Abstract
Photodynamic therapy (PDT) is a promising treatment strategy where activation of photosensitizer drugs with specific wavelengths of light results in energy transfer cascades that ultimately yield cytotoxic reactive oxygen species which can render apoptotic and necrotic cell death. Without light the photosensitizer drugs are minimally toxic and the photoactivating light itself is non-ionizing. Therefore, harnessing this mechanism in tumors provides a safe and novel way to selectively eradicate tumor with reduced systemic toxicity and side effects on healthy tissues. For successful PDT of solid tumors, it is necessary to ensure tumor-selective delivery of the photosensitizers, as well as, the photoactivating light and to establish dosimetric correlation of light and drug parameters to PDT-induced tumor response. To this end, the nanomedicine approach provides a promising way towards enhanced control of photosensitizer biodistribution and tumor-selective delivery. In addition, refinement of nanoparticle designs can also allow incorporation of imaging agents, light delivery components and dosimetric components. This review aims at describing the current state-of-the-art regarding nanomedicine strategies in PDT, with a comprehensive narrative of the research that has been carried out in vitro and in vivo, with a discussion of the nanoformulation design aspects and a perspective on the promise and challenges of PDT regarding successful translation into clinical application.
Keywords: nanomedicine, drug delivery, photodynamic therapy, cancer, targeted nanoparticles
1. Introduction
The global burden of cancer-related morbidity and mortality continues to be significant, with close to 8 million deaths annually [1]. Current clinical strategies for treating cancer include surgery, radiation therapy, chemotherapy, and more recently, immunotherapy and other small molecule-based therapies, along with combination of these strategies. Surgery is often the first attempted strategy though it may not be a stand-alone option if metastasis has occurred. Surgical removal of tumor is also not a viable method in cases where the tumor is located in sensitive areas, for example, in the vicinity of the spinal cord or near other vital organs. Radiation therapy damages the DNA of cells due to the ionizing nature of the radiation, which can prevent tumor cell replication and possibly shrink the tumor, if selectively focused on the tumor. However, radiotherapy frequently leads to long term side effects including damage to normal neighboring cells, formation of scar tissue, and immunosuppression. Chemotherapeutic agents are either cytostatic or cytotoxic molecules that can arrest or kill quickly dividing cell lines within the body. A chemotherapeutic agent is usually intravenously delivered yielding systemic effects. This approach may be advantageous when dealing with metastasized tumors but the concomitant challenge involves balancing the therapeutic effect of the drug with undesirable systemic toxic sideeffects [2]. In recent years the benefits of chemotherapy and other small molecule drugs have been significantly enhanced via the nanomedicine approach where the drug molecules are packaged within nanovehicles that keep the drug in circulation for longer periods of time (by preventing renal clearance and non-specific uptake) and allow increased uptake within the tumors via extravasation through the tumor-associated leaky vasculature (the enhanced permeation and retention or EPR effect) [3–5]. Immunotherapy works by enabling the patient's natural immune system to fight the cancer or reducing inherent signaling cascade mechanisms that promote cancer proliferation and aggression, by using cancer vaccines and cancer cell receptor-targeted monoclonal antibodies. However, cancer cells are prone to mutation leading to limited effectiveness of highly specific immunotherapies. Nevertheless, immunotherapy strategies in combination with chemo- or radiotherapy have recently shown clinical benefit in certain cancers [6–8].
While refinement of the above described conventional cancer treatment modalities to improve their tumorselectivity is a significant part of current cancer research, investigations have also focused on developing alternate treatment modalities that may be safer or more beneficial in cases where extensive surgery, chemotherapy or radiotherapy may be limited due to risk of cosmetic and functional tissue damage. To this end, a promising strategy is photodynamic therapy (PDT). PDT involves three components: a photosensitizer (PS) drug, a specific wavelength of drug-activating light and oxygen. Light activation of a photosensitizer results in energy transfer cascades that ultimately yield cytotoxic reactive oxygen species (Figure 1) which can then render apoptotic and necrotic cell death. Since the first modern demonstration of harnessing this mechanism to kill tumor cells by Dougherty et al. in 1978, PDT has undergone extensive investigations in cancer treatment [9] (Figure 2). A major advantage of PDT over conventional chemotherapy is that both the PS itself is minimally toxic in the absence of light and hence PS accumulation in non-specific tissues confers minimal systemic toxicity. Furthermore, compared to radiotherapy, the activating light is non-ionizing and hence its effect on tissues without the PS drug is not harmful. As a result, PDT has the potential to be repeated safely if needed without the risk of harming neighboring healthy tissue [10]. This makes PDT very attractive for tumors where loco-regionally recurrent treatment may be needed. By virtue of these characteristics, PDT can offer the possibility of dualselectivity in cancer therapy by developing technologies that ensure PS accumulation selectively in the tumor and light irradiation selectively on the tumor [11]. The clinical interest in PDT is exhibited by health agency approvals with the PS formulations Photofrin®, Levulan®, and Visudyne® in the US, various other formulations (Foscan®, Photosense®, Metvix®) in other countries, and ongoing clinical trials [12, 13]. Table 1 provides a listing of some of the current clinically approved uses of PDT. As evident from Table 1, PDT is currently being used mainly to treat skin diseases and easily accessible malignant and pre-malignant lesions. One reason for this limited clinical repertoire is the challenge associated with PS delivery. Most PS molecules are highly hydrophobic and hence difficult to be incorporated into intravenously deliverable formulations. In most current clinical applications the PS is formulated in lipidic (e.g. Cremophor) or organic (e.g. dextrose, propylene glycol etc.) excipients which are reasonable for topical and local administration, but can lead to unpredictable biodistribution profiles, allergy, hypersensitivity and toxicity issues if used intravenously, especially in multiple doses. The unpredictable biodistribution can lead to PS accumulation in easily light-exposed uninvolved tissues like the eyes and skin, which in turn result in prolonged phototoxicity and photosensitivity in patients when exposed to ambient light. One typical example is Foscan®, an ethanol/propylene glycol formulation of the PS temoporfin, which had failed to achieve FDA approval for treatment of head-and-neck cancers due to poor tumor selectivity, high plasma retention, and serious burns arising from skin photosensitivity [14–17]. As for excipients like Cremophor, the issues with allergy, hypersensitivity and toxicity are well known [18,19]. The other challenge in enhancing the clinical repertoire of PDT is appropriate delivery of the drug-activating light. Easily accessible tissues can undergo convenient illumination for PDT, but deep tissue tumors may present limitations to light penetration. For example, blue light (400-450 nm) penetrates < 1 mm into tissue while orange light (590-620 nm) penetrates approximately 1.5 mm and red light (620-750 nm) penetrates even further up to 3 mm [20].
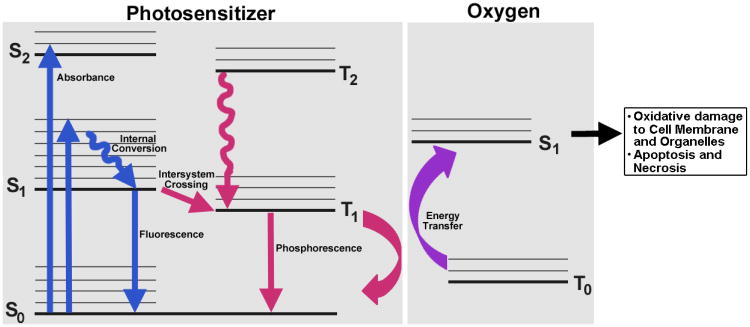
Figure 1.
Jablonski diagram depicting the electronic transition states and energy transfer phenomena between the photosensitizer molecule and oxygen in photodynamic therapy that ultimately leads to oxidative cell damage.
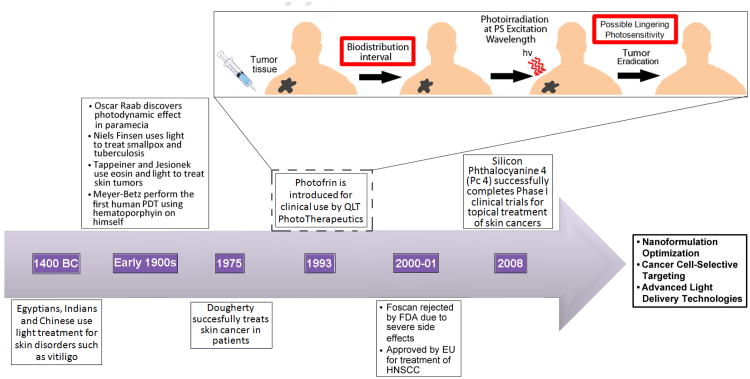
Figure 2. The history, development and current state-of-the-art for PDT.
Table 1. Current clinically-approved photosensitizer formulations and their corresponding applications.
| Trade Name | Drug | Company | Approval Information | Application | Excipient Formulation | Activation Wavelength | Chemical Structure | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Photofrin® | Hematoporphyrin derivative | Axcan Pharm | Canada, 1993 Japan, 1994 USA, 1995 | Bladder cancer, lung cancer, esophageal cancer, Barrett'sesophagus | Dextrose or NaCl injection | 630 nm |
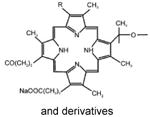
|
||||
| Metvixia® | Methyl aminolevulinate | Photocure/Galderma | USA,2004 EU, 2001 New Zealand, 2002 Australia, 2003 | Actinic keratosis, basal cell carcinoma, non-melanoma skin cancer | Topical cream (includes cetostearyl alcohol and peanut oil) | 630 nm |
|
||||
| Foscan® | Temeporfin/m-THPC | Biolitec Pharma | EU, 2001 | Head and neck cancer | Ethanol/Propylene Glycol | 652 nm |
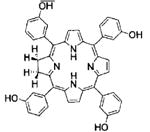
|
||||
| Visudyne® | Verteporfin/benzoporphyrin derivative monoacid | Novartis | USA, 2000 Canada, 2000 EU, 2000 Japan, 2003 | Wet age-related macular degeneration, pathologic myopia, histoplasmosis | Liposomal | 690 nm |
|
||||
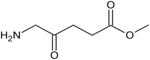
| Levulan® | Aminolevulinic acid | DUSA Pharma | USA, 1999 | Actinic keratosis, esophageal dysplasia | Topical cream | 417 nm |
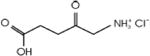
|
||||
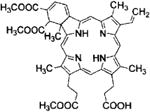
| Laserphyrin® | Talaporfin/Mono-L-aspartyl chlorin e6 | Meiji Seika Pharma | Japan, 2004 | Lung cancer | Saline injection | 664 nm |
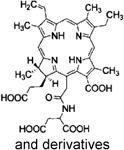
|
||||
| Photosens® | Sulfonated aluminum phthalocyanine | NIOPIK | Russia, 2001 | Various cancers | NaCl injection | 675 nm |
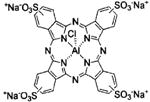
|
||||
While advances in fiber-optic based laser technologies can provide novel avenues to deliver the drug-activating light interstitially and intra-tumorally, the nanomedicine approach can provide an effective way to resolve the challenges associated with intravenous administration and selective delivery of the PS to deep tissue tumors. Several recent reviews have highlighted the various nanoparticle designs that are being investigated for the encapsulation and controlled release of photosensitizers [21–25]. Some other review articles have summarized the progress of PDT as such, in the clinical arena of cancer treatment [26–28]. However, a state-of-the-art review of in vitro and in vivo PDT studies using nanovehicle-based photosensitizer delivery, along with a critical discussion of design parameters for efficient nanoformulation of photosensitizers, perspectives and challenges regarding in vivo drug distribution and tumor-selective light delivery is not currently available. Therefore, we have attempted to provide this comprehensive information, to aid future research in photodynamic nanomedicine.
2. In vitro and in vivo investigations in nanoparticle-based PDT
2.1. In vitro studies in photodynamic nanomedicine
Nanoparticle-mediated PDT of cancer has been studied using a wide variety of photosensitizers and nanovehicles. The majority of published reports in this area focus on in vitro studies, most likely due to several in vivo challenges such as ensuring site-selectivity of drug delivery, difficulties in light delivery and variabilities in oxygen levels due to tumor hypoxia. These challenges and ongoing research approaches to resolve them are discussed later in this article after reviewing the in vivo studies. Table 2 provides a comprehensive list of reported in vitro studies, including the various photosensitizers, the corresponding nanovehicles used for their formulation and the corresponding cancer cell lines that these formulations have been tested on, along with appropriate references. As evident from Table 2, most photosensitizers tested fall under a few main categories, namely, porphyrins, chlorins and phthalocyanines. In addition, some other dyes like hypericin, hypocrellin, indocyanine and methylene blue have been studied as photosensitizers. In the following sections we describe the various in vitro nanomedicine studies that have been carried out under these categories of photosensitizers.
