skin drops off significantly over time through 1–5 hrs. In contrast, in the tumor all formulations lead to similar levels of Pc 4 uptake initially, but over time the EGFR-targeted nanoformulation is taken up at a higher level compared to the nontargeted formulation. The magnified inset for the first 6 hrs in the tumor shows significantly increasing uptake of Pc 4 for the EGFR-targeted nanoformulation group, which promisingly corresponds to the trend seen in our in vitro studies. Also, this higher level of Pc 4 uptake is maintained over the longer time periods (shown up to 5 days). Such enhanced intra-tumoral uptake and retention of Pc 4 for the EGFR-targeted nanoformulation is possibly due to receptor-mediated rapid internalization of the drug by the cancer cells, as was indicated in our in vitro studies.
Figure 6.
(A) shows PDT schematic for the in vivo studies, (B) shows OPS-measured skin concentrations of Pc 4 over 5 days (with the first 6 hrs in magnified inset) while (C) shows OPS-measured tumor concentrations of Pc 4 over 5 days (with the first 6 hrs in magnified inset) when delivered intravenously by the different formulations; the EGFR-targeted Pc 4 formulation shows higher intra-tumoral uptake and retention of Pc 4 over time compared to the non-targeted and the Intralipid-based Pc 4 formulations.
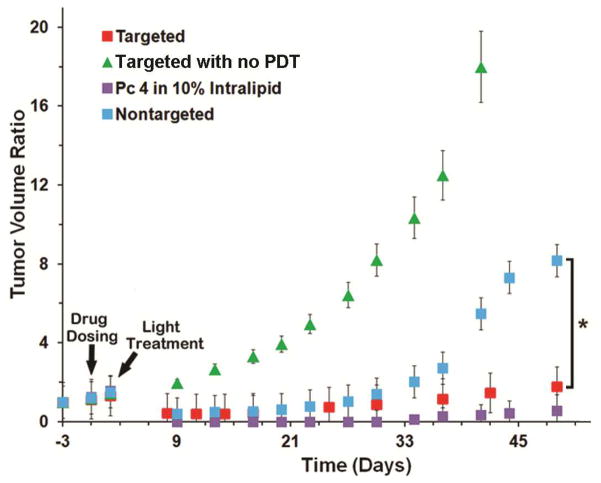
Figure 7 shows normalized tumor volume as a function of time following systemic administration of Pc 4 formulation at Day 0 and tumor photoirradiation at Day 2 for the four study groups. Tumor volume ratio was calculated by normalizing with respect to the original tumor volume three days (Day -3) before drug dosing (Day 0). Pc 4 formulation in 10% Intralipid (a clinically used excipient made of soybean oil, egg yolk phospholipids, glycerin and water) was used as a comparison control. As evident from the results, following photoirradiation tumors treated with targeted and nontargeted Pc 4 nanoformulations, as well as, those treated with the 10% Intralipid-based formulation all showed initial regression (red, blue, and violet squares in Figure 7) whereas, the control tumors without photoirradiation continued to grow (green triangles in Figure 7). The mice in the ‘no PDT’ (green triangles) group had to be euthanized by day 45 due to advanced tumor burden. For both the targeted and the nontargeted formulation injection groups, the tumor regression seemed to be similar until Day 30. However beyond this time point, the nontargeted formulation injection group began to re-grow considerably. In contrast, the EGFR-targeted nanoformulation injection group maintained significantly regressed tumors and this trend was similar to the animals treated with the Intralipid-based Pc 4 formulation. It is important to note here that the Intralipid formulation is effective in small animal model studies but have limitations in clinical translation because of the similar uncontrolled biodistribution, hypersensitivity and toxicity issues as Cremophor43–48. Our EGFR-targeted Pc 4 nanoformulation shows an in vivo PDT response in SCC-15 xenografts as effective as the Intralipid formulation, indicating that the nanoformulation holds significant promise towards clinical translation in the future.
Figure 7.
PDT efficacy as measured by post-PDT normalized tumor volume ratio; following PDT, the animals injected with the EGFR-targeted formulation exhibited regressed tumor volume over longer periods of time (greater PDT response), compared to the non-targeted formulation.
Discussion
In the light of the aggressive and recurrent nature of H&N cancers, a treatment strategy that can be safely repeated without significant functional and cosmetic damage to critical neighboring healthy tissue while effectively eradicating malignant tissue, is of great clinical significance. To this end, PDT holds excellent promise, as evident from several clinical perspectives 11,13,49–51. In the context of the drug-light-oxygen triad management for PDT of H&N tumors, the tumor-selective delivery of photoactivating light can be conveniently achieved via specialized fiber-optic devices 12,52–54. However, challenges still remain regarding the choice of ideal photosensitizer drugs that have chemical purity of composition and high molar absorptivity at tissue-penetrating light wavelengths, as well as, regarding effective formulation of the photosensitizer that allows systemic administration yet tumor-selective preferential uptake.
Compared to the clinically approved (FDA and EU-approved) PDT formulations like Photofrin®, Foscan® and Laserphyrin® which consist of porphyrin and chlorin based photosensitizers, Pc 4 has higher chemical purity and is activcated at deeper red wavelengths (almost near infra-red) which allows for use of light with deeper tissue penetration. Pc 4 has high molar absorptivity at this deep red wavelength (ε = 2.4 × 105 M−1 cm−1 at an absorption maximum of 670 nm), compared to Photofrin (ε = 1.2 × 103 M−1 cm−1 at 630 nm), Foscan (ε = 2.2 × 104 M−1 cm−1 at 652 nm) and Laserphyrin (ε = 4.0 × 104 M−1 cm−1 at 654 nm) 55. This makes Pc 4 potentially a superior photosensitizer compared to the current clinical porphyrin and chlorin molecules. Pc 4-PDT efficacy has been recently demonstrated in several human cancers in vitro and model xenograftsin vivo40,56. Also, Pc 4 allows the red fluorescence during photoirradiation to be used as a surrogate measure of delivered dose in tissues and cells 57. Pc 4-PDT acts by strong induction of apoptosis, with the reactive oxygen species causing significant damage to mitochondria and endoplasmic reticulum 58. A clinical trial of topically applied Pc 4 for PDT of dermal cancers has yielded promising results at CWRU 8. In the Department of Head and Neck Surgery at Case Western, preclinical studies on Pc 4-PDT of selected H&N tumor lines have shown promise 59,60. Therefore, there is significant clinical interest in enhancing Pc 4-PDT efficacy in H&N cancers by improving tumor-selective delivery and intracellular uptake.
Regarding formulation of photosensitizers that allow for safe systemic administration yet preferential or enhanced tumor uptake, all current clinical formulations are in lipidic or organic surfactant systems which do not have any active mechanism for tumor-specific uptake. As mentioned earlier, tumoral uptake of the photosensitizer for these formulations are attributed to hydrophobic partitioning of the drug into plasma proteins and subsequent uptake of the protein-drug complexes by lipoprotein receptors and tumor stromal matrix. For systemic delivery, these mechanisms increase the risk of unpredictable pharmacokinetics and biodistribution. In contrast, the targeted nanomedicine strategies that have shown considerable benefit in tumor-selective delivery of chemotherapy agents might allow for similar delivery pathways for photosensitizers and significantly improve pharmacokinetics and resultant PDT efficacy. Utilization of such nanomedicine approaches for PDT is gaining momentum, as evident from recent research efforts in formulation of various PS in a variety of nanovehicle platforms and investigation of cell-specific targeted delivery of the various PS-loaded nanovehicle formulations by using vehicle surface-decoration with receptor-targeted antibodies, peptides and other small molecule ligands 22,23,61–63. Research on nanomedicine strategies specifically for PDT of H&N cancers is a developing field, as evident from some recent reports using iron oxide particles, PEG-PLA based polymeric micelles and mesoporous silica nanoparticles 64–66. These nanovehicle platforms were not reported to incorporate targeting moieties that facilitate receptor-mediated active uptake in the H&N tumor cells and possibly relied on EPR-mediated passive uptake. We report a polymeric micelle-based nanoformulation of the photosensitizer Pc 4 that is surface-decorated with GE11 peptides that actively target and bind to EGFRs upregulated on H&N tumor cells and can thereby undergo receptor-mediated internalization to render high uptake of Pc 4. Our in vitro data suggests that the rapid intracellular uptake of Pc 4 is due to receptor-mediated active internalization of the Pc 4-loaded micelle nanovehicles triggered by the binding of the vehicle surface decoration of GE11 peptides to the high EGFR expression on the surface of SCC-15 cells. It has been recently reported elsewhere that GE11 based EGFR-binding results in clathrin mediated endocytosis without induction of EGFR-signaling 67. This is a significant advantage compared to EGFR-targeting using endogenous molecules like EGF, which may trigger EGFR-based signaling cascades that are known to promote tumor cell survival and growth. We have demonstrated that our targeted nanomedicine approach results in enhanced intra-tumoral Pc 4 delivery and resultant improved PDT efficacy by both in vitro and in vivo. Therefore our nanomedicine strategy can lead to a significant advancement in PDT technologies targeted to H&N cancers.