Table 2. In vitro PDT studies reported on nanoparticle-based photosensitizer formulations.
| Photosensitizer | NP Vehicle | Targeting Motif | Cell Type | Ref. |
|---|---|---|---|---|
| Porphyrin derivatives (e.g. hematoporphyrin, porfimer sodium etc.) | Human serum albumin nanoparticles | N/A (Passive) | Jurkat (human T-cell leukemia) | [29–31] |
| Metal oxide nanopartices | N/A (Passive) | A549 (human lung) NIH:OVCAR-3 (human ovarian) |
[32, 33] | |
| AS1411 aptamer | MCF-7 (human breast) | [34] | ||
| Magnetic chitosan nanoparticles | N/A (Passive) | SW480 (human colon) | [35] | |
| Silica nanoparticles | N/A (Passive) | SW480 (human colon) HCT 116 (human colon) HT-29 (human colon) MCF-7 (human breast) MDA-MB-231(human breast) A431 (human epidermoid) LLBC37 (lymphoblastoid) HeLa (human cervical) |
[36–38] | |
| Mannose | MDA-MB-231 (human breast) | [39, 40] | ||
| Virus-like particles | Glycan-targeting of CD22 | CHO (Chinese hamster ovary) | [54] | |
| Polymeric nanoparticles | N/A (Passive) | SW480 (human colon) H2009 (human lung) HeLa (human cervical) HepG2 (human liver) C6 (rat glioma) |
[41–43, 45, 50] | |
| Folic acid | HeLa (human cervical) | [44] | ||
| F3 peptide | MDA-MB-435 (human breast) F98 (rat glioma) MCF-7 (human breast) 9L(rat gliosarcoma) |
[46–49] | ||
| Liposomes | N/A (Passive) | HCT-116 (human colorectal) DU 145 (human prostate) |
[93] | |
| Fluorescence organic nanoparticles | N/A (Passive) | CL1-0 (human lung) HeLa (human cervical) |
[94] | |
| Marine atelocollagen nanocapsules | N/A (Passive) | HeLa (human cervical) | [95] | |
| Gold nanoparticles | N/A (Passive) | Jurkat (human T-cell leukemia) K562 (human leukemia) MDA-MB-231 (human breast) |
[51–53] | |
| Chemiluminescence nanoparticles | N/A (Passive) | C6 (rat glioma) LoVo (human colon) |
[96] | |
| Chlorin derivatives(chlorin e6, mTHPC, pheophorbide etc) | Chitosan nanoparticles | N/A (Passive) | MDA-MB-231 (human breast) HeLa (human cervical) SCC-7 (murine squamous cell carcinoma) |
[97, 98] |
| Human serum albumin nanoparticles | N/A (Passive) | HeLa (human cervical) | [99] | |
| N/A (Passive) | Jurkat (human T-cell leukemia) | [29–31] | ||
| NaYF4:Yb,Er/NaGdF4 nanoparticles | N/A (Passive) | U87MG (human glioma) | [64] | |
| N/A (Passive) | HeLa (human cervical) 4T1 (murine breast) |
[65] | ||
| Iron oxide nanoparticles | N/A (Passive) | MGC-803 (human gastric) | [63] | |
| Mesoporous silica nanoparticles and Hyaluronic acid nanoparticles | Hyaluronic acid targeting of CD44 | HT-29 (human colon) HCT-116 (human colorectal) |
[61, 100] | |
| Polymeric nanoparticles | N/A (Passive) | EMT-6 (murine breast) Colon-26 (murine colon) C6 (rat glioma) |
[101–103] | |
| Folate | HeLa (human cervical) | [62] | ||
| Neuropilin-I targeting peptide | MDA-MB-231 (human breast) | [60] | ||
| Silica nanoparticles | N/A (Passive) | RIF-1 (murine fibrosarcoma) | [104, 105] | |
| Liposomes | Cetuximab (C225)Ki-67 antibody | OVCAR-5 (human ovarian) A431 (human epidermoid) |
[106, 107] | |
| Phthalocyanine derivatives(zinc (II) phthalocyanine, silicon phthalocyanine 4 etc.) | NaGdF4:Yb,Er/NaGdF4 nanoparticles | N/A (Passive) | MEAR (murine hepatoma) HeLa (human cervical) |
[77, 78] |
| MB49 (murine bladder) | [55] | |||
| Polymeric nanoparticles | GE11 peptide | MCF-7 (human breast) A431 (human epidermoid) |
[56, 69, 70] | |
| Gold nanoparticles | EGF | HeLa (human cervical) 9L (rat glioma) |
[71, 72] | |
| T antigen-specific lectin | HT-29 (human colon) | [74] | ||
| Anti-HER2 antibody | SK-BR-3 (human breast) MDA-MB-231 (human breast) |
[75] | ||
| N/A (Passive) | MCF-7 (human breast) | [76] | ||
| Mesoporous silica nanoparticles | N/A (Passive) | H22 (murine hepatoma) | [108] | |
| PLGA nanoparticles | N/A (Passive) | P388-D1 (murine lymphoma) | [68] | |
| Dendrimers | N/A (Passive) | A549 (human lung) | [109] | |
| Liposomes | N/A (Passive) | MCF-7 (human breast) | [76] | |
| LDL nanoparticles | N/A (Passive) | HepG2 (human liver) | [110] | |
| Cyanine IR-768 | Poly(n-butyl cyanoacrylate) nanocapsules | N/A (Passive) | MCF-7 (human breast) | [79] |
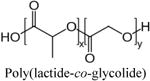
| Hypericin | PLA and PLGA nanoparticles | N/A (Passive) | NuTu-19 (rat ovarian) | [59] |
| Hypocrellin A | Silica nanoparticles | N/A (Passive) | HeLa (human cervical) | [85] |
| Indocyanine green | Poly(propargyl acrylate) | N/A (Passive) | HepG2 (human liver) P388-D1 (murine lymphoma) |
[80, 81] |
| ORMOSIL | Anti-HER-2 antibody | LNCaP (human prostate) | [82] | |
| Gold nanoparticles | Anti-EGFR antibody | A549 (human lung) | [83] | |
| Calcium phosphosilicate | Anti-CD117 antibody (anti-SCFR) | 32D-p210-GFP (murine CML) Human patient AML cells |
[84] | |
| C60 and C70 (fullerene cages) | Viral nanoparticles | N/A (Passive) | PC-3 (human prostate) | [86] |
| Cyclodextrin nanoparticles | N/A (Passive) | HeLa (human cervical) | [87] | |
| Fullerene nanocages | N/A (Passive) | HeLa (human cervical) | [88] | |
| Methylene blue | Apoferritin nanocages | N/A (Passive) | MCF-7 (human breast) | [89] |
| Dioctyl sodium sulfosuccinate (aerosol OT; AOT) alginate nanoparticles | N/A (Passive) | MCF-7 (human breast) and 4T1 (murine metastatic breast) NCI/ADR-RES (human ovarian) |
[57, 92] | |
| Phosphonate-terminated silica nanoparticles | N/A (Passive) | HeLa (human cervical) | [91] | |
| Polyacrylamide, sol-gel silica and ORMOSIL nanoparticles | N/A (Passive) | C6 (rat glioma) | [58, 90] |
Abbreviations
AML: Acute myeloid leukemia
CML: Chronic myeloid leukemia
EGF: Epidermal Growth Factor
LDL: low density lipoprotein
ORMOSIL: Organically modified silica
SCFR: Stem Cell Growth Factor Receptor
2.1.1. Porphyrin-based nanoformulations
Porphyrin derivatives are a major class of photosensitizer. Porfimer sodium or Photofrin is a member of this class and has been clinically approved for PDT of several pre-cancerous lesions and malignancies in the USA. The current clinical formulation does not involve a nanoparticle vehicle; however several promising pre-clinical studies have been carried out with nanoformulations of porphyrin photosensitizers. For example, Chen et al have used human serum albumin nanoparticles for delivery of 5,10,15,20-tetrakis(m-hydroxyphenyl)porphyrine (mTHPP; a porphyrin derivative) and pheophorbides (chlorin derivatives) to leukemia cells [29–31]. It was found that the nanoparticles were taken up through lysosomal mechanisms and caused ∼50% cell death due to apoptosis [31]. Porphyrin derivatives such as Photofrin and protoporphyrin IX have been formulated in several different nanoparticle systems such as metal oxide, chitosan, polymeric, silica and gold nanoparticles (examples shown in Figures 3A and 3B) [32–53]. While majority of these formulations have envisioned the EPR mechanism to be utilized to passively target solid tumors, some have utilized receptor-mediated active targeting to enhance cell-selective delivery. For example, Yin et al conjugated the AS1411 aptamer to multifunctional iron oxide nanoparticles packaging 5,10,15,20-tetrakis(1-methylpyridinium-4-yl) porphyrin (TMPyP4) for multimodal imaging and subsequent PDT of breast cancer [34]. Other examples where porphyrin PS have been packaged in nanoparticles capable of active targeting of cancer cells include mannose receptor-targeted silica nanoparticles, CD22-targeted viral nanoparticles and folate receptor-targeted polymeric nanoparticles [39, 40, 44, 54].
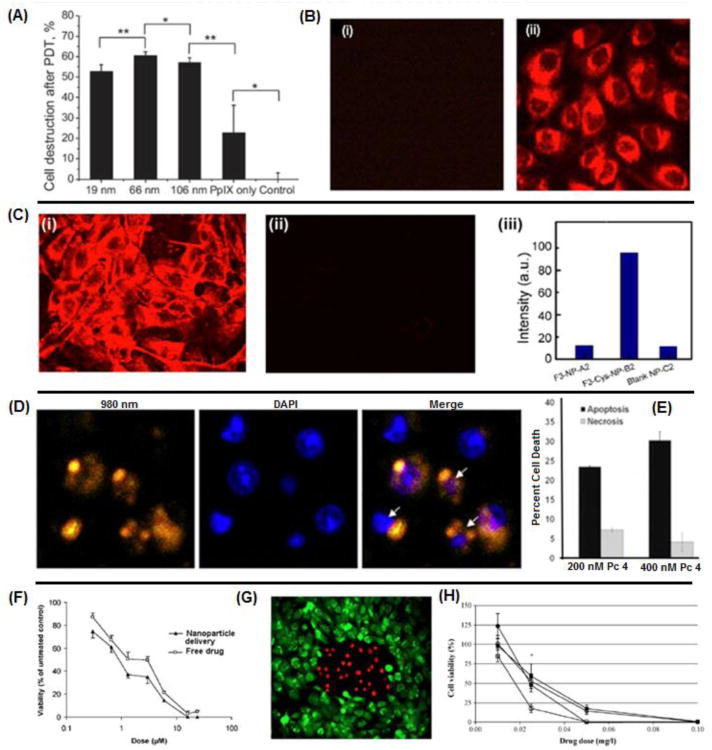
Figure 3.
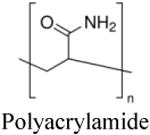
Representative images showing the feasibility of packaging photosensitizers in nanoparticle vehicles for delivery to cancer cells, in vitro; (A) percentage of breast cancer cell death caused by PDT with protoporphyrin IX loaded gold nanoparticles of different sizes compared to free drug; (B) intracellular uptake of porphyrin-derivative loaded silica nanoparticles after 4 hours incubation (ii) compared to cells alone (i); (C) F3 peptide-targeted polyacrylamide nanoparticles (i) loaded with 2-devinyl-2-(1- hexyloxyethyl) pyropheophorbide (HPPH), a chlorin-derivative, are taken up into 9L gliosarcoma cells faster than nontargeted analogs (ii), as corroborated by the quantitative fluorescence data (iii); (D) confocal microscopy of bladder carcinoma cells incubated with zinc (II) phthalocyanine loaded silica-coated upconversion nanoparticles, apoptotic cells are indicated by arrows; (E) increased percentage of cell death due to apoptosis and necrosis in breast cancer cells when treated with polymeric micelle-encapsulated silicon phthalocyanine photosensitizer Pc 4; (F) dose-response curve in breast cancer cells shows enhanced PDT efficacy when treated with nanoparticle encapsulated methylene blue compared to free drug; (G) cell death (red) following 2 hrs of incubation in glioma cells with nanoparticle-encapsulated methylene blue and irradiation compared to nonirradiated cells (green) and (H) decrease in ovarian cancer cell viability following treatment with free hypericin (circles) compared to nanoparticle-encapsulated hypericin (squares) at various drug dosages.