It is to be noted that our in vivo pilot studies were carried out in a subcutaneous xenograft model of SCC-15 in mice and hence the photoirradiation of the tumor could be achieved relatively conveniently using an external laser. The mice that underwent PDT using the targeted nanoformulation showed significantly regressed tumor for over two months. These studies have demonstrated the in vivo feasibility and efficacy of our photodynamic nanomedicine strategy, and have provided the basis for continued studies focused on PDT efficacy in not only regressing tumors, but also on long term cure and survival. An interesting observation is that the tumor regression results for the EGFR-targeted nanoformulation injected group were quite similar to the Intralipid formulation injected group, although the uptake data suggests that the Intralipid formulation results in lower amounts of intra-tumoral Pc 4 delivery compared to the EGFR-targeted formulation. We speculate that the pathway of cellular internalization and subsequent pattern of intracellular distribution of Pc 4 formulated in Intralipid is very different from that formulated in the EGFR-targeted (as well as non-targeted) polymeric micelles, and this may have an effect on the subsequent PDT response upon photoirradiation. The actual mechanisms of intracellular uptake and distribution of drugs formulated in Intralipid (and other lipid emulsion systems) is not at all well-documented in literature and most studies in this context focus on in vivo overall biodistribution and pharmacokinetics at the tissue level. The general consensus regarding such lipid emulsion formulated drugs is that in vivo the drugs can partition from the emulsions into lipoproteins that are then subsequently bound and internalized by lipoprotein receptors (e.g. LDL receptors) upregulated on target cells. Such uptake and distribution patterns of Pc 4 in Intralipid formulations may result in increased localization of Pc 4 in cellular organelles that allow for increased PDT effect with lower amount of Pc 4, leading to comparable cell-killing with higher amount of Pc 4 nanoformulation that is taken up via EGFR-mediated lysosomalendocytotic pathway. Our ongoing studies are focused on elucidating such mechanistic possibilities and the results from these studies will be part of future publications. Furthermore, future studies will be focused on orthotopic models of H&N SCCs where targeted nanomedicine strategies for photosensitizer delivery can be integrated with specialized fiber-optic technologies for light delivery, to evaluate the translational capabilities. In addition, our EGFR-targeted photodynamic nanomedicine formulation can become potentially applicable toward developing PDT strategies for several other EGFR-overexpressing tumors 36.
Conclusion
PDT can become a safe and effective treatment modality for H&N cancers, especially where repeated treatment is required due to the aggressive and recurrent nature of the cancers. To achieve this, in parallel with the ongoing development of tumor-selective light delivery technologies, it is necessary to develop photosensitizer formulations that can be administered systemically but can render enhanced preferential uptake of the drug in the tumor. To this end, we have developed a polymeric micelle-based nanoformulation of the photosensitizer Pc 4 that is targeted via GE-11 peptide decorations to EGFRs upregulated on H&N cancer cells. Our in vitro studies show that this EGFR-targeted Pc 4 nanoformulation results in faster and higher uptake in EGFR-overexpressing H&N SCC15 cells and renders significant cell-killing with drastic reduction of clonogenictity. Our pilot in vivo studies in H&N SCC15 xenografts in mice demonstrate the ability of this EGFR-targeted Pc 4 formulation to render high intra-tumoral uptake and post-PDT significant tumor response. Altogether, these results show the feasibility and promise of a cell-targeted photodynamic nanomedicine strategy that can be effective for treatment of H&N cancers.
Acknowledgments
The authors would like to acknowledge the help of Dr. Z.R. Lu of the CWRU Department of Biomedical Engineering at Case Western Reserve University to allow the use of the confocal microscope. The authors would also like to thank Dr. N.L. Oleinick of the Case Comprehensive Cancer Center at Case Western School of Medicine for her expert opinions and insights on the potential of PDT in various cancers including H&N carcinomas. A. Master is funded by an F31-DE019998 award from the NIDCR.