2.1.2. Chlorin-based nanoformulations
Chlorins form another major class of photosensitizers with a chemical structure similar to chlorophyll. The most popular drug in this class is chlorin e6, which has been studied in a variety of nanovehicles including chitosan, human serum albumin, silica, iron oxide, hyaluronic acid and various polymeric nanoparticles. Similar to the porphyrin-based nanoformulations, these chlorin-loaded nanoparticles were largely designed to utilize the EPR-based passive targeting mechanisms in breast, cervical, colon and brain cancers. However, a few approaches have also utilized active receptor-mediated targeting. For example, Benachour et al used a neuropilin-I targeting peptide on silica nanoparticles loaded with 5-(4-carboxyphenyl)-10,15,20-triphenylchlorin (TPC) to target tumorangiogenic vessels to achieve close to 100% cell death in vitro [60]. Yoon et al developed CD44 tumor-targeting hyaluronic acid nanoparticles (HANPs) as the carrier for chlorin e6 to achieve nearly 100% cell death in two different human colon cancer cell lines [61]. Similarly, folate receptor targeting was utilized by Li et al on pheophorbide A loaded heparin nanoparticles for delivery to cervical cancer [62]. More recent research with chlorin-based nanoformulations involves nanoparticles designed for combining imaging and PDT. For example, Huang et al have reported on chlorin e6 conjugated magnetic nanoparticles for concomitant MR imaging and PDT of gastric cancer [63]. Similarly, Wang et al developed tumor homing F3 peptide functionalized polyacrylamide nanoparticles loaded with 2-devinyl-2-(1- hexyloxyethyl) pyropheophorbide (HPPH) for the “see and treat” strategy through concurrent fluorescence imaging and PDT of gliosarcoma and breast carcinomas (example shown in Figure 3C) [49]. Another recent nanovehicle formulation of chlorins involve a unique platform called ‘upconversion nanoparticles’, that has been studied by a few groups for PDT of glioma, cervical and breast cancers [64, 65]. Upconversion is a process where sequential absorption of multiple photons can lead to emission of light at wavelengths shorter than the excitation wavelength (usually excitation in the infra-red range to render emission in the visible range) [66]. This emitted light can in turn activate the photosensitizer if upconversion elements (e.g. rare earth metals like Er3+) are formulated along with photosensitizers within the same vehicle. Hence this approach is potentially promising towards resolving challenges of ensuring light delivery in vivo.
2.1.3. Phthalocyanine-based nanoformulations
Phthalocyanine based photosensitizers, such as silicon phthalocyanine 4 (Pc 4) and zinc (II) phthalocyanine have been studied in several nanovehicles such as micelles, gold nanoparticles and silica nanoparticles. Phthalocyanine derivatives have also been packaged in mesoporous silica nanoparticles, PLGA nanoparticles, dendrimers, liposomes and LDL nanoparticles, without any receptor-targeted ligand decorations and tested on a variety of cancer cells in vitro. For example, Ricci-Junior et al have studied the delivery of zinc (II) phthalocyanine to murine lymophama cells via nontargeted PLGA nanoparticles to yield 60% cell death in vitro [67, 68]. Due to the lack of active targeting abilities and limited tumor selectivity, many of these non-targeted nanoparticle formulations have not progressed into efficient in vivo studies. In our research we have packaged Pc 4 in passively targeted polymeric nanoparticles for treatment of human breast and epidermoid carcinomas [56]. We later refined the nanoformulation with EGFR-specific peptides for active-targeting of head and neck carcinomas (Figure 3E) [69, 70]. Due to the high PDT-potency of Pc 4 and the superior cell-targeting capability of the nanoparticle platform, we were able to achieve high intracellular uptake and close to 100% cell death in vitro with drug dosages on the nanomolar scale [56, 69, 70]. Pc 4 has also been used in EGF-targeted gold nanoparticles by Cheng et al to target human cervical and rat glioma. However, it was found that to achieve effective levels of PDT in vitro, much higher doses of Pc 4 was required. This is possibly due to the fact that the drug was adsorbed on the nanoparticle surface where it is exposed to the plasma environment and prone to desorptive loss, rather than packaged within the nanoparticle [71–73]. Gold nanoparticles have also been used to package other phthalocyanine photosensitizers, and have been decorated with targeting moieties such as T-antigen specific lectins and anti-HER2 antibodies for selective delivery to colon and breast cancer [74–76]. Upconverting nanoparticles have been used for passive targeting of murine hepatoma and bladder cancer as well as human cervical cancer using PS such as zinc (II) phthalocyanine (e.g. Figure 3D) [55, 77, 78]. Zhao et al were able to achieve only 40% in vitro cell death on murine liver cancer cells but were able to successfully use the lanthanide-doped upconversion nanoparticles as a successful T1 and T2 contrast agent [77]. Qiao used similar nanoparticles but varied drug concentration, irradiation power and duration to yield up to 80% cell death along with successful imaging. However, it is unclear how much toxicity is due to the nanoparticle vehicle rather than the PDT induced effect. For example, Guo et al showed 50% cell death was caused by the upconversion nanoparticles alone [55]. These toxicity issues could prevent the nanoparticles from being used in vivo, and therefore more studies with these particles are needed to establish safety.
2.1.4. Other miscellaneous photosensitizers
Besides the above-described major classes of photosensitizers, several other photosensitizer molecules have also been investigated in nanoparticle-based formulations. Among these, cyanine IR-768 and indocyanine green (ICG) are cyanine compounds that can be activated by a higher wavelength (near infra-red, NIR) than most other photosensitizers, thus allowing for higher tissue penetration of the activating light. Pietkiewicz et al used oilcored poly(n-butyl cyanoacrylate) nanocapsules to deliver cyanine IR-768 to doxorubicin-sensitive and –resistant human breast cancer cell lines. They were able to show almost 100% cell death in vitro in both cell lines which shows promise towards using PDT in chemotherapy resistant cancers [79]. One limitation of the NIR or IRactivable photosensitizers is that due to their higher wavelength of activation, the energy transferred to the photosensitizer is lower resulting in a lower singlet oxygen yield. Despite this, several groups have studied ICG loaded nanoparticles for treatment of deep tissue cancers [80–84]. For example, Barth et al developed ICGloaded calcium phophosilicate nanoparticles conjugated with anti-CD117 antibody for active-receptor mediated targeting of leukemia stem cells. They were able to achieve 50% cell death in vitro before moving on to in vivo work [84]. Hypericin is a natural extract of St. John's wort previously used in cancer detection. It has recently gained popularity in PDT research due to its ability to cause photosensitivity when ingested past a threshold concentration. Like many photosensitizers, hypericin is highly hydrophobic. Zeisser-Labouebe et al have demonstrated formulation of hypericin in poly(lactic acid) (PLA) and poly(lactic-co-glycolic acid) (PLGA) nanoparticles for passive delivery to ovarian cancer (e.g. Figure 3H) [59]. In their studies it was found that they were able to use lower dosages to achieve 100% PDT-induced cell death in vitro when drug was delivered via nanoformulation compared to free drug [59]. Zhou et al experimented with the concept of heavy atom addition to photosensitizer-loaded silica nanoparticles to increase singlet oxygen production for enhanced PDT of cervical cancer. They used another less studied photosensitizer, hypocrellin A, and found that they could get up 90% cell death in vitro [85]. Fullerene cages such as C60 are a relatively new class of photosensitizers known for their carbon-only closed-cage polyhedron shape. Due to their plethora of pi-bond electrons, they can successfully emit fluorescence and participate in the cascade of energy transfer events that leads to the creation of singlet oxygen. They have been conjugated to the surface of viral nanoparticles, cyclodextrin nanoparticles and other fullerene nanocages and have been studied for the treatment of cervical and prostate cancer [86–88]. Methylene blue (MB) is another biological dye that has gained recent popularity as a photosensitizer due to its strong absorption band at 630 nm. It is often used as a model photosensitizer because its absorption wavelength is similar to Photofrin. A variety of nanoparticles have been studied with MB including apoferritin nanocages, polyacrylamide nanoparticles and silica nanoparticles (Figure 3G) [58, 89–91]. Khdair et al have developed aerosol alginate nanoparticles for simultaneous delivery of doxorubicin and MB for a chemotherapy-PDT combination treatment strategy (e.g. Figure 3F). Though lacking any active targeting motif, they were able to successfully enhance cytotoxicity in drug-resistant ovarian cancer cells [57, 92].
2.2. In vivo studies in photodynamic nanomedicine
As stated previously, majority of research reported regarding in nanoparticle-formulation based PDT involves in vitro studies, probably because many of the in vivo challenges regarding consistent levels of drug delivery, siteselective light delivery and sufficient oxygen levels in tumors have not been completely resolved yet. Nevertheless, several preclinical studies have been carried out using appropriate in vivo animal models, in subcutaneous and orthotopic xenografts, showing promising results.
Porphyrin derivatives, particularly first generation photosensitizers like porfimer sodium (Photofrin), have been studied in various murine models with different nanoparticles. Hu et al developed meso-tetra (carboxyphenyl) porphyrin (TCPP) -loaded PLGA nanoparticles and TCPP-nanoparticles to treat human colon cancer xenografts in mice [41]. It was found that mice treated with TCPP-nanoparticles and the TCPP-loaded PLGA nanoparticles demonstrated significant tumor regression over time though the tumors did eventually grow back to their original volume or higher after 25 days [41]. In a similar study, Reddy et al used F3 peptide-targeted iron oxide nanoparticles loaded with Photofrin to increase the median survival of mice with orthotopic rat gliomas from 7 days (with saline treatment) to 33 days [46]. Some groups have attempted to develop multifunctional porphyrin derivative loaded nanoparticles for dual imaging and PDT. For example, Sun et al used magnetic chitosan nanoparticles conjugated to 2,7,12,18-tetramethyl-3,8-di-(1-propoxyethyl)- 13,17-bis-(3-hydroxypropyl) porphyrin (PHPP) to treat mice infected with human colon cancer xenografts [35]. Following magnetically induced targeting, they were able to induce significant reduction in tumor size compared with the nontargeted tumors (Figure 4A) [35].
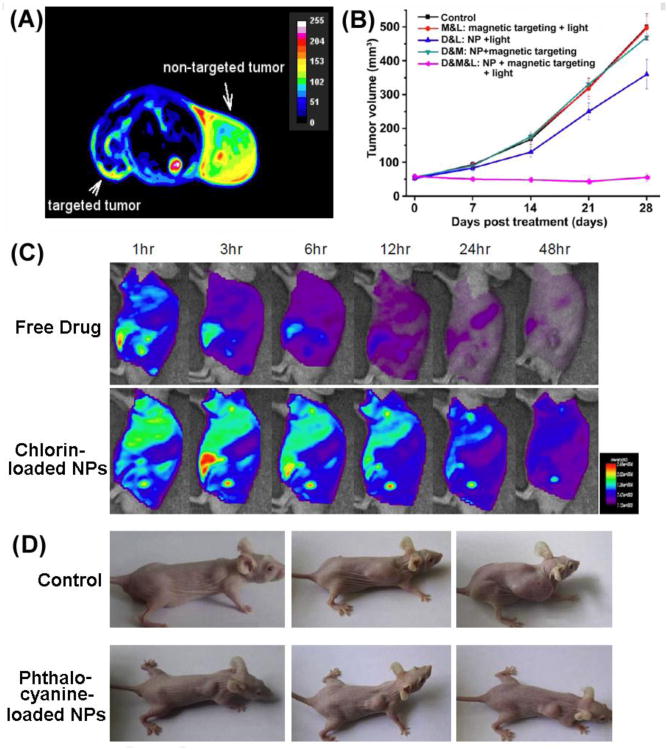
Figure 4.