References
- 1.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steel G. Basic Clinical Radiobiology. Arnold; London: 1997. The growth rate of tumours. [Google Scholar]
- 3.Kim S, Grandis J, Rinaldo A, Takes R. Emerging Perspectives in Epidermal Growth Factor Receptor Targeting in Head and Neck Cancer. Head & neck. 2008:667–674. doi: 10.1002/hed.20859. [DOI] [PubMed] [Google Scholar]
- 4.Foote CS. Definition of type I and type II photosensitized oxidation. Photochem Photobiol. 1991;54:659. doi: 10.1111/j.1751-1097.1991.tb02071.x. [DOI] [PubMed] [Google Scholar]
- 5.Dougherty TJ, Grindey GB, Fiel R, Weishaupt KR, Boyle DG. Photoradiation therapy. II. Cure of animal tumors with hematoporphyrin and light. J Natl Cancer Inst. 1975;55:115–21. doi: 10.1093/jnci/55.1.115. [DOI] [PubMed] [Google Scholar]
- 6.Triesscheijn M, Baas P, Schellens J, Stewart F. Photodynamic therapy in oncology. Oncologist. 2006;11:1034–1044. doi: 10.1634/theoncologist.11-9-1034. [DOI] [PubMed] [Google Scholar]
- 7.Dougherty T, Kaufman J, Goldfarb A, Weishaupt K, Boyle D, Mittleman A. Photoradiation therapy for treatment of malignant tumors. Cancer Res. 1978;38:2628–2635. [PubMed] [Google Scholar]
- 8.Miller JD, Baron ED, Scull H, Hsia A, Berlin JC, McCormick T, Colussi V, Kenney ME, Cooper KD, Oleinick NL. Photodynamic therapy with the phthalocyanine photosensitizer Pc 4: the case experience with preclinical mechanistic and early clinical-translational studies. Toxicology and Applied Pharmacology. 2007;224:290–299. doi: 10.1016/j.taap.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sibata CH, Colussi VC, Oleinick NL, Kinsella TJ. Photodynamic therapy in oncology. Expert Opin Pharmacother. 2001;2:917–27. doi: 10.1517/14656566.2.6.917. [DOI] [PubMed] [Google Scholar]
- 10.Huang Z. A review of progress in clinical photodynamic therapy. Technol Cancer Res Treat. 2005;4:283. doi: 10.1177/153303460500400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biel MA. Photodynamic therapy treatment of early oral and laryngeal cancers. Photochemistry and Photobiology. 2007;83:1063–1068. doi: 10.1111/j.1751-1097.2007.00153.x. [DOI] [PubMed] [Google Scholar]
- 12.Copper MP, Triesscheijn M, Tan IB, Ruevekamp MC, Stewart F. A Photodynamic therapy in the treatment of multiple primary tumours in the head and neck, located to the oral cavity and oropharynx. Clin Otolaryngol. 2007;32:185–9. doi: 10.1111/j.1365-2273.2007.01441.x. [DOI] [PubMed] [Google Scholar]
- 13.Biel M. Advances in photodynamic therapy for the treatment of head and neck cancers. Lasers Surg Med. 2006;38:349–55. doi: 10.1002/lsm.20368. [DOI] [PubMed] [Google Scholar]
- 14.Betz CS, Rauschning W, Stranadko EP, Riabov MV, Albrecht V, Nifantiev NE, Hopper C. Optimization of treatment parameters for Foscan-PDT of basal cell carcinomas. 2008;40:300–311. doi: 10.1002/lsm.20632. [DOI] [PubMed] [Google Scholar]
- 15.Allison R, Mota H, Sibata CH. Clinical PD/PDT in North America: An historical review. Photodiagnosis and Photodynamic Therapy. 2004;1:263–277. doi: 10.1016/S1572-1000(04)00084-5. [DOI] [PubMed] [Google Scholar]
- 16.Solban N, Rizvi I, Hasan T. Targeted photodynamic therapy. Lasers Surg Med. 2006;38:522–31. doi: 10.1002/lsm.20345. [DOI] [PubMed] [Google Scholar]
- 17.Egorin MJ, Zuhowski EG, Sentz DL, Dobson JM, Callery PS, Eiseman JL. Plasma pharmacokinetics and tissue distribution in CD2F1 mice of Pc4 (NSC 676418), a silicone phthalocyanine photodynamic sensitizing agent. Cancer Chemother Pharmacol. 1999;44:283–94. doi: 10.1007/s002800050979. [DOI] [PubMed] [Google Scholar]