Representative images of in vivo studies of nanoformulation-mediated PDT; (A) T2 weighted image of porphyrin derivative loaded magnetic chitosan NPs showing reduction in magnetically targeted tumor (left) compared to nontargeted tumor (right); (B) Tumor volume after treatment with chlorin-conjugated iron oxide NPs showing tumor stagnation in the group receiving magnetically localized NPs followed by subsequent PDT; (C) Tumor targeting ability of chlorin-loaded NPs compared to free drug as seen by fluorescence imaging of the tumor hotspot and (D) tumor growth suppression caused by phthalocyanine-loaded NPs in hepatoma xenografts.
Chlorin-based PDT nanoformulations have also progressed into in vivo studies. Chlorin e6 (Ce6) has been loaded inside human serum albumin (HSA), glycol chitosan (HGC), iron oxide, hyaluronic acid (HA) and upconversion nanoparticles for in vivo PDT investigations [61, 63, 64, 98, 99]. Several research groups have developed Ce6-HSA nanoparticles, Ce6-chitosan nanoparticles and Ce6-hyaluronic acid nanoparticles for the study of colon cancer. The nanoparticle formulations exhibit significant growth inhibition of colon cancer xenografts in mouse models compared to treatment with free Ce6 (Figure 4C) [61, 98, 99]. Another commonly used chlorin derivative is 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH). HPPH-loaded polyacrylamide nanoparticles (PAA) developed by Wang et al and Gupta et al have been studied in multiple murine models [102, 111]. Wang et al demonstrated that the rate of growth inhibition in a murine colon cancer xenograft model was dependent on both the HPPH dosage and light fluence [102]. Using the same tumor model, Gupta et al showed that formulations of HPPH-loaded PAA nanoparticles significantly inhibited the growth of the tumors compared to saline treatment [111]. Similar to the work discussed previously with porphyrin derivatives, iron oxide nanoparticles have been studied for concomitant imaging and PDT. Huang et al developed a single platform consisting of Ce6 conjugated iron oxide nanoparticles for magnetically guided drug delivery and subsequent PDT. It was found that tumor size stayed relatively stagnant over a twenty eight day period following nanoparticle administration and PDT (Figure 4B) [63]. A newer area of research involves upconversion nanoparticles which convert lower level excitation photons to higher energy NIR photons for enhanced PDT. Park et al used Ce6-conjugated upconversion nanoparticles (UCNPs) to treat human glioma xenografts in mice [64]. IV injection of UCNPs resulted in significant tumor regression compared to controls.
Phthalocyanine photosensitizers have also been studied in several animal models. For example, Tu et al developed zinc-phthalocyanine (ZnPc) loaded mesoporous silica nanoparticles (MSNs) to treat human hepatoma xenografts in mice (Figure 4D) [108]. They were able to increase the lifetime of the treated animals from 16 days to 40 days. Cheng et al used silicon phthalocyanine 4 (Pc 4)-conjugated gold nanoparticles to treat orthotopic human gliomas in a murine model and found that they were able to induce tumor necrosis [73]. Using a different approach, Nishiyama et al loaded phthalocyanine dendrimers inside of polymeric micelles and used them to treat human lung cancer xenografts in mice [109]. Thirty days after treatment, tumors treated with nanoparticles had only grown to 10 times their starting size, while tumors treated with a control grew to 25 times their starting size. As stated previously, we have developed EGFR-targeted Pc 4-loaded polymeric micelles and have demonstrated their capability for tumor-targeted delivery of Pc 4 for enhanced PDT. Our ongoing studies are focused on evaluating this Pc 4 formulation for PDT of head and neck carcinomas in mouse models.
Among other photosensitizer nanoformulations, indocyanine green (ICG)-loaded calcium phosphosilicate nanoparticles developed by Barth et al were tested in a mouse model to determine treatment efficacy against orthotopic murine chronic myeloid leukemia [84]. This formulation was found to both decrease the size of the tumors and increase the life span of the mice tested. He et al found methylene blue-loaded phosponateterminated silica nanoparticles led to tumor necrosis and shrinkage in mice upon light activation [91].
3. Current state-of-the-art and challenges for nanoparticle-based PDT
3.1. Nanoparticle design parameters
As evident from Table 2 and Table 3, nanoparticles provide an effective way to package a variety of photosensitizer molecules and deliver them via passive and active targeting pathways to tumor cells. Table 5 lists the various nanoparticle systems (along with representative construct components) that have been studied for photosensitizer delivery. In this context, it is also important to recognize the critical design parameters for nanoparticulate vehicles, as listed in Table 4, that influence efficient packaging and enhanced delivery of photosensitizers to deep tissue tumors in vivo. The plasma half-life of the photosensitizer-loaded nanovehicles is dependent upon renal clearance extent and also on the rate of reticuloendothelial system (RES) uptake-based clearance (e.g. in the liver). The renal elimination can be avoided by fabricating nanovehicles that are bigger than renal filtration threshold of 15 nm in diameter [113, 114]. The RES uptake can be avoided by minimizing plasma protein adsorption (opsonization) on the nanovehicle surface. It has been shown that making nanovehicle surface hydrophilic (e.g. by grafting polyethylene glycol (PEG) brushes onto the surface) or modulating the geometric parameters (size and shape) of the vehicle, can modulate the protein adsorption and macrophagic interaction, as well as, biotransport (e.g. margination to the wall and extravasation) properties, and thereby can enhance plasma half-life [115–122]. The increased half-life can allow for increased number of passages of the PS-loaded nanovehicles past the tumor site, thereby enhancing the possibility of EPR-mediated increased accumulation within the tumor stroma, resultant increased bioavailability of the encapsulated photosensitizer drug within the tumor, and significantly enhanced PDT efficacy, while avoiding side-effects in healthy tissues. An additional level of tumor-selectivity can be achieved by modifying the PS-loaded nanovehicle surface with antibodies, antibody fragments and ligands that bind to specific receptors upregulated on the cancer cells surface and thereby facilitate receptor-mediated cellular internalization of the payload [46–49, 56, 69, 70, 123–126].
Table 3. In vivo PDT studies reported on nanoparticle-based photosensitizer formulations.
| Photosensitizer | NP Vehicle | Targeting Motif | Cell Type | Ref. |
|---|---|---|---|---|
| Porphyrin derivatives (hematoporphyrin, porfimer sodium etc.) | PLGA nanoparticles | N/A (Passive) | SW480 (human colon cancer) | [41] |
| Polyacrylamide nanoparticles | F3 peptide | 9L (orthotopic rat glioma) | [46, 112] | |
| Magnetic chitosan nanoparticles | N/A (Passive) | SW480 (xenograft human colon) | [35] | |
| Silica nanoparticles | N/A (Passive) | HeLa (human colon cancer) HCT 116 (human colon cancer) A549 (human lung cancer) |
[37, 38] | |
| Chlorin derivatives (chlorin e6, mTHPC, pheophorbide etc) | Human serum albumin nanoparticles | N/A (Passive) | HT-29 (human colon cancer) | [99] |
| Chitosan nanoparticles | N/A (Passive) | HT-29 (human colon cancer) | [98] | |
| Iron oxide nanoparticles | N/A (Passive) | MGC-803 (human gastric cancer) | [63] | |
| NaYF4:Yb,Er/NaGdF4 nanoparticles | N/A (Passive) | U87MG (human glioma) | [64] | |
| Hyaluronic acid nanoparticles | Hyaluronic acid targeted to CD44 | HT-29 (human colon cancer) | [61] | |
| PLGA nanoparticles | N/A (Passive) | Rhabdomyosarcoma (orthotopic) | [101] | |
| Polyacrylamide nanoparticles | N/A (Passive) | Colon-26 (murine colon cancer) | [102, 111] | |
| Phthalocyanine derivatives (zinc (II) phthalocyanine, silicon phthalocyanine 4 etc.) | Mesoporous silica nanoparticles | N/A (Passive) | H22 (human hepatoma) | [108] |
| Dendrimers | N/A (Passive) | A549 (human lung cancer) | [109] | |
| Gold nanoparticles | EGF | Gli36 (orthotopic human glioma) | [73] | |
| Indocyanine green | Calcium phosphosilicate | Anti-CD117 antibody | 32D-p210-GFP (orthotopic murine chronic myeloid leukemia) | [84] |
| Methylene blue | Phosphonate-terminated silica nanoparticles | N/A (Passive) | HeLa (human cervical cancer) | [91] |
Table 5. Examples of nanoparticles systems along with components currently being studied for photosensitizer formulations.
| Type of Nanoparticle | Example of Nanoparticle Components | Reference | |
|---|---|---|---|

|
|
Master et al [56, 70] | |
|
|
Zhao et al [44] | ||
|
|
Sibata et al [136] | ||

|
|
Ichikawa et al [137] | |
|
Nawalany et al [93] | ||

|
|
Ricci-Junior et al [68] Hu et al [41] Tang et al [90] Tang et al [58] |
|
|
Wang et al [102] Gao et al [50] Reddy et al [46] Hah et al [47] Wang et al [49] |
||

|
|
Chen et al [36] Simon et al [37] Brevet et al [39] |
|

|
|
Cheng et al [71] Khaing et al [53] Gamaleia et al [51] Obaid et al [74] |
|
|
|
Zhao et al [77] Park et al [64] Qiao et al [78] Guo et al [55] |
|

|
|
Chen et al [96] |
Table 4. Design Parameters for Nanoparticle vehicles for Photosensitizer Delivery to Solid Tumors.
| Criteria | Rationale |
|---|---|
| Size and Shape |
|
| Plasma Half-Life |
|
| Drug Encapsulation Efficiency |
|
| Surface Charge (zeta potential) |
|
| Controlled Release Kinetics |
|
| Active Cell-Targeting Ability |
|
| Ease of Formulation and Scale Up |
|
| Possible Imaging Component |
|
3.2. Photosensitizer encapsulation considerations
Regarding encapsulation of photosensitizers in nanovehicles, it is important to note that unlike loading of chemotherapeutic drugs where higher encapsulation correlates to higher cytotoxic dose, for effective PDT the ideal dose may not be necessarily the maximum amount of photosensitizer that can be loaded. If the photosensitizer molecules aggregate extensively within the nanovehicle, upon photoirradiation they can self-quench the reactive oxygen species and thus reduce the photodynamic efficacy. Hence formulation of photosensitizers within nanovehicles should ensure sufficient loading that is photodynamicaly active but not aggregating and self-quenching [69]. Also, photosensitizer loading within nanovehicles should also ensure that while in circulation the drug molecules do not leak out or partition into the plasma. For this purpose, nanovehicle designs where the drug is physically trapped within the vehicle core (e.g. in micelles) or chemically conjugated to the vehicle (e.g. in certain polymer or silica based nanoparticles) may have advantage over systems where the drug molecules are adsorbed or conjugated onto the vehicle surface (e.g. in certain gold based nanoparticles).
Vehicle surface-adsorbed drug molecules can potentially desorb either by physical partitioning or chemical/enzymatic cleavage in the plasma environment in vivo, leading to premature loss of the drug even before the vehicle can reach its target site. It is interesting to note here that for chemotherapeutic drugs encapsulated or conjugated within a vehicle core, therapeutic effects may get affected due to challenges in release kinetics of the drug from the vehicle, but for PDT actual release of the photosensitizers from the vehicle may not be necessary, since the actual cytotoxic component is the reactive oxygen species. Oxygen can easily diffuse within the vehicle core, interact with the photoactivated PS, and get converted into reactive oxygen species, which can then diffuse out of the vehicle to cause cytotoxic damage. Therefore, nanoparticle vehicles that enable encapsulation of photosensitizers within the vehicle core, can potentially avoid premature drug loss and inactivation of the drug by plasma components, while allowing drug accumulation preferentially within the tumor for subsequent PDT. The versatile nature of nanoparticulate delivery vehicles also allows for multifunctional modifications that can incorporate imaging agents for diagnostic functionalities, along with photosensitizers for PDT [127–130]. For example, magnetic resonance imaging (MRI) agents like Gadolinium (T1 agent) or iron oxide (T2 agent) can be incorporated in photosensitizer-loaded nanovehicles to allow MRI guidance of tumor volume to be photoirradiated or MRI-assisted evaluation of PDT effect [131, 132]. Following similar rationale, optical probes (e.g. fluorescent molecules) can be incorporated in the vehicle for optical imaging [133–135].