- 18.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 19.Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63:136–51. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Farokhzad OC, Karp JM, Langer R. Nanoparticle-aptamer bioconjugates for cancer targeting. Expert Opinion On Drug Delivery. 2006;3:311–324. doi: 10.1517/17425247.3.3.311. [DOI] [PubMed] [Google Scholar]
- 21.Schneider R, Schmitt F, Frochot C, Fort Y, Lourette N, Guillemin F, Müller JF, Barberi-Heyob M. Design, synthesis, and biological evaluation of folic acid targeted tetraphenylporphyrin as novel photosensitizers for selective photodynamic therapy. Bioorganic & Medicinal Chemistry. 2005;13:2799–2808. doi: 10.1016/j.bmc.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee DK, Fong LS, Zhang Y. Nanoparticles in photodynamic therapy: an emerging paradigm. Adv Drug Deliv Rev. 2008;60:1627–37. doi: 10.1016/j.addr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Allison RR, Mota HC, Bagnato VS, Sibata CH. Bio-nanotechnology and photodynamic therapy--state of the art review. Photodiagnosis Photodyn Ther. 2008;5:19–28. doi: 10.1016/j.pdpdt.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Marur S, Forastiere A. A Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83:489–501. doi: 10.4065/83.4.489. [DOI] [PubMed] [Google Scholar]
- 25.Oda K, Matsuoka Y, Funahashi A, Kitano H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol. 2005;1:2005.0010. doi: 10.1038/msb4100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993;53:3579–3584. [PubMed] [Google Scholar]
- 27.Zimmermann M, Zouhair A, Azria D, Ozsahin M. The epidermal growth factor receptor (EGFR) in head and neck cancer: its role and treatment implications. Radiation oncology London England. 2006;1:11. doi: 10.1186/1748-717X-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Astsaturov I, Cohen RB, Harari PM. Clinical application of EGFR inhibitors in head and neck squamous cell cancer. Cancer Treat Res. 2008;139:135–52. [PubMed] [Google Scholar]
- 29.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–74. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 30.Master AM, Rodriguez ME, Kenney ME, Oleinick NL, Sen Gupta A. Delivery of the photosensitizer Pc 4 in PEG–PCL micelles for in vitro PDT studies. J Pharm Sci. 2010;99:2386–2398. doi: 10.1002/jps.22007. [DOI] [PubMed] [Google Scholar]
- 31.Master AM, Qi Y, Oleinick NL, Sen Gupta A. EGFR-mediated Intracellular Delivery of Pc 4 Nanoformulation for Targeted Photodynamic Therapy of Cancer: In Vitro Studies. Nanomedicine. 2011;8:655–64. doi: 10.1016/j.nano.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Master AM, Livingston M, Oleinick NL, Sen Gupta A. Optimization of a Nanomedicine-Based Silicon Phthalocyanine 4 Photodynamic Therapy (Pc 4-PDT) Strategy for Targeted Treatment of EGFR-Overexpressing Cancers. Mol Pharm. 2012 doi: 10.1021/mp300256e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mamot C, Drummond DC, Greiser U, Hong K, Kirpotin DB, Marks JD, Park JW, Cells T. Epidermal growth factor receptor (EGFR)-targeted immunoliposomes mediate specific and efficient drug delivery to EGFR- and EGFRvIII-overexpressing tumor cells. Cancer Res. 2003;63:3154–61. [PubMed] [Google Scholar]
- 34.Kanick SC, Eiseman JL, Joseph E, Guo J, Parker RS. Noninvasive and nondestructive optical spectroscopic measurement of motexafin gadolinium in mouse tissues: comparison to high-performance liquid chromatography. J Photochem Photobiol B, Biol. 2007;88:90–104. doi: 10.1016/j.jphotobiol.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Oleinick NL, Antunez AR, Clay ME, Rihter BD, Kenney ME. New phthalocyanine photosensitizers for photodynamic therapy. Photochemistry and Photobiology. 1993;57:242–247. doi: 10.1111/j.1751-1097.1993.tb02282.x. [DOI] [PubMed] [Google Scholar]