3.3. Tumor characteristics
While design of passively and actively targeted nanoparticle delivery systems with appropriate size, shape, charge, surface-modification and drug-loading can significantly improve the tumor-selective delivery and release of the photosensitizers, certain challenges regarding the other two components of PDT, namely, oxygen and photoactivating light, need to be resolved in parallel to improve the clinical repertoire of PDT. The presence of sufficient molecular oxygen throughout the tumor volume is crucial for effective PDT. The mechanism of PDT requires the creation of reactive oxygen species (e.g. singlet oxygen) from tissue oxygen via interaction with the photoactivated drug. However, the oxygen levels within a tumor tissue can be highly heterogenous due to dysregulated vascular distribution, leading to severely hypoxic regions [138–140]. Tumor cells in these hypoxic regions are resistant to PDT due to lack of sufficient molecular oxygen. Also, the heterogenous vascular distribution can affect the vascularly-mediated delivery of PS-loaded nanovehicles uniformly throughout the tumor volume. Furthermore, tumor cells in hypoxic regions can also become more aggressive due to various signaling mechanisms, for example induced by hypoxia inducible transcription factor 1 (HIF 1) [140]. Hence strategies are required to restore normal oxygen environment (normoxia) in such tumor regions, as well as, to achieve uniform tissue penetration of the PS-loaded nanovehicles, to make the tumors amenable to PDT and other therapies. Various therapeutic and interventional strategies, for example, molecular inhibition of HIF-1 pathways, hyperbaric oxygen therapy, ozone therapy, etc. are currently under research to restore normoxia in oxygen-deprived tumor regions [141, 142]. In parallel, interesting research is going on in the area of nanoparticle design where particle morphology, charge and tumor environment-selective degradation capabilities are being utilized to enhance the tumor penetration and uniform distribution of payload [121, 143–150]. Integration of these strategies can significantly improve the efficacy of PDT in various tumors [151, 152].
3.4. Light delivery technologies
Challenges also exist regarding the delivery of appropriate light dose throughout the entire tumor volume, partly due to the limited tissue penetration parameters of the photoactivating wavelengths and also due to the difficulty in dosimetric correlations between the extent of photoirradiation and corresponding PDT effect [26]. As mentioned previously, light with wavelengths is the deep red to near infra-red (NIR) regions have higher tissue penetration capability. Therefore photosensitizers that can be activated by wavelengths ≥ 650 nm may have advantage in deep tissue tumor applications in vivo. Regarding technological components of light delivery, significant advancements have been made in the area of laser-based illuminators and light diffusers using fiberoptic arrangements and various geometries [153–165]. Table 6 shows selected examples of lasers used for clinical PDT. Lasers have been the preferred light source due to their high power and wavelength range. Additionally, it is relatively easy to couple them to optical fibers for endoscopic use. One of the more widely used systems is the argon laser or argon laser pumped dye laser system. The laser dyes (rhodamine derivatives) can be chosen to alter the wavelength range to match the absorption of the photosensitizer being used. Argon lasers do have a disadvantage of requiring high levels of adjustment and technical support [166]. Argon-pumped dye lasers are often coupled to optical fibers for treatment of lung cancer, oral cancers and Barrett's esophageous [167–170]. These lasers are indicated for use with fiber optics due to their very small beam cross-section. At the same time, this small beam cross-section complicates their use with larger legions (e.g. skin cancers). A diffuser is often put at the end of the fiber to yield uniform distribution of photoirradiation. Semiconductor diode lasers are another type of continuous wave laser though they can also be used in pulsed mode. They have compact equipment enclosures, are generally cheaper require less maintenance and are air-cooled which makes them portable and easier to use. They are usually coupled to optical fibers for endoscopic use but the fiber output can also be expanded for use on larger legions such as the skin. Metal vapor lasers and metal vapor laser pumped dye lasers are a variation on the argon laser systems. The gold and copper vapor lasers are the most popular in this group. They have an advantage of enhanced portability over the argon lasers because they do not require specialized cooling equipment or power supplies. However, at the same time, they require long warm-up and cool-down times. Additionally, they are often considerably more expensive. Metal vapor laser systems produced pulsed outputs as opposed to continuous wave outputs which causes changes in the light delivery dynamics though this has not been shown to cause a difference in PDT response [162]. Solid state lasers are pulsed laser technology that often emit in the near infrared region. For example, the frequently used Nd:YAG laser emits at 1064 nm though frequency doubling can allow for emission at other wavelengths. The frequency-doubled emission can be coupled with pumped dye lasers to yield output in the region of photosensitizer absorption. These light delivery technologies can provide effective ways to modulate illuminator geometry and in vivo placement customized to the target tumor tissue volume. Some interesting technological advancements have also occurred in the field of nanoparticle systems that allow improved strategies for photosensitizer activation with appropriate wavelength. An interesting example is the class of ‘upconversion nanoparticles’ that were described previously in Section 2.1.2. [55, 171–173], where irradiation of tissue-penetrating infra-red wavelengths on certain rare earth elements results in their emission in the visible range which can then activate photosensitizers in their proximity. Although the PDT promise of such particle formulations has been demonstrated, the biosafety of these upconverting rare earth elements need to undergo further research to guarantee clinical translation [174].
Table 6. Examples of laser technologies currently used for PDT.
| Laser Type | Wavelength Range | Fluence Rate (mW/cm2) | Method of Light Delivery |
|---|---|---|---|
| Continuous Wave Lasers | |||
| Argon laser | 488 and 515 nm | 500-1000 | Direct or fiber optics |
| Argon laser pumped dye laser | 500-750 nm | 10-200 | Direct or fiber optics |
| Semiconductor diode lasers | 600-950 nm | ≤ 700 | Fiber optics |
| Pulsed Lasers | |||
| Metal vapor laser | UV/Vis | ≤ 10 | Direct or fiber optics |
| Metal vapor laser pumped dye laser | 500-750 nm | 10-500 | Direct or fiber optics |
| Solid state laser | Usually 1064 nm but can be frequency doubled to 532, 355 and 266 nm | ≤ 10 | Direct or fiber optics |
3.5. PDT dosimetry parameters
For efficient clinical translation of PDT technologies where nanoparticle-based tumor-targeted photosensitizer delivery is integrated with tumor-selective light delivery, it is crucial to correlate PDT parameters with the corresponding biological effect (dosimetry). According to the American Association of Physicists in Medicine (AAPM) report No. 88, for successful PDT-induced treatment (effective tumor killing), it should be ensured that the product of photoirradiation fluence rate and photoirradiation exposure time should exceed the threshold required to induce cell killing [175]. Establishing this threshold and hence the corresponding photoirradiation parameters is often a challenge, especially for deep tissue tumors where drug distribution and light distribution throughout the entire tumor volume may not be uniform. Various dosimetric techniques, for example, measurement of singlet oxygen luminescence, image-assisted evaluation of PDT-induced biological effect, measurement of fluorescence photobleaching of the photosensitizers as a surrogate parameter and measurement of total light energy absorbed by the drug per unit tissue volume, are being investigated to establish precise correlation between PDT parameters and treatment effects [176–189]. A recent interesting nanoparticle-based development in this area is the fabrication of chemoluminescent probe-incorporated nanoparticle systems that allow chemoluminescence-based monitoring of reactive oxygen species levels produced upon photoirradiation [96, 190]. Efficient integration of appropriate drug delivery mechanisms, light delivery technologies and dosimetric approaches can be envisioned to significantly enhance the translational promise of nanoparticle-based photosensitizer formulations.
Conclusion
Photodynamic Therapy (PDT) continues to gain momentum as a clinically translatable stand-alone, alternative or adjunctive treatment modality for various cancers. Successful PDT of deep tissue solid tumors will require optimization of tumor-targeted drug delivery, tumor-selective photoirradiation and dosimetric correlation of PDT-induced tumor killing effect. Nanoparticles can provide a multitude of ways to enhance the tumor-targeted photosensitizer delivery component, by enabling optimum photosensitizer encapsulation, protection from plasmainduced drug inactivation or premature drug leakage, enhanced uptake within the tumor tissue and cell, and tumor environment-specific drug release and distribution. In addition, refinement of nanoparticle designs can also allow incorporation of imaging agents, dosimetric components and light delivery components. With continued research in optimizing the drug-light-oxygen triad, clinical applications of photodynamic nanomedicine strategies customized towards various deep tissue solid tumors can be envisioned in near future.
Acknowledgments
A.M.M. is funded by an F31-DE019998 award from the NIDCR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cancer Fact Sheet. http://www.who.int/mediacentre/factsheets/fs297/en/index.html.
- 2.Darzynkiewicz Z, et al. Impaired DNA damage response--an Achilles' heel sensitizing cancer to chemotherapy and radiotherapy. Eur J Pharmacol. 2009;625:143–50. doi: 10.1016/j.ejphar.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maeda H, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 4.Fang J, et al. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63:136–51. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 6.Ramakrishnan R, Gabrilovich DI. Mechanism of synergistic effect of chemotherapy and immunotherapy of cancer. Cancer Immunol Immunother. 2011;60:419–23. doi: 10.1007/s00262-010-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott AM, et al. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–87. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 8.Kwitniewski M, et al. Immunotherapy: a way to improve the therapeutic outcome of photodynamic therapy? Photochemical photobiological sciences Official journal of the European Photochemistry Association and the European Society for Photobiology. 2008;7:1011–1017. doi: 10.1039/b806710d. [DOI] [PubMed] [Google Scholar]
- 9.Dougherty T, et al. Photoradiation therapy for treatment of malignant tumors. Cancer Res. 1978;38:2628–2635. [PubMed] [Google Scholar]
- 10.Miller JD, et al. Photodynamic therapy with the phthalocyanine photosensitizer Pc 4: the case experience with preclinical mechanistic and early clinical-translational studies. Toxicology and Applied Pharmacology. 2007;224:290–299. doi: 10.1016/j.taap.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milane L, et al. Development of EGFR-Targeted Polymer Blend Nanocarriers for Combination Paclitaxel/Lonidamine Delivery To Treat Multi-Drug Resistance in Human Breast and Ovarian Tumor Cells. Mol Pharmaceutics. 2011;8:185–203. doi: 10.1021/mp1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allison RR, Sibata CH. Oncologic photodynamic therapy photosensitizers: a clinical review. Photodiagnosis and Photodynamic Therapy. 2010;7:61–75. doi: 10.1016/j.pdpdt.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Detty MR, et al. Current clinical and preclinical photosensitizers for use in photodynamic therapy. J Med Chem. 2004;47:3897–915. doi: 10.1021/jm040074b. [DOI] [PubMed] [Google Scholar]
- 14.Senge MO, Brandt JC. Temoporfin (Foscan®, 5,10,15,20-tetra(m-hydroxyphenyl)chlorin)--a second-generation photosensitizer. Photochem Photobiol. 87:1240–96. doi: 10.1111/j.1751-1097.2011.00986.x. [DOI] [PubMed] [Google Scholar]
- 15.FDA. Foscan FDA Hearings. 2005 [Google Scholar]
- 16.Betz CS, et al. Optimization of treatment parameters for Foscan-PDT of basal cell carcinomas. 2008 doi: 10.1002/lsm.20632. [DOI] [PubMed] [Google Scholar]
- 17.Allison R, et al. Clinical PD/PDT in North America: An historical review. Photodiagnosis and Photodynamic Therapy. 2004;1:263–277. doi: 10.1016/S1572-1000(04)00084-5. [DOI] [PubMed] [Google Scholar]
- 18.Weiss R, et al. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8:1263–1268. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- 19.Price KS, Castells MC. Taxol reactions. Allergy Asthma Proc. 23:205–8. [PubMed] [Google Scholar]
- 20.Barolet D. Light-emitting diodes (LEDs) in dermatology. Semin Cutan Med Surg. 2008;27:227–38. doi: 10.1016/j.sder.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Bechet D, et al. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008;26:612–21. doi: 10.1016/j.tibtech.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee DK, et al. Nanoparticles in photodynamic therapy: an emerging paradigm. Adv Drug Deliv Rev. 2008;60:1627–37. doi: 10.1016/j.addr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Juzenas P, et al. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv Drug Deliv Rev. 2008;60:1600–14. doi: 10.1016/j.addr.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu MK, et al. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics. 2012;2:3–44. doi: 10.7150/thno.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olivo M, et al. Targeted Therapy of Cancer Using Photodynamic Therapy in Combination with Multi-faceted Anti-Tumor Modalities. Pharmaceuticals. 2010;3:1507–1529. doi: 10.3390/ph3051507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Z, et al. Photodynamic therapy for treatment of solid tumors--potential and technical challenges. Technol Cancer Res Treat. 2008;7:309–20. doi: 10.1177/153303460800700405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Z. A review of progress in clinical photodynamic therapy. Technol Cancer Res Treat. 2005;4:283. doi: 10.1177/153303460500400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson BC, Patterson MS. The physics, biophysics and technology of photodynamic therapy. Phys Med Biol. 2008;53:R61–109. doi: 10.1088/0031-9155/53/9/R01. [DOI] [PubMed] [Google Scholar]
- 29.Wacker M, et al. Photosensitizer loaded HSA nanoparticles. I: Preparation and photophysical properties. Int J Pharm. 2010;393:253–62. doi: 10.1016/j.ijpharm.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 30.Chen K, et al. Photophysical evaluation of mTHPC-loaded HSA nanoparticles as novel PDT delivery systems. J Photochem Photobiol B, Biol. 2010;101:340–7. doi: 10.1016/j.jphotobiol.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Chen K, et al. Novel photosensitizer-protein nanoparticles for photodynamic therapy: photophysical characterization and in vitro investigations. J Photochem Photobiol B Biol. 2009;96:66–74. doi: 10.1016/j.jphotobiol.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Fakhar-e-Alam M, et al. Sensitivity of A-549 human lung cancer cells to nanoporous zinc oxide conjugated with Photofrin. Lasers Med Sci. 2012;27:607–14. doi: 10.1007/s10103-011-0989-8. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, et al. Optical behaviors of ZnO-porphyrin conjugates and their potential applications for cancer treatment. Applied Physics Letters. 2008;92:143901. [Google Scholar]
- 34.Yin M, et al. Photosensitizer-incorporated G-quadruplex DNA-functionalized magnetofluorescent nanoparticles for targeted magnetic resonance/fluorescence multimodal imaging and subsequent photodynamic therapy of cancer. Chem Commun (Camb) 2012;48:6556–8. doi: 10.1039/c2cc32129g. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, et al. Magnetic chitosan nanoparticles as a drug delivery system for targeting photodynamic therapy. Nanotechnology. 2009;20:135102. doi: 10.1088/0957-4484/20/13/135102. [DOI] [PubMed] [Google Scholar]
- 36.Chen ZL, et al. Studies on Preparation of Photosensitizer Loaded Magnetic Silica Nanoparticles and Their Anti-Tumor Effects for Targeting Photodynamic Therapy. Nanoscale Research Letters. 2009;4:400–408. doi: 10.1007/s11671-009-9254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon V, et al. Pp IX silica nanoparticles demonstrate differential interactions with in vitro tumor cell lines and in vivo mouse models of human cancers. Photochem Photobiol. 2010;86:213–22. doi: 10.1111/j.1751-1097.2009.00620.x. [DOI] [PubMed] [Google Scholar]
- 38.Qian J, et al. Photosensitizer encapsulated organically modified silica nanoparticles for direct two-photon photodynamic therapy and in vivo functional imaging. Biomaterials. 2012;33:4851–60. doi: 10.1016/j.biomaterials.2012.02.053. [DOI] [PubMed] [Google Scholar]
- 39.Brevet D, et al. Mannose-targeted mesoporous silica nanoparticles for photodynamic therapy. Chem Commun (Camb) 2009:1475–7. doi: 10.1039/b900427k. [DOI] [PubMed] [Google Scholar]
- 40.Hocine O, et al. Silicalites and Mesoporous Silica Nanoparticles for photodynamic therapy. Int J Pharm. 2010;402:221–30. doi: 10.1016/j.ijpharm.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Hu Z, et al. Meso-tetra (carboxyphenyl) porphyrin (TCPP) nanoparticles were internalized by SW480 cells by a clathrin-mediated endocytosis pathway to induce high photocytotoxicity. Biomedicine & pharmacotherapy. 2009;63:155–64. doi: 10.1016/j.biopha.2008.07.087. [DOI] [PubMed] [Google Scholar]
- 42.Ding H, et al. Nanoscopic micelle delivery improves the photophysical properties and efficacy of photodynamic therapy of protoporphyrin IX. J Control Release. 2011;151:271–7. doi: 10.1016/j.jconrel.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kojima C, et al. Preparation of poly(ethylene glycol)-attached dendrimers encapsulating photosensitizers for application to photodynamic therapy. Bioconjug Chem. 2007;18:663–70. doi: 10.1021/bc060244u. [DOI] [PubMed] [Google Scholar]
- 44.Zhao L, et al. Self-assembled photosensitizer-conjugated nanoparticles for targeted photodynamic therapy. J Biomater Appl. 2012;0:1–14. doi: 10.1177/0885328212459777. [DOI] [PubMed] [Google Scholar]
- 45.Shen X, et al. Photosensitizer-doped conjugated polymer nanoparticles for simultaneous two-photon imaging and two-photon photodynamic therapy in living cells. Nanoscale. 2011;3:5140–6. doi: 10.1039/c1nr11104c. [DOI] [PubMed] [Google Scholar]
- 46.Reddy GR, et al. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin Cancer Res. 2006;12:6677–86. doi: 10.1158/1078-0432.CCR-06-0946. [DOI] [PubMed] [Google Scholar]
- 47.Hah HJ, et al. Methylene blue-conjugated hydrogel nanoparticles and tumor-cell targeted photodynamic therapy. Macromol Biosci. 2011;11:90–9. doi: 10.1002/mabi.201000231. [DOI] [PubMed] [Google Scholar]
- 48.Qin M, et al. Methylene blue covalently loaded polyacrylamide nanoparticles for enhanced tumor-targeted photodynamic therapy. Photochem Photobiol Sci. 2011;10:832–41. doi: 10.1039/c1pp05022b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang S, et al. Multifunctional biodegradable polyacrylamide nanocarriers for cancer theranostics--a “see and treat” strategy. ACS Nano. 2012;6:6843–51. doi: 10.1021/nn301633m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao D, et al. Nanoparticles for two-photon photodynamic therapy in living cells. Nano Lett. 2006;6:2383–2386. doi: 10.1021/nl0617179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gamaleia N, et al. Photodynamic activity of hematoporphyrin conjugates with gold nanoparticles: experiments in vitro. Experimental Oncology. 2010;32:44–47. [PubMed] [Google Scholar]
- 52.Xu H, et al. Effects of light irradiation upon photodynamic therapy based on 5-aminolevulinic acid-gold nanoparticle conjugates in K562 cells via singlet oxygen generation. Int J Nanomedicine. 2012;7:5029–38. doi: 10.2147/IJN.S33261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khaing Oo MK, et al. Gold nanoparticle-enhanced and size-dependent generation of reactive oxygen species from protoporphyrin IX. ACS Nano. 2012;6:1939–47. doi: 10.1021/nn300327c. [DOI] [PubMed] [Google Scholar]
- 54.Rhee JK, et al. Glycan-targeted virus-like nanoparticles for photodynamic therapy. Biomacromolecules. 2012;13:2333–8. doi: 10.1021/bm300578p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo H, et al. Singlet oxygen-induced apoptosis of cancer cells using upconversion fluorescent nanoparticles as a carrier of photosensitizer. Nanomedicine. 2010;6:486–95. doi: 10.1016/j.nano.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Master AM, et al. Delivery of the photosensitizer Pc 4 in PEG–PCL micelles for in vitro PDT studies. J Pharm Sci. 2010;99:2386–2398. doi: 10.1002/jps.22007. [DOI] [PubMed] [Google Scholar]
- 57.Khdair A, et al. Surfactant-polymer nanoparticles enhance the effectiveness of anticancer photodynamic therapy. Mol Pharm. 2008;5:795–807. doi: 10.1021/mp800026t. [DOI] [PubMed] [Google Scholar]
- 58.Tang W, et al. Encapsulation of methylene blue in polyacrylamide nanoparticle platforms protects its photodynamic effectiveness. Biochem Biophys Res Commun. 2008;369:579–583. doi: 10.1016/j.bbrc.2008.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeisser-Labouèbe M, et al. Hypericin-loaded nanoparticles for the photodynamic treatment of ovarian cancer. Int J Pharm. 2006;326:174–81. doi: 10.1016/j.ijpharm.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 60.Benachour H, et al. Multifunctional Peptide-conjugated hybrid silica nanoparticles for photodynamic therapy and MRI. Theranostics. 2012;2:889–904. doi: 10.7150/thno.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoon HY, et al. Tumor-targeting hyaluronic acid nanoparticles for photodynamic imaging and therapy. Biomaterials. 2012;33:3980–9. doi: 10.1016/j.biomaterials.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 62.Li L, et al. Self-quenchable biofunctional nanoparticles of heparin–folate-photosensitizer conjugates for photodynamic therapy. Carbohydrate Polymers. 2011;86:708–715. [Google Scholar]
- 63.Huang P, et al. Photosensitizer-conjugated magnetic nanoparticles for in vivo simultaneous magnetofluorescent imaging and targeting therapy. Biomaterials. 2011;32:3447–58. doi: 10.1016/j.biomaterials.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 64.Park Y Il, et al. Theranostic Probe Based on Lanthanide-Doped Nanoparticles for Simultaneous In Vivo Dual-Modal Imaging and Photodynamic Therapy. Adv Mater Weinheim. 2012:1–7. doi: 10.1002/adma.201202433. [DOI] [PubMed] [Google Scholar]
- 65.Wang C, et al. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials. 2011;32:6145–54. doi: 10.1016/j.biomaterials.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 66.Ungun B, et al. Nanofabricated upconversion nanoparticles for photodynamic therapy. Opt Express. 2009;17:80–6. doi: 10.1364/oe.17.000080. [DOI] [PubMed] [Google Scholar]
- 67.Ricci-Júnior E, Marchetti JM. Zinc(II) phthalocyanine loaded PLGA nanoparticles for photodynamic therapy use. Int J Pharm. 2006;310:187–95. doi: 10.1016/j.ijpharm.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 68.Ricci-Júnior E, Marchetti JM. Preparation, characterization, photocytotoxicity assay of PLGA nanoparticles containing zinc (II) phthalocyanine for photodynamic therapy use. J Microencapsul. 2006;23:523–538. doi: 10.1080/02652040600775525. [DOI] [PubMed] [Google Scholar]
- 69.Master AM, et al. Optimization of a Nanomedicine-Based Silicon Phthalocyanine 4 Photodynamic Therapy (Pc 4-PDT) Strategy for Targeted Treatment of EGFR-Overexpressing Cancers. Mol Pharm. 2012 doi: 10.1021/mp300256e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Master AM, et al. EGFR-mediated Intracellular Delivery of Pc 4 Nanoformulation for Targeted Photodynamic Therapy of Cancer: In Vitro Studies. Nanomedicine. 2011;8:655–64. doi: 10.1016/j.nano.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng Y, et al. Delivery and efficacy of a cancer drug as a function of the bond to the gold nanoparticle surface. Langmuir. 2010;26:2248–55. doi: 10.1021/la902390d. [DOI] [PubMed] [Google Scholar]