- 36.Siavash H, Nikitakis NG, Sauk JJ. Targeting of epidermal growth factor receptor by cyclopentenone prostaglandin 15-Deoxy-Delta12,14-prostaglandin J2 in human oral squamous carcinoma cells. Cancer Lett. 2004;211:97–103. doi: 10.1016/j.canlet.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Zhu TF, Szostak JW. Exploding vesicles. Journal of Systems Chemistry. 2011;2:4. [Google Scholar]
- 38.Patito IA, Rothmann C, Malik Z. Nuclear transport of photosensitizers during photosensitization and oxidative stress. Biology of the Cell. 2001;93:285–291. doi: 10.1016/s0248-4900(01)01118-2. [DOI] [PubMed] [Google Scholar]
- 39.Colussi VC, Feyes DK, Mulvihill JW, Li YS, Kenney ME, Elmets CA, Oleinick NL, Mukhtar H. Phthalocyanine 4 (Pc 4) photodynamic therapy of human OVCAR-3 tumor xenografts. Photochemistry and Photobiology. 1999;69:236–241. [PubMed] [Google Scholar]
- 40.George JE, Ahmad Y, Varghai D, Li X, Berlin J, Jackowe D, Jungermann M, Wolfe MS, Lilge L, Totonchi A, Morris RL, Peterson A, Lust WD, Kenney ME, Hoppel CL, Sun J, Oleinick NL, Dean D. Pc 4 photodynamic therapy of U87-derived human glioma in the nude rat. Lasers in Surgery and Medicine. 2005;36:383–389. doi: 10.1002/lsm.20185. [DOI] [PubMed] [Google Scholar]
- 41.Bareford LM, Swaan PW. Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev. 2007;59:748–58. doi: 10.1016/j.addr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franken NAP, Rodermond HM, Stap J, Haveman J, Van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–9. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 43.Weiss R, Donehower R, Wiernik P, Ohnuma T, Gralla R, Trump D, Baker J, Van Echo D, Von Hoff D, Leyland-Jones B. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8:1263–1268. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- 44.Price KS, Castells MC. Taxol reactions. Allergy Asthma Proc. 23:205–8. [PubMed] [Google Scholar]
- 45.Fader AN, Rose PG. Abraxane for the treatment of gynecologic cancer patients with severe hypersensitivity reactions to paclitaxel. Int J Gynecol Cancer. 2009;19:1281–3. doi: 10.1111/IGC.0b013e3181a38e2f. [DOI] [PubMed] [Google Scholar]
- 46.Bass J, Friedl W, Jeranek W. Intralipid causing adult respiratory distress syndrome. J Natl Med Assoc. 1984;76:401–3. 407. [PMC free article] [PubMed] [Google Scholar]
- 47.Kamath KR, Berry A, Cummins G. Acute hypersensitivity reaction to Intralipid. N Engl J Med. 1981;304:360. [PubMed] [Google Scholar]
- 48.Buchman A, Ament M. Comparative hypersensitivity in intravenous lipid emulsions. Journal of Parenteral and Enteral Nutrition. 1991;15:345–346. doi: 10.1177/0148607191015003345. [DOI] [PubMed] [Google Scholar]
- 49.D’Cruz AK, Robinson MH, Biel M. A mTHPC-mediated photodynamic therapy in patients with advanced, incurable head and neck cancer: a multicenter study of 128 patients. Head Neck. 2004;26:232–40. doi: 10.1002/hed.10372. [DOI] [PubMed] [Google Scholar]
- 50.Allison RR, Cuenca RE, Downie GH, Camnitz P, Brodish B, Sibata CH. Clinical photodynamic therapy of head and neck cancers—A review of applications and outcomes. Photodiagnosis and Photodynamic Therapy. 2005;2:205–222. doi: 10.1016/S1572-1000(05)00092-X. [DOI] [PubMed] [Google Scholar]
- 51.Senge MO, Brandt JC. Temoporfin (Foscan®, 5,10,15,20-tetra(m-hydroxyphenyl)chlorin)--a second-generation photosensitizer. Photochem Photobiol. 87:1240–96. doi: 10.1111/j.1751-1097.2011.00986.x. [DOI] [PubMed] [Google Scholar]
- 52.Biel M. Photodynamic therapy and the treatment of head and neck cancers. J Clin Laser Med Surg. 1996;14:239–244. doi: 10.1089/clm.1996.14.239. [DOI] [PubMed] [Google Scholar]
- 53.Betz CS, Jäger HR, Brookes JAS, Richards R, Leunig A, Hopper C. Interstitial photodynamic therapy for a symptom-targeted treatment of complex vascular malformations in the head and neck region. Lasers Surg Med. 2007;39:571–82. doi: 10.1002/lsm.20535. [DOI] [PubMed] [Google Scholar]