- 72.Cheng Y, et al. Addressing Brain Tumors with Targeted Gold Nanoparticles: A New Gold Standard for Hydrophobic Drug Delivery? Small. 2011:2301–2306. doi: 10.1002/smll.201100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng Y, et al. Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. J Am Chem Soc. 2008;130:10643–7. doi: 10.1021/ja801631c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Obaid G, et al. Targeting the oncofetal Thomsen-Friedenreich disaccharide using jacalin-PEG phthalocyanine gold nanoparticles for photodynamic cancer therapy. Angew Chem Int Ed Engl. 2012;51:6158–62. doi: 10.1002/anie.201201468. [DOI] [PubMed] [Google Scholar]
- 75.Stuchinskaya T, et al. Targeted photodynamic therapy of breast cancer cells using antibody-phthalocyanine-gold nanoparticle conjugates. Photochem Photobiol Sci. 2011;10:822–31. doi: 10.1039/c1pp05014a. [DOI] [PubMed] [Google Scholar]
- 76.Nombona N, et al. Synthesis of phthalocyanine conjugates with gold nanoparticles and liposomes for photodynamic therapy. J Photochem Photobiol B Biol. 2012;107:35–44. doi: 10.1016/j.jphotobiol.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 77.Zhao Z, et al. Multifunctional core-shell upconverting nanoparticles for imaging and photodynamic therapy of liver cancer cells. Chem Asian J. 2012;7:830–7. doi: 10.1002/asia.201100879. [DOI] [PubMed] [Google Scholar]
- 78.Qiao XF, et al. Triple-functional core-shell structured upconversion luminescent nanoparticles covalently grafted with photosensitizer for luminescent, magnetic resonance imaging and photodynamic therapy in vitro. Nanoscale. 2012;4:4611–23. doi: 10.1039/c2nr30938f. [DOI] [PubMed] [Google Scholar]
- 79.Pietkiewicz J, et al. New approach to hydrophobic cyanine-type photosensitizer delivery using polymeric oil-cored nanocarriers: hemolytic activity, in vitro cytotoxicity and localization in cancer cells. European journal of pharmaceutical sciences. 2010;39:322–35. doi: 10.1016/j.ejps.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 80.Rungta P, et al. Selective imaging and killing of cancer cells with protein-activated near-infrared fluorescing nanoparticles. Macromol Biosci. 2011;11:927–37. doi: 10.1002/mabi.201100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gomes A, Lunardi L. Indocyanine green nanoparticles useful for photomedicine. Photomedicine and …. 2006;24:514–521. doi: 10.1089/pho.2006.24.514. [DOI] [PubMed] [Google Scholar]
- 82.Kim G, et al. Indocyanine-green-embedded PEBBLEs as a contrast agent for photoacoustic imaging. J Biomed Opt. 2011;12:044020. doi: 10.1117/1.2771530. [DOI] [PubMed] [Google Scholar]
- 83.Kuo WS, et al. Gold nanomaterials conjugated with indocyanine green for dual-modality photodynamic and photothermal therapy. Biomaterials. 2012;33:3270–8. doi: 10.1016/j.biomaterials.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 84.Barth BM, et al. Targeted indocyanine-green-loaded calcium phosphosilicate nanoparticles for in vivo photodynamic therapy of leukemia. ACS Nano. 2011;5:5325–37. doi: 10.1021/nn2005766. [DOI] [PubMed] [Google Scholar]
- 85.Zhou L, et al. External heavy-atomic construction of photosensitizer nanoparticles for enhanced in vitro photodynamic therapy of cancer. J Phys Chem B. 2012;116:12744–9. doi: 10.1021/jp305137j. [DOI] [PubMed] [Google Scholar]
- 86.Wen AM, et al. Photodynamic activity of viral nanoparticles conjugated with C60. Chem Commun (Camb) 2012;48:9044–6. doi: 10.1039/c2cc34695h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iohara D, Hiratsuka M. Evaluation of photodynamic activity of C60/2― hydroxypropyl― Î2― cyclodextrin nanoparticles. Journal of …. 2012;101:3390–3397. doi: 10.1002/jps.23045. [DOI] [PubMed] [Google Scholar]
- 88.Wang M, et al. Synthesis and photodynamic effect of new highly photostable decacationically armed [60]- and [70]fullerene decaiodide monoadducts to target pathogenic bacteria and cancer cells. J Med Chem. 2012;55:4274–85. doi: 10.1021/jm3000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yan F, et al. Cellular uptake and photodynamic activity of protein nanocages containing methylene blue photosensitizing drug. Photochem Photobiol. 2010;86:662–6. doi: 10.1111/j.1751-1097.2009.00696.x. [DOI] [PubMed] [Google Scholar]
- 90.Tang W, et al. Photodynamic characterization and in vitro application of methylene blue-containing nanoparticle platforms. Photochemistry and Photobiology. 2005;81:242–249. doi: 10.1562/2004-05-24-RA-176.1. [DOI] [PubMed] [Google Scholar]
- 91.He X, et al. Methylene blue-encapsulated phosphonate-terminated silica nanoparticles for simultaneous in vivo imaging and photodynamic therapy. Biomaterials. 2009;30:5601–9. doi: 10.1016/j.biomaterials.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 92.Khdair A, et al. Nanoparticle-mediated combination chemotherapy and photodynamic therapy overcomes tumor drug resistance in vitro. European Journal of Pharmaceutics …. 2009;71:214–222. doi: 10.1016/j.ejpb.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 93.Nawalany K, et al. Novel nanostructural photosensitizers for photodynamic therapy: in vitro studies. Int J Pharm. 2012;430:129–40. doi: 10.1016/j.ijpharm.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 94.Chang CC, et al. Selective photodynamic therapy based on aggregation-induced emission enhancement of fluorescent organic nanoparticles. Biomaterials. 2012;33:897–906. doi: 10.1016/j.biomaterials.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 95.Deda DK, et al. A new micro/nanoencapsulated porphyrin formulation for PDT treatment. Int J Pharm. 2009;376:76–83. doi: 10.1016/j.ijpharm.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 96.Chen R, et al. Chemiluminescent nanomicelles for imaging hydrogen peroxide and self-therapy in photodynamic therapy. J Biomed Biotechnol. 2011;2011:679492. doi: 10.1155/2011/679492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lim CK, et al. Iodinated Photosensitizing Chitosan: Self-Assembly into Tumor-Homing Nanoparticles with Enhanced Singlet Oxygen Generation. Bioconjug Chem. 2012 doi: 10.1021/bc300012g. [DOI] [PubMed] [Google Scholar]
- 98.Lee SJ, et al. Comparative study of photosensitizer loaded and conjugated glycol chitosan nanoparticles for cancer therapy. J Control Release. 2011;152:21–9. doi: 10.1016/j.jconrel.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 99.Jeong H, et al. Photosensitizer-conjugated human serum albumin nanoparticles for effective photodynamic therapy. Theranostics. 2011;1:230–9. doi: 10.7150/thno/v01p0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gary-Bobo M, et al. Hyaluronic acid-functionalized mesoporous silica nanoparticles for efficient photodynamic therapy of cancer cells. Photodiagnosis Photodyn Ther. 2012;9:256–60. doi: 10.1016/j.pdpdt.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 101.Konan-Kouakou YN, et al. In vitro and in vivo activities of verteporfin-loaded nanoparticles. J Control Release. 2005;103:83–91. doi: 10.1016/j.jconrel.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 102.Wang S, et al. Novel methods to incorporate photosensitizers into nanocarriers for cancer treatment by photodynamic therapy. Lasers Surg Med. 2011;43:686–95. doi: 10.1002/lsm.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gao D, Xu H. Ultrafine hydrogel nanoparticles: synthetic approach and therapeutic application in living cells. Angewandte Chemie …. 2007:2224–2227. doi: 10.1002/anie.200603927. [DOI] [PubMed] [Google Scholar]
- 104.Kim S, et al. Organically Modified Silica Nanoparticles with Intraparticle Heavy-Atom Effect on the Encapsulated Photosensitizer for Enhanced Efficacy of Photodynamic Therapy. The Journal of Physical Chemistry C. 2009;113:12641–12644. doi: 10.1021/jp900573s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roy I, et al. Ceramic-based nanoparticles entrapping water-insoluble photosensitizing anticancer drugs: a novel drug-carrier system for photodynamic therapy. J Am Chem Soc. 2003;125:7860–7865. doi: 10.1021/ja0343095. [DOI] [PubMed] [Google Scholar]
- 106.Savellano MD, Hasan T. Photochemical targeting of epidermal growth factor receptor: a mechanistic study. Clinical cancer research. 2005;11:1658. doi: 10.1158/1078-0432.CCR-04-1902. [DOI] [PubMed] [Google Scholar]
- 107.Rahmanzadeh R, et al. Ki-67 as a molecular target for therapy in an in vitro three-dimensional model for ovarian cancer. Cancer Res. 2010;70:9234–42. doi: 10.1158/0008-5472.CAN-10-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tu J, et al. Multifunctional ZnPc-loaded mesoporous silica nanoparticles for enhancement of photodynamic therapy efficacy by endolysosomal escape. Biomaterials. 2012;33:7903–14. doi: 10.1016/j.biomaterials.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 109.Nishiyama N, et al. Enhanced photodynamic cancer treatment by supramolecular nanocarriers charged with dendrimer phthalocyanine. J Control Release. 2009;133:245–51. doi: 10.1016/j.jconrel.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 110.Li H, et al. High payload delivery of optical imaging and photodynamic therapy agents to tumors using phthalocyanine-reconstituted low-density lipoprotein nanoparticles. J Biomed Opt. 2011;10:41203. doi: 10.1117/1.2011429. [DOI] [PubMed] [Google Scholar]
- 111.Gupta A, et al. Multifunctional nanoplatforms for fluorescence imaging and photodynamic therapy developed by post-loading photosensitizer and fluorophore to polyacrylamide nanoparticles. Nanomedicine. 2012;8:941–50. doi: 10.1016/j.nano.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Koo YEL, et al. Photonic explorers based on multifunctional nanoplatforms for biosensing and photodynamic therapy. Appl Opt. 2007;46:1924–30. doi: 10.1364/ao.46.001924. [DOI] [PubMed] [Google Scholar]
- 113.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653–64. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang L, et al. Nanoparticles in Medicine: Therapeutic Applications and Developments. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 115.Moghimi SM, et al. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 116.Moghimi S, et al. Nanomedicine: current status and future prospects. FASEB. 2005;19:311–330. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 117.Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58:1532–55. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 118.Decuzzi P, et al. Size and shape effects in the biodistribution of intravascularly injected particles. J Control Release. 2010;141:320–7. doi: 10.1016/j.jconrel.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 119.Nel AE, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–57. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 120.Huang RB, et al. Dynamic and cellular interactions of nanoparticles in vascular-targeted drug delivery. Mol Membr Biol. 2010;27:312–27. doi: 10.3109/09687688.2010.522117. [DOI] [PubMed] [Google Scholar]
- 121.Champion J, et al. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J Control Release. 2007;121:3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Caldorera-Moore M. Designer nanoparticles: incorporating size, shape and triggered release into nanoscale drug carriers. Expert Opinion on Drug Delivery. 2010;7:479–495. doi: 10.1517/17425240903579971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rosenkranz AA, et al. Targeted intracellular delivery of photosensitizers to enhance photodynamic efficiency. Immunology and Cell Biology. 2000;78:452–464. doi: 10.1046/j.1440-1711.2000.00925.x. [DOI] [PubMed] [Google Scholar]
- 124.Paszko E, et al. Nanodrug applications in photodynamic therapy. Photodiagnosis Photodyn Ther. 2011;8:14–29. doi: 10.1016/j.pdpdt.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 125.Sharman WM, et al. Targeted photodynamic therapy via receptor mediated delivery systems. Advanced Drug Delivery Reviews. 2004;56:53–76. doi: 10.1016/j.addr.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 126.Master AM, Sen Gupta A. EGFR-Targeted Nanocarriers for Enhanced Cancer Treatment. Nanomedicine (Lond) 2012 doi: 10.2217/nnm.12.160. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lucignani G. Nanoparticles for concurrent multimodality imaging and therapy: the dawn of new theragnostic synergies. Eur J Nucl Med Mol Imaging. 2009;36:869–74. doi: 10.1007/s00259-009-1104-2. [DOI] [PubMed] [Google Scholar]
- 128.Xie J, et al. Nanoparticle-based theranostic agents. Adv Drug Deliv Rev. 2010;62:1064–79. doi: 10.1016/j.addr.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sumer B, Gao J. Theranostic nanomedicine for cancer. Nanomedicine (Lond) 2008;3:137–40. doi: 10.2217/17435889.3.2.137. [DOI] [PubMed] [Google Scholar]
- 130.Torchilin VP. Multifunctional Pharmaceutical Nanocarriers: Development of the Concept Multifunctionality of Pharmaceutical Carriers: What to Expect? 2008 [Google Scholar]
- 131.Kopelman R, et al. Multifunctional nanoparticle platforms for in vivo MRI enhancement and photodynamic therapy of a rat brain cancer. J Magn Magn Mater. 2005;293:404–410. [Google Scholar]
- 132.Van Vlerken LE, Amiji MM. Multi-functional polymeric nanoparticles for tumour-targeted drug delivery. Expert Opinion on Drug Delivery. 2006;3:205–216. doi: 10.1517/17425247.3.2.205. [DOI] [PubMed] [Google Scholar]
- 133.Liong M, et al. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2:889–96. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Barenholz Y. (Chezy),Doxil®— The First FDA-Approved Nano-Drug: Lessons Learned. J Control Release. 2012 doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 135.Ye Y, Chen X. Integrin targeting for tumor optical imaging. Theranostics. 2011;1:102–26. doi: 10.7150/thno/v01p0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sibata MN, et al. Photophysicals and photochemicals studies of zinc(II) phthalocyanine in long time circulation micelles for photodynamic therapy use. European journal of pharmaceutical sciences official journal of the European Federation for Pharmaceutical Sciences. 2004;23:131–138. doi: 10.1016/j.ejps.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 137.Ichikawa K, et al. Antiangiogenic photodynamic therapy (PDT) by using long-circulating liposomes modified with peptide specific to angiogenic vessels. Biochimica et Biophysica Acta. 2005;1669:69–74. doi: 10.1016/j.bbamem.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 138.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–47. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 139.Vaupel P, et al. Oxygenation status of malignant tumors: Pathogenesis of hypoxia and significance for tumor therapy. Seminars in Oncology. 2001;28:29–35. doi: 10.1016/s0093-7754(01)90210-6. [DOI] [PubMed] [Google Scholar]
- 140.Vaupel P, et al. Treatment resistance of solid tumors. Medical oncology. 2001;18:243–259. doi: 10.1385/MO:18:4:243. [DOI] [PubMed] [Google Scholar]
- 141.Bennewith KL, Dedhar S. Targeting hypoxic tumour cells to overcome metastasis. BMC Cancer. 2011;11:504. doi: 10.1186/1471-2407-11-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kunz M, Ibrahim SM. Molecular responses to hypoxia in tumor cells. Mol Cancer. 2003;2:23. doi: 10.1186/1476-4598-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Doshi N, Mitragotri S. Macrophages recognize size and shape of their targets. PLoS ONE. 2010;5:e10051. doi: 10.1371/journal.pone.0010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu Y, et al. The shape of things to come: importance of design in nanotechnology for drug delivery. Ther Deliv. 2012;3:181–194. doi: 10.4155/tde.11.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kamaly N, et al. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tan J, et al. The influence of size, shape and vessel geometry on nanoparticle distribution. Microfluidics and Nanofluidics. 2012;14:77–87. doi: 10.1007/s10404-012-1024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Perrault SD, et al. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009;9:1909–15. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 149.Albanese A, et al. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 150.Arvizo RR, et al. Modulating pharmacokinetics, tumor uptake and biodistribution by engineered nanoparticles. PLoS ONE. 2011;6:e24374. doi: 10.1371/journal.pone.0024374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bocci V, et al. Restoration of normoxia by ozone therapy may control neoplastic growth: a review and a working hypothesis. J Altern Complement Med. 2005;11:257–65. doi: 10.1089/acm.2005.11.257. [DOI] [PubMed] [Google Scholar]
- 152.Daruwalla J, Christophi C. Hyperbaric oxygen therapy for malignancy: a review. World J Surg. 2006;30:2112–31. doi: 10.1007/s00268-006-0190-6. [DOI] [PubMed] [Google Scholar]
- 153.Jankun J, et al. Diverse optical characteristic of the prostate and light delivery system: implications for computer modelling of prostatic photodynamic therapy. BJU Int. 2005;95:1237–44. doi: 10.1111/j.1464-410X.2005.05512.x. [DOI] [PubMed] [Google Scholar]
- 154.Wilson BC, et al. Instrumentation and light dosimetry for intra-operative photodynamic therapy (PDT) of malignant brain tumours. Phys Med Biol. 1986;31:125–33. doi: 10.1088/0031-9155/31/2/002. [DOI] [PubMed] [Google Scholar]
- 155.Roche JVE, et al. Photodynamic Therapy (PDT) of Gastrointestinal Tumours: A New Light Delivery System. Lasers in Medical Science. 1998;13:137–142. [Google Scholar]
- 156.Rendon A, et al. Treatment planning using tailored and standard cylindrical light diffusers for photodynamic therapy of the prostate. Phys Med Biol. 2008;53:1131–49. doi: 10.1088/0031-9155/53/4/021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nyst HJ, et al. Performance of a dedicated light delivery and dosimetry device for photodynamic therapy of nasopharyngeal carcinoma: phantom and volunteer experiments. Lasers Surg Med. 2007;39:647–53. doi: 10.1002/lsm.20536. [DOI] [PubMed] [Google Scholar]
- 158.Weersink RA, et al. Techniques for delivery and monitoring of TOOKAD (WST09)-mediated photodynamic therapy of the prostate: clinical experience and practicalities. J Photochem Photobiol B Biol. 2005;79:211–22. doi: 10.1016/j.jphotobiol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 159.Samkoe KS, et al. Complete blood vessel occlusion in the chick chorioallantoic membrane using two-photon excitation photodynamic therapy: implications for treatment of wet age-related macular degeneration. J Biomed Opt. 12:034025. doi: 10.1117/1.2750663. [DOI] [PubMed] [Google Scholar]
- 160.Drobizhev M, et al. Near-infrared two-photon absorption in phthalocyanines: enhancement of lowest gerade-gerade transition by symmetrical electron-accepting substitution. J Chem Phys. 2006;124:224701. doi: 10.1063/1.2200355. [DOI] [PubMed] [Google Scholar]
- 161.Juzeniene A, et al. Effectiveness of different light sources for 5-aminolevulinic acid photodynamic therapy. Lasers Med Sci. 2004;19:139–49. doi: 10.1007/s10103-004-0314-x. [DOI] [PubMed] [Google Scholar]
- 162.Mang TS. Lasers and light sources for PDT: past, present and future. Photodiagnosis and Photodynamic Therapy. 2004;1:43–48. doi: 10.1016/S1572-1000(04)00012-2. [DOI] [PubMed] [Google Scholar]
- 163.Schmidt MH, et al. Light-emitting diodes as a light source for intraoperative photodynamic therapy. Neurosurgery. 1996;38:552–6. doi: 10.1097/00006123-199603000-00025. discussion 556–7. [DOI] [PubMed] [Google Scholar]
- 164.Lustig RA, et al. A multicenter Phase I safety study of intratumoral photoactivation of talaporfin sodium in patients with refractory solid tumors. Cancer. 2003;98:1767–71. doi: 10.1002/cncr.11708. [DOI] [PubMed] [Google Scholar]
- 165.Chen J, et al. New Technology for Deep Light Distribution in Tissue for Phototherapy. Cancer J. 8:154–63. doi: 10.1097/00130404-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 166.Brancaleon L, Moseley H. Laser and Non-laser Light Sources for. 2002:173–186. doi: 10.1007/s101030200027. [DOI] [PubMed] [Google Scholar]
- 167.Kübler A, et al. Treatment of oral leukoplakia by topical application of 5-aminolevulinic acid. Int J Oral Maxillofac Surg. 1998;27:466–9. doi: 10.1016/s0901-5027(98)80040-4. [DOI] [PubMed] [Google Scholar]
- 168.Okunaka T, et al. Photodynamic therapy for multiple primary bronchogenic carcinoma. Cancer. 1991;68:253–8. doi: 10.1002/1097-0142(19910715)68:2<253::aid-cncr2820680206>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 169.Marijnissen JP, et al. Pilot study on light dosimetry for endobronchial photodynamic therapy. Photochem Photobiol. 1993;58:92–9. doi: 10.1111/j.1751-1097.1993.tb04908.x. [DOI] [PubMed] [Google Scholar]
- 170.Krishnadath KK, et al. Persistent genetic abnormalities in Barrett's esophagus after photodynamic therapy. Gastroenterology. 2000;119:624–30. doi: 10.1053/gast.2000.18012. [DOI] [PubMed] [Google Scholar]
- 171.Chatterjee DK, Yong Z. Upconverting nanoparticles as nanotransducers for photodynamic therapy in cancer cells. Nanomedicine (Lond) 2008;3:73–82. doi: 10.2217/17435889.3.1.73. [DOI] [PubMed] [Google Scholar]
- 172.Cui S, et al. Amphiphilic chitosan modified upconversion nanoparticles for in vivo photodynamic therapy induced by near-infrared light. J Mater Chem. 2012;22:4861. [Google Scholar]
- 173.Idris NM, et al. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat Med. 2012;18:1580–5. doi: 10.1038/nm.2933. [DOI] [PubMed] [Google Scholar]
- 174.Chen J, Zhao JX. Upconversion nanomaterials: synthesis, mechanism, and applications in sensing. Sensors Basel Sensors. 2012;12:2414–35. doi: 10.3390/s120302414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.American Association of Physicists in Medicine. AAPM Report No 88: Photodynamic Therapy Dosimetry. 2005 [Google Scholar]
- 176.Bai L, et al. The relationship of phthalocyanine 4 (pc 4) concentrations measured noninvasively to outcome of pc 4 photodynamic therapy in mice. Photochem Photobiol. 2009;85:1011–9. doi: 10.1111/j.1751-1097.2009.00542.x. [DOI] [PubMed] [Google Scholar]
- 177.Niedre MJ, et al. In vitro tests of the validity of singlet oxygen luminescence measurements as a dose metric in photodynamic therapy. Cancer Res. 2003;63:7986. [PubMed] [Google Scholar]
- 178.Davis SJ, et al. Proceedings of SPIE. SPIE; 2003. Ultrasensitive diode-laser-based monitor for singlet oxygen; pp. 140–148. [Google Scholar]
- 179.Tempel-Brami C, et al. Detection of light images by simple tissues as visualized by photosensitized magnetic resonance imaging. PLoS ONE. 2007;2:e1191. doi: 10.1371/journal.pone.0001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gross S, et al. Monitoring photodynamic therapy of solid tumors online by BOLD-contrast MRI. Nat Med. 2003;9:1327–31. doi: 10.1038/nm940. [DOI] [PubMed] [Google Scholar]
- 181.Ivan Yeung WT, et al. In vivo CT measurement of blood-brain transfer constant of iopamidol in human brain tumors. Journal of Neuro-Oncology. 1992;14:177–187. doi: 10.1007/BF00177622. [DOI] [PubMed] [Google Scholar]
- 182.Herman MA, et al. Tumor blood-flow changes following protoporphyrin IX-based photodynamic therapy in mice and humans. J Photochem Photobiol B Biol. 52:99–104. doi: 10.1016/s1011-1344(99)00109-8. [DOI] [PubMed] [Google Scholar]
- 183.Berard V, et al. Positron emission tomography imaging of tumor response after photodynamic therapy. J Environ Pathol Toxicol Oncol. 2006;25:239–49. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.150. [DOI] [PubMed] [Google Scholar]
- 184.Molckovsky A, Wilson BC. Monitoring of cell and tissue responses to photodynamic therapy by electrical impedance spectroscopy. Phys Med Biol. 2001;46:983–1002. doi: 10.1088/0031-9155/46/4/306. [DOI] [PubMed] [Google Scholar]
- 185.Moriyama EH, et al. Bioluminescence imaging of the response of rat gliosarcoma to ALA-PpIX-mediated photodynamic therapy. Photochem Photobiol. 80:242–9. doi: 10.1562/2004-02-20-RA-088. [DOI] [PubMed] [Google Scholar]
- 186.Wang KKH, et al. Irradiation-induced enhancement of Pc 4 fluorescence and changes in light scattering are potential dosimeters for Pc 4-PDT. Photochemistry and Photobiology. 2007;83:1056–1062. doi: 10.1111/j.1751-1097.2007.00128.x. [DOI] [PubMed] [Google Scholar]
- 187.Wilson BC, et al. Implicit and explicit dosimetry in photodynamic therapy: a New paradigm. Lasers Med Sci. 1997;12:182–99. doi: 10.1007/BF02765099. [DOI] [PubMed] [Google Scholar]
- 188.Dysart J, et al. Calculation of Singlet Oxygen Dose from Photosensitizer Fluorescence and Photobleaching During mTHPC Photodynamic Therapy of MLL Cells. Photochemistry and 81. 2005:196–205. doi: 10.1562/2004-07-23-RA-244. [DOI] [PubMed] [Google Scholar]
- 189.Svaasand L, Potter W. The implications of photobleaching for photodynamic therapy. In: Henderson BW, Dougherty TJ, editors. Photodynamic therapy: basic principles and clinical applications1. Marcel Dekker; New York: 1992. pp. 369–385. [Google Scholar]
- 190.Chen W. Nanoparticle self-lighting photodynamic therapy for cancer treatment. J Biomed Nanotechnol. 2008;4:369–376. [Google Scholar]