- 54.Fan KF, Hopper C, Speight PM, Buonaccorsi G, MacRobert AJ, Bown SG. Photodynamic therapy using 5-aminolevulinic acid for premalignant and malignant lesions of the oral cavity. Cancer. 1996;78:1374–83. doi: 10.1002/(SICI)1097-0142(19961001)78:7<1374::AID-CNCR2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 55.Detty MR, Gibson SL, Wagner SJ. Current clinical and preclinical photosensitizers for use in photodynamic therapy. J Med Chem. 2004;47:3897–915. doi: 10.1021/jm040074b. [DOI] [PubMed] [Google Scholar]
- 56.Fei B, Wang H, Meyers JD, Feyes DK, Oleinick NL, Duerk JL. High-field magnetic resonance imaging of the response of human prostate cancer to Pc 4-based photodynamic therapy in an animal model. Lasers Surg Med. 2007;39:723–30. doi: 10.1002/lsm.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bai L, Guo J, FAB, Eiseman JL, Bontempo F. A The relationship of phthalocyanine 4 (pc 4) concentrations measured noninvasively to outcome of pc 4 photodynamic therapy in mice. Photochem Photobiol. 2009;85:1011–9. doi: 10.1111/j.1751-1097.2009.00542.x. [DOI] [PubMed] [Google Scholar]
- 58.Xue L, Chiu S, Fiebig A, Andrews DW, Oleinick NL. Photodamage to multiple Bcl-xL isoforms by photodynamic therapy with the phthalocyanine photosensitizer Pc 4. Oncogene. 2003;22:9197–204. doi: 10.1038/sj.onc.1207019. [DOI] [PubMed] [Google Scholar]
- 59.Megerian CA, Ziadi S, Sprecher R, Setrakian S, Stepnick D, Oleinick N, Mukhtar H. Photodynamic Therapy of Human Squamous Cell Carcinoma In Vitro and in Xenografts in Nude Mice. Laryngoscope. 1993;103:967. doi: 10.1288/00005537-199309000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Lee RG, Vecchiotti MA, Heaphy J, Panneerselvam A, Schluchter MD, Oleinick NL, Lavertu P, Alagramam KN, Arnold JE, Sprecher RC. Photodynamic therapy of cottontail rabbit papillomavirus-induced papillomas in a severe combined immunodeficient mouse xenograft system. Laryngoscope. 2010;120:618–24. doi: 10.1002/lary.20709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeisser-Labouèbe M. Nanoparticles for photodynamic therapy of cancer. In: Kumar C, editor. Nanotechnologies for the. Vol. 6. Wiley; 2007. pp. 40–86. [Google Scholar]
- 62.Wang S, Gao R, Zhou F, Selke M. Nanomaterials and singlet oxygen photosensitizers: potential applications in photodynamic therapy. J Mater Chem. 2004;14:487. [Google Scholar]
- 63.Bechet D, Couleaud P, Frochot C, Viriot ML, Guillemin F, Barberi-Heyob M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008;26:612–21. doi: 10.1016/j.tibtech.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 64.Peng XH, Qian X, Mao H, Wang AY, Chen ZG, Nie S, Shin DM. Targeted magnetic iron oxide nanoparticles for tumor imaging and therapy. Int J Nanomedicine. 2008;3:311–21. doi: 10.2147/ijn.s2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen EM, Ding H, Kessinger CW, Khemtong C, Gao J, Sumer BD. Polymeric micelle nanoparticles for photodynamic treatment of head and neck cancer cells. Otolaryngol Head Neck Surg. 2010;143:109–15. doi: 10.1016/j.otohns.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 66.Besic Gyenge E, Darphin X, Wirth A, Pieles U, Walt H, Bredell M, Maake C. Uptake and fate of surface modified silica nanoparticles in head and neck squamous cell carcinoma. J Nanobiotechnology. 2011;9:32. doi: 10.1186/1477-3155-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mickler FM, Möckl L, Ruthardt N, Ogris M, Wagner E, Bräuchle C. Tuning nanoparticle uptake: live-cell imaging reveals two distinct endocytosis mechanisms mediated by natural and artificial EGFR targeting ligand. Nano Lett. 2012;12:3417–23. doi: 10.1021/nl300395q. [DOI] [PubMed] [Google Scholar